Physiological and Transcriptome Analyses Offer Insights into Revealing the Mechanisms of Red Tilapia (Oreochromis spp.) in Response to Carbonate Alkalinity Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Culture and Experimental Design
2.2. Collection of Experimental Samples
2.3. Morphological Analysis of Gills
2.4. Enzyme Activity Assays of Gills
2.5. Transcriptome Sequencing of Gills
2.5.1. RNA Extraction and Transcriptome Sequencing
2.5.2. Data Assembly and Identification of Differentially Expressed Genes
2.5.3. Weighted Gene Co-Expression Network Analysis (WGCNA)
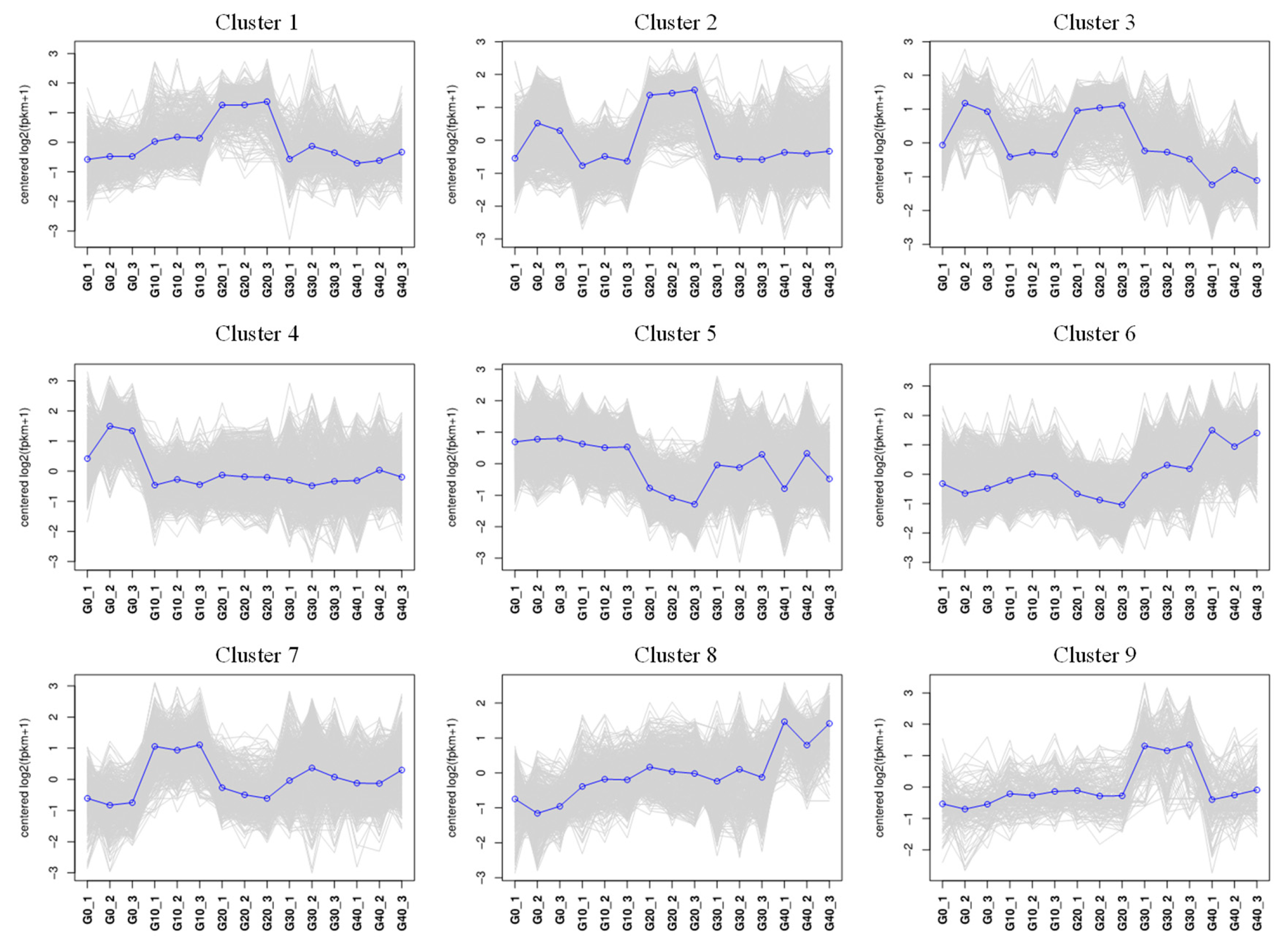
2.5.4. Fuzzy C-Means Cluster Analysis
2.5.5. Quantitative Real-Time PCR (qPCR) Verification
2.6. Statistical Analysis
3. Results
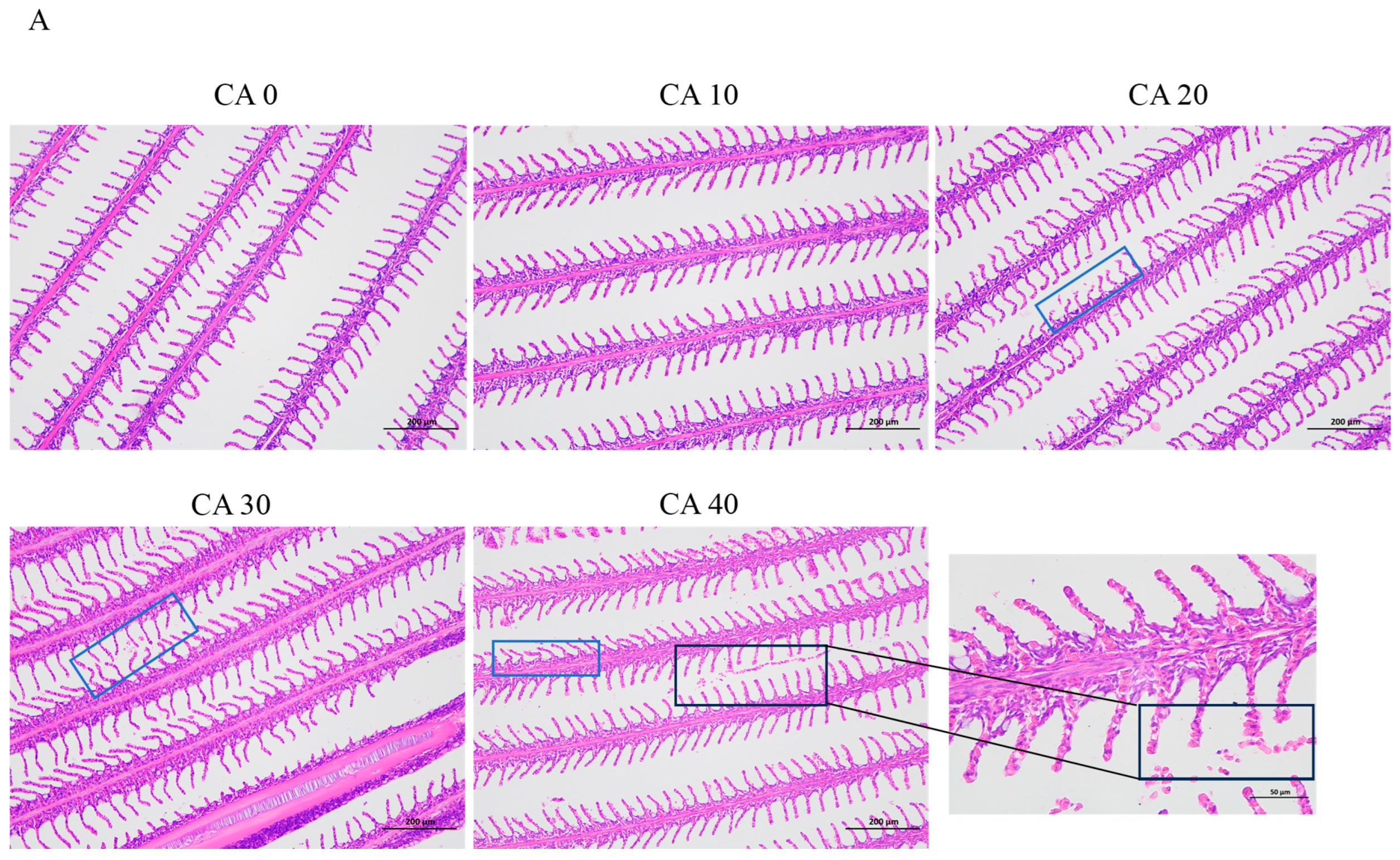
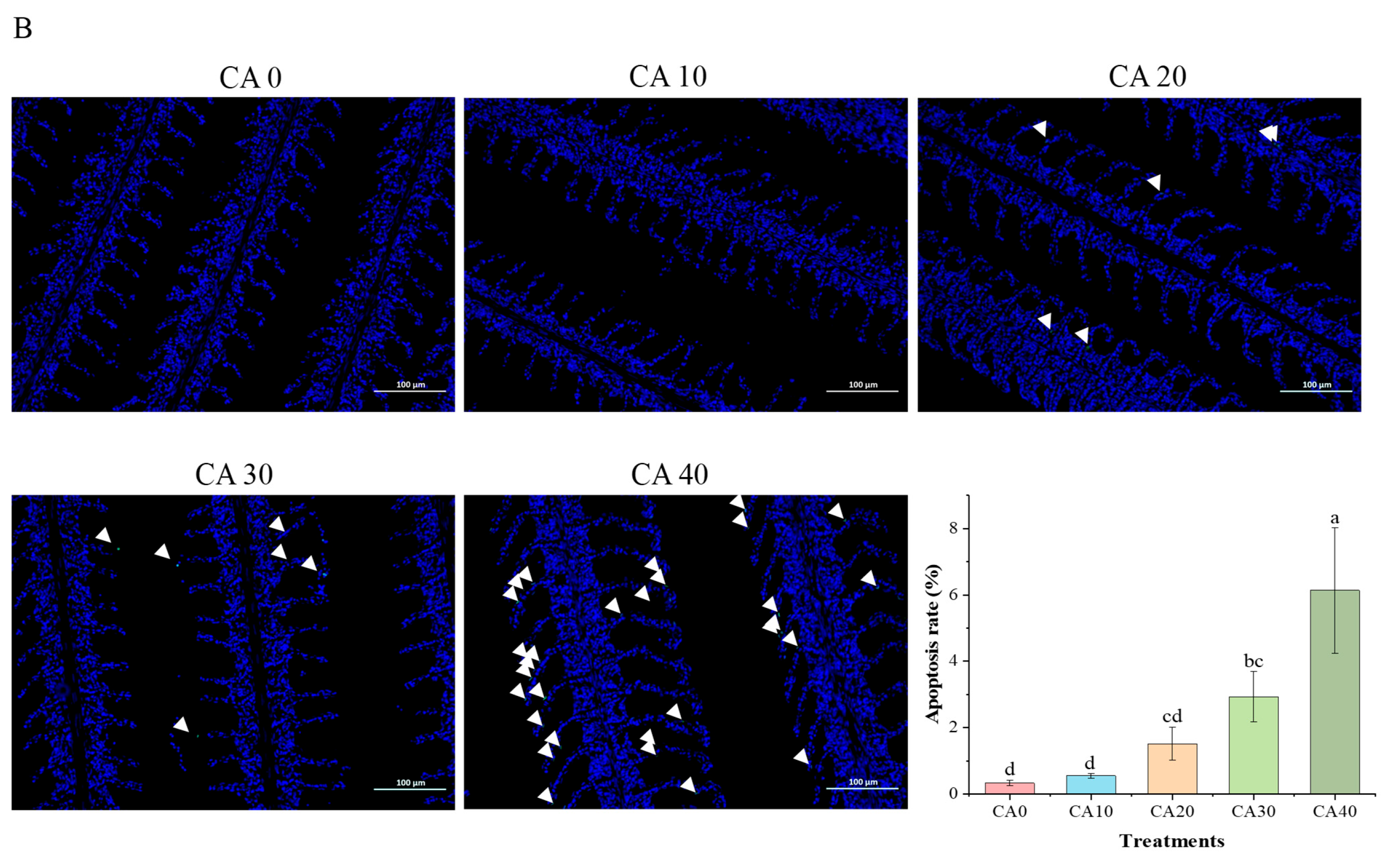
3.1. Histopathological Observations
3.2. Enzymatic Activities Assays
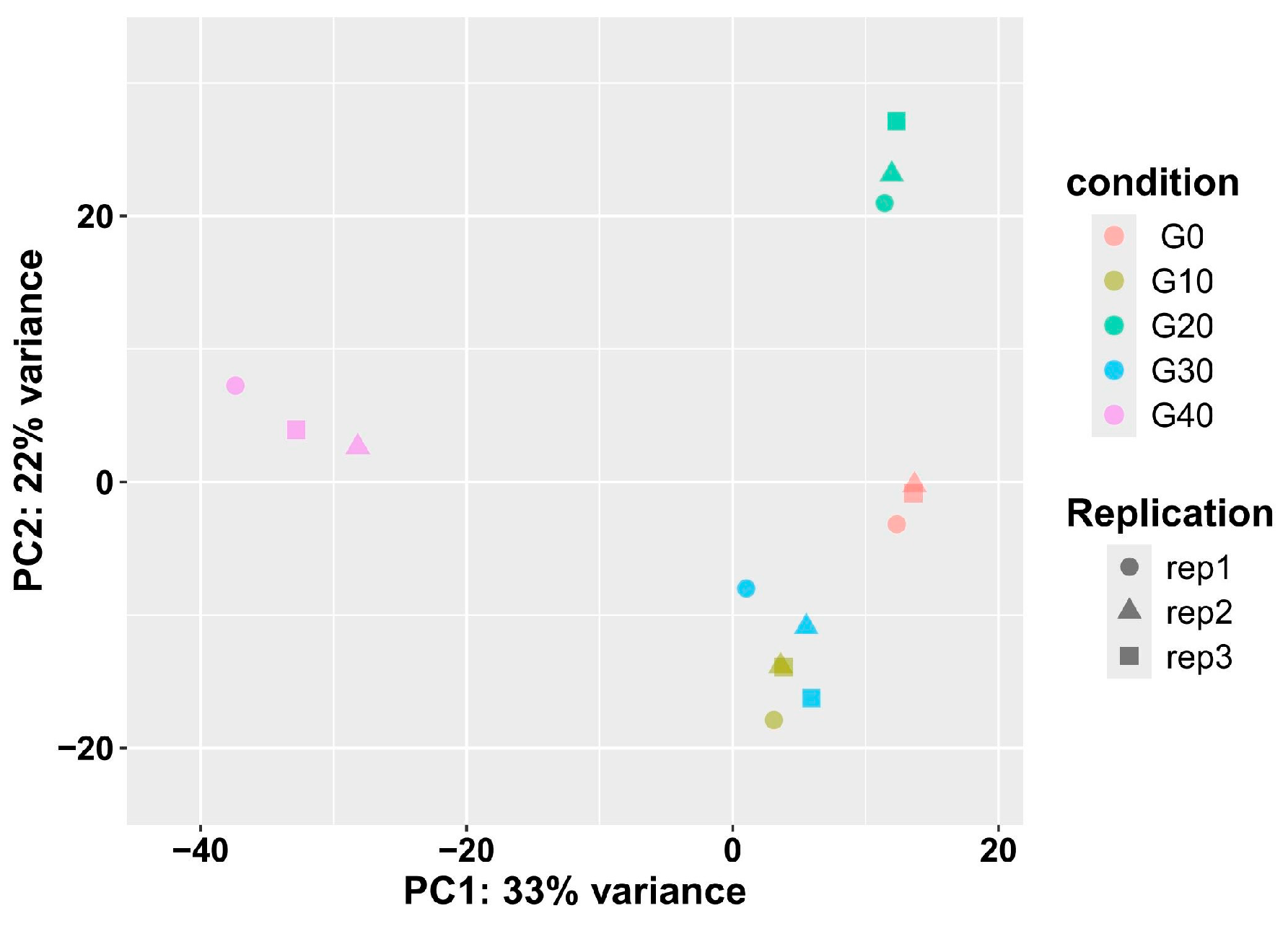
3.3. Overview of Sequencing Data
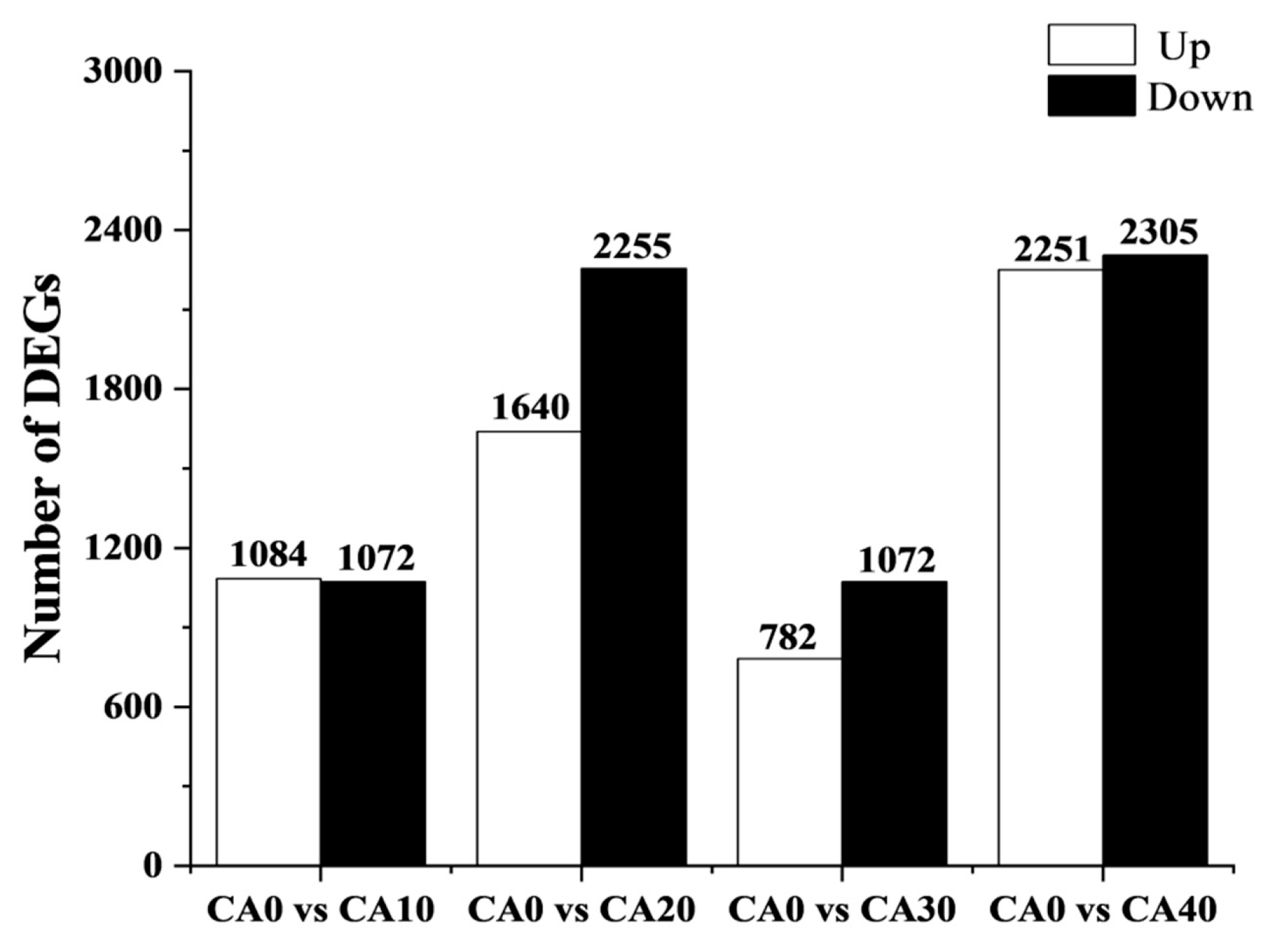
3.4. Analysis of the Differentially Expressed Genes
3.5. Gene Co-Expression Network Analysis
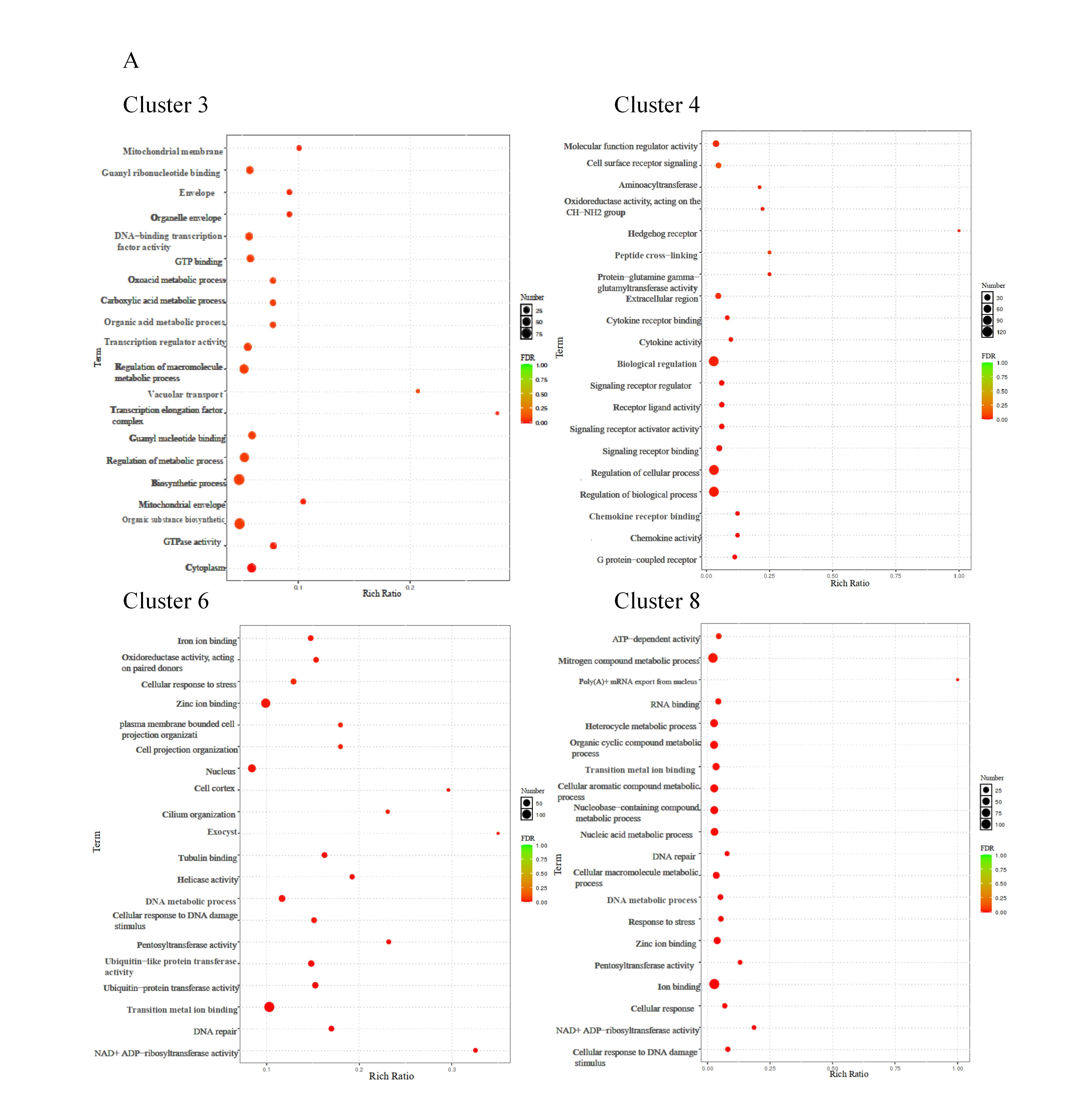
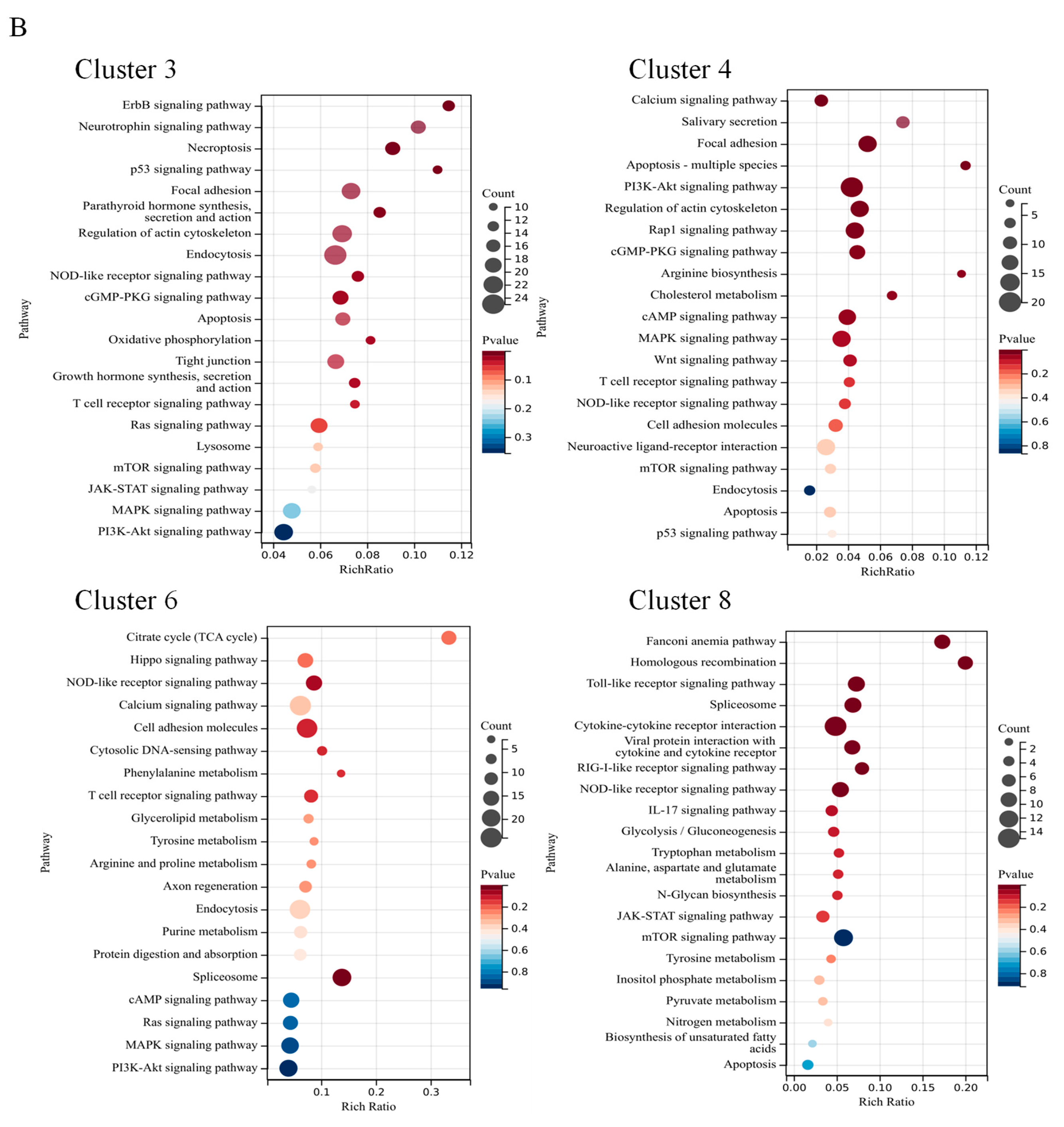
3.6. Correlation of Gene Expression Patterns with the CA Concentration
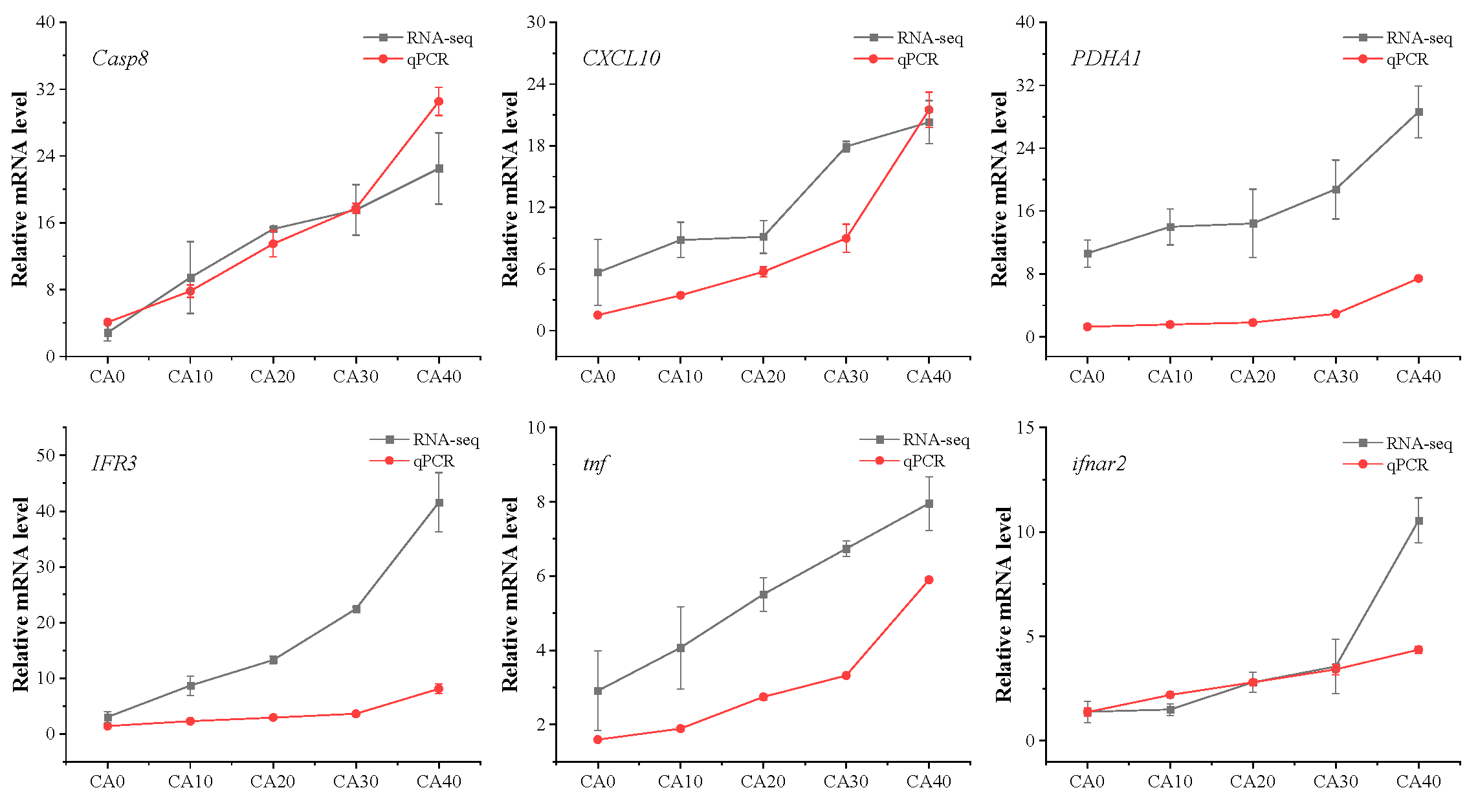
3.7. qPCR Verification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Q.R.; Zhang, F.; Li, R.N.; Li, E.C.; Qin, J.G.; Chen, L.Q.; Wang, X.D. Growth performance, antioxidant capacity, intestinal microbiota, and metabolomics analysis of Nile tilapia (Oreochromis niloticus) under carbonate alkalinity stress. Aquaculture 2025, 595, 741675. [Google Scholar] [CrossRef]
- Hua, J.X.; Tao, Y.F.; Lu, S.Q.; Li, Y.; Dong, Y.L.; Jiang, B.J.; Xi, B.W.; Qiang, J. Integration of transcriptome, histopathology, and physiological indicators reveals regulatory mechanisms of largemouth bass (Micropterus salmoides) in response to carbonate alkalinity stress. Aquaculture 2025, 596, 741883. [Google Scholar] [CrossRef]
- Lin, T.T.; Lai, Q.F.; Yao, Z.L.; Lu, J.X.; Zhou, K.; Wang, H. Combined effects of carbonate alkalinity and pH on survival, growth and haemocyte parameters of the Venus clam Cyclina sinensis. Fish Shellfish Immun. 2013, 35, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zheng, J.B.; Xu, Y.; Li, F.; Liu, S.L.; Jiang, W.P.; Chi, M.L.; Zhang, H.Q. Changes in the physiological indicators and gene expression for grass carp (Ctenopharyngodon idella) in response to alkaline stress. Aquac. Int. 2025, 33, 329. [Google Scholar] [CrossRef]
- Wang, Y.S.; Gonzalez, R.J.; Patrick, M.L.; Grosell, M.; Zhang, C.G.; Feng, Q.; Du, J.Z.; Walsh, P.J.; Wood, C.M. Unusual physiology of scale-less carp, Gymnocypris przewalskii, in Lake Qinghai: A high altitude alkaline saline lake. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 134, 409–421. [Google Scholar] [CrossRef]
- Liu, Y.J.; Yao, M.Z.; Li, S.W.; Wei, X.F.; Ding, L.; Han, S.C.; Wang, P.; Lv, B.C.; Chen, Z.X.; Sun, Y.C. Integrated application of multi-omics approach and biochemical assays provides insights into physiological responses to saline-alkaline stress in the gills of crucian carp (Carassius auratus). Sci. Total Environ. 2022, 822, 153622. [Google Scholar] [CrossRef]
- Yao, Z.L.; Lai, Q.F.; Zhou, K.; Rizalita, R.E.; Wang, H. Developmental biology of medaka fish (Oryzias latipes) exposed to alkalinity stress. J. Appl. Ichthyol. 2010, 26, 397–402. [Google Scholar] [CrossRef]
- Zhang, R.; Zhao, Z.G.; Li, M.S.; Luo, L.; Wang, S.H.; Guo, K.; Xu, W. Effects of saline-alkali stress on the tissue structure, antioxidation, immunocompetence and metabolomics of Eriocheir sinensis. Sci. Total Environ. 2023, 871, 162109. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wen, H.S.; Liu, Y.; Qin, X.; Sun, D.L.; Zhang, C.; Zhang, K.Q.; Zhang, M.Z.; Li, J.F.; Li, Y. Gill histological and transcriptomic analysis provides insights into the response of spotted sea bass (Lateolabrax maculatus) to alkalinity stress. Aquaculture 2023, 563, 738945. [Google Scholar] [CrossRef]
- Sun, Y.C.; Wu, S.; Du, N.N.; Song, Y.; Xu, W. High-throughput metabolomics enables metabolite biomarkers and metabolic mechanism discovery of fish in response to alkalinity stress. RSC Adv. 2018, 8, 14983–14990. [Google Scholar] [CrossRef]
- Su, H.H.; Ma, D.M.; Zhu, H.P.; Liu, Z.G.; Gao, F.Y. Transcriptomic response to three osmotic stresses in gills of hybrid tilapia (Oreochromis mossambicus female × O. urolepis hornorum male). BMC Genom. 2020, 21, 110. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Randall, D.J.; Tsui, T.K.N. Tribute to R. G. Boutilier: Acid-base transfer across fish gills. J. Exp. Biol. 2006, 209, 1179–1184. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.M.; Bergman, H.L.; Bianchini, A.; Laurent, P.; Maina, J.; Johannsson, O.E.; Bianchini, L.F.; Chevalier, C.; Kavembe, G.D.; Papah, M.B.; et al. Transepithelial potential in the Magadi tilapia, a fish living in extreme alkalinity. J. Comp. Physiol. B 2012, 182, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.A.; Iwama, G.K.; Wood, C.M. Ammonia and urea excretion in Lahontan cutthroat trout (Oncorhynchus clarki henshawi) adapted to the highly alkaline Pyramid Lake. J. Exp. Biol. 1993, 175, 153–172. [Google Scholar] [CrossRef]
- Shen, L.; Hao, Z.R.; Zhou, K.; Lai, Q.F.; Wang, H.P.; Yao, Z.L.; Lin, T. Tolerability studies of Carassius auratus gibelio to salinity and carbonate alkalinity. Mar. Fish. 2014, 36, 445–452. [Google Scholar]
- Song, L.Y.; Zhao, Y.; Song, Y.D.; Zhao, L.L.; Ma, C.X.; Zhao, J.L. Effects of saline-alkaline water on growth performance, nutritional processing, and immunity in Nile tilapia (Oreochromis niloticus). Aquaculture 2011, 544, 737036. [Google Scholar] [CrossRef]
- A, L.L.; Zhang, Y.J.; Xu, B.K.; Zhang, H.C.; Li, Y.X.; Wang, L.; Liang, J.; Zhou, W.G.; Feng, Z.H.; Zhang, H. Comprehensive analyses of annexins in naked carp (Gymnocypris przewalskii) unveil their roles in saline-alkaline stress. Aquaculture 2024, 579, 740175. [Google Scholar] [CrossRef]
- Marshall, W.S.; Grosell, M. Ion transport, osmoregulation, and acid-base balance. In The Physiology of Fishes; CRC Press: Boca Raton, FL, USA, 2005; pp. 177–230. [Google Scholar]
- Evans, D.H. (Ed.) Osmotic and Ionic Regulation: Cells and Animals, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Ni, Q.; Li, W.Q.; Liang, X.F.; Liu, J.L.; Ge, H.X.; Dong, Z.G. Gill transcriptome analysis reveals the molecular response to the acute low-salinity stress in Cyclina sinensis. Aquac. Rep. 2021, 19, 100564. [Google Scholar] [CrossRef]
- Viviana, L.; Fernanda, B.I.; André, S.L.; Adalto, B. Effect of salinity on survival, growth and biochemical parameters in juvenile Lebranch mullet Mugil liza (Perciformes: Mugilidae). Neotrop. Ichthyol. 2015, 13, 447–452. [Google Scholar] [CrossRef]
- Ma, Z.H.; Guo, H.Y.; Zheng, P.L.; Wang, L.; Jiang, S.G.; Zhang, D.C.; Qin, J.G. Effect of salinity on the rearing performance of juvenile golden pompano Trachinotus ovatus (Linnaeus 1758). Aquac. Res. 2016, 47, 1761–1769. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, J.S.; Yan, M.Z.; Tang, S.S.; Wang, X.D.; Qin, J.G.; Chen, L.Q.; Li, E.C. Inulin alleviates hypersaline-stress induced oxidative stress and dysbiosis of gut microbiota in Nile tilapia (Oreochromis niloticus). Aquaculture 2020, 529, 735681. [Google Scholar] [CrossRef]
- Li, Y.; Gao, P.C.; Zhou, K.; Sun, Z.; Qin, H.C.; Lai, Q.F. Effects of saline and alkaline stresses on the survival, growth, and physiological responses in juvenile mandarin fish (Siniperca chuatsi). Aquaculture 2024, 591, 741143. [Google Scholar] [CrossRef]
- Liu, W.; Xu, C.; Li, Z.; Chen, L.Q.; Wang, X.D.; Li, E.C. Reducing Dietary Protein Content by Increasing Carbohydrates Is More Beneficial to the Growth, Antioxidative Capacity, Ion Transport, and Ammonia Excretion of Nile Tilapia (Oreochromis niloticus) under Long-Term Alkalinity Stress. Aquac. Nutr. 2023, 16, 9775823. [Google Scholar] [CrossRef]
- Zhang, L.S.; Zhao, Y.; Xiong, W.K.; Cao, M.H.; Zhao, J.L.; Wu, J.K.; Zhang, J.L. Lysine deacetylase inhibitors (KDACi) enhance the adaptability of tilapia (Oreochromis niloticus) to carbonate alkalinity stress. Aquaculture 2025, 604, 742492. [Google Scholar] [CrossRef]
- Jinagool, P.L.; Wipassa, V.; Chaiyasing, R.; Chukanhom, K.; Aengwanich, W. Effect of increasing ambient temperature on physiological changes, oxidative stress, nitric oxide, total antioxidant power, and mitochondrial activity of Nile tilapia (Oreochromis niloticus Linn.). Aquaculture 2024, 589, 741017. [Google Scholar] [CrossRef]
- Wen, J.; Chen, S.L.; Xu, W.Y.; Zheng, G.D.; Zou, S.M. Effects of high NaHCO3 alkalinity on growth, tissue structure, digestive enzyme activity, and gut microflora of grass carp juvenile. Environ. Sci. Pollut. Res. Int. 2023, 30, 85223–85236. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; Tao, Y.F.; Zhu, J.H.; Lu, S.Q.; Cao, Z.M.; Ma, J.L.; He, J.; Xu, P. Effects of heat stress on follicular development and atresia in Nile tilapia (Oreochromis niloticus) during one reproductive cycle and its potential regulation by autophagy and apoptosis. Aquaculture 2022, 555, 738171. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.H.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Andrew, M.; Owen, O.; Nitin, S.B.; Jonathan, T.W.; Daniel, R.; Nada, A.; Benno, S.; Trey, I. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Futschik, M.E. Mfuzz: A software package for soft clustering of microarray data. Bioinformation 2007, 2, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, L.; Jiang, G.; Li, Z.; Bi, Y.H.; Zhou, Z.G. Characterization of a novel γ-type carbonic anhydrase, Sjγ-CA2, in Saccharina japonica: Insights into carbon concentration mechanism in macroalgae. Int. J. Biol. Macromol. 2024, 263, 130506. [Google Scholar] [CrossRef]
- Fan, Z.; Wu, D.; Li, J.N.; Li, C.H.; Zheng, X.H.; Wang, L.S. Phosphorus nutrition in Songpu Mirror carp (Cyprinus carpio Songpu) during chronic carbonate alkalinity stress: Effects on growth, intestinal immunity, physical barrier function, and intestinal microflora. Front. Immunol. 2022, 13, 900793. [Google Scholar] [CrossRef]
- Wang, L.Y.; Tian, Y.; Wen, H.S.; Yu, P.; Liu, Y.; Qi, X.; Gao, Z.C.; Zhang, K.Q.; Li, Y. Slc4 Gene Family in Spotted Sea Bass (Lateolabrax maculatus): Structure, Evolution, and Expression Profiling in Response to Alkalinity Stress and Salinity Changes. Genes 2020, 11, 1271. [Google Scholar] [CrossRef]
- Gao, S.; Chan, Y.M.; Zhao, X.F.; Sun, B.; Zhang, L.M.; Liang, L.Q.; Dong, Z.G. The effect of different bicarbonate alkalinity on the gill structure of amur ide (Leuciscus waleckii). Acta Hydrobiol. Sin. 2020, 44, 736–743. [Google Scholar] [CrossRef]
- Souza, M.S.; Modenutti, B.E.; Balseiro, E.G. Antioxidant Defences in Planktonic Crustaceans Exposed to Different Underwater Light Irradiances in Andean Lakes. Water Air Soil Pollut. 2007, 183, 49–57. [Google Scholar] [CrossRef]
- Lawniczak, M.; Romestaing, C.; Roussel, D.; Maazouzi, C.; Renault, D.; Hervant, F. Preventive antioxidant responses to extreme oxygen level fluctuation in a subterranean crustacean. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 165, 299–303. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide Radical and Superoxide Dismutases. Annu. Rev. Biochem. 1995, 64, 97–112. [Google Scholar] [CrossRef]
- Weihrauch, D.; Joseph, G.; Allen, G.J.P. Ammonia excretion in aquatic invertebrates: New insights and questions. J. Exp. Biol. 2018, 221, jeb169219. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.J.P.; Shin, D.S.; Getzoff, E.D.; Tainer, J.A. The structural biochemistry of the superoxide dismutases. Biochim. Biophys. Acta 2010, 1804, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Toppo, S.; Orian, L. The glutathione peroxidase family: Discoveries and mechanism. Free Radic. Biol. Med. 2022, 187, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Panday, S.; Talreja, R.; Kavdia, M. The role of glutathione and glutathione peroxidase in regulating cellular level of reactive oxygen and nitrogen species. Microvasc. Res. 2020, 131, 104010. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Dyk, J.C.V.; Pieterse, G.M.; Vuren, J.H.J.V. Histological changes in the liver of Oreochromis mossambicus (Cichlidae) after exposure to cadmium and zinc. Ecotoxicol. Environ. Saf. 2007, 66, 432–440. [Google Scholar] [CrossRef]
- Li, D.; Chen, Q.; Cao, J.L.; Chen, H.X.; Li, L.X.; Cedergreen, N.; Xie, H.B.; Xie, L.T. The chronic effects of lignin-derived bisphenol and bisphenol A in Japanese medaka Oryzias latipes. Aquat. Toxicol. 2016, 170, 199–207. [Google Scholar] [CrossRef]
- Jezierska, B.; Ługowska, K.; Witeska, M. The effects of heavy metals on embryonic development of fish (a review). Fish Physiol. Biochem. 2009, 35, 625–640. [Google Scholar] [CrossRef]
- Queiroz, E.C.; da Silva, B.F.; Salla, R.V. Genotoxic Damages and Bioaccumulation of Cadmium in Geophagus Brasiliensis (Quoy & Gaimard, 1824). Bull. Environ. Contam. Toxicol. 2019, 102, 181–185. [Google Scholar] [CrossRef]
- Annabi, A.; Said, K.; Messaoudi, I. Cadmium: Bioaccumulation, histopathology and detoxifying mechanisms in fish. Am. J. Res. Commun. 2013, 1, 60–79. [Google Scholar]
- Wang, C.; Dong, X.S.; Yang, Y.Y.; Xu, G.J.; Wu, M.M.; Yan, F.J.; Zhang, L.G.; An, L.; Fu, P.S.; Wang, X.R.; et al. Metabolites in the TCA Cycle Promote Resistance to Chloramphenicol of Edwardsiella tarda. J. Proteome Res. 2021, 20, 972–981. [Google Scholar] [CrossRef]
- Yang, Z.G.; Zhou, J.Y.; Wei, B.H.; Cheng, Y.X.; Zhang, L.; Zhen, X.M. Comparative trans-criptome analysis reveals osmotic-regulated genes in the gill of Chinese mitten crab (Eriocheir sinensis). PLoS ONE 2019, 14, e0210469. [Google Scholar] [CrossRef]
- Hardie, G.D. AMPK: A key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. 2008, 32, S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Hardie, D.G. AMPK: Sensing Glucose as well as Cellular Energy Status. Cell Metab. 2018, 27, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Vancura, A.; Nagar, S.; Kaur, P.; Bu, P.; Bhagwat, M.; Vancurova, I. Reciprocal Regulation of AMPK/SNF1 and Protein Acetylation. Int. J. Mol. Sci. 2018, 19, 3314. [Google Scholar] [CrossRef] [PubMed]
- Dziewulska, A.; Dobosz, A.M.; Dobrzyn, A.; Smolinska, A.; Kolczynska, K.; Ntambi, J.M.; Dobrzyn, P. SCD1 regulates the AMPK/SIRT1 pathway and histone acetylation through changes in adenine nucleotide metabolism in skeletal muscle. J. Cell. Physiol. 2020, 235, 1129–1140. [Google Scholar] [CrossRef]
- Hardie, D.G.; Ross, F.A.; Hawley, S.A. AMPK: A nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012, 13, 251–262. [Google Scholar] [CrossRef]
- Salminen, A.; Hyttinen, J.M.T.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signalingand inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef]
- Qin, Z.; Ge, Q.; Wang, J.J.; Li, M.D.; Zhang, X.H.; Li, J.; Li, J.T. Metabolomic responses based on transcriptome of the hepatopancreas in Exopalaemon carinicauda under carbonate alkalinity stress. Ecotoxicol. Environ. Saf. 2023, 268, 115723. [Google Scholar] [CrossRef]
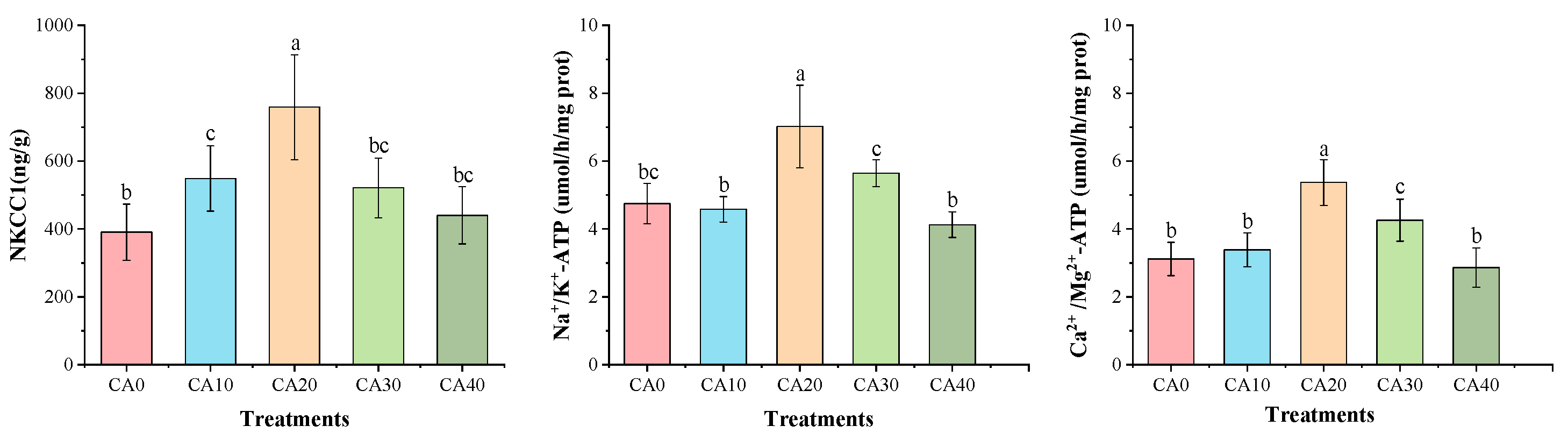



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, W.; Wang, W.; Hua, J.; Xu, D.; Qiang, J. Physiological and Transcriptome Analyses Offer Insights into Revealing the Mechanisms of Red Tilapia (Oreochromis spp.) in Response to Carbonate Alkalinity Stress. Antioxidants 2025, 14, 1112. https://doi.org/10.3390/antiox14091112
Ye W, Wang W, Hua J, Xu D, Qiang J. Physiological and Transcriptome Analyses Offer Insights into Revealing the Mechanisms of Red Tilapia (Oreochromis spp.) in Response to Carbonate Alkalinity Stress. Antioxidants. 2025; 14(9):1112. https://doi.org/10.3390/antiox14091112
Chicago/Turabian StyleYe, Wei, Wen Wang, Jixiang Hua, Dongpo Xu, and Jun Qiang. 2025. "Physiological and Transcriptome Analyses Offer Insights into Revealing the Mechanisms of Red Tilapia (Oreochromis spp.) in Response to Carbonate Alkalinity Stress" Antioxidants 14, no. 9: 1112. https://doi.org/10.3390/antiox14091112
APA StyleYe, W., Wang, W., Hua, J., Xu, D., & Qiang, J. (2025). Physiological and Transcriptome Analyses Offer Insights into Revealing the Mechanisms of Red Tilapia (Oreochromis spp.) in Response to Carbonate Alkalinity Stress. Antioxidants, 14(9), 1112. https://doi.org/10.3390/antiox14091112





