Protective Effect of Fenofibrate on Oxidative Stress-Induced Apoptosis in Retinal–Choroidal Vascular Endothelial Cells: Implication for Diabetic Retinopathy Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Fenofibrate Pretreatment
2.2. Cell Viability Assay
2.3. Analysis of Apoptosis by Flow Cytometry
2.4. Detection of Intracellular ROS
2.5. Quantitative Detection of ROS-Induced Cellular Oxidation
2.6. Determination of Mitochondrial Dysfunction
2.7. Preparation of RNA and cDNA
2.8. Analysis of mRNA Expression Levels
2.9. Protein Extractions and Western Blot Analysis
2.10. Caspase-3 Activity Assay
2.11. Statistical Analyses
3. Results
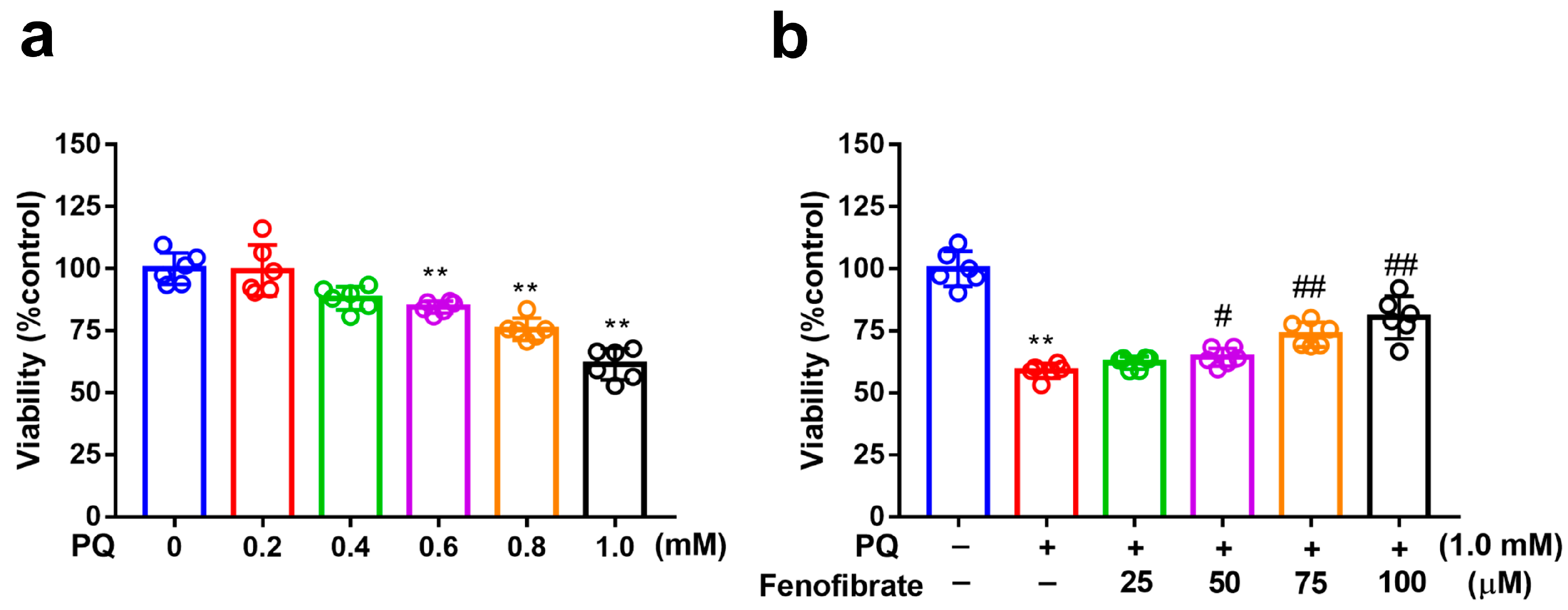
3.1. Fenofibrate Treatment Decreased PQ-Induced RF/6A Cell Death
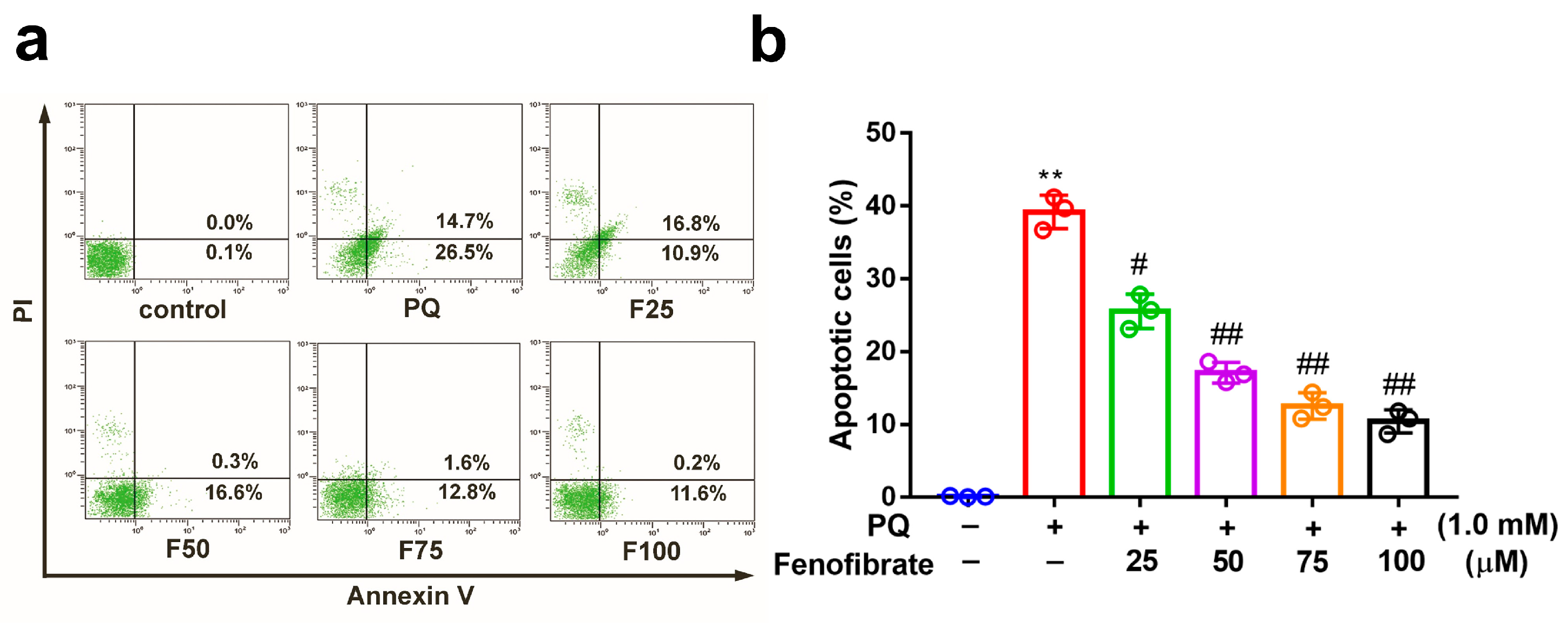
3.2. Fenofibrate Treatment Suppressed PQ-Induced Apoptosis in RF/6A Cells
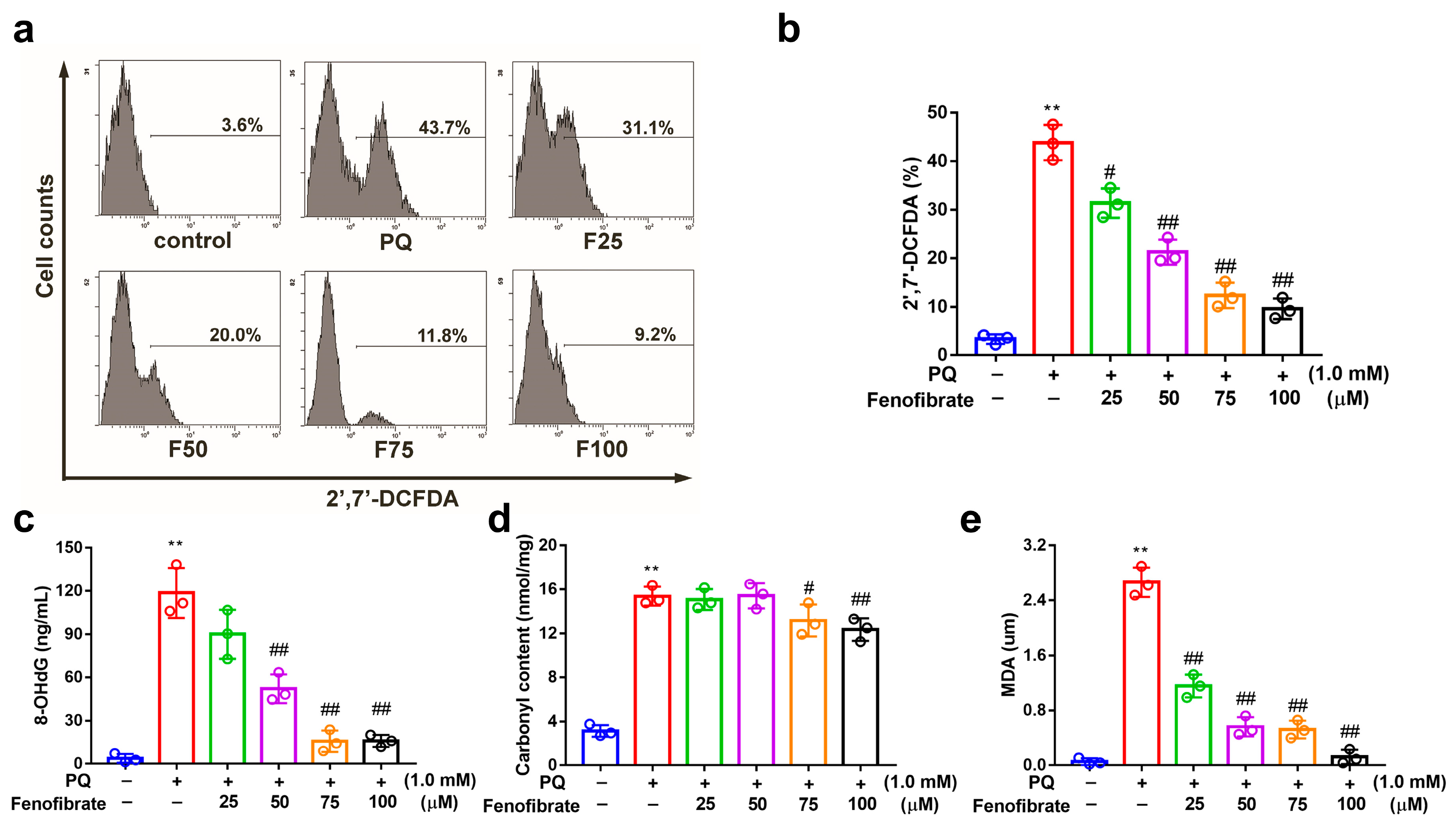
3.3. Fenofibrate Treatment Suppressed PQ-Induced ROS, 8-OHdG, Malondialdehyde, and Protein Carbonyl Content Production in RF/6A Cells
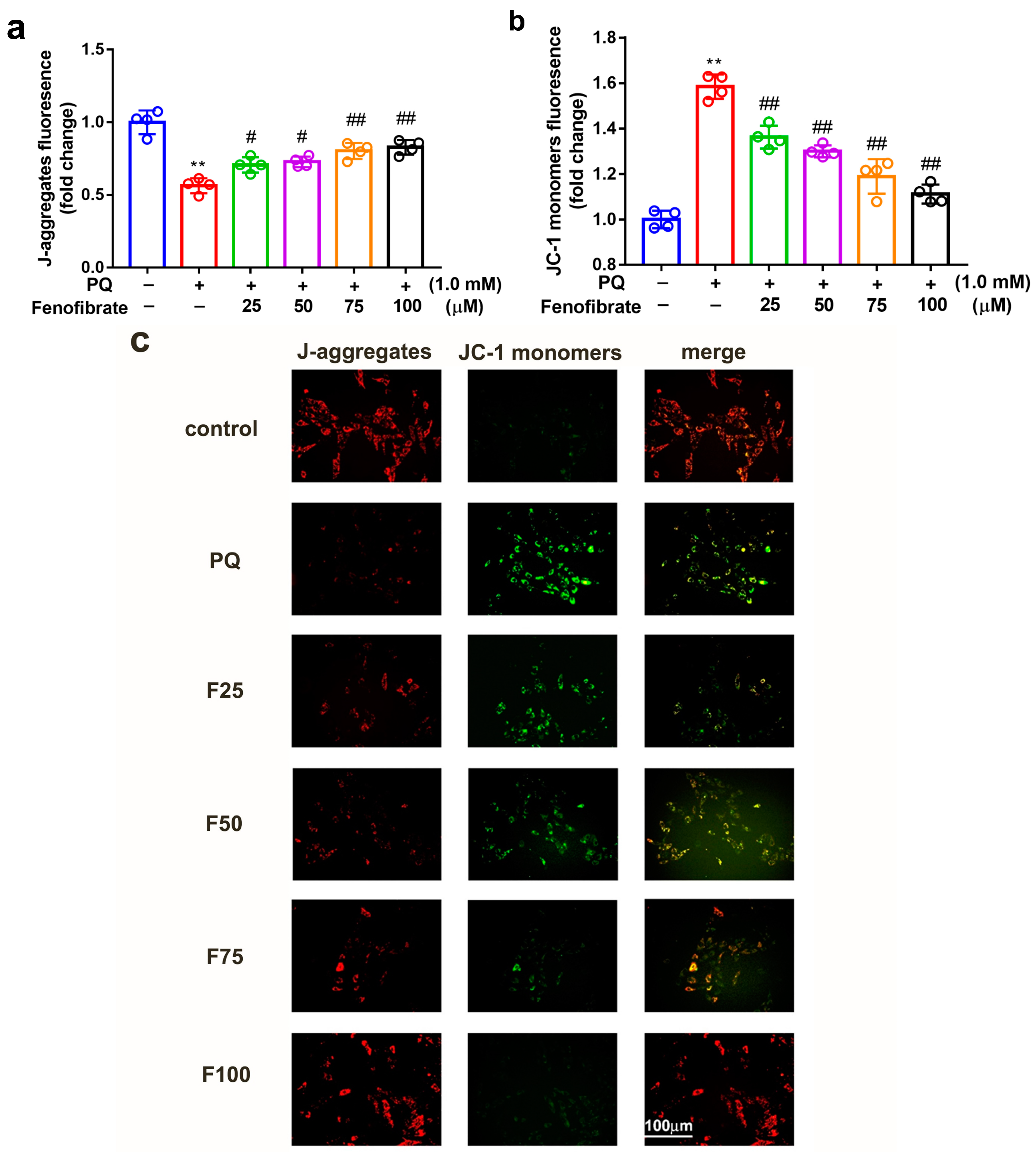
3.4. Fenofibrate Treatment Diminished Mitochondrial Damage in PQ-Induced RF/6A Cell
3.5. Effects of Fenofibrate on PQ-Induced Oxidative Stress-Related mRNA Levels in RF/6A Cells
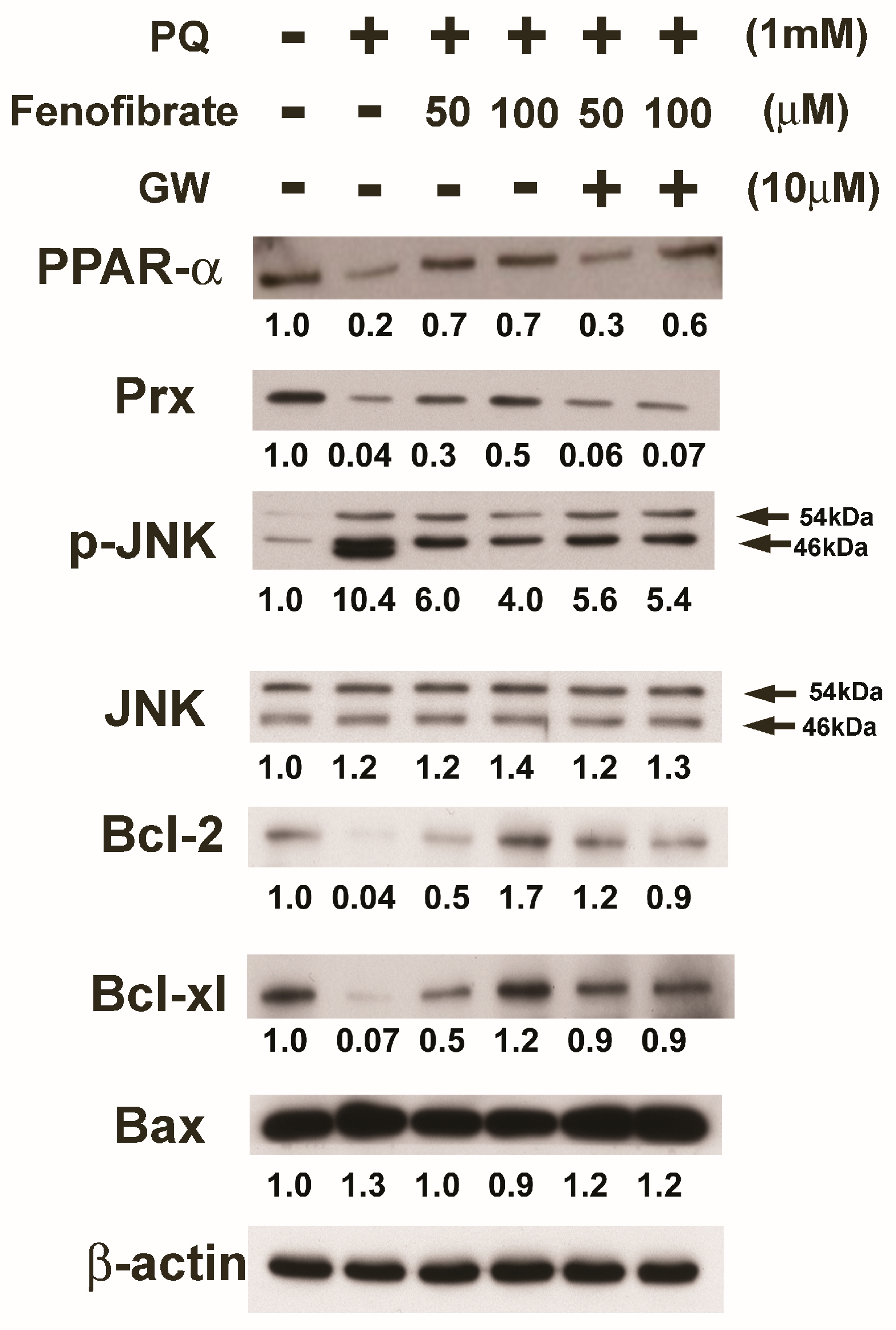
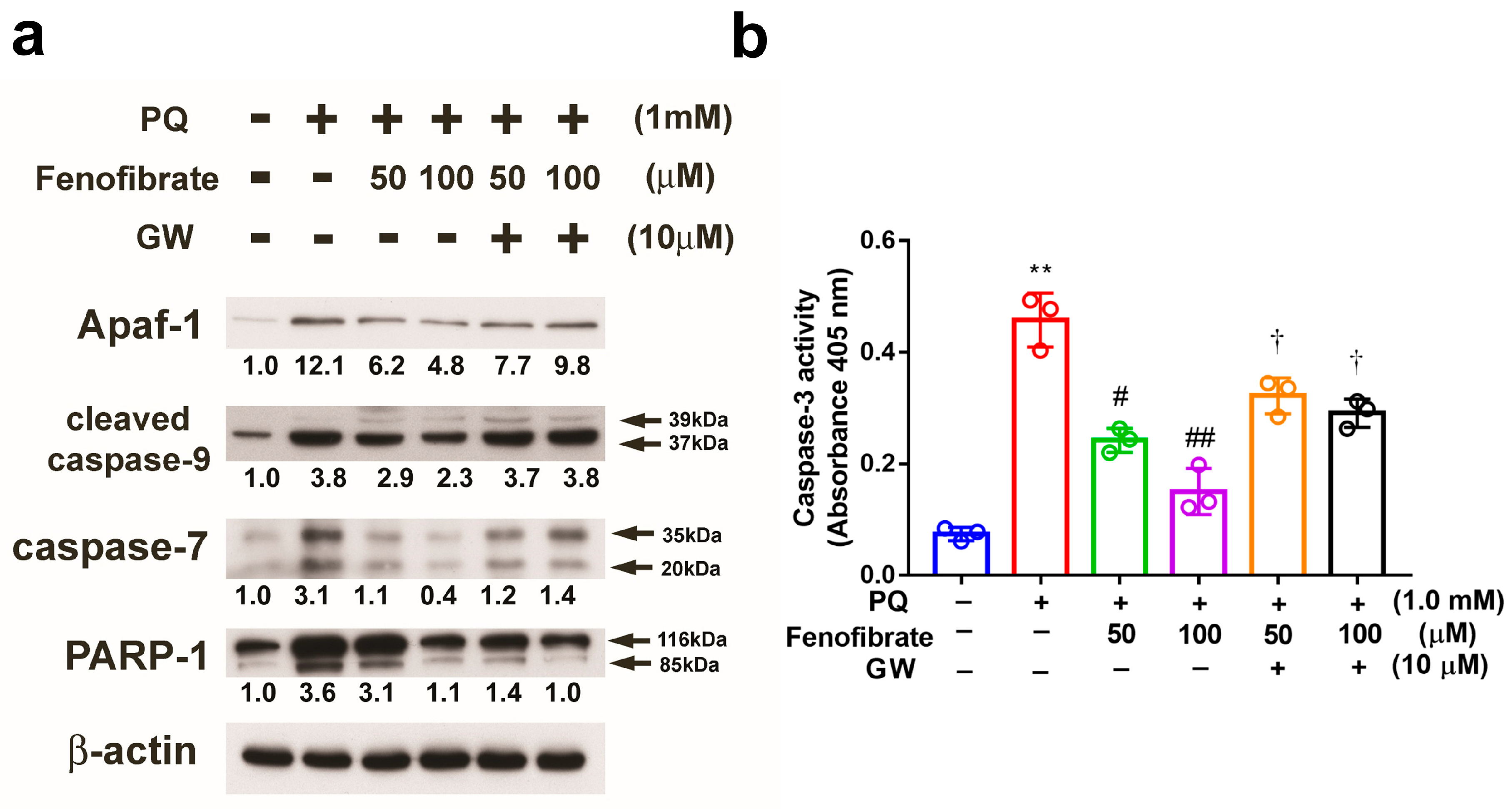
3.6. Effects of Fenofibrate on PQ-Induced Apoptosis and Stress-Signaling Pathway-Related Proteins in RF/6A Cells
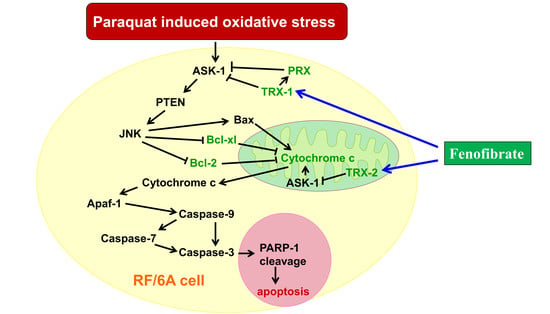
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simó, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Martinez-Zapata, M.J.; Marti-Carvajal, A.J.; Sola, I.; Pijoan, J.I.; Buil-Calvo, J.A.; Cordero, J.A.; Evans, J.R. Anti-vascular endothelial growth factor for proliferative diabetic retinopathy. Cochrane Database Syst. Rev. 2014, 11, Cd008721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fu, Y.; Xu, X.; Li, M.; Du, L.; Han, Y.; Ge, Y. PERK pathway are involved in NO-induced apoptosis in endothelial cells cocultured with RPE under high glucose conditions. Nitric Oxide 2014, 40, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L. Oxidative stress and diabetic neuropathy: A new understanding of an old problem. J. Clin. Investig. 2003, 111, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. The pathobiology of diabetic complications: A unifying mechanism. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Park, S.W.; Cho, C.S.; Jun, H.O.; Ryu, N.H.; Kim, J.H.; Yu, Y.S.; Kim, J.S.; Kim, J.H. Anti-angiogenic effect of luteolin on retinal neovascularization via blockade of reactive oxygen species production. Investig. Ophthalmol. Vis. Sci. 2012, 53, 7718–7726. [Google Scholar] [CrossRef]
- Feenstra, D.J.; Yego, E.C.; Mohr, S. Modes of Retinal Cell Death in Diabetic Retinopathy. J. Clin. Exp. Ophthalmol. 2013, 4, 298. [Google Scholar] [CrossRef]
- Schoonjans, K.; Martin, G.; Staels, B.; Auwerx, J. Peroxisome proliferator-activated receptors, orphans with ligands and functions. Curr. Opin. Lipidol. 1997, 8, 159–166. [Google Scholar] [CrossRef]
- Keech, A.C.; Mitchell, P.; Summanen, P.A.; O’Day, J.; Davis, T.M.; Moffitt, M.S.; Taskinen, M.R.; Simes, R.J.; Tse, D.; Williamson, E.; et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet 2007, 370, 1687–1697. [Google Scholar] [CrossRef]
- Chew, E.Y.; Ambrosius, W.T.; Davis, M.D.; Danis, R.P.; Gangaputra, S.; Greven, C.M.; Hubbard, L.; Esser, B.A.; Lovato, J.F.; Perdue, L.H.; et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N. Engl. J. Med. 2010, 363, 233–244. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Y.; Ding, L.; He, X.; Takahashi, Y.; Gao, Y.; Shen, W.; Cheng, R.; Chen, Q.; Qi, X.; et al. Pathogenic role of diabetes-induced PPAR-alpha down-regulation in microvascular dysfunction. Proc. Natl. Acad. Sci. USA 2013, 110, 15401–15406. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Gonzalez-Rodriguez, A.; Garcia-Ramirez, M.; Revuelta-Cervantes, J.; Hernandez, C.; Simo, R.; Valverde, A.M. Beneficial effects of fenofibrate in retinal pigment epithelium by the modulation of stress and survival signaling under diabetic conditions. J. Cell. Physiol. 2012, 227, 2352–2362. [Google Scholar] [CrossRef] [PubMed]
- Yeh, P.T.; Wang, L.C.; Chang, S.W.; Yang, W.S.; Yang, C.M.; Yang, C.H. Effect of Fenofibrate on the Expression of Inflammatory Mediators in a Diabetic Rat Model. Curr. Eye Res. 2019, 44, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lu, C.; Li, F.; Wang, H.; He, L.; Hao, Y.; Chen, A.F.; An, H.; Wang, X.; Hong, T.; et al. PPAR-alpha Agonist Fenofibrate Upregulates Tetrahydrobiopterin Level through Increasing the Expression of Guanosine 5′-Triphosphate Cyclohydrolase-I in Human Umbilical Vein Endothelial Cells. PPAR Res. 2011, 2011, 523520. [Google Scholar] [CrossRef]
- Kim, J.; Ahn, J.H.; Kim, J.H.; Yu, Y.S.; Kim, H.S.; Ha, J.; Shinn, S.H.; Oh, Y.S. Fenofibrate regulates retinal endothelial cell survival through the AMPK signal transduction pathway. Exp. Eye Res. 2007, 84, 886–893. [Google Scholar] [CrossRef]
- Ding, L.; Cheng, R.; Hu, Y.; Takahashi, Y.; Jenkins, A.J.; Keech, A.C.; Humphries, K.M.; Gu, X.; Elliott, M.H.; Xia, X.; et al. Peroxisome proliferator-activated receptor α protects capillary pericytes in the retina. Am. J. Pathol. 2014, 184, 2709–2720. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Lin, M.; Jenkins, A.J.; Keech, A.C.; Mott, R.; Lyons, T.J.; Ma, J.X. Therapeutic effects of PPARalpha agonists on diabetic retinopathy in type 1 diabetes models. Diabetes 2013, 62, 261–272. [Google Scholar] [CrossRef]
- Trudeau, K.; Roy, S.; Guo, W.; Hernandez, C.; Villarroel, M.; Simo, R.; Roy, S. Fenofibric acid reduces fibronectin and collagen type IV overexpression in human retinal pigment epithelial cells grown in conditions mimicking the diabetic milieu: Functional implications in retinal permeability. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6348–6354. [Google Scholar] [CrossRef]
- Watson, W.H.; Yang, X.; Choi, Y.E.; Jones, D.P.; Kehrer, J.P. Thioredoxin and its role in toxicology. Toxicol. Sci. 2004, 78, 3–14. [Google Scholar] [CrossRef]
- Powis, G.; Montfort, W.R. Properties and biological activities of thioredoxins. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 261–295. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.G.; Kang, S.W.; Chang, T.S.; Jeong, W.; Kim, K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life 2001, 52, 35–41. [Google Scholar] [CrossRef]
- Tanaka, T.; Hosoi, F.; Yamaguchi-Iwai, Y.; Nakamura, H.; Masutani, H.; Ueda, S.; Nishiyama, A.; Takeda, S.; Wada, H.; Spyrou, G.; et al. Thioredoxin-2 (TRX-2) is an essential gene regulating mitochondria-dependent apoptosis. EMBO J. 2002, 21, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Saxena, G.; Chen, J.; Shalev, A. Intracellular shuttling and mitochondrial function of thioredoxin-interacting protein. J. Biol. Chem. 2010, 285, 3997–4005. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Oshima, M.; Oshima, H.; Takaku, K.; Maruyama, T.; Yodoi, J.; Taketo, M.M. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev. Biol. 1996, 178, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Nonn, L.; Williams, R.R.; Erickson, R.P.; Powis, G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol. Cell. Biol. 2003, 23, 916–922. [Google Scholar] [CrossRef]
- Tobiume, K.; Matsuzawa, A.; Takahashi, T.; Nishitoh, H.; Morita, K.; Takeda, K.; Minowa, O.; Miyazono, K.; Noda, T.; Ichijo, H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001, 2, 222–228. [Google Scholar] [CrossRef]
- Liu, G.H.; Qu, J.; Shen, X. Thioredoxin-mediated negative autoregulation of peroxisome proliferator-activated receptor alpha transcriptional activity. Mol. Biol. Cell. 2006, 17, 1822–1833. [Google Scholar] [CrossRef]
- Billiet, L.; Furman, C.; Cuaz-Perolin, C.; Paumelle, R.; Raymondjean, M.; Simmet, T.; Rouis, M. Thioredoxin-1 and its natural inhibitor, vitamin D3 up-regulated protein 1, are differentially regulated by PPARalpha in human macrophages. J. Mol. Biol. 2008, 384, 564–576. [Google Scholar] [CrossRef]
- Fang, I.M.; Yang, C.H.; Yang, C.M.; Chen, M.S. Chitosan oligosaccharides attenuates oxidative-stress related retinal degeneration in rats. PLoS ONE 2013, 8, e77323. [Google Scholar] [CrossRef]
- Chen, M.; Luo, C.; Penalva, R.; Xu, H. Paraquat-induced retinal degeneration is exaggerated in CX3CR1-deficient mice and is associated with increased retinal inflammation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 682–690. [Google Scholar] [CrossRef]
- Lederman, M.; Hagbi-Levi, S.; Grunin, M.; Obolensky, A.; Berenshtein, E.; Banin, E.; Chevion, M.; Chowers, I. Degeneration modulates retinal response to transient exogenous oxidative injury. PLoS ONE 2014, 9, e87751. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Tawara, S.; Igarashi, K.; Takenaka, A. Effect of various radical generators on insulin-dependent regulation of hepatic gene expression. Biosci. Biotechnol. Biochem. 2007, 71, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Katsumata, Y.; Ozawa, T.; Tawara, S.; Igarashi, K.; Cho, Y.; Shibata, N.; Hakuno, F.; Takahashi, S.; Takenaka, A. Effect of paraquat-induced oxidative stress on insulin regulation of insulin-like growth factor-binding protein-1 gene expression. J. Clin. Biochem. Nutr. 2010, 46, 157–167. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shibata, M.; Hakuno, F.; Yamanaka, D.; Okajima, H.; Fukushima, T.; Hasegawa, T.; Ogata, T.; Toyoshima, Y.; Chida, K.; Kimura, K.; et al. Paraquat-induced oxidative stress represses phosphatidylinositol 3-kinase activities leading to impaired glucose uptake in 3T3-L1 adipocytes. J. Biol. Chem. 2010, 285, 20915–20925. [Google Scholar] [CrossRef] [PubMed]
- Gendron, R.; Good, W.; Adams, L.; Paradis, H. Suppressed expression of tubedown-1 in retinal neovascularization of proliferative diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3000–3007. [Google Scholar]
- Lukiw, W.J.; Ottlecz, A.; Lambrou, G.; Grueninger, M.; Finley, J.; Thompson, H.W.; Bazan, N.G. Coordinate activation of HIF-1 and NF-κB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4163–4170. [Google Scholar] [CrossRef]
- You, J.J.; Yang, C.M.; Chen, M.S.; Yang, C.H. Regulation of Cyr61/CCN1 expression by hypoxia through cooperation of c-Jun/AP-1 and HIF-1a in retinal vascular endothelial cells. Exp. Eye Res. 2010, 91, 825–836. [Google Scholar] [CrossRef]
- You, J.J.; Yang, C.H.; Yang, C.M.; Chen, M.S. Cyr61 induces the expression of monocyte chemoattractant protein-1 via the integrin ανβ3, FAK, PI3K/Akt, and NF-κB pathways in retinal vascular endothelial cells. Cell. Signal. 2014, 26, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Xu, W.; Wang, Y.; Tao, M.; Hu, Z.; Lv, B.; Hui, Y.; Du, H. OxLDL enhances choroidal neovascularization lesion through inducing vascular endothelium to mesenchymal transition process and angiogenic factor expression. Cell. Signal. 2020, 70, 109571. [Google Scholar] [CrossRef]
- Warden, C.; Barnett, J.M.; Brantley, M.A., Jr. Taurocholic acid inhibits features of age-related macular degeneration in vitro. Exp. Eye Res. 2020, 193, 107974. [Google Scholar] [CrossRef]
- Xie, W.; Zhou, P.; Qu, M.; Dai, Z.; Zhang, X.; Zhang, C.; Dong, X.; Sun, G.; Sun, X. Ginsenoside Re Attenuates High Glucose-Induced RF/6A Injury via Regulating PI3K/AKT Inhibited HIF-1α/VEGF Signaling Pathway. Front. Pharmacol. 2020, 11, 695. [Google Scholar] [CrossRef]
- Li, J.; He, J.; Zhang, X.; Li, J.; Zhao, P.; Fei, P. TSP1 ameliorates age-related macular degeneration by regulating the STAT3-iNOS signaling pathway. Exp. Cell Res. 2020, 388, 111811. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Li, R.; Du, J.; Yao, Y. Angiogenic factor with G patch and FHA domains 1 protects retinal vascular endothelial cells under hyperoxia by inhibiting autophagy. J. Biochem. Mol. Toxicol. 2020, e22572. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, Y.; Fu, Y.; Zhang, D.; Xin, Y. MiR-221–3p regulates the microvascular dysfunction in diabetic retinopathy by targeting TIMP3. Pflugers Arch. 2020. [Google Scholar] [CrossRef]
- Bordet, R.; Ouk, T.; Petrault, O.; Gele, P.; Gautier, S.; Laprais, M.; Deplanque, D.; Duriez, P.; Staels, B.; Fruchart, J.C.; et al. PPAR: A new pharmacological target for neuroprotection in stroke and neurodegenerative diseases. Biochem. Soc. Trans. 2006, 34, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Simo, R.; Mitchell, P. Fenofibrate—A potential systemic treatment for diabetic retinopathy? Am. J. Ophthalmol. 2012, 154, 6–12. [Google Scholar] [CrossRef]
- Pearsall, E.A.; Cheng, R.; Matsuzaki, S.; Zhou, K.; Ding, L.; Ahn, B.; Kinter, M.; Humphries, K.M.; Quiambao, A.B.; Farjo, R.A.; et al. Neuroprotective effects of PPARα in retinopathy of type 1 diabetes. PLoS ONE 2019, 14, e0208399. [Google Scholar] [CrossRef]
- Kadian, S.; Mahadevan, N.; Balakumar, P. Differential effects of low-dose fenofibrate treatment in diabetic rats with early onset nephropathy and established nephropathy. Eur. J. Pharmacol. 2013, 698, 388–396. [Google Scholar] [CrossRef]
- Quagliaro, L.; Piconi, L.; Assaloni, R.; Martinelli, L.; Motz, E.; Ceriello, A. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: The role of protein kinase C and NAD(P)H-oxidase activation. Diabetes 2003, 52, 2795–2804. [Google Scholar] [CrossRef]
- Chang, Y.; Chang, T.C.; Lee, J.J.; Chang, N.C.; Huang, Y.K. Sanguis draconis, a dragon’s blood resin, attenuates high glucose-induced oxidative stress and endothelial dysfunction in human umbilical vein endothelial cells. Sci. World J. 2014, 2014, 423259. [Google Scholar] [CrossRef]
- Yang, T.L.; Chen, M.F.; Luo, B.L.; Yu, J.; Jiang, J.L.; Li, Y.J. Effect of fenofibrate on LDL-induced endothelial dysfunction in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 370, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Uppalapati, D.; Das, N.R.; Gangwal, R.P.; Damre, M.V.; Sangamwar, A.T.; Sharma, S.S. Neuroprotective Potential of Peroxisome Proliferator Activated Receptor- alpha Agonist in Cognitive Impairment in Parkinson’s Disease: Behavioral, Biochemical, and PBPK Profile. PPAR Res. 2014, 2014, 753587. [Google Scholar] [CrossRef]
- Madsen-Bouterse, S.A.; Kowluru, R.A. Oxidative stress and diabetic retinopathy: Pathophysiological mechanisms and treatment perspectives. Rev. Endocr. Metab. Disord. 2008, 9, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Tomizawa, A.; Hattori, Y.; Inoue, T.; Hattori, S.; Kasai, K. Fenofibrate suppresses microvascular inflammation and apoptosis through adenosine monophosphate-activated protein kinase activation. Metabolism 2011, 60, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, P.; Hernandez, C.; Corraliza, L.; Carvalho, A.R.; Simo, R. Effect of fenofibrate on retinal neurodegeneration in an experimental model of type 2 diabetes. Acta Diabetol. 2015, 52, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Garrido, E.O.; Grant, C.M. Role of thioredoxins in the response of Saccharomyces cerevisiae to oxidative stress induced by hydroperoxides. Mol. Microbiol. 2002, 43, 993–1003. [Google Scholar] [CrossRef]
- Niso-Santano, M.; Gonzalez-Polo, R.A.; Bravo-San Pedro, J.M.; Gomez-Sanchez, R.; Lastres-Becker, I.; Ortiz-Ortiz, M.A.; Soler, G.; Moran, J.M.; Cuadrado, A.; Fuentes, J.M. Activation of apoptosis signal-regulating kinase 1 is a key factor in paraquat-induced cell death: Modulation by the Nrf2/Trx axis. Free Radic. Biol. Med. 2010, 48, 1370–1381. [Google Scholar] [CrossRef]
- Huh, K.H.; Cho, Y.; Kim, B.S.; Do, J.H.; Park, Y.J.; Joo, D.J.; Kim, M.S.; Kim, Y.S. The role of thioredoxin 1 in the mycophenolic acid-induced apoptosis of insulin-producing cells. Cell Death Dis. 2013, 4, e721. [Google Scholar] [CrossRef]
- Fiuza, B.; Subelzu, N.; Calcerrada, P.; Straliotto, M.R.; Piacenza, L.; Cassina, A.; Rocha, J.B.; Radi, R.; de Bem, A.F.; Peluffo, G. Impact of SIN-1-derived peroxynitrite flux on endothelial cell redox homeostasis and bioenergetics: Protective role of diphenyl diselenide via induction of peroxiredoxins. Free Radic. Res. 2015, 49, 122–132. [Google Scholar] [CrossRef]
- Devi, T.S.; Hosoya, K.; Terasaki, T.; Singh, L.P. Critical role of TXNIP in oxidative stress, DNA damage and retinal pericyte apoptosis under high glucose: Implications for diabetic retinopathy. Exp. Cell Res. 2013, 319, 1001–1012. [Google Scholar] [CrossRef]
- Singh, L.P. Thioredoxin Interacting Protein (TXNIP) and Pathogenesis of Diabetic Retinopathy. J. Clin. Exp. Ophthalmol. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. The Bcl-2 protein family: Arbiters of cell survival. Science 1998, 281, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.S.; Choi, H.Y.; Choi, T.W.; Kim, B.W.; Kim, J.H.; Lee, E.R.; Cho, S.G. Differential regulation of the antiapoptotic action of B-cell lymphoma 2 (Bcl-2) and B-cell lymphoma extra long (Bcl-xL) by c-Jun N-terminal protein kinase (JNK) 1-involved pathway in neuroglioma cells. Biol. Pharm. Bull. 2008, 31, 1686–1690. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, L.; Wang, J.; Xiao, H.; Wu, W.; Wang, Y.; Liu, X. MAPK signaling pathways regulate mitochondrial-mediated apoptosis induced by isoorientin in human hepatoblastoma cancer cells. Food Chem. Toxicol. 2013, 53, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Al-Lamki, R.; Bai, L.; Streb, J.W.; Miano, J.M.; Bradley, J.; Min, W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ. Res. 2004, 94, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, R.M. Apoptosis and caspases in neurodegenerative diseases. N. Engl. J. Med. 2003, 348, 1365–1375. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, Y.-J.; Lin, C.-W.; Cho, S.-L.; Yang, W.-S.; Yang, C.-M.; Yang, C.-H. Protective Effect of Fenofibrate on Oxidative Stress-Induced Apoptosis in Retinal–Choroidal Vascular Endothelial Cells: Implication for Diabetic Retinopathy Treatment. Antioxidants 2020, 9, 712. https://doi.org/10.3390/antiox9080712
Hsu Y-J, Lin C-W, Cho S-L, Yang W-S, Yang C-M, Yang C-H. Protective Effect of Fenofibrate on Oxidative Stress-Induced Apoptosis in Retinal–Choroidal Vascular Endothelial Cells: Implication for Diabetic Retinopathy Treatment. Antioxidants. 2020; 9(8):712. https://doi.org/10.3390/antiox9080712
Chicago/Turabian StyleHsu, Ying-Jung, Chao-Wen Lin, Sheng-Li Cho, Wei-Shiung Yang, Chung-May Yang, and Chang-Hao Yang. 2020. "Protective Effect of Fenofibrate on Oxidative Stress-Induced Apoptosis in Retinal–Choroidal Vascular Endothelial Cells: Implication for Diabetic Retinopathy Treatment" Antioxidants 9, no. 8: 712. https://doi.org/10.3390/antiox9080712
APA StyleHsu, Y.-J., Lin, C.-W., Cho, S.-L., Yang, W.-S., Yang, C.-M., & Yang, C.-H. (2020). Protective Effect of Fenofibrate on Oxidative Stress-Induced Apoptosis in Retinal–Choroidal Vascular Endothelial Cells: Implication for Diabetic Retinopathy Treatment. Antioxidants, 9(8), 712. https://doi.org/10.3390/antiox9080712