Microwave-Assisted Extraction of Ricinine from Ricinus communis Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Plant Material
2.2. Extraction of Ricinine
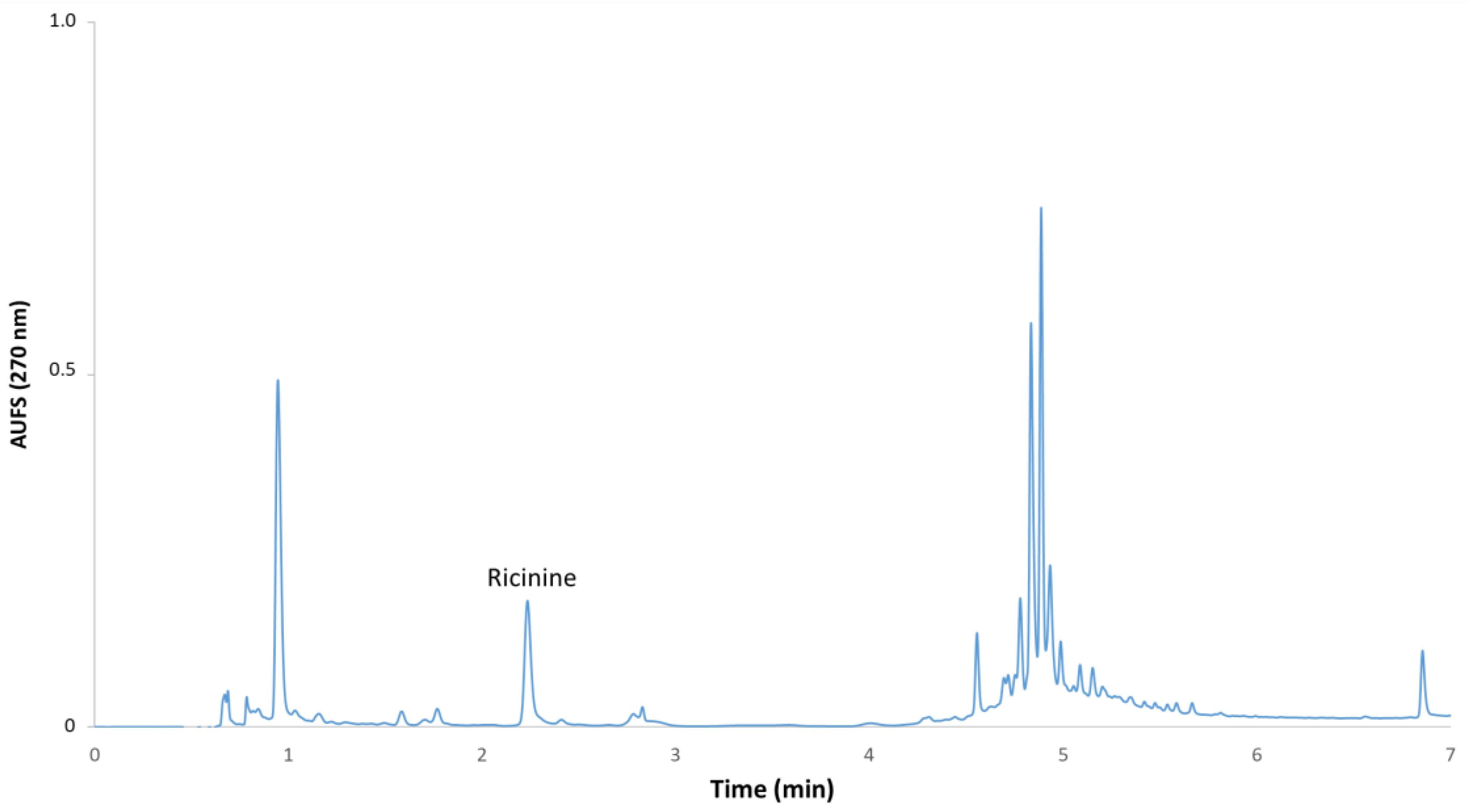
2.3. Determination of Ricinine
2.4. Experimental Design for the Extractions
2.5. Data Analysis
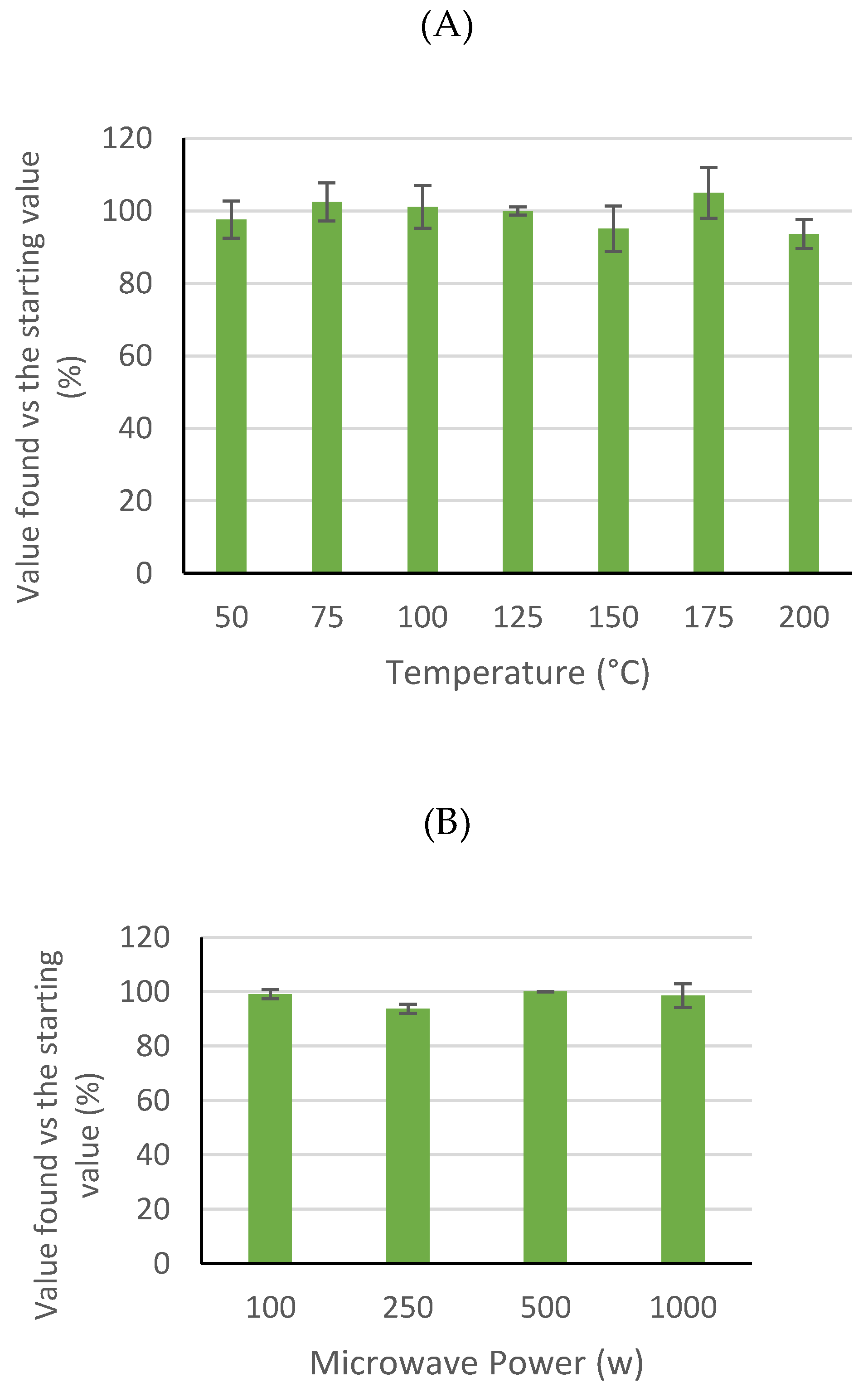
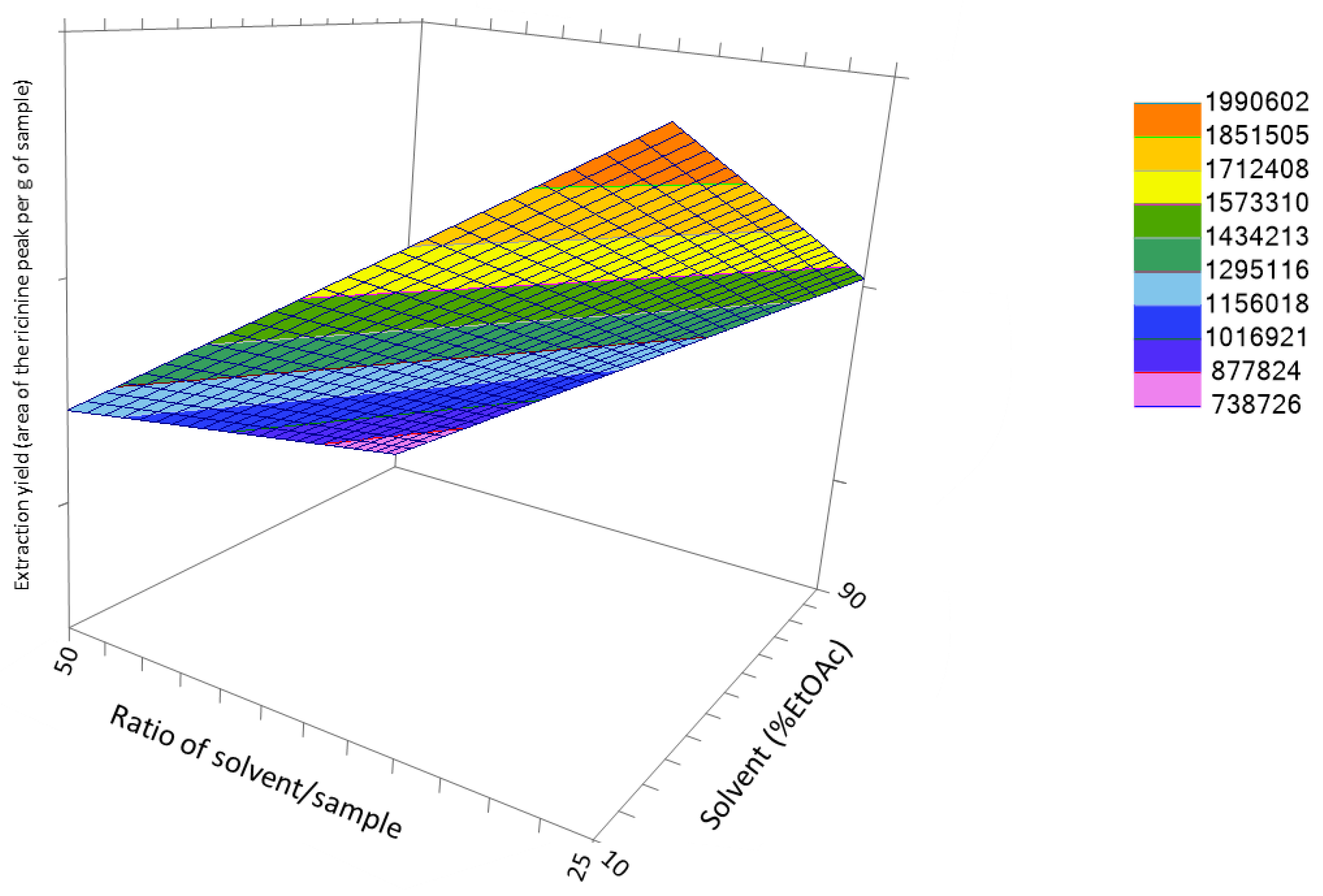
3. Results and Discussion
3.1. Ricinine Stability at Different Extraction Temperatures and Microwave Powers
3.2. Development of the Method
3.3. Optimization of the Extraction Conditions
3.4. Method Validation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Arruda Rodrigues, H.C.; Carvalho, S.P.; Souza, H.A.; Carvalho, A.A. Castor bean cultivars and nitrogen fertilization in the formation of seedlings. Acta Sci.-Agron. 2010, 32, 471–476. [Google Scholar] [CrossRef][Green Version]
- Tripathi, A.C.; Gupta, R.; Saraf, S.K. Phytochemical investigation characterisation and anticonvulsant activity of Ricinus communis seeds in mice. Nat. Prod. Res. 2011, 25, 1881–1884. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, A.C.; Angelucci, M.E.M.; Da Costa, M.L.; Batista, I.R.; De Oliveira, B.H.; Da Cunha, C. Pharmacological evaluation of ricinine, a central nervous system stimulant isolated from Ricinus communis. Pharmacol. Biochem. Behav. 1999, 63, 367–375. [Google Scholar] [CrossRef]
- Bullangpoti, V.; Khumrungsee, N.; Pluempanupat, W.; Kainoh, Y.; Saguanpong, U. Toxicity of ethyl acetate extract and ricinine from Jatropha gossypifolia senescent leaves against Spodoptera exigua Hubner (Lepidoptera: Noctuidae). J. Pestic. Sci. 2011, 36, 260–263. [Google Scholar] [CrossRef]
- Bigi, M.; Torkomian, V.L.; de Groote, S.T.; Hebling, M.J.A.; Bueno, O.C.; Pagnocca, F.C.; Fernandes, J.B.; Vieira, P.C.; da Silva, M. Activity of Ricinus communis (Euphorbiaceae) and ricinine against the leaf-cutting ant Atta sexdens rubropilosa (Hymenoptera: Formicidae) and the symbiotic fungus Leucoagaricus gongylophorus. Pest Manag. Sci. 2004, 60, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Lopez, M.A.; Perez, S.; Rodriguez-Hernandez, G.C.; Guevara-Fefer, P.; Zavala-Sanchez, M.A. Activity of Ricinus communis (Euphorbiaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae). Afr. J. Biotechnol. 2010, 9, 1359–1365. [Google Scholar]
- El-Naggar, M.H.; Elgaml, A.; Abdel Bar, F.M.; Badria, F.A. Antimicrobial and antiquorum-sensing activity of Ricinus communis extracts and ricinine derivatives. Nat. Prod. Res. 2019, 33, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.M.; Batista, D.L.J.; Ribeiro, L.A.F.; Boffo, E.F.; de Cerqueira, M.D.; Martins, D.; de Castro, R.D.; de Souza-Neta, L.C.; Pinto, E.; Zambotti-Villela, L.; et al. Identification of antioxidant and antimicrobial compounds from the oilseed crop Ricinus communis using a multiplatform metabolite profiling approach. Ind. Crops Prod. 2018, 124, 834–844. [Google Scholar] [CrossRef]
- Zoumpoulakis, P.; Sinanoglou, V.J.; Siapi, E.; Heropoulos, G.; Proestos, C. Evaluating Modern Techniques for the Extraction and Characterisation of Sunflower (Hellianthus annus L.) Seeds Phenolics. Antioxidants 2017, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Le, B.; Golokhvast, K.S.; Yang, S.H.; Sun, S. Optimization of Microwave-Assisted Extraction of Polysaccharides from Ulva pertusa and Evaluation of Their Antioxidant Activity. Antioxidants 2019, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Quiroz, J.Q.; Torres, A.C.; Ramirez, L.M.; Garcia, M.S.; Gomez, G.C.; Rojas, J. Optimization of the Microwave-Assisted Extraction Process of Bioactive Compounds from Annatto Seeds (Bixa orellana L.). Antioxidants 2019, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Microwave assisted extraction of soy isoflavones. Anal. Chim. Acta 2007, 588, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.-F.; Zhang, X.; Yang, X.-H.; Qiu, N.-X.; Wang, Y.; Wang, Z.-Z. Microwave assisted extraction of flavonoids from cultivated Epimedium sagittatum: Extraction yield and mechanism, antioxidant activity and chemical composition. Ind. Crop. Prod. 2013, 50, 857–865. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Optimisation and validation of the microwave-assisted extraction of phenolic compounds from rice grains. Food Chem. 2015, 169, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Destandau, E.; Michel, T.; Elfakir, C. Microwave-assisted Extraction. In Natural Product Extraction: Principles and Applications; Rostagno, M.A., Prado, J.M., Eds.; Royal Society of Chemistry: London, UK, 2013; pp. 113–156. [Google Scholar] [CrossRef]
- Setyaningsih, W.; Palma, M.; Barroso, C.G. A new microwave-assisted extraction method for melatonin determination in rice grains. J. Cereal Sci. 2012, 56, 340–346. [Google Scholar] [CrossRef]
- Cai, M.; Chen, X.; Wei, X.; Pan, S.; Zhao, Y.; Jin, M. Dispersive solid-phase extraction followed by high-performance liquid chromatography/tandem mass spectrometry for the determination of ricinine in cooking oil. Food Chem. 2014, 158, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gu, D.; Liu, Y.; Huang, F.; Yang, Y. Large-scale separation of ricinine from a by-product of Ricinus communis L. by pH-zone-refining counter-current chromatography. Ind. Crops Prod. 2013, 49, 160–163. [Google Scholar] [CrossRef]
- Cazal, C.d.M.; Batalhao, J.R.; Domingues, V.d.C.; Bueno, O.C.; Rodrigues Filho, E.; Forim, M.R.; Fernandes da Silva, M.F.G.; Vieira, P.C.; Fernandes, J.B. High-speed counter-current chromatographic isolation of ricinine, an insecticide from Ricinus communis. J. Chromatogr. A 2009, 1216, 4290–4294. [Google Scholar] [CrossRef] [PubMed]
- Campana, A.M.G.; Rodriguez, L.C.; Barrero, F.A.; Ceba, M.R. ALAMIN: A chemometric program to check analytical method performance and to assess the trueness by standard addition methodology. TrAC-Trends Anal. Chem. 1997, 16, 381–385. [Google Scholar] [CrossRef]
| Independent Variables | Codes | Variables Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Temperature (°C) | X1 | 125 | 150 | 175 |
| Power (W) | X2 | 500 | 750 | 1000 |
| Extraction time (min) | X3 | 5 | 10 | 15 |
| Solvent (%EtOAc) | X4 | 10 | 50 | 90 |
| Ratio of solvent/sample | X5 | 25 | 37.5 | 50 |
| Extraction Variable 1 | Resulting Values for Ricinine (mg of Ricinine/g of Sample) | Relative Error (%) | |||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | X5 | Experimental Values | Predicted Values | |
| 175 | 500 | 5 | 10 | 50 | 0.483 | 0.496 | 3 |
| 175 | 500 | 5 | 10 | 25 | 0.870 | 0.901 | 4 |
| 175 | 1000 | 15 | 90 | 37.5 | 0.411 | 0.622 | 41 |
| 125 | 1000 | 5 | 10 | 50 | 0.390 | 0.545 | 33 |
| 150 | 1000 | 15 | 50 | 50 | 0.615 | 0.440 | 33 |
| 175 | 1000 | 5 | 10 | 25 | 0.820 | 0.948 | 15 |
| 125 | 1000 | 5 | 50 | 25 | 0.995 | 0.777 | 25 |
| 125 | 500 | 15 | 50 | 25 | 1.037 | 0.761 | 31 |
| 175 | 1000 | 15 | 50 | 25 | 1.003 | 0.845 | 17 |
| 175 | 500 | 15 | 90 | 37.5 | 0.422 | 0.599 | 35 |
| 125 | 750 | 15 | 90 | 25 | 0.641 | 0.649 | 1 |
| 175 | 1000 | 15 | 10 | 50 | 0.780 | 0.561 | 33 |
| 125 | 1000 | 10 | 90 | 37.5 | 0.303 | 0.513 | 52 |
| 150 | 1000 | 10 | 10 | 25 | 0.907 | 0.896 | 1 |
| 125 | 500 | 15 | 10 | 50 | 0.473 | 0.519 | 9 |
| 150 | 1000 | 5 | 90 | 25 | 0.683 | 0.685 | 0 |
| 150 | 500 | 15 | 90 | 50 | 0.415 | 0.336 | 21 |
| 175 | 500 | 10 | 90 | 25 | 0.776 | 0.706 | 9 |
| 175 | 1000 | 5 | 90 | 50 | 0.417 | 0.417 | 0 |
| 175 | 750 | 5 | 90 | 25 | 0.721 | 0.719 | 0 |
| 125 | 500 | 5 | 90 | 37.5 | 0.279 | 0.503 | 57 |
| 125 | 500 | 5 | 10 | 25 | 0.892 | 0.771 | 15 |
| 175 | 500 | 5 | 90 | 50 | 0.390 | 0.400 | 2 |
| 175 | 500 | 15 | 10 | 25 | 0.882 | 0.918 | 4 |
| 125 | 1000 | 15 | 10 | 37.5 | 0.482 | 0.723 | 40 |
| 125 | 750 | 10 | 90 | 50 | 0.327 | 0.272 | 18 |
| 150 | 750 | 10 | 50 | 37.5 | 0.582 | 0.615 | 5 |
| 150 | 750 | 10 | 50 | 37.5 | 0.561 | 0.615 | 9 |
| 150 | 750 | 10 | 50 | 37.5 | 0.585 | 0.614 | 5 |
| Model Term | Estimate | p-Value |
|---|---|---|
| b0 | 4.0787 | |
| b1 | 0.1944 | 0.3025 |
| b2 | 0.0417 | 0.8330 |
| b3 | 0.0360 | 0.8492 |
| b4 | −0.4011 | 0.0049 |
| b5 | −0.6843 | 0.0001 |
| Ratio (mL of Solvent g of Sample−1) | ||
| 25 | 20 | 10 |
| 1.359 a ± 0.0079 | 1.173 b ± 0.0018 | 0.560 c ± 0.0371 |
| Percentage of ethyl acetate in methanol | ||
| 10 | 5 | 0 |
| 1.416 a ± 0.0059 | 1.263 b ± 0.0021 | 0.525 c ± 0.0262 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nebo, L.; Varela, R.M.; Fernandes, J.B.; Palma, M. Microwave-Assisted Extraction of Ricinine from Ricinus communis Leaves. Antioxidants 2019, 8, 438. https://doi.org/10.3390/antiox8100438
Nebo L, Varela RM, Fernandes JB, Palma M. Microwave-Assisted Extraction of Ricinine from Ricinus communis Leaves. Antioxidants. 2019; 8(10):438. https://doi.org/10.3390/antiox8100438
Chicago/Turabian StyleNebo, Liliane, Rosa M. Varela, João B. Fernandes, and Miguel Palma. 2019. "Microwave-Assisted Extraction of Ricinine from Ricinus communis Leaves" Antioxidants 8, no. 10: 438. https://doi.org/10.3390/antiox8100438
APA StyleNebo, L., Varela, R. M., Fernandes, J. B., & Palma, M. (2019). Microwave-Assisted Extraction of Ricinine from Ricinus communis Leaves. Antioxidants, 8(10), 438. https://doi.org/10.3390/antiox8100438





