Endotoxin Tolerance in Abdominal Aortic Aneurysm Macrophages, In Vitro: A Case–Control Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Eligibility Criteria
2.2. Monocyte Isolation Protocol
2.3. Macrophage Maturation and Activation
2.4. Measurement of Free 8-Isoprostane and a Prostaglandin E2 Metabolite
2.5. Cytokine and MMP-9 Assays
2.6. Antioxidant Assays
2.7. Western Blot Analysis
2.8. Confocal Microscopy
2.9. RNA Isolation and Real-Time Quantitative PCR
2.10. Data Analysis
3. Results
3.1. AAA Was Associated with Systemic Inflammation
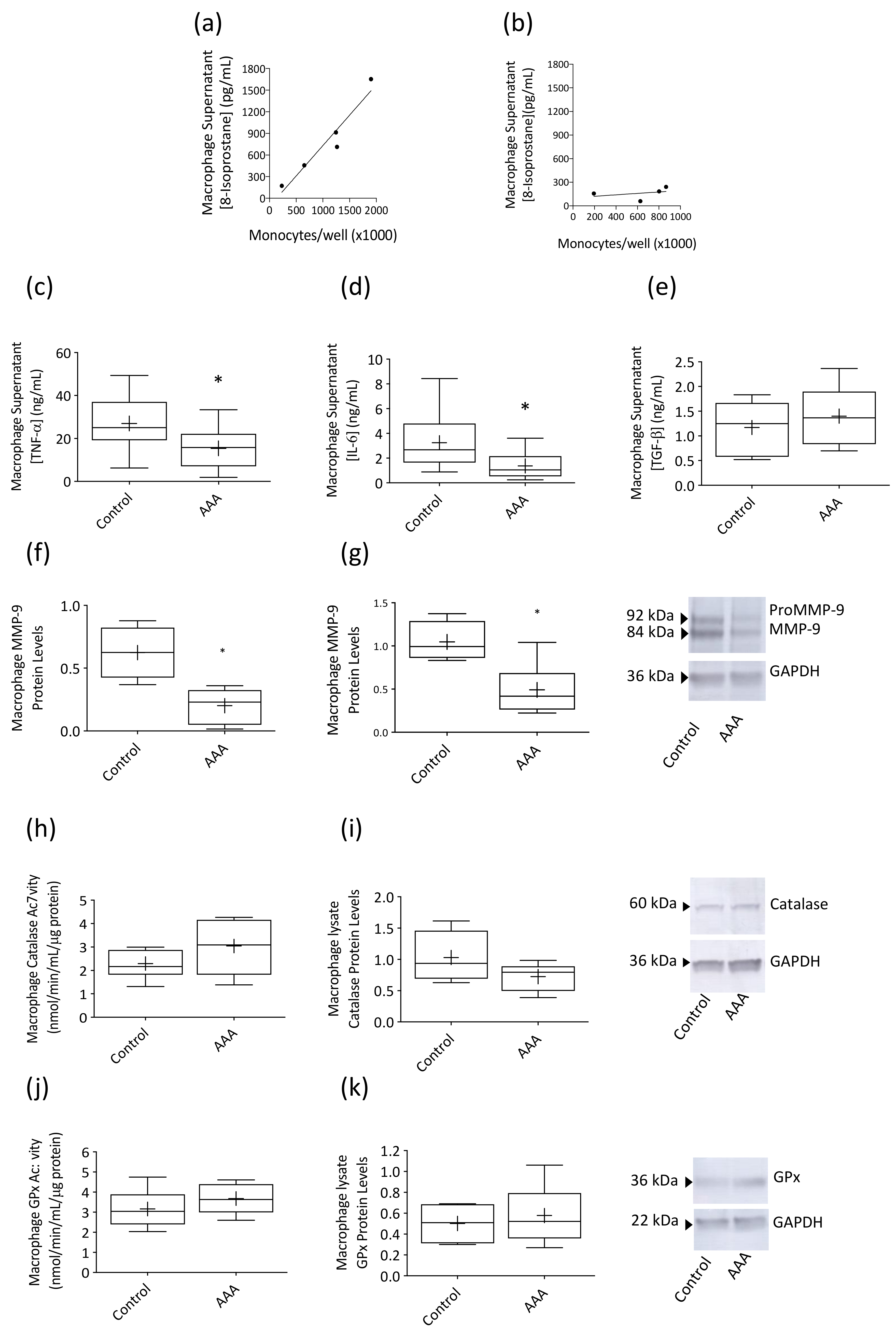
3.2. Macrophage Responses to LPS Stimulation Were Suppressed in AAA
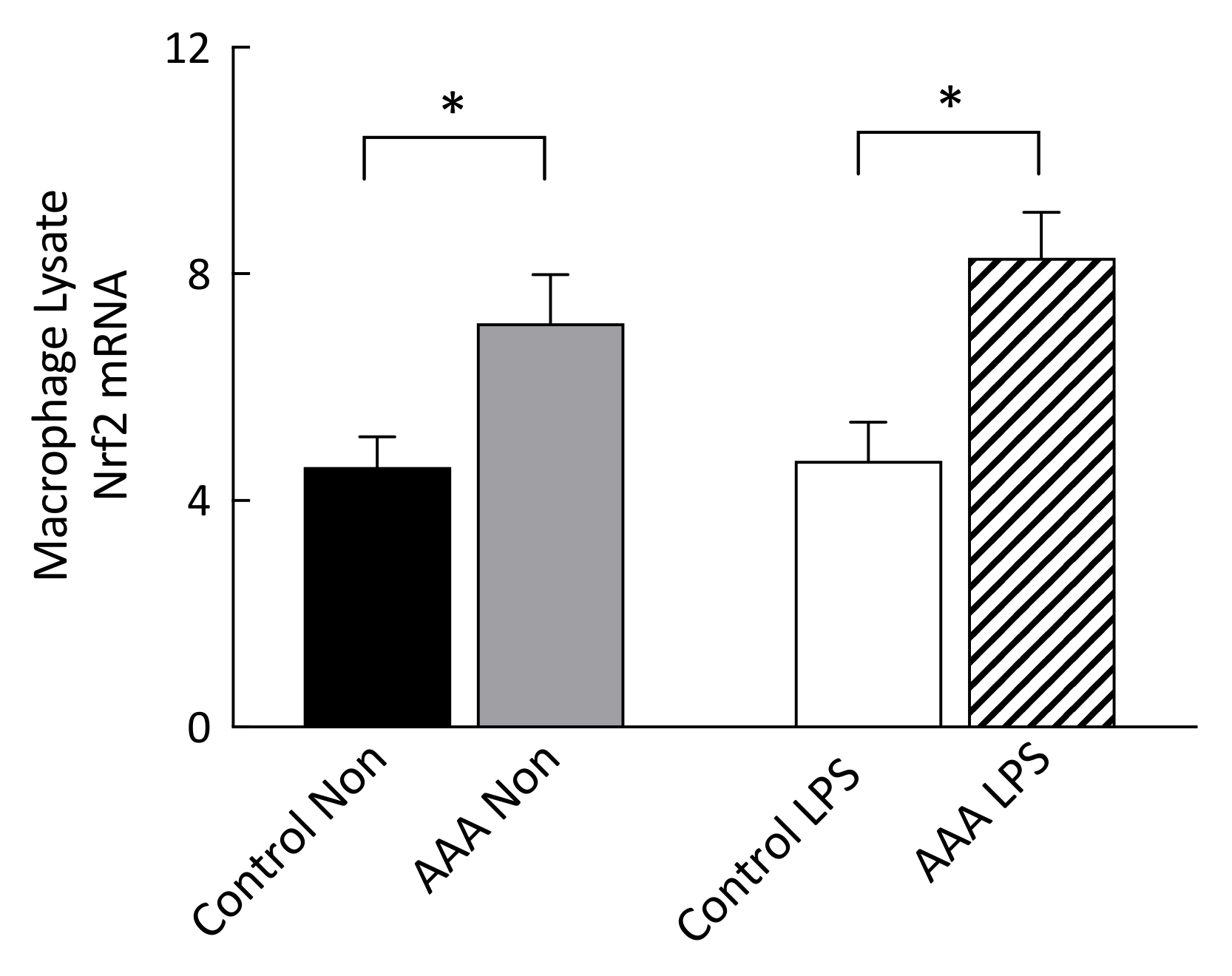
3.3. Nrf2 mRNA Transcripts Were Increased in AAA Macrophages
3.4. Lipid Raft and TLR4 Internalization Was Observed in AAA Macrophages
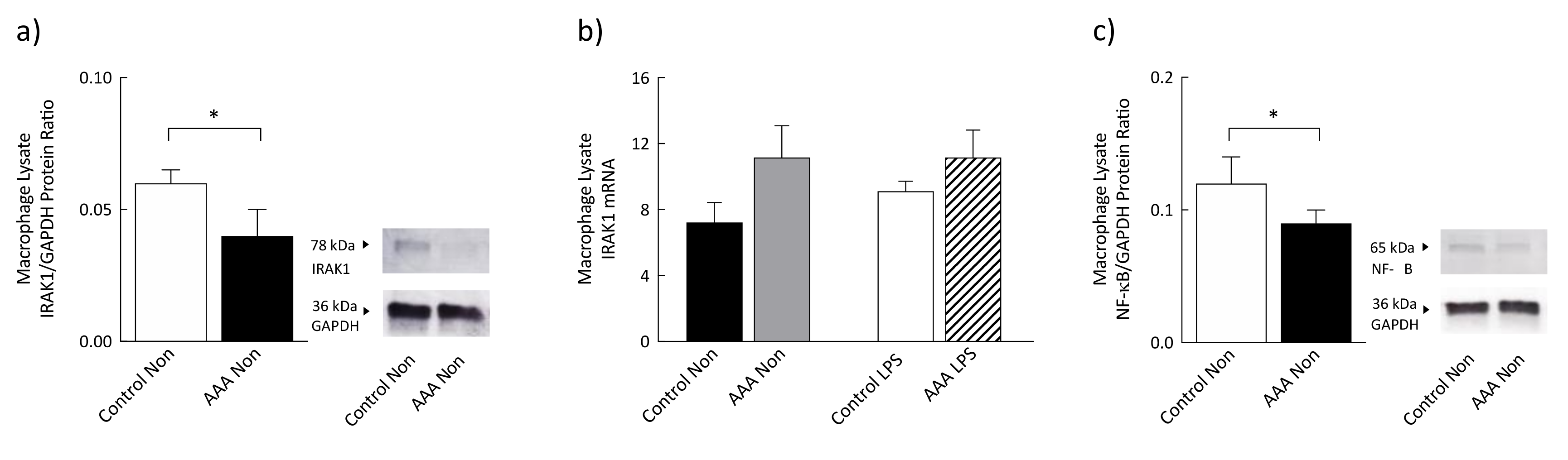
3.5. Levels of IRAK1 and Phosphorylated NF-κB Were Significantly Decreased in AAA
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAA | Abdominal aortic aneurysm |
| BHT | Butylhydroxytoluene |
| DPBS | Dulbecco’s phosphate buffered solution |
| EDTA | Ethylenediaminetetraacetic acid |
| ELISA | Enzyme-linked immunosorbent assay |
| GPx | Glutathione peroxidase |
| IFN- | Interferon gamma |
| IL-1 | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IMDM | Iscove’s Modified Dulbecco’s Medium |
| IRAK1 | Interleukin-1 receptor-associated kinase 1 |
| LPS | Lipopolysaccharide |
| M-CSF | Macrophage colony-stimulating factor |
| MMP-9 | Matrix metalloproteinase 9 |
| mRNA | Messenger RNA |
| NF-B | Nuclear factor kappa B |
| Nrf2 | Nuclear factor E2-related factor 2 |
| NSAID | Non-steroidal anti-inflammatory drug |
| PGE2 | Prostaglandin E2 |
| qPCR | Quantitative polymerase chain reaction |
| RIPA | Radioimmunoprecipitation assay |
| TGF- | Transforming growth factor beta |
| TLR4 | Toll-like receptor 4 |
| TNF- | Tumor necrosis factor alpha |
References
- Nordon, I.M.; Hinchliffe, R.J.; Loftus, I.M.; Thompson, M.M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011, 8, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.-B.; Martin-Ventura, J.-L.; Egido, J.; Sakalihasan, N.; Treska, V.; Lindholt, J.; Allaire, E.; Thorsteinsdottir, U.; Cockerill, G.; Swedenborg, J. Novel aspects of the pathogenesis of aneurysms of the abdominal aorta in humans. Cardiovasc. Res. 2011, 90, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul-Hussien, H.; Hanemaaijer, R.; Kleemann, R.; Verhaaren, B.F.; van Bockel, J.H.; Lindeman, J.H. The pathophysiology of abdominal aortic aneurysm growth: Corresponding and discordant inflammatory and proteolytic processes in abdominal aortic and popliteal artery aneurysms. J. Vasc. Surg. 2010, 51, 1479–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dale, M.A.; Ruhlman, M.K.; Baxter, B.T. Inflammatory cell phenotypes in AAAs: Their role and potential as targets for therapy. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1746–1755. [Google Scholar] [CrossRef] [Green Version]
- Canton, J.; Khezri, R.; Glogauer, M.; Grinstein, S. Contrasting phagosome pH regulation and maturation in human M1 and M2 macrophages. Mol. Biol. Cell 2014, 25, 3330–3341. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Invest. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Boytard, L.; Spear, R.; Chinetti-Gbaguidi, G.; Acosta-Martin, A.E.; Vanhoutte, J.; Lamblin, N.; Staels, B.; Amouyel, P.; Haulon, S.; Pinet, F. Role of proinflammatory CD68+ mannose receptor−macrophages in peroxiredoxin-1 expression and in abdominal aortic aneurysms in humans. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Lindqvist, M.; Wallinder, J.; Henriksson, A.E. Soluble urokinase plasminogen activator receptor in patients with abdominal aortic aneurysm. Thromb. Res. 2012, 130, 511–513. [Google Scholar] [CrossRef]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889. [Google Scholar] [CrossRef]
- Kadl, A.; Meher, A.K.; Sharma, P.R.; Lee, M.Y.; Doran, A.C.; Johnstone, S.R.; Elliott, M.R.; Gruber, F.; Han, J.; Chen, W. Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circ. Res. 2010, 107, 737–746. [Google Scholar] [CrossRef]
- Espey, M.G. Tumor macrophage redox and effector mechanisms associated with hypoxia. Free Radic. Biol. Med. 2006, 41, 1621–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meital, L.T.; Coward, A.S.; Windsor, M.; Bailey, T.G.; Kuballa, A.; Russell, F.D. A simple and effective method for the isolation and culture of human monocytes from small volumes of peripheral blood. J. Immunol. Methods 2019, 472, 75–78. [Google Scholar] [CrossRef]
- Repnik, U.; Knezevic, M.; Jeras, M. Simple and cost-effective isolation of monocytes from buffy coats. J. Immunol. Methods 2003, 278, 283–292. [Google Scholar] [CrossRef]
- Meital, L.T.; Windsor, M.T.; Perissiou, M.; Schulze, K.; Magee, R.; Kuballa, A.; Golledge, J.; Bailey, T.G.; Askew, C.D.; Russell, F.D. Omega-3 fatty acids decrease oxidative stress and inflammation in macrophages from patients with small abdominal aortic aneurysm. Sci. Rep. 2019, 9, 12978. [Google Scholar] [CrossRef]
- Song, Y.; Hou, M.; Li, Z.; Luo, C.; Ou, J.-S.; Yu, H.; Yan, J.; Lu, L. TLR4/NF-κB/Ceramide signaling contributes to Ox-LDL-induced calcification of human vascular smooth muscle cells. Eur. J. Pharmacol. 2017, 794, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.L.; Cintron, M.; Wu, T.Y.; Guo, Y.; Huang, Y.; Jeong, W.S.; Kong, A.N.T. Pharmacodynamics of dietary phytochemical indoles I3C and DIM: Induction of Nrf2-mediated phase II drug metabolizing and antioxidant genes and synergism with isothiocyanates. Biopharm. Drug Dispos. 2011, 32, 289–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, J.; Zhao, J.; Yan, Z.; Liu, Z.; Wang, N. Antitumor effects of a novel sulfur-containing hydroxamate histone deacetylase inhibitor H40. Int. J. Cancer 2009, 124, 1235–1244. [Google Scholar] [CrossRef]
- Lee, E.E.; Eyler, L.T.; Wolkowitz, O.M.; Martin, A.S.; Reuter, C.; Kraemer, H.; Jeste, D.V. Elevated plasma F2-isoprostane levels in schizophrenia. Schizophr. Res. 2016, 176, 320–326. [Google Scholar] [CrossRef] [Green Version]
- Lindsay, T.F.; Luo, X.P.; Lehotay, D.C.; Rubin, B.B.; Anderson, M.; Walker, P.M.; Romaschin, A.D. Ruptured abdominal aortic aneurysm, a “two-hit” ischemia/reperfusion injury: Evidence from an analysis of oxidative products. J. Vasc. Surg. 1999, 30, 219–228. [Google Scholar] [CrossRef] [Green Version]
- Rosner, B. Percentage points for a generalized ESD many-outlier procedure. Technometrics 1983, 25, 165–172. [Google Scholar] [CrossRef]
- Guzik, B.; Sagan, A.; Ludew, D.; Mrowiecki, W.; Chwała, M.; Bujak-Gizycka, B.; Filip, G.; Grudzien, G.; Kapelak, B.; Żmudka, K. Mechanisms of oxidative stress in human aortic aneurysms—association with clinical risk factors for atherosclerosis and disease severity. Int. J. Cardiol. 2013, 168, 2389–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindeman, J.H.; Abdul-Hussien, H.; Schaapherder, A.F.; Bockel, J.H.V.; Thüsen, J.H.V.D.; Roelen, D.L.; Kleemann, R. Enhanced expression and activation of pro-inflammatory transcription factors distinguish aneurysmal from atherosclerotic aorta: IL-6-and IL-8-dominated inflammatory responses prevail in the human aneurysm. Clin. Sci. 2008, 114, 687–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahnström, J.; Gottsäter, A.; Lindblad, B.; Dahlbäck, B. Plasma concentrations of apolipoproteins AI, B and M in patients with abdominal aortic aneurysms. Clin. Biochem. 2010, 43, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Juvonen, J.; Surcel, H.-M.; Satta, J.; Teppo, A.-M.; Bloigu, A.; Syrjälä, H.; Airaksinen, J.; Leinonen, M.; Saikku, P.; Juvonen, T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2843–2847. [Google Scholar] [CrossRef] [PubMed]
- Hamano, K.; Li, T.-S.; Takahashi, M.; Kobayashi, T.; Shirasawa, B.; Ito, H.; Zempo, N. Enhanced tumor necrosis factor-α expression in small sized abdominal aortic aneurysms. World J. Surg. 2003, 27, 476–480. [Google Scholar] [CrossRef]
- Morimoto, K.; Hasegawa, T.; Tanaka, A.; Wulan, B.; Yu, J.; Morimoto, N.; Okita, Y.; Okada, K. Free-radical scavenger edaravone inhibits both formation and development of abdominal aortic aneurysm in rats. J. Vasc. Surg. 2012, 55, 1749–1758. [Google Scholar] [CrossRef] [Green Version]
- Yajima, N.; Masuda, M.; Miyazaki, M.; Nakajima, N.; Chien, S.; Shyy, J.Y. Oxidative stress is involved in the development of experimental abdominal aortic aneurysm: A study of the transcription profile with complementary DNA microarray. J. Vasc. Surg. 2002, 36, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Pincemail, J.; Defraigne, J.-O.; Cheramy–Bien, J.; Dardenne, N.; Donneau, A.-F.; Albert, A.; Labropoulos, N.; Sakalihasan, N. On the potential increase of the oxidative stress status in patients with abdominal aortic aneurysm. Redox Rep. 2012, 17, 139–144. [Google Scholar] [CrossRef]
- Ramos-Mozo, P.; Madrigal-Matute, J.; Martinez-Pinna, R.; Blanco-Colio, L.M.; Lopez, J.A.; Camafeita, E.; Meilhac, O.; Michel, J.-B.; Aparicio, C.; de Ceniga, M.V. Proteomic analysis of polymorphonuclear neutrophils identifies catalase as a novel biomarker of abdominal aortic aneurysm: Potential implication of oxidative stress in abdominal aortic aneurysm progression. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 3011–3019. [Google Scholar] [CrossRef] [Green Version]
- Seeley, J.J.; Ghosh, S. Molecular mechanisms of innate memory and tolerance to LPS. J. Leukoc. Biol. 2017, 101, 107–119. [Google Scholar] [CrossRef]
- Vikatmaa, P.; Lajunen, T.; Ikonen, T.S.; Pussinen, P.J.; Lepäntalo, M.; Leinonen, M.; Saikku, P. Chlamydial lipopolysaccharide (cLPS) is present in atherosclerotic and aneurysmal arterial wall—cLPS levels depend on disease manifestation. Cardiovasc. Pathol. 2010, 19, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Moure, J.S.; Vykoukal, D.; Davies, M.G. Biology of aortic aneurysms and dissections. Methodist Debakey Cardiovasc. J. 2011, 7, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Manabe, H.; Kawai, N.; Goto, S.; Umemoto, T. Plasma fibrinogen and D-dimer concentrations are associated with the presence of abdominal aortic aneurysm: A systematic review and meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 273–277. [Google Scholar] [CrossRef] [Green Version]
- del Fresno, C.; García-Rio, F.; Gómez-Piña, V.; Soares-Schanoski, A.; Fernández-Ruíz, I.; Jurado, T.; Kajiji, T.; Shu, C.; Marín, E.; del Arroyo, A.G. Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: Demonstration in isolated monocytes from cystic fibrosis patients. J. Immunol. 2009, 182, 6494–6507. [Google Scholar] [CrossRef] [PubMed]
- Escoll, P.; del Fresno, C.; Garcia, L.; Vallés, G.; Lendinez, M.J.; Arnalich, F.; López-Collazo, E. Rapid up-regulation of IRAK-M expression following a second endotoxin challenge in human monocytes and in monocytes isolated from septic patients. Biochem. Biophys. Res. Commun. 2003, 311, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Foster, S.L.; Hargreaves, D.C.; Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 2007, 447, 972–978. [Google Scholar] [CrossRef]
- Mages, J.; Dietrich, H.; Lang, R. A genome-wide analysis of LPS tolerance in macrophages. Immunobiology 2008, 212, 723–737. [Google Scholar] [CrossRef]
- Rajaiah, R.; Perkins, D.J.; Polumuri, S.K.; Zhao, A.; Keegan, A.D.; Vogel, S.N. Dissociation of endotoxin tolerance and differentiation of alternatively activated macrophages. J. Immunol. 2013, 190, 4763–4772. [Google Scholar] [CrossRef] [Green Version]
- Yang, N.-B.; Ni, S.-L.; Li, S.-S.; Zhang, S.-N.; Hu, D.-P.; Lu, M.-Q. Endotoxin tolerance alleviates experimental acute liver failure via inhibition of high mobility group box 1. Int. J. Clin. Exp. Pathol. 2015, 8, 9062. [Google Scholar]
- Döcke, W.-D.; Randow, F.; Syrbe, U.; Krausch, D.; Asadullah, K.; Reinke, P.; Volk, H.-D.; Kox, W. Monocyte deactivation in septic patients: Restoration by IFN-γ treatment. Nat. Med. 1997, 3, 678–681. [Google Scholar] [CrossRef]
- Santos, S.S.; Carmo, A.M.; Brunialti, M.K.; Machado, F.R.; Azevedo, L.C.; Assunção, M.; Trevelin, S.C.; Cunha, F.Q.; Salomao, R. Modulation of monocytes in septic patients: Preserved phagocytic activity, increased ROS and NO generation, and decreased production of inflammatory cytokines. Intensive Care Med. Exp. 2016, 4, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sáenz, J.; Izura, J.; Manrique, A.; Sala, F.; Gaminde, I. Early prognosis in severe sepsis via analyzing the monocyte immunophenotype. Intensive Care Med. 2001, 27, 970–977. [Google Scholar] [CrossRef] [PubMed]
- Landelle, C.; Lepape, A.; Voirin, N.; Tognet, E.; Venet, F.; Bohé, J.; Vanhems, P.; Monneret, G. Low monocyte human leukocyte antigen-DR is independently associated with nosocomial infections after septic shock. Intensive Care Med. 2010, 36, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Kokje, V.B.; Gäbel, G.; Koole, D.; Northoff, B.H.; Holdt, L.M.; Hamming, J.F.; Lindeman, J.H. IL-6: A Janus-like factor in abdominal aortic aneurysm disease. Atherosclerosis 2016, 251, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englesbe, M.J.; Wu, A.H.; Clowes, A.W.; Zierler, R.E. The prevalence and natural history of aortic aneurysms in heart and abdominal organ transplant patients. J. Vasc. Surg. 2003, 37, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Lindeman, J.H.; Rabelink, T.J.; van Bockel, J.H. Immunosuppression and the abdominal aortic aneurysm. Circulation 2011, 124, e463–e465. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, E.H.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef]
- Olsson, S.; Sundler, R. The role of lipid rafts in LPS-induced signaling in a macrophage cell line. Mol. Immunol. 2006, 43, 607–612. [Google Scholar] [CrossRef]
- Medvedev, A.E.; Lentschat, A.; Wahl, L.M.; Golenbock, D.T.; Vogel, S.N. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J. Immunol. 2002, 169, 5209–5216. [Google Scholar] [CrossRef] [Green Version]
- Li, C.H.; Wang, J.H.; Redmond, H.P. Bacterial lipoprotein-induced self-tolerance and cross-tolerance to LPS are associated with reduced IRAK-1 expression and MyD88-IRAK complex formation. J. Leukoc. Biol. 2006, 79, 867–875. [Google Scholar] [CrossRef]
- Goode, B.; Joseph, N.M.; Stevers, M.; van Ziffle, J.; Onodera, C.; Talevich, E.; Grenert, J.P.; Yeh, I.; Bastian, B.C.; Phillips, J.J. Adenomatoid tumors of the male and female genital tract are defined by TRAF7 mutations that drive aberrant NF-kB pathway activation. Mod. Pathol. 2018, 31, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swantek, J.L.; Tsen, M.F.; Cobb, M.H.; Thomas, J.A. IL-1 receptor-associated kinase modulates host responsiveness to endotoxin. J. Immunol. 2000, 164, 4301–4306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef] [PubMed]
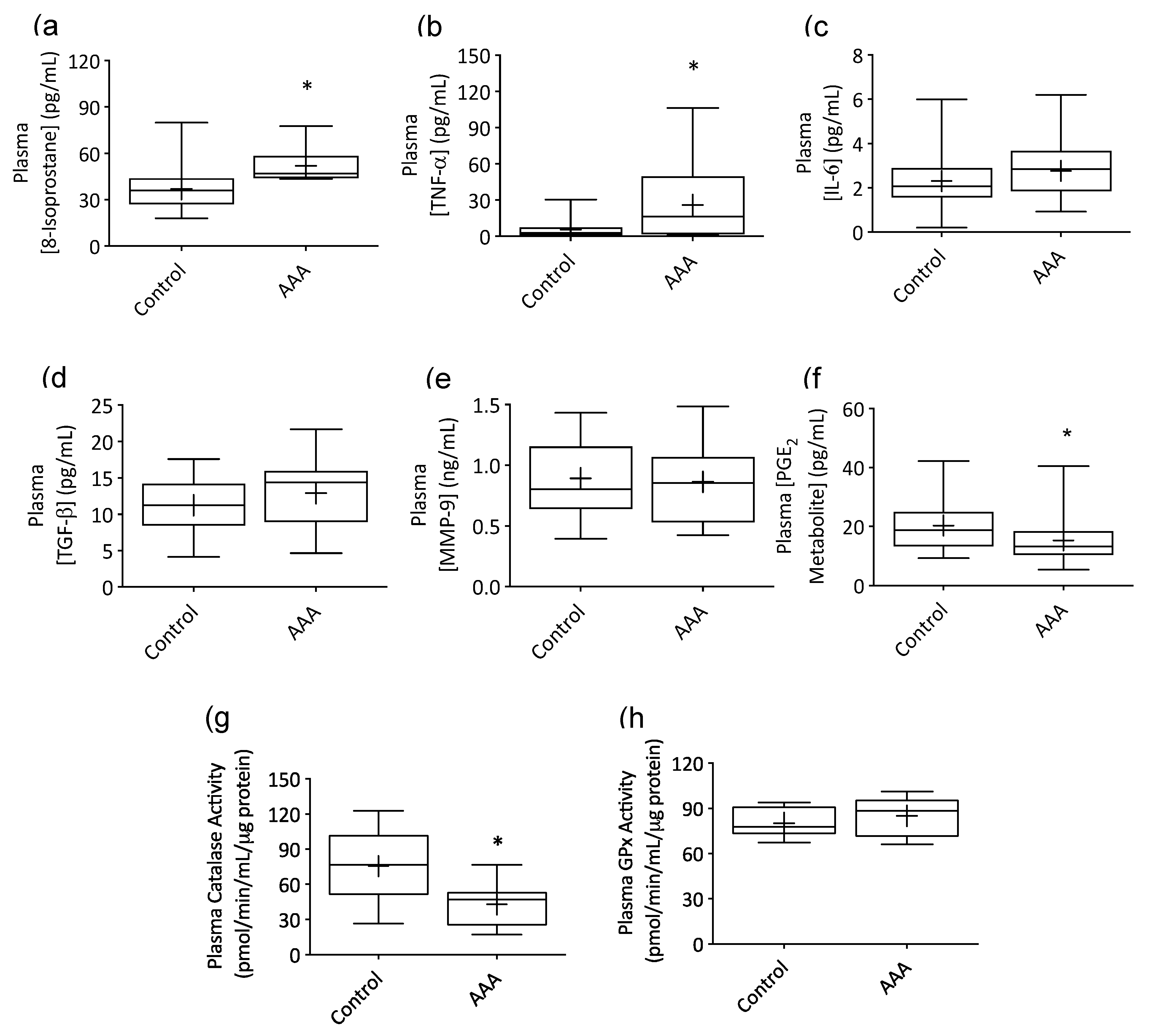

| Biomarker Experiments | Confocal Experiments | |||
|---|---|---|---|---|
| Variable | AAA Patients (n = 19) | Control Participants (n = 36) | AAA Patients (n = 14) | Control Participants (n = 8) |
| Gender (Male/Female) | 19/0 | 36/0 | 14/0 | 8/0 |
| Age (years) | 74.6 ± 5.8 | 71.7 ± 5.2 | 73.0 ± 4.92 | 69.7 ± 5.15 |
| AAA size (mm) | 37.8 ± 5.3 | 37.0 ± 5.5 | ||
| Smoking: Never | 7 | 16 | 3 | 3 |
| Past | 10 | 20 | 9 | 4 |
| Current | 2 | 0 | 2 | 1 |
| BMI (kg/m2) | 26.9 ± 2.9 | 25.8 ± 3.4 | 27.5 ± 3.6 | 24.9 ± 4.0 |
| Systolic blood pressure (mmHg) | 136.5 ± 13.6 | 135.9 ± 11.3 | 142.1 ± 14.7 | 131.3 ± 14.4 |
| Diastolic blood pressure (mmHg) | 78.6 ± 6.1 | 79.6 ± 8.3 | 81.0 ± 7.6 | 84.0 ± 4.2 |
| Coronary heart disease | 12 (63%) | 3 (8%) | 5 (35%) | 0 (0%) |
| Diabetes | 2 (11%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Dyslipidemia | 15(79%) | 9 (25%) | 9 (64%) | 2 (25%) |
| Hypertension | 12 (63%) | 13 (36%) | 9 (64%) | 3 (38%) |
| NSAIDs | 5 (26%) | 3 (8%) | 2 (14%) | 0 (0%) |
| Ace inhibitors | 2 (11%) | 2 (6%) | 2 (14%) | 1 (13%) |
| Alpha-1 blockers | 0 (0%) | 2 (6%) | 0 (0%) | 0 (0%) |
| AT receptor antagonists | 6 (32%) | 6 (17%) | 3 (21%) | 2 (25%) |
| Beta blockers | 8 (42%) | 4 (11%) | 2 (14%) | 2 (25%) |
| Calcium channel blockers | 2 (11%) | 3 (8%) | 3 (21%) | 2 (25%) |
| Diuretics | 2 (11%) | 1 (3%) | 0 (0%) | 0 (0%) |
| Anti-platelet drugs | 10 (53%) | 2 (6%) | 7 (50%) | 1 (13%) |
| Statins | 14 (74%) | 10 (28%) | 9 (64%) | 2 (25%) |
| Endpoint | Variable | Chi Square | d.f. | p | Confounder |
|---|---|---|---|---|---|
| TNF-α | Beta blockers | 0.19 | 1 | 0.66 | No |
| TNF-α | Statins | 1.18 | 1 | 0.28 | No |
| TNF-α | Low dose Aspirin | 0.10 | 1 | 0.75 | No |
| TNF-α | Hyperlipidemia | 0.56 | 1 | 0.46 | No |
| TNF-α | Hypertension | 0.01 | 1 | 0.91 | No |
| IL-6 | Beta blockers | 0.23 | 1 | 0.63 | No |
| IL-6 | Statins | 0.00 | 1 | 0.96 | No |
| IL-6 | Hyperlipidemia | 0.12 | 1 | 0.73 | No |
| IL-6 | Hypertension | 0.17 | 1 | 0.68 | No |
| IL-6 | Coronary heart disease | 0.66 | 1 | 0.42 | No |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meital, L.T.; Windsor, M.T.; Maynard, A.E.; Schulze, K.; Magee, R.; O’Donnell, J.; Jha, P.; Meital, C.Y.; Perissiou, M.; Coverdale, S.; et al. Endotoxin Tolerance in Abdominal Aortic Aneurysm Macrophages, In Vitro: A Case–Control Study. Antioxidants 2020, 9, 896. https://doi.org/10.3390/antiox9090896
Meital LT, Windsor MT, Maynard AE, Schulze K, Magee R, O’Donnell J, Jha P, Meital CY, Perissiou M, Coverdale S, et al. Endotoxin Tolerance in Abdominal Aortic Aneurysm Macrophages, In Vitro: A Case–Control Study. Antioxidants. 2020; 9(9):896. https://doi.org/10.3390/antiox9090896
Chicago/Turabian StyleMeital, Lara T., Mark T. Windsor, Alesiya E. Maynard, Karl Schulze, Rebecca Magee, Jill O’Donnell, Pankaj Jha, Chaim Y. Meital, Maria Perissiou, Steven Coverdale, and et al. 2020. "Endotoxin Tolerance in Abdominal Aortic Aneurysm Macrophages, In Vitro: A Case–Control Study" Antioxidants 9, no. 9: 896. https://doi.org/10.3390/antiox9090896
APA StyleMeital, L. T., Windsor, M. T., Maynard, A. E., Schulze, K., Magee, R., O’Donnell, J., Jha, P., Meital, C. Y., Perissiou, M., Coverdale, S., Golledge, J., Kuballa, A. V., Bailey, T. G., Askew, C. D., & Russell, F. D. (2020). Endotoxin Tolerance in Abdominal Aortic Aneurysm Macrophages, In Vitro: A Case–Control Study. Antioxidants, 9(9), 896. https://doi.org/10.3390/antiox9090896








