Hercynine, Ergothioneine and Redox State in Stallion’s Seminal Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Semen and Seminal Plasma Samplings
2.2. Quantification of Ergothioneine (ERT) in Seminal Plasma
2.3. Quantification of Hercynine (ERY) in Seminal Plasma
2.4. Quantification of Glutathione (GSH) and Cysteine (Cys) in Seminal Plasma
2.5. Quantification of Free Thiol Groups in Seminal Plasma (DTNB)
2.6. Trolox Equivalent Antioxidant Capacity Assay (TEAC)
2.7. Statistical Analysis
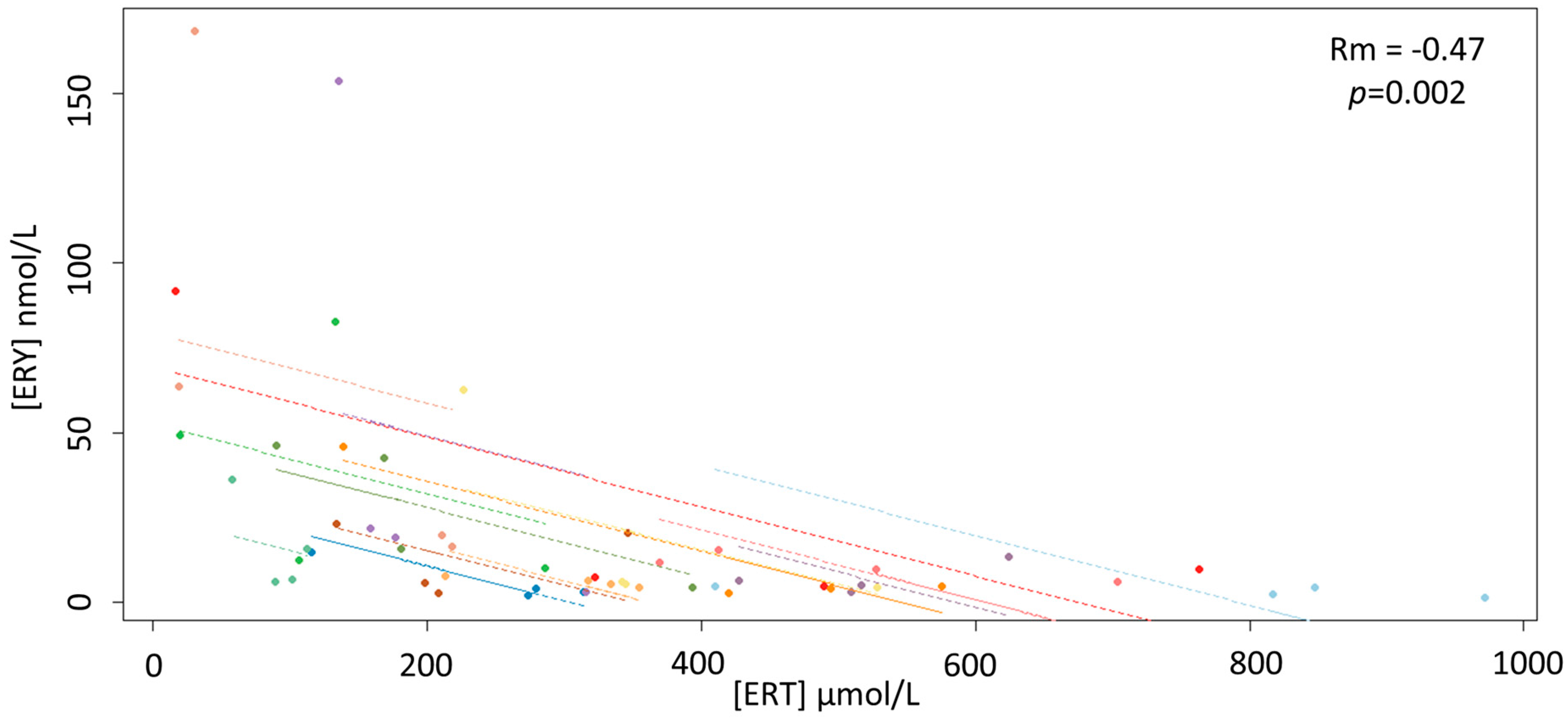
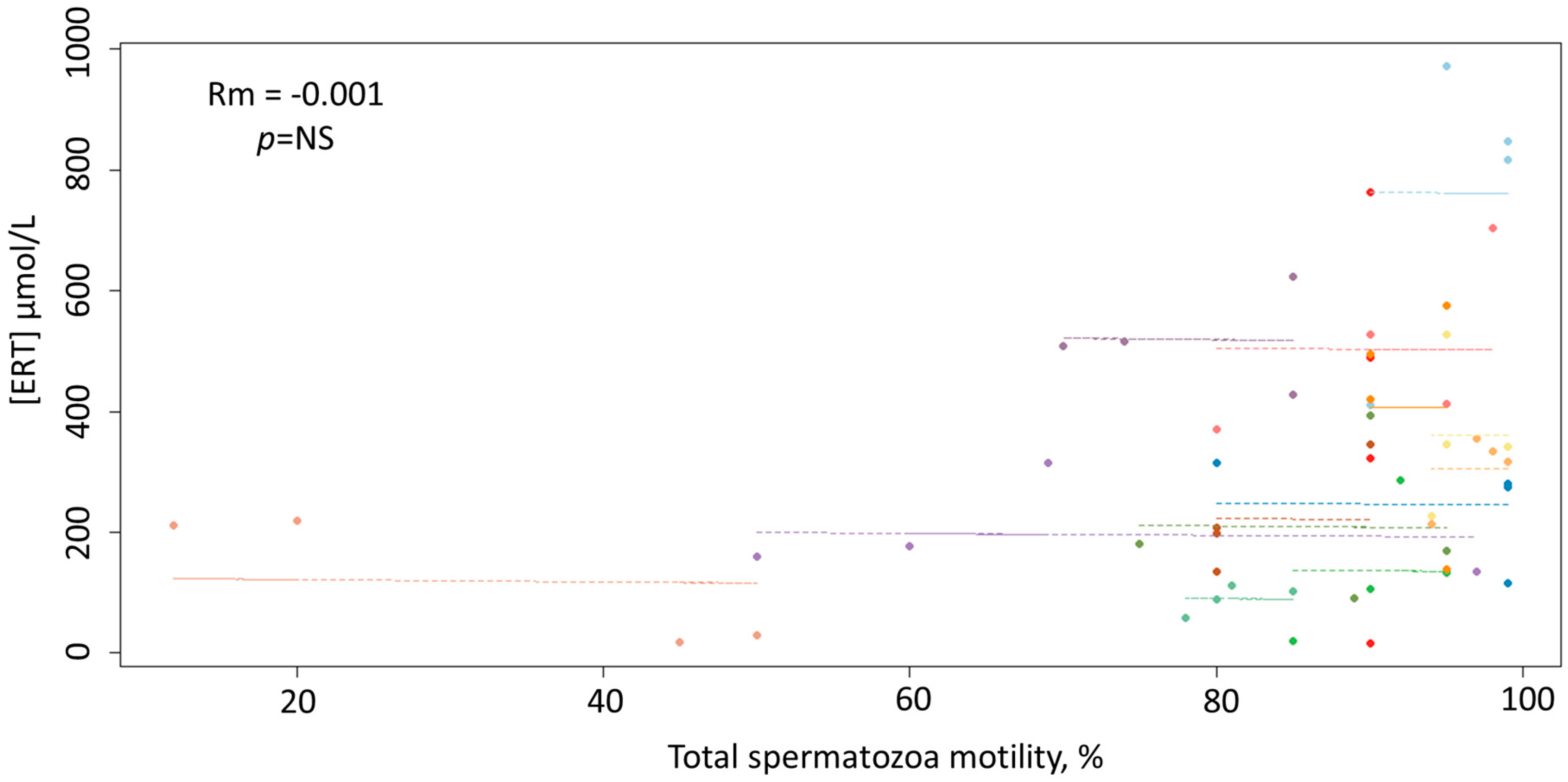
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Storey, B.T. Mammalian sperm metabolism: Oxygen and sugar, friend and foe. Int. J. Dev. Biol. 2008, 52, 427–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibb, Z.; Lambourne, S.R.; Aitken, R.J. The paradoxical relationship between stallion fertility and oxidative stress. Biol. Reprod. 2014, 91, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ferrusola, C.O.; Fernandez, L.G.; Sandoval, C.S.; García, B.M.; Martínez, H.R.; Tapia, J.A.; Peña, F.J. Inhibition of the mitochondrial permeability transition pore reduces ‘apoptosis like’ changes during cryopreservation of stallion spermatozoa. Theriogenology 2010, 74, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Johannisson, A.; Dalin, A.M.; Hammar, L.; Sandebert, T.; Rodriguez-Martinez, H. Sperm morphology and chromatin integrity in Swedish warmblood stallions and their relationship to pregnancy rates. Acta Vet. Scand. 2008, 50, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Ball, B.A.; Vo, A.T.; Baumber, J. Generation of reactive oxygen species by equine spermatozoa. Am. J. Vet. Res. 2001, 62, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, C.; Kharche, S.D.; Ranjan, R.; Kumar, S.; Goel, A.K.; Jindal, S.K.; Agarwal, S.K. Effect of vitamin C supplementation on freezability of Barbari buck semen. Small Rumin. Res. 2015, 129, 104–107. [Google Scholar] [CrossRef]
- Vernet, P.; Aitken, R.J.; Drevet, J.R. Antioxidant strategies in the epididymis. Mol. Cell Endocrinol. 2004, 216, 31–39. [Google Scholar] [CrossRef]
- Garcia, E.M.; Vázquez, J.M.; Calvete, J.J.; Sanz, L.; Caballero, I.; Parrilla, I.; Gil, M.A.; Roca, J.; Martinez, E.A. Dissecting the protective effect of the seminal plasma spermadhesin PSP-I/PSP-II on boar sperm functionality. J. Androl. 2006, 27, 434–443. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Spencer, J.P.E.; Mahmood, N. Protection against oxidative damage and cell death by the natural antioxidant ergothioneine. Food Chem. Toxicol. 1999, 37, 1043–1053. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. The unusual amino acid l-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 2010, 17, 1134–1140. [Google Scholar] [CrossRef] [Green Version]
- Ey, J.; Schömig, E.; Taubert, D. Dietary sources and antioxidant effects of ergothioneine. J. Agric. Food Chem. 2007, 55, 6466–6474. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, C.; Bauer, T.; Surek, B.; Schömig, E.; Gründemann, D. Cyanobacteria produce high levels of ergothioneine. Food Chem. 2001, 129, 1766–1769. [Google Scholar] [CrossRef]
- Gründemann, D. The ergothioneine transporter controls and indicates ergothioneine activity—A review. Prev. Med. 2012, 54, S71–S74. [Google Scholar] [CrossRef] [PubMed]
- Nikodemus, D.; Lazic, D.; Bach, M.; Bauer, T.; Pfeiffer, C.; Wiltzer, L.; Lain, E.; Schömig, E.; Gründemann, D. Paramount levels of ergothioneine transporter SLC22A4 mRNA in boar seminal vesicles and cross-species analysis of ergothioneine and glutathione in seminal plasma. J. Physiol. Pharmacol. 2011, 62, 411–419. [Google Scholar]
- Leone, E. Ergothioneine in the equine ampullar secretion. Nature 1954, 174, 404–405. [Google Scholar] [CrossRef]
- Mann, T. Biochemistry of stallion semen. J. Reprod. Fertil. Suppl. 1975, 23, 47–52. [Google Scholar]
- Gründemann, D.; Harlfinger, S.; Golz, S.; Geerts, A.; Lazar, A.; Berkels, R.; Jung, N.; Rubbert, A.; Schömig, E. Discovery of the ergothioneine transporter. Proc. Natl. Acad. Sci. USA. 2005, 102, 5256–5261. [Google Scholar] [CrossRef] [Green Version]
- Cheah, I.K.; Halliwell, B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim. Biophys. Acta. 2012, 1822, 784–793. [Google Scholar] [CrossRef] [Green Version]
- Servillo, L.; Castaldo, D.; Casale, R.; D’Onofrio, N.; Giovane, A.; Cautela, D.; Balestrieri, M.L. An uncommon redox behavior sheds light on the cellular antioxidant properties of ergothioneine. Free Radic. Biol. Med. 2015, 79, 228–236. [Google Scholar] [CrossRef]
- Melville, D.B. Ergothioneine. Vitam. Horm. 1958, 17, 155–204. [Google Scholar]
- Servillo, L.; D’Onofrio, N.; Balestrieri, M.L. Ergothioneine antioxidant function: From chemistry to cardiovascular therapeutic potential. J. Cardiovasc. Pharmacol. 2017, 69, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Najafi, A.; Kia, H.D.; Mohammadi, H.; Najafi, M.H.; Zanganeh, Z.; Sharafi, M.; Martinez-Pastor, F.; Adeldust, H. Different concentrations of cysteamine and ergothioneine improve microscopic and oxidative parameters in ram semen frozen with a soybean lecithin extender. Cryobiology 2014, 69, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Reddy, I.J.; Dhali, A.; Javvaji, P.K. l-Ergothioneine improves the developmental potential of in vitro sheep embryos without influencing OCTN1-mediated cross-membrane transcript expression. Zygote 2018, 26, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, S.; Zinellu, A.; Arru, D.; Nieddu, S.; Strina, A.; Ariu, F.; Pintus, G.; Carru, C.; Bogliolo, L.; Ledda, S. Amniotic fluid l-ergothioneine concentrations in pregnant sheep after natural mating and transfer of vitrified/thawed in-vitro produced embryos. Res. Vet. Sci. 2015, 102, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, S.; Pisanu, E.; Cambedda, D.; Pintus, G.; Carru, C.; Zinellu, A. Ultra-performance liquid chromatographic determination of l-ergothioneine in commercially available classes of cow milk. J. Food Sci. 2014, 79, C1683–C1687. [Google Scholar] [CrossRef] [PubMed]
- Sotgia, S.; Zinellu, A.; Pisanu, E.; Vlahopoulou, G.; Ariu, F.; Ledda, S.; Pintus, G.; Carru, C.; Bogliolo, L. Concentrations of l-ergothioneine in follicular fluids of farm animals. Comp. Clin. Pathol. 2015, 24, 1261–1265. [Google Scholar] [CrossRef]
- Marden, W.; Werthessen, N.T. Influence of seminal fluid on sperm motility. Fertil. Steril. 1956, 7, 508–515. [Google Scholar] [CrossRef]
- Haag, F.M.; MacLeod, J. Relationship between nonprotein sulfhydryl concentration of seminal fluid and motility of spermatozoa in man. J. Appl. Physiol. 1959, 14, 27–30. [Google Scholar] [CrossRef]
- Sotgia, S.; Pisanu, E.; Pintus, G.; Erre, G.L.; Pinna, G.A.; Deiana, L.; Carru, C.; Zinellu, A. Plasma L-ergothioneine measurement by high-performance liquid chromatography and capillary electrophoresis after a pre-column derivatization with 5-Iodoacetamidofluorescein (5-IAF) and fluorescence detection. PLoS ONE 2013, 8, e70374. [Google Scholar] [CrossRef] [Green Version]
- Sotgia, S.; Murphy, R.B.; Zinellu, A.; Elliot, D.; Paliogiannis, P.; Pinna, G.A.; Carru, C.; Mangoni, A.A. Development of an LC–tandem mass spectrometry method for the quantitative analysis of hercynine in human whole blood. Molecules 2018, 23, 3326. [Google Scholar] [CrossRef] [Green Version]
- Carru, C.; Deiana, L.; Sotgia, S.; Pes, G.M.; Zinellu, A. Plasma thiols redox status by laser-induced fluorescence capillary electrophoresis. Electrophoresis 2004, 25, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Heuchel, V.; Schwartz, D.; Czyglik, F. Between and within subject correlations and variances for certain semen characteristics in fertile men. Andrologia 1983, 15, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Calculating correlation coefficients with repeated observations: Part 1—Correlation within subjects. BMJ 1995, 310, 446. [Google Scholar] [CrossRef] [Green Version]
- Bakdash, J.Z.; Marusich, L.R. Repeated measures correlation. Front. Psychol. 2017, 8, 456. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine—A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef] [Green Version]
- Gamal, A.; El-Maaty, A.M.A.; Rawash, Z.M. Comparative blood and seminal plasma oxidant/antioxidant status of Arab stallions with different ages and their relation to semen quality. Asian Pac. J. Reprod. 2016, 5, 428–433. [Google Scholar]
- Sotgia, S.; Mangoni, A.A.; Forteschi, M.; Murphy, R.B.; Elliot, D.; Sotgiu, E.; Pintus, G.; Carru, C.; Zinellu, A. Identification of the main intermediate precursor of l-ergothioneine biosynthesis in human biological specimens. Molecules 2016, 21, 1298. [Google Scholar] [CrossRef] [Green Version]

| Withdrawal #1 | Withdrawal #2 | Withdrawal #3 | Withdrawal #4 | |
|---|---|---|---|---|
| ERT µmol/L ± SD | 234.59 ± 156.13 | 310.61 ± 225.76 | 369.91 ± 260.70 | 363.01 ± 232.34 |
| ERY nmol/L (IQR) | 6.31 (16.10) | 6.41 (11.21) | 5.33 (15.43) | 11.54 (12.66) |
| GSH µmol/L (IQR) | 1.71 (1.29) | 1.28 (0.99) | 1.11 (0.44) | 0.98 (1.05) |
| Cys µmol/L (IQR) | 40.23 (34.08) | 32.47 (22.09) | 25.38 (33.09) | 29.90 (35.65) |
| DTNB µmol/L (IQR) | 3.36 (1.24) | 4.20 (3.01) | 3.18 (2.09) | 3.63 (1.27) |
| TEAC μmolTE/100 g (IQR) | 7.46 (1.36) | 6.33 (2.97) | 8.19 (5.98) | 6.10 (4.59) |
| Sperm Volume mL ± SD | 25 ± 12 | 32 ± 13 | 30 ± 11 | 33 ± 14 |
| Sperm Concentration million per mL (IQR) | 360 (288) | 358 (169) | 415 (231) | 402 (169) |
| Total Motility % (IQR) | 83 (10) | 93 (17) | 90 (15) | 91 (5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotgia, S.; Taras, A.; Zinellu, A.; Cherchi, R.; Mangoni, A.A.; Carru, C.; Bogliolo, L. Hercynine, Ergothioneine and Redox State in Stallion’s Seminal Plasma. Antioxidants 2020, 9, 855. https://doi.org/10.3390/antiox9090855
Sotgia S, Taras A, Zinellu A, Cherchi R, Mangoni AA, Carru C, Bogliolo L. Hercynine, Ergothioneine and Redox State in Stallion’s Seminal Plasma. Antioxidants. 2020; 9(9):855. https://doi.org/10.3390/antiox9090855
Chicago/Turabian StyleSotgia, Salvatore, Andrea Taras, Angelo Zinellu, Raffaele Cherchi, Arduino A Mangoni, Ciriaco Carru, and Luisa Bogliolo. 2020. "Hercynine, Ergothioneine and Redox State in Stallion’s Seminal Plasma" Antioxidants 9, no. 9: 855. https://doi.org/10.3390/antiox9090855
APA StyleSotgia, S., Taras, A., Zinellu, A., Cherchi, R., Mangoni, A. A., Carru, C., & Bogliolo, L. (2020). Hercynine, Ergothioneine and Redox State in Stallion’s Seminal Plasma. Antioxidants, 9(9), 855. https://doi.org/10.3390/antiox9090855







