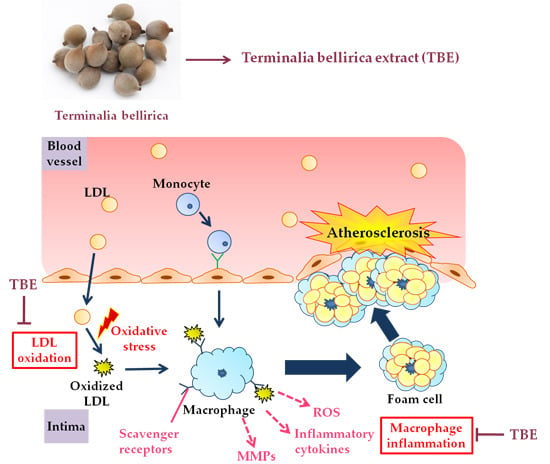
Terminalia bellirica Extract Inhibits Low-Density Lipoprotein Oxidation and Macrophage Inflammatory Response in Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Determination of Total Polyphenol Content
2.3. 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Radical Scavenging Activity
2.4. Isolation of LDL from Human Subjects
2.5. LDL Lag Time Assay
2.6. 15-Lipoxygenase Inhibitory Activity
2.7. Cell Culture and Treatment
2.8. Real-Time RT-PCR
2.9. Detection of MMP-9 Activity by Gelatin Zymography
2.10. Measurement of Intracellular ROS Production
2.11. Statistical Analysis
3. Results
3.1. The Total Polyphenol Content of TBE
3.2. The DPPH Radical Scavenging Activity of TBE
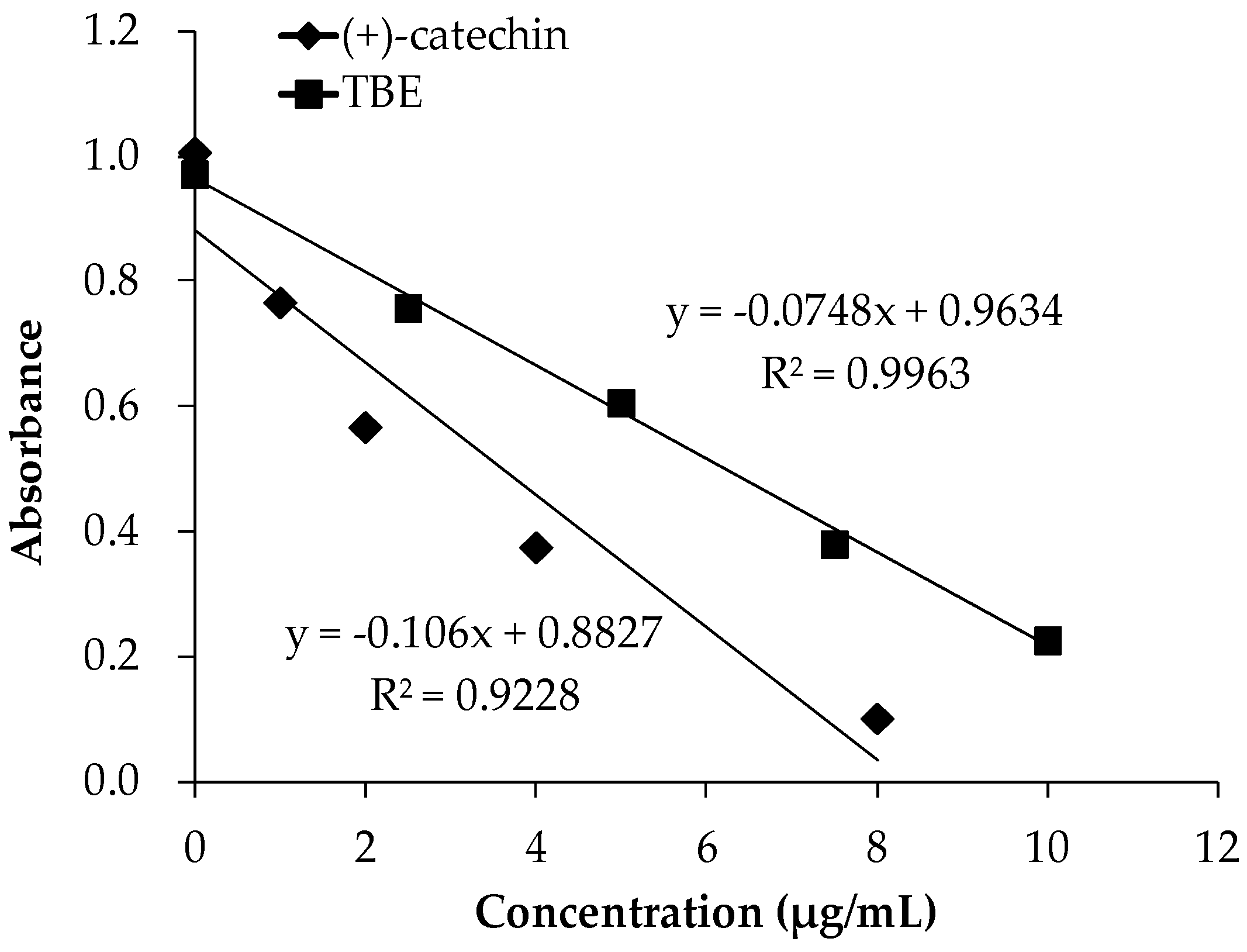
3.3. TBE Slowed LDL Oxidation In Vitro
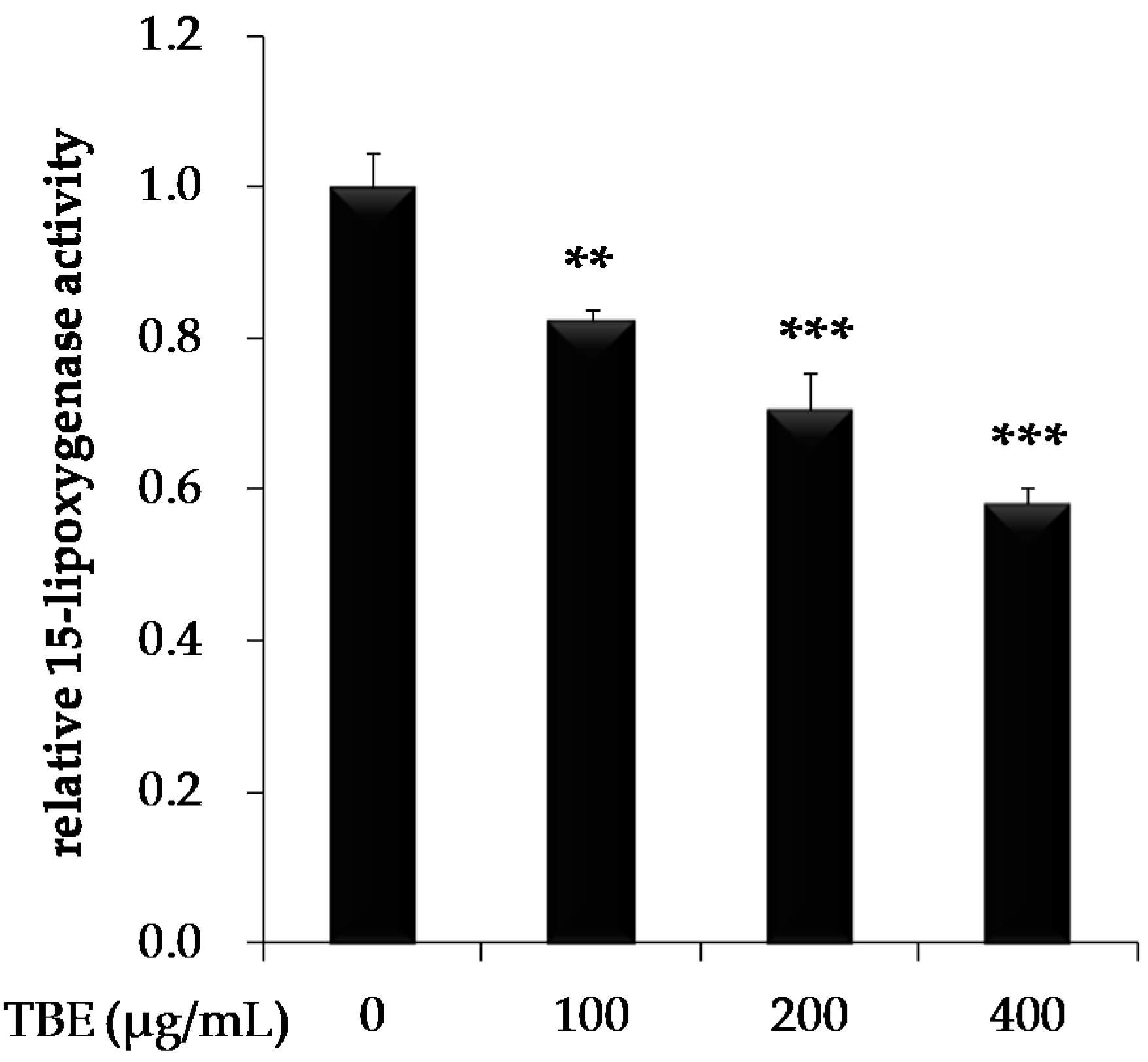
3.4. TBE Inhibited 15-Lipoxygenase (15-LOX) Activity
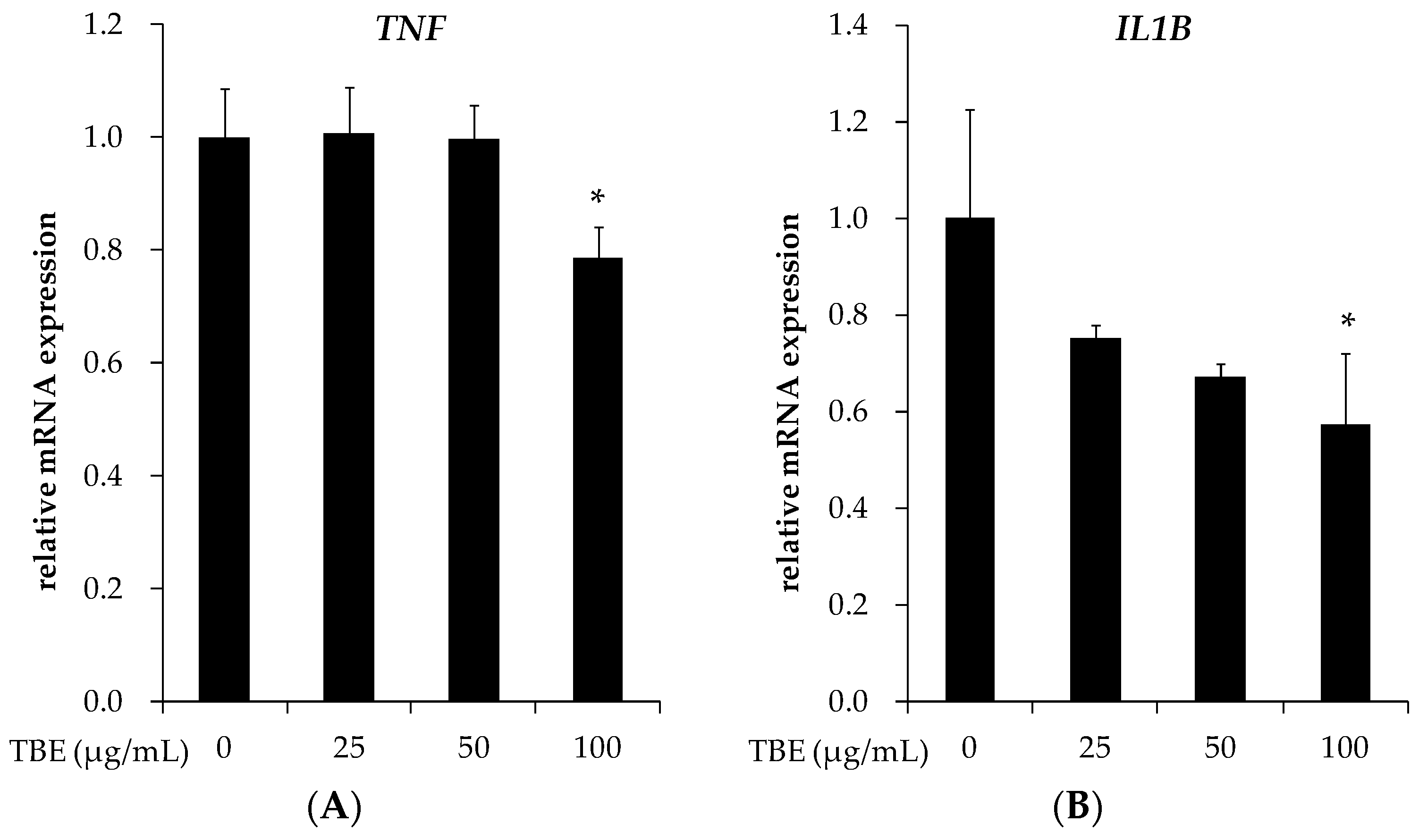
3.5. TBE Reduced the mRNA Expression of TNF-α and IL-1β in THP-1 Macrophages
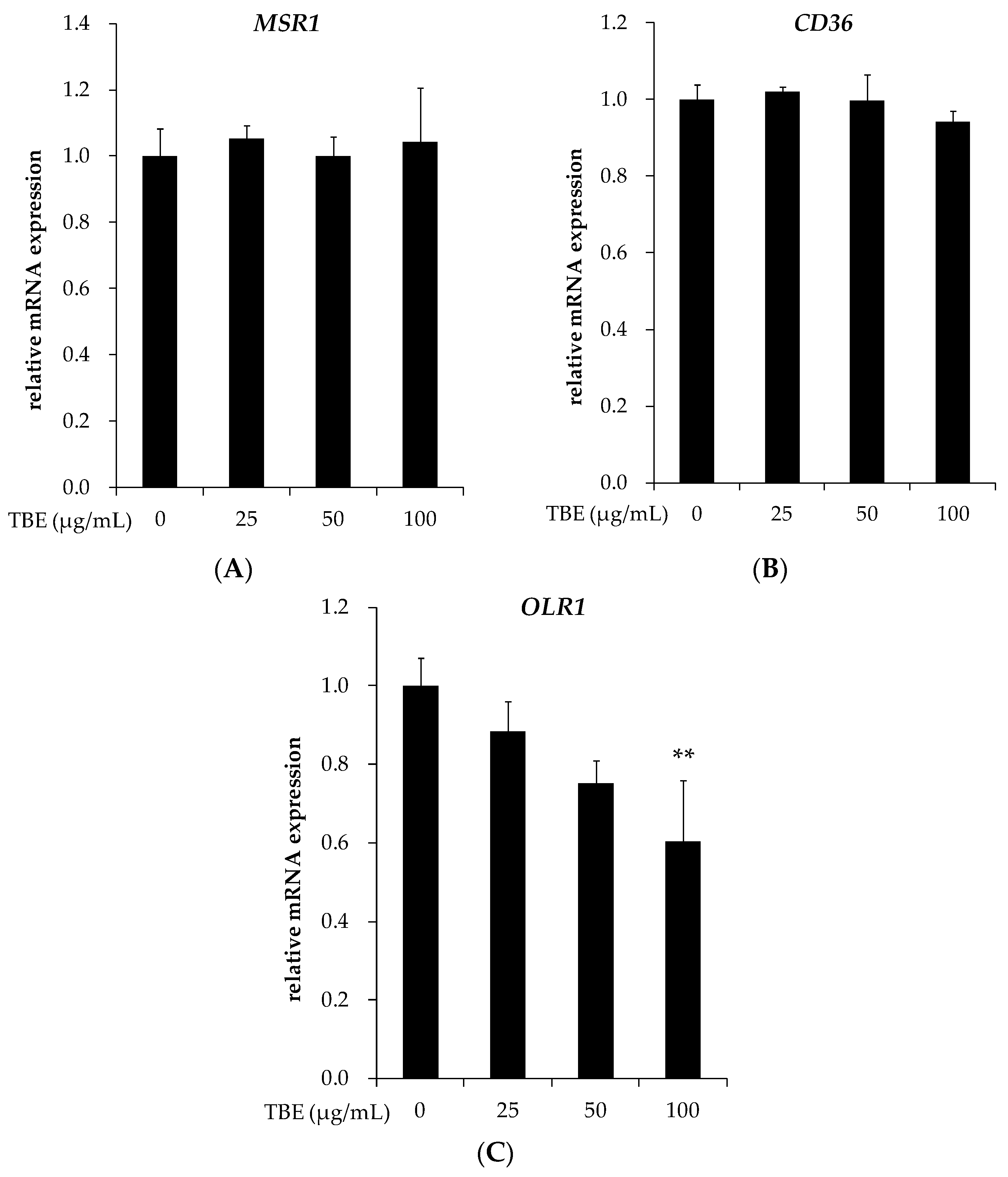
3.6. TBE Suppressed the mRNA Expression of LOX-1 in THP-1 Macrophages
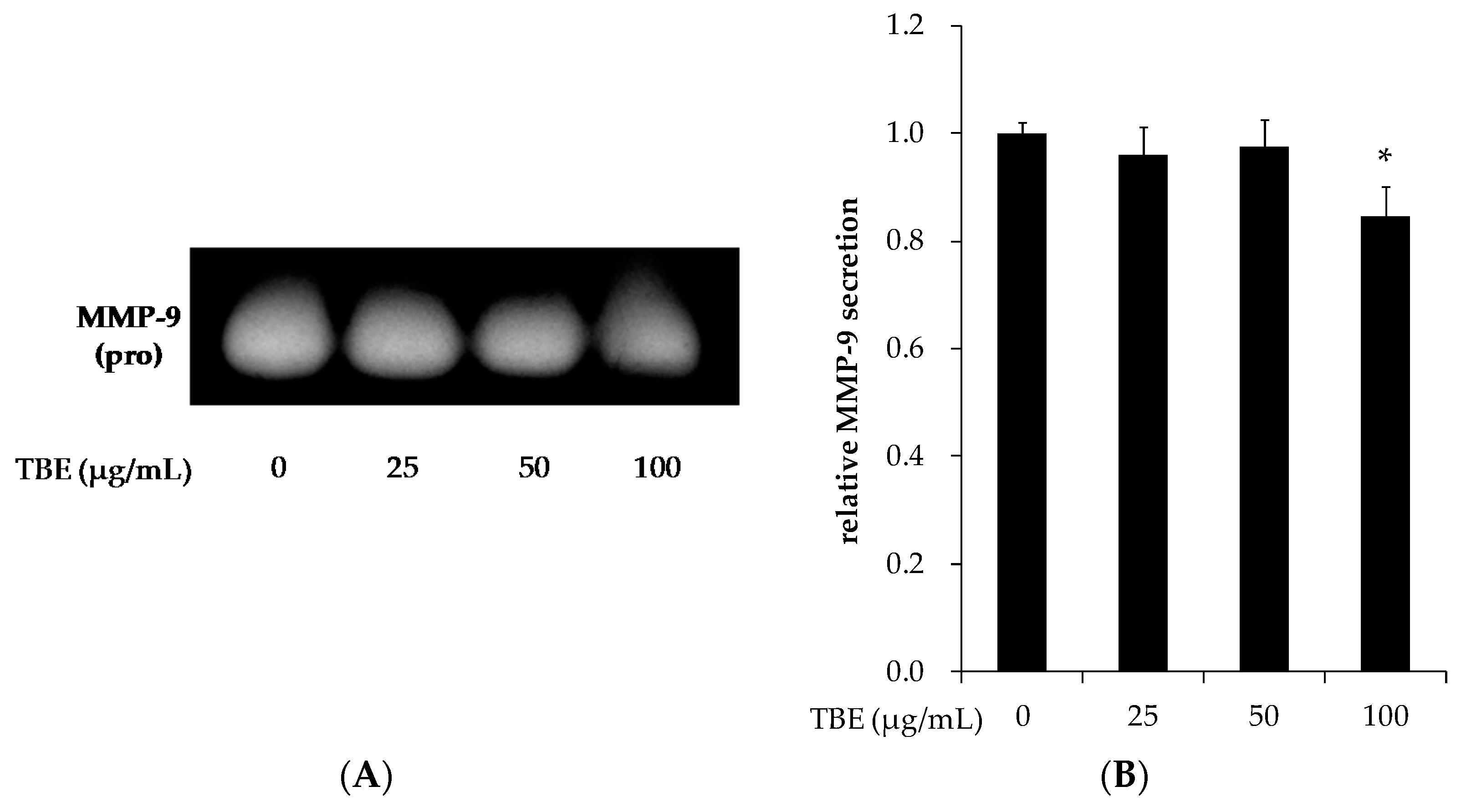
3.7. TBE Reduced the MMP-9 Secretion in THP-1 Macrophages
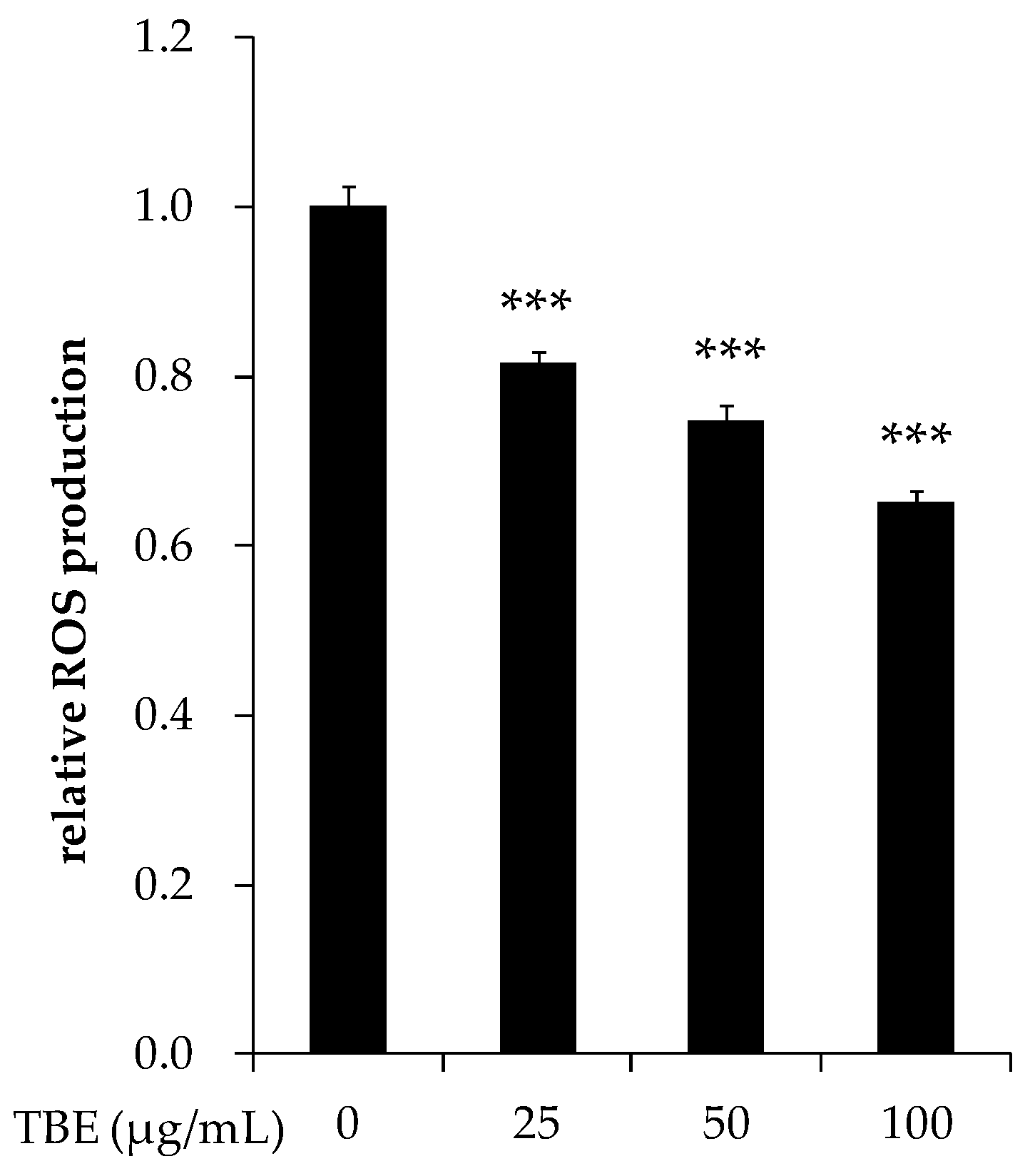
3.8. TBE Decreased the ROS Production in THP-1 Macrophages
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Modak, M.; Dixit, P.; Londhe, J.; Ghaskadbi, S.; Devasagayam, T.P. Indian herbs and herbal drugs used for the treatment of diabetes. J. Clin. Biochem. Nutr. 2007, 40, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Shaila, H.P.; Udupa, S.L.; Udupa, A.L. Hypolipidemic activity of three indigenous drugs in experimentally induced atherosclerosis. Int. J. Cardiol. 1998, 67, 119–124. [Google Scholar] [CrossRef]
- Makihara, H.; Shimada, T.; Machida, E.; Oota, M.; Nagamine, R.; Tsubata, M.; Kinoshita, K.; Takahashi, K.; Aburada, M. Preventive effect of Terminalia bellirica on obesity and metabolic disorders in spontaneously obese type 2 diabetic model mice. J. Nat. Med. 2012, 66, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Gilani, A. Pharmacodynamic evaluation of Terminalia bellerica for its antihypertensive effect. J. Food Drug Anal. 2008, 16, 6–14. [Google Scholar]
- Kaplan, M.; Aviram, M. Oxidized low density lipoprotein: Atherogenic and proinflammatory characteristics during macrophage foam cell formation. An inhibitory role for nutritional antioxidants and serum paraoxonase. Clin. Chem. Lab. Med. 1999, 37, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Bobryshev, Y.V.; Orekhov, A.N. Macrophage-mediated cholesterol handling in atherosclerosis. J. Cell. Mol. Med. 2016, 20, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Burchardt, P.; Zurawski, J.; Zuchowski, B.; Kubacki, T.; Murawa, D.; Wiktorowicz, K.; Wysocki, H. Low-density lipoprotein, its susceptibility to oxidation and the role of lipoprotein-associated phospholipase A2 and carboxyl ester lipase lipases in atherosclerotic plaque formation. Arch. Med. Sci. AMS 2013, 9, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation in atherosclerosis. Nature 2002, 420, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Galis, Z.S.; Sukhova, G.K.; Lark, M.W.; Libby, P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J. Clin. Investig. 1994, 94, 2493–2503. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000, 279, L1005–L1028. [Google Scholar] [PubMed]
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Eriksson, P.; Hansson, G.K.; Herzfeld, I.; Klein, M.; Hansson, L.O.; Valen, G. Expression of matrix metalloproteinase 9 and its regulators in the unstable coronary atherosclerotic plaque. Int. J. Mol. Med. 2005, 15, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Ikeda, U. Matrix metalloproteinases and atherosclerosis. Curr. Atheroscler. Rep. 2004, 6, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N.; Kanner, J.; German, J.B.; Parks, E.; Kinsella, J.E. Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet 1993, 341, 454–457. [Google Scholar] [CrossRef]
- Miura, S.; Watanabe, J.; Sano, M.; Tomita, T.; Osawa, T.; Hara, Y.; Tomita, I. Effects of various natural antioxidants on the Cu2+-mediated oxidative modification of low density lipoprotein. Biol. Pharm. Bull. 1995, 18, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The zutphen elderly study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- Knekt, P.; Jarvinen, R.; Reunanen, A.; Maatela, J. Flavonoid intake and coronary mortality in finland: A cohort study. BMJ 1996, 312, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Neil, H.A. The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2003, 57, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using folin-ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Hirano, R.; Kondo, K.; Iwamoto, T.; Igarashi, O.; Itakura, H. Effects of antioxidants on the oxidative susceptibility of low-density lipoprotein. J. Nutr. Sci. Vitaminol. 1997, 43, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Tani, M.; Uto-Kondo, H.; Iizuka, M.; Saita, E.; Sone, H.; Kurata, H.; Kondo, K. Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur. J. Nutr. 2010, 49, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Kume, N.; Cybulsky, M.I.; Gimbrone, M.A., Jr. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J. Clin. Investig. 1992, 90, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Kume, N.; Gimbrone, M.A., Jr. Lysophosphatidylcholine transcriptionally induces growth factor gene expression in cultured human endothelial cells. J. Clin. Investig. 1994, 93, 907–911. [Google Scholar] [CrossRef] [PubMed]
- Rajavashisth, T.B.; Liao, J.K.; Galis, Z.S.; Tripathi, S.; Laufs, U.; Tripathi, J.; Chai, N.N.; Xu, X.P.; Jovinge, S.; Shah, P.K.; et al. Inflammatory cytokines and oxidized low density lipoproteins increase endothelial cell expression of membrane type 1-matrix metalloproteinase. J. Biol. Chem. 1999, 274, 11924–11929. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Kisugi, R. Mechanisms of LDL oxidation. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Middha, S.K.; Goyal, A.K.; Lokesh, P.; Yardi, V.; Mojamdar, L.; Keni, D.S.; Babu, D.; Usha, T. Toxicological evaluation of emblica officinalis fruit extract and its anti-inflammatory and free radical scavenging properties. Pharmacogn. Mag. 2015, 11, S427–S433. [Google Scholar] [PubMed]
- Eshwarappa, R.S.; Ramachandra, Y.L.; Subaramaihha, S.R.; Subbaiah, S.G.; Austin, R.S.; Dhananjaya, B.L. Anti-lipoxygenase activity of leaf gall extracts of Terminalia chebula (gaertn.) retz. (combretaceae). Pharmacogn. Res. 2016, 8, 78–82. [Google Scholar] [CrossRef]
- Ngoc, T.M.; Hung, T.M.; Thuong, P.T.; Kim, J.C.; Choi, J.S.; Bae, K.; Hattori, M.; Choi, C.S.; Lee, J.S.; Min, B.S. Antioxidative activities of galloyl glucopyranosides from the stem-bark of juglans mandshurica. Biosci. Biotechnol. Biochem. 2008, 72, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Ait-Oufella, H.; Taleb, S.; Mallat, Z.; Tedgui, A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Ohta, H.; Wada, H.; Niwa, T.; Kirii, H.; Iwamoto, N.; Fujii, H.; Saito, K.; Sekikawa, K.; Seishima, M. Disruption of tumor necrosis factor-alpha gene diminishes the development of atherosclerosis in ApoE-deficient mice. Atherosclerosis 2005, 180, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Merhi-Soussi, F.; Kwak, B.R.; Magne, D.; Chadjichristos, C.; Berti, M.; Pelli, G.; James, R.W.; Mach, F.; Gabay, C. Interleukin-1 plays a major role in vascular inflammation and atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc. Res. 2005, 66, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Norata, G.D.; Catapano, A.L. LOX-1, OxLDL, and atherosclerosis. Mediat. Inflamm. 2013. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Arai, Y.; Kurihara, H.; Kita, T.; Sawamura, T. Overexpression of lectin-like oxidized low-density lipoprotein receptor-1 induces intramyocardial vasculopathy in apolipoprotein E-null mice. Circ. Res. 2005, 97, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.L.; Sanada, N.; Hu, C.P.; Chen, J.; Dandapat, A.; Sugawara, F.; Satoh, H.; Inoue, K.; Kawase, Y.; Jishage, K.; et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ. Res. 2007, 100, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Honjo, M.; Nakamura, K.; Yamashiro, K.; Kiryu, J.; Tanihara, H.; McEvoy, L.M.; Honda, Y.; Butcher, E.C.; Masaki, T.; Sawamura, T. Lectin-like oxidized LDL receptor-1 is a cell-adhesion molecule involved in endotoxin-induced inflammation. Proc. Natl. Acad. Sci. USA 2003, 100, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ogura, S.; Chen, J.; Little, P.J.; Moss, J.; Liu, P. LOX-1 in atherosclerosis: Biological functions and pharmacological modifiers. Cell. Mol. Life Sci. CMLS 2013, 70, 2859–2872. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Uboldi, P.; Ferri, N.; Corsini, A.; Kuhn, H.; Catapano, A.L. Upregulation of lectin-like oxidized low density lipoprotein receptor 1 (LOX-1) expression in human endothelial cells by modified high density lipoproteins. Biochem. Biophys. Res. Commun. 2012, 428, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Radhika, A.; Jacob, S.S.; Sudhakaran, P.R. Influence of oxidatively modified LDL on monocyte-macrophage differentiation. Mol. Cell. Biochem. 2007, 305, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Nissinen, L.; Kahari, V.M. Matrix metalloproteinases in inflammation. Biochim. Biophys. Acta 2014, 1840, 2571–2580. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Wen, M.H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 2002, 277, 22131–22139. [Google Scholar] [CrossRef] [PubMed]
- Pendyala, S.; Usatyuk, P.V.; Gorshkova, I.A.; Garcia, J.G.; Natarajan, V. Regulation of NADPH oxidase in vascular endothelium: The role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid. Redox Signal. 2009, 11, 841–860. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Yu, Y.; Haigh, S.; Johnson, J.; Lucas, R.; Stepp, D.W.; Fulton, D.J. Regulation of NADPH oxidase 5 by protein kinase c isoforms. PLoS ONE 2014, 9, e88405. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Yang, C.M. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem. Pharmacol. 2012, 84, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Luo, S.F.; Lee, C.W.; Wang, S.W.; Lin, C.C.; Chang, C.C.; Chen, Y.L.; Chau, L.Y.; Yang, C.M. Overexpression of HO-1 protects against TNF-alpha-mediated airway inflammation by down-regulation of TNFR1-dependent oxidative stress. Am. J. Pathol. 2009, 175, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Wei, H.; Frei, B. Genetic deficiency of NADPH oxidase does not diminish, but rather enhances, LPS-induced acute inflammatory responses in vivo. Free Radic. Biol. Med. 2009, 46, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Barry-Lane, P.A.; Patterson, C.; van der Merwe, M.; Hu, Z.; Holland, S.M.; Yeh, E.T.; Runge, M.S. P47phox is required for atherosclerotic lesion progression in ApoE−/− mice. J. Clin. Investig. 2001, 108, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Huang, P.J.; Liu, Y.R.; Kumar, K.J.; Hsu, L.S.; Lu, T.L.; Chia, Y.C.; Takajo, T.; Kazunori, A.; Hseu, Y.C. Toona sinensis inhibits LPS-induced inflammation and migration in vascular smooth muscle cells via suppression of reactive oxygen species and NF-κB signaling pathway. Oxid. Med. Cell. Longev. 2014. [Google Scholar] [CrossRef] [PubMed]

| Gene | Sequence (5′ to 3′) |
|---|---|
| TNF | Forward: TGGAGAAGGGTGACCGACTC |
| Reverse: TCCTCACAGGGCAATGATCC | |
| IL1B | Forward: CTGTACGATCACTGAACTGC |
| Reverse: CACCACTTGTTGCTCCATACT | |
| MSR1 | Forward: AGGCCCTCTTAAGATCAGG |
| Reverse: ACAACACGGGAACCAAAGTC | |
| CD36 | Forward: CAATTAAAAAGCAAGTTGTCCTCGA |
| Reverse: ATCACTTCCTGTGGATTTTGCA | |
| OLR1 | Forward: ACAGATCTCAGCCCGGCAACAAGCA |
| Reverse: GGGAGACAGCGCCTCGGACTCTAAAT | |
| GAPDH | Forward: TGCACCACCAACTGCTTAGC |
| Reverse: GGCATGGACTGTGGTCATGAG |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, M.; Kishimoto, Y.; Saita, E.; Suzuki-Sugihara, N.; Kamiya, T.; Taguchi, C.; Iida, K.; Kondo, K. Terminalia bellirica Extract Inhibits Low-Density Lipoprotein Oxidation and Macrophage Inflammatory Response in Vitro. Antioxidants 2016, 5, 20. https://doi.org/10.3390/antiox5020020
Tanaka M, Kishimoto Y, Saita E, Suzuki-Sugihara N, Kamiya T, Taguchi C, Iida K, Kondo K. Terminalia bellirica Extract Inhibits Low-Density Lipoprotein Oxidation and Macrophage Inflammatory Response in Vitro. Antioxidants. 2016; 5(2):20. https://doi.org/10.3390/antiox5020020
Chicago/Turabian StyleTanaka, Miori, Yoshimi Kishimoto, Emi Saita, Norie Suzuki-Sugihara, Tomoyasu Kamiya, Chie Taguchi, Kaoruko Iida, and Kazuo Kondo. 2016. "Terminalia bellirica Extract Inhibits Low-Density Lipoprotein Oxidation and Macrophage Inflammatory Response in Vitro" Antioxidants 5, no. 2: 20. https://doi.org/10.3390/antiox5020020
APA StyleTanaka, M., Kishimoto, Y., Saita, E., Suzuki-Sugihara, N., Kamiya, T., Taguchi, C., Iida, K., & Kondo, K. (2016). Terminalia bellirica Extract Inhibits Low-Density Lipoprotein Oxidation and Macrophage Inflammatory Response in Vitro. Antioxidants, 5(2), 20. https://doi.org/10.3390/antiox5020020