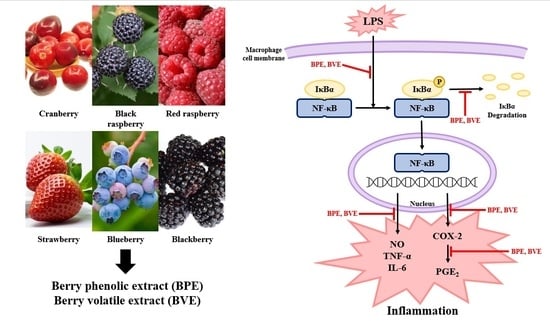
Berry Phenolic and Volatile Extracts Inhibit Pro-Inflammatory Cytokine Secretion in LPS-Stimulated RAW264.7 Cells through Suppression of NF-κB Signaling Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Materials
2.3. Extraction of Berry Phenolics
2.4. HPLC Analysis of Berry Phenolics
2.5. Extraction of Berry Volatiles
2.6. GC/GC-MS Analysis of Berry Volatiles
2.7. Determination of Total Phenolics, Total Anthocyanins and Antioxidant Capacity
2.8. Cell Culture and Treatment
2.9. Cell Viability Assay and Nitric Oxide Determination
2.10. ELISA for IL-6, TNF-α and PGE2
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Results
3.1. Composition of Berry Phenolic Extracts
3.1.1. Cranberries
3.1.2. Black Raspberries
3.1.3. Red Raspberries
3.1.4. Strawberries
3.1.5. Blueberries
3.1.6. Blackberries
3.2. Composition of Berry Volatile Extracts
3.2.1. Cranberries
3.2.2. Black Raspberries
3.2.3. Red Raspberries
3.2.4. Strawberries
3.2.5. Blueberries
3.2.6. Blackberries
3.3. Antioxidant Capacity of Phenolic and Volatile Extracts
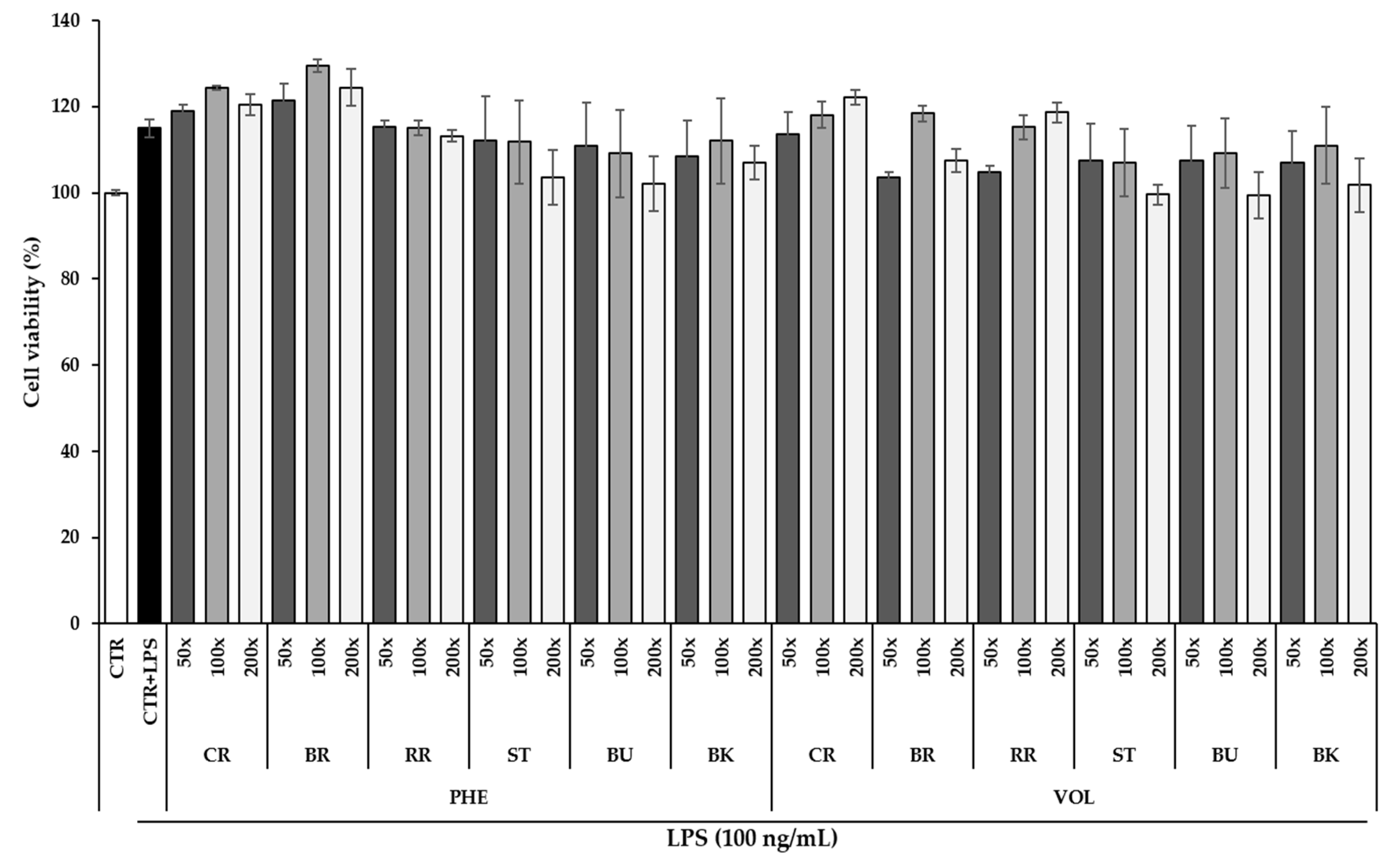
3.4. Effect of Berry Phenolic and Volatile Extracts on RAW264.7 Cell Viability
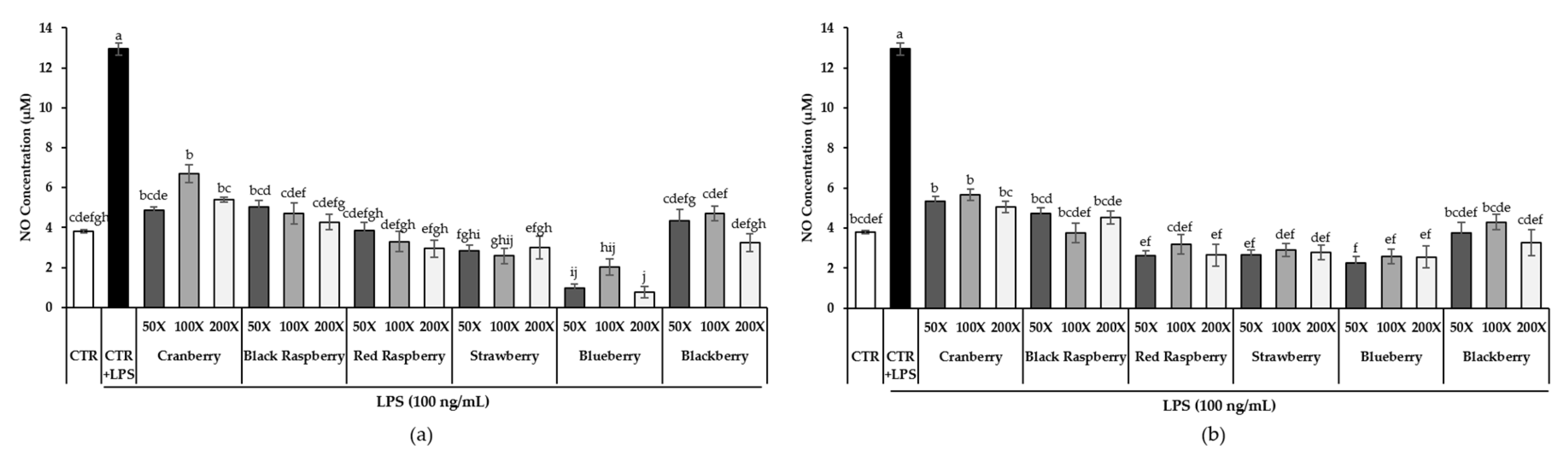
3.5. Effect of Berry Phenolic and Volatile Extracts on LPS-Induced NO Production
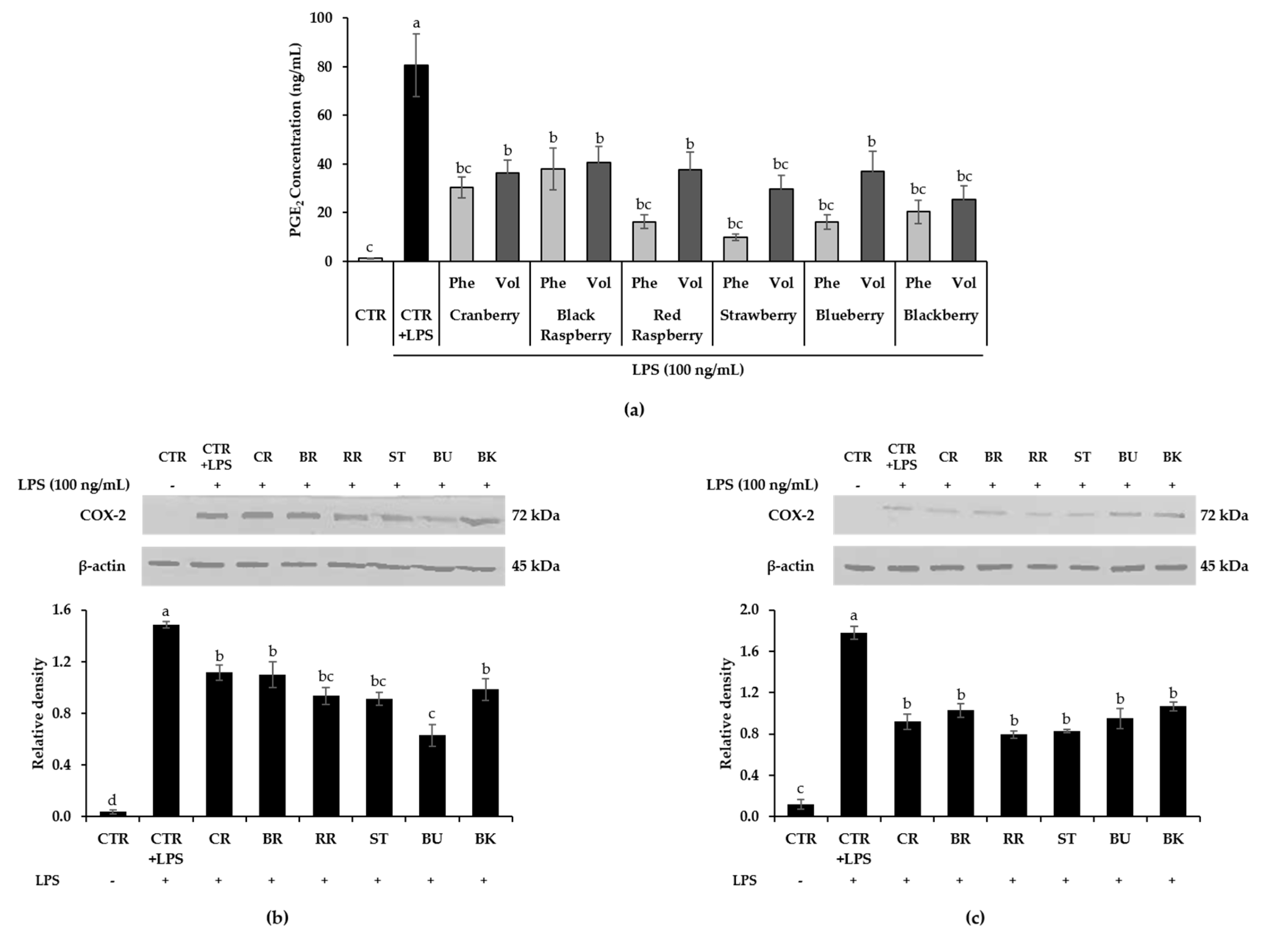
3.6. Effect of Berry Phenolic and Volatile Extracts on LPS-Stimulated PGE2/COX-2 Production
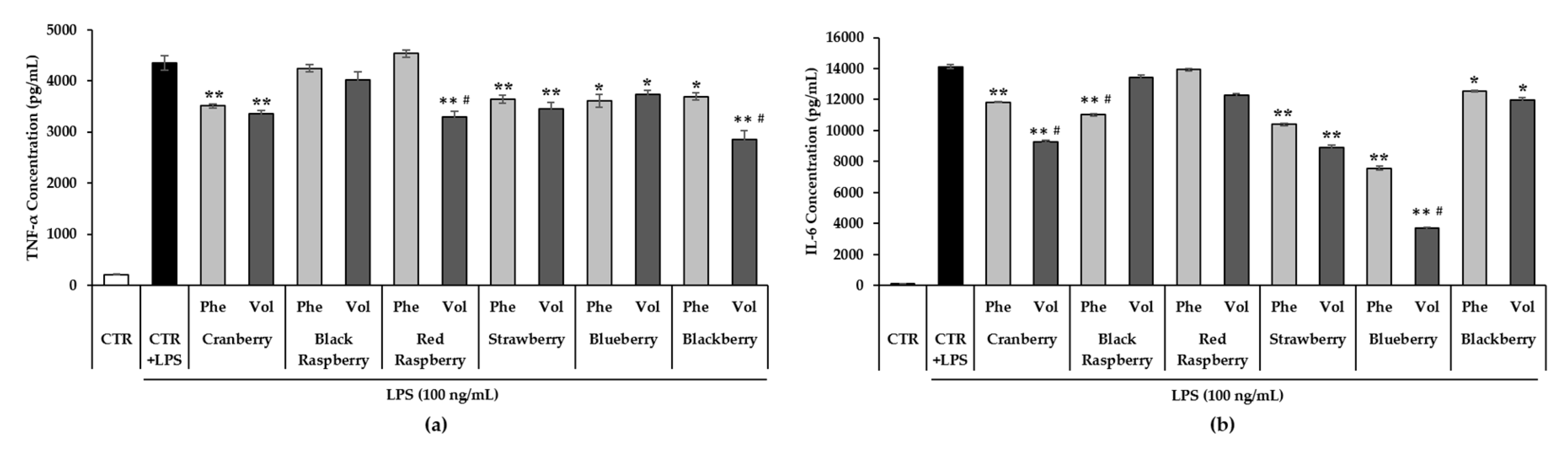
3.7. Effect of Berry Phenolic and Volatile Extracts on LPS-Induced Pro-Inflammatory Cytokines Production
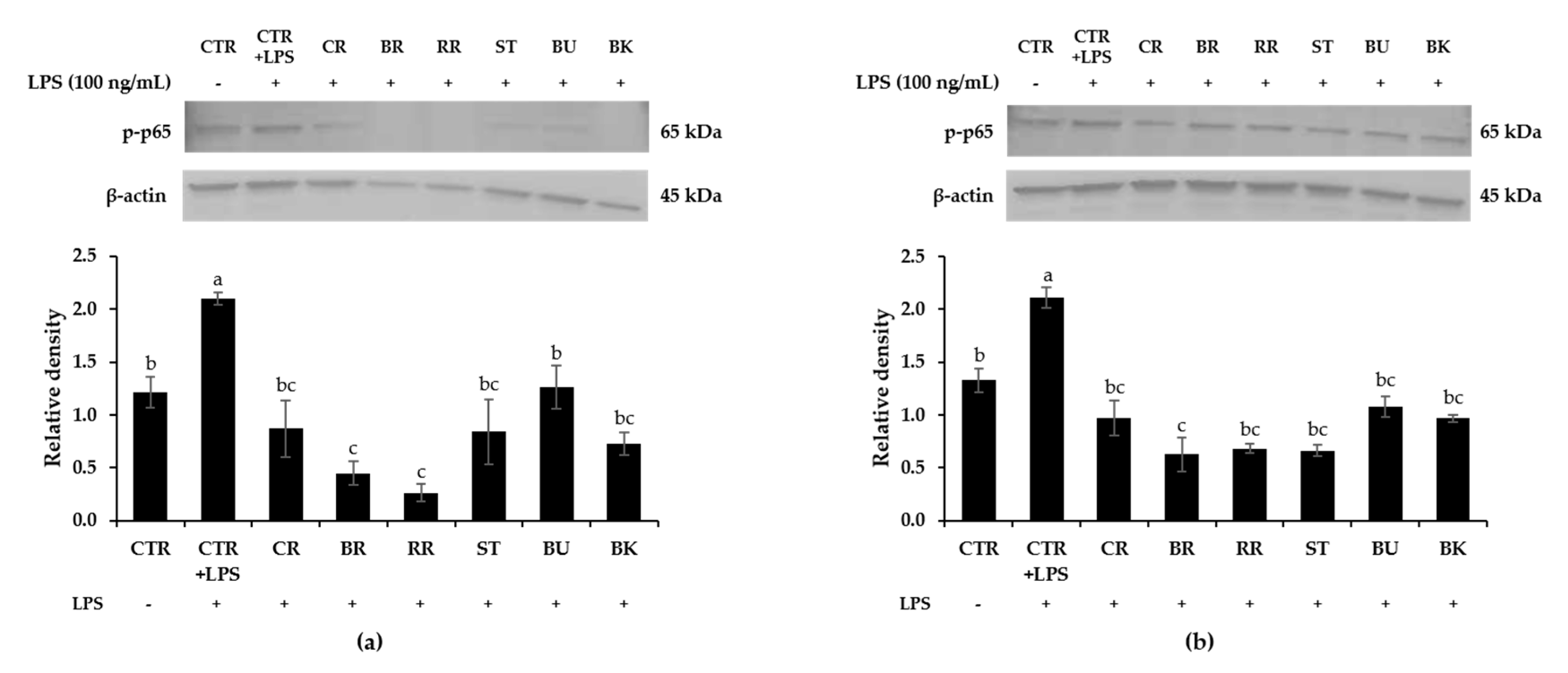
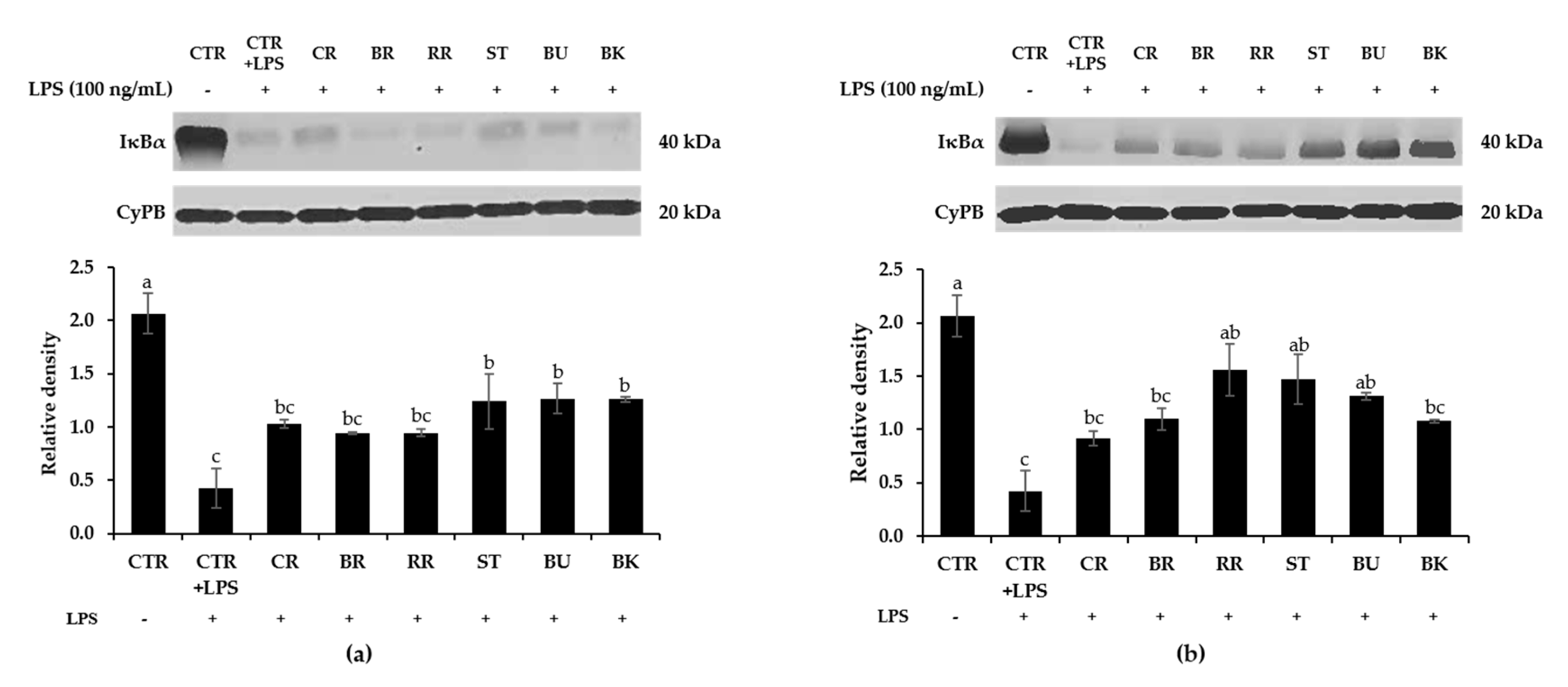
3.8. Effect of Berry Phenolic and Volatile Extracts on LPS-Stimulated NF-κB Activation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kader, A.A. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Varela-López, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and therapeutic effects of edible berries: A focus on colon cancer prevention and treatment. Molecules 2016, 21, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farzaei, M.H.; Rahimi, R.; Abdollahi, M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr. Pharm. Biotechnol. 2015, 16, 196–210. [Google Scholar] [CrossRef]
- Martin, D.A.; Bolling, B.W. A review of the efficacy of dietary polyphenols in experimental models of inflammatory bowel diseases. Food Funct. 2015, 6, 1773–1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Fruit polyphenols: A review of anti-inflammatory effects in humans. Crit. Rev. Food Sci. Nutr. 2016, 56, 419–444. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.S.; Wu, X.; Patterson, K.M.; Barnes, J.; Carter, S.G.; Scherwitz, L.; Beaman, R.; Endres, J.R.; Schauss, A.G. In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J. Agric. Food Chem. 2008, 56, 8326–8333. [Google Scholar] [CrossRef] [Green Version]
- Kristo, A.S.; Klimis-Zacas, D.; Sikalidis, A.K. Protective role of dietary berries in cancer. Antioxidants 2016, 5, 37. [Google Scholar] [CrossRef] [Green Version]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.; Wu, Z.; Huang, L.; Qiu, H.; Wang, L.; Li, L.; Yao, L.; Kang, K.; Qu, J.; Wu, Y.; et al. Mulberry fruit prevents LPS-induced NF-κB/pERK/MAPK signals in macrophages and suppresses acute colitis and colorectal tumorigenesis in mice. Sci. Rep. 2015, 5, 17348. [Google Scholar] [CrossRef] [Green Version]
- Goff, S.A.; Klee, H.J. Plant volatile compounds: Sensory cues for health and nutritional value? Science 2006, 311, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Hui, Y.H.; Chen, F.; Nollet, L.M.; Guiné, R.P.; Martín-Belloso, O.; Mínguez-Mosquera, M.I.; Paliyath, G.; Pessoa, F.L.; Le Quéré, J.L.; Sidhu, J.S.; et al. (Eds.) Handbook of Fruit and Vegetable Flavors; John Wiley and Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Forney, C.F.; Song, J. Flavors and aromas: Chemistry and biological functions. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; pp. 515–540. [Google Scholar]
- Fenoll, J.; Manso, A.; Hellín, P.; Ruiz, L.; Flores, P. Changes in the aromatic composition of the Vitis vinifera grape Muscat Hamburg during ripening. Food Chem. 2009, 114, 420–428. [Google Scholar] [CrossRef]
- Dymerski, T.; Namieśnik, J.; Vearasilp, K.; Arancibia-Avila, P.; Toledo, F.; Weisz, M.; Katrich, E.; Gorinstein, S. Comprehensive two-dimensional gas chromatography and three-dimensional fluorometry for detection of volatile and bioactive substances in some berries. Talanta 2015, 134, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Paré, P.W.; Tumlinson, J.H. Plant volatiles as a defense against insect herbivores. Plant Physiol. 1999, 121, 325–332. [Google Scholar] [CrossRef] [Green Version]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Kang, J.; Li, Z.; Schauss, A.G.; Badger, T.M.; Nagarajan, S.; Wu, T.; Wu, X. The açaí flavonoid velutin is a potent anti-inflammatory agent: Blockade of LPS-mediated TNF-α and IL-6 production through inhibiting NF-κB activation and MAPK pathway. J. Nutr. Biochem. 2012, 23, 1184–1191. [Google Scholar] [CrossRef]
- Agrawal, N.K.; Kant, S. Targeting inflammation in diabetes: Newer therapeutic options. World J. Diabetes 2014, 5, 697. [Google Scholar] [CrossRef]
- Jang, K.J.; Choi, S.H.; Yu, G.J.; Hong, S.H.; Chung, Y.H.; Kim, C.H.; Yoon, H.M.; Kim, G.Y.; Kim, B.W.; Choi, Y.H. Anti-inflammatory potential of total saponins derived from the roots of Panax ginseng in lipopolysaccharide-activated RAW 264.7 macrophages. Exp. Ther. Med. 2016, 11, 1109–1115. [Google Scholar] [CrossRef]
- Dmitrieva, O.S.; Shilovskiy, I.P.; Khaitov, M.R.; Grivennikov, S.I. Interleukins 1 and 6 as main mediators of inflammation and cancer. Biochemistry (Moscow) 2016, 81, 80–90. [Google Scholar] [CrossRef]
- Cheng, A.; Yan, H.; Han, C.; Wang, W.; Tian, Y.; Chen, X. Polyphenols from blueberries modulate inflammation cytokines in LPS-induced RAW264. 7 macrophages. Int. J. Biol. Macromol. 2014, 69, 382–387. [Google Scholar] [CrossRef]
- Lee, W.S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1β, IL-6 and TNF-α production by AP-1 and NF-κB inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Carrillo-Perdomo, E.; Aller, A.; Giampieri, F.; Gasparrini, M.; González-Pérez, L.; Beltrán-Ayala, P.; Battino, M. Anti-inflammatory effect of Capuli cherry against LPS-induced cytotoxic damage in RAW 264.7 macrophages. Food Chem. Toxicol. 2017, 102, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R.; Liyanage, R.; Lay, J.O.; Prior, R.L. Ellagitannin composition of blackberry as determined by HPLC-ESI-MS and MALDI-TOF-MS. J. Agric. Food Chem. 2008, 56, 661–669. [Google Scholar] [CrossRef]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonol glycosides and antioxidant capacity of various blackberry and blueberry genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2005, 85, 2149–2158. [Google Scholar] [CrossRef]
- Moore, K.; Howard, L.; Brownmiller, C.; Gu, I.; Lee, S.O.; Mauromoustakos, A. Inhibitory effects of cranberry polyphenol and volatile extracts on nitric oxide production in LPS activated RAW 264.7 macrophages. Food Funct. 2019, 10, 7091–7102. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Akkari, H.; Hajaji, S.; B’chir, F.; Rekik, M.; Gharbi, M. Correlation of polyphenolic content with radical-scavenging capacity and anthelmintic effects of Rubus ulmifolius (Rosaceae) against Haemonchus contortus. Vet. Parasitol. 2016, 221, 46–53. [Google Scholar] [CrossRef]
- Griess, P. Bemerkungen zu der abhandlung der HH. Weselsky und Benedikt Ueber einige Azoverbindungen. Ber. Dtsch. Chem. Ges. 1879, 12, 426–428. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.Y.; Stretch, A.W. Antioxidant capacity in cranberry is influenced by cultivar and storage temperature. J. Agric. Food Chem. 2001, 49, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Viskelis, P.; Rubinskienė, M.; Jasutienė, I.; Šarkinas, A.; Daubaras, R.; Česonienė, L. Anthocyanins, antioxidative, and antimicrobial properties of American cranberry (Vaccinium macrocarpon Ait.) and their press cakes. J. Food Sci. 2009, 74, C157–C161. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef]
- Vvedenskaya, I.O.; Rosen, R.T.; Guido, J.E.; Russell, D.J.; Mills, K.A.; Vorsa, N. Characterization of flavonols in cranberry (Vaccinium macrocarpon) powder. J. Agric. Food Chem. 2004, 52, 188–195. [Google Scholar] [CrossRef] [PubMed]
- White, B.L.; Howard, L.R.; Prior, R.L. Impact of different stages of juice processing on the anthocyanin, flavonol, and procyanidin contents of cranberries. J. Agric. Food Chem. 2011, 59, 4692–4698. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Tulio, A.Z., Jr.; Reese, R.N.; Wyzgoski, F.J.; Rinaldi, P.L.; Fu, R.; Scheerens, J.C.; Miller, A.R. Cyanidin 3-rutinoside and cyanidin 3-xylosylrutinoside as primary phenolic antioxidants in black raspberry. J. Agric. Food Chem. 2008, 56, 1880–1888. [Google Scholar] [CrossRef]
- Anttonen, M.J.; Karjalainen, R.O. Environmental and genetic variation of phenolic compounds in red raspberry. J. Food Compos. Anal. 2005, 18, 759–769. [Google Scholar] [CrossRef]
- Connor, A.M.; Stephens, M.J.; Hall, H.K.; Alspach, P.A. Variation and heritabilities of antioxidant activity and total phenolic content estimated from a red raspberry factorial experiment. J. Am. Soc. Hortic. Sci. 2005, 130, 403–411. [Google Scholar] [CrossRef] [Green Version]
- Mullen, W.; McGinn, J.; Lean, M.E.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef]
- Mullen, W.; Yokota, T.; Lean, M.E.; Crozier, A. Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC–MSn. Phytochemistry 2003, 64, 617–624. [Google Scholar] [CrossRef]
- Rekika, D.; Khanizadeh, S.; Deschênes, M.; Levasseur, A.; Charles, M.T.; Tsao, R.; Yang, R. Antioxidant capacity and phenolic content of selected strawberry genotypes. HortScience 2005, 40, 1777–1781. [Google Scholar] [CrossRef] [Green Version]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Lee, R.; Scheuller, H.S.; Heber, D. Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chem. 2006, 97, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Törrönen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef]
- Sellappan, S.; Akoh, C.C.; Krewer, G. Phenolic compounds and antioxidant capacity of Georgia-grown blueberries and blackberries. J. Agric. Food Chem. 2002, 50, 2432–2438. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant capacity as influenced by total phenolic and anthocyanin content, maturity, and variety of Vaccinium species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Masuero, D.; Palmieri, L.; Mattivi, F. Identification and quantification of flavonol glycosides in cultivated blueberry cultivars. J. Food Compos. Anal. 2012, 25, 9–16. [Google Scholar] [CrossRef]
- Siriwoharn, T.; Wrolstad, R.E. Polyphenolic composition of Marion and Evergreen blackberries. J. Food Sci. 2004, 69, FCT233–FCT240. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, F.; Wang, L.; Niu, Y.; Chen, H.; Wang, H.; Xiao, Z. Characterization of the key aroma volatile compounds in cranberry (Vaccinium macrocarpon Ait.) using gas chromatography–olfactometry (GC-O) and odor activity value (OAV). J. Agric. Food Chem. 2016, 64, 4990–4999. [Google Scholar] [CrossRef] [PubMed]
- Hirvi, T.; Honkanen, E.; Pyysalo, T. xThe aroma of cranberries. Z. Lebensm. Unters. Forsch. 1981, 172, 365–367. [Google Scholar] [CrossRef]
- Anjou, K.L.A.S.; von Sydow, E.R.I.K. The aroma of cranberries. II. Vaccinium macrocarpon Ait. Acta Chem. Scand. 1967, 21, 2076–2082. [Google Scholar] [CrossRef]
- Croteau, R.J.; Fagerson, I.S. Major volatile components of the juice of American cranberry. J. Food Sci. 1968, 33, 386–389. [Google Scholar] [CrossRef]
- Ruse, K.; Sabovics, M.; Rakcejeva, T.; Dukalska, L.; Galoburda, R.; Berzina, L. The effect of drying conditions on the presence of volatile compounds in cranberries. World Acad. Sci. Eng. Technol. 2012, 64, 854–860. [Google Scholar]
- Kim, B.H.; Park, S.K. Volatile aroma and sensory analysis of black raspberry wines fermented by different yeast strains. J. Inst. Brew. 2015, 121, 87–94. [Google Scholar] [CrossRef]
- Du, X.; Qian, M. Flavor chemistry of small fruits: Blackberry, raspberry, and blueberry. In Flavor and Health Benefits of Small Fruits; American Chemical Society: Washington, DC, USA, 2010; pp. 27–43. [Google Scholar]
- Klesk, K.; Qian, M.; Martin, R.R. Aroma extract dilution analysis of cv. Meeker (Rubus idaeus L.) red raspberries from Oregon and Washington. J. Agric. Food Chem. 2004, 52, 5155–5161. [Google Scholar] [CrossRef]
- Aprea, E.; Biasioli, F.; Gasperi, F. Volatile compounds of raspberry fruit: From analytical methods to biological role and sensory impact. Molecules 2015, 20, 2445–2474. [Google Scholar] [CrossRef] [Green Version]
- Malowicki, S.M.; Martin, R.; Qian, M.C. Comparison of sugar, acids, and volatile composition in raspberry bushy dwarf virus-resistant transgenic raspberries and the wild type ‘Meeker’(Rubus Idaeus L.). J. Agric. Food Chem. 2008, 56, 6648–6655. [Google Scholar] [CrossRef]
- Pabst, A.; Barron, D.; Etievant, P.; Schreier, P. Studies on the enzymic hydrolysis of bound aroma constituents from raspberry fruit pulp. J. Agric. Food Chem. 1991, 39, 173–175. [Google Scholar] [CrossRef]
- Zabetakis, I.; Holden, M.A. Strawberry flavour: Analysis and biosynthesis. J. Sci. Food Agric. 1997, 74, 421–434. [Google Scholar] [CrossRef]
- Pyysalo, T.; Honkanen, E.; Hirvi, T. Volatiles of wild strawberries, Fragaria vesca L., compared to those of cultivated berries, Fragaria. times. ananassa cv Senga Sengana. J. Agric. Food Chem. 1979, 27, 19–22. [Google Scholar] [CrossRef]
- Hakala, M.A.; Lapveteläinen, A.T.; Kallio, H.P. Volatile compounds of selected strawberry varieties analyzed by purge-and-trap headspace GC-MS. J. Agric. Food Chem. 2002, 50, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Misran, A.; Padmanabhan, P.; Sullivan, J.A.; Khanizadeh, S.; Paliyath, G. Composition of phenolics and volatiles in strawberry cultivars and influence of preharvest hexanal treatment on their profiles. Can. J. Plant Sci. 2015, 95, 115–126. [Google Scholar] [CrossRef]
- Farneti, B.; Khomenko, I.; Grisenti, M.; Ajelli, M.; Betta, E.; Algarra, A.A.; Cappellin, L.; Aprea, E.; Gasperi, F.; Biasioli, F.; et al. Exploring blueberry aroma complexity by chromatographic and direct-injection spectrometric techniques. Front. Plant Sci. 2017, 8, 617. [Google Scholar] [CrossRef]
- Du, X.; Rouseff, R. Aroma active volatiles in four southern highbush blueberry cultivars determined by gas chromatography–olfactometry (GC-O) and gas chromatography–mass spectrometry (GC-MS). J. Agric. Food Chem. 2014, 62, 4537–4543. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.C.; Stein-Chisholm, R.E.; Boykin, D.L. Qualitative analysis of volatiles in rabbiteye blueberry cultivars at various maturities using rapid solid-phase microextraction. J. Am. Soc. Hortic. Sci. 2014, 139, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, J.L.; Schwieterman, M.L.; Colquhoun, T.A.; Clark, D.G.; Olmstead, J.W. Potential for increasing southern highbush blueberry flavor acceptance by breeding for major volatile components. HortScience 2013, 48, 835–843. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, M.F.; Sanz, J.; Martínez-Castro, I.; Giuffrè, A.M.; Sicari, V.; Soria, A.C. Statistical analysis for improving data precision in the SPME GC–MS analysis of blackberry (Rubus ulmifolius Schott) volatiles. Talanta 2014, 125, 248–256. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, M.F.; Sanz, J.; Sanz, M.L.; Giuffrè, A.M.; Sicari, V.; Soria, A.C. Optimization of a solid-phase microextraction method for the gas chromatography–mass spectrometry analysis of blackberry (Rubus ulmifolius Schott) fruit volatiles. Food Chem. 2015, 178, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Humpf, H.U.; Schreier, P. Bound aroma compounds from the fruit and the leaves of blackberry (Rubus laciniata L.). J. Agric. Food Chem. 1991, 39, 1830–1832. [Google Scholar] [CrossRef]
- Klesk, K.; Qian, M. Aroma extract dilution analysis of cv. Marion (Rubus s hyb) and cv. Evergreen (R. laciniatus L.) blackberries. J. Agric. Food Chem. 2003, 51, 3436–3441. [Google Scholar] [CrossRef] [PubMed]
- Jacques, A.C.; Chaves, F.C.; Zambiazi, R.C.; Brasil, M.C.; Caramão, E.B. Bioactive and volatile organic compounds in Southern Brazilian blackberry (Rubus fruticosus) fruit cv. Tupy. Food Sci. Technol. 2014, 34, 636–643. [Google Scholar] [CrossRef] [Green Version]
- Paredes-López, O.; Cervantes-Ceja, M.L.; Vigna-Pérez, M.; Hernández-Pérez, T. Berries: Improving human health and healthy aging, and promoting quality life—A review. Plant Foods Hum. Nutr. 2010, 65, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Battino, M.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Mezzetti, B.; Giampieri, F. The effects of strawberry bioactive compounds on human health. In Proceedings of the VIII International Strawberry Symposium, Québec City, QC, Canada, 13–17 August 2016; Volume 1156, pp. 355–362. [Google Scholar]
- Seeram, N.P.; Heber, D. Impact of berry phytochemicals on human health: Effects beyond antioxidation. In Antioxidant Measurement and Applications; American Chemical Society: Washington, DC, USA, 2006. [Google Scholar]
- Koli, R.; Erlund, I.; Jula, A.; Marniemi, J.; Mattila, P.; Alfthan, G. Bioavailability of various polyphenols from a diet containing moderate amounts of berries. J. Agric. Food Chem. 2010, 58, 3927–3932. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The blackberry fruit: A review on its composition and chemistry, metabolism and bioavailability, and health benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and bioavailability of anthocyanins and phenolic acids from grape/blueberry juice and smoothie in vitro and in vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef] [Green Version]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef]
- Giampieri, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Kohlert, C.; Van Rensen, I.; März, R.; Schindler, G.; Graefe, E.U.; Veit, M. Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Med. 2000, 66, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, A.U. An overview of inflammation: Mechanism and consequences. Front. Biol. 2011, 6, 274. [Google Scholar] [CrossRef]
- Duarte, J.; Francisco, V.; Perez-Vizcaino, F. Modulation of nitric oxide by flavonoids. Food Funct. 2014, 5, 1653–1668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzik, T.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Bogdan, C. Nitric oxide synthase in innate and adaptive immunity: An update. Trends Immunol. 2015, 36, 161–178. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Wu, Z.; Yao, L.; Wu, Y.; Huang, L.; Liu, K.; Zhou, X.; Gou, D. Anthocyanin-rich fractions from red raspberries attenuate inflammation in both RAW264. 7 macrophages and a mouse model of colitis. Sci. Rep. 2014, 4, 6234. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.; Han, C.; Fang, X.; Sun, J.; Chen, X.; Wan, F. Extractable and non-extractable polyphenols from blueberries modulate LPS-induced expression of iNOS and COX-2 in RAW264. 7 macrophages via the NF-κB signalling pathway. J. Sci. Food Agric. 2016, 96, 3393–3400. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef]
- Jeong, J.B.; Jeong, H.J. Rheosmin, a naturally occurring phenolic compound inhibits LPS-induced iNOS and COX-2 expression in RAW264. 7 cells by blocking NF-κB activation pathway. Food Chem. Toxicol. 2010, 48, 2148–2153. [Google Scholar] [CrossRef]
- Hwang, D.; Kang, M.J.; Jo, M.J.; Seo, Y.B.; Park, N.G.; Kim, G.D. Anti-inflammatory activity of β-thymosin peptide derived from pacific oyster (crassostrea gigas) on no and pge2 production by down-regulating nf-κb in lps-induced raw264. 7 macrophage cells. Mar. Drugs 2019, 17, 129. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zou, L.; Li, T.; Lai, D.; Wu, Y.; Qin, S. Mogroside V inhibits LPS-induced COX-2 expression/ROS production and overexpression of HO-1 by blocking phosphorylation of AKT1 in RAW264. 7 cells. Acta Biochim. Biophys. Sin. 2019, 51, 365–374. [Google Scholar] [CrossRef]
- Turini, M.E.; DuBois, R.N. Cyclooxygenase-2: A therapeutic target. Annu. Rev. Med. 2002, 53, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Yao, F.; Xue, Q.; Li, K.; Cao, X.; Sun, L.; Liu, Y. Phenolic compounds and ginsenosides in ginseng shoots and their antioxidant and anti-inflammatory capacities in LPS-Induced RAW264. 7 Mouse Macrophages. Int. J. Mol. Sci. 2019, 20, 2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aoki, T.; Narumiya, S. Prostaglandins and chronic inflammation. Trends Pharmacol. Sci. 2012, 33, 304–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Velde, F.; Grace, M.H.; Esposito, D.; Pirovani, M.É.; Lila, M.A. Quantitative comparison of phytochemical profile, antioxidant, and anti-inflammatory properties of blackberry fruits adapted to Argentina. J. Food Compos. Anal. 2016, 47, 82–91. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, H.; Kim, S.A.; Park, H.K.; Kim, W. Anti-inflammatory and anti-superbacterial activity of polyphenols isolated from black raspberry. Korean J. Physiol. Pharmacol. 2013, 17, 73–79. [Google Scholar] [CrossRef]
- Soromou, L.W.; Zhang, Z.; Li, R.; Chen, N.; Guo, W.; Huo, M.; Guan, S.; Lu, J.; Deng, X. Regulation of inflammatory cytokines in lipopolysaccharide-stimulated RAW 264.7 murine macrophage by 7-O-methyl-naringenin. Molecules 2012, 17, 3574–3585. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Ahn, C.B.; Je, J.Y. Anti-inflammatory action of high molecular weight Mytilus edulis hydrolysates fraction in LPS-induced RAW264.7 macrophage via NF-κB and MAPK pathways. Food Chem. 2016, 202, 9–14. [Google Scholar] [CrossRef]
- Yeganeh Montakhab, H.; Dousti, M.; Ahmadi Rezaie, H.B. Assessment of the relative level of TNF-α gene expression in RAW264. 7 Cells. Mod. Med Lab. J. 2017, 1, 23–28. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, S.; Ciechomska, M.; Cant, R.; Hügle, T.; van Laar, J.M. Interleukin-6, its role in fibrosing conditions. Cytokine Growth Factor Rev. 2012, 23, 99–107. [Google Scholar] [CrossRef]
- Shirota, Y.; Yarboro, C.; Fischer, R.; Pham, T.H.; Lipsky, P.; Illei, G.G. Impact of anti-interleukin-6 receptor blockade on circulating T and B cell subsets in patients with systemic lupus erythematosus. Ann. Rheum. Dis. 2013, 72, 118–128. [Google Scholar] [CrossRef]
- Rincon, M.; Irvin, C.G. Role of IL-6 in asthma and other inflammatory pulmonary diseases. Int. J. Biol. Sci. 2012, 8, 1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zhang, J.; Wang, H.; Xu, J.; He, J.; Liu, L.; Zhang, T.; Chen, R.; Kang, J. Phenolic acid profiling, antioxidant, and anti-inflammatory activities, and miRNA regulation in the polyphenols of 16 blueberry samples from China. Molecules 2017, 22, 312. [Google Scholar] [CrossRef] [PubMed]
- Parelman, M.A.; Storms, D.H.; Kirschke, C.P.; Huang, L.; Zunino, S.J. Dietary strawberry powder reduces blood glucose concentrations in obese and lean C57BL/6 mice, and selectively lowers plasma C-reactive protein in lean mice. Br. J. Nutr. 2012, 108, 1789–1799. [Google Scholar] [CrossRef] [Green Version]
- Jo, Y.H.; Park, H.C.; Choi, S.; Kim, S.; Bao, C.; Kim, H.W.; Choi, H.K.; Lee, H.J.; Auh, J.H. Metabolomic analysis reveals cyanidins in black raspberry as candidates for suppression of lipopolysaccharide-induced inflammation in murine macrophages. J. Agric. Food Chem. 2015, 63, 5449–5458. [Google Scholar] [CrossRef]
- Khan, H.; Ullah, H.; Castilho, P.C.M.F.; Gomila, A.S.; D’Onofrio, G.; Filosa, R.; Wang, F.; Nabavi, S.M.; Daglia, M.; Silva, A.S.; et al. Targeting NF-κB signaling pathway in cancer by dietary polyphenols. Crit. Rev. Food Sci. Nutr. 2019, 1–11. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Christian, F.; Smith, E.L.; Carmody, R.J. The regulation of NF-κB subunits by phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.R.; Moreira, F.V.; Fraga, B.P.; Souza, D.P.D.; Bonjardim, L.R.; Quintans-Junior, L.J. Cardiovascular effects of monoterpenes: A review. Rev. Bras. Farmacogn. 2011, 21, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Silva, R.O.; Salvadori, M.S.; Sousa, F.B.M.; Santos, M.S.; Carvalho, N.S.; Sousa, D.P.; Gomes, B.S.; Oliveira, F.A.; Barbosa, A.L.R.; Freitas, R.M.; et al. Evaluation of the anti-inflammatory and antinociceptive effects of myrtenol, a plant-derived monoterpene alcohol, in mice. Flavour Fragr. J. 2014, 29, 184–192. [Google Scholar] [CrossRef]
- Viana, A.F.S.C.; da Silva, F.V.; Fernandes, H.D.B.; Oliveira, I.S.; Braga, M.A.; Nunes, P.I.G.; Viana, D.D.A.; de Sousa, D.P.; Rao, V.S.; Oliveira, R.D.C.M.; et al. Gastroprotective effect of (-)-myrtenol against ethanol-induced acute gastric lesions: Possible mechanisms. J. Pharm. Pharmacol. 2016, 68, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Cui, X.; Xue, J.; Chi, G.; Gao, R.; Deng, X.; Guan, S.; Wei, J.; Soromou, L.W.; Feng, H.; et al. Anti-inflammatory effects of linalool in RAW 264.7 macrophages and lipopolysaccharide-induced lung injury model. J. Surg. Res. 2013, 180, e47–e54. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.B.; Gali-Muhtasib, H.; Göransson, H.; Larsson, R. Alpha terpineol: A potential anticancer agent which acts through suppressing NF-κB signalling. Anticancer Res. 2010, 30, 1911–1919. [Google Scholar] [PubMed]
| Berry | Phenolic Extract | Total Volatiles | Volatile Extract | ||
|---|---|---|---|---|---|
| TPH 1 (mg GAE/100 g) | TACY 2 (mg/100 g) | DPPH (µM TE/100 g) | (µg/kg) | DPPH (µM TE/kg) | |
| Cranberry | 421.3 b 3 | 98.6 c | 1806.5 bc | 2864.9 c | 4.93 a |
| Black raspberry | 1186.4 a | 342.0 a | 2761.8 a | 8556.1 b | 7.73 a |
| Red raspberry | 208.1 c | 19.1 e | 1474.8 d | 2055.5 cd | 6.57 a |
| Strawberry | 125.4 d | 8.7 e | 643.6 e | 2989.7 c | 8.62 a |
| Blueberry | 389.5 b | 184.9 b | 1674.8 c | 1424.1 d | ND |
| Blackberry | 386.2 b | 70.2 d | 1980.2 b | 12,212.9 a | ND |
| Class | Cranberry | Black Raspberry | Red Raspberry | Strawberry | Blueberry | Blackberry |
|---|---|---|---|---|---|---|
| Monoterpene | 1727.1 ± 129.3 1 | 5217.6 ± 562.9 | 958.7 ± 240.3 | 1293.9 ± 144 | 639.6 ± 129.9 | 1163.1 ± 150.6 |
| Alcohol | 176.6 ± 50.6 | 1163.5 ± 183.3 | 89.9 ± 27.5 | 85.4 ± 22.9 | 247.1 ± 96 | 2198.1 ± 121.4 |
| Aldehyde | 158.6 ± 23.4 | 647 ± 315.8 | 106.4 ± 9.8 | 158.9 ± 29.9 | 110.2 ± 31.3 | 39.4 ± 8.1 |
| Ester | 413.6 ± 46.3 | 836.6 ± 189 | 42.5 ± 9.4 | 414.4 ± 108.6 | 93.8 ± 27.8 | 1240.8 ± 201.4 |
| Ketone | 50.3 ± 2.6 | 78 ± 6.2 | 52.9 ± 14.7 | 84.1 ± 24.6 | 62.8 ± 13.4 | 159.9 ± 13.5 |
| Acid | 338.7 ± 39.4 | 312.9 ± 51.4 | 489.7 ± 231 | 597.1 ± 251.9 | 33.4 ± 15 | 6967.2 ± 503.7 |
| C13 norisoprenoid | 126 ± 30.5 | 186.3 ± 33.5 | 11.7 ± 1.1 | 96.2 ± 9.2 | 90.8 ± 61.4 | |
| Sesquiterpene | 43.4 ± 11.2 | 8.6 ± 2.2 | ||||
| Phenolic | 32.3 ± 12 | 78.8 ± 8.7 | 10.6 ± 2.3 | 32 ± 0.8 | 4.6 ± 4.6 | |
| Lactone | 24.4 ± 3.9 | 30.9 ± 3.8 | 8.8 ± 1.1 | 55.1 ± 27 | ||
| Alkylbenzene | 34.7 ± 5.1 | 4.4 ± 1.1 | 99.5 ± 13.8 | |||
| Furan | 38.5 ± 19.3 | 206.8 ± 34.8 | 67 ± 26.3 | 293.9 ± 38.5 | ||
| Furanone | 8.9 ± 4.3 | 33.4 ± 11.2 | ||||
| Hydrocarbon | 1.2 ± 1.2 | 6.1 ± 1.4 | 11.8 ± 3.0 | |||
| Benzothiazole | 6.7 ± 0.9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, I.; Brownmiller, C.; Stebbins, N.B.; Mauromoustakos, A.; Howard, L.; Lee, S.-O. Berry Phenolic and Volatile Extracts Inhibit Pro-Inflammatory Cytokine Secretion in LPS-Stimulated RAW264.7 Cells through Suppression of NF-κB Signaling Pathway. Antioxidants 2020, 9, 871. https://doi.org/10.3390/antiox9090871
Gu I, Brownmiller C, Stebbins NB, Mauromoustakos A, Howard L, Lee S-O. Berry Phenolic and Volatile Extracts Inhibit Pro-Inflammatory Cytokine Secretion in LPS-Stimulated RAW264.7 Cells through Suppression of NF-κB Signaling Pathway. Antioxidants. 2020; 9(9):871. https://doi.org/10.3390/antiox9090871
Chicago/Turabian StyleGu, Inah, Cindi Brownmiller, Nathan B. Stebbins, Andy Mauromoustakos, Luke Howard, and Sun-Ok Lee. 2020. "Berry Phenolic and Volatile Extracts Inhibit Pro-Inflammatory Cytokine Secretion in LPS-Stimulated RAW264.7 Cells through Suppression of NF-κB Signaling Pathway" Antioxidants 9, no. 9: 871. https://doi.org/10.3390/antiox9090871