At physiological pH (7.40), LA and DHLA are mostly deprotonated (shown as LA
−, DHLA
−) because their p
Ka values in aqueous solution at 298.15 K are 4.76 and 4.85, respectively [
46]. Consequently, these species can coordinate through one of the carbonyl oxygen atoms (CO) or with both oxygen atoms (COO) of the carboxylate group, as well as with one or both sulfur atoms (S1, S2). In the discussion that follows, only 1:1 complexes have been considered (complexes in which only one organic ligand is present) and numeric labels are used to identify each complex. Iron complexes in each group have been labelled in sequence starting with the most stable one. The coordination sites of the organic ligand are explicitly indicated, together with their geometric distribution (
cis or
trans) when applicable. Each complex formation equilibrium considered has as many species in the reactant and product side so that reference state conversions or other corrections are not necessary.
The M06(SMD)/6-31++G(d,p) Cartesian coordinates, as well as the absolute enthalpies and Gibbs free energies of the different species considered in this study in water at 298.15 K are reported in the
Supporting Information (Table S1). Fe(III) complexes tend to be octahedral (hexa-coordinated), but tetra- and penta-coordinated complexes are also possible [
47], and they are explored in
Section 3.2. Test calculations were performed for low- and high-spin complexes. The latter ones were significantly more stable in all cases, which shows that the ligands studied (LA
−, DHLA
− and DHLA
2−) are weak-field ligands. Some of these test results are shown in
Table S2. The iron complexes discussed in the sections that follow are high spin.
3.1. Octahedral Complexes with Fe(III)
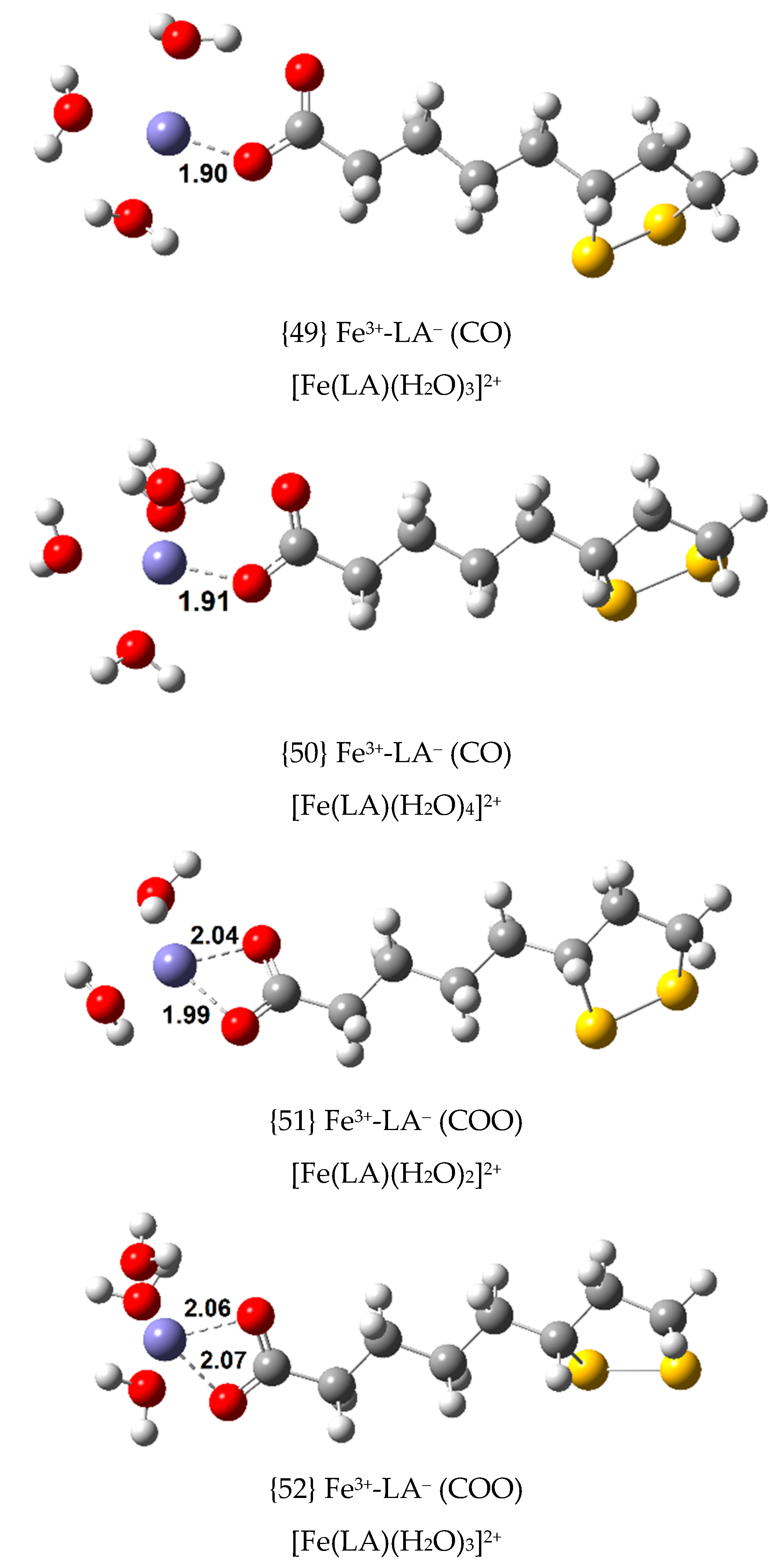
Four Fe(III) complexes with LA
− were calculated. Their
,
and
values are displayed in
Table 1, following the equilibrium indicated in Equation (4), and their structures are shown in
Figure 2. In this equilibrium, and in others that will be presented in the following sections, (6 − n)H
2O identifies an optimized cluster of no more than five water molecules. In the present study, clusters with up to five water molecules were calculated.
Two of the four Fe(III) complexes with LA
−, {1} and {2}, were found to be exergonic, with
values of −18.1 and −16.4 kcal/mol, respectively. The most stable complex has one of the carboxylate oxygen atoms coordinating, while complex {2} has both oxygen atoms coordinating the central ion. Further, the formation constant of complex {2} is eighteen times less than that of complex {1}. When coordination occurs via the sulfur atoms of LA
− (or DHLA
−), thermodynamic stability is greatly reduced. This was also observed when studying LA
− and DHLA
− coordination to Cu(II) [
10]. Coordination bond distances between Fe(III) and LA
− are always slightly larger than their Cu(II) counterparts with similar coordination patterns, in agreement with a larger atomic radius for Fe(III) (78.5 pm when 6-coordinated, octahedral, high spin) relative to that of Cu(II) (71 pm, when square planar). Optimizations of Fe(III) complexes with simultaneous oxygen and sulfur coordination from LA
−, previously reported for Cu(II), were unsuccessful; the sulfur coordination is lost in every attempt.
Seven Fe(III) complexes with DHLA
− were optimized. Their structures, with relevant bond distances indicated, are displayed in
Figure 3.
Table 2 contains their
,
and
values as per the complex formation equilibrium shown in Equation (5). Four of these complexes are exergonic. The two most stable complexes also have DHLA
− coordinating via CO (monodentate), {5}, and COO (bidentate), {6}. These are 2–3 kcal/mol less stable than their LA
- counterparts, complexes {1} and {2}, respectively. However, their coordinating bond distances to Fe(III) are very similar to the ones found with LA
− (see
Figure 2). Similar observations can be made on these last two points when inspecting the Cu(II) complexes with LA
− and DHLA
− [
10]. The other two exergonic Fe(III) complexes with DHLA
−, {7} and {8}, seem to be penta-coordinated. Both contain CO coordination from DHLA
− and each of the sulfur atoms are nearby, but too far away (3.96–3.98 Å) from the central ion to coordinate to it. These complexes are 1.3–2.0 kcal/mol less stable than the equivalent octahedral complex {5}. Coordination via S1 and S2 led to complex {11}, which is less stable than when only S2 or S1 coordinates to the central ion, {9} and {10}. These three complexes, {9}, {10} and {11} are endergonic. Complex {11}, the least stable of all, seems to be too strained and entropically unfavourable; it exhibits the shortest Fe(III)-S coordinating distances (2.58 and 2.63 Å) when compared to other LA
− and DHLA
− complexes.
To further explore other possible Fe(III) complexes, the doubly deprotonated DHLA anion, DHLA
2−, was also considered as a ligand with a second deprotonation in the thiol group closer to the carboxylate group (S1) and in the thiol group which is farther apart (S2) (see
Figure 1). Four Fe(III) complexes were calculated with S1-deprotonated DHLA
2− (see
Figure 4) and five with the S2-deprotonated anion (see
Figure 5). Thermodynamic calculations were performed to determine their
,
and
values as shown in
Table S3, according to the formation equilibrium shown in Equation (6).
As previously illustrated in our study of the Cu(II) complexes with DHLA
2− [
10], transforming Equation (6) into Equation (7) implies adding 4.50 kcal/mol to the
values reported in
Table S3. This value, 4.50 kcal/mol, is the thermodynamic cost of forming DHLA
2− in water under physiological pH conditions. The new thermodynamic results, displayed in
Table 3, can be properly compared to those related to the formation equilibria shown in Equations (4) and (5).
It can be observed that relative to the new set of reactant species (DHLA
− and [Fe(H
2O)
6]
3+), the nine complexes with DHLA
2− are significantly exergonic, with
values from −21.9 to −37.3 kcal/mol. They are much more thermodynamically stable than the previously studied complexes with LA
− and DHLA
− as ligands. A similar situation was observed in the study of the Cu(II) complexes with the M06-2X functional; however, the complexes with DHLA
2− had smaller
values, ranging from –3.6 to –21.8 kcal/mol [
10].
The most stable complex with DHLA2− in each group involves coordination through an oxygen atom of the carboxylate group and the deprotonated sulphur atom in cis configuration. That is the case of complex {12}, with = –30.6 kcal/mol, and {16}, with = –37.3 kcal/mol. Considering all the complexes with DHLA2− in which the deprotonated sulphur atom is a coordination centre, the sulphur-central ion distance (which goes from 2.29 to 2.33 Å) is much shorter than for any complex with LA− and DHLA−, which is reasonable given its negative charge and much greater basicity. The two most stable Fe(III) complexes involve S2-deprotonated DHLA2−, {16} and {17}, which are followed by two complexes with S1-deprotonated DHLA2−, {12} and {13}. Complex {17} is the only one in which the organic ligand studied coordinates to Fe(III) through three atomic centres, the carboxylate group and the deprotonated sulphur atom (S2).
3.3. Reduction of Fe(III) to Fe(II): The First Step of the Haber–Weiss Cycle
When Fe(III) is reduced by a strong reducing agent (the superoxide radical anion,
, or ascorbate,
) to Fe(II), very reactive (and harmful) hydroxyl radicals are formed as a product of the Haber–Weiss cycle. In the first step of the cycle, Fe(III) is reduced to Fe(II). In the next step, known as the Fenton reaction, Fe(II) is oxidized back to Fe(III) and
•OH radicals are formed, as shown in Equations (8) and (9).
Since the ions Fe(III) and
are solvated in water, a hydrated octahedral complex is a more realistic representation of these species. The most stable hydrated complexes calculated are shown in
Figure 8. Therefore, the actual reference reactions considered for the kinetic study of the first step of the Haber–Weiss cycle are shown in Equations (10) and (11).
Investigating the secondary antioxidant capacity of LA and DHLA relative to the Fe(III)/Fe(II) reduction involves finding out if any of the possible complexes with Fe(III) could significantly slow down the reduction of this ion when in the presence of
or
, as represented by Equations (12) and (13) that focus on DHLA
2− as organic ligand. If this occurs, the potential damage caused by
•OH radical formation could be greatly reduced.
Hence, with initial focus on the exergonic Fe(III) complexes previously calculated (twenty-four of them), the rate constants of their reduction to Fe(II) by reaction with
and with
were calculated. This required the optimization of the corresponding Fe(II) complex from each Fe(III) complex. The structures of six of these Fe(II) complexes are shown in
Figure 9 with a clear indication of the Fe(III) complex related to them. The Fe(II) complexes calculated are high spin (see
Table S2) and preserve the coordination number of the corresponding Fe(III) complex, with the exception of {40} that is penta-coordinated but it is the reduction product of the octahedral complex {17}, and {66} that is tetra-coordinated and comes from the penta-coordinated Fe(III) complex {57}. In all cases, the coordinating bond distances between the organic ligand and the central ion, Fe(II), became slightly larger relative to the same distances in the initial Fe(III) complexes, with the exception of complex {66}, for which the CO-Fe and S-Fe distances were slightly decreased.
Standard Gibbs free energies of reaction (Δ
G°, kcal/mol) and activation (Δ
G≠, kcal/mol), as well as various rate constants (
k,
kD and
kapp, M
–1 s
–1) for the single-electron transfer (SET) reactions with
and with ascorbate are shown in
Tables S4 and S5, respectively. Reactions in each table have been listed in ascending order of their calculated apparent rate constant (
kapp). In general, the more exergonic the SET reaction, the smaller the calculated Δ
G≠ value and the larger the rate constant. Plots of
kapp versus Δ
G°, which are displayed in
Figure S1 for both sets of reactions, show this tendency.
The calculated k values for the reactions with , including that of the reference reaction involving the hydrated ions, are larger than 1.0 × 108 M−1 s−1 and required diffusion corrections. The calculated kapp values of these reaction, which are exergonic, are in in the order of 109 M−1 s−1. Only the kapp for the {12} {35} reaction (7.08 × 109 M−1 s−1) is marginally smaller than that of the reference reaction (7.71 × 109 M−1 s−1). This shows that relative to the Fe(III)/Fe(II) reduction with , LA and DHLA do not possess secondary antioxidant activity.
Focusing on the reactions with ascorbate, very different results are observed. Reduced kinetic information for the six SET reactions with ascorbate having the smallest rate constants (those from complexes {12}, {16}, {14}, {55}, {13} and {18}, with DHLA
2− as ligand) is displayed in
Table 5, together with the kinetic information for the reaction of these Fe(III) complexes with
. The structures of the Fe(II) complexes involved in these reactions are those shown in
Figure 9. The Δ
G° and Δ
G≠ values of these reaction (see
Table S5) are very similar.
The first thirteen of the twenty-four reactions with ascorbate listed in
Table S5 are endergonic and show
k values between 70.8 and 1.16 × 10
8 M
-1 s
-1, which are significantly smaller relative to the reference reaction with
kapp of 7.36 × 10
9 M
–1 s
–1. The eleven most thermodynamically stable Fe(III) complexes (in order: {16} > {55} > {17} > {12} > {54} > {13} > {53} > {57} > {14} > {18} > {19}) are included in this group of reactions, but the
values of the Fe(III) complexes and the rate constant of their SET reactions are not 100% correlated. When the
kapp values of the reactions with
and with ascorbate are plotted versus the
of the Fe(III) complexes involved, a slight correlation can be observed (see
Figure S2). More stable Fe(III) complexes tend to have smaller SET rate constants, but complex {12}, the fourth most stable complex, breaks this general tendency with both reactions. The remaining eleven reactions are slightly exergonic and their
k values, being larger than 1.0 × 10
9 M
−1 s
−1, required diffusion corrections and lead to
kapp values from 2.41 × 10
9 to 7.47 × 10
9 M
−1 s
−1. All these reactions are slower than the reference reaction except for the reaction from complex {49}.
Hence, relative to the Fe(III)/Fe(II) reduction with ascorbate, DHLA shows significant secondary antioxidant activity to the point of being able to fully inhibit the formation of
•OH radicals in the Fenton reaction. The most stable Fe(III) complex, {16}, displays a SET rate constant that is 3.6 × 10
6 times smaller than the reference reaction. However, LA (see the kinetic results for the reactions from complexes {1} and {2} in
Table S5) displays some (minor) degree of secondary antioxidant activity being able to slow the first step of the Haber–Weiss cycle up to three times.
3.4. Copper (II) Complexes and Their Comparison with Fe(III) Complexes
To compare the secondary antioxidant activity of LA and DHLA with respect to the Cu(II)/Cu(I) and Fe(III)/Fe(II) reductions, some of the calculations previously reported in Reference 10 for the Cu(II) complexes at the M06-2X(SMD)/6-31++G(d,p) level of theory have been re-done using the M06 functional. The three most stable Cu(II) complexes (previously reported as complexes {23}, {24} and {25} in Reference 10; now labelled {1B}, {2B} and {3B}), all with the S2-deprotonated DHLA
2− ligand, were re-optimized and their corresponding linear Cu(I) complexes (labelled {4B}, {5B} and {6B}) were calculated. Reference 10 only reports the Cu(I) complex related to complex {1B}. The new M06 structures are shown in
Figure 10.
The most stable Cu(II)-LA
- complex (reported as {1} in Reference 10; now labelled {9B}) was also recalculated, and its Cu(I) reduction product was optimized, {10B}. These structures are reported in the
Supporting Information. The M06 coordination distances from the Cu(II) central ion to the organic ligand are slightly shorter than in the M06-2X study in most cases, and they become shorter in the corresponding Cu(I) complex. Thermodynamic calculations related to the formation of the Cu(II)-DHLA
2− complexes at physiological pH, as shown in Equation (14), are reported in
Table 6.
The relative thermodynamic stability order of complexes {1B}, {2B} and {3B}, previously reported with (M06-2X)
values of −21.8, −18.26 and −18.0 kcal/mol (see
Table 6 of Reference 10) respectively, is preserved with new (M06) values of −31.4, −30.0 and −18.6 kcal/mol, respectively, that are significantly more negative for {1B} and {2B}. These complexes are predicted to be more stable with the M06 functional than with the M06-2X functional, with
values in line with those obtained for the Fe(III) complexes with similar coordination patterns. For example, {16} Fe
3+-DHLA
2− (CO, S2
cis), the most stable Fe(III) complex calculated in the present study with
of −37.3 kcal/mol, compares to the most stable Cu(II) complex, {1B} Cu
2+-DHLA
2− (CO, S2
cis), with
of −31.4 kcal/mol. Complex {2B} has no equivalent Fe(III) complex, but {3B}, with
of −18.6 kcal/mol, is once again about 6 kcal/mol less stable that its Fe(III) counterpart, {19} Fe
3+-DHLA
2− (CO), with
of −24.5 kcal/mol. These results seem to indicate that the Fe(III) complexes with DHLA
2− are more thermodynamically stable than the Cu(II) complexes.
The same is observed when comparing the M06-2X ( = −13.1 kcal/mol) and M06 ( = −13.4 kcal/mol) stability of the most stable Cu(II)-LA− complex, which is also more stable than the Cu(II)-DHLA− complexes. Similarly, the most stable Fe(III)-LA- complex ( = −18.1 kcal/mol) is about 4.7 kcal/mol more stable than its Cu(II) counterpart. Hence, the Fe(III) complexes appear to be more thermodynamically stable than their equivalent Cu(II) complexes.
The kinetic study of the SET reactions of {1B}, {2B}, {3B} and {9B} with the superoxide radical anion and ascorbate was also performed. Standard Gibbs free energies of reaction (ΔG°, kcal/mol) and activation (Δ
G≠, kcal/mol), as well as various rate constants (k, k
D and k
app, M
–1 s
–1) are shown in
Table S6. Reduced kinetic information is displayed in
Table 7.
The reactions of the Cu(II) complexes with
lead to
kapp values that are 1.2 to 3.4 times smaller than the
kapp value of the reference reaction (7.43 × 10
9 M
–1 s
–1). The experimentally measured rate constant for the reference reaction, 8.1 × 10
9 M
–1 s
–1, is in excellent agreement with our calculation [
48]. The most stable complex, {1B}, leads to the greatest rate constant reduction (2.19 × 10
9 M
–1 s
–1), which is almost non-existent when considering the Fe(III) reactions (see
Table 5). This shows that relative to the Cu(II)/Cu(I) reduction with
, DHLA and LA possess some (minor) degree of secondary antioxidant activity, which is non-existent relative to the Fe(III)/Fe(II) reduction.
In the previous study [
10], the M06-2X rate constant of the reference reaction was calculated to be 1.29 × 10
8 M
−1 s
−1, and the rate constant for the reduction of the most stable complex, {1B}, with
was almost 100 times less (
k = 1.33 × 10
6 M
−1 s
−1). The k values with the M06 functional are larger (6.50 × 10
11 M
−1 s
−1 for the reference reaction and 3.00 x 10
9 M
-1 s
-1 for the reduction of {1B}, which is slightly over 200 times slower), but the diffusion corrections applied reduce the difference between the final
kapp values obtained, which are both in the order of 10
9 M
−1 s
−1.
Similarly to what was found with the Fe(III) reactions (see
Table S5), the reactions of the Cu(II) complexes with ascorbate have smaller rate constants that those of the reactions with
and are significantly slower, by as much as 7.6 × 10
3 times, than the reference reaction. The rate constant of the reference reaction is 2.51 × 10
7 M
−1 s
−1, and the rate constant for the reduction of the most stable complex, {1B}, is 3.32 × 10
3 M
−1 s
−1. This shows that relative to the Cu(II)/Cu(I) reduction with ascorbate, DHLA and LA possess significant secondary antioxidant activity. However, relative to the Fe(III)/Fe(II) reduction (see
Table S5), DHLA exhibits a very high secondary antioxidant character (with rate constant reduction of up to 10
8 times), and LA plays a minor role this way.