Effects of Antioxidants in Human Cancers: Differential Effects on Non-Coding Intronic RNA Expression
Abstract
:1. Introduction
2. Materials and Methods
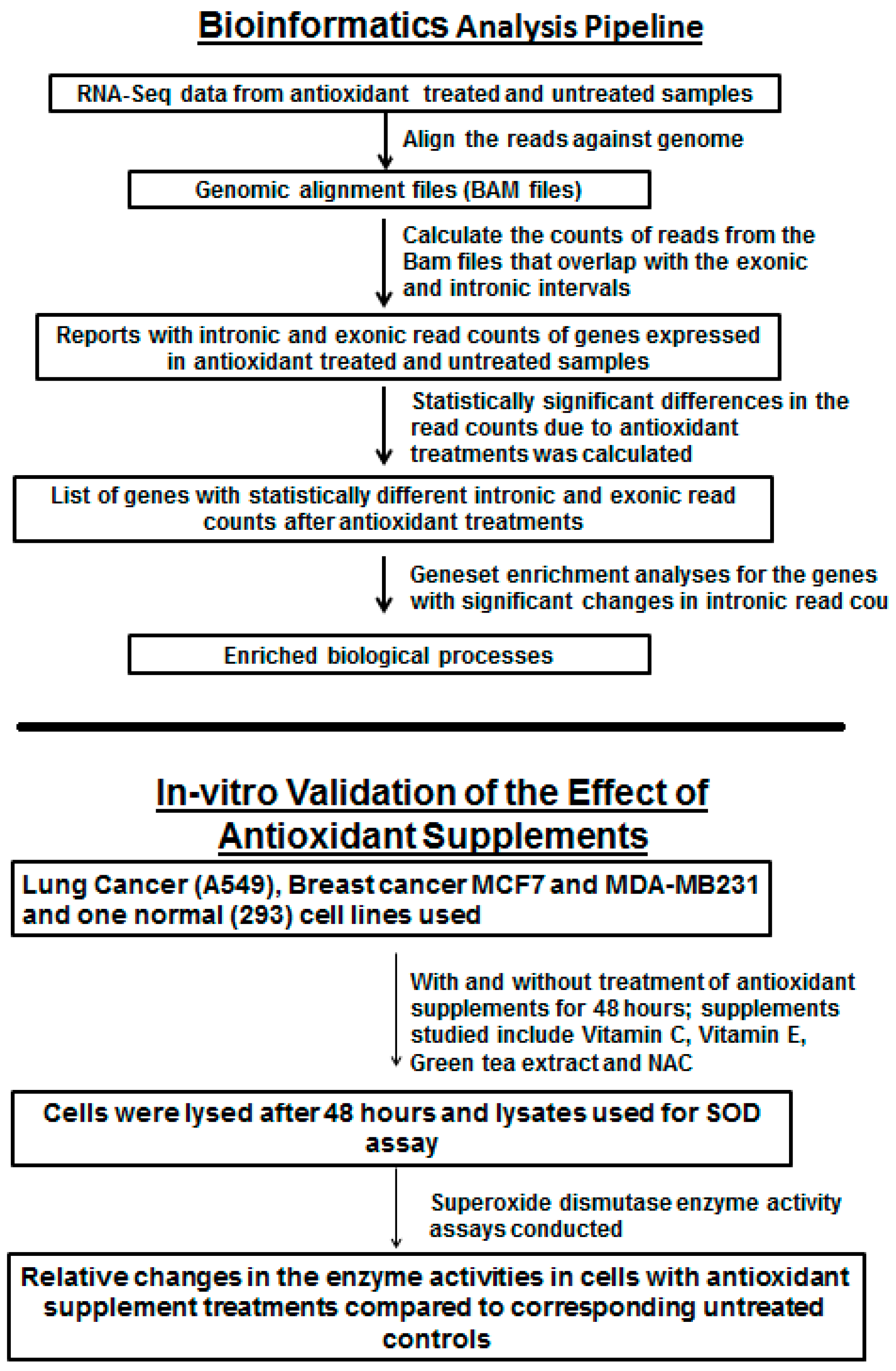
2.1. RNA-Seq Data Analysis
- Murine lung cancer dataset (Accession: E-GEOD-52594): RNA-Seq data were downloaded from ArrayExpress. The objective of the original study was to study the impact of Vitamin E and NAC supplementation in murine models of KRAS-induced lung cancer [27]. Antioxidants were administered 1 week after the induction of lung cancer, and the mice were euthanized 8 to 10 weeks later. There were 3 experimental groups (untreated, NAC-treated and Vitamin E-treated. Each group consisted of 5 animals, and from each animal two tumor samples were harvested for analysis. A total of 30 samples were profiled by RNA-Seq analyses.
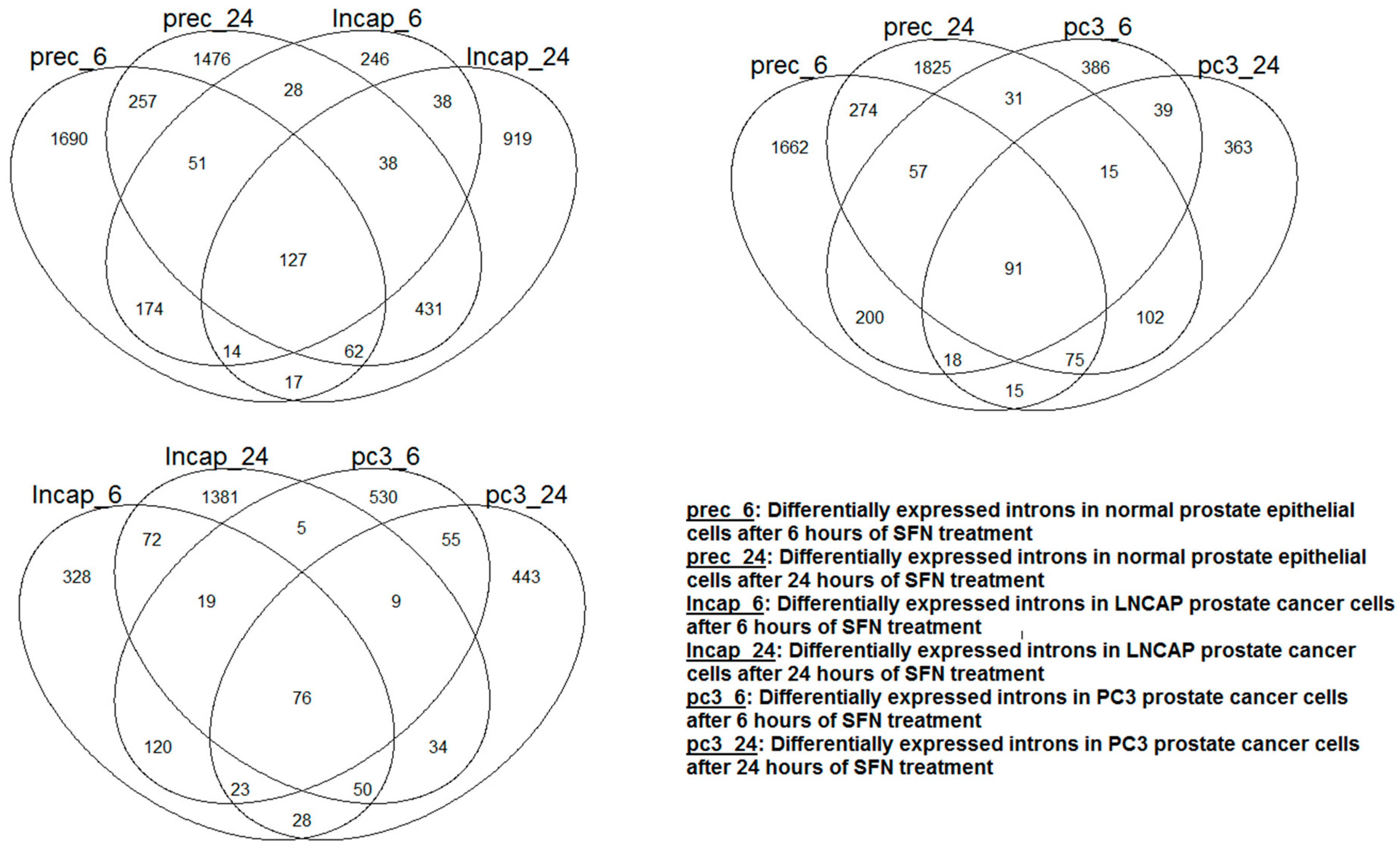
- Human normal and prostate cancer cells dataset (SRP027258): RNA-Seq data were downloaded from NCBI SRA. Normal prostate epithelial cells and androgen-dependent and androgen-independent prostate cancer cells were treated with 15 µM sulforaphane (SFN), a phytochemical derived from cruciferous vegetables, and the transcriptome was determined at 6 and 24 h time points [28].
| Antioxidant Supplements Studied | Organism | NCBI/Arrayexpress Accession | Brief Description of The Original Study |
|---|---|---|---|
| Vitamin E, N-acetyl cysteine (NAC) | Mus musculus (mouse) | E-GEOD-52594 | The original study [27] found that both antioxidants increase tumor cell proliferation by reducing ROS, DNA damage, and p53 expression. |
| Sulforaphane (SFN) | Human prostate normal and cancer cell lines | SRP027258 | SFN influenced the expression of genes in functional groups and pathways that are critical in cancer including cell cycle, apoptosis and angiogenesis, but the specific effects of SFN differed depending on the state of cancer progression [28]. |
2.2. Intronic Count Analysis
2.3. In Vitro Analysis on the Effect of Antioxidant Supplements on Superoxide Dismutase Activity on Established Human Cell Lines
2.4. RNA-Isolation and RT-PCR
3. Results
3.1. Murine Lung Cancer Dataset
| a. Summary of Results from Murine Model for KRAS-Induced Lung Cancer Data Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Number of Genes with Significant Differences in the Intronic Read Counts from Antioxidant Supplement (NAC, Vitamin E) Treated Tumor Tissues Compared to Control Tumor Tissue | ||||||||||
| NAC | Vitamin E | |||||||||
| Down | Up | Down | Up | |||||||
| Number of genes | 459 | 86 | 1143 | 315 | ||||||
| b. Summary of Results from Human Prostate Cancer Cells Data Analysis | ||||||||||
| Total Number of Genes with Significant Differences in the Intronic Read Counts in Sulforaphane Treated Cells Compared to Untreated Cells | ||||||||||
| Normal Prostate Epithelial Cells | LNCAP (Prostate Cancer Cells, Hormone, Dependent) | PC3 (Prostate Cancer Cells, Hormone Independent) | ||||||||
| Down | Up | Down | Up | Down | Up | |||||
| 6 h | 1680 | 1971 | 428 | 575 | 569 | 707 | ||||
| 24 h | 1522 | 1437 | 1313 | 476 | 496 | 457 | ||||
3.2. Functional Annotation of Genes with Differentially Expressed Intronic RNA
| Gene Symbols | ||||
|---|---|---|---|---|
| ALDOC | F7 * | IL18 | MRC1 * | SOCS3 |
| AREG | FCGR2B | ITGAX * | MSR1 | STARD10 |
| AXL | GAPDH | ITGB2 | PTGS1 | TNFSF9 |
| CAMSAP1 | GJA1 | ITIH4 * | ROS1 * | TYROBP |
| CD68 | HDC | KRAS | RPL3 | |
| CRLF1 | HK1 * | LCP1 | SERPINE1 | |
| CTSK | HK2 * | LRG1 * | SH3RF1 | |
| ELL2 | HSPA1B | LRP2 * | SIRPA | |
| EPHA7 | HSPA8 | ME1 | SLAIN1 | |
| F10 | HSPH1 | MMP12 | SLC38A2 | |
| For Genes with Increased Expression of Introns |
|---|
| Fructose and mannose metabolism |
| Ensemble of genes encoding ECM-associated proteins including ECM-affiliated proteins, ECM regulators and secreted factors |
| Ensemble of genes encoding extracellular matrix and extracellular matrix-associated proteins |
| Genes involved in Transmembrane transport of small molecules |
| Type II diabetes mellitus |
| For Genes with Decreased Expression of Introns |
| Drug metabolism—cytochrome P450 |
| Genes involved in Biological oxidations |
| Genes involved in Muscle contraction |
| Metabolism of xenobiotics by cytochrome P450 |
| Glutathione metabolism |
| Enriched Canonical Pathways |
|---|
| For genes with Increased Expression of Introns |
| Ensemble of genes encoding extracellular matrix and extracellular matrix-associated proteins |
| Genes involved in Adaptive Immune System |
| Beta2 integrin cell surface interactions |
| Ensemble of genes encoding ECM-associated proteins including ECM-affiliated proteins, ECM regulators and secreted factors |
| Genes involved in Transmembrane transport of small molecules |
| For Genes with Decreased Expression of Introns |
| Drug metabolism—cytochrome P450 |
| Genes involved in Biological oxidations |
| Metabolism of xenobiotics by cytochrome P450 |
| Ensemble of genes encoding extracellular matrix and extracellular matrix-associated proteins |
| Genes involved in Phase 1—Functionalization of compounds |

3.3. Human Normal and Prostate Cancer Cells Dataset
| Enriched Canonical Pathways |
|---|
| Normal Prostate Epithelial Cells with 6 h of SFN Treatment |
| Ensemble of genes encoding extracellular matrix and extracellular matrix-associated proteins |
| Genes involved in Developmental Biology |
| Genes involved in Transmission across Chemical Synapses |
| Genes involved in Immune System |
| Genes involved in Axon guidance |
| Normal Prostate Epithelial Cells with 24 h of SFN Treatment |
| Genes involved in Transmembrane transport of small molecules |
| Arrhythmogenic right ventricular cardiomyopathy (ARVC) |
| Focal adhesion |
| Genes involved in Immune System |
| Genes involved in Developmental Biology |
| LNCAP Prostate Cancer Cells with 6 h of SFN Treatment |
| Caspase cascade in apoptosis |
| Endocytosis |
| Genes involved in Developmental Biology |
| Peroxisome |
| Genes involved in Signaling by Rho GTPases |
| LNCAP Prostate Cancer cells with 24 h of SFN Treatment |
| Genes involved in Collagen formation |
| Genes involved in Developmental Biology |
| Genes involved in Neuronal System |
| Genes involved in Metabolism of lipids and lipoproteins |
| Genes involved in Extracellular matrix organization |
| PC3 Prostate Cancer Cells with 6 h of SFN Treatment |
| Genes involved in Collagen formation |
| Genes involved in Extracellular matrix organization |
| Regulation of RhoA activity |
| Genes involved in Metabolism of lipids and lipoproteins |
| Ensemble of genes encoding extracellular matrix and extracellular matrix-associated proteins |
| PC3 Prostate Cancer Cells with 24 h of SFN Treatment |
| Genes involved in Axon guidance |
| Genes involved in Immune System |
| Genes involved in NRAGE signals death through JNK |
| Genes involved in Signaling by Rho GTPases |
| Genes involved in Signaling by NGF |
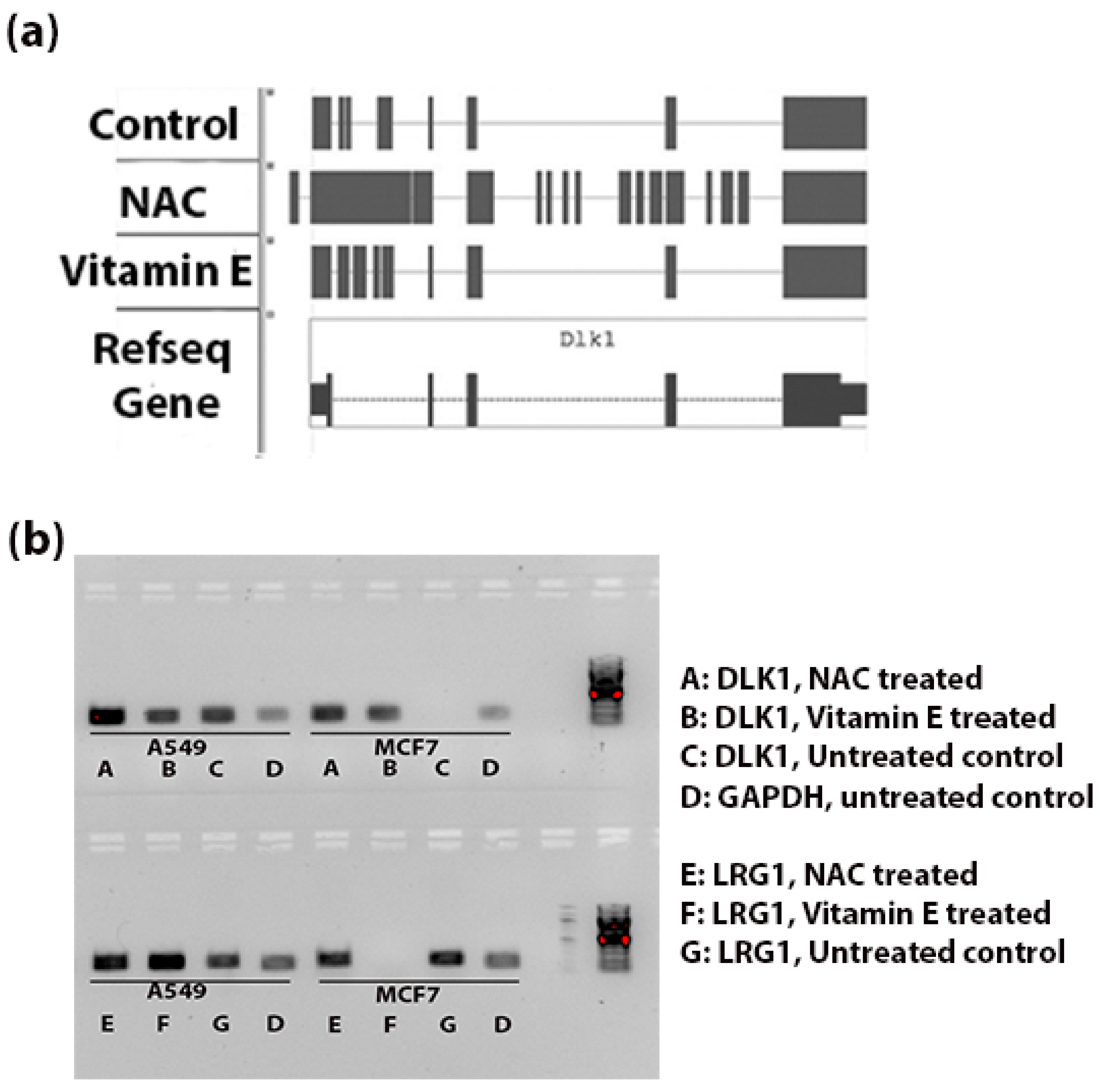
3.4. Validation of Intronic RNA in DLK1 and LRG1

4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.M.; Perez-Gomez, C.; Nunez de Castro, I. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Hercberg, S.; Galan, P.; Preziosi, P.; Bertrais, S.; Mennen, L.; Malvy, D.; Roussel, A.M.; Favier, A.; Briancon, S. The SU.VI.MAX Study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch. Intern. Med. 2004, 164, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Ushakova, T.; Melkonyan, H.; Nikonova, L.; Afanasyev, V.; Gaziev, A.I.; Mudrik, N.; Bradbury, R.; Gogvadze, V. Modification of gene expression by dietary antioxidants in radiation-induced apoptosis of mice splenocytes. Free Radic. Biol. Med. 1999, 26, 887–891. [Google Scholar] [CrossRef]
- Kunsch, C.; Medford, R.M. Oxidative stress as a regulator of gene expression in the vasculature. Circ. Res. 1999, 85, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Canali, R.; Natarelli, L.; Leoni, G.; Azzini, E.; Comitato, R.; Sancak, O.; Barella, L.; Virgili, F. Vitamin C supplementation modulates gene expression in peripheral blood mononuclear cells specifically upon an inflammatory stimulus: A pilot study in healthy subjects. Genes Nutr. 2014, 9, 390. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Meng, N.; Fu, J.; Chen, X.; Xu, X.; Feng, Q.; Jiang, H.; Dai, J.; Yuan, X.; Lu, Y.; et al. Genome-wide transcriptome and antioxidant analyses on gamma-irradiated phases of deinococcus radiodurans R1. PLoS ONE 2014, 9, e85649. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Mason, S.R.; Flatscher-Bader, T.; Ward, L.C.; Marsh, S.A.; Wilce, P.A.; Fassett, R.G.; de Haan, J.B.; Coombes, J.S. Effects of exercise and antioxidant supplementation on endothelial gene expression. Int. J. Cardiol. 2012, 158, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.H.; Liu, J.J.; Yeh, C.L.; Chiu, W.C.; Yeh, S.L. Effects of glutamine supplementation on oxidative stress-related gene expression and antioxidant properties in rats with streptozotocin-induced type 2 diabetes. Br. J. Nutr. 2012, 107, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Louro, R.; Smirnova, A.S.; Verjovski-Almeida, S. Long intronic noncoding RNA transcription: Expression noise or expression choice? Genomics 2009, 93, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Martianov, I.; Ramadass, A.; Serra Barros, A.; Chow, N.; Akoulitchev, A. Repression of the human dihydrofolate reductase gene by a non-coding interfering transcript. Nature 2007, 445, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Brito, G.C.; Fachel, Â.A.; Vettore, A.L.; Vignal, G.M.; Gimba, E.R.P.; Campos, F.S.; Barcinski, M.A.; Verjovski-Almeida, S.; Reis, E.M. Identification of protein-coding and intronic noncoding RNAs down-regulated in clear cell renal carcinoma. Mol. Carcinog. 2008, 47, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.M.; Nakaya, H.I.; Louro, R.; Canavez, F.C.; Flatschart, A.V.F.; Almeida, G.T.; Egidio, C.M.; Paquola, A.C.; Machado, A.A.; Festa, F.; et al. Antisense intronic non-coding RNA levels correlate to the degree of tumor differentiation in prostate cancer. Oncogene 2004, 23, 6684–6692. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.H.; Erie, J.C.; Bakri, S.J. Nutritional supplementation and age-related macular degeneration. Semin. Ophthalmol. 2011, 26, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.C.; Ervin, A.M.; Tao, J.; Davis, R.M. Antioxidant vitamin supplementation for preventing and slowing the progression of age-related cataract. Cochrane Database Syst. Rev. 2012, 6, Cd004567. [Google Scholar] [PubMed]
- Tarozzi, A.; Angeloni, C.; Malaguti, M.; Morroni, F.; Hrelia, S.; Hrelia, P. Sulforaphane as a Potential Protective Phytochemical against Neurodegenerative Diseases. Oxidative Med. Cell. Longevity 2013, 2013, 10. [Google Scholar] [CrossRef] [PubMed]
- Grabacka, M.M.; Gawin, M.; Pierzchalska, M. Phytochemical modulators of mitochondria: The search for chemopreventive agents and supportive therapeutics. Pharmaceuticals (Basel) 2014, 7, 913–942. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, C. Antioxidant supplements to prevent mortality. JAMA 2013, 310, 1178–1179. [Google Scholar] [CrossRef] [PubMed]
- Sablina, A.A.; Budanov, A.V.; Ilyinskaya, G.V.; Agapova, L.S.; Kravchenko, J.E.; Chumakov, P.M. The antioxidant function of the p53 tumor suppressor. Nat. Med. 2005, 11, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Sultan, M.; Schulz, M.H.; Richard, H.; Magen, A.; Klingenhoff, A.; Scherf, M.; Seifert, M.; Borodina, T.; Soldatov, A.; Parkhomchuk, D.; et al. A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science 2008, 321, 956–960. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Gaidatzis, D.; Burger, L.; Florescu, M.; Stadler, M.B. Analysis of intronic and exonic reads in RNA-seq data characterizes transcriptional and post-transcriptional regulation. 2015; 33, 722–729. [Google Scholar]
- Li, L.; Tan, J.; Zhang, Y.; Han, N.; Di, X.; Xiao, T.; Cheng, S.; Gao, Y.; Liu, Y. DLK1 promotes lung cancer cell invasion through upregulation of MMP9 expression depending on Notch signaling. PLoS ONE 2014, 9, e91509. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Y.; Qiu, F.; Qiu, Z. Proteomic identification of exosomal LRG1: A potential urinary biomarker for detecting NSCLC. Electrophoresis 2011, 32, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants Accelerate Lung Cancer Progression in Mice. Sci. Transl. Med. 2014, 6, 221ra15. [Google Scholar] [CrossRef] [PubMed]
- Beaver, L.M.; Buchanan, A.; Sokolowski, E.I.; Riscoe, A.N.; Wong, C.P.; Chang, J.H.; Lohr, C.V.; Williams, D.E.; Dashwood, R.H.; Ho, E. Transcriptome analysis reveals a dynamic and differential transcriptional response to sulforaphane in normal and prostate cancer cells and suggests a role for Sp1 in chemoprevention. Mol. Nutr. Food Res. 2014, 58, 2001–2013. [Google Scholar] [CrossRef] [PubMed]
- Illumina iGenomes. Available online: https://support.illumina.com/sequencing/sequencing_software/igenome.html (accessed on 26 December 2014).
- Kim, D.; Pertea, G.; Trapnell, C.; Pimentel, H.; Kelley, R.; Salzberg, S.L. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013, 14, R36. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Trapnell, C.; Pop, M.; Salzberg, S.L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009, 10, R25. [Google Scholar] [CrossRef] [PubMed]
- SAMtools. Available online: http://samtools.sourceforge.net/ (accessed on 26 December 2014).
- UCSC Genome Bioinformatics. Available online: https://genome.ucsc.edu/cgi-bin/hgTables (accessed on 15 December 2014).
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- P.adjust {stats}. Available online: https://stat.ethz.ch/R-manual/R-devel/library/stats/html/p.adjust.html (accessed on 15 January 2015).
- GSEA Gene Set Enrichment Analysis. Available online: http://software.broadinstitute.org/gsea/msigdb (accessed on 30 January 2015).
- Yu, Y.; Bae, S.; Kim, H.; Kim, Y.; Chu, N.B.; Chu, N.K.; Kang, J.S.; Lee, W.J. The Anti-tumor Activity of Vitamin C via the Increase of Fas (CD95) and MHC I Expression on Human Stomach Cancer Cell Line, SNU1. Immune Netw. 2011, 11, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.H.; Knoff, J.; Yeh, W.H.; Yang, B.; Wang, C.; Kim, Y.S.; Kim, T.W.; Wu, T.C.; Hung, C.F. Treatment of tumors with vitamin E suppresses myeloid derived suppressor cells and enhances CD8+ T cell-mediated antitumor effects. PLoS ONE 2014, 9, e103562. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Lee, Y.K.; Geyer, S.M.; Grote, D.; Stenson, M.; Zincke, S.; Ansell, S.M.; Witzig, T.E.; Kay, N.E. The Green Tea Extract Epigallocatechin Induces in Vitro Cell Death in Primary Human Lymphoma Cells through an ROS Dependent Mechanism. ASH Annu. Meet. Abstr. 2006, 108, 234. [Google Scholar]
- Vahdati-Mashhadian, N.; Jafari, M.R.; Sharghi, N.; Sanati, T. Protective Effects of Vitamin C and NAC on the Toxicity of Rifampin on Hepg2 Cells. Iran J. Pharm. Res. 2013, 12, 141–146. [Google Scholar] [PubMed]
- Bio-Rad Protein Assay. Available online: http://www.bio-rad.com/LifeScience/pdf/Bulletin_9004.pdf (accessed on 10 February 2015).
- Superoxide Dismutase Assay Kit. Available online: https://www.caymanchem.com/pdfs/706002.pdf (accessed on 10 February 2015).
- Primer3 (v0.4.0). Available online: http://bioinfo.ut.ee/primer3-0.4.0/ (accessed on 25 May 2015).
- Sweet-Cordero, A.; Mukherjee, S.; Subramanian, A.; You, H.; Roix, J.J.; Ladd-Acosta, C.; Mesirov, J.; Golub, T.R.; Jacks, T. An oncogenic KRAS2 expression signature identified by cross-species gene-expression analysis. Nat. Genet. 2005, 37, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Klein, E.A.; Thompson, I.M., Jr.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Jerome-Morais, A.; Diamond, A.M.; Wright, M.E. Dietary supplements and human health: For better or for worse? Mol. Nutr. Food Res. 2011, 55, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Crowe, F.L.; Roddam, A.W.; Key, T.J.; Appleby, P.N.; Overvad, K.; Jakobsen, M.U.; Tjonneland, A.; Hansen, L.; Boeing, H.; Weikert, C.; et al. Fruit and vegetable intake and mortality from ischaemic heart disease: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heart study. Eur. Heart J. 2011, 32, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J. Is cancer a disease of abnormal cellular metabolism?: New angles on an old idea. Genet. Med.: Off. J. Am. Coll. Med. Genet. 2008, 10, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Takashima, Y.; Ohtsuka, T.; Gonzalez, A.; Miyachi, H.; Kageyama, R. Intronic delay is essential for oscillatory expression in the segmentation clock. Proc. Natl. Acad. Sci. USA 2011, 108, 3300–3305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chorev, M.; Carmel, L. The Function of Introns. Front. Genet. 2012, 3, 55. [Google Scholar] [CrossRef] [PubMed]
- Nakaya, H.I.; Amaral, P.P.; Louro, R.; Lopes, A.; Fachel, A.A.; Moreira, Y.B.; El-Jundi, T.A.; da Silva, A.M.; Reis, E.M.; Verjovski-Almeida, S. Genome mapping and expression analyses of human intronic noncoding RNAs reveal tissue-specific patterns and enrichment in genes related to regulation of transcription. Genome Biol. 2007, 8, R43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdi, M.H.; Mahdavi, M.; Kheradmand, E.; Shahverdi, A.R. The preventive oral supplementation of a selenium nanoparticle-enriched probiotic increases the immune response and lifespan of 4T1 breast cancer bearing mice. Arzneimittelforschung 2012, 62, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.Y.; Ou, N.; Lu, Q.B. Antioxidant induces DNA damage, cell death and mutagenicity in human lung and skin normal cells. Sci. Rep. 2013, 3, 3169. [Google Scholar] [CrossRef] [PubMed]
- Watson, J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol. 2013, 3, 120144. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Luo, X.; Hu, H.; Wang, R.; Sun, Y.; Zeng, R.; Chen, H. Integrative proteomics and tissue microarray profiling indicate the association between overexpressed serum proteins and non-small cell lung cancer. PLoS ONE 2012, 7, e51748. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menon, S.; Lu, C.; Menon, R.; Schwartz, J.; Guan, Y. Effects of Antioxidants in Human Cancers: Differential Effects on Non-Coding Intronic RNA Expression. Antioxidants 2016, 5, 1. https://doi.org/10.3390/antiox5010001
Menon S, Lu C, Menon R, Schwartz J, Guan Y. Effects of Antioxidants in Human Cancers: Differential Effects on Non-Coding Intronic RNA Expression. Antioxidants. 2016; 5(1):1. https://doi.org/10.3390/antiox5010001
Chicago/Turabian StyleMenon, Shreya, Chunxia Lu, Rajasree Menon, Jessica Schwartz, and Yuanfang Guan. 2016. "Effects of Antioxidants in Human Cancers: Differential Effects on Non-Coding Intronic RNA Expression" Antioxidants 5, no. 1: 1. https://doi.org/10.3390/antiox5010001
APA StyleMenon, S., Lu, C., Menon, R., Schwartz, J., & Guan, Y. (2016). Effects of Antioxidants in Human Cancers: Differential Effects on Non-Coding Intronic RNA Expression. Antioxidants, 5(1), 1. https://doi.org/10.3390/antiox5010001





