Cytokine and Lymphocyte Profiles in Dogs with Atopic Dermatitis after Allergen-Specific Immunotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. cAD Diagnosis and Sample Collection
2.3. Allergen-Specific Immunotherapy (ASIT)
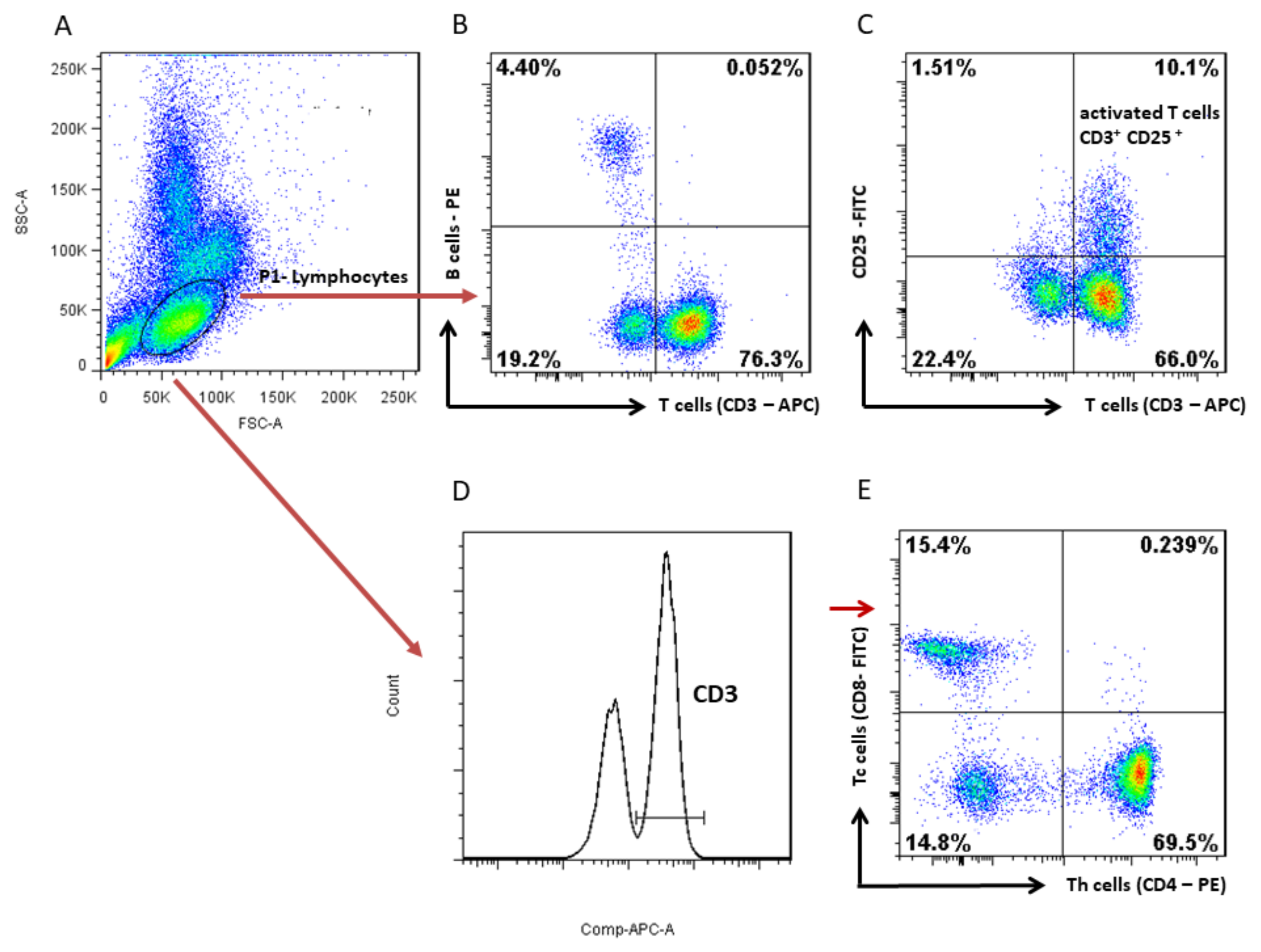
2.4. Flow Cytometric Analysis of Peripheral Blood Mononuclear Cells (PBMC)
2.4.1. Analysis of Lymphocyte Subpopulations with Flow Cytometry
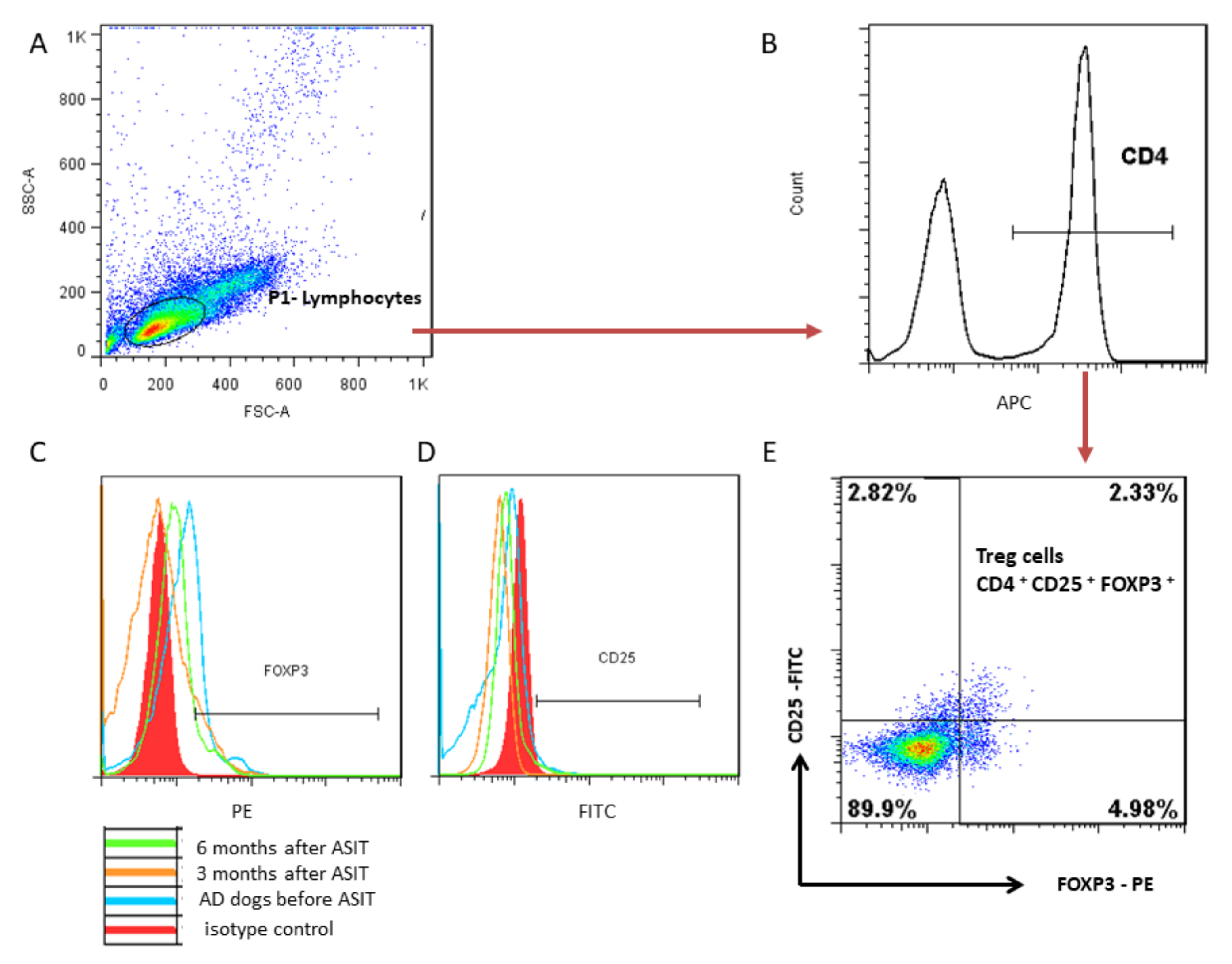
2.4.2. Treg Cell Analysis with Flow Cytometry
2.5. Measurement of Cytokine Concentration in Plasma by ELISA
2.6. Statistical Analysis
3. Results
3.1. Patients
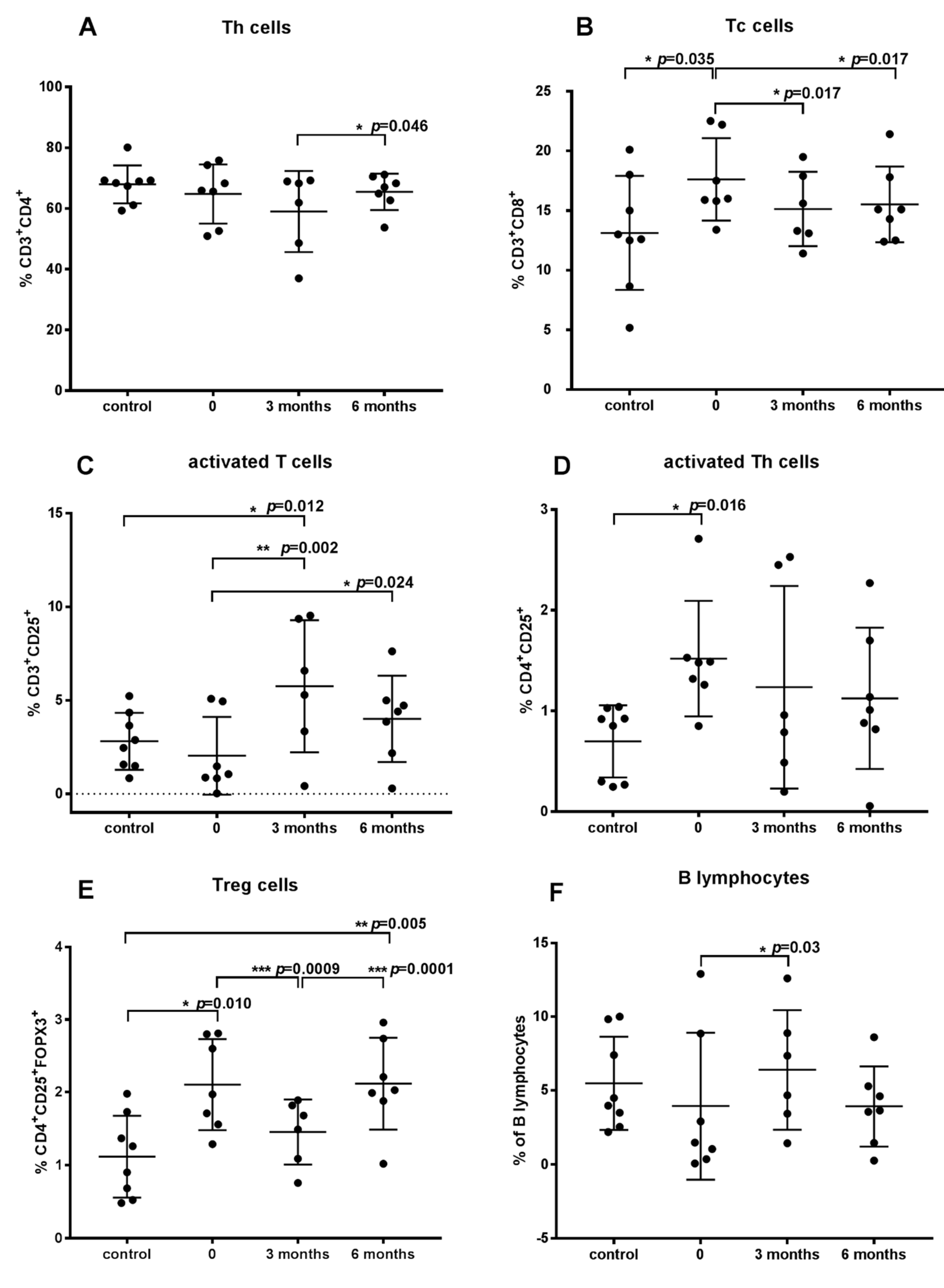
3.2. Effect of ASIT Treatment on Percentages of Lymphocyte Subpopulations and on Level of Cytokines in AD Dogs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Halliwell, R. Revised nomenclature for veterinary allergy. Vet. Immunol. Immunopathol. 2006, 114, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.E.; DeBoer, D.J. The ACVD task force on canine atopic dermatitis (XIV): Clinical manifestations of canine atopic dermatitis. Vet. Immunol. Immunopathol. 2001, 81, 255–269. [Google Scholar] [CrossRef]
- Bensignor, E.; Marignac, G.; Crosaz, O.; Cavana, P. Pruritus in dogs. Vet. Dermatol. 2013, 24, 292. [Google Scholar] [CrossRef]
- Hensel, P.; Santoro, D.; Favrot, C.; Hill, P.; Griffin, C. Canine atopic dermatitis: Detailed guidelines for diagnosis and allergen identification. BMC Vet. Res. 2015, 11, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivry, T.; Banovic, F. Treatment of canine atopic dermatitis: Time to revise our strategy? Vet. Dermatol. 2019, 30, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Favrot, C. Clinical signs and diagnosis of canine atopic dermatitis. In Proceedings of the 3rd Congresso Latinoamericano de Dermatologia Veterinaria, Buenos Aires, Argentina, 26–27 November 2015. [Google Scholar]
- Bieber, T. Atopic dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, T.J.; Knight, P.A.; McAleese, S.M.; Lamb, J.R.; Hill, P.B. Expression of Th1, Th2 and immunosuppressive cytokine gene transcripts in canine atopic dermatitis. Clin. Exp. Allergy 2002, 32, 789–795. [Google Scholar] [CrossRef]
- Schlotter, Y.M.; Rutten, V.P.; Riemers, F.M.; Knol, E.F.; Willemse, T. Lesional skin in atopic dogs shows a mixed Type-1 and Type-2 immune responsiveness. Vet. Immunol. Immunopathol. 2011, 143, 20–26. [Google Scholar] [CrossRef]
- Olivry, T.; Naydan, D.K.; Moore, P.F. Characterization of the cutaneous inflammatory infiltrate in canine atopic dermatitis. Am. J. Dermatopathol. 1997, 19, 477–486. [Google Scholar] [CrossRef]
- Sinke, J.D.; Thepen, T.; Bihari, I.C.; Rutten, V.P.; Willemse, T. Immunophenotyping of skin infiltrating T-cell subsets in dogs with atopic dermatitis. Vet. Immunol. Immunopathol. 1997, 57, 13–23. [Google Scholar] [CrossRef]
- Hennino, A.; Vocanson, M.; Toussaint, Y.; Rodet, K.; Benetière, J.; Schmitt, A.M.; Aries, M.F.; Bérard, F.; Rozières, A.; Nicolas, J.F. Skin-infiltrating CD8+ T cells initiate atopic dermatitis lesions. J. Immunol. 2007, 178, 5571–5577. [Google Scholar] [CrossRef] [Green Version]
- Hennino, A.; Jean-Decoster, C.; Giordano-Labadie, F.; Debeer, S.; Vanbervliet, B.; Rozières, A.; Schmitt, A.M.; Nicolas, J.F. CD8+ T cells are recruited early to allergen exposure sites in atopy patch test reactions in human atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 1064–1067. [Google Scholar] [CrossRef]
- Jassies-van der Lee, A.; Rutten, V.P.; Bruijn, J.; Willemse, T.; Broere, F. CD4+ and CD8+ skin-associated T lymphocytes in canine atopic dermatitis produce interleukin-13, interleukin-22 and interferon-γ and contain a CD25+FoxP3+ subset. Vet. Dermatol. 2014, 25, 456–472. [Google Scholar] [CrossRef]
- Majewska, A.; Gajewska, M.; Dembele, K.; Maciejewski, H.; Prostek, A.; Jank, M. Lymphocytic, cytokine and transcriptomic profiles in peripheral blood of dogs with atopic dermatitis. BMC Vet. Res. 2016, 12, 174. [Google Scholar] [CrossRef] [Green Version]
- Martín-Orozco, E.; Norte-Muñoz, M.; Martínez-García, J. Regulatory T Cells in Allergy and Asthma. Front. Pediatr. 2017, 23, 117. [Google Scholar] [CrossRef]
- Beccati, M.; Martini, V.; Comazzi, S.; Fanton, N.; Cornegliani, L. Lymphocyte subpopulations and Treg cells in dogs with atopic dermatitis receiving ciclosporin therapy: A prospective study. Vet. Dermatol. 2016, 27, 17–25. [Google Scholar] [CrossRef]
- Hauck, V.; Hügli, P.; Meli, M.L.; Rostaher, A.; Fischer, N.; Hofmann-Lehmann, R.; Favrot, C. Increased numbers of FoxP3-expressing CD4+ CD25+ regulatory T cells in peripheral blood from dogs with atopic dermatitis and its correlation with disease severity. Vet. Dermatol. 2016, 27, 26-e9. [Google Scholar] [CrossRef] [Green Version]
- Olivry, T.; Foster, A.P.; Mueller, R.S.; McEwan, N.A.; Chesney, C.; Williams, H.C. Interventions for atopic dermatitis in dogs: A systematic review of randomized controlled trials. Vet. Dermatol. 2010, 21, 4–22. [Google Scholar] [CrossRef]
- Gedon, N.K.; Müller, R.S. Atopic dermatitis in cats and dogs: A difficult disease for animals and owners. Clin. Transl. Allergy 2018, 8, 41. [Google Scholar] [CrossRef]
- Fischer, N.M.; Müller, R.S. Allergen Specific Immunotherapy in Canine Atopic Dermatitis: An Update. Curr. Derm. Rep. 2019, 8, 297–302. [Google Scholar] [CrossRef]
- Frew, A.J. Allergen immunotherapy. J. Allergy Clin. Immunol. 2010, 125 (Suppl. S2), S306–S313. [Google Scholar] [CrossRef]
- Canadian Society of Allergy and Clinical Immunology. Immunotherapy Manual; Canadian Society of Allergy and Clinical Immunology: Orleans, ON, Canada, 2016. [Google Scholar]
- Moote, W.; Kim, H.; Ellis, A.K. Allergen-specific immunotherapy. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. S2), 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akdis, C.A.; Akdis, M. Mechanisms of allergen-specific immunotherapy. J. Allergy Clin. Immunol. 2011, 127, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immunotherapy: Multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 2014, 133, 621–631. [Google Scholar] [CrossRef]
- Jutel, M.; Kosowska, A.; Smolinska, S. Allergen Immunotherapy: Past, Present, and Future. Allergy Asthma Immunol. Res. 2016, 8, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirvent, S.; Soria, I.; Cirauqui, C.; Cases, B.; Manzano, A.I.; Diez-Rivero, C.M.; Reche, P.A.; López-Relaño, J.; Martínez-Naves, E.; Cañada, F.J.; et al. Novel vaccines targeting dendritic cells by coupling allergoids to nonoxidized mannan enhance allergen uptake and induce function-al regulatory T cells through programmed death ligand 1. J. Allergy Clin. Immunol. 2016, 138, 558–567. [Google Scholar] [CrossRef] [Green Version]
- Van de Veen, W.; Wirz, O.F.; Globinska, A.; Akdis, M. Novel mechanisms in immune tolerance to allergens during natural allergen exposure and allergen-specific immunotherapy. Curr. Opin. Immunol. 2017, 48, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.P.; Serra-Caetano, A.; Pedro, E.; Melo, A.; Caramalho, I.; Barbosa, M.P.; Victorino, R.M.M.; Sousa, A.E. Expansion of FOXP3+ regulatory CD4 T cells upon exposure to hymenoptera venom during the beekeeping season. Allergy 2019, 74, 1182–1184. [Google Scholar] [CrossRef]
- Keppel, K.E.; Campbell, K.L.; Zuckermann, F.A.; Greeley, E.A.; Schaeffer, D.J.; Husmann, R.J. Quantitation of canine regulatory T cell populations, serum interleukin-10 and allergen-specific IgE concentrations in healthy control dogs and canine atopic dermatitis patients receiving allergen-specific immunotherapy. Vet. Immunol. Immunopathol. 2008, 123, 337–344. [Google Scholar] [CrossRef]
- Martini, F.; Rostaher, A.; Favrot, C.; Fischer, N. Interleukin 10 and transforming growth factor-beta 1 plasma levels in atopic dogs before and during immunotherapy. Vet. Rec. 2022, 190, e1270. [Google Scholar] [CrossRef]
- Akdis, C.A.; Akdis, M. Mechanisms of immune tolerance to allergens: Role of IL-10 and Tregs. J. Clin. Investig. 2014, 124, 4678–4680. [Google Scholar] [CrossRef] [Green Version]
- Kucuksezer, U.C.; Ozdemir, C.; Cevhertas, L.; Ogulur, I.; Akdis, M.; Akdis, C.A. Mechanisms of allergen-specific immuno-therapy and allergen tolerance. Allergol. Int. 2020, 69, 549–560. [Google Scholar] [CrossRef]
- Shida, M.; Kadoya, M.; Park, S.J.; Nishifuji, K.; Momoi, Y.; Iwasaki, T. Allergen-specific immunotherapy induces Th1 shift in dogs with atopic dermatitis. Vet. Immunol. Immunopathol. 2004, 102, 19–31. [Google Scholar] [CrossRef]
- Létourneau, S.; Krieg, C.; Pantaleo, G.; Boyman, O. IL-2- and CD25-dependent immunoregulatory mechanisms in the ho-meostasis of T-cell subsets. J. Allergy Clin. Immunol. 2009, 123, 758–762. [Google Scholar] [CrossRef]
- Almeida, A.R.; Borghans, J.A.; Freitas, A.A. T cell homeostasis: Thymus regeneration and peripheral T cell restoration in mice with a reduced fraction of competent precursors. J. Exp. Med. 2001, 194, 591–599. [Google Scholar] [CrossRef] [Green Version]
- Curotto de Lafaille, M.A.; Lino, A.C.; Kutchukhidze, N.; Lafaille, J.J. CD25−T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J. Immunol. 2004, 173, 7259–7268. [Google Scholar] [CrossRef] [Green Version]
- Zorn, E.; Nelson, E.A.; Mohseni, M.; Porcheray, F.; Kim, H.; Litsa, D.; Bellucci, R.; Raderschall, E.; Canning, C.; Soiffer, R.J.; et al. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood 2006, 108, 1571–1579. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.P.; Kim, Y.S.; Kim, O.Y.; Kim, Y.M.; Jeon, S.G.; Roh, T.Y.; Park, J.S.; Gho, Y.S.; Kim, Y.K. TNF-alpha is a key mediator in the development of Th2 cell response to inhaled allergens induced by a viral PAMP double-stranded RNA. Allergy 2012, 67, 1138–1148. [Google Scholar] [CrossRef]
- Ahmad, S.; Azid, N.A.; Boer, J.C.; Lim, J.; Chen, X.; Plebanski, M.; Mohamud, R. The Key Role of TNF-TNFR2 Interactions in the Modulation of Allergic Inflammation: A Review. Front. Immunol. 2018, 9, 2572. [Google Scholar] [CrossRef]
- Verde, M.T.; Villanueva-Saz, S.; Loste, A.; Marteles, D.; Pereboom, D.; Conde, T.; Fernández, A. Comparison of circulating CD4+, CD8+ lymphocytes and cytokine profiles between dogs with atopic dermatitis and healthy dogs. Res. Vet. Sci. 2022, 145, 13–20. [Google Scholar] [CrossRef]

| Breeds | Age Years/ Months | Clinical Symptoms | 1st Stage of Immunotherapy after 13 Weeks | 2nd Stage of Immunotherapy after 29 Weeks | Commentary |
|---|---|---|---|---|---|
| Labrador retriever | 4/5 | Dermatitis on the bridge of the nose; severe seasonal pruritus; no other skin lesions | No improvement—the dog was still itchy and rubbing his face | Significant improvement was observed at the end of this phase; no additional treatment was needed | No antipruritic drugs were used during Phases I and II of immunotherapy |
| American Staffordshire terrier | 2/2 | Recurrent papular dermatitis in the lumbosacral area; erythema and papules in the groin region and on the lateral surface of hind limbs; severe pruritus; good response to steroid treatment | Hypersensitivity reactionafter each dose of the allergen extract, which showed follicular dermatitis, erythema, severe pruritus | Similar reactions as in Phase I | The duration of whole immunotherapy was prolonged due to hypersensitivity reactions that occurred after each allergen dose and required treatment. The interval between allergen doses was extended by the time of each treatment |
| American Staffordshire terrier | 6/5 | Erythema in the groin area and on the medial surface of front limbs | Continuation of immunotherapy; clinical examination of the patient was not possible during this time; lack of feedback from the owner | There was significant improvement after discontinuation of immunotherapy | No antipruritic drugs were used during the whole immunotherapy |
| Labrador retriever | 2 | Severe pruritus; seborrheic interdigital dermatitis (front limbs) | Persisting pruritus and seborrheic dermatitis | Initial improvement was observed—the dog stopped licking his paws excessively and was less pruritic. Good response to immunotherapy—the dog was no longer pruritic, even after discontinuation of immunotherapy | No antipruritic drugs were used during the whole immunotherapy |
| Small Münsterländer | 3 | Recurrent pododermatitis of the front limbs (interdigital spaces); recurrent ceruminous otitis; erythema of the concave pinna surface in both ears; no pustules or papules on the skin | No changes in the clinical picture were observed (no improvement or exacerbation of clinical signs) | There was significant reduction in pruritus; no skin changes were noted after the last allergen dose. Good response to immunotherapy | No antipruritic drugs were used during the whole immunotherapy |
| Golden retriever | 5 | Erythema and crusts on the lower abdomen; chronic ceruminous otitis; epidermal collarettes; severe pruritus | Epidermal collarettes in the groin area as well as ceruminous otitis were still observed | Persisting epidermal collarettes; recurrent otitis externa; seborrheic dermatitis. However, there was significant reduction in pruritus after discontinuation of immunotherapy | There was a need to use steroids (dexamethasone injections) and/or antibiotics (cefalexin) to treat pruritic skin lesions during both stages of immunotherapy |
| Golden retriever | 3/6 | Erythema of interdigital spaces and dorsal surface of front limbs; lower abdomen erythema; pustules on the skin of right thigh; otitis externa (only right ear) | Initial exacerbation of pruritus and dermatitis. Spontaneous resolution of clinical signs was observed later without any treatment needed | Occasional erythema of the inner surface of front limbs was observed. Significant improvement in dog’s general skin condition | The dog was less pruritic and did not need any additional antipruritic treatment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majewska, A.; Dembele, K.; Dziendzikowska, K.; Prostek, A.; Gajewska, M. Cytokine and Lymphocyte Profiles in Dogs with Atopic Dermatitis after Allergen-Specific Immunotherapy. Vaccines 2022, 10, 1037. https://doi.org/10.3390/vaccines10071037
Majewska A, Dembele K, Dziendzikowska K, Prostek A, Gajewska M. Cytokine and Lymphocyte Profiles in Dogs with Atopic Dermatitis after Allergen-Specific Immunotherapy. Vaccines. 2022; 10(7):1037. https://doi.org/10.3390/vaccines10071037
Chicago/Turabian StyleMajewska, Alicja, Kourou Dembele, Katarzyna Dziendzikowska, Adam Prostek, and Małgorzata Gajewska. 2022. "Cytokine and Lymphocyte Profiles in Dogs with Atopic Dermatitis after Allergen-Specific Immunotherapy" Vaccines 10, no. 7: 1037. https://doi.org/10.3390/vaccines10071037
APA StyleMajewska, A., Dembele, K., Dziendzikowska, K., Prostek, A., & Gajewska, M. (2022). Cytokine and Lymphocyte Profiles in Dogs with Atopic Dermatitis after Allergen-Specific Immunotherapy. Vaccines, 10(7), 1037. https://doi.org/10.3390/vaccines10071037






