COVID-19 Vaccination and Rates of Infections, Hospitalizations, ICU Admissions, and Deaths in the European Economic Area during Autumn 2021 Wave of SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
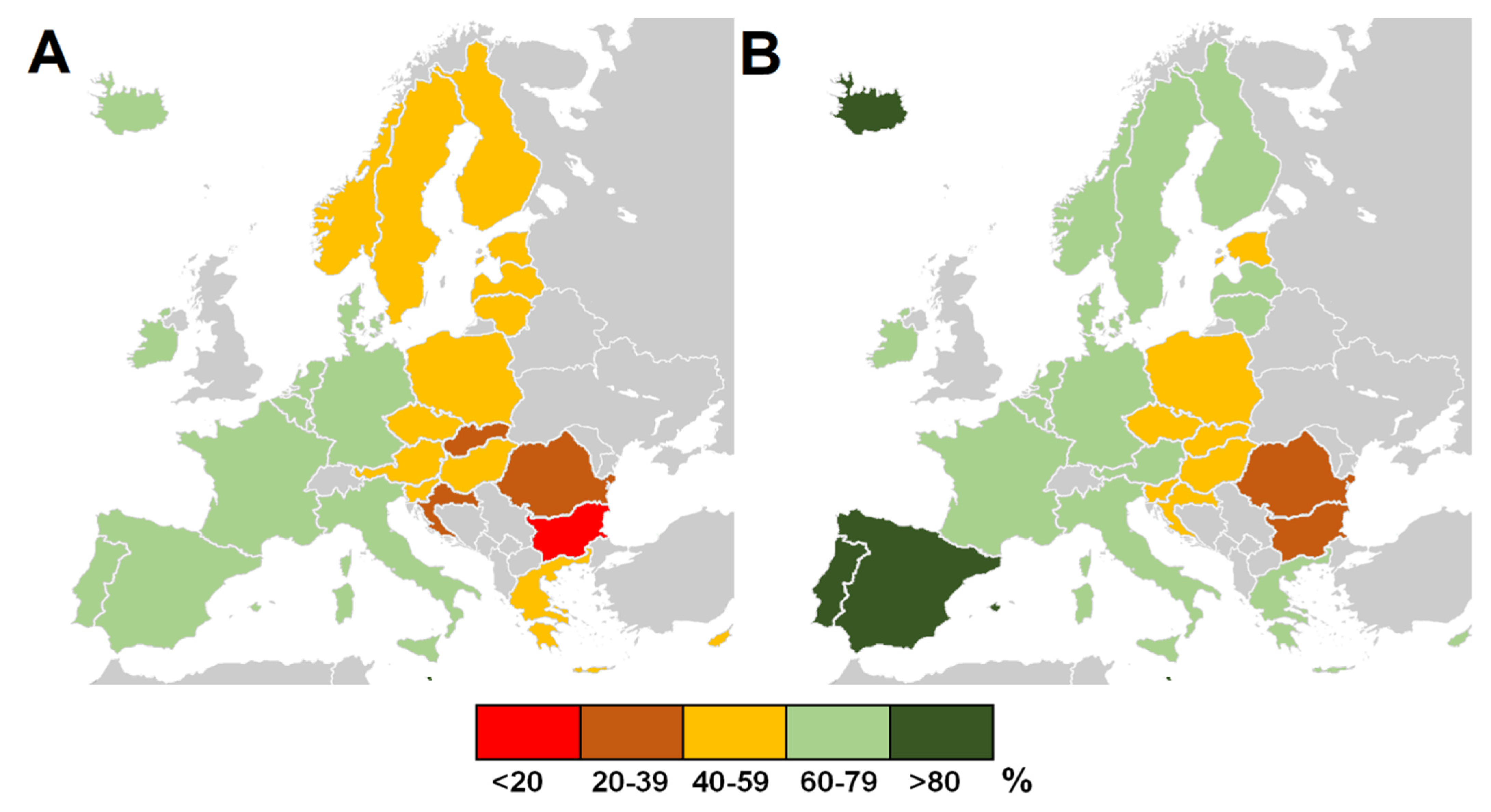
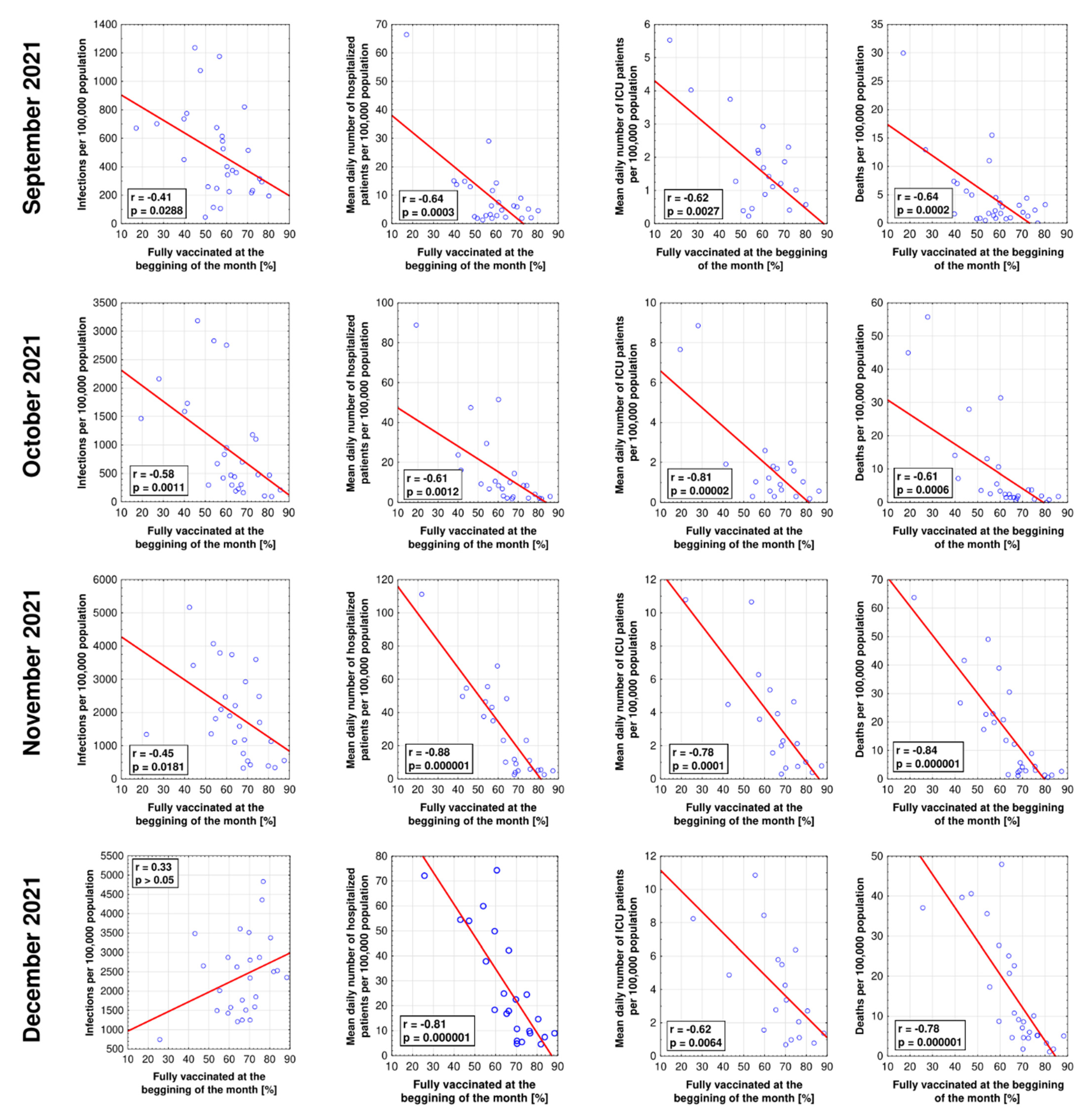
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nowakowska, J.; Sobocińska, J.; Lewicki, M.; Lemańska, Ż.; Rzymski, P. When Science Goes Viral: The Research Response during Three Months of the COVID-19 Outbreak. Biomed. Pharmacother. 2020, 129, 110451. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, K.; Rzymski, P.; Islam, M.S.; Makuku, R.; Mushtaq, A.; Khan, A.; Ivanovska, M.; Makka, S.A.; Hashem, F.; Marquez, L.; et al. COVID-19 Vaccinations: The Unknowns, Challenges, and Hopes. J. Med. Virol. 2021, 94, 1336–1349. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccine Tracker. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID-19/vaccine-tracker.html (accessed on 1 February 2022).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and Efficacy of the ChAdOx1 NCoV-19 Vaccine (AZD1222) against SARS-CoV-2: An Interim Analysis of Four Randomised Controlled Trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- SARS-COV-2 Delta Variant Now Dominant in Much of the European Region and Efforts Must Be Reinforced to Prevent Transmission, Warn WHO/Europe and ECDC. Available online: https://www.ecdc.europa.eu/en/news-events/sars-cov-2-delta-variant-now-dominant-european-region (accessed on 1 February 2022).
- Grannis, S.J.; Rowley, E.A.; Ong, T.C.; Stenehjem, E.; Klein, N.P.; DeSilva, M.B.; Naleway, A.L.; Natarajan, K.; Thompson, M.G.; VISION Network. Interim Estimates of COVID-19 Vaccine Effectiveness against COVID-19-Associated Emergency Department or Urgent Care Clinic Encounters and Hospitalizations among Adults during SARS-CoV-2 B.1.617.2 (Delta) Variant Predominance—Nine States, June-August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Scobie, H.M.; Johnson, A.G.; Suthar, A.B.; Severson, R.; Alden, N.B.; Balter, S.; Bertolino, D.; Blythe, D.; Brady, S.; Cadwell, B.; et al. Monitoring Incidence of COVID-19 Cases, Hospitalizations, and Deaths, by Vaccination Status—13 U.s. Jurisdictions, April 4-July 17, 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1284–1290. [Google Scholar] [CrossRef]
- Bajema, K.L.; Dahl, R.M.; Prill, M.M.; Meites, E.; Rodriguez-Barradas, M.C.; Marconi, V.C.; Beenhouwer, D.O.; Brown, S.T.; Holodniy, M.; Lucero-Obusan, C.; et al. Effectiveness of COVID-19 MRNA Vaccines against COVID-19-Associated Hospitalization—Five Veterans Affairs Medical Centers, United States, 1 February–6 August 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1294–1299. [Google Scholar] [CrossRef]
- Rzymski, P.; Pazgan-Simon, M.; Simon, K.; Łapiński, T.; Zarębska-Michaluk, D.; Szczepańska, B.; Chojnicki, M.; Mozer-Lisewska, I.; Flisiak, R. Clinical Characteristics of Hospitalized COVID-19 Patients Who Received at Least One Dose of COVID-19 Vaccine. Vaccines 2021, 9, 781. [Google Scholar] [CrossRef]
- Hsu, L.; Grüne, B.; Buess, M.; Joisten, C.; Klobucnik, J.; Nießen, J.; Patten, D.; Wolff, A.; Wiesmüller, G.A.; Kossow, A.; et al. COVID-19 Breakthrough Infections and Transmission Risk: Real-World Data Analyses from Germany’s Largest Public Health Department (Cologne). Vaccines 2021, 9, 1267. [Google Scholar] [CrossRef]
- Eyre, D.W.; Taylor, D.; Purver, M.; Chapman, D.; Fowler, T.; Pouwels, K.; Walker, A.S.; Peto, T.E.A. The Impact of SARS-CoV-2 Vaccination on Alpha and Delta Variant Transmission. medRxiv 2021. [Google Scholar] [CrossRef]
- Chia, P.Y.; Ong, S.W.X.; Chiew, C.J.; Ang, L.W.; Chavatte, J.-M.; Mak, T.-M.; Cui, L.; Kalimuddin, S.; Chia, W.N.; Tan, C.W.; et al. Virological and Serological Kinetics of SARS-CoV-2 Delta Variant Vaccine Breakthrough Infections: A Multicentre Cohort Study. Clin. Microbiol. Infect. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gavenčiak, T.; Monrad, J.T.; Leech, G.; Sharma, M.; Mindermann, S.; Brauner, J.M.; Bhatt, S.; Kulveit, J. Seasonal Variation in SARS-CoV-2 Transmission in Temperate Climates. medRxiv 2021. [Google Scholar] [CrossRef]
- Audi, A.; AlIbrahim, M.; Kaddoura, M.; Hijazi, G.; Yassine, H.M.; Zaraket, H. Seasonality of Respiratory Viral Infections: Will COVID-19 Follow Suit? Front. Public Health 2020, 8, 567184. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Huang, J.; Li, C.; Zhao, Y.; Wang, D.; Huang, Z.; Yang, K. The Role of Seasonality in the Spread of COVID-19 Pandemic. Environ. Res. 2021, 195, 110874. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E.; Hasell, J.; Macdonald, B.; Beltekian, D.; Roser, M. Coronavirus Pandemic (COVID-19). Our World Data 2020. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 1 February 2022).
- Warren, G.W.; Lofstedt, R. COVID-19 Vaccine Rollout Risk Communication Strategies in Europe: A Rapid Response. J. Risk Res. 2021, 24, 369–379. [Google Scholar] [CrossRef]
- Rzymski, P.; Borkowski, L.; Drąg, M.; Flisiak, R.; Jemielity, J.; Krajewski, J.; Mastalerz-Migas, A.; Matyja, A.; Pyrć, K.; Simon, K.; et al. The Strategies to Support the COVID-19 Vaccination with Evidence-Based Communication and Tackling Misinformation. Vaccines 2021, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Karafillakis, E.; Van Damme, P.; Hendrickx, G.; Larson, H.J. COVID-19 in Europe: New Challenges for Addressing Vaccine Hesitancy. Lancet 2022, 399, 699–701. [Google Scholar] [CrossRef]
- Data on the Daily Number of New Reported COVID-19 Cases and Deaths by EU/EEA Country. Available online: https://www.ecdc.europa.eu/en/publications-data/data-daily-new-cases-covid-19-eueea-country (accessed on 31 January 2022).
- Data on Hospital and ICU Admission Rates and Current Occupancy for COVID-19. Available online: https://www.ecdc.europa.eu/en/publications-data/download-data-hospital-and-icu-admission-rates-and-current-occupancy-covid-19 (accessed on 1 February 2022).
- SARS-CoV-2 Variants Dashboard. Available online: https://www.ecdc.europa.eu/en/covid-19/situation-updates/variants-dashboard (accessed on 1 February 2022).
- Kuhlmann, C.; Mayer, C.K.; Claassen, M.; Maponga, T.; Burgers, W.A.; Keeton, R.; Riou, C.; Sutherland, A.D.; Suliman, T.; Shaw, M.L.; et al. Breakthrough Infections with SARS-CoV-2 Omicron despite MRNA Vaccine Booster Dose. Lancet 2022, 399, 625–626. [Google Scholar] [CrossRef]
- Pulliam, J.R.C.; van Schalkwyk, C.; Govender, N.; von Gottberg, A.; Cohen, C.; Groome, M.J.; Dushoff, J.; Mlisana, K.; Moultrie, H. Increased Risk of SARS-CoV-2 Reinfection Associated with Emergence of the Omicron Variant in South Africa. medRxiv 2021. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- El Sahly, H.M.; Baden, L.R.; Essink, B.; Doblecki-Lewis, S.; Martin, J.M.; Anderson, E.J.; Campbell, T.B.; Clark, J.; Jackson, L.A.; Fichtenbaum, C.J.; et al. Efficacy of the MRNA-1273 SARS-CoV-2 Vaccine at Completion of Blinded Phase. N. Engl. J. Med. 2021, 385, 1774–1785. [Google Scholar] [CrossRef] [PubMed]
- Shrotri, M.; Navaratnam, A.M.D.; Nguyen, V.; Byrne, T.; Geismar, C.; Fragaszy, E.; Beale, S.; Fong, W.L.E.; Patel, P.; Kovar, J.; et al. Spike-Antibody Waning after Second Dose of BNT162b2 or ChAdOx1. Lancet 2021, 398, 385–387. [Google Scholar] [CrossRef]
- Gruell, H.; Vanshylla, K.; Tober-Lau, P.; Hillus, D.; Schommers, P.; Lehmann, C.; Kurth, F.; Sander, L.E.; Klein, F. mRNA Booster Immunization Elicits Potent Neutralizing Serum Activity against the SARS-CoV-2 Omicron Variant. Nat. Med. 2022, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Pajon, R.; Doria-Rose, N.A.; Shen, X.; Schmidt, S.D.; O’Dell, S.; McDanal, C.; Feng, W.; Tong, J.; Eaton, A.; Maglinao, M.; et al. SARS-CoV-2 Omicron Variant Neutralization after MRNA-1273 Booster Vaccination. N. Engl. J. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Stokel-Walker, C. What Do We Know about Covid Vaccines and Preventing Transmission? BMJ 2022, 376, o298. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Camargo, C.A.; Fal, A.; Flisiak, R.; Gwenzi, W.; Kelishadi, R.; Leemans, A.; Nieto, J.J.; Ozen, A.; Perc, M.; et al. COVID-19 Vaccine Boosters: The Good, the Bad, and the Ugly. Vaccines 2021, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.C.; Shin, B.-H.; Gadsden, T.-A.M.; Chu, M.; Petrosyan, A.; Le, C.N.; Zabner, R.; Oft, J.; Pedraza, I.; Cheng, S.; et al. T Cell Immune Responses to SARS-CoV-2 and Variants of Concern (Alpha and Delta) in Infected and Vaccinated Individuals. Cell. Mol. Immunol. 2021, 18, 2554–2556. [Google Scholar] [CrossRef]
- Liu, J.; Chandrashekar, A.; Sellers, D.; Barrett, J.; Jacob-Dolan, C.; Lifton, M.; McMahan, K.; Sciacca, M.; VanWyk, H.; Wu, C.; et al. Vaccines Elicit Highly Conserved Cellular Immunity to SARS-CoV-2 Omicron. Nature 2022, 31, 732. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikora, D.; Rzymski, P. COVID-19 Vaccination and Rates of Infections, Hospitalizations, ICU Admissions, and Deaths in the European Economic Area during Autumn 2021 Wave of SARS-CoV-2. Vaccines 2022, 10, 437. https://doi.org/10.3390/vaccines10030437
Sikora D, Rzymski P. COVID-19 Vaccination and Rates of Infections, Hospitalizations, ICU Admissions, and Deaths in the European Economic Area during Autumn 2021 Wave of SARS-CoV-2. Vaccines. 2022; 10(3):437. https://doi.org/10.3390/vaccines10030437
Chicago/Turabian StyleSikora, Dominika, and Piotr Rzymski. 2022. "COVID-19 Vaccination and Rates of Infections, Hospitalizations, ICU Admissions, and Deaths in the European Economic Area during Autumn 2021 Wave of SARS-CoV-2" Vaccines 10, no. 3: 437. https://doi.org/10.3390/vaccines10030437
APA StyleSikora, D., & Rzymski, P. (2022). COVID-19 Vaccination and Rates of Infections, Hospitalizations, ICU Admissions, and Deaths in the European Economic Area during Autumn 2021 Wave of SARS-CoV-2. Vaccines, 10(3), 437. https://doi.org/10.3390/vaccines10030437






