Influence of Cooking and Ingredients on the Antioxidant Activity, Phenolic Content and Volatile Profile of Different Variants of the Mediterranean Typical Tomato Sofrito
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Tomato Sofrito and Dry-Matter Determination
2.3. Preparation of Antioxidant Extracts
2.4. Antioxidant In Vitro Activity
2.4.1. DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Method
2.4.2. ABTS (Diammonium Salt) Method
2.4.3. FRAP (Ferric-Reducing Antioxidant Power) Method
2.4.4. Total Polyphenols Content (TPC)
2.5. Analysis of Volatile Compounds by Headspace Solid-Phase Microextraction-Gas Chromatography Mass Spectrometry (HS-SPME-GC-MS)
2.5.1. HS-SPME Sampling
2.5.2. Box–Behnken Experimental Design (BBD)
2.5.3. Gas Chromatography-Mass Spectrometry (GC–MS) Analysis
2.5.4. Volatiles Quantification Validation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Dry Matter
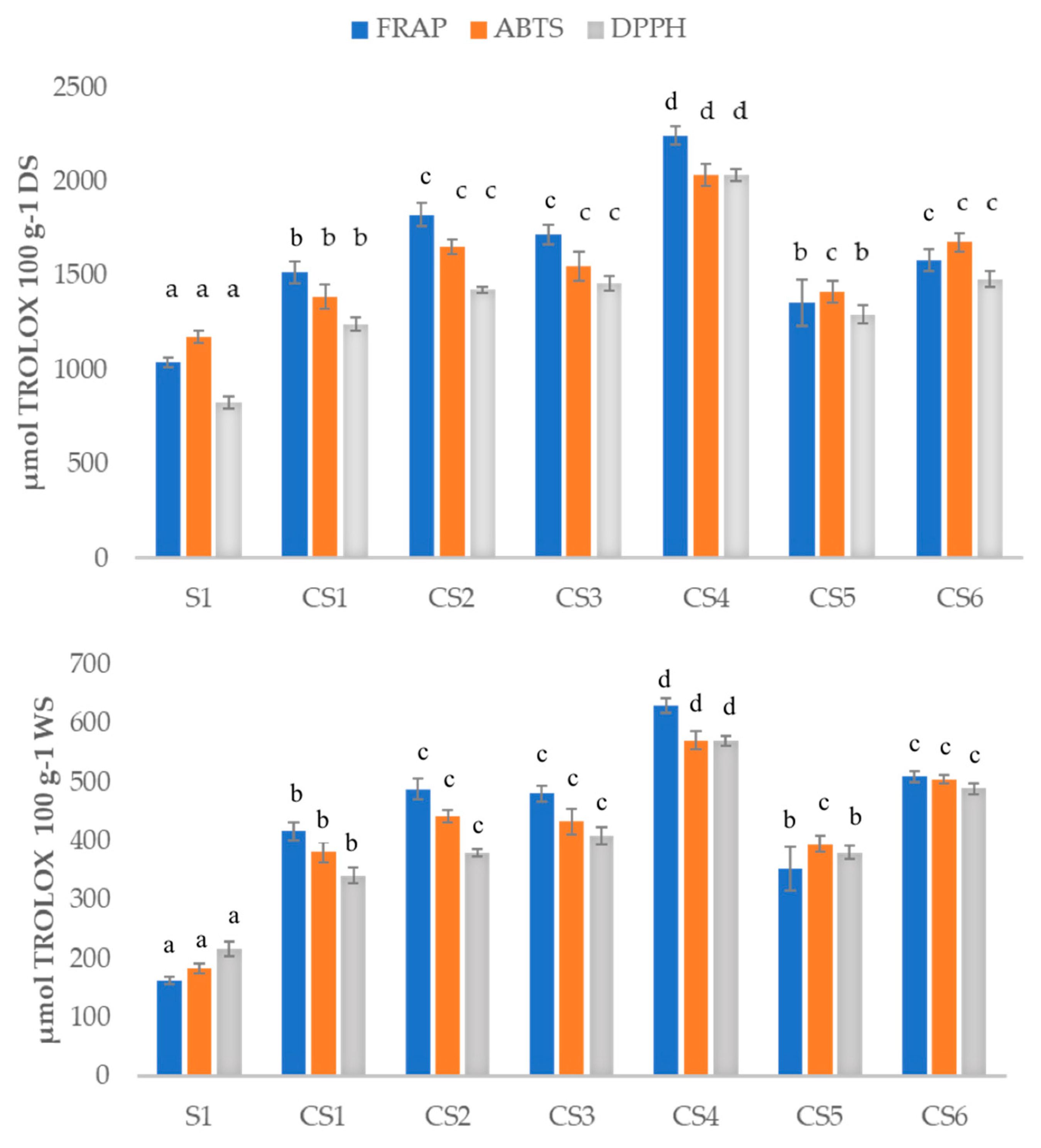
3.2. Analysis of Total Antioxidant Capacity
3.3. Experimental Design for Optimization of HS-SPME Procedure for Volatiles Extraction
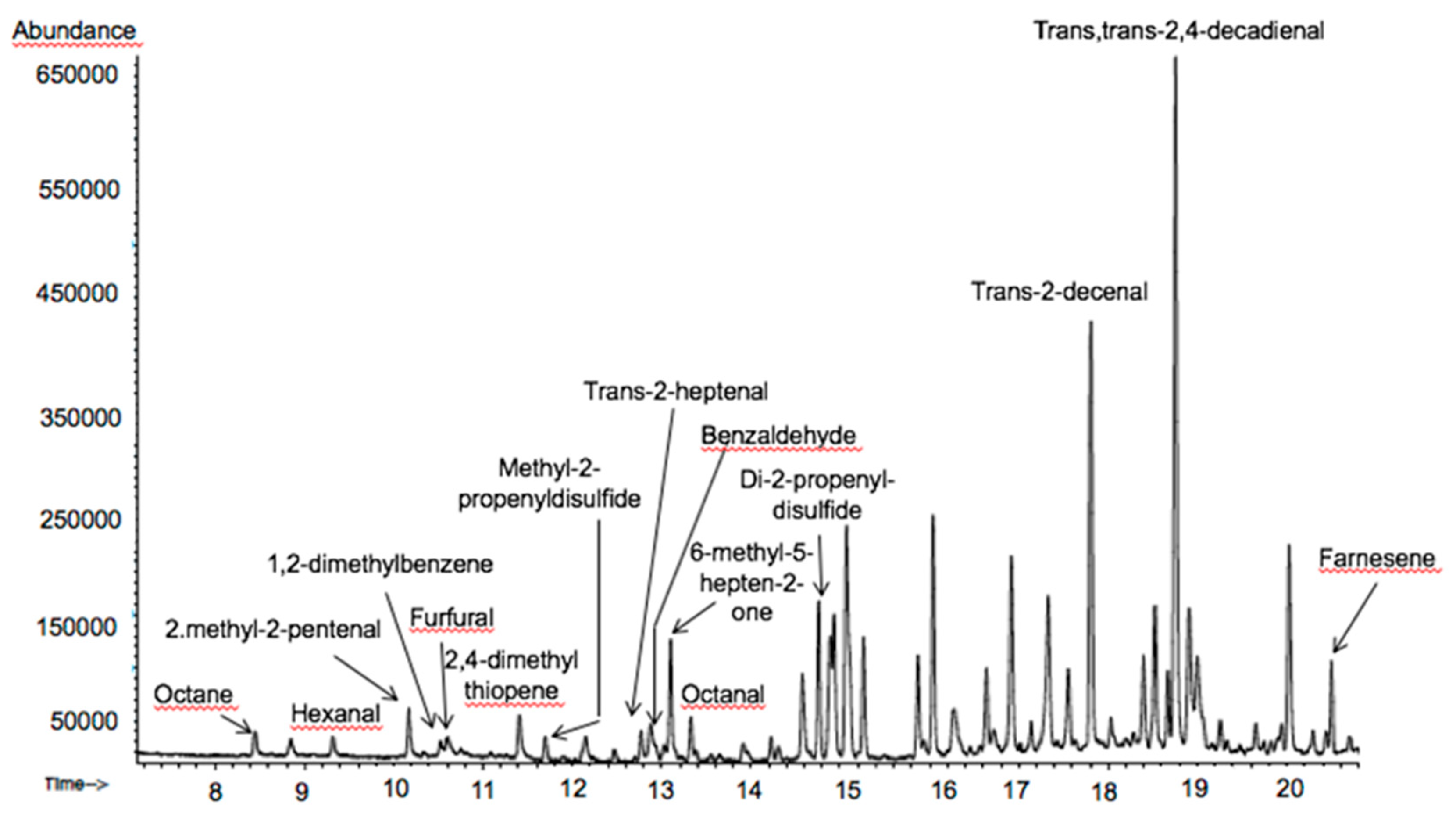
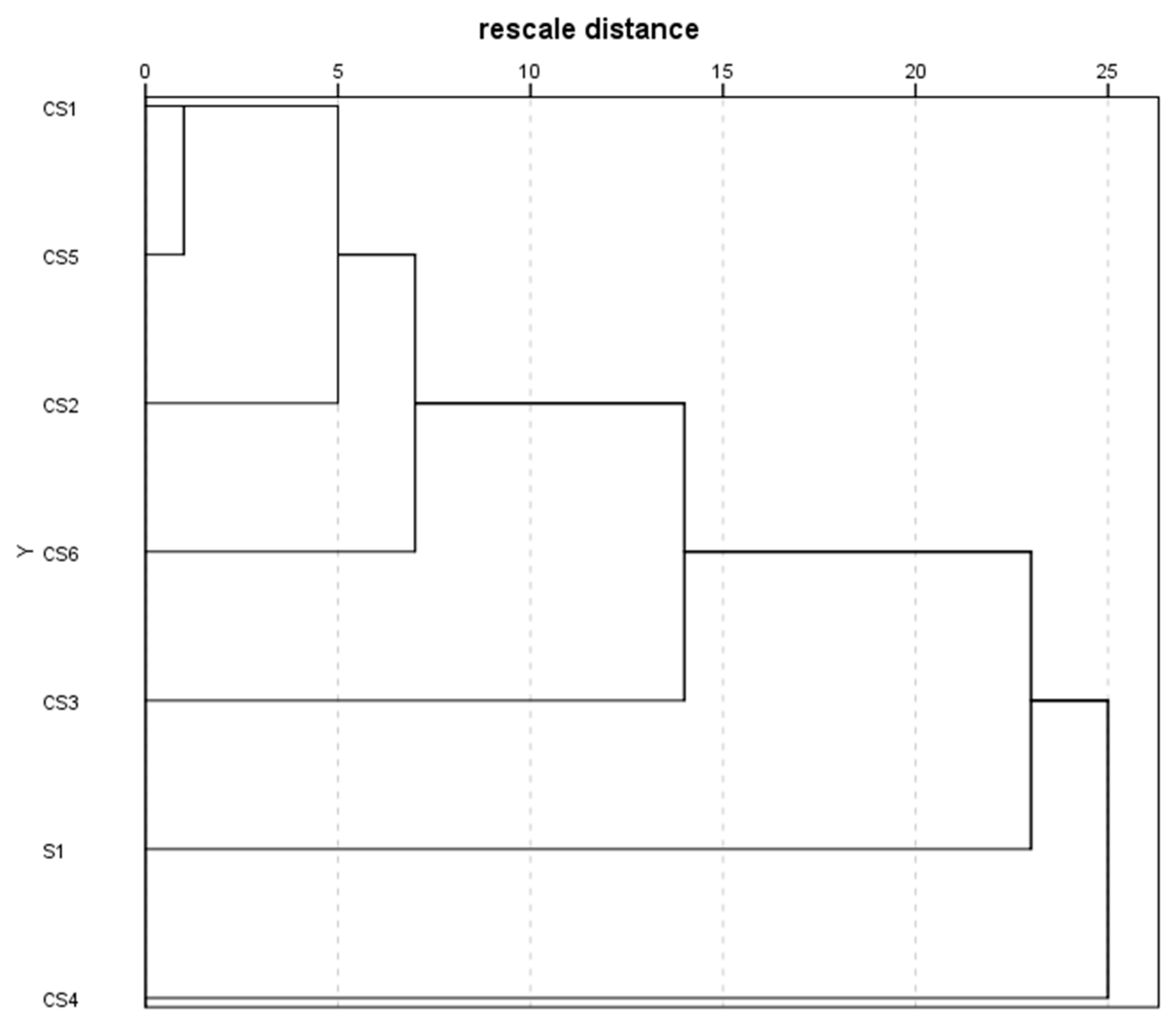
3.4. Volatile Compounds Profile Identification
3.5. Validation of the Quantification Method for the Volatile Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Estruch, R.; Salas-Salvadó, J. Towards an even healthier mediterranean diet. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1163–1166. [Google Scholar] [CrossRef]
- Rinaldi de Alvarenga, J.F.; Tran, C.; Hurtado-Barroso, S.; Martinez-Huélamo, M.; Illan, M.; Lamuela-Raventos, R.M. Home cooking and ingredient synergism improve lycopene isomer production in Sofrito. Food Res. Int. 2017, 99, 851–861. [Google Scholar] [CrossRef]
- McGee, H. On Food and Cooking: The Science and Lore of the Kitchen; Scribner: New York, NY, USA, 2004; ISBN 0684800012. [Google Scholar]
- Gahler, S.; Otto, K.; Böhm, V. Alterations of vitamin C, total phenolics, and antioxidant capacity as affected by processing tomatoes to different products. J. Agric. Food Chem. 2003, 51, 7962–7968. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; De Alvarenga, J.F.R.; Estruch, R.; Lamuela-Raventos, R.M. Bioactive compounds present in the Mediterranean sofrito. Food Chem. 2013, 141, 3366–3372. [Google Scholar] [CrossRef]
- Pinto, M.P.; Henriquesa, C.; Lima, G.; Fátima, Q. Lycopene content and antioxidant capacity of portuguese watermelon fruits. Electron. J. Environ. Agric. Food Chem. 2001, 10, 2090–2097. [Google Scholar]
- Tomas, M.; Beekwilder, J.; Hall, R.D.; Sagdic, O.; Boyacioglu, D.; Capanoglu, E. Industrial processing versus home processing of tomato sauce: Effects on phenolics, flavonoids and in vitro bioaccessibility of antioxidants. Food Chem. 2017, 220, 51–58. [Google Scholar] [CrossRef]
- Bendini, A.; Vallverdú-Queralt, A.; Valli, E.; Palagano, R.; Lamuela-Raventos, R.M.; Toschi, T.G. Italian and Spanish commercial tomato sauces for pasta dressing: Study of sensory and head-space profiles by flash profiling and solid-phase microextraction-gas chomatography-mass spectrometry. J. Sci. Food Agric. 2017, 97, 3261–3267. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Olalere, O.A. Optimization of microwave-assisted extraction of flavonoids and antioxidants from Vernonia amygdalina leaf using response surface methodology. Food Bioprod. Process. 2018, 107, 36–48. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; Da Silva, E.G.P.; Portugal, L.A.; Dos Reis, P.S.; Souza, A.S.; et al. Box-behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Hou, F.; Mu, T.; Ma, M.; Blecker, C. Optimization of processing technology using response surface methodology and physicochemical properties of roasted sweet potato. Food Chem. 2019, 278, 136–143. [Google Scholar] [CrossRef]
- D’Agostino, M.F.; Sanz, J.; Sanz, M.L.; Giuffrè, A.M.; Sicari, V.; Soria, A.C. Optimization of a solid-phase microextraction method for the gas chromatography-mass spectrometry analysis of blackberry (Rubus ulmifolius Schott) fruit volatiles. Food Chem. 2015, 178, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Reinivuo, H.; Bell, S.; Ovaskainen, M.L. Harmonisation of recipe calculation procedures in European food composition databases. J. Food Compos. Anal. 2009, 22, 410–413. [Google Scholar] [CrossRef]
- Capanoglu, E.; Beekwilder, J.; Boyacioglu, D.; De Vos, R.C.H.; Hall, R.D. The effect of industrial food processing on potentially health-beneficial tomato antioxidants. Crit. Rev. Food Sci. Nutr. 2010, 50, 919–930. [Google Scholar] [CrossRef] [PubMed]
- Moharram, H.A.; Youssef, M.M. Methods for determining the antioxidant activity: A review. Alexandria J. Food Sci. Technol. 2014, 11, 31–42. [Google Scholar] [CrossRef]
- Thanuja, S.; Sivakanthan, S.; Vasantharuba, S.V. Effect of different cooking methods on antioxidant properties of Tomato (Lycopersicon esculentum). Ceylon J. Sci. 2019, 48, 85. [Google Scholar] [CrossRef]
- Masci, A.; Coccia, A.; Lendaro, E.; Mosca, L.; Paolicelli, P.; Cesa, S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016, 202, 59–69. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Valdés, A.; Vidal, L.; Beltrán, A.; Canals, A.; Garrigós, M.C. Microwave-assisted extraction of phenolic compounds from almond skin byproducts (Prunus amygdalus): A multivariate analysis approach. J. Agric. Food Chem. 2015, 63, 5395–5402. [Google Scholar] [CrossRef]
- Marengo, E.; Mazzucco, E.; Robotti, E.; Gosetti, F.; Manfredi, M.; Calabrese, G. Characterization study of tomato sauces stored in different packaging materials. Curr. Anal. Chem. 2016, 13, 187–201. [Google Scholar] [CrossRef]
- D’Auria, M.; Racioppi, R. HS-SPME-GC-MS Analysis of onion (Allium cepa L.) and shallot (Allium ascalonicum L.). Food Res. 2017, 1, 161–165. [Google Scholar] [CrossRef]
- Cherfaoui, M.; Cecchi, T.; Keciri, S.; Boudriche, L. Volatile compounds of Algerian extra-virgin olive oils: Effects of cultivar and ripening stage. Int. J. Food Prop. 2018, 21, 36–49. [Google Scholar] [CrossRef]
- McGorrin, R.J.; Gimelfarb, L. Comparison of flavor components in fresh and cooked tomatillo with red plum tomato. Dev. Food Sci. 1998, 40, 295–313. [Google Scholar]
- Clarke, H.J.; Mannion, D.T.; O’Sullivan, M.G.; Kerry, J.P.; Kilcawley, K.N. Development of a headspace solid-phase microextraction gas chromatography mass spectrometry method for the quantification of volatiles associated with lipid oxidation in whole milk powder using response surface methodology. Food Chem. 2019, 292, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- van Boekel, M.; Fogliano, V.; Pellegrini, N.; Stanton, C.; Scholz, G.; Lalljie, S.; Somoza, V.; Knorr, D.; Jasti, P.R.; Eisenbrand, G. A review on the beneficial aspects of food processing. Mol. Nutr. Food Res. 2010, 54, 1215–1247. [Google Scholar] [CrossRef] [PubMed]
- Sahlin, E.; Savage, G.P.; Lister, C.E. Investigation of the antioxidant properties of tomatoes after processing. J. Food Compos. Anal. 2004, 17, 635–647. [Google Scholar] [CrossRef]
- Mieres-Castro, D.; Schmeda-Hirschmann, G.; Theoduloz, C.; Gómez-Alonso, S.; Pérez-Navarro, J.; Márquez, K.; Jiménez-Aspee, F. Antioxidant activity and the isolation of polyphenols and new iridoids from Chilean Gaultheria phillyreifolia and G. poeppigii berries. Food Chem. 2019, 291, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Köksal, E.; Bursal, E.; Gülçin, İ.; Korkmaz, M.; Çağlayan, C.; Gören, A.C.; Alwasel, S.H. Antioxidant activity and polyphenol content of Turkish thyme (Thymus vulgaris) monitored by liquid chromatography and tandem mass spectrometry. Int. J. Food Prop. 2017, 20, 514–525. [Google Scholar] [CrossRef]
- Celano, R.; Piccinelli, A.L.; Pagano, I.; Roscigno, G.; Campone, L.; De Falco, E.; Russo, M.; Rastrelli, L. Oil distillation wastewaters from aromatic herbs as new natural source of antioxidant compounds. Food Res. Int. 2017, 99, 298–307. [Google Scholar] [CrossRef]
- Base de datos Española de composición de alimentos (BEDCA). Available online: https://www.bedca.net/ (accessed on 2 November 2019).
- Locatelli, D.A.; Nazareno, M.A.; Fusari, C.M.; Camargo, A.B. Cooked garlic and antioxidant activity: Correlation with organosulfur compound composition. Food Chem. 2017, 220, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Anaya, J.D.P.; Samaniego-Sánchez, C.; Castañeda-Saucedo, M.C.; Villalón-Mir, M.; De La Serrana, H.L.G. Phenols and the antioxidant capacity of Mediterranean vegetables prepared with extra virgin olive oil using different domestic cooking techniques. Food Chem. 2015, 188, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Antonini, E.; Ninfali, P. Homemade tomato sauce in the Mediterranean diet: A rich source of antioxidants. Ital. J. Food Sci. 2017, 30, 37–49. [Google Scholar]
- Pawliszyn, J. Theory of Solid-Phase Microextraction. J. Chromatogr. Sci. 2000, 38, 270–278. [Google Scholar] [CrossRef]
- Ho, C.W.; Wan Aida, W.M.; Maskat, M.Y.; Osman, H. Optimization of headspace solid phase microextraction (HS-SPME) for gas chromatography mass spectrometry (GC-MS) analysis of aroma compound in palm sugar (Arenga pinnata). J. Food Compos. Anal. 2006, 19, 822–830. [Google Scholar] [CrossRef]
- Lanzotti, V. The analysis of onion and garlic. J. Chromatogr. A 2006, 1112, 3–22. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Bendini, A.; Barbieri, S.; Di Lecce, G.; Martin-Belloso, O.; Toschi, T.G. Volatile profile and sensory evaluation of tomato juices treated with pulsed electric fields. J. Agric. Food Chem. 2013, 61, 1977–1984. [Google Scholar] [CrossRef]
- Dresow, J.F.; Böhm, H. The Influence of Volatile Compounds of the Flavour of Raw, Boiled and Baked Potatoes: Impact of Agricultural Measures on the Volatile Components. Landbauforschung. Appl. Agric. For. Res. 2009, 59, 309–338. [Google Scholar]
- da Silva, M.D.R.G.; Costa Freitas, A.M.; Cabrita, M.J.; Garci, R. Olive oil composition: Volatile compounds. In Olive Oil—Constituents, Quality, Health Properties and Bioconversions; InTech: London, UK, 2012. [Google Scholar]
- Marković, K.; Vahčić, M.; Kovačević Ganić, K.; Banovć, M. Aroma volatiles of tomatoes and tomato products evaluated by solid-phase microextraction. Flavour Fragr. J. 2007, 22, 395–400. [Google Scholar] [CrossRef]
- Tschiggerl, C.; Bucar, F. Investigation of the volatile fraction of rosemary infusion extracts. Sci. Pharm. 2010, 78, 483–492. [Google Scholar] [CrossRef]
- Gonçalves, G.M.S.; Srebernich, S.M.; Bragagnolo, N.; Madalozzo, E.S.; Merhi, V.L.; Pires, D.C. Study of the composition of Thymus vulgaris essential oil, developing of topic formulations and evaluation of antimicrobial efficacy. J. Med. Plants Res. 2014, 7, 1736–1745. [Google Scholar] [CrossRef]
- Nishimura, H.; Higuchi, O.; Tateshita, K.; Tomobe, K.; Okuma, Y.; Nomura, Y. Antioxidative activity and ameliorative effects of memory impairment of sulfur-containing compounds in Allium species. BioFactors 2006, 26, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Nieto, G.; Huvaere, K.; Skibsted, L.H. Antioxidant activity of rosemary and thyme by-products and synergism with added antioxidant in a liposome system. Eur. Food Res. Technol. 2011, 233, 11–18. [Google Scholar] [CrossRef]
- Woo, K.S.; Yoon, H.S.; Lee, Y.R.; Lee, J.; Kim, D.J.; Hong, J.T.; Jeong, H.S. Characteristics and antioxidative activity of volatile compounds in heated garlic (Allium sativum). Food Sci. Biotechnol. 2007, 16, 822–827. [Google Scholar]

| Recipe * | Tomato | Onion | Garlic | Olive Oil | Salt | Thyme | Rosemary | Dry Matter % ** | YF |
|---|---|---|---|---|---|---|---|---|---|
| CS1 | 400 | 150 | 15 | 50 | 2 | 27.4 ± 0.2a | 0.54 ± 0.1 | ||
| CS2 | 400 | 150 | 30 | 50 | 2 | 26.7 ± 0.1b | 0.56 ± 0.2 | ||
| CS3 | 400 | 150 | 15 | 50 | 2 | 1 | 28.0 ± 0.1c | 0.54 ± 0.1 | |
| CS4 | 400 | 150 | 15 | 50 | 2 | 1 | 27.9 ± 0.1c | 0.56 ± 0.1 | |
| CS5 | 400 | 150 | 0 | 50 | 2 | 26.0 ± 0.1d | 0.55 ± 0.1 | ||
| CS6 | 400 | 0 | 15 | 50 | 2 | 33.2 ± 0.4e | 0.50 ± 0.2 | ||
| S1 | 400 | 150 | 15 | 50 | 2 | 17.3 ± 0.2f | |||
| Raw onion | 8.6 ± 0.2g | ||||||||
| Raw garlic | 33.8 ± 0.2e |
| Validation Parameter | Hexanal | Trans-2-Heptenal | Octanal | Trans-2-Decenal |
|---|---|---|---|---|
| Linear range (mg Kg−1) | 0.001–1.096 | 0.001–1.760 | 0.002–0.880 | 0.004–0.073 |
| R2 value | 0.998 | 0.997 | 0.998 | 0.990 |
| Limits of detection (LOD) (µg Kg−1) | 0.89 ± 0.06 | 1.03 ± 0.53 | 1.55 ± 0.07 | 18.48 ± 0.65 |
| Limits of quantitation (LOQ) (µg Kg−1) | 2.97 ± 0.20 | 5.76 ± 0.78 | 5.15 ± 0.24 | 61.59 ± 2.18 |
| Intra-day repeatability * | 1.9 | 4.2 | 1.4 | 3.5 |
| Inter-day repeatability * | 6.7 | 4.9 | 6.5 | 7.9 |
| Compound | Rt (min) | S1 | CS1 | CS2 | CS3 | CS4 | CS5 | CS6 |
|---|---|---|---|---|---|---|---|---|
| trans-2-heptenal | 12.77 | 0.66 ± 0.07a | 0.83 ± 0.36b | 0.73 ± 0.14b | 0.75 ± 017b | 0.88 ± 0.36b | 0.88 ± 0.11b | 1.11 ± 0.21b |
| 2-hexenal | 10.75 | 0.67 ± 0.04 | Nd | Nd | Nd | Nd | Nd | Nd |
| 1-octanol | 14.58 | 1.26 ± 0.21 | Nd | Nd | Nd | Nd | Nd | Nd |
| 2,4-dimehyl-thiophene | 11.41 | 1.34 ± 0.31a | 1.64 ± 0.14a | 1.36 ± 0.14a | 1.26 ± 0.21a | 1.43 ± 0.33a | 1.76 ± 0.25a | 0.28 ± 0.01b |
| furfural | 10.59 | 1.36 ± 0.23 | 0.84 ± 0.11 | 1.02 ± 0.21 | 1,34 ±0.14 | 1.26 ± 27 | 1.35 ± 0.12 | Nd |
| hexanal | 9.32 | 1.70 ± 0.32a | 0.53 ± 0.06b | 0.60 ±0.11b | 0.51 ± 0.05b | 0.45 ± 0.25b | 0.70 ± 0.10b | 0.89 ± 0.12c |
| benzaldehyde | 13.01 | 1.70 ± 0.42a | 0.44 ± 08b | 0.39 ± 0.10b | 0.44 ± 0.07b | 0.42 ± 0.07b | 0.33 ± 0.03b | 0.38 ± 0.04b |
| 6-methyl-5-hepten-2-one | 13.10 | 1.82 ± 0.45 | 2.16 ± 0.84 | 3.55 ± 0.85 | 2.21 ± 0.74 | 3.04 ± 0.73 | 3.24 ± 0.40 | 3.63 ± 0.35 |
| alpha-farnesene | 20.51 | 1.99 ± 0.56 | 0.89 ± 0.18 | 0.71 ± 0.33 | 1.03 ± 0.19 | 1.08 ± 0.54 | 0.47 ± 0.20 | Nd |
| di-2-propenyltrisulfide | 18.38 | 3.05 ± 0.46 | Nd | Nd | Nd | Nd | Nd | Nd |
| methyl-2-propenyldisulfide | 11.70 | 3.68 ± 0.65a | 0.50 ± 0.06b | 0.85 ± 0.18b | 0.65 ± 0.15b | 0.64 ± 0.28b | Nd | 0.22 ±0.02c |
| 2-methyl-2-pentenal | 10.16 | 5.40 ± 0.70a | 2.21 ± 0.69b | 1.99 ± 0.16b | 1.49 ± 0.38b | 1.18 ± 0.40b | 1.52 ± 0.30b | 0.34 ± 0.01c |
| 3,4-dihydro-3-vinyl-1,2-dithiin | 16.92 | 26.34 ± 3.13 | Nd | Nd | Nd | Nd | Nd | Nd |
| di-2-propenyldisulfide | 14.76 | 47.12 ± 5.20a | 2.84 ± 0.57b | 16.76 ± 1.30c | 4.94 ± 0.91b | 2.99 ± 1.31b | 0.58 ± 0.10d | 6.36 ± 0.39b |
| 1,2-dimethyl-benzene | 10.52 | Nd | 0.55 ± 0.26 | 0.33 ± 0.07 | 0.41 ± 0.08 | 0.47 ± 0.18 | 0.60 ± 0.18 | Nd |
| benzeneacetaldehyde | 14.57 | Nd | 2.27 ± 0.48 | 1.14 ± 0.23 | 2.69 ± 0.46 | 3.02 ± 0.33 | 2.36 ± 0.54 | Nd |
| limonene | 13.38 | Nd | 0.33 ± 0.05 | 0.33 ± 0.07 | 0.37 ± 0.03 | 2.15 ± 0.61 | 0.25 ± 0.23 | 0.23 ± 0.02 |
| octanal | 13.32 | Nd | 1.18 ± 0.26 | 1.14 ± 0.23 | 1.39 ± 0.27 | 1.09 ± 0.44 | 0.97 ± 0.26 | 1.41 ± 0.17 |
| octane | 8.43 | Nd | 1.30 ± 0.44a | 1.01 ± 0.16a | 0.82 ± 25a | 1.04 ± 0.33a | 2.47 ± 0.47b | 4.16 ± 0.14c |
| trans,trans-2,4-decadienal | 18.75 | Nd | 10.60 ± 3.82 | 10.73 ± 2.25 | 10.30 ± 1.91 | 10.91 ± 3.4 | 2.84 ± 0.04 | 1.89 ± 0.11 |
| trans-2-decenal | 17.81 | Nd | 7.05 ± 1.00 | 7.09 ± 2.39 | 7.84 ± 1.48 | 7.00 ± 3.60 | 5.26 ± 1.62 | 5.44 ± 0.48 |
| dipropyldisulfide | 15.08 | Nd | 14.88 ± 1.87 | 14.88 ± 1.87 | 12.01 ± 0.19 | 11.07 ± 2.54 | Nd | Nd |
| trans-Citral | 18.03 | Nd | 0.71 ± 0.29 | 0.71 ± 0.29 | Nd | Nd | Nd | Nd |
| trans-propenylpropyldisulfide | 15.27 | Nd | 2.42 ± 0.39 | 2.42 ± 0.39 | 2.62 ± 0.54 | 2.96 ± 1.07 | Nd | Nd |
| Eucalyptol | 13.61 | Nd | Nd | Nd | Nd | 16.37 ± 1.47 | Nd | Nd |
| 1-methyl-4-(1-methylethyl)-benzene | 13.46 | Nd | Nd | Nd | Nd | 2.52 ± 0.44 | Nd | Nd |
| 2,4-heptadienal | 13.91 | Nd | Nd | Nd | 1.66 ± 0.54 | 0.63 ± 0.08 | 0.48 ± 0.11 | Nd |
| 2-methyl-5-(1-methylethyl)-phenol | 18.83 | Nd | Nd | Nd | 14.62 ± 4.14 | Nd | Nd | Nd |
| 4-terpineol | 16.46 | Nd | Nd | Nd | 1.71 ± 0 41 | 2.5 2± 0.30 | Nd | Nd |
| alpha-amorphene | 20.44 | Nd | Nd | Nd | Nd | 0.63 ± 0.07 | Nd | Nd |
| alpha-copaene | 19.07 | Nd | Nd | Nd | Nd | 2.65 ± 0.37 | Nd | Nd |
| alpha-humulne | 20.40 | Nd | Nd | Nd | Nd | 0.48 ± 0.19 | Nd | Nd |
| alpha-terpineol | 16.76 | Nd | Nd | Nd | 2.46 ± 0.09 | 7.66 ± 1.32 | Nd | Nd |
| beta-caryophyllene | 19.92 | Nd | Nd | Nd | 3.69 ± 0.39 | Nd | Nd | Nd |
| borneol L | 16.61 | Nd | Nd | Nd | 21.40 ± 3.19 | 5.98 ± 1.07 | Nd | Nd |
| camphene | 11.95 | Nd | Nd | Nd | Nd | 0.59 ± 0.23 | Nd | Nd |
| camphor | 16.31 | Nd | Nd | Nd | Nd | 12.27 ± 2.62 | Nd | Nd |
| delta-cadinene | 20.98 | Nd | Nd | Nd | 0.91 ± 18 | 1.02 ± 0.42 | Nd | Nd |
| linalool | 15.01 | Nd | Nd | Nd | 4.43 ± 0.24 | 0.86 ± 0.34 | Nd | Nd |
| myrcene | 12.54 | Nd | Nd | Nd | Nd | 0.71 ± 0.16 | Nd | Nd |
| thymol | 18.60 | Nd | Nd | Nd | 1.47 ± 0.44 | Nd | nd | Nd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beltrán Sanahuja, A.; De Pablo Gallego, S.L.; Maestre Pérez, S.E.; Valdés García, A.; Prats Moya, M.S. Influence of Cooking and Ingredients on the Antioxidant Activity, Phenolic Content and Volatile Profile of Different Variants of the Mediterranean Typical Tomato Sofrito. Antioxidants 2019, 8, 551. https://doi.org/10.3390/antiox8110551
Beltrán Sanahuja A, De Pablo Gallego SL, Maestre Pérez SE, Valdés García A, Prats Moya MS. Influence of Cooking and Ingredients on the Antioxidant Activity, Phenolic Content and Volatile Profile of Different Variants of the Mediterranean Typical Tomato Sofrito. Antioxidants. 2019; 8(11):551. https://doi.org/10.3390/antiox8110551
Chicago/Turabian StyleBeltrán Sanahuja, Ana, Saray López De Pablo Gallego, Salvador E. Maestre Pérez, Arantzazu Valdés García, and María Soledad Prats Moya. 2019. "Influence of Cooking and Ingredients on the Antioxidant Activity, Phenolic Content and Volatile Profile of Different Variants of the Mediterranean Typical Tomato Sofrito" Antioxidants 8, no. 11: 551. https://doi.org/10.3390/antiox8110551
APA StyleBeltrán Sanahuja, A., De Pablo Gallego, S. L., Maestre Pérez, S. E., Valdés García, A., & Prats Moya, M. S. (2019). Influence of Cooking and Ingredients on the Antioxidant Activity, Phenolic Content and Volatile Profile of Different Variants of the Mediterranean Typical Tomato Sofrito. Antioxidants, 8(11), 551. https://doi.org/10.3390/antiox8110551










