Effect of Roux-en-Y Bariatric Bypass Surgery on Subclinical Atherosclerosis and Oxidative Stress Markers in Leukocytes of Obese Patients: A One-Year Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Biochemical Determinations
2.3. Evaluation of Cellular Adhesion Molecules (CAMs) and Myeloperoxidase (MPO)
2.4. SOD Activity Assay and Carbonylation of Serum Protein
2.5. Isolation of Leukocytes from Blood Samples
2.6. Fluorescence Imaging of Superoxide Production
2.7. Western Blotting
2.8. Dynamic Flow-Chamber-Based Adhesion Assay
2.9. LDL and HDL Subfractions
2.10. Statistical Analysis
3. Results
3.1. Systemic and Leukocyte Oxidative Stress Parameters
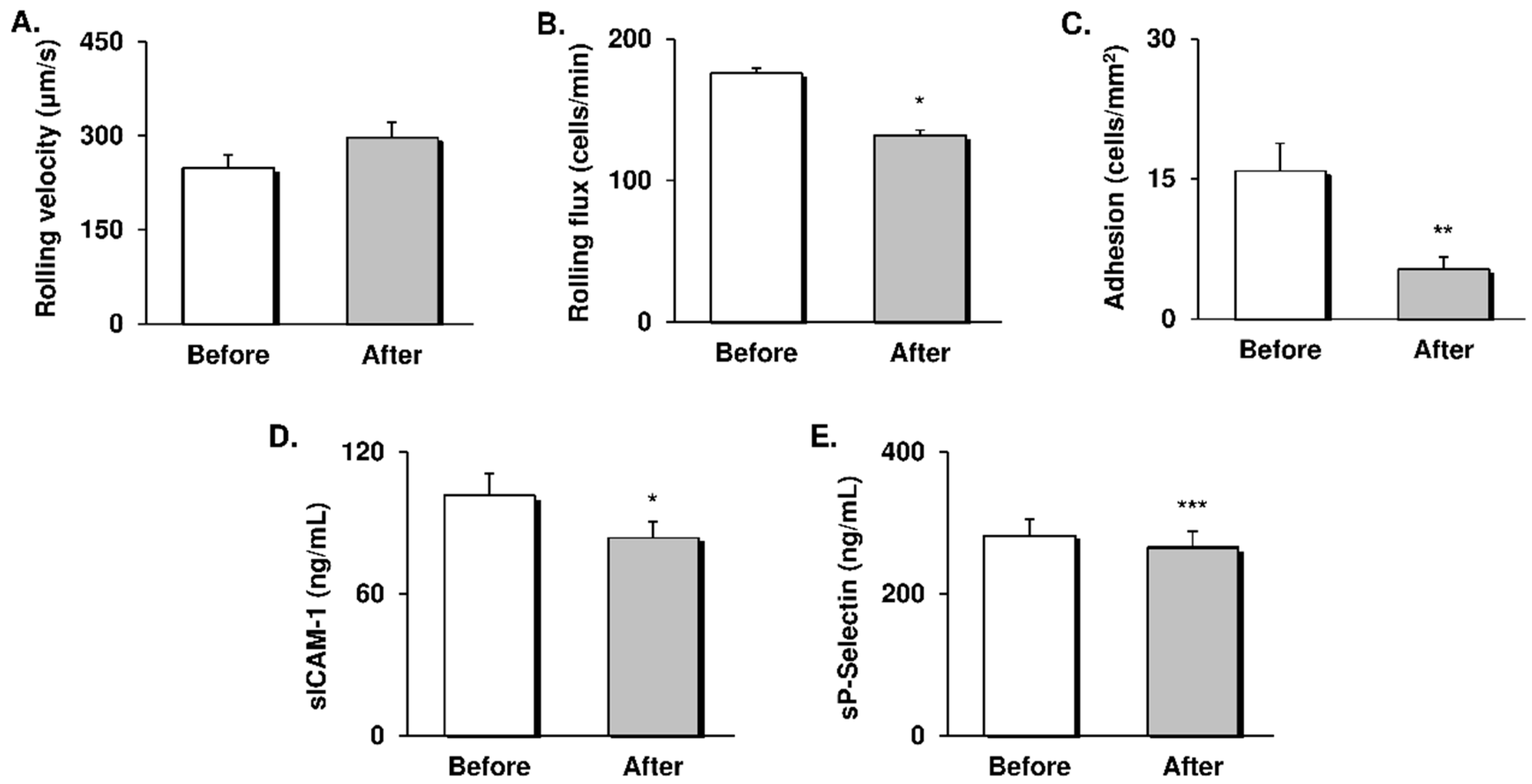
3.2. Leukocyte-Endothelial Cell Interactions and CAMs
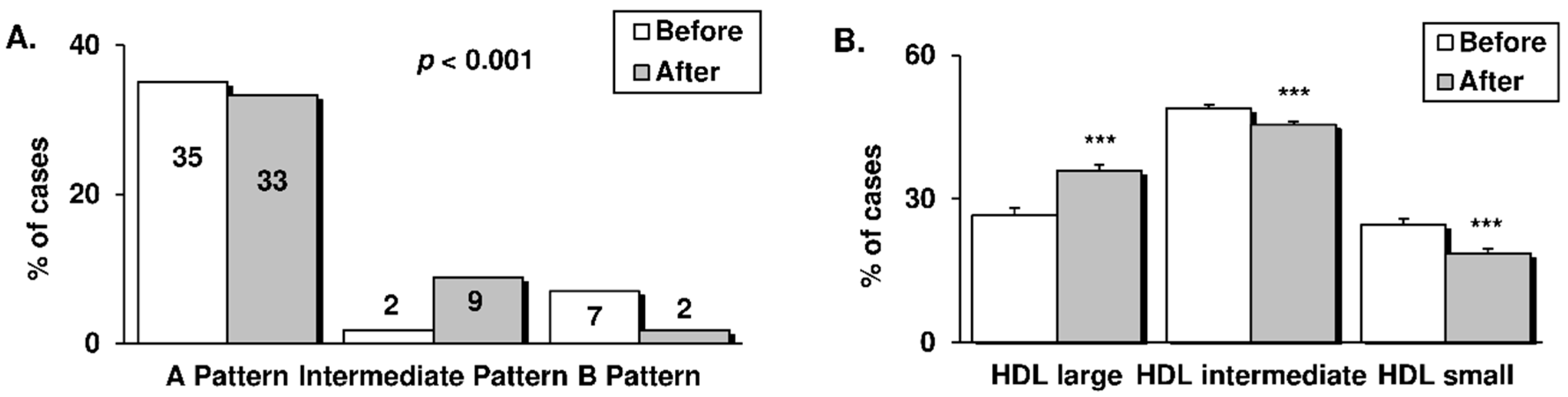
3.3. LDL and HDL Subfractions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferrante, A.W., Jr. Obesity-induced inflammation: A metabolic dialogue in the language of inflammation. J. Intern. Med. 2007, 262, 408–414. [Google Scholar] [CrossRef]
- Grundy, S.M. Obesity, metabolic syndrome, and cardiovascular disease. J. Clin. Endocrinol. Metab. 2004, 89, 2595–2600. [Google Scholar] [CrossRef]
- Prospective Studies Collaboration; Whitlock, G.; Lewington, S.; Sherliker, P.; Clarke, R.; Emberson, J.; Halsey, J.; Qizilbash, N.; Collins, R.; Peto, R. Body-mass index and cause-specific mortality in 900,000 adults: Collaborative analyses of 57 prospective studies. Lancet 2009, 373, 1083–1096. [Google Scholar] [CrossRef]
- Despres, J.P. Body fat distribution and risk of cardiovascular disease: An update. Circulation 2012, 126, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Mangalat, D.; Korbut, R. Adipocytokines—Novel link between inflammation and vascular function? J. Physiol. Pharmacol. 2006, 57, 505–528. [Google Scholar] [PubMed]
- Fontana, L.; Eagon, J.C.; Trujillo, M.E.; Scherer, P.E.; Klein, S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 2007, 56, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Reho, J.J.; Rahmouni, K. Oxidative and inflammatory signals in obesity-associated vascular abnormalities. Clin. Sci. 2017, 131, 1689–1700. [Google Scholar] [CrossRef]
- Lopez-Domenech, S.; Bañuls, C.; Diaz-Morales, N.; Escribano-Lopez, I.; Morillas, C.; Veses, S.; Orden, S.; Alvarez, A.; Victor, V.M.; Hernandez-Mijares, A.; et al. Obesity impairs leukocyte-endothelium cell interactions and oxidative stress in humans. Eur. J. Clin. Investig. 2018, 48, e12985. [Google Scholar] [CrossRef]
- Chattopadhyay, M.; Khemka, V.K.; Chatterjee, G.; Ganguly, A.; Mukhopadhyay, S.; Chakrabarti, S. Enhanced ROS production and oxidative damage in subcutaneous white adipose tissue mitochondria in obese and type 2 diabetes subjects. Mol. Cell. Biochem. 2015, 399, 95–103. [Google Scholar] [CrossRef]
- Olusi, S.O. Obesity is an independent risk factor for plasma lipid peroxidation and depletion of erythrocyte cytoprotectic enzymes in humans. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 1159–1164. [Google Scholar] [CrossRef]
- Ozata, M.; Mergen, M.; Oktenli, C.; Aydin, A.; Sanisoglu, S.Y.; Bolu, E.; Yilmaz, M.I.; Sayal, A.; Isimer, A.; Ozdemir, I.C. Increased oxidative stress and hypozincemia in male obesity. Clin. Biochem. 2002, 35, 627–631. [Google Scholar] [CrossRef]
- Wu, M.Y.; Li, C.J.; Hou, M.F.; Chu, P.Y. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Fernando, S.; Schwarz, N.; Tan, J.T.; Bursill, C.A.; Psaltis, P.J. Inflammation as a Therapeutic Target in Atherosclerosis. J. Clin. Med. 2019, 8, 1109. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Aljada, A.; Hofmeyer, D.; Syed, T.; Mohanty, P.; Dandona, P. Circulating mononuclear cells in the obese are in a proinflammatory state. Circulation 2004, 110, 1564–1571. [Google Scholar] [CrossRef]
- Hernandez-Mijares, A.; Rocha, M.; Rovira-Llopis, S.; Banuls, C.; Bellod, L.; de Pablo, C.; Alvarez, A.; Roldan-Torres, I.; Sola-Izquierdo, E.; Victor, V.M. Human leukocyte/endothelial cell interactions and mitochondrial dysfunction in type 2 diabetic patients and their association with silent myocardial ischemia. Diabetes Care 2013, 36, 1695–1702. [Google Scholar] [CrossRef]
- Bañuls, C.; Rovira-Llopis, S.; Marañon, A.M.d.; Veses, S.; Jover, A.; Gomez, M.; Rocha, M.; Hernandez-Mijares, A.; Victor, V.M. Metabolic syndrome enhances endoplasmic reticulum, oxidative stress and leukocyte-endothelium interactions in PCOS. Metab. Clin. Exp. 2017, 71, 153. [Google Scholar] [CrossRef]
- Ikramuddin, S.; Korner, J.; Lee, W.J.; Connett, J.E.; Inabnet, W.B.; Billington, C.J.; Thomas, A.J.; Leslie, D.B.; Chong, K.; Jeffery, R.W.; et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: The Diabetes Surgery Study randomized clinical trial. JAMA 2013, 309, 2240–2249. [Google Scholar] [CrossRef]
- Sjostrom, L.; Peltonen, M.; Jacobson, P.; Sjostrom, C.D.; Karason, K.; Wedel, H.; Ahlin, S.; Anveden, A.; Bengtsson, C.; Bergmark, G.; et al. Bariatric surgery and long-term cardiovascular events. JAMA 2012, 307, 56–65. [Google Scholar] [CrossRef]
- Lupoli, R.; Di Minno, M.N.; Guidone, C.; Cefalo, C.; Capaldo, B.; Riccardi, G.; Mingrone, G. Effects of bariatric surgery on markers of subclinical atherosclerosis and endothelial function: A meta-analysis of literature studies. Int. J. Obes. 2016, 40, 395–402. [Google Scholar] [CrossRef]
- Joao Cabrera, E.; Valezi, A.C.; Delfino, V.D.; Lavado, E.L.; Barbosa, D.S. Reduction in plasma levels of inflammatory and oxidative stress indicators after Roux-en-Y gastric bypass. Obes. Surg. 2010, 20, 42–49. [Google Scholar] [CrossRef]
- Puzziferri, N.; Roshek, T.B., 3rd; Mayo, H.G.; Gallagher, R.; Belle, S.H.; Livingston, E.H. Long-term follow-up after bariatric surgery: A systematic review. JAMA 2014, 312, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Franssen, R.; Monajemi, H.; Stroes, E.S.; Kastelein, J.J. Obesity and dyslipidemia. Endocrinol. Metab. Clin. N. Am. 2008, 37, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Minervino, D.; Gumiero, D.; Nicolazzi, M.A.; Carnicelli, A.; Fuorlo, M.; Guidone, C.; Di Gennaro, L.; Fattorossi, A.; Mingrone, G.; Landolfi, R. Leukocyte Activation in Obese Patients: Effect of Bariatric Surgery. Medicine 2015, 94, e1382. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Abril, S.A.; Morillas-Ariño, C.; Ponce-Marco, J.L.; Torres-Sanchez, T.; Delgado-Gomis, F.; Hernandez-Mijares, A.; Rocha, M. Short- and Long-Term Effects of Weight Loss on the Complement Component C3 After Laparoscopic Gastric Bypass in Obese Patients. Obes. Surg. 2016, 26, 2756–2763. [Google Scholar] [CrossRef] [PubMed]
- Al-Zoairy, R.; Melmer, A.; Ress, C.; Laimer, M.; Kaser, S.; Ebenbichler, C. Lipid profile changes after pronounced weight loss induced by bariatric surgery. Clin. Lipidol. 2012, 7, 163–175. [Google Scholar] [CrossRef]
- Kjellmo, C.A.; Karlsson, H.; Nestvold, T.K.; Ljunggren, S.; Cederbrant, K.; Marcusson-Stahl, M.; Mathisen, M.; Lappegard, K.T.; Hovland, A. Bariatric surgery improves lipoprotein profile in morbidly obese patients by reducing LDL cholesterol, apoB, and SAA/PON1 ratio, increasing HDL cholesterol, but has no effect on cholesterol efflux capacity. J. Clin. Lipidol. 2018, 12, 193–202. [Google Scholar] [CrossRef]
- Kontush, A. HDL particle number and size as predictors of cardiovascular disease. Front. Pharmacol. 2015, 6, 218. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Rose, L.; Buring, J.E.; Cook, N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 2002, 347, 1557–1565. [Google Scholar] [CrossRef]
- Badimon, L.; Pena, E.; Arderiu, G.; Padro, T.; Slevin, M.; Vilahur, G.; Chiva-Blanch, G. C-Reactive Protein in Atherothrombosis and Angiogenesis. Front. Immunol. 2018, 9, 430. [Google Scholar] [CrossRef]
- Csanyi, G.; Taylor, W.R.; Pagano, P.J. NOX and inflammation in the vascular adventitia. Free Radic. Biol. Med. 2009, 47, 1254–1266. [Google Scholar] [CrossRef]
- Roberts, H.M.; Grant, M.M.; Hubber, N.; Super, P.; Singhal, R.; Chapple, I.L.C. Impact of Bariatric Surgical Intervention on Peripheral Blood Neutrophil (PBN) Function in Obesity. Obes. Surg. 2018, 28, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Monzo-Beltran, L.; Vazquez-Tarragon, A.; Cerda, C.; Garcia-Perez, P.; Iradi, A.; Sanchez, C.; Climent, B.; Tormos, C.; Vazquez-Prado, A.; Girbes, J.; et al. One-year follow-up of clinical, metabolic and oxidative stress profile of morbid obese patients after laparoscopic sleeve gastrectomy. 8-oxo-dG as a clinical marker. Redox Biol. 2017, 12, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; McCall, M.R.; Frei, B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: Reaction pathways and antioxidant protection. Arterioscler. Thromb. Vasc Biol. 2000, 20, 1716–1723. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.L.; Hazen, S.L. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Curr. Opin. Lipidol. 2003, 14, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; Baldus, S. Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol. Ther. 2006, 111, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Domenech, S.; Martinez-Herrera, M.; Abad-Jimenez, Z.; Morillas, C.; Escribano-Lopez, I.; Diaz-Morales, N.; Banuls, C.; Victor, V.M.; Rocha, M. Dietary weight loss intervention improves subclinical atherosclerosis and oxidative stress markers in leukocytes of obese humans. Int. J. Obes (Lond) 2019, 43, 2200–2209. [Google Scholar] [CrossRef]
- Da Silva, V.R.; Moreira, E.A.; Wilhelm-Filho, D.; de Miranda, J.X.; Beninca, J.P.; Vigil, S.V.; Moratelli, A.M.; Garlet, T.R.; de Souza Meirelles, M.S.; Vannucchi, H.; et al. Proinflammatory and oxidative stress markers in patients submitted to Roux-en-Y gastric bypass after 1 year of follow-up. Eur. J. Clin. Nutr. 2012, 66, 891–899. [Google Scholar] [CrossRef]
- Bielinski, S.J.; Berardi, C.; Decker, P.A.; Kirsch, P.S.; Larson, N.B.; Pankow, J.S.; Sale, M.; de Andrade, M.; Sicotte, H.; Tang, W.; et al. P-selectin and subclinical and clinical atherosclerosis: The Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 2015, 240, 3–9. [Google Scholar] [CrossRef]
- Klinke, A.; Nussbaum, C.; Kubala, L.; Friedrichs, K.; Rudolph, T.K.; Rudolph, V.; Paust, H.J.; Schroder, C.; Benten, D.; Lau, D.; et al. Myeloperoxidase attracts neutrophils by physical forces. Blood 2011, 117, 1350–1358. [Google Scholar] [CrossRef]
- Garg, R.; Kumbkarni, Y.; Aljada, A.; Mohanty, P.; Ghanim, H.; Hamouda, W.; Dandona, P. Troglitazone reduces reactive oxygen species generation by leukocytes and lipid peroxidation and improves flow-mediated vasodilatation in obese subjects. Hypertension 2000, 36, 430–435. [Google Scholar] [CrossRef][Green Version]
- Escribano-Lopez, I.; Diaz-Morales, N.; Iannantuoni, F.; Lopez-Domenech, S.; de Maranon, A.M.; Abad-Jimenez, Z.; Banuls, C.; Rovira-Llopis, S.; Herance, J.R.; Rocha, M.; et al. The mitochondrial antioxidant SS-31 increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Sci. Rep. 2018, 8, 15862. [Google Scholar] [CrossRef] [PubMed]
- Borzi, A.M.; Buscemi, C.; Corleo, D.; Randazzo, C.; Rosafio, G.; Pantuso, G.; Buscemi, S. Endothelial Function in Obese Patients Treated with Bariatric Surgery. Diabetes Metab. Syndr. Obes 2020, 13, 247–256. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Before | After |
|---|---|---|
| n (females %) | 57 (84.5) | |
| Age (years) | 45.39 ± 10.49 | |
| Weight (kg) | 109.3 ± 16.1 | 78.6 ± 13.0 *** |
| BMI (kg/m2) | 39.8 ± 5.3 | 28.9 ± 4.3 *** |
| Waist (cm) | 115.5 ± 11.2 | 89.0 ± 11.8 *** |
| EWL (%) | - | 80.4 ± 29.0 |
| SBP (mmHg) | 131.8 ± 15.9 | 122.4 ± 17.6 *** |
| DBP (mmHg) | 81.3 ± 10.3 | 74.1 ± 10.6 ** |
| Glucose (mg/dL) | 98.6 ± 23.6 | 85.8 ± 11.4 *** |
| Insulin (μU/mL) | 14.7 ± 7.6 | 6.9 ± 3.0 *** |
| HOMA-IR | 3.8 ± 3.2 | 1.45 ± 0.7 *** |
| HbA1c (%) | 5.5 ± 0.7 | 5.2 ± 0.4 *** |
| TC (mg/dL) | 187.7 ± 34.5 | 166.6 ± 26.4 *** |
| HDLc (mg/dL) | 46.5 ± 8.8 | 55.0 ± 9.6 *** |
| LDLc (mg/dL) | 122.0 ± 39.7 | 95.9 ± 21.0 *** |
| TG (mg/dL) | 98.5 (77, 144) | 75 (55, 100) *** |
| hsCRP (mg/L) | 3.7 (2.0, 5.5) | 0.6 (0.2, 1.2) *** |
| C3c (mg/L) | 126.7 ± 22.9 | 95.6 ± 17.6 *** |
| Leukocytes (cells × 103/μL) | 7.6 ± 2.3 | 6.3 ± 1.9 *** |
| Treatment | ||
| Hypertension % (n) | 37 (21) | 16 (9) |
| Hyperlipidemia % (n) | 23 (13) | 9 (5) |
| T2D % (n) | 30 (17) | 4 (2) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abad-Jiménez, Z.; López-Domènech, S.; Gómez-Abril, S.Á.; Periañez-Gómez, D.; de Marañón, A.M.; Bañuls, C.; Morillas, C.; Víctor, V.M.; Rocha, M. Effect of Roux-en-Y Bariatric Bypass Surgery on Subclinical Atherosclerosis and Oxidative Stress Markers in Leukocytes of Obese Patients: A One-Year Follow-Up Study. Antioxidants 2020, 9, 734. https://doi.org/10.3390/antiox9080734
Abad-Jiménez Z, López-Domènech S, Gómez-Abril SÁ, Periañez-Gómez D, de Marañón AM, Bañuls C, Morillas C, Víctor VM, Rocha M. Effect of Roux-en-Y Bariatric Bypass Surgery on Subclinical Atherosclerosis and Oxidative Stress Markers in Leukocytes of Obese Patients: A One-Year Follow-Up Study. Antioxidants. 2020; 9(8):734. https://doi.org/10.3390/antiox9080734
Chicago/Turabian StyleAbad-Jiménez, Zaida, Sandra López-Domènech, Segundo Ángel Gómez-Abril, Dolores Periañez-Gómez, Aranzazu M. de Marañón, Celia Bañuls, Carlos Morillas, Víctor M. Víctor, and Milagros Rocha. 2020. "Effect of Roux-en-Y Bariatric Bypass Surgery on Subclinical Atherosclerosis and Oxidative Stress Markers in Leukocytes of Obese Patients: A One-Year Follow-Up Study" Antioxidants 9, no. 8: 734. https://doi.org/10.3390/antiox9080734
APA StyleAbad-Jiménez, Z., López-Domènech, S., Gómez-Abril, S. Á., Periañez-Gómez, D., de Marañón, A. M., Bañuls, C., Morillas, C., Víctor, V. M., & Rocha, M. (2020). Effect of Roux-en-Y Bariatric Bypass Surgery on Subclinical Atherosclerosis and Oxidative Stress Markers in Leukocytes of Obese Patients: A One-Year Follow-Up Study. Antioxidants, 9(8), 734. https://doi.org/10.3390/antiox9080734










