Cancer Chemotherapy and Chemiluminescence Detection of Reactive Oxygen Species in Human Semen
Abstract
1. Introduction
2. Materials and Methods
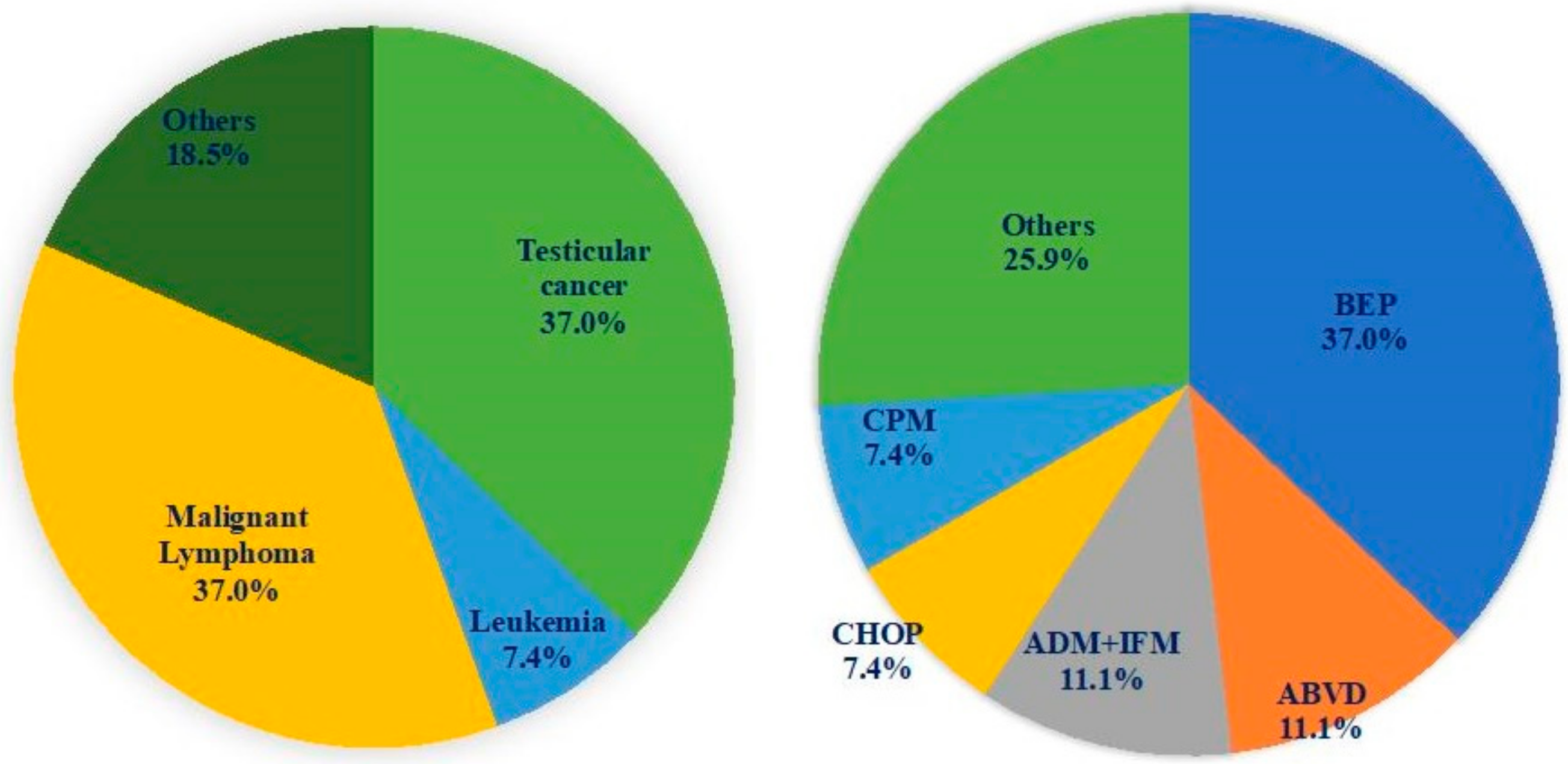
2.1. Subjects
2.2. Semen Collection and Assessment of Semen Features
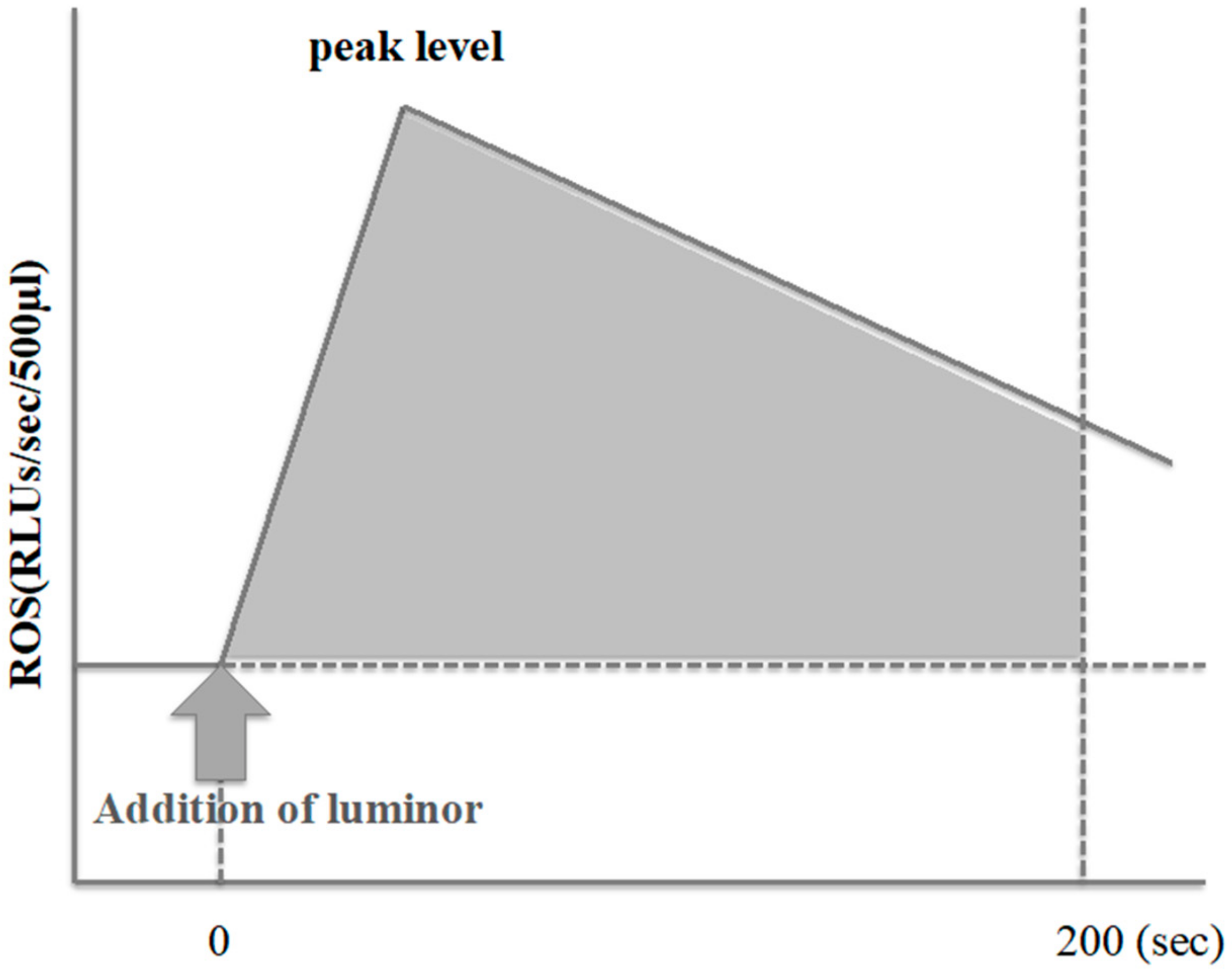
2.3. ROS Measurement
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ito, Y.; Miyashiro, I.; Ito, H.; Hosono, S.; Chihara, D.; Nakata-Yamada, K.; Nakayama, M.; Matsuzaka, M.; Hattori, M.; Sugiyama, H.; et al. Long-term survival and conditional survival of cancer patients in Japan using population-based cancer registry data. Cancer Sci. 2014, 105, 1480–1486. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Suzuki, K.; Iwasaki, A.; Yumura, Y.; Kubota, Y. Sperm cryopreservation before cancer chemotherapy helps in the emotional battle against cancer. Cancer 2005, 104, 521–524. [Google Scholar] [CrossRef] [PubMed]
- Schrader, M.; Müller, M.; Straub, B.; Miller, K. The impact of chemotherapy on male fertility: A survey of the biologic basis and clinical aspects. Reprod. Toxicol. 2001, 15, 611–617. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive Oxygen Species and Sperm Function—In Sickness and In Health. J. Androl. 2012, 33, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G.N. Oxidative stress and male reproductive health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kardeh, S.; Ashkani-Esfahani, S.; Alizadeh, A.M. Paradoxical action of reactive oxygen species in creation and therapy of cancer. Eur. J. Pharmacol. 2014, 735, 150–168. [Google Scholar] [CrossRef]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Shi, X.; Liu, K.J. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell. Biochem. 2004, 255, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Yen, Y.-P.; Tsai, K.-S.; Chen, Y.-W.; Huang, C.-F.; Yang, R.-S.; Liu, S.-H. Arsenic induces apoptosis in myoblasts through a reactive oxygen species-induced endoplasmic reticulum stress and mitochondrial dysfunction pathway. Arch. Toxicol. 2012, 86, 923–933. [Google Scholar] [CrossRef]
- Marullo, R.; Werner, E.; Degtyareva, N.; Moore, B.; Altavilla, G.; Ramalingam, S.S.; Doetsch, P.W. Cisplatin Induces a Mitochondrial-ROS Response That Contributes to Cytotoxicity Depending on Mitochondrial Redox Status and Bioenergetic Functions. PLoS ONE 2013, 8, e81162. [Google Scholar] [CrossRef] [PubMed]
- Yumura, Y.; Takeshima, T.; Kawahara, T.; Sanjo, H.; Kuroda, S.; Asai, T.; Mori, K.; Kondou, T.; Uemura, H.; Iwasaki, A. Reactive oxygen species measured in the unprocessed semen samples of 715 infertile patients. Reprod. Med. Biol. 2017, 16, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, T.; Yumura, Y.; Yasuda, K.; Sanjo, H.; Kuroda, S.; Yamanaka, H.; Iwasaki, A. Inverse correlation between reactive oxygen species in unwashed semen and sperm motion parameters as measured by a computer-assisted semen analyzer. Asian J. Androl. 2017, 19, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef]
- Ollero, M.; Gil-Guzman, E.; Lopez, M.C.; Sharma, R.K.; Agarwal, A.; Larson, K.; Evenson, D.; Thomas, A.J., Jr.; Alvarez, J.G. Characterization of subsets of human spermatozoa at different stages of maturation: Implications in the diagnosis and treatment of male infertility. Hum. Reprod. 2001, 16, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Tesarik, J.; Greco, E.; Mendoza, C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum. Reprod. 2004, 19, 611–615. [Google Scholar] [CrossRef]
- Sakkas, D.; Alvarez, J.G. Sperm DNA fragmentation: Mechanisms of origin, impact on reproductive outcome, and analysis. Fertil. Steril. 2010, 93, 1027–1036. [Google Scholar] [CrossRef]
- Yumura, Y.; Iwasaki, A.; Saito, K.; Ogawa, T.; Hirokawa, M. Effect of reactive oxygen species in semen on the pregnancy of infertile couples. Int. J. Urol. 2009, 16, 202–207. [Google Scholar] [CrossRef]
- O’Flaherty, C.; Hales, B.F.; Chan, P.; Robaire, B. Impact of chemotherapeutics and advanced testicular cancer or Hodgkin lymphoma on sperm deoxyribonucleic acid integrity. Fertil. Steril. 2010, 94, 1374–1379. [Google Scholar] [CrossRef]
- Dragovich, T.; Gordon, M.; Mendelson, D.; Wong, L.; Modiano, M.; Chow, H.-H.S.; Samulitis, B.; O’Day, S.; Grenier, K.; Hersh, E.; et al. Phase I Trial of Imexon in Patients with Advanced Malignancy. J. Clin. Oncol. 2007, 25, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Berretta, M.; Bottacin, A.; Palego, P.; Sartini, B.; Cosci, I.; Finos, L.; Selice, R.; Foresta, C.; Garolla, A. Impact of Bep or Carboplatin Chemotherapy on Testicular Function and Sperm Nucleus of Subjects with Testicular Germ Cell Tumor. Front. Pharmacol. 2016, 7, 673. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Schover, L.R.; Partridge, A.H.; Patrizio, P.; Wallace, W.H.; Hagerty, K.; Beck, L.N.; Brennan, L.V.; Oktay, K. American Society of Clinical Oncology Recommendations on Fertility Preservation in Cancer Patients. J. Clin. Oncol. 2006, 24, 2917–2931. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.; Van Casteren, N.J.; Wildhagen, M.F.; Romijn, J.C.; Dohle, G.R. Sperm DNA integrity in cancer patients before and after cytotoxic treatment. Hum. Reprod. 2010, 25, 1877–1883. [Google Scholar] [CrossRef]
- Guerriero, G.; D’Errico, G.; Di Giaimo, R.; Rabbito, D.; Olanrewaju, O.S.; Ciarcia, G. Reactive oxygen species and glutathione antioxidants in the testis of the soil biosentinel Podarcis sicula (Rafinesque 1810). Environ. Sci. Pollut. Res. Int. 2018, 25, 18286–18296. [Google Scholar] [CrossRef]
- Showell, M.G.; Mackenzie-Proctor, R.; Brown, J.; Yazdani, A.; Stankiewicz, M.T.; Hart, R.J. Antioxidants for male subfertility. Cochrane Database Syst. Rev. 2014, 12, CD007411. [Google Scholar] [CrossRef]
Data Availability: The data used to support the findings of this study are available from the corresponding author upon request. |
| Characteristic | Postchemotherapy (A) | Idiopathic (B) | Control (C) | p | |
|---|---|---|---|---|---|
| A–B | A–C | ||||
| n | 64 | 467 | 402 | ||
| Age (years) | 34.9 ± 7.46 | 37.02 ± 7.00 | 37.40 ± 6.28 | 0.015 | 0.004 |
| Sperm concentration (million /mL) | 22.10 ± 31.32 | 34.75 ± 34.98 | 44.18 ± 28.93 | 0.003 | <0.001 |
| Sperm motility (%) | 23.85 ± 20.63 | 29.75 ± 21.17 | 26.90 ± 16.08 | 0.018 | 0.03 |
| ROS-positive rate (%) | 42.2 (27/64) | 34.4 (161/467) | 20.4 (82/402) | 0.226 | <0.001 |
| Parameter | Postchemotherapy (A) | Idiopathic (B) | Control (C) | p | |
|---|---|---|---|---|---|
| A–B | A–C | ||||
| n | 27 | 161 | 82 | ||
| Sperm concentration (million/mL) | 28.66 ± 37.19 | 28.97 ± 31.55 | 43.48 ± 24.70 | 0.482 | 0.010 |
| Sperm motility (%) | 19.36 ± 20.89 | 26.37 ± 19.89 | 29.03 ± 14.17 | 0.047 | 0.004 |
| ROS level (RLUs) | 81032.2 ± 139294.0 | 76906.7 ± 155068.0 | 79374.3 ± 273599.9 | 0.448 | 0.488 |
| ROS level per million spermatozoa (RLUs) | 25937.0 ± 62187.3 | 21389.6 ± 134787.0 | 6696.7 ± 26468.2 | 0.432 | 0.013 |
| Parameter | ROS-positive | ROS-negative | p |
|---|---|---|---|
| n | 27 | 37 | |
| Age | 35.1 ± 7.3 | 34.9 ± 7.7 | 0.456 |
| Sperm concentration (million/mL) | 28.66 ± 37.19 | 17.31 ± 25.74 | 0.077 |
| Sperm motility (%) | 19.36 ± 20.89 | 21.14 ± 20.08 | 0.069 |
| The period from treatment to the first visit (month) | 34.67 ± 52.57 | 30.07 ± 70.02 | 0.387 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeshima, T.; Kuroda, S.; Yumura, Y. Cancer Chemotherapy and Chemiluminescence Detection of Reactive Oxygen Species in Human Semen. Antioxidants 2019, 8, 449. https://doi.org/10.3390/antiox8100449
Takeshima T, Kuroda S, Yumura Y. Cancer Chemotherapy and Chemiluminescence Detection of Reactive Oxygen Species in Human Semen. Antioxidants. 2019; 8(10):449. https://doi.org/10.3390/antiox8100449
Chicago/Turabian StyleTakeshima, Teppei, Shinnosuke Kuroda, and Yasushi Yumura. 2019. "Cancer Chemotherapy and Chemiluminescence Detection of Reactive Oxygen Species in Human Semen" Antioxidants 8, no. 10: 449. https://doi.org/10.3390/antiox8100449
APA StyleTakeshima, T., Kuroda, S., & Yumura, Y. (2019). Cancer Chemotherapy and Chemiluminescence Detection of Reactive Oxygen Species in Human Semen. Antioxidants, 8(10), 449. https://doi.org/10.3390/antiox8100449





