Factors Influencing Men’s Attitudes toward HPV Vaccination in Males Included in the Chinese National Immunization Program
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Measures
2.3. Statistical Analyses
3. Results
Socioeconomic Characteristics and Influencing Factors
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munoz, N.; Bosch, F.X.; de Sanjose, S.; Herrero, R.; Castellsague, X.; Shah, K.V.; Snijders, P.J.; Meijer, C.J.; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N. Engl. J. Med. 2003, 348, 518–527. [Google Scholar] [CrossRef] [Green Version]
- Kombe Kombe, A.J.; Li, B.; Zahid, A.; Mengist, H.M.; Bounda, G.A.; Zhou, Y.; Jin, T. Epidemiology and Burden of Human Papillomavirus and Related Diseases, Molecular Pathogenesis, and Vaccine Evaluation. Front. Public Health 2020, 8, 552028. [Google Scholar] [CrossRef] [PubMed]
- Ortu, G.; Barret, A.S.; Danis, K.; Duchesne, L.; Levy-Bruhl, D.; Velter, A. Low vaccination coverage for human papillomavirus disease among young men who have sex with men, France, 2019. Eurosurveillance 2021, 26, 2001965. [Google Scholar] [CrossRef] [PubMed]
- Chesson, H.W.; Dunne, E.F.; Hariri, S.; Markowitz, L.E. The estimated lifetime probability of acquiring human papillomavirus in the United States. Sex. Transm. Dis. 2014, 41, 660–664. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Available online: https://www.iarc.fr/faq/latest-global-cancer-data-2020-qa/ (accessed on 7 April 2022).
- Jasrotia, R.; Dhanjal, D.S.; Bhardwaj, S.; Sharma, P.; Chopra, C.; Singh, R.; Kumar, A.; Mubayi, A.; Kumar, D.; Kumar, R.; et al. Nanotechnology based vaccines: Cervical cancer management and perspectives. J. Drug Deliv. Sci. Technol. 2022, 71, 103351. [Google Scholar] [CrossRef]
- Buchanan, T.R.; Graybill, W.S.; Pierce, J.Y. Morbidity and mortality of vulvar and vaginal cancers: Impact of 2-, 4-, and 9-valent HPV vaccines. Hum. Vaccin. Immunother. 2016, 12, 1352–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gargano, J.W.; Unger, E.R.; Liu, G.; Steinau, M.; Meites, E.; Dunne, E.; Markowitz, L.E. Prevalence of Genital Human Papillomavirus in Males, United States, 2013–2014. J. Infect. Dis. 2017, 215, 1070–1079. [Google Scholar] [CrossRef]
- Wei, F.; Yin, K.; Wu, X.; Lan, J.; Huang, S.; Sheng, W.; Zhao, J.; Su, Y.; Wang, Y.; Li, Y.; et al. Human papillomavirus prevalence and associated factors in women and men in south China: A population-based study. Emerg. Microbes Infect. 2016, 5, e119. [Google Scholar] [CrossRef]
- Olesen, T.B.; Sand, F.L.; Rasmussen, C.L.; Albieri, V.; Toft, B.G.; Norrild, B.; Munk, C.; Kjaer, S.K. Prevalence of human papillomavirus DNA and p16(INK4a) in penile cancer and penile intraepithelial neoplasia: A systematic review and meta-analysis. Lancet Oncol. 2019, 20, 145–158. [Google Scholar] [CrossRef]
- Lin, C.; Franceschi, S.; Clifford, G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: A systematic review and meta-analysis. Lancet Infect. Dis. 2018, 18, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Serrano, B.; Brotons, M.; Bosch, F.X.; Bruni, L. Epidemiology and burden of HPV-related disease. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 14–26. [Google Scholar] [CrossRef]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, W.; Pan, H.; Lin, B.; Zhong, X. Analysis of HPV Vaccination Willingness amongst HIV-Negative Men Who Have Sex with Men in China. Vaccines 2021, 9, 1069. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, L.E.; Schiller, J.T. Human Papillomavirus Vaccines. J. Infect. Dis. 2021, 224 (Suppl. S2), S367–S378. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.G.; Samakoses, R.; Block, S.L.; Lazcano-Ponce, E.; Restrepo, J.A.; Mehlsen, J.; Chatterjee, A.; Iversen, O.E.; Joshi, A.; Chu, J.L.; et al. 4-Valent Human Papillomavirus (4vHPV) Vaccine in Preadolescents and Adolescents After 10 Years. Pediatrics 2017, 140, e20163947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliano, A.R.; Palefsky, J.M.; Goldstone, S.; Moreira, E.D., Jr.; Penny, M.E.; Aranda, C.; Vardas, E.; Moi, H.; Jessen, H.; Hillman, R.; et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N. Engl. J. Med. 2011, 364, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Castellsague, X.; Giuliano, A.R.; Goldstone, S.; Guevara, A.; Mogensen, O.; Palefsky, J.M.; Group, T.; Shields, C.; Liu, K.; Maansson, R.; et al. Immunogenicity and safety of the 9-valent HPV vaccine in men. Vaccine 2015, 33, 6892–6901. [Google Scholar] [CrossRef]
- Van Damme, P.; Meijer, C.; Kieninger, D.; Schuyleman, A.; Thomas, S.; Luxembourg, A.; Baudin, M. A phase III clinical study to compare the immunogenicity and safety of the 9-valent and quadrivalent HPV vaccines in men. Vaccine 2016, 34, 4205–4212. [Google Scholar] [CrossRef] [Green Version]
- Drolet, M.; Benard, E.; Boily, M.C.; Ali, H.; Baandrup, L.; Bauer, H.; Beddows, S.; Brisson, J.; Brotherton, J.M.; Cummings, T.; et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: A systematic review and meta-analysis. Lancet Infect. Dis. 2015, 15, 565–580. [Google Scholar] [CrossRef] [Green Version]
- De Sanjose, S.; Serrano, B.; Tous, S.; Alejo, M.; Lloveras, B.; Quiros, B.; Clavero, O.; Vidal, A.; Ferrandiz-Pulido, C.; Pavon, M.A.; et al. Burden of Human Papillomavirus (HPV)-Related Cancers Attributable to HPVs 6/11/16/18/31/33/45/52 and 58. JNCI Cancer Spectr 2018, 2, pky045. [Google Scholar] [CrossRef] [Green Version]
- Markowitz, L.E.; Dunne, E.F.; Saraiya, M.; Chesson, H.W.; Curtis, C.R.; Gee, J.; Bocchini, J.A., Jr.; Unger, E.R. Human papillomavirus vaccination: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2014, 63, 1–30. [Google Scholar] [PubMed]
- Mennini, F.S.; Bonanni, P.; Bianic, F.; de Waure, C.; Baio, G.; Plazzotta, G.; Uhart, M.; Rinaldi, A.; Largeron, N. Cost-effectiveness analysis of the nine-valent HPV vaccine in Italy. Cost Eff. Resour. Alloc. 2017, 15, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Largeron, N.; Petry, K.U.; Jacob, J.; Bianic, F.; Anger, D.; Uhart, M. An estimate of the public health impact and cost-effectiveness of universal vaccination with a 9-valent HPV vaccine in Germany. Expert. Rev. Pharmacoecon. Outcomes Res. 2017, 17, 85–98. [Google Scholar] [CrossRef] [PubMed]
- De La Fuente, J.; Hernandez Aguado, J.J.; San Martin, M.; Ramirez Boix, P.; Cedillo Gomez, S.; Lopez, N. Estimating the epidemiological impact and cost-effectiveness profile of a nonavalent HPV vaccine in Spain. Hum. Vaccin. Immunother. 2019, 15, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Si, M.; Su, X.; Wang, W.; Zhang, X.; Gu, X.; Ma, L.; Li, J.; Zhang, S.; Ren, Z.; et al. Willingness to human papillomavirus (HPV) vaccination and influencing factors among male and female university students in China. J. Med. Virol. 2022, 94, 2776–2786. [Google Scholar] [CrossRef]
- Bruni, L.; Saura-Lazaro, A.; Montoliu, A.; Brotons, M.; Alemany, L.; Diallo, M.S.; Afsar, O.Z.; LaMontagne, D.S.; Mosina, L.; Contreras, M.; et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010–2019. Prev. Med. 2021, 144, 106399. [Google Scholar] [CrossRef]
- Deng, C.; Chen, X.; Liu, Y. Human papillomavirus vaccination: Coverage rate, knowledge, acceptance, and associated factors in college students in mainland China. Hum. Vaccin. Immunother. 2021, 17, 828–835. [Google Scholar] [CrossRef]
- Kwan, T.T.; Chan, K.K.; Yip, A.M.; Tam, K.F.; Cheung, A.N.; Young, P.M.; Lee, P.W.; Ngan, H.Y. Barriers and facilitators to human papillomavirus vaccination among Chinese adolescent girls in Hong Kong: A qualitative-quantitative study. Sex. Transm. Infect. 2008, 84, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Colombara, D.V.; Wang, S.M. The impact of HPV vaccination delays in China: Lessons from HBV control programs. Vaccine 2013, 31, 4057–4059. [Google Scholar] [CrossRef] [Green Version]
- Viscidi, R.P.; Schiffman, M.; Hildesheim, A.; Herrero, R.; Castle, P.E.; Bratti, M.C.; Rodriguez, A.C.; Sherman, M.E.; Wang, S.; Clayman, B.; et al. Seroreactivity to human papillomavirus (HPV) types 16, 18, or 31 and risk of subsequent HPV infection: Results from a population-based study in Costa Rica. Cancer Epidemiol. Biomark. Prev. 2004, 13, 324–327. [Google Scholar] [CrossRef] [Green Version]
- Olsson, S.E.; Kjaer, S.K.; Sigurdsson, K.; Iversen, O.E.; Hernandez-Avila, M.; Wheeler, C.M.; Perez, G.; Brown, D.R.; Koutsky, L.A.; Tay, E.H.; et al. Evaluation of quadrivalent HPV 6/11/16/18 vaccine efficacy against cervical and anogenital disease in subjects with serological evidence of prior vaccine type HPV infection. Hum. Vaccin. 2009, 5, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Huh, W.K.; Joura, E.A.; Giuliano, A.R.; Iversen, O.E.; de Andrade, R.P.; Ault, K.A.; Bartholomew, D.; Cestero, R.M.; Fedrizzi, E.N.; Hirschberg, A.L.; et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: A randomised, double-blind trial. Lancet 2017, 390, 2143–2159. [Google Scholar] [CrossRef]
- Castellsague, X.; Munoz, N.; Pitisuttithum, P.; Ferris, D.; Monsonego, J.; Ault, K.; Luna, J.; Myers, E.; Mallary, S.; Bautista, O.M.; et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24-45 years of age. Br. J. Cancer 2011, 105, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Mascaro, V.; Pileggi, C.; Curra, A.; Bianco, A.; Pavia, M. HPV vaccination coverage and willingness to be vaccinated among 18-30year-old students in Italy. Vaccine 2019, 37, 3310–3316. [Google Scholar] [CrossRef] [PubMed]
- Henrikson, N.B.; Tuzzio, L.; Gilkey, M.B.; McRee, A.L. “You’re never really off time”: Healthcare providers’ interpretations of optimal timing for HPV vaccination. Prev. Med. Rep. 2016, 4, 94–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, H.; He, W.; Lin, B.; Zhong, X. Factors influencing HPV vaccination willingness among men who have sex with men in China: A structural equation modeling analysis. Hum. Vaccin. Immunother. 2022, 18, 2038504. [Google Scholar] [CrossRef]
- Nadarzynski, T.; Smith, H.; Richardson, D.; Jones, C.J.; Llewellyn, C.D. Human papillomavirus and vaccine-related perceptions among men who have sex with men: A systematic review. Sex. Transm. Infect. 2014, 90, 515–523. [Google Scholar] [CrossRef]
- Chelimo, C.; Wouldes, T.A.; Cameron, L.D.; Elwood, J.M. Risk factors for and prevention of human papillomaviruses (HPV), genital warts and cervical cancer. J. Infect. 2013, 66, 207–217. [Google Scholar] [CrossRef]
- Linertová, R.; Guirado-Fuentes, C.; Mar Medina, J.; Imaz-Iglesia, I.; Rodríguez-Rodríguez, L.; Carmona-Rodríguez, M. Cost-effectiveness of extending the HPV vaccination to boys: A systematic review. J. Epidemiol. Community Health 2021, 75, 910–916. [Google Scholar] [CrossRef]
- Datta, S.; Pink, J.; Medley, G.F.; Petrou, S.; Staniszewska, S.; Underwood, M.; Sonnenberg, P.; Keeling, M.J. Assessing the cost-effectiveness of HPV vaccination strategies for adolescent girls and boys in the UK. BMC Infect. Dis. 2019, 19, 552. [Google Scholar] [CrossRef] [Green Version]
- Moreira, E.D., Jr.; Block, S.L.; Ferris, D.; Giuliano, A.R.; Iversen, O.E.; Joura, E.A.; Kosalaraksa, P.; Schilling, A.; Van Damme, P.; Bornstein, J.; et al. Safety Profile of the 9-Valent HPV Vaccine: A Combined Analysis of 7 Phase III Clinical Trials. Pediatrics 2016, 138, e20154387. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.R.; Hashibe, M.; Bodson, J.; Gren, L.H.; Taylor, B.A.; Greenwood, J.; Jackson, B.R.; She, R.; Egger, M.J.; Kepka, D. Factors related to HPV vaccine uptake and 3-dose completion among women in a low vaccination region of the USA: An observational study. BMC Womens Health 2016, 16, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce Campbell, C.M.; Lin, H.Y.; Fulp, W.; Papenfuss, M.R.; Salmeron, J.J.; Quiterio, M.M.; Lazcano-Ponce, E.; Villa, L.L.; Giuliano, A.R. Consistent condom use reduces the genital human papillomavirus burden among high-risk men: The HPV infection in men study. J. Infect. Dis. 2013, 208, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Burd, E.M.; Dean, C.L. Human Papillomavirus. Microbiol. Spectr. 2016, 4, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Aung, E.T.; Fairley, C.K.; Tabrizi, S.N.; Danielewski, J.A.; Ong, J.J.; Chen, M.Y.; Bradshaw, C.S.; Chow, E.P.F. Detection of human papillomavirus in urine among heterosexual men in relation to location of genital warts and circumcision status. Sex. Transm. Infect. 2018, 94, 222–225. [Google Scholar] [CrossRef]
- Koene, F.; Wolffs, P.; Brink, A.; Dukers-Muijrers, N.; Quint, W.; Bruggeman, C.; Hoebe, C. Comparison of urine samples and penile swabs for detection of human papillomavirus in HIV-negative Dutch men. Sex. Transm. Infect. 2016, 92, 467–469. [Google Scholar] [CrossRef]
- Choi, E.P.H.; Wong, J.Y.H.; Lau, A.Y.Y.; Fong, D.Y.T. Gender and Sexual Orientation Differences in Human Papillomavirus (HPV) Vaccine Uptake among Chinese Young Adults. Int. J. Environ. Res. Public Health 2018, 15, 1099. [Google Scholar] [CrossRef] [Green Version]
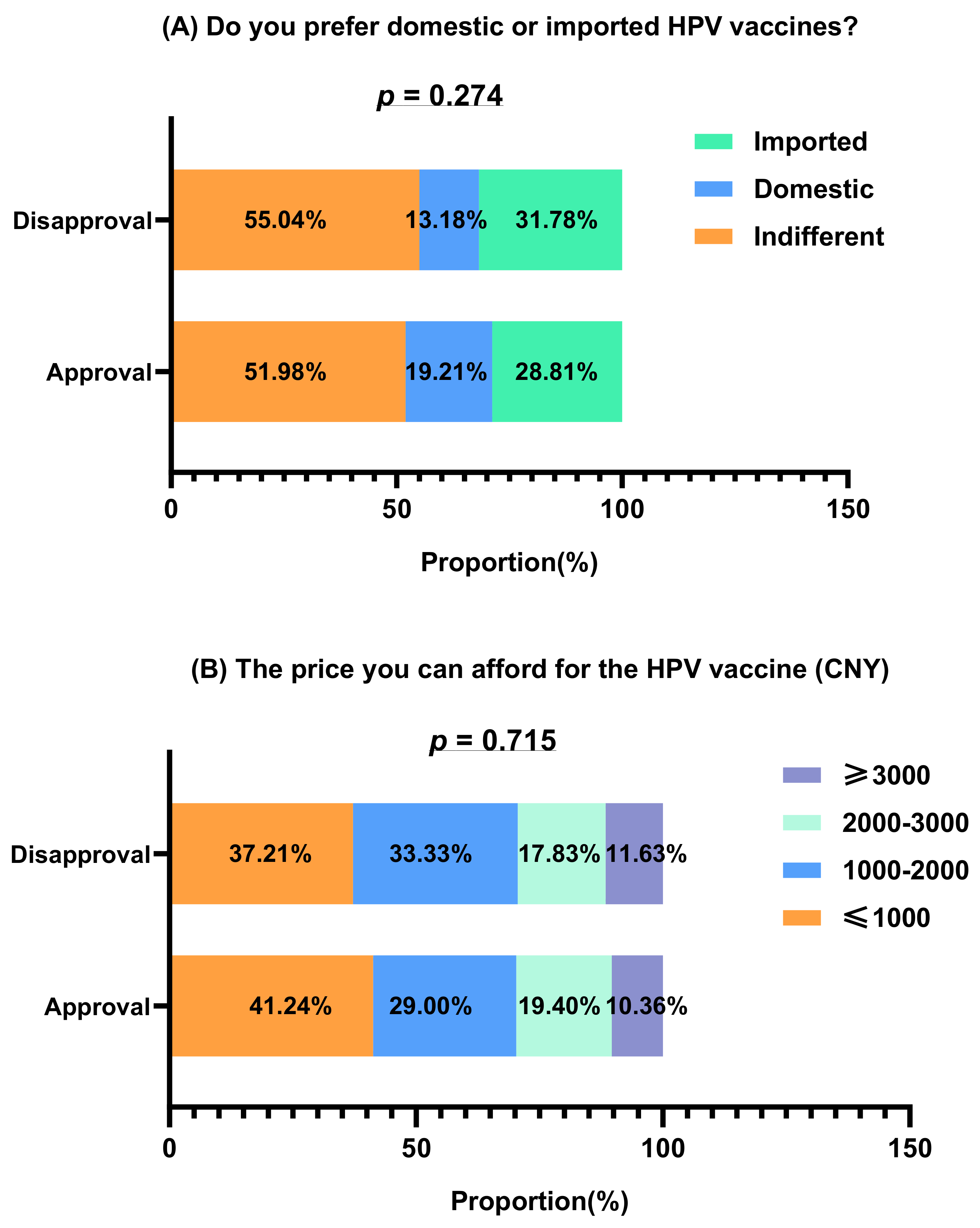
| Variable | Total (n = 660) | Approval (n = 531) | Disapproval (n = 129) | p |
|---|---|---|---|---|
| Age | 28.04 ± 5.27 | 27.94 ± 5.32 | 28.43 ± 5.05 | 0.878 |
| Ethnicity | 0.451 | |||
| Han | 623 (94.39%) | 503 (94.73%) | 120 (93.02%) | |
| Other | 37 (5.61%) | 28 (5.27%) | 9 (6.98%) | |
| Household registration | 0.032 | |||
| Urban | 404 (61.21%) | 314 (59.13%) | 90 (69.77%) | |
| Rural | 256 (38.79%) | 217 (40.87%) | 39 (30.23%) | |
| Education level | 0.651 | |||
| Junior college and below | 171 (25.91%) | 140 (26.37%) | 31 (24.03%) | |
| Undergraduate | 320 (48.48%) | 259 (48.78%) | 61 (47.29%) | |
| Postgraduate and above | 169 (25.61%) | 132 (20.72%) | 37 (28.68%) | |
| Occupation | 0.434 | |||
| Public institutions | 307 (46.52%) | 258 (48.59%) | 49 (37.98%) | |
| Private enterprise | 81 (12.27%) | 70(13.18%) | 11 (8.53%) | |
| Student | 150 (22.73%) | 134 (25.24%) | 16 (12.40%) | |
| Other | 83 (12.58%) | 69 (12.99%) | 14 (10.85%) | |
| Marriage | 0.027 | |||
| Unmarried | 468 (70.91%) | 382 (71.94%) | 86 (66.67%) | |
| Married | 192 (29.09%) | 149 (28.06%) | 43 (33.33%) | |
| Annual income | 0.014 | |||
| CNY ≤ 100,000 | 363 (55.00%) | 305 (57.44%) | 58 (44.96%) | |
| CNY 100,000–200,000 | 234 (35.45%) | 182 (34.27%) | 52 (40.31%) | |
| CNY ≥ 200,000 | 63 (9.55%) | 44 (8.29%) | 19 (14.73%) | |
| History of HPV infection | 0.000 | |||
| No | 390 (59.09%) | 295 (55.56%) | 95 (73.64%) | |
| Yes | 270 (40.91%) | 236 (44.44%) | 34 (26.36%) |
| Variable | Total (n = 660) | Approval (n = 531) | Disapproval (n = 129) | p |
|---|---|---|---|---|
| Number of sexual partners | 0.132 | |||
| 0 | 179 (27.12%) | 152 (28.63%) | 27 (20.93%) | |
| 1–2 | 466 (70.61%) | 367 (69.11%) | 99 (76.74%) | |
| 3–6 | 9 (1.36%) | 7 (1.32%) | 2 (1.55%) | |
| >6 | 6 (0.91%) | 5 (0.94%) | 1 (0.78%) | |
| Sexual partner in the last 6 months | 0.047 | |||
| No | 179 (27.12%) | 152 (28.63%) | 27 (20.93%) | |
| Yes | 481 (72.88%) | 379 (71.37%) | 102 (79.07%) | |
| Frequency of condom use | 0.160 | |||
| Frequently | 367 (55.61%) | 295 (55.56%) | 72 (55.81%) | |
| Occasionally | 83 (12.58%) | 61 (11.49%) | 22 (17.05%) | |
| Never | 210 (31.82%) | 175 (32.96%) | 35 (27.13%) | |
| STD examination | 0.791 | |||
| No | 487 (73.79%) | 393(74.01%) | 94 (72.87%) | |
| Yes | 173 (26.21%) | 138 (25.99%) | 35 (27.13%) | |
| HIV testing | 0.876 | |||
| No | 431 (65.30%) | 346 (65.16%) | 85 (65.89%) | |
| Yes | 229 (34.70%) | 185 (34.84%) | 44 (34.11%) |
| Variable | Total (n = 660) | Approval (n = 531) | Disapproval (n = 129) | p |
|---|---|---|---|---|
| Have you ever heard of HPV? | 0.131 | |||
| No | 55 (8.33%) | 40 (7.53%) | 15 (11.63%) | |
| Yes | 605 (91.67%) | 491 (92.47%) | 114 (88.37%) | |
| Both men and women can be infected with HPV | 0.819 | |||
| No | 78 (11.82%) | 62 (11.68%) | 16 (12.40%) | |
| Yes | 582 (88.18%) | 469 (88.32%) | 113 (87.60%) | |
| Diseases associated with HPV infection (scores) | <0.001 | |||
| 0–1 | 84 (12.73%) | 54 (10.17%) | 30 (23.26%) | |
| 2–3 | 335 (50.76%) | 275 (51.79%) | 60 (46.51%) | |
| 4 | 241 (36.52%) | 202 (38.04%) | 39 (30.23%) | |
| High-risk factors of HPV (scores) | <0.001 | |||
| 1–2 | 146 (22.12%) | 95 (17.89%) | 51 (39.53%) | |
| 3 | 192 (29.09%) | 155 (29.19%) | 37 (28.68%) | |
| 4–6 | 322 (48.79%) | 281 (52.92%) | 41 (31.78%) | |
| Transmission routes of HPV (scores) | 0.003 | |||
| 1 | 153 (23.18%) | 114 (21.47%) | 39 (30.23%) | |
| 2 | 343 (51.97%) | 271 (51.04%) | 72 (55.81%) | |
| 3 | 164 (24.85%) | 146 (27.50%) | 18 (13.95%) | |
| Preventive methods of HPV infection F (scores) | 0.003 | |||
| 1–2 | 76 (11.52%) | 53 (9.98%) | 23 (17.83%) | |
| 3–4 | 242 (36.67%) | 187 (35.22%) | 55 (42.64%) | |
| 5–6 | 342 (51.82%) | 291 (54.80%) | 51 (39.53%) |
| Variable | Approval (n = 531) | Disapproval (n = 129) | Total (n = 660) | p |
|---|---|---|---|---|
| Can the HPV vaccine prevent malignancies? | 0.761 | |||
| No | 41 (7.72%) | 11 (8.53%) | 41 (7.88%) | |
| Yes | 490 (92.28%) | 118 (91.47%) | 490 (92.12%) | |
| When should the HPV vaccine be given? | 0.005 | |||
| Unknown | 103(19.40%) | 41 (31.78%) | 144(21.82%) | |
| After first sexual activity | 14 (2.64%) | 1 (0.78%) | 15 (2.27%) | |
| Before first sexual activity | 414 (77.97%) | 87 (67.44%) | 501 (75.91%) | |
| Can men be vaccinated? | 0.521 | |||
| No | 28 (5.27%) | 9 (6.98%) | 37 (5.61%) | |
| Yes | 503 (94.73%) | 120 (93.02%) | 623 (94.39%) | |
| Does the HPV vaccine protect against all types of HPV infection? | 0.287 | |||
| Unknown | 83 (15.63%) | 26 (20.16%) | 109 (16.52%) | |
| Yes | 73 (13.75%) | 21 (16.28%) | 94 (14.24%) | |
| No | 375 (70.62%) | 82 (63.57%) | 457 (69.24%) |
| Factors | β-Coefficient | OR (95%CI) | p |
|---|---|---|---|
| Household registration | 0.142 | ||
| Urban | reference | 1 | |
| Rural | 0.34 | 1.40 (0.89–2.20) | |
| Education level | 0.189 | ||
| Junior college and below | reference | 1 | |
| Undergraduate | −0.02 | 0.98 (0.57–1.70) | 0.950 |
| Postgraduate and above | −0.46 | 0.63 (0.34–1.18) | 0.149 |
| Marriage status | 0.331 | ||
| Unmarried | reference | 1 | |
| Married | 0.24 | 1.27 (0.78–2.07) | |
| Annual income (CNY) | 0.007 | ||
| ≤100,000 | reference | 1 | |
| 100–200,000 | −0.47 | 0.63 (0.39–1.00) | 0.051 |
| ≥200,000 | −1.08 | 0.34 (0.17–0.68) | 0.002 |
| History of HPV infection | 0.008 | ||
| No | reference | 1 | |
| Yes | 0.61 | 1.84 (1.17–2.90) | |
| Sexual partner in the last 6 months | 0.233 | ||
| No | reference | 1 | |
| Yes | −0.32 | 0.73 (0.43-1.23) | |
| Diseases associated with HPV infection | 0.12 | 1.13 (0.91–1.39) | 0.269 |
| High-risk factors of HPV | 0.37 | 1.44 (1.17–1.79) | 0.001 |
| Transmission routes of HPV | 0.10 | 1.10 (0.78–1.56) | 0.574 |
| Preventive methods of HPV infection | −0.09 | 0.91 (0.75–1.11) | 0.363 |
| Timing of vaccine administration | 0.049 | ||
| Unknown | reference | 1 | |
| After first sex | 1.71 | 5.51 (0.67–45.10) | 0.112 |
| Before first sex | 0.56 | 1.75 (1.04–2.95) | 0.036 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, Y.; Shao, H.; Zhang, T.; Pu, J.; Tang, C. Factors Influencing Men’s Attitudes toward HPV Vaccination in Males Included in the Chinese National Immunization Program. Vaccines 2022, 10, 1054. https://doi.org/10.3390/vaccines10071054
Tao Y, Shao H, Zhang T, Pu J, Tang C. Factors Influencing Men’s Attitudes toward HPV Vaccination in Males Included in the Chinese National Immunization Program. Vaccines. 2022; 10(7):1054. https://doi.org/10.3390/vaccines10071054
Chicago/Turabian StyleTao, Yi, Huarui Shao, Ting Zhang, Junliang Pu, and Chengyong Tang. 2022. "Factors Influencing Men’s Attitudes toward HPV Vaccination in Males Included in the Chinese National Immunization Program" Vaccines 10, no. 7: 1054. https://doi.org/10.3390/vaccines10071054
APA StyleTao, Y., Shao, H., Zhang, T., Pu, J., & Tang, C. (2022). Factors Influencing Men’s Attitudes toward HPV Vaccination in Males Included in the Chinese National Immunization Program. Vaccines, 10(7), 1054. https://doi.org/10.3390/vaccines10071054





