Novel Hybrid Peptide DEFB126 (1-39)-TP5 Inhibits LPS-Induced Inflammatory Responses and Oxidative Stress by Neutralizing LPS and Blocking the TLR4/MD2-NFκB Signaling Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Hybrid Peptide Design
2.2. Peptide Synthesis
2.3. Circular Dichroism Analysis
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Ex Vivo Stability of DTP
2.7. Animal Model
2.8. Histopathology and Immunohistochemistry
2.9. Histopathology and Immunohistochemistry ELISA
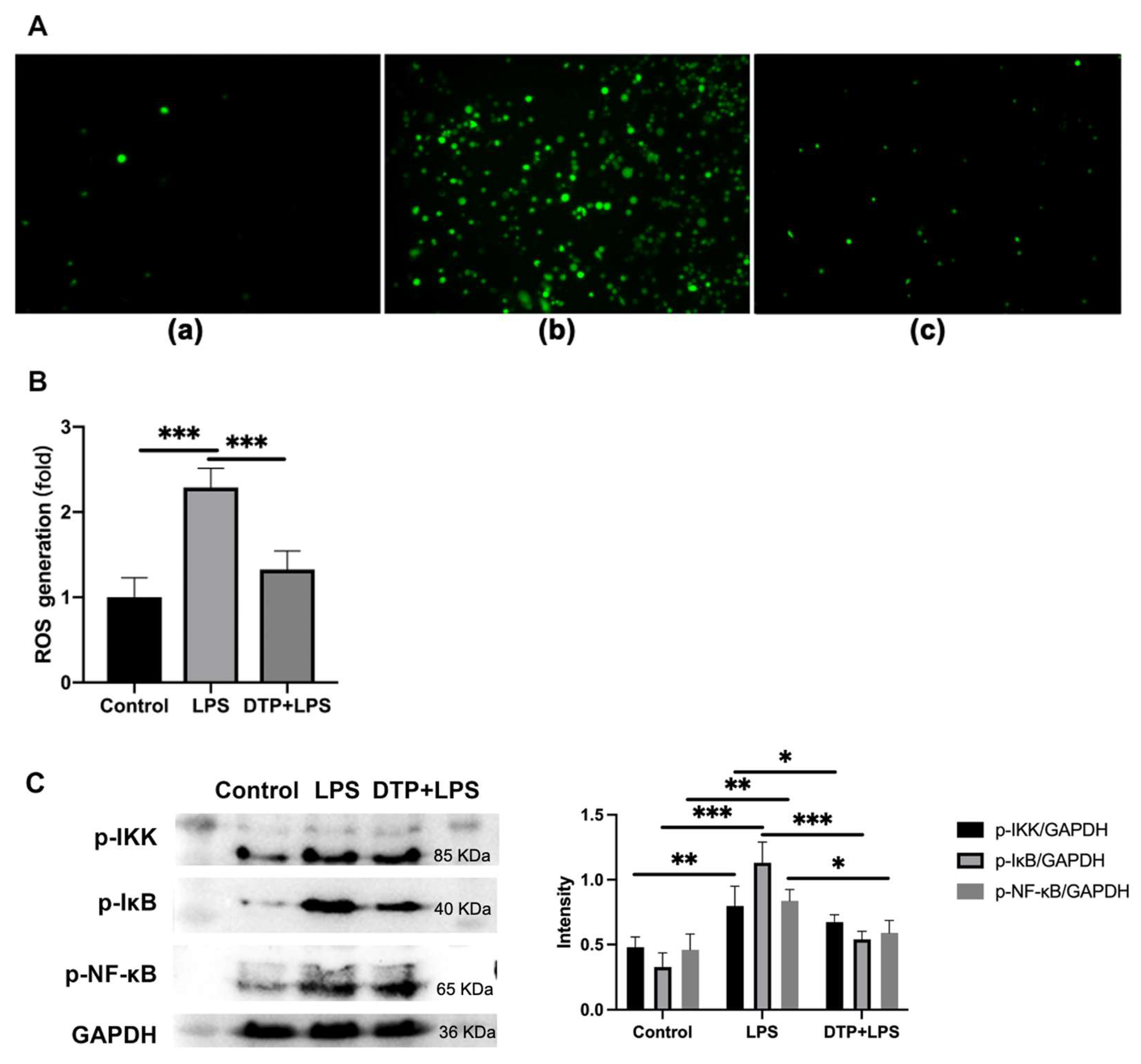
2.10. The Detection of Reactive Oxygen Species (ROS) Generation
2.11. Neutralization of LPS
2.12. Molecular Docking
2.13. Surface Plasmon Resonance (SPR)
2.14. Western Blotting
2.15. Statistics
3. Results
3.1. Peptide Design and Characterization
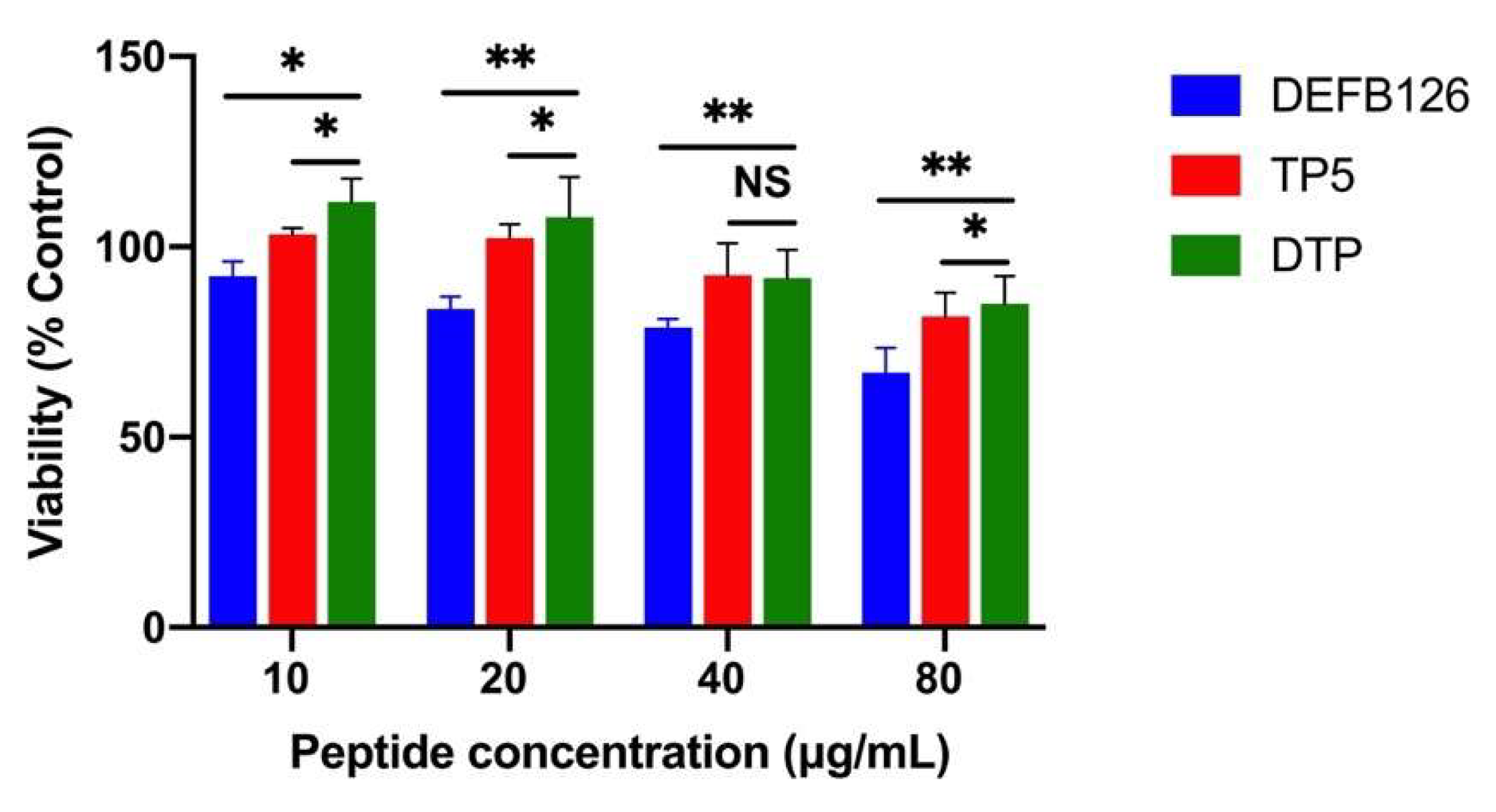
3.2. Cytotoxicity in RAW264.7 Macrophage Cells
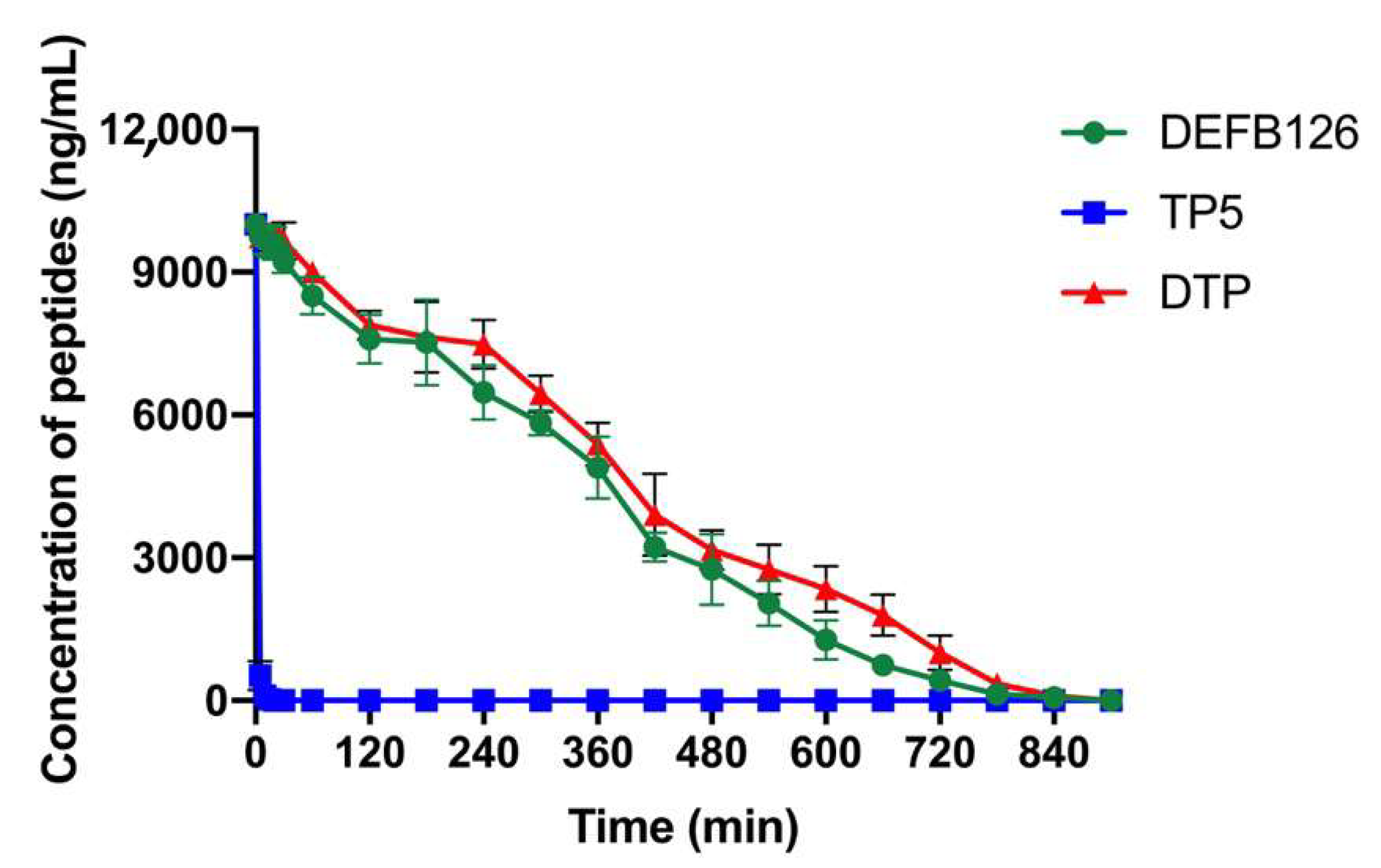
3.3. Ex Vivo Stability of DTP in Plasma
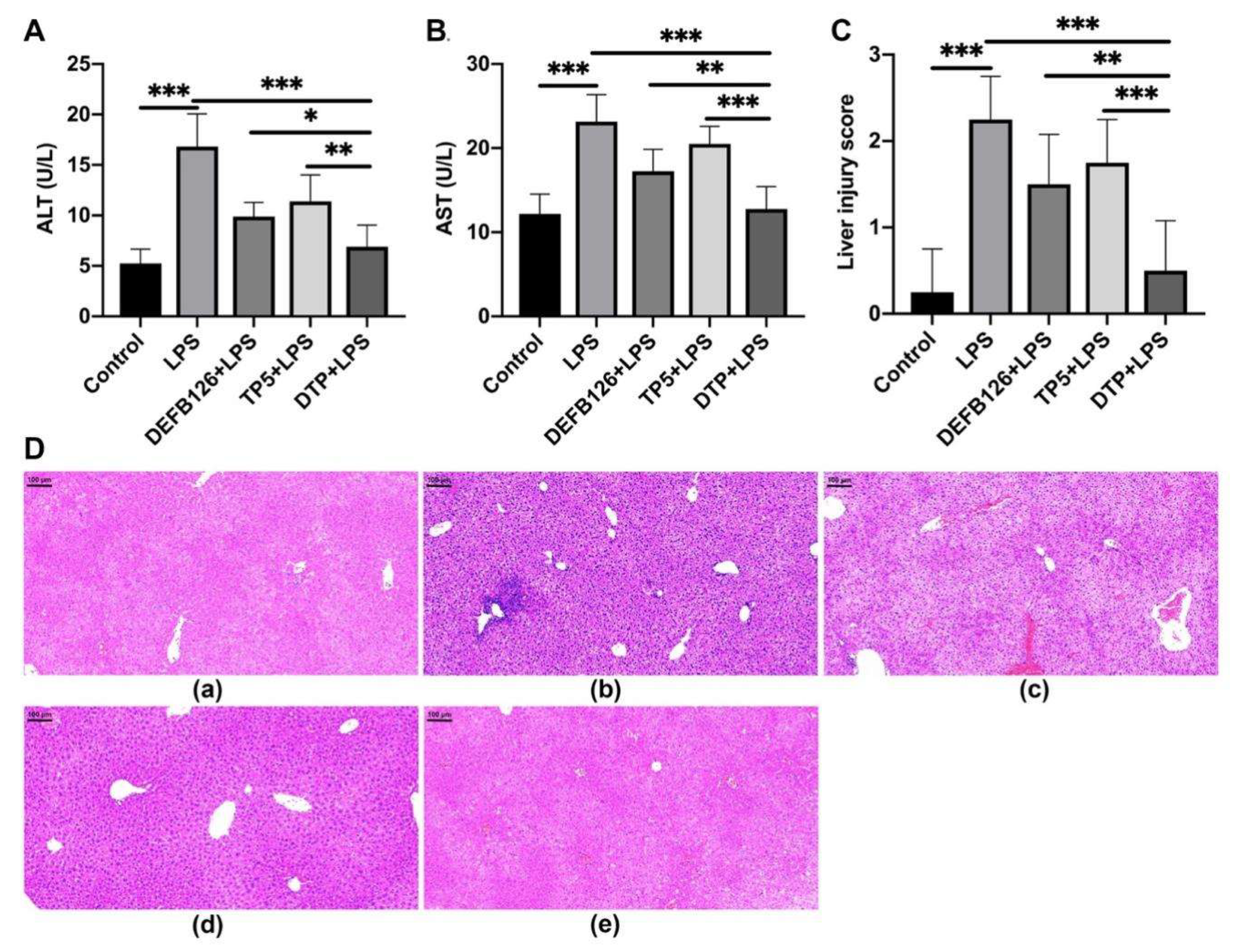
3.4. Protective Effects of DTP Against LPS-Induced Liver Injury
3.5. The Effects of DTP on LPS-Induced Inflammatory Response
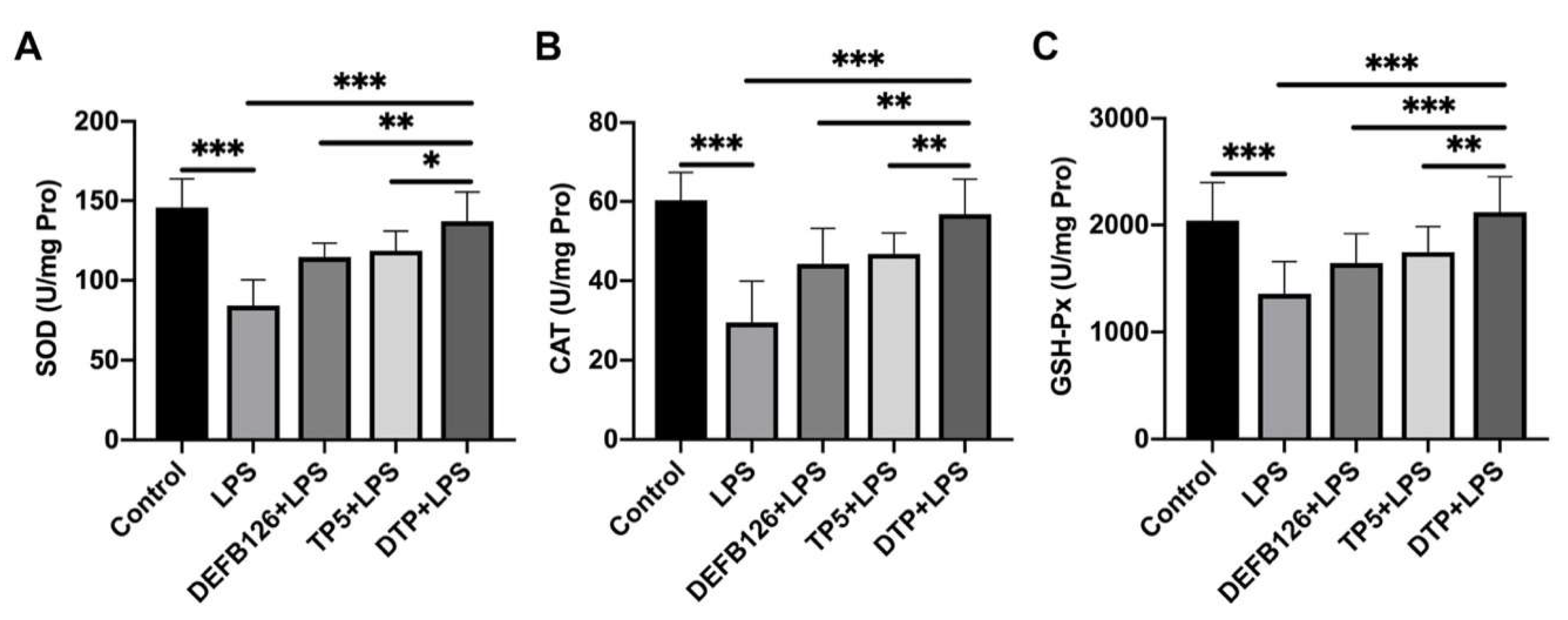
3.6. The Effects of DTP on LPS-Induced Oxidative Stress
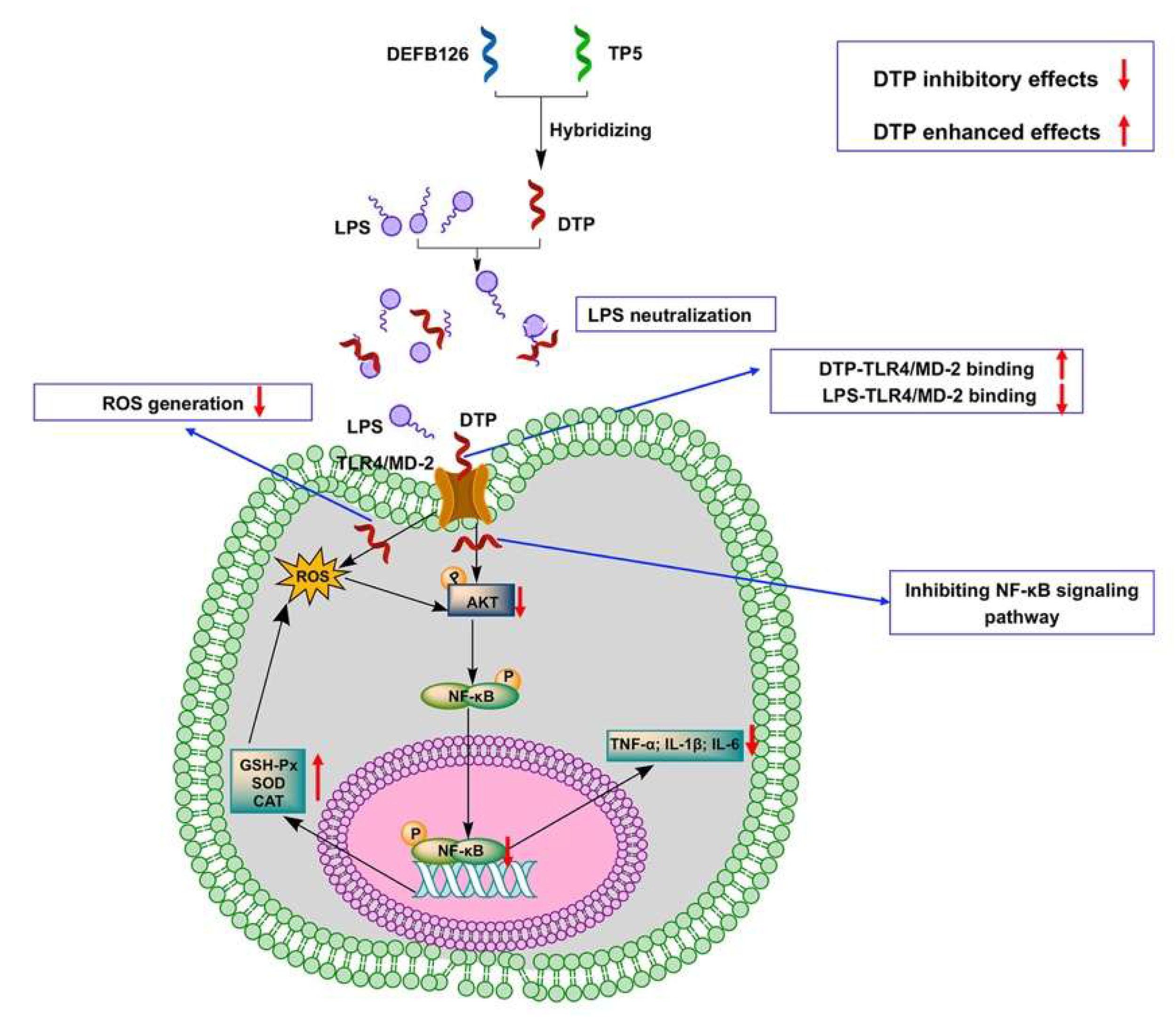
3.7. DTP Exerts Anti-Inflammatory and Antioxidant Activities by Neutralizing LPS, Binding to TLR4/MD-2, Reducing ROS Content and Inhibiting the Activation of the NF-κB Signaling Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zong, X.; Hu, W.; Song, D.; Li, Z.; Du, H.; Lu, Z.; Wang, Y. Porcine lactoferrin-derived peptide LFP-20 protects intestinal barrier by maintaining tight junction complex and modulating inflammatory response. Biochem. Pharmacol. 2016, 104, 74–82. [Google Scholar] [CrossRef]
- Fessler, M.B.; Malcolm, K.C.; Duncan, M.W.; Worthen, G.S. A genomic and proteomic analysis of activation of the human neutrophil by lipopolysaccharide and its mediation by p38 mitogen-activated protein kinase. J. Biol. Chem. 2002, 277, 31291–31302. [Google Scholar] [CrossRef]
- Ren, J.; Wu, X.; Jiang, R.; Hao, D.; Liu, Y. Molecular hydrogen inhibits lipopolysaccharide-triggered NLRP3 inflammasome activation in macrophages by targeting the mitochondrial reactive oxygen species. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 50–55. [Google Scholar] [CrossRef]
- Gao, X.; Yang, X.; Deng, C.; Chen, Y.; Bian, Y.; Zhang, X.; Jin, Y.; Zhang, J.; Liang, X.J. A mitochondria-targeted nanozyme with enhanced antioxidant activity to prevent acute liver injury by remodeling mitochondria respiratory chain. Biomaterials 2025, 318, 123133. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Rios, F.J.; Alves-Lopes, R.; Neves, K.B.; Camargo, L.L.; Montezano, A.C. Oxidative Stress: A Unifying Paradigm in Hypertension. Can. J. Cardiol. 2020, 36, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Liang, Y.; Du, X.; Ye, R.D. Serum amyloid A promotes LPS clearance and suppresses LPS-induced inflammation and tissue injury. EMBO Rep. 2018, 19, e45517. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, T.; Chen, X.; Song, Y. Changes of Lipopolysaccharide-Induced Acute Kidney and Liver Injuries in Rats Based on Metabolomics Analysis. J. Inflamm. Res. 2021, 14, 1807–1825. [Google Scholar] [CrossRef]
- Mohapatra, S.S.; Dwibedy, S.K.; Padhy, I. Polymyxins, the last-resort antibiotics: Mode of action, resistance emergence, and potential solutions. J. Biosci. 2021, 46, 85. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, R.; Wang, J.; Tong, Y.; Zhang, J.; Li, Z.; Zhang, H.; Abbas, Z.; Si, D.; Wei, X. Isolation, Characterization, and Functional Properties of Antioxidant Peptides from Mulberry Leaf Enzymatic Hydrolysates. Antioxidants 2024, 13, 854. [Google Scholar] [CrossRef]
- Liu, H.; Li, B. Separation and identification of collagen peptides derived from enzymatic hydrolysate of Salmo salar skin and their anti-inflammatory activity in lipopolysaccharide (LPS)-induced RAW264.7 inflammatory model. J. Food Biochem. 2022, 46, e14122. [Google Scholar] [CrossRef]
- Tonolo, F.; Coletta, S.; Fiorese, F.; Grinzato, A.; Albanesi, M.; Folda, A.; Ferro, S.; De Mario, A.; Piazza, I.; Mammucari, C.; et al. Sunflower seed-derived bioactive peptides show antioxidant and anti-inflammatory activity: From in silico simulation to the animal model. Food Chem. 2024, 439, 138124. [Google Scholar] [CrossRef] [PubMed]
- Sreelekshmi, P.J.; Devika, V.; Aiswarya, L.S.; Jeevan, S.R.; Ramanunni, K.; Nair, P.B.; Sadanandan, S. Recent Advances in Bioactive Peptides as Functional Food for Health Promotions and Medicinal Applications. Protein Pept. Lett. 2023, 30, 626–639. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhang, L.; Yang, Y.; Hou, Y.; Xu, Y.; Wang, Z.; Su, H.; Han, F.; Han, J.; Liu, P.; et al. LL-37 transports immunoreactive cGAMP to activate STING signaling and enhance interferon-mediated host antiviral immunity. Cell Rep. 2022, 39, 110880. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhu, S.; Wang, R.; Chen, X.; Cai, K. Isolation and Identification of Anti-Inflammatory Peptide from Goose Blood Hydrolysate to Ameliorate LPS-Mediated Inflammation and Oxidative Stress in RAW264.7 Macrophages. Molecules 2022, 27, 8816. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H.; Gu, Y.; Xin, A.; Zhang, Y.; Diao, H.; Lin, D. Human beta-defensin DEFB126 is capable of inhibiting LPS-mediated inflammation. Appl. Microbiol. Biotechnol. 2013, 97, 3395–3408. [Google Scholar] [CrossRef]
- Peng, S.J.; Feng, Y.; Li, X.; Wang, X.X.; Wang, Y.; Zhou, B.T.; Liu, Y.; Liu, T.; Wu, Y.C. Thymopentin (TP-5) prevents lipopolysaccharide-induced neuroinflammation and dopaminergic neuron injury by inhibiting the NF-κB/NLRP3 signaling pathway. Int. Immunopharmacol. 2023, 119, 110109. [Google Scholar] [CrossRef]
- Lunin, S.M.; Novoselova, T.V.; Khrenov, M.O.; Glushkova, O.V.; Parfeniuk, S.B.; Smolikhina, T.I.; Fesenko, E.E.; Novoselova, E.G. Immunomodulatory effects of thymopentin under acute and chronic inflammations in mice. Biofizika 2009, 54, 260–266. [Google Scholar] [CrossRef]
- Semple, F.; Dorin, J.R. β-Defensins: Multifunctional Modulators of Infection, Inflammation and More? J. Innate Immun. 2012, 4, 337–348. [Google Scholar] [CrossRef]
- Corrales-Garcia, L.L.; Possani, L.D.; Corzo, G. Expression systems of human β-defensins: Vectors, purification and biological activities. Amino Acids 2011, 40, 5–13. [Google Scholar] [CrossRef]
- Huang, L.; Leong, S.S.J.; Jiang, R. Soluble fusion expression and characterization of bioactive human beta-defensin 26 and 27. Appl. Microbiol. Biotechnol. 2009, 84, 301–308. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, S.; Wang, S.; Liu, Y. Human β-defensins: The multi-functional natural peptide. Biochem. Pharmacol. 2024, 227, 116451. [Google Scholar] [CrossRef]
- Cieślik, M.; Bagińska, N.; Górski, A.; Jończyk-Matysiak, E. Human β-Defensin 2 and Its Postulated Role in Modulation of the Immune Response. Cells 2021, 10, 2991. [Google Scholar] [CrossRef] [PubMed]
- Koeninger, L.; Armbruster, N.S.; Brinch, K.S.; Kjaerulf, S.; Andersen, B.; Langnau, C.; Autenrieth, S.E.; Schneidawind, D.; Stange, E.F.; Malek, N.P.; et al. Human β-Defensin 2 Mediated Immune Modulation as Treatment for Experimental Colitis. Front. Immunol. 2020, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Ke, P.; Bao, X.; Wu, H.; Xia, Y.; Zhang, Z.; Zhong, H.; Dai, Q.; Wu, L.; Wang, T.; et al. Peptide nano-blanket impedes fibroblasts activation and subsequent formation of pre-metastatic niche. Nat. Commun. 2022, 13, 2906. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Li, H.; Zhou, C.; Chen, L.; Zhang, L.; Chen, Y.; Zhang, S.; Pan, X.; Huang, S.; Shang, W.; et al. Thymopentin plays a key role in restoring the function of macrophages to alleviate the sepsis process. Int. Immunopharmacol. 2024, 126, 111295. [Google Scholar] [CrossRef]
- Novoselova, E.G.; Lunin, S.M.; Khrenov, M.O.; Novoselova, T.V.; Fesenko, E.E. Involvement of NF-kappaB transcription factor in the antiinflammatory activity of thymic peptides. Dokl. Biol. Sci. 2009, 428, 484–486. [Google Scholar] [CrossRef]
- Zou, Q.; Zhang, L.; Sun, F. The effect of thymopentin on immune function and inflammatory levels in end-stage renal disease patients with maintenance hemodialysis. Am. J. Transl. Res. 2022, 14, 414–420. [Google Scholar]
- Han, Y.R.; Wang, T.H.; Gong, W.P.; Liang, J.Q.; An, H.R. Clinical Efficacy of a Combination of Thymopentin and Antituberculosis Drugs in Treating Drug-Resistant Pulmonary Tuberculosis: Meta Analysis. Ther. Clin. Risk Manag. 2022, 18, 287–298. [Google Scholar] [CrossRef]
- Liu, X.; Xi, R.; Du, X.; Wang, Y.; Cheng, L.; Yan, G.; Lu, H.; Liu, T.; Li, F. Thymopentapeptide Affects T-Cell Subsets by Modulating the Flora of the Skin Surface to Alleviate Psoriasis. Drug Des. Dev. Ther. 2024, 18, 2775–2791. [Google Scholar] [CrossRef]
- Zhang, T.; Qin, X.Y.; Cao, X.; Li, W.H.; Gong, T.; Zhang, Z.R. Thymopentin-loaded phospholipid-based phase separation gel with long-lasting immunomodulatory effects: In vitro and in vivo studies. Acta Pharmacol. Sin. 2019, 40, 514–521. [Google Scholar] [CrossRef]
- Gonser, S.; Crompton, N.E.; Folkers, G.; Weber, E. Increased radiation toxicity by enhanced apoptotic clearance of HL-60 cells in the presence of the pentapeptide thymopentin, which selectively binds to apoptotic cells. Mutat. Res. 2004, 558, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Zhou, T.; Zhang, J.; Xu, J.; Guo, X.; Hu, H.; Zhang, X.; Hu, M.; Li, J.; Yang, W.; et al. Enhanced cell selectivity of hybrid peptides with potential antimicrobial activity and immunomodulatory effect. Biochim. Biophys. Acta-Gen. Subj. 2020, 1864, 129532. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.A.; Thwaite, J.E.; Ulaeto, D.O.; Atkins, T.P.; Atkins, H.S. Design and characterization of novel hybrid antimicrobial peptides based on cecropin A, LL-37 and magainin II. Peptides 2012, 33, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, R.; Zhao, X.; Zhang, J.; Tong, Y.; Abbas, Z.; Li, Z.; Zhang, H.; Si, D.; Wei, X. Hybridization Design and High-Throughput Screening of Peptides with Immunomodulatory and Antioxidant Activities. Int. J. Mol. Sci. 2025, 26, 505. [Google Scholar] [CrossRef]
- Wang, J.; Tang, Y.; Zhao, X.; Ding, Z.; Ahmat, M.; Si, D.; Zhang, R.; Wei, X. Molecular hybridization modification improves the stability and immunomodulatory activity of TP5 peptide. Front. Immunol. 2024, 15, 1472839. [Google Scholar] [CrossRef]
- Tischio, J.P.; Patrick, J.E.; Weintraub, H.S.; Chasin, M.; Goldstein, G. Short in vitro half-life of thymopoietin32–36 pentapeptide in human plasma. Int. J. Pept. Protein Res. 1979, 14, 479–484. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, W.; Wu, C.; Pan, Z.; Yao, G.; Fang, L.; Su, W. Myristic acid-modified thymopentin for enhanced plasma stability and immune-modulating activity. Int. Immunopharmacol. 2017, 47, 88–94. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, X.; Zhang, J.; Cao, J.; Wang, F. Expression of thymosin alpha1-thymopentin fusion peptide in Pichia pastoris and its characterization. Arch. Pharm. Res. 2008, 31, 1471–1476. [Google Scholar] [CrossRef]
- Zhang, L.; Wei, X.; Wang, Z.; Liu, P.; Hou, Y.; Xu, Y.; Su, H.; Koci, M.D.; Yin, H.; Zhang, C. NF-κB activation enhances STING signaling by altering microtubule-mediated STING trafficking. Cell Rep. 2023, 42, 112185. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Z.; Liu, P.; Wei, X.; Zhang, Z.; Fan, S.; Zhang, L.; Han, F.; Song, Y.; Chu, L.; et al. SMPDL3A is a cGAMP-degrading enzyme induced by LXR-mediated lipid metabolism to restrict cGAS-STING DNA sensing. Immunity 2023, 56, 2492–2507. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Saredy, J.; Yuan, Z.; Yang, X.; Wang, H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020, 37, 101759. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, C. A universe of second messengers for cGLR-STING signaling. Immunity 2023, 56, 1975–1977. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Song, E.; Song, Y. Co-administration of lipopolysaccharide and d-galactosamine induces genotoxicity in mouse liver. Sci. Rep. 2021, 11, 1733. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lin, Y.; Miao, L.; Pan, W.; Jiang, W.; Qian, L.; Hao, J.; Xi, B.; Liu, B.; Ge, X. Ferulic acid alleviates lipopolysaccharide-induced acute liver injury in Megalobrama amblycephala. Aquaculture 2021, 532, 735972. [Google Scholar] [CrossRef]
- Zhu, F.; Ma, J.; Li, W.; Liu, Q.; Qin, X.; Qian, Y.; Wang, C.; Zhang, Y.; Li, Y.; Jiang, D.; et al. The orphan receptor Nur77 binds cytoplasmic LPS to activate the non-canonical NLRP3 inflammasome. Immunity 2023, 56, 753–767. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Zhao, T.; Zhang, Q.; Liu, Y.; Sun, B.; Su, G.; Zhao, M. Inhibitory Effects of Walnut (Juglans regia) Peptides on Neuroinflammation and Oxidative Stress in Lipopolysaccharide-Induced Cognitive Impairment Mice. J. Agric. Food. Chem. 2020, 68, 2381–2392. [Google Scholar] [CrossRef]
- Wen, L.; Bi, H.; Zhou, X.; Jiang, Y.; Zhu, H.; Fu, X.; Yang, B. Structure characterization of soybean peptides and their protective activity against intestinal inflammation. Food Chem. 2022, 387, 132868. [Google Scholar] [CrossRef]
- Bamdad, F.; Shin, S.H.; Suh, J.W.; Nimalaratne, C.; Sunwoo, H. Anti-Inflammatory and Antioxidant Properties of Casein Hydrolysate Produced Using High Hydrostatic Pressure Combined with Proteolytic Enzymes. Molecules 2017, 22, 609. [Google Scholar] [CrossRef]
- Liu, Y.; Xia, X.; Xu, L.; Wang, Y. Design of hybrid β-hairpin peptides with enhanced cell specificity and potent anti-inflammatory activity. Biomaterials 2013, 34, 237–250. [Google Scholar] [CrossRef]
- Ma, Z.; Wei, D.; Yan, P.; Zhu, X.; Shan, A.; Bi, Z. Characterization of cell selectivity, physiological stability and endotoxin neutralization capabilities of α-helix-based peptide amphiphiles. Biomaterials 2015, 52, 517–530. [Google Scholar] [CrossRef]
- Decker, A.P.; Mechesso, A.F.; Zhou, Y.; Xu, C.; Wang, G. Hydrophobic diversification is the key to simultaneously increased antifungal activity and decreased cytotoxicity of two ab initio designed peptides. Peptides 2022, 158, 170880. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, S.; Chen, L.; Pan, X.; Wen, Z.; Chen, Y.; Zhang, L.; Liu, J.; Chen, D. Lipopolysaccharide induced intestinal epithelial injury: A novel organoids-based model for sepsis in vitro. Chin. Med. J. 2022, 135, 2232–2239. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, H.; Luo, Y.; Yi, X.; Zhou, Z.; Guo, F.; Yi, L. Lipopolysaccharide-induced active telocyte exosomes alleviate lipopolysaccharide-induced vascular barrier disruption and acute lung injury via the activation of the miRNA-146a-5p/caspase-3 signaling pathway in endothelial cells. Burn. Trauma 2025, 13, tkae74. [Google Scholar] [CrossRef]
- Cui, A.; Huang, T.; Li, S.; Ma, A.; Pérez, J.L.; Sander, C.; Keskin, D.B.; Wu, C.J.; Fraenkel, E.; Hacohen, N. Dictionary of immune responses to cytokines at single-cell resolution. Nature 2024, 625, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Kureshi, C.T.; Dougan, S.K. Cytokines in cancer. Cancer Cell 2025, 43, 15–35. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Ouyang, Y.; Zhang, Y.; Pan, P. Exploration of the role of oxidative stress-related genes in LPS-induced acute lung injury via bioinformatics and experimental studies. Sci. Rep. 2023, 13, 21804. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Chen, H.; Du, D.; Lv, W.; Li, S.; Li, D.; Xu, Z.; Gao, M.; Hu, H.; Liu, D. β-Glucan from Saccharomyces cerevisiae alleviates oxidative stress in LPS-stimulated RAW264.7 cells via Dectin-1/Nrf2/HO-1 signaling pathway. Cell Stress Chaperones 2021, 26, 629–637. [Google Scholar] [CrossRef]
- Averill-Bates, D.A. The antioxidant glutathione. Vitam. Horm. 2023, 121, 109–141. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Han, M.; Matsumoto, A.; Wang, Y.; Thompson, D.C.; Vasiliou, V. Glutathione and Transsulfuration in Alcohol-Associated Tissue Injury and Carcinogenesis. Adv. Exp. Med. Biol. 2018, 1032, 37–53. [Google Scholar] [CrossRef]
- Gil, D.; Rodriguez, J.; Ward, B.; Vertegel, A.; Ivanov, V.; Reukov, V. Antioxidant Activity of SOD and Catalase Conjugated with Nanocrystalline Ceria. Bioengineering 2017, 4, 18. [Google Scholar] [CrossRef]
- Tomasi, M.L.; Ryoo, M.; Yang, H.; Iglesias, A.A.; Ko, K.S.; Lu, S.C. Molecular mechanisms of lipopolysaccharide-mediated inhibition of glutathione synthesis in mice. Free Radic. Biol. Med. 2014, 68, 148–158. [Google Scholar] [CrossRef]
- El-Agamy, D.S.; Shebl, A.M.; Shaaban, A.A. Modulation of d-galactosamine/lipopolysacharride-induced fulminant hepatic failure by nilotinib. Hum. Exp. Toxicol. 2018, 37, 51–60. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, L.; Zhang, R.; Wu, R.; Si, D.; Ahmad, B.; Petitte, J.N.; Mozdziak, P.E.; Li, Z.; Guo, H.; et al. A highly efficient hybrid peptide ameliorates intestinal inflammation and mucosal barrier damage by neutralizing lipopolysaccharides and antagonizing the lipopolysaccharide-receptor interaction. Fed. Am. Soc. Exp. Biol. J. 2020, 34, 16049–16072. [Google Scholar] [CrossRef]
- Sun, Y.; Shang, D. Inhibitory Effects of Antimicrobial Peptides on Lipopolysaccharide-Induced Inflammation. Mediat. Inflamm. 2015, 2015, 167572. [Google Scholar] [CrossRef]
- Jiang, Y.; Yan, S.; Zhang, S.; Wang, M.; Diao, W.; Li, J.; Fang, X.; Yin, H. Discovery of pyrazolo[1,5-a]pyrimidine derivatives targeting TLR4−TLR4∗ homodimerization via AI-powered next-generation screening. Eur. J. Med. Chem. 2024, 280, 116945. [Google Scholar] [CrossRef] [PubMed]
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef] [PubMed]
- Menu, N.M.I.; Arachchilage, H.M.K.W.; Lee, M.H.; Kang, C.H.; Tae, L.K.; Hyun, C.Y.; Lee, S.; Kim, G.Y. Acertannin attenuates LPS-induced inflammation by interrupting the binding of LPS to the TLR4/MD2 complex and activating Nrf2-mediated HO-1 activation. Int. Immunopharmacol. 2022, 113, 109344. [Google Scholar] [CrossRef] [PubMed]
- Garate, J.A.; Oostenbrink, C. Lipid A from lipopolysaccharide recognition: Structure, dynamics and cooperativity by molecular dynamics simulations. Proteins 2013, 81, 658–674. [Google Scholar] [CrossRef]
- Rahman, I.; Macnee, W. Regulation of redox glutathione levels and gene transcription in lung inflammation: Therapeutic approaches. Free Radic. Biol. Med. 2000, 28, 1405–1420. [Google Scholar] [CrossRef]
- Akter, K.A.; Sharma, S.; Sifat, A.E.; Zhang, Y.; Patel, D.K.; Cucullo, L.; Abbruscato, T.J. Metformin ameliorates neuroinflammatory environment for neurons and astrocytes during in vitro and in vivo stroke and tobacco smoke chemical exposure: Role of Nrf2 activation. Redox Biol. 2024, 75, 103266. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, T.; Kumar, N.; Chawla, M.; Philpott, D.J.; Basak, S. The NF-κB signaling system in the immunopathogenesis of inflammatory bowel disease. Sci. Signal. 2024, 17, eadh1641. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB signaling in inflammation and cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef] [PubMed]



| Peptides | Sequence | Molecular Weight | Net Charge a | H b |
|---|---|---|---|---|
| DEFB126 | NWYVKKCLNDVGICKKKCKPEEMHVKNGWAMCGKQRDCCVPAD | 4957.9 | +4 | −0.638 |
| TP5 | RKDVY | 679.8 | +1 | −1.680 |
| DTP | NWYVKKCLNDVGICKKKCKPEEMHVKNGWAMCGKQRDCCRKDVY | 5237.2 | +6 | −1.483 |
| Peptides | DEFB126 | TP5 | DTP |
|---|---|---|---|
| t1/2 (min) | 324.39 ± 53.09 a | 1.57 ± 0.35 b | 349 ± 69.17 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Zhao, X.; Ding, Z.; Wang, J.; Zhang, J.; Zhou, Y.; Ahmat, M.; Wang, H.; Zhu, Y.; Ahmad, B.; et al. Novel Hybrid Peptide DEFB126 (1-39)-TP5 Inhibits LPS-Induced Inflammatory Responses and Oxidative Stress by Neutralizing LPS and Blocking the TLR4/MD2-NFκB Signaling Axis. Antioxidants 2025, 14, 1117. https://doi.org/10.3390/antiox14091117
Tang Y, Zhao X, Ding Z, Wang J, Zhang J, Zhou Y, Ahmat M, Wang H, Zhu Y, Ahmad B, et al. Novel Hybrid Peptide DEFB126 (1-39)-TP5 Inhibits LPS-Induced Inflammatory Responses and Oxidative Stress by Neutralizing LPS and Blocking the TLR4/MD2-NFκB Signaling Axis. Antioxidants. 2025; 14(9):1117. https://doi.org/10.3390/antiox14091117
Chicago/Turabian StyleTang, Yuan, Xuelian Zhao, Zetao Ding, Junyong Wang, Jing Zhang, Yichen Zhou, Marhaba Ahmat, Hao Wang, Yang Zhu, Baseer Ahmad, and et al. 2025. "Novel Hybrid Peptide DEFB126 (1-39)-TP5 Inhibits LPS-Induced Inflammatory Responses and Oxidative Stress by Neutralizing LPS and Blocking the TLR4/MD2-NFκB Signaling Axis" Antioxidants 14, no. 9: 1117. https://doi.org/10.3390/antiox14091117
APA StyleTang, Y., Zhao, X., Ding, Z., Wang, J., Zhang, J., Zhou, Y., Ahmat, M., Wang, H., Zhu, Y., Ahmad, B., Abbas, Z., Si, D., Zhang, R., & Wei, X. (2025). Novel Hybrid Peptide DEFB126 (1-39)-TP5 Inhibits LPS-Induced Inflammatory Responses and Oxidative Stress by Neutralizing LPS and Blocking the TLR4/MD2-NFκB Signaling Axis. Antioxidants, 14(9), 1117. https://doi.org/10.3390/antiox14091117







