Healthcare Workers Occupationally Exposed to Ionizing Radiation Exhibit Altered Levels of Inflammatory Cytokines and Redox Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Blood Collection
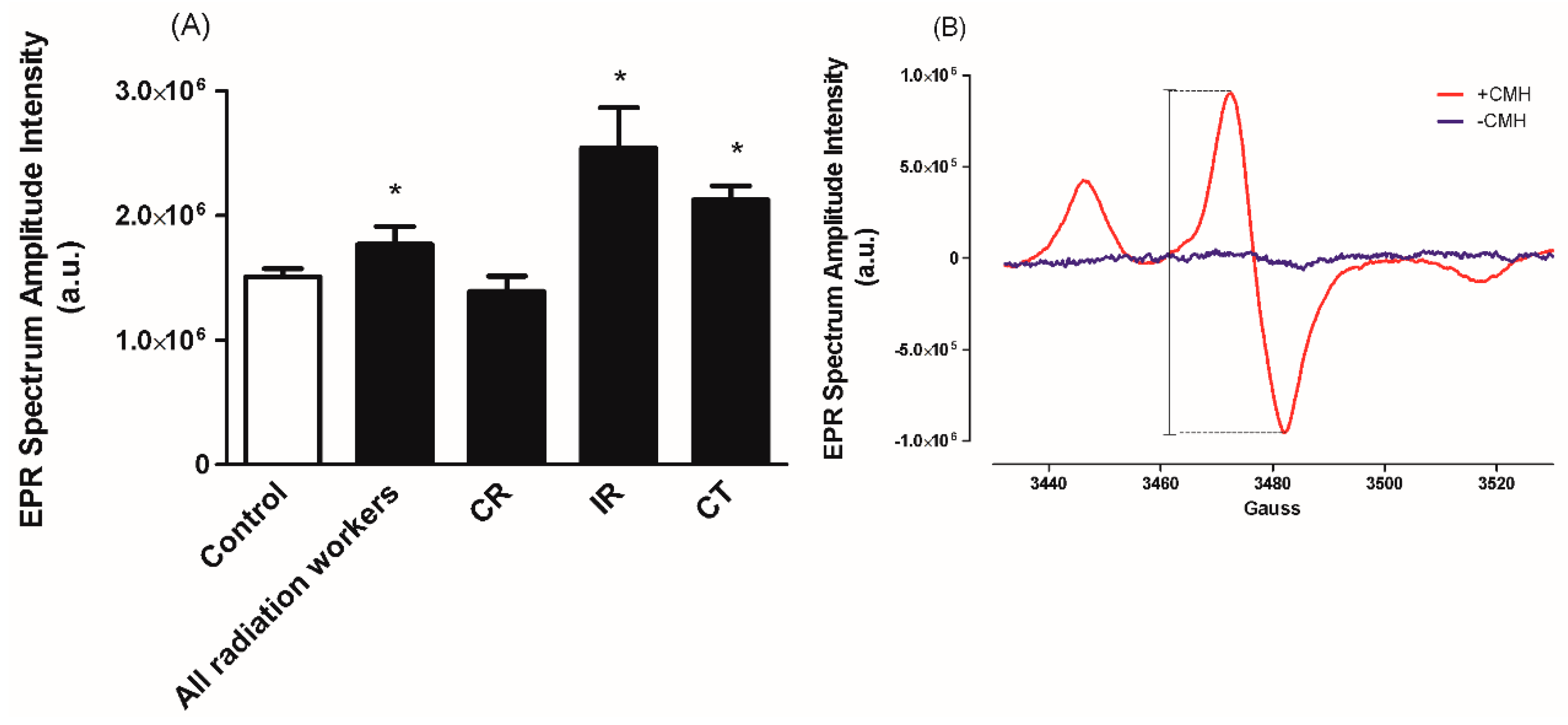
2.3. Superoxide Measurement
2.4. Assessment of DNA Oxidation
2.5. Cytokine Levels
2.6. Extracellular Superoxide Dismutase (EcSOD) and Glutathione Levels
2.7. Statistical Analysis
3. Results
3.1. Study Participants
3.2. Superoxide (O2•−) Level
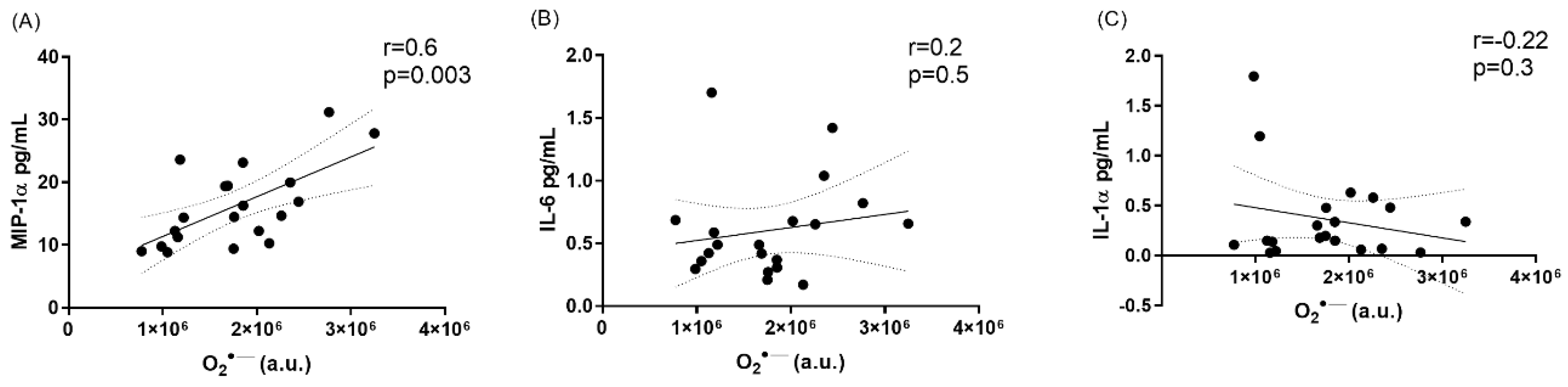
3.3. Systemic Inflamzmatory Marker Analysis
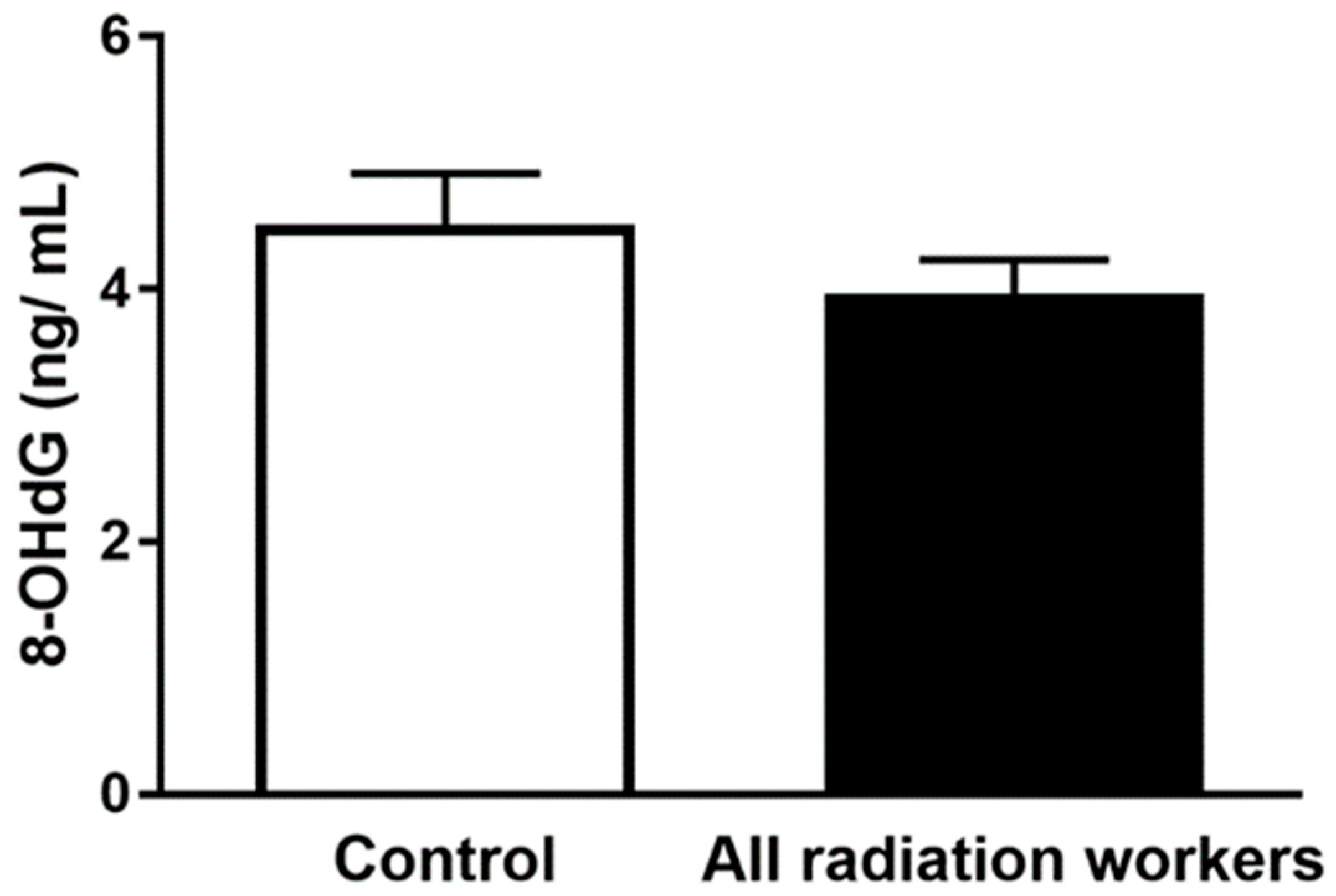
3.4. Plasma 8-OHdG Concentration
3.5. Antioxidants Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| O2•− | superoxide |
| EcSOD | extracellular superoxide dismutase |
| GSH/GSSG | reduced/oxidized glutathione ratio |
| ICRP | International Commission of Radiation Protection |
| ROS | reactive oxygen species |
| mSV | millisieverts |
| EPR | electron paramagnetic resonance |
| a.u. | arbitrary units |
| CR | conventional radiography |
| CT | computed tomography |
| IR | interventional radiography |
| 8-OHdG | 8-hydroxydeoxyguanosine |
| IL | interleukin |
| MIP | macrophage inflammatory protein |
References
- ICRP. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP publication 103. Ann. ICRP 2007, 37, 1–332. [Google Scholar] [CrossRef]
- Rajaraman, P.; Doody, M.M.; Yu, C.L.; Preston, D.L.; Miller, J.S.; Sigurdson, A.J.; Freedman, D.M.; Alexander, B.H.; Little, M.P.; Miller, D.L.; et al. Cancer Risks in U.S. Radiologic Technologists Working With Fluoroscopically Guided Interventional Procedures, 1994–2008. Am. J. Roentgenol. 2016, 206, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Hayata, I. Chromosomal mutations by low dose radiation vs. those by other mutagenic factors. Int. Congr. Ser. 2005, 1276, 17–20. [Google Scholar] [CrossRef]
- Hei, T.K.; Zhou, H.; Suzuki, M. Extranuclear target and low dose radiation risk assessment. Int. Congr. Ser. 2005, 1276, 21–24. [Google Scholar] [CrossRef]
- Liu, S.Z. On radiation hormesis expressed in the immune system. Crit. Rev. Toxicol. 2003, 33, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Meeran, S.M.; Katiyar, S.; Elmets, C.A.; Katiyar, S.K. Silymarin inhibits UV radiation-induced immunosuppression through augmentation of interleukin-12 in mice. Mol. Cancer Ther. 2006, 5, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.M.; Temme, J.B.; Abdalla, M.Y.; Zimmerman, M.C. Redox status in workers occupationally exposed to long-term low levels of ionizing radiation: A pilot study. Redox Rep. 2016, 21, 139–145. [Google Scholar] [CrossRef]
- Spitz, D.R.; Azzam, E.I.; Li, J.J.; Gius, D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifying concept in stress response biology. Cancer Metastasis Rev. 2004, 23, 311–322. [Google Scholar] [CrossRef]
- Zakeri, F.; Hirobe, T. A cytogenetic approach to the effects of low levels of ionizing radiations on occupationally exposed individuals. Eur. J. Radiol. 2010, 73, 191–195. [Google Scholar] [CrossRef]
- Jacob, P.; Ruhm, W.; Walsh, L.; Blettner, M.; Hammer, G.; Zeeb, H. Is cancer risk of radiation workers larger than expected? Occup. Environ. Med. 2009, 66, 789–796. [Google Scholar] [CrossRef]
- Zielinski, J.M.; Garner, M.J.; Band, P.R.; Krewski, D.; Shilnikova, N.S.; Jiang, H.; Ashmore, P.J.; Sont, W.N.; Fair, M.E.; Letourneau, E.G.; et al. Health outcomes of low-dose ionizing radiation exposure among medical workers: A cohort study of the Canadian national dose registry of radiation workers. Int. J. Occup. Med. Environ. Health 2009, 22, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, I.V.; Nikitaki, Z.; Souli, M.P.; Aziz, A.; Nowsheen, S.; Aziz, K.; Rogakou, E.; Georgakilas, A.G. Complex DNA Damage: A Route to Radiation-Induced Genomic Instability and Carcinogenesis. Cancers 2017, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Godekmerdan, A.; Ozden, M.; Ayar, A.; Gursu, M.F.; Ozan, A.T.; Serhatlioglu, S. Diminished cellular and humoral immunity in workers occupationally exposed to low levels of ionizing radiation. Arch. Med. Res. 2004, 35, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Hrycek, A.; Czernecka-Micinska, A.; Klucinski, P.; Badowski, R. Peripheral blood lymphocytes and selected serum interleukins in workers operating X-ray equipment. Toxicol. Lett. 2002, 132, 101–107. [Google Scholar] [CrossRef]
- McDermott, C.E.; Gengozian, N. The effect of low exposure-rate gamma irradiation on T and B lymphocyte function in the mouse. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1980, 37, 415–428. [Google Scholar] [CrossRef]
- NCRP Report No. 122. Use of Personal Monitors to Estimate Effective Dose Equivalent and Effective Dose to Workers for External Exposure to Low-LET Radiation; Report No. 122; The National Council on Radiation Protection and Measurements (NCRP): Bethesda, MD, USA, 1995. [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation. Ionizing Radiation: Sources and Biological Effects (Report to the General Assembly); United Nations Scientific Committee on the Effects of Atomic Radiation: Vienna, Austria, 1982. [Google Scholar]
- Efstathopoulos, E.P.; Makrygiannis, S.S.; Kottou, S.; Karvouni, E.; Giazitzoglou, E.; Korovesis, S.; Tzanalaridou, E.; Raptou, P.D.; Katritsis, D.G. Medical personnel and patient dosimetry during coronary angiography and intervention. Phys. Med. Biol. 2003, 48, 3059–3068. [Google Scholar] [CrossRef]
- Kim, K.P.; Miller, D.L.; Balter, S.; Kleinerman, R.A.; Linet, M.S.; Kwon, D.; Simon, S.L. Occupational radiation doses to operators performing cardiac catheterization procedures. Health Phys. 2008, 94, 211–227. [Google Scholar] [CrossRef]
- Alshkhrah, I.A.; Abu-Khaled, Y.S. Determination of occupational effective dose in angiocardiography in Jordan. Radiat. Prot. Manag. 1999, 16, 41–47. [Google Scholar]
- Linet, M.S.; Kim, K.P.; Miller, D.L.; Kleinerman, R.A.; Simon, S.L.; Berrington de Gonzalez, A. Historical review of occupational exposures and cancer risks in medical radiation workers. Radiat. Res. 2010, 174, 793–808. [Google Scholar] [CrossRef]
- Linet, M.S.; Freedman, D.M.; Mohan, A.K.; Doody, M.M.; Ron, E.; Mabuchi, K.; Alexander, B.H.; Sigurdson, A.; Hauptmann, M. Incidence of haematopoietic malignancies in US radiologic technologists. Occup. Environ. Med. 2005, 62, 861–867. [Google Scholar] [CrossRef]
- Riley, P.A. Free radicals in biology: Oxidative stress and the effects of ionizing radiation. Int. J. Radiat. Biol. 1994, 65, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Zaider, M.; Bardash, M.; Fung, A. Molecular damage induced directly and indirectly by ionizing radiation in DNA. Int. J. Radiat. Biol. 1994, 66, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.Q.; Aw, T.Y.; Jones, D.P. Glutathione-dependent protection against oxidative injury. Pharmacol. Ther. 1990, 47, 61–71. [Google Scholar] [CrossRef]
- Weiss, J.F.; Landauer, M.R. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology 2003, 189, 1–20. [Google Scholar] [CrossRef]
- Moss, R.B.; Moll, T.; El-Kalay, M.; Kohne, C.; Soo Hoo, W.; Encinas, J.; Carlo, D.J. Th1/Th2 cells in inflammatory disease states: Therapeutic implications. Expert Opin. Biol. Ther. 2004, 4, 1887–1896. [Google Scholar] [CrossRef]
- Wall, B.F.; Kendall, G.M.; Edwards, A.A.; Bouffler, S.; Muirhead, C.R.; Meara, J.R. What are the risks from medical X-rays and other low dose radiation? Br. J. Radiol. 2006, 79, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, F.; Hirobe, T.; Akbari Noghabi, K. Biological effects of low-dose ionizing radiation exposure on interventional cardiologists. Occup. Med. 2010, 60, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Z.; Jin, S.Z.; Liu, X.D.; Sun, Y.M. Role of CD28/B7 costimulation and IL-12/IL-10 interaction in the radiation-induced immune changes. BMC Immunol. 2001, 2, 8. [Google Scholar] [CrossRef]
- Ibuki, Y.; Goto, R. Contribution of inflammatory cytokine release to activation of resident peritoneal macrophages after in vivo low-dose gamma-irradiation. J. Radiat. Res. 1999, 40, 253–262. [Google Scholar] [CrossRef]
- Shieh, M.C.; Su, Y.C.; Wu, M.F. The Study on Biological Effect of Low Dose Radiation in Taiwan. In Proceedings of the 4th International Nuclear Energy Symposium on Energy Future in the Asia/Pacific Region, Taipei, Taiwan, 15–16 March 1999. [Google Scholar]
- Sajous, L.; Botta, A.; Sari-Minodier, I. Urinary 8-hydroxy-2′-deoxyguanosine: A biomarker of environmental oxidative stress. Ann. Biol. Clin. 2008, 66, 19–29. [Google Scholar] [CrossRef]
- El-Benhawy, S.A.; Sadek, N.A.; Behery, A.K.; Issa, N.M.; Ali, O.K. Chromosomal aberrations and oxidative DNA adduct 8-hydroxy-2-deoxyguanosine as biomarkers of radiotoxicity in radiation workers. J. Radiat. Res. Appl. Sci. 2016, 9, 249–258. [Google Scholar] [CrossRef]
- Chatzinikolaou, G.; Karakasilioti, I.; Garinis, G.A. DNA damage and innate immunity: Links and trade-offs. Trends Immunol. 2014, 35, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y. DNA damage: A trigger of innate immunity but a requirement for adaptive immune homeostasis. Nat. Rev. Immunol. 2006, 6, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Rodier, F.; Coppe, J.P.; Patil, C.K.; Hoeijmakers, W.A.; Munoz, D.P.; Raza, S.R.; Freund, A.; Campeau, E.; Davalos, A.R.; Campisi, J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat. Cell Biol. 2009, 11, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Limon-Pacheco, J.; Gonsebatt, M.E. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutat. Res. 2009, 674, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Hernanz, A.; Fernandez-Vivancos, E.; Montiel, C.; Vazquez, J.J.; Arnalich, F. Changes in the intracellular homocysteine and glutathione content associated with aging. Life Sci. 2000, 67, 1317–1324. [Google Scholar] [CrossRef]
- Herzenberg, L.A.; De Rosa, S.C.; Dubs, J.G.; Roederer, M.; Anderson, M.T.; Ela, S.W.; Deresinski, S.C.; Herzenberg, L.A. Glutathione deficiency is associated with impaired survival in HIV disease. Proc. Natl. Acad. Sci. USA 1997, 94, 1967–1972. [Google Scholar] [CrossRef] [PubMed]
- Asensi, M.; Sastre, J.; Pallardo, F.V.; Lloret, A.; Lehner, M.; Garcia-de-la Asuncion, J.; Vina, J. Ratio of reduced to oxidized glutathione as indicator of oxidative stress status and DNA damage. Methods Enzymol. 1999, 299, 267–276. [Google Scholar]
- Kleinman, W.A.; Richie, J.P., Jr. Status of glutathione and other thiols and disulfides in human plasma. Biochem. Pharmacol. 2000, 60, 19–29. [Google Scholar] [CrossRef]
- Lang, C.A.; Mills, B.J.; Mastropaolo, W.; Liu, M.C. Blood glutathione decreases in chronic diseases. J. Lab. Clin. Med. 2000, 135, 402–405. [Google Scholar] [CrossRef]
- Owen, J.B.; Butterfield, D.A. Measurement of oxidized/reduced glutathione ratio. Methods Mol. Biol. 2010, 648, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Durovic, B.; Spasic-Jokic, V.; Durovic, B. Influence of occupational exposure to low-dose ionizing radiation on the plasma activity of superoxide dismutase and glutathione level. Vojnosanit. Pregl. 2008, 65, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumari, S.; Salian, S.R.; Uppangala, S.; Kalthur, G.; Challapalli, S.; Chandraguthi, S.G.; Kumar, P.; Adiga, S.K. Genetic Instability in Lymphocytes is Associated With Blood Plasma Antioxidant Levels in Health Care Workers Occupationally Exposed to Ionizing Radiation. Int. J. Toxicol. 2016, 35, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.L.; Tedesco, I.; Russo, M.; Cioppa, A.; Andreassi, M.G.; Picano, E. Cellular adaptive response to chronic radiation exposure in interventional cardiologists. Eur. Heart J. 2012, 33, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of oxidative damage in human disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Fratini, E.; Carbone, C.; Capece, D.; Esposito, G.; Simone, G.; Tabocchini, M.A.; Tomasi, M.; Belli, M.; Satta, L. Low-radiation environment affects the development of protection mechanisms in V79 cells. Radiat. Environ. Biophys. 2015, 54, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.; Ericsson, M.; Lindgren, M.; Gustafsson, H. A high precision method for quantitative measurements of reactive oxygen species in frozen biopsies. PLoS ONE 2014, 9, e90964. [Google Scholar] [CrossRef]
- Dikalov, S.I.; Kirilyuk, I.A.; Voinov, M.; Grigor’ev, I.A. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic. Res. 2011, 45, 417–430. [Google Scholar] [CrossRef]

| Characteristics | Control (n = 40) | All radiation workers (n = 20) | p-Value |
|---|---|---|---|
| Age (Mean ± standard error of the mean (SEM)) | 41.1 ± 1.8 | 39.4 ± 2.2 | 0.57 |
| Gender | 1 | ||
| Male (%) | 5 (12.5) | 3 (15) | |
| Female (%) | 35 (87.5) | 17 (85) | |
| Alcohol intake (%) | 0.4 | ||
| Yes | 28 (70) | 17 (85) | |
| No | 12 (30) | 3 (15) | |
| Smoking | |||
| Yes | 0 | 0 | |
| No | 40 (100%) | 20 (100%) | |
| Dietary Supplements (%) | 0.2 | ||
| Yes | 18 (45) | 16 (80) | |
| No | 22 (55) | 4 (20) | |
| Mean dose (millisieverts (mSv)/year)—(SEM) | 0 | 2.03 (0.4) | |
| Duration of radiation exposure, years (Mean ± SEM) | NA | 16 ± 2 | |
| GLTEQ total-mean (SEM) | 35.6 (3.3) | 38 (6.4) | 0.7 |
| GLTEQ sweat/heart beat | 0.15 | ||
| (i) Never | 10 | 1 | |
| (ii) Sometimes | 20 | 14 | |
| (iii) Often | 10 | 5 |
| Cytokines | Control | All Radiation Workers | p-Value |
|---|---|---|---|
| IFN-γ | 10.2 ± 1.84 | 6.56 ± 0.84 | 0.24 |
| IL-10 | 0.51 ± 0.18 | 0.38 ± 0.07 | 0.67 |
| IL-12p70 | 0.15 ± 0.05 | 0.12 ± 0.03 | 0.64 |
| IL-13 | 0.09 ± 0.05 | 0.1 ± 0.04 | 0.61 |
| IL-1β | 0.14 ± 0.13 | 0.01 ± 0.01 | 0.94 |
| IL-2 | 0.12 ± 0.02 | 0.30 ± 0.21 | 0.91 |
| IL-4 | 0.12 ± 0.08 | 0.01 ± 0.00 | 0.44 |
| IL-6 | 0.44 ± 0.08 | 0.60 ± 0.08 * | 0.04 |
| IL-8 | 5.28 ± 0.49 | 4.85 ± 0.54 | 0.62 |
| TNF-α | 2.45 ± 0.31 | 2.16 ± 0.16 | 0.64 |
| Eotaxin | 742 ± 60.1 | 711 ± 49.1 | 0.70 |
| Eotaxin-3 | 105 ± 7.45 | 110 ± 9.88 | 0.74 |
| IL-8 (HA) | 27.4 ± 15.6 | 13.7 ± 4.78 | 0.96 |
| IP-10 | 478 ± 210 | 273 ± 35.7 | 0.55 |
| MCP-1 | 103 ± 9.51 | 97.8 ± 5.06 | 0.76 |
| MDC | 613 ± 33.4 | 599 ± 58.8 | 0.43 |
| MIP-1α | 12.0 ± 1.67 | 16.2 ± 1.44 * | 0.01 |
| MIP-1β | 46.4 ± 5.71 | 35.5 ± 2.62 | 0.37 |
| TARC | 49.4 ± 6.28 | 46.8 ± 4.45 | 0.39 |
| GM-CSF | 0.2 ± 0.15 | 0.08 ± 0.02 | 0.41 |
| IL-12p40 | 113 ± 10.14 | 116 ± 14.7 | 0.71 |
| IL-15 | 2.17 ± 0.09 | 2.40 ± 0.27 | 0.74 |
| IL-16 | 298 ± 30.5 | 284 ± 26.2 | 0.91 |
| IL-17A | 2.2 ± 0.37 | 1.51 ± 0.18 | 0.05 |
| IL-1α | 0.24 ± 0.07 | 0.36 ± 0.10 * | 0.03 |
| IL-5 | 0.32 ± 0.09 | 0.41 ± 0.17 | 0.42 |
| IL-7 | 4.29 ± 0.29 | 4.11 ± 0.28 | 0.93 |
| TNF-β | 0.30 ± 0.02 | 0.37± 0.03 | 0.06 |
| VEGF-A | 44.7 ± 3.75 | 36.5 ± 3.09 | 0.28 |
| Cytokines | Unexposed Workers | Radiation Workers | |||
|---|---|---|---|---|---|
| Control (n = 40) | All (n = 20) | Conventional Radiography (CR) | Interventional Radiography (IR) | Computed Tomography (CT) | |
| IL-6 | |||||
| Range | 0.05–2.3 | 0.17–1.70 | 0.17–1.70 | 0.42–1.42 | 0.3–1.04 |
| Mean ± SEM | 0.44 ± 0.08 | 0.60 ± 0.08 a | 0.50 ± 0.12 | 0.83 ± 0.21 b | 0.67 ± 0.15 |
| MIP-1α | |||||
| Range | 0–46.70 | 8.86–31.19 | 8.86–23.62 | 16.9–31.19 | 12.25–19.99 |
| Mean ± SEM | 12.0 ± 1.67 | 16.2 ± 1.44 a | 13.82 ± 1.56 | 23.84 ± 3.38 b | 15.80 ± 1.62 |
| IL-1α | |||||
| Range | 0–1.77 | 0.03–1.79 | 0.03–1.79 | 0.03–0.48 | 0.07–0.63 |
| Mean ± SEM | 0.24 ± 0.07 | 0.36 ± 0.10 a | 0.40 ± 0.16 | 0.26 ± 0.10 | 0.36 ± 0.14 |
| Plasma 8-OHdG | Unexposed Workers | Radiation Workers | |||
|---|---|---|---|---|---|
| Control (n = 40) | All (n = 20) | Conventional Radiography (CR) | Interventional Radiography (IR) | Computed Tomography (CT) | |
| 8-OHdG concentration | |||||
| Range | 0.92–8.11 | 2.22–7.63 | 2.22–7.63 | 2.86–5.08 | 2.89–5.04 |
| Mean ± SEM | 4.51 ± 0.40 | 3.97 ± 0.27 | 3.92 ± 0.39 | 4.16 ± 0.49 | 3.93 ± 0.57 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, I.M.; Abdalla, M.Y.; Moore, T.A.; Bartenhagen, L.; Case, A.J.; Zimmerman, M.C. Healthcare Workers Occupationally Exposed to Ionizing Radiation Exhibit Altered Levels of Inflammatory Cytokines and Redox Parameters. Antioxidants 2019, 8, 12. https://doi.org/10.3390/antiox8010012
Ahmad IM, Abdalla MY, Moore TA, Bartenhagen L, Case AJ, Zimmerman MC. Healthcare Workers Occupationally Exposed to Ionizing Radiation Exhibit Altered Levels of Inflammatory Cytokines and Redox Parameters. Antioxidants. 2019; 8(1):12. https://doi.org/10.3390/antiox8010012
Chicago/Turabian StyleAhmad, Iman M., Maher Y. Abdalla, Tiffany A. Moore, Lisa Bartenhagen, Adam J. Case, and Matthew C. Zimmerman. 2019. "Healthcare Workers Occupationally Exposed to Ionizing Radiation Exhibit Altered Levels of Inflammatory Cytokines and Redox Parameters" Antioxidants 8, no. 1: 12. https://doi.org/10.3390/antiox8010012
APA StyleAhmad, I. M., Abdalla, M. Y., Moore, T. A., Bartenhagen, L., Case, A. J., & Zimmerman, M. C. (2019). Healthcare Workers Occupationally Exposed to Ionizing Radiation Exhibit Altered Levels of Inflammatory Cytokines and Redox Parameters. Antioxidants, 8(1), 12. https://doi.org/10.3390/antiox8010012






