Antioxidant Potential of Plumieride against CCl4-Induced Peroxidative Damage in Rats
Abstract
:1. Introduction
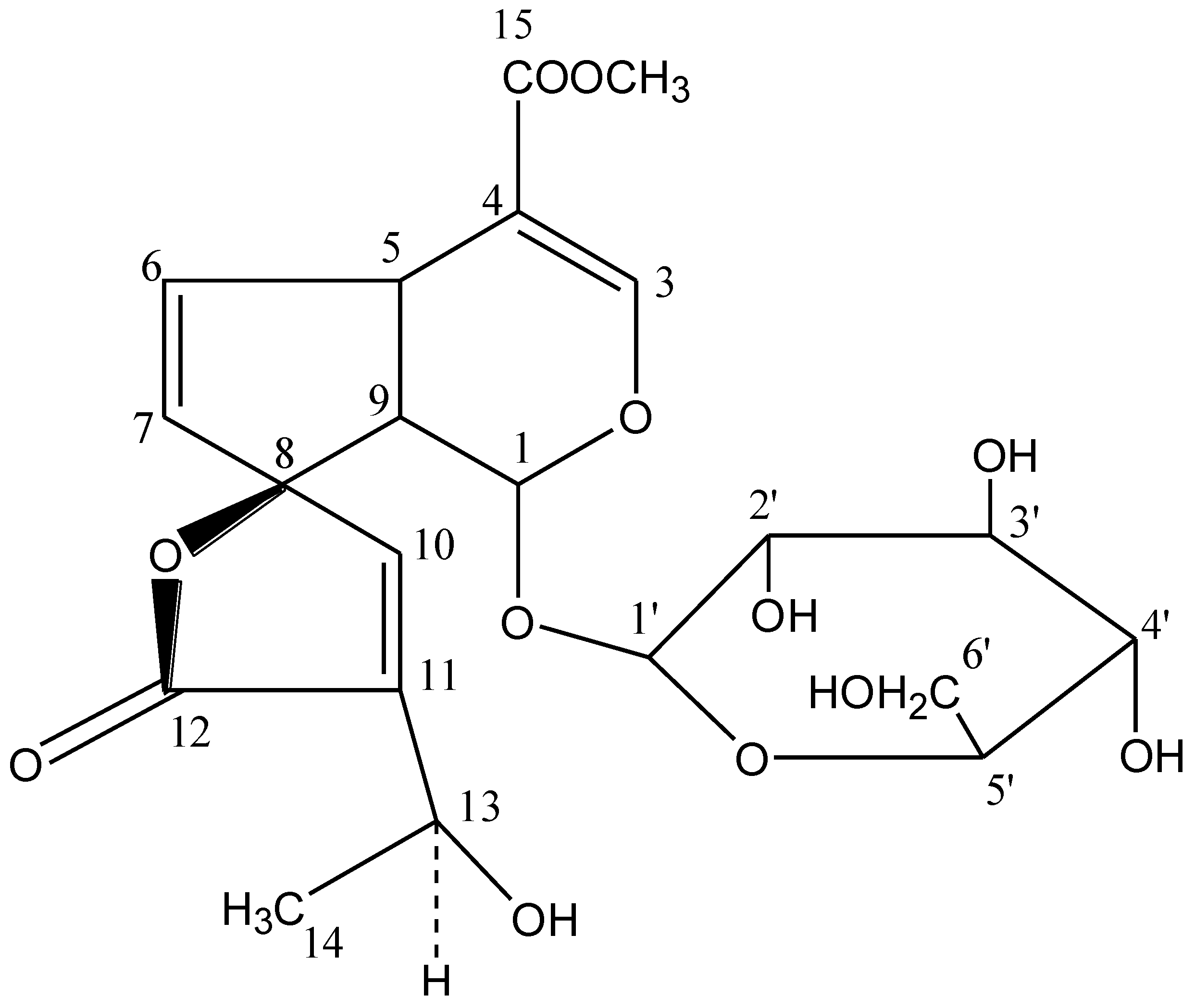
2. Materials and Methods
2.1. Animal Model
2.2. Plant Material
2.3. Plant Extraction and Isolation of Active Compounds
2.4. Purification, Identification and Structure Elucidation of Active Compound
2.5. Standard Drug
2.6. Chemicals
2.7. Ethical Aspects
2.8. Behavioral and Toxic Effects
2.9. Experimental Protocol
- Group I: Vehicle treated rats were kept on normal diet and served as control for 30 days.
- Group II: Rats were intoxicated with carbon tetrachloride at 0.3 mL/kg body weight/twice a week with olive oil (1:1), intra-peritoneally for 30 days.
- Group III: Rats orally received plumieride at 5 mg/kg body weight/day with olive oil, and CCl4 as Group II for 30 days, simultaneously.
- Group IV: Rats orally received plumieride at 10 mg/kg body weight/day with olive oil, and CCl4 as Group II for 30 days, simultaneously.
- Group V: Rats orally received plumieride at 20 mg/kg body weight/day with olive oil, and CCl4 as Group II for 30 days, simultaneously.
- Group VI: Rats orally received silymarin at 20 mg/kg body weight/day with olive oil, and CCl4 as Group II for 30 days, simultaneously.
2.10. Assessment of Liver Function
2.11. Histopathology
2.12. Statistical Process
3. Results
| Treatment Design | AST (IU/L) | ALT (IU/L) | GGT (IU/L) | ALP (KAU) | Total Bilirubin (mg/100 mL) | Total Protein (mg/dL) |
|---|---|---|---|---|---|---|
| Control (vehicle treated) Group I | 122.27 ± 1.37 | 104.33 ± 2.45 | 8.24 ± 0.28 | 19.37 ± 1.19 | 0.82 ± 0.05 | 6.32 ± 0.29 |
| CCl4 (0.3 mL/kg body weight/twice a week with olive oil, intra-peritoneally, 1:1) Group II | 356.45 ± 3.42 *** | 330.72 ± 4.57 *** | 43.92 ± 2.10 *** | 65.33 ± 2.19 *** | 2.85 ± 0.14 *** | 2.69 ± 0.14 *** |
| CCl4 + plumieride (5 mg/kg body weight/day, orally) Group III | 275.50 ± 2.66 a | 262.14 ± 2.59 a | 35.10 ± 1.35 b | 56.48 ± 1.59 b | 2.10 ± 0.15 b | 3.12 ± 0.15 ns |
| CCl4 + plumieride (10 mg/kg body weight/day, orally) Group IV | 224.26 ± 1.98 a,c | 201.21 ± 1.92 a,c | 28.05 ± 0.72 a,c | 48.10 ± 1.37 a,d | 1.75 ± 0.16 a,ns | 4.56 ± 0.10 a,c |
| CCl4 + plumieride (20 mg/kg body weight/day, orally) Group V | 172.24 ± 1.49 a,c,e | 157.20 ± 1.37 a,c,e | 19.10 ± 0.65 a,c,e | 35.15 ± 1.18 a,c,e | 1.27 ± 0.12 a,d,f | 6.42 ± 0.17 a,c,e |
| CCl4 + silymarin (20 mg/kg body weight/day, orally) Group VI | 145.18 ± 1.55 a,c,e,g | 128.37 ± 1.92 a,c,e,g | 15.75 ± 1.77 a,c,e,h | 30.47 ± 1.72 a,c,e,i | 1.22 ± 0.08 a,c,f,ns | 6.19 ± 0.18 a,c,e,ns |
| Treatment Design | LPO (n mol TBARS/mg Tissue) | SOD (μmol/mg Protein) | CAT (μmol H2O2 Consumed/min/mg Protein) | GSH (n mol/g Tissue) | GPx (n mol NADPH Consumed/min/mg Protein) | GR (n mol NADPH Consumed/min/mg Protein) |
|---|---|---|---|---|---|---|
| Control (vehicle treated) Group I | 3.04 ± 0.21 | 10.24 ± 0.42 | 62.22 ± 2.33 | 4.52 ± 0.19 | 12.18 ± 0.42 | 18.27 ± 0.62 |
| CCl4 (0.3 mL/kg b. wt/twice a week with olive oil, intra-peritoneally, 1:1) Group II | 11.23 ± 0.89 *** | 4.10 ± 0.13 *** | 26.21 ± 1.94 *** | 1.72 ± 0.09 *** | 5.09 ± 0.25 *** | 8.82 ± 0.15 *** |
| CCl4 + plumieride (5 mg/kg body weight/day, orally) Group III | 8.18 ± 0.32 a | 5.32 ± 0.14 a | 32.10 ± 1.52 b | 2.05 ± 0.10 b | 6.62 ± 0.21 a | 10.52 ± 0.17 a |
| CCl4 + plumieride (10 mg/kg body weight/day, orally) Group IV | 6.22 ± 0.29 a,c | 6.89 ± 0.16 a,c | 40.21 ± 1.89 a,d | 3.06 ± 0.14 a,c | ai8.35 ± 0.18 a,c | 12.82 ± 0.22 a,c |
| CCl4 + plumieride (20 mg/kg body weight/day, orally) Group V | 3.95 ± 0.35 a,c,e | 7.83 ± 0.18 a,c,f | 48.58 ± 1.23 a,c,f | 3.95 ± 0.13 a,c,e | 10.10 ± 0.15 a,c,e | 16.17 ± 0.21 a,c,e |
| CCl4 + silymarin (20 mg/kg body weight/day, orally) Group VI | 3.68 ± 0.42 a,c,e,ns | 8.14 ± 0.33 a,c,f,ns | 51.40 ± 1.67 a,c,f,ns | 3.63 ± 0.12 a,c,e,ns | 10.21 ± 0.24 a,c,e,ns | 15.28 ± 0.33 a,c,e,g |
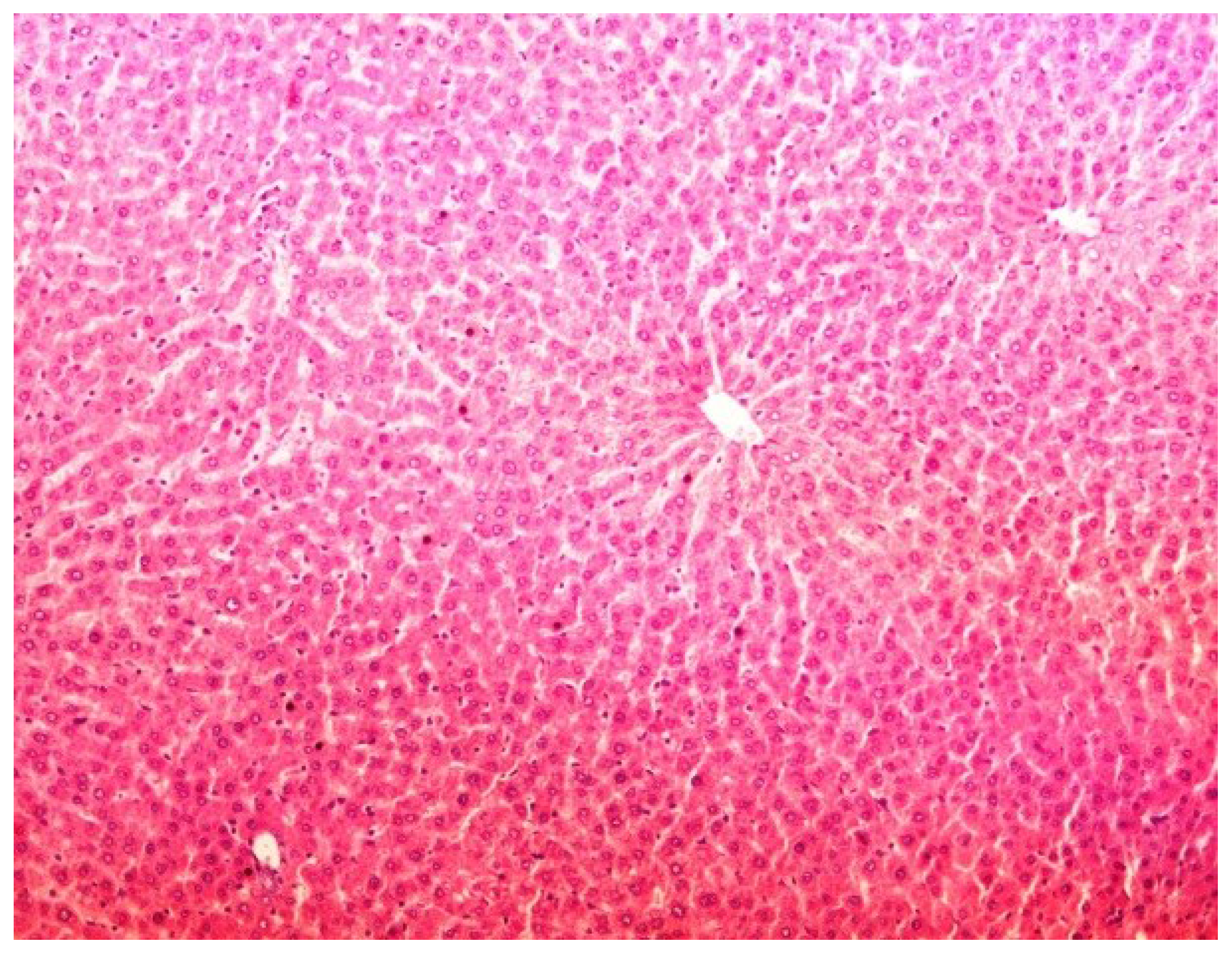




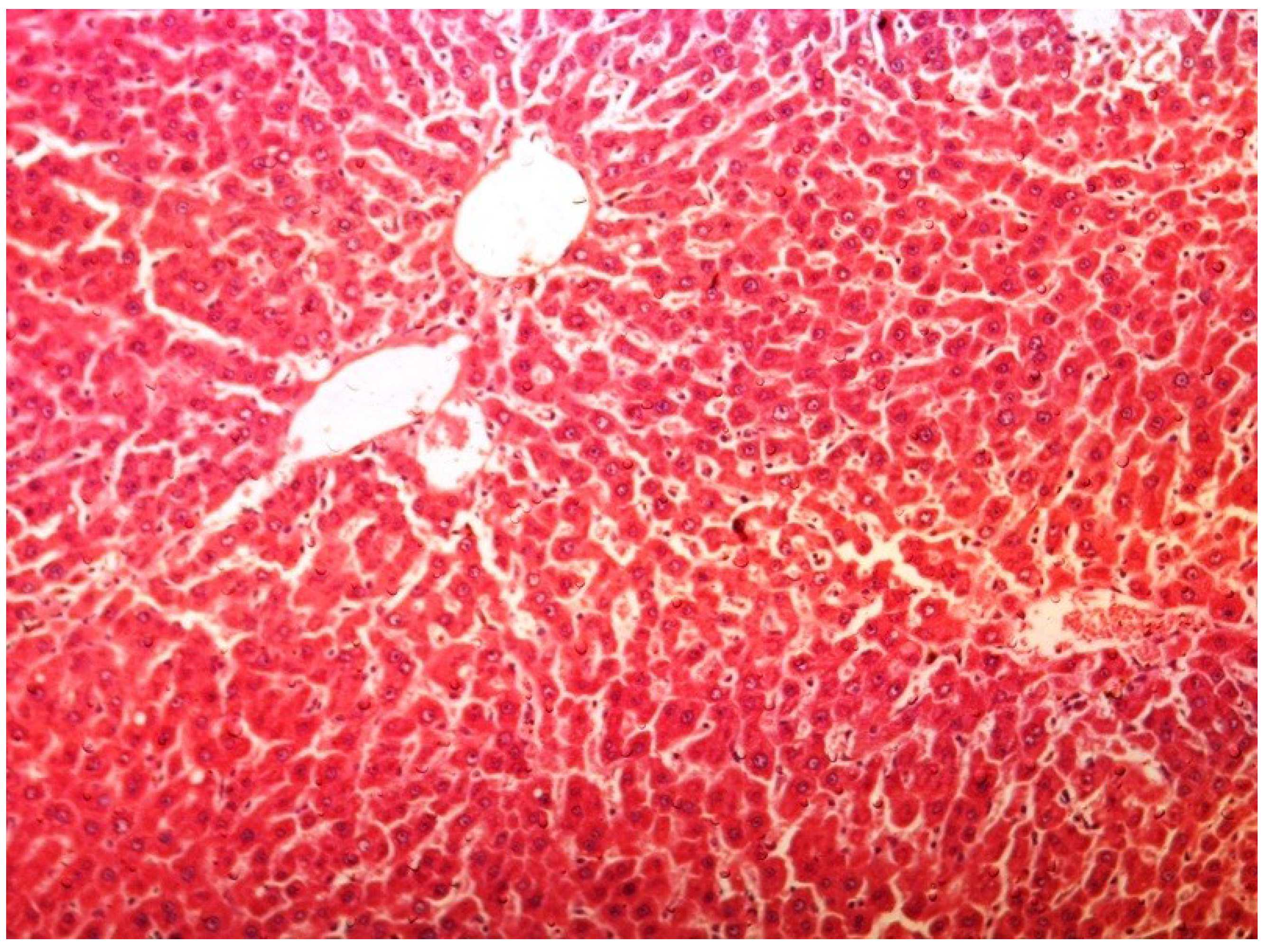
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Singh, D.; Singh, R.; Singh, P.; Gupta, R.S. Effects of embelin on lipid peroxidation and free radical scavenging activity against liver damage in rats. Basic Clin. Pharmacol. Toxicol. 2009, 105, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Park, J.S.; Chae, S.; Kim, S.; Moon, C.; Hyun, J.W.; Shin, T. Hepatoprotective effects of Lycium chinense Miller fruit and its constituent betaine in CCl4-induced hepatic damage in rats. Acta Histochem. 2014, 116, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Matés, J.M.; Segura, J.A.; Alonso, F.J.; Márquez, J. Natural antioxidants: Therapeutic prospects for cancer and neurological diseases. Mini Rev. Med. Chem. 2009, 9, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Manubolu, M.; Goodla, L.; Ravilla, S.; Thanasekaran, J.; Dutta, P.; Malmlöf, K.; Obulum, V.R. Protective effect of Actiniopteris radiata (Sw.) Link. against CCl4 induced oxidative stress in albino rats. J. Ethnopharmacol. 2014, 153, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Huo, H.Z.; Wang, B.; Liang, Y.K.; Bao, Y.Y.; Gu, Y. Hepatoprotective and antioxidant effects of licorice extract against CCl4-induced oxidative damage in rats. Int. J. Mol. Sci. 2011, 12, 6529–6543. [Google Scholar] [CrossRef] [PubMed]
- Boonclarm, D.; Sornwatana, T.; Arthan, D.; Kongsaeree, P.; Svasti, J. β-Glucosidase catalyzing specific hydrolysis of an iridoid β-glucoside from Plumeria obtusa. Acta Biochim. Biophys. Sin. 2006, 38, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Akhtar, N.; Riaz, N.; Ali, M.S.; Jabbar, A. Isolation and characterization of secondary metabolites from Plumeria obtusa. J. Asian Nat. Prod. Res. 2011, 13, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Dobhal, M.P.; Li, G.; Gryshuk, A.; Graham, A.; Bhatanager, A.K.; Khaja, S.D.; Joshi, Y.C.; Sharma, M.C.; Oseroff, A.; Pandey, P.K.; et al. Structural modification of plumieride isolated from Plumeria bicolor and the effect of these modification on in vitro anticancer activity. J. Org. Chem. 2004, 69, 6165–6172. [Google Scholar] [CrossRef] [PubMed]
- Kuigoua, G.M.; Kouam, S.F.; Ngadjui, B.T.; Schulz, B.; Green, I.R.; Choudhary, M.I.; Krohn, K. Minor secondary metabolic products from the stem bark of Plumeria rubra Linn. displaying antimicrobial activities. Planta Med. 2010, 76, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Sharma, U.; Kumar, P.; Gupta, Y.K.; Dobhal, M.P.; Singh, S. Antifungal activity of plumericin and isoplumericin. Nat. Prod. Commun. 2011, 6, 1567–1568. [Google Scholar] [PubMed]
- Sharma, U.; Singh, D.; Kumar, P.; Dobhal, M.P.; Singh, S. Antiparasitic activity of plumericin and isoplumericin isolated from Plumeria bicolor against Leishmania donovani. Indian J. Med. Res. 2011, 134, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Singh, A.; Sharma, U.; Singh, D.; Dobhal, M.P.; Singh, S. Anti-mycobacterial activity of plumericin and isoplumericin against MDR Mycobacterium tuberculosis. Pulm. Pharmacol. Ther. 2013, 26, 332–325. [Google Scholar] [CrossRef] [PubMed]
- Rebouças Sde, O.; da Silva, J.; Bertoni, R.S.; Decker, N.; Santos, M.S.; Rossatto, R.R.; Corrêa, D.S.; Ferraz, A.B. Assessment of the genotoxic and mutagenic properties of Himatanthus articulatus bark extracts used as phytotherapeutic drug in the Amazon. J. Ethnopharmacol. 2013, 147, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, R.K.; Afifi-Yazar, F.U.; Sticher, O.; Winkler, T. 13C NMR spectroscopy of naturally occurring iridoid glucosides and their acylated derivatives. Tetrahedron 1980, 36, 2317–2326. [Google Scholar] [CrossRef]
- Yamauchi, T.; Abe, F.; Taki, M. Protoplumericin, an iridoid bis-glucoside in Allamanda neriifolia. Chem. Pharm. Bull. 1981, 29, 3051–3055. [Google Scholar] [CrossRef]
- Abe, F.; Mori, T.; Yamauchi, T. Iridoids of Apocynaceae. III. Minor iridoids from Allamanda neriifolia. Chem. Pharm. Bull. 1984, 32, 2947–2956. [Google Scholar] [CrossRef]
- Porchezhian, E.; Ansari, S.H. Hepatoprotective activity of Abutilon indicum on experimental liver damage in rats. Phytomedicine 2005, 12, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxidation in animal tissue by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of superoxide anion radical in auto-oxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Moron, M.S.; Dipierre, J.W.; Mannervick, B. Levels of glutathione, glutathione reductase and glutathione-s-transferase activities in rat lung and liver. Biochem. Biophys. Acta 1979, 582, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, I.; Mennervick, B. Glutathione reductase. In Methods in Enzymology; Academic Press: New York, NY, USA, 1985; Volume 113, pp. 484–490. [Google Scholar]
- Paglia, D.E.; Velentine, W.M. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Pradeep, K.; Rajmohan, C.V.; Anand, K.G.; Karthikayan, S. Effect of pretreatment of Cassia fistula Linn.leaf extract against subacute CCl4 induced hepatotoxicity in rats. Indian J. Exp. Biol. 2005, 43, 526–530. [Google Scholar] [PubMed]
- Saikia, L.R.; Upadhyaya, S. Antioxidant activity, phenol and flavonoid content of some less known medicinal plants of Assam. Int. J. Pharm. Bio. Sci. 2011, 2, 383–388. [Google Scholar]
- Basu, S. Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicology 2003, 169, 113–117. [Google Scholar]
- Zeashan, H.; Amresh, G.; Singh, S.; Rao, C.V. Hepatoprotective activity of Amaranthus spinosus in experimental animals. J. Food Chem. Toxicol. 2008, 46, 3417–3421. [Google Scholar] [CrossRef]
- Kaplowitz, N.; Aw, T.Y.; Simon, F.R.; Stolz, A. Drug induced hepatotoxicity. Ann. Intern. Med. 1986, 104, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Kalegari, M.; Gemin, C.A.; Araújo-Silva, G.; Brito, N.J.; López, J.A.; Oliveira Tozetto, S.D.; das Graças Almeida, M.; Miguel, M.D.; Stien, D.; Miguel, O.G.; et al. Chemical composition, antioxidant activity and hepatoprotective potential of Rourea induta Planch. (Connaraceae) against CCl4-induced liver injury in female rats. Nutrition 2014, 6, 713–718. [Google Scholar]
- Bhakta, T.; Mukherjee, P.K.; Mukherjee, K.; Banerjee, S.; Mandal, S.C.; Maity, T.K.; Pal, M.; Saha, B.P. Evaluation of hepatoprotective activity of Cassia fistula leaf extract. J. Ethnopharmacol. 1999, 66, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Woo, E.; Choi, C.Y.; Shin, D.W.; Lee, D.G.; You, H.J.; Jeong, H.G. Protective effect of acteoside on carbon tetrachloride-induced hepatotoxicity. Life Sci. 2004, 74, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.A.; Khan, M.R.; Ahmad, B.; Noureen, F.; Rashid, U.; Khan, R.A. Investigation on flavonoid composition and anti-free radical potential of Sida cordata. BMC Complement. Altern. Med. 2013, 13, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Banu, G.S.; Kannan, V.; Pandian, M.R. Antihepatotoxic effect of β-carotene on paracetamol induced hepatic damage in rats. Indian J. Exp. Biol. 2005, 43, 351–355. [Google Scholar] [PubMed]
- Mandal, P.K.; Bishayee, A.; Chatterjee, M. Stimulation of tissue repair by Mikania cordata root extract in carbon tetrachloride-induced liver injury in mice. Phytother. Res. 1993, 7, 103–105. [Google Scholar] [CrossRef]
- Salvi, M.; Battaglia, V.; Brunati, A.M.; LaRocca, N.; Tibaldi, E.; Pietrangeli, P.; Marcocci, L.; Mondovi, B.; Rossi, C.A.; Toninello, A.; et al. Catalase takes part in rat liver mitochondria oxidative stress defense. J. Biol. Chem. 2007, 282, 24407–24415. [Google Scholar] [CrossRef] [PubMed]
- Parimoo, H.A.; Sharma, R.; Patil, R.D.; Sharma, O.P.; Kumar, P.; Kumar, N. Hepatoprotective effect of Ginkgo biloba leaf extract on lantadenes-induced hepatotoxicity in guinea pigs. Toxicon 2014, 81, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Matsuki, O.; Nomura, T.; Kubodera, A.; Yamaoka, K. Elevation of mouse liver glutathione level by low-dose gamma ray irradiation and its effect on CCl4-induced liver damage. Anticancer Res. 1998, 18, 2471–2476. [Google Scholar] [PubMed]
- Meister, A. Glutathione. In The Liver, Biology and Pathobiology; Arias, I.M., Boyer, J.L., Jakoby, W.B., Fausto, D., Schacter, D., Shafritz, D.A., Eds.; Ravan Press: New York, NY, USA, 1994; pp. 401–417. [Google Scholar]
- Okuno, H.; Hazama, H.; Mutazo, T.; Shiozaki, Y.; Somoshima, Y.T. Drug metabolizing activity in rats with chronic liver injury, induced by carbon tetrachloride relationship with the hydroxyproline content in liver. Jpn. J. Pharmacol. 1998, 41, 363–369. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, D.; Arya, P.V.; Sharma, A.; Aggarwal, V.P.; Dobhal, M.P.; Gupta, R.S. Antioxidant Potential of Plumieride against CCl4-Induced Peroxidative Damage in Rats. Antioxidants 2014, 3, 798-813. https://doi.org/10.3390/antiox3040798
Singh D, Arya PV, Sharma A, Aggarwal VP, Dobhal MP, Gupta RS. Antioxidant Potential of Plumieride against CCl4-Induced Peroxidative Damage in Rats. Antioxidants. 2014; 3(4):798-813. https://doi.org/10.3390/antiox3040798
Chicago/Turabian StyleSingh, Dharmendra, Priya Vrat Arya, Ashutosh Sharma, Ved Prakash Aggarwal, Mahabeer Prasad Dobhal, and Radhey Shyam Gupta. 2014. "Antioxidant Potential of Plumieride against CCl4-Induced Peroxidative Damage in Rats" Antioxidants 3, no. 4: 798-813. https://doi.org/10.3390/antiox3040798
APA StyleSingh, D., Arya, P. V., Sharma, A., Aggarwal, V. P., Dobhal, M. P., & Gupta, R. S. (2014). Antioxidant Potential of Plumieride against CCl4-Induced Peroxidative Damage in Rats. Antioxidants, 3(4), 798-813. https://doi.org/10.3390/antiox3040798




