Management and Prevention of Neurodegenerative Disorders: Can Antioxidant-Rich Dietary Interventions Help?
Abstract
1. Introduction
2. Pharmacological Considerations in Brain Disorders
2.1. Mitochondrial Dysfunction in Brain Disorders
2.2. Mitophagy
2.3. Glymphatic Clearance for Brain Health
2.4. Neuroinflammation
2.5. Brain–Body Interactions in Brain Health
2.5.1. Gut–Brain Axis, Microbiome, and Virome
2.5.2. Heart–Brain Axis
2.5.3. Other Organs
2.6. Vagus Nerve Stimulation Impacted by Food
2.7. Ferroptosis
2.8. Impact of Caloric Restriction on Brain Health
3. Diets and Their Mechanism in Preventing Neurodegenerative Diseases
3.1. Foods That Worsen Neurodegenerative Diseases
3.2. Diets for Prevention and Management
3.2.1. Paleolithic Diet
3.2.2. Mediterranean Diet
3.2.3. DASH Diets
3.2.4. MIND Diet
3.2.5. Ketogenic Diet
3.2.6. Modified Atkins Diet
3.2.7. Swank Diet
4. Clinical Studies on the Management of Neurodegenerative Disease with Diet Intervention
4.1. Parkinson’s Disease
| Food as Medicine | Mechanism of Action | Bioactive Compounds/Chemical Constituents | Outcome/Results/Efficacy | Ref. |
|---|---|---|---|---|
| Mediterranean diet containing vegetables (6 servings daily), fruits (3 servings daily), legumes (3–4 servings weekly), unsaturated fatty acids (olive oil), and fish (5–6 servings weekly). | Antioxidants and anti-inflammatory effects. | Vitamin C, vitamin E, β-carotenoids, protein, polyphenols, and omega-3-fatty acids. | Improves total antioxidant serum levels and prevent neurodegeneration. | [241] |
| Dietary antioxidants. | Counteract oxygen free radicals, thus decreasing oxidative damage. | Vitamin E and β-carotenoids. | A diet containing antioxidants is associated with fewer incidences of PD. | [242] |
| Nutritional Diet containing whey protein twice daily for 30 days. | Potentiate Muscle protein synthesis. | Leucine and vitamin D. | Increases muscle mass and improves motor function. | [252] |
| Probiotics. | Rehabilitate gut microbiota, and promote bowel. | Lactobacilli and Bifidobacterium. | Improves constipation and alleviates symptoms of PD. | [249] |
| Probiotics (2 × 109 CFU/g for 12 weeks). | Antioxidants and anti-inflammatory; modulate insulin resistance. | L. reuteri, L. fermentum, L. acidophilus, and B. bifidum. | Potentiate clinical and biochemical profiles in people with PD. | [250] |
| Mediterranean diet. | Antioxidant, anti-inflammatory, neuroprotective, and improve gut–brain signaling pathway | Protein, carbohydrate, lipid, and alcohol. | Consumption of the Mediterranean diet reduces early symptoms of prodromal PD. | [247] |
| Mediterranean diet. | Improve bowel movement and intestinal barrier function and decrease inflammation. | Short-chain fatty acids (butyrate, propionate, and acetate). | Improves gut microbiota, constipation, and symptoms of PD. | [246] |
| Ovo-lacto vegetarian diet containing ghee, vegetables, fruits, nuts, seeds, milk, and egg products (3 meals per day). | Neuroprotective and anti-inflammatory. | Short-chain fatty acids (butyric acid and propionate). | Improves gut microbiota and bowel clearance, reducing motor symptoms associated with PD. | [251] |
4.2. Alzheimer’s Disease
| Culinary Medicine | Mechanism | Active Chemical | Outcome/Result/Efficacy | Ref. |
|---|---|---|---|---|
| Mediterranean diet containing 40 mL of coconut oil (20 mL during breakfast and lunch each) for only 21 consecutive days | Medium-chain triglycerides are metabolized to produce ketone bodies, which exerts neuroprotective effects. | Medium-chain triglyceride containing lauric acid, caprylic acid, and linolenic acid. | Effective on temporal orientation, visuospatial memory, and semantic memory. | [271] |
| High glycemic diet containing potatoes, starchy vegetables, refined grains, whole grains, lean and fatty meats, and added sugar (negative effect) | Impaired glucose metabolism, insulin resistance, and type 2 diabetes are factors for amyloid deposition in the brain. | Sugar and carbohydrate. | In cognitively normal adults, a high-glycemic diet raises global and regional cerebral amyloid burden. | [272] |
| Modified Atkins contains fat (large amount), carbohydrates (low amount), and protein (moderate). | Promote metabolic ketosis. | Carbohydrates, fat, and proteins. | Enhances cognition, episodic memory, and mood Improves liveliness in patients with AD. | [260] |
| Supplement containing probiotics and selenium: selenium (200 μg/day) with a probiotic (2 × 109 CFU/day each) for 12 weeks. | Reduces tau protein, enhances gut bacterial composition, enhances blood levels of GSH and TAC, and decreases hs-CRP protein levels. | Probiotics containing Lactobacillus acidophilus, Bifidobacterium bifidum, and Bifidobacterium longum. | Significant increase in cognitive function. | [250] |
| Vitamin E supplementation. | Suppresses cyclooxygenase, associates with antioxidant and anti-inflammatory activity, and inhibits secretase, which is responsible for amyloid production. | Alpha, beta, and gamma tocopherols. | Meta-analyses indicate that individuals with low serum levels of vitamin E are prone to the development of AD. | [266] |
| Lipid-based diet containing Souvenaid (125 mL per day). | Neuroprotective. | DHA, eicosapentaenoic acid, choline, uridine monophosphate, vitamin B12, B6, C, and E, folic acid, phospholipids, and selenium. | Improves cognition, reduces brain atrophy, and slows disease progression in AD. | [267] |
| Mediterranean diet containing extra virgin olive 20–30 g per day. | Neuroprotective. | Extra virgin olive oil polyphenols (280 ppm). | Individuals who adhere to a Mediterranean diet plus extra virgin olive oil increase scores of short-term improvements than those following the med diet alone. | [273] |
| Modified ketogenic diet containing green vegetables, meats, eggs, nuts, seeds, creams, and natural oils. | Promotes physiological ketosis. | 58% Fat (26% saturated, 32% non-saturated), 29% protein, 7% fiber, and 6% net carbohydrate by weight. | Compared with a regular diet, patients adhered to a ketogenic diet improves daily function and quality of life in hospitalized patients with AD. | [261] |
| Mediterranean diet containing Walnut oil. | Enhances mitochondrial function and reduces β-amyloid (1–40). | Linoleic acid, oleic acid, α-linolenic acid, and gamma and beta tocopherol. | Raises ATP production, increases neuronal function, and decreases AD. | [262] |
| MIND diet containing green leafy vegetables, nuts, berries, beans/legumes, whole grains, fish, poultry, olive oil, and wine. | Anti-inflammatory and antioxidant effects. | Nutrients like folate, lutein-zeaxanthin, vitamin E, and flavonoids. | Improves cognition function and brain plasticity. | [263] |
4.3. Multiple Sclerosis
| Culinary Medicine | Mechanism | Active Chemical | Outcome/Result/Efficacy | Ref. |
|---|---|---|---|---|
| Mediterranean diet containing Epigallocatechin gallate (EGCG) and coconut oil (800 mg of EGCG, 60 mL of coconut oil). | Neuroprotective antioxidant, anti-inflammatory agent, and anxiolytic agent. | Coconut oil: palmitic acid, myristic acid, lauric acid, stearic acid, and oleic acid; Epigallocatechin gallate (EGCG): polyphenol. | Improves state anxiety and functional capacity; decreases IL-6. | [287] |
| Polysaccharide-based multi-nutrient dietary supplements (3 times per day). | Increases IL-2, TNF-α, EGF, CD95+, andCD34+ but decreases IL-1b. | Polysaccharides, antioxidants, phytochemicals, vitamins, and minerals. | Improves immune functions and reduces infection rates. | [291] |
| Modified Paleolithic diet (Two servings per week). | Paleolithic diet increased key nutrients targeting brain function including omega-3-fatty acids, vitamin B, antioxidants, and coenzyme Q10. | Modified Paleolithic diet-contains fibers, potassium, and essential fatty acids. | Modified Paleolithic diet induces clinical improvements in patients with MS. | [259] |
| Medium-chain triglyceride (MCT)-based ketogenic diet containing coconut oil. | Improved mitochondrial function; decreased oxidative stress. | High fat and low carbohydrate. | MCT-based ketogenic diet induces ketosis but does not induce clinical improvement. | [298,299] |
| MIND diet containing fruits, vegetables, legumes, nuts, whole grains, dairy, fried foods, processed meats, and fat intake (Servings/day). | Neuroprotection, encourages remyelination and acts as a disease modifier. | High intake of omega-3-fatty acids and polyunsaturated fatty acids. | MIND diet score is linked to thalamic volume; individuals in the highest quartile of MIND diet scores had more significant thalamic volumes versus those in the lowest quartile. For individual food/nutrients, higher intakes of full-fat dairy were associated with lower T2 lesion volumes. Higher intakes of marine omega-3 fatty acids were associated with greater NAWM microstructural integrity. | [293] |
| Protein source containing red meat (g/week), poultry (serves/week), fish (serves/week), shrimp, and eggs; fruit and vegetable (g/day); butter; and dietary supplements, multi-vitamins, calcium, fish oil, iron, folic acid, protein, vegetable, and dairy products. | Fruits and vegetables have anti-inflammatory properties; red meat causes inflammation. | ------- | Subjects with a higher diet-related inflammatory index has a higher risk for MS onset compared with those adhering to a more anti-inflammatory diet regimen. Improving nutritional a pattern through educational programs is likely to reduce MS risk. | [300] |
| High-fat diet (HFD diet) (Negative effect). | HFD diet causes the production of proinflammatory cytokines, IL-6, IL-1β, and Th17, which causes gut inflammation and CNS autoimmunity, finally leading to MS. | 45 kcal% Fat and dextrose. | HFD diet induces obesity, leading to the production of pathogenic bacteria, which can induce inflammation via the induction of gut permeability and pro-inflammatory mediators. | [292] |
| Mediterranean diet. | Improves lipid profile, modulates inflammation, and increases antioxidant property. | Fruits, vegetables, unprocessed cereals, unsaturated fats, and wine (300 mL/day) | Significant effects on MS are probably mediated by modulation of the gut microbiota and low-grade chronic systemic inflammation. | [301] |
| Calorie restriction (CR) diets. | Decreases body fat and weight; decreases oxidative stress and inflammation. | Polysaccharide-based multinutrient formula. | CR diets are beneficial for achieving weight loss and modulating emotional health. | [291,302] |
| Modified Paleolithic elimination (Wahls) diet (4 servings); low-saturated-fat (Swank) diets (6–9 servings). | Modifies neuroinflammation and oxidative stress; boosts mass and diversity of gut microbiota. | 5 g of cod liver oil and vegetable oil or fish oil (10–15 g). | Both diets significantly reduced fatigue and improved quality of life in patients with relapsing-remitting MS. | [232] |
| Calorie restriction (CR) diets (5 days per week). | Anti-inflammatory and neuroprotective. | Carbohydrate, protein, and fat. | CR diets are safe and effective ways to achieve weight loss in people with MS, altering the circulating metabolome and cell subsets, but without change in adipokine levels. | [303] |

4.4. Amyotrophic Lateral Sclerosis
| Culinary Medicine | Mechanism of Action | Bioactive Compounds/Chemical Constitutes | Outcome/Results/Efficacy | Ref. |
|---|---|---|---|---|
| Essential vitamins containing vitamin D and vitamin-E. | Neuroprotective and antioxidant effects. | 25-OHD and tocopherols. | Vitamin D and vitamin E are defensive in the case of ALS. | [314] |
| High-caloric fatty diet (HCFD) (405 kcal/day). | Stabilizes body weight. | 30mL of HCFD three times a day. | Diet intake decreases weight loss and increases survival of individuals with ALS. | [317] |
| Coconut oil. | Neuroprotective. | Medium-chain triglycerides. | Retards disease symptoms, increases motor function, increases survival, and stabilizes body weight in mice. | [316] |
4.5. Huntington’s Disease
4.6. Other Neurodegenerative Conditions
5. Future Perspective and Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Businaro, R.; Vauzour, D.; Sarris, J.; Münch, G.; Gyengesi, E.; Brogelli, L.; Zuzarte, P. Therapeutic Opportunities for Food Supplements in Neurodegenerative Disease and Depression. Front. Nutr. 2021, 8, 669846. [Google Scholar] [CrossRef]
- Mostile, G.; Terranova, R.; Carlentini, G.; Contrafatto, F.; Terravecchia, C.; Donzuso, G.; Sciacca, G.; Cicero, C.E.; Luca, A.; Nicoletti, A.; et al. Differentiating neurodegenerative diseases based on EEG complexity. Sci. Rep. 2024, 14, 24365. [Google Scholar] [CrossRef]
- Dauncey, M.J. Nutrition, the Brain and Cognitive Decline: Insights from Epigenetics. Eur. J. Clin. Nutr. 2014, 68, 1179–1185. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- World Health Organization. Intersectoral Global Action Plan on Epilepsy and Other Neurological Disorders 2022–2031. Available online: https://www.who.int/news/item/28-04-2022-draft-intersectoral-global-action-plan-on-epilepsy-and-other-neurological-disorders-2022-2031 (accessed on 17 February 2025).
- World Failing to Address Dementia Challenge. Available online: https://www.who.int/news/item/02-09-2021-world-failing-to-address-dementia-challenge (accessed on 27 August 2025).
- Zhao, B. Natural Antioxidants for Neurodegenerative Diseases. Mol. Neurobiol. 2005, 31, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A. Multiple Sclerosis Is Primarily a Neurodegenerative Disease. J. Neural Transm. 2013, 120, 1463–1466. [Google Scholar] [CrossRef]
- Grayson, M. Parkinson’s Disease. Nature 2016, 538, S1. [Google Scholar] [CrossRef]
- Gaba, A. Nutrition and Huntington’s Disease—A Review of Current Practice and Theory. Curr. Nutr. Rep. 2025, 14, 18. [Google Scholar] [CrossRef]
- Morgan, S.; Orrell, R.W. Pathogenesis of Amyotrophic Lateral Sclerosis. Br. Med. Bull. 2016, 119, 87–97. [Google Scholar] [CrossRef]
- Arnaoutoglou, N.A.; O’Brien, J.T.; Underwood, B.R. Dementia with Lewy Bodies—From Scientific Knowledge to Clinical Insights. Nat. Rev. Neurol. 2019, 15, 103–112. [Google Scholar] [CrossRef]
- Gorman, A.M. Neuronal Cell Death in Neurodegenerative Diseases: Recurring Themes around Protein Handling: Apoptosis Review Series. J. Cell Mol. Med. 2008, 12, 2263–2280. [Google Scholar] [CrossRef]
- 2023 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M.; Yi, H.; Akao, Y.; Tanaka, M. Oxidative Stress in Mitochondria: Decision to Survival and Death of Neurons in Neurodegenerative Disorders. Mol. Neurobiol. 2005, 31, 81–93. [Google Scholar] [CrossRef]
- Armstrong, J.S. The Role of the Mitochondrial Permeability Transition in Cell Death. Mitochondrion 2006, 6, 225–234. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Trichopoulou, A.; Panza, F. Cross-Sectional and Longitudinal Associations between Adherence to Mediterranean Diet with Physical Performance and Cognitive Function in Older Adults: A Systematic Review and Meta-Analysis. Ageing Res. Rev. 2021, 70, 101395. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Levine, H. Alzheimer’s Disease and the Amyloid-β Peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Novoa, C.; Salazar, P.; Cisternas, P.; Gherardelli, C.; Vera-Salazar, R.; Zolezzi, J.M.; Inestrosa, N.C. Inflammation Context in Alzheimer’s Disease, a Relationship Intricate to Define. Biol. Res. 2022, 55, 39. [Google Scholar] [CrossRef]
- Gonsette, R.E. Neurodegeneration in Multiple Sclerosis: The Role of Oxidative Stress and Excitotoxicity. J. Neurol. Sci. 2008, 274, 48–53. [Google Scholar] [CrossRef]
- Watson, R.R.; Preedy, V.R. Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease: Prevention and Therapy; Academic Press: Cambridge, MA, USA, 2014; pp. 1–500. [Google Scholar] [CrossRef]
- Ari, C.; Pilla, R.; D’Agostino, D. Nutritional/Metabolic Therapies in Animal Models of Amyotrophic Lateral Sclerosis, Alzheimer’s Disease, and Seizures. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease: Prevention and Therapy; Academic Press: Cambridge, MA, USA, 2015; pp. 449–459. [Google Scholar] [CrossRef]
- Wilson, K.A.; Bar, S.; Dammer, E.B.; Carrera, E.M.; Hodge, B.A.; Hilsabeck, T.A.U.; Bons, J.; Brownridge, G.W.; Beck, J.N.; Rose, J.; et al. OXR1 Maintains the Retromer to Delay Brain Aging under Dietary Restriction. Nat. Commun. 2024, 15, 467. [Google Scholar] [CrossRef]
- Amato, A.; Terzo, S.; Mulè, F. Natural Compounds as Beneficial Antioxidant Agents in Neurodegenerative Disorders: A Focus on Alzheimer’s Disease. Antioxidants 2019, 8, 608. [Google Scholar] [CrossRef]
- Pandey, M.C.; Tyagi, P. Antioxidants and Free Radicals: Human and Food System. J. Crit. Rev. 2020, 7, 753–756. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The Diagnosis of Mild Cognitive Impairment Due to Alzheimer’s Disease: Recommendations from the National Institute on Aging-Alzheimer’s Association Workgroups on Diagnostic Guidelines for Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Pogačnik, L.; Ota, A.; Ulrih, N.P. An Overview of Crucial Dietary Substances and Their Modes of Action for Prevention of Neurodegenerative Diseases. Cells 2020, 9, 576. [Google Scholar] [CrossRef]
- Sintzel, M.B.; Rametta, M.; Reder, A.T. Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 2018, 7, 59–85. [Google Scholar] [CrossRef]
- Wingerchuk, D.M. Smoking: Effects on Multiple Sclerosis Susceptibility and Disease Progression. Ther. Adv. Neurol. Disord. 2012, 5, 13–22. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the Clinical Course of Multiple Sclerosis: The 2013 Revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- Thacker, E.L.; Mirzaei, F.; Ascherio, A. Infectious Mononucleosis and Risk for Multiple Sclerosis: A Meta-Analysis. Ann. Neurol. 2006, 59, 499–503. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Tumer, T.B.; Moreira, A.C.; et al. Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef]
- Poddar, J.; Pradhan, M.; Ganguly, G.; Chakrabarti, S. Biochemical Deficits and Cognitive Decline in Brain Aging: Intervention by Dietary Supplements. J. Chem. Neuroanat. 2019, 95, 70–80. [Google Scholar] [CrossRef]
- Naoi, M.; Inaba-Hasegawa, K.; Shamoto-Nagai, M.; Maruyama, W. Neurotrophic Function of Phytochemicals for Neuroprotection in Aging and Neurodegenerative Disorders: Modulation of Intracellular Signaling and Gene Expression. J. Neural Transm. 2017, 124, 1515–1527. [Google Scholar] [CrossRef]
- Cencic, A.; Chingwaru, W. The Role of Functional Foods, Nutraceuticals, and Food Supplements in Intestinal Health. Nutrients 2010, 2, 611–625. [Google Scholar] [CrossRef]
- Thomas, C.; Mayegowda Shilpa, B.; Babu Mythri, R. Disease Modifying Potential of Functional Foods for Neurodegenerative Disorders: Status Update on Regulatory Compliance. In Functional Foods—Phytochemicals and Health Promoting Potential; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P. Nutrients and Oxidative Stress: Friend or Foe? Oxidative Med. Cell. Longev. 2018, 2018, 9719584. [Google Scholar] [CrossRef]
- Soni, M.; Kos, K.; Lang, I.A.; Jones, K.; Melzer, D.; Llewellyn, D.J. Vitamin D and Cognitive Function. Scand. J. Clin. Lab. Investig. 2012, 72, 79–82. [Google Scholar] [CrossRef]
- Wood, A.H.R.; Chappell, H.F.; Zulyniak, M.A. Dietary and Supplemental Long-Chain Omega-3 Fatty Acids as Moderators of Cognitive Impairment and Alzheimer’s Disease. Eur. J. Nutr. 2022, 61, 589–604. [Google Scholar] [CrossRef]
- Sydenham, E.; Dangour, A.D.; Lim, W.-S. Omega 3 Fatty Acid for the Prevention of Cognitive Decline and Dementia. Sao Paulo Med. J. 2012, 130, 419. [Google Scholar] [CrossRef]
- Lim, W.-S.; Gammack, J.K.; Van Niekerk, J.K.; Dangour, A. Omega 3 Fatty Acid for the Prevention of Dementia. Cochrane Database Syst. Rev. 2006, 139, 416. [Google Scholar] [CrossRef]
- Mao, P.; Reddy, P.H. Is Multiple Sclerosis a Mitochondrial Disease? Biochim. Biophys. Acta Mol. Basis Dis. 2010, 1802, 66–79. [Google Scholar] [CrossRef]
- Salvati, S.; Di Biase, A.; Attorri, L.; Di Benedetto, R.; Sanchez, M.; Lorenzini, L.; Alessandri, M.; Calzà, L. Ethyl-Eicosapentaenoic Acid Ameliorates the Clinical Course of Experimental Allergic Encephalomyelitis Induced in Dark Agouti Rats. J. Nutr. Biochem. 2013, 24, 1645–1654. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective Natural Products for Alzheimer’s Disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef]
- Phan, C.W.; David, P.; Naidu, M.; Wong, K.H.; Sabaratnam, V. Therapeutic Potential of Culinary-Medicinal Mushrooms for the Management of Neurodegenerative Diseases: Diversity, Metabolite, and Mechanism. Crit. Rev. Biotechnol. 2015, 35, 355–368. [Google Scholar] [CrossRef]
- Varga, J.; Dér, N.P.; Zsindely, N.; Bodai, L. Green Tea Infusion Alleviates Neurodegeneration Induced by Mutant Huntingtin in Drosophila. Nutr. Neurosci. 2020, 23, 183–189. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Duennwald, M.; Markovic, P.; Wacker, J.L.; Engemann, S.; Roark, M.; Legleiter, J.; Marsh, J.L.; Thompson, L.M.; Lindquist, S.; et al. Green Tea (−)-Epigallocatechin-Gallate Modulates Early Events in Huntingtin Misfolding and Reduces Toxicity in Huntington’s Disease Models. Hum. Mol. Genet. 2006, 15, 2743–2751. [Google Scholar] [CrossRef]
- Irish, A.K.; Erickson, C.M.; Wahls, T.L.; Snetselaar, L.G.; Darling, W.G. Randomized Control Trial Evaluation of a Modified Paleolithic Dietary Intervention in the Treatment of Relapsing-Remitting Multiple Sclerosis: A Pilot Study. Degener. Neurol. Neuromuscul. Dis. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- Li, R.-L.; Wang, L.-Y.; Duan, H.-X.; Zhang, Q.; Guo, X.; Wu, C.; Peng, W. Regulation of Mitochondrial Dysfunction Induced Cell Apoptosis Is a Potential Therapeutic Strategy for Herbal Medicine to Treat Neurodegenerative Diseases. Front. Pharmacol. 2022, 13, 937289. [Google Scholar] [CrossRef]
- Nolan, J.M.; Mulcahy, R.; Power, R.; Moran, R.; Howard, A.N. Nutritional Intervention to Prevent Alzheimer’s Disease: Potential Benefits of Xanthophyll Carotenoids and Omega-3 Fatty Acids Combined. J. Alzheimer’s Dis. 2018, 64, 367–378. [Google Scholar] [CrossRef]
- Muldoon, M.F.; Ryan, C.M.; Yao, J.K.; Conklin, S.M.; Manuck, S.B. Long-Chain Omega-3 Fatty Acids and Optimization of Cognitive Performance. Mil. Med. 2014, 179, 95–105. [Google Scholar] [CrossRef]
- Gillette-Guyonnet, S.; Secher, M.; Vellas, B. Nutrition and Neurodegeneration: Epidemiological Evidence and Challenges for Future Research. Br. J. Clin. Pharmacol. 2013, 75, 738–755. [Google Scholar] [CrossRef]
- Silva, R.F.M.; Pogačnik, L. Food, Polyphenols and Neuroprotection. Neural Regen. Res. 2017, 12, 582–583. [Google Scholar] [CrossRef]
- Verma, R.; Rao, L.; Nagpal, D.; Yadav, M.; Kumar, M.; Mittal, V.; Kaushik, D. Exploring the Prospective of Curcumin-Loaded Nanomedicine in Brain Cancer Therapy: An Overview of Recent Updates and Patented Nanoformulations. Recent Pat. Nanotechnol. 2023, 18, 278–294. [Google Scholar] [CrossRef]
- Jayaraman, A.; Pike, C.J. Alzheimer’s Disease and Type 2 Diabetes: Multiple Mechanisms Contribute to Interactions Topical Collection on Pathogenesis of Type 2 Diabetes and Insulin Resistance. Curr. Diabetes Rep. 2014, 14, 476. [Google Scholar] [CrossRef] [PubMed]
- Claudino, P.A.; Bueno, N.B.; Piloneto, S.; Halaiko, D.; Azevedo de Sousa, L.P.; Barroso Jara Maia, C.H.; Netto, B.D.M. Consumption of Ultra-Processed Foods and Risk for Alzheimer’s Disease: A Systematic Review. Front. Nutr. 2024, 10, 1288749. [Google Scholar] [CrossRef]
- Wiȩckowska-Gacek, A.; Mietelska-Porowska, A.; Chutorański, D.; Wydrych, M.; Długosz, J.; Wojda, U. Western Diet Induces Impairment of Liver-Brain Axis Accelerating Neuroinflammation and Amyloid Pathology in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 136. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND Diet Associated with Reduced Incidence of Alzheimer’s Disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef]
- Fadel, J.R.; Jolivalt, C.G.; Reagan, L.P. Food for Thought: The Role of Appetitive Peptides in Age-Related Cognitive Decline. Ageing Res. Rev. 2013, 12, 764–776. [Google Scholar] [CrossRef]
- Więckowska-Gacek, A.; Mietelska-Porowska, A.; Wydrych, M.; Wojda, U. Western Diet as a Trigger of Alzheimer’s Disease: From Metabolic Syndrome and Systemic Inflammation to Neuroinflammation and Neurodegeneration. Ageing Res. Rev. 2021, 70, 101397. [Google Scholar] [CrossRef]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2022, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Food as Medicine: Translating the Evidence. Nat. Med. 2023, 29, 753–754. [CrossRef]
- Berkowitz, S.A.; Terranova, J.; Randall, L.; Cranston, K.; Waters, D.B.; Hsu, J. Association between Receipt of a Medically Tailored Meal Program and Health Care Use. JAMA Intern. Med. 2019, 179, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Downer, S.; Berkowitz, S.A.; Berkowitz, S.A.; Harlan, T.S.; Olstad, D.L.; Mozaffarian, D. Food Is Medicine: Actions to Integrate Food and Nutrition into Healthcare. BMJ 2020, 369, m2482. [Google Scholar] [CrossRef]
- Song, N.; Mei, S.; Wang, X.; Hu, G.; Lu, M. Focusing on Mitochondria in the Brain: From Biology to Therapeutics. Transl. Neurodegener. 2024, 13, 23. [Google Scholar] [CrossRef]
- Mosharov, E.V.; Rosenberg, A.M.; Monzel, A.S.; Osto, C.A.; Stiles, L.; Rosoklija, G.B.; Dwork, A.J.; Bindra, S.; Junker, A.; Zhang, Y.; et al. A Human Brain Map of Mitochondrial Respiratory Capacity and Diversity. Nature 2025, 641, 749–758. [Google Scholar] [CrossRef]
- Rosenberg, A.M.; Saggar, M.; Monzel, A.S.; Devine, J.; Rogu, P.; Limoges, A.; Junker, A.; Sandi, C.; Mosharov, E.V.; Dumitriu, D.; et al. Brain Mitochondrial Diversity and Network Organization Predict Anxiety-like Behavior in Male Mice. Nat. Commun. 2023, 14, 4726. [Google Scholar] [CrossRef]
- Wen, P.; Sun, Z.; Gou, F.; Wang, J.; Fan, Q.; Zhao, D.; Yang, L. Oxidative Stress and Mitochondrial Impairment: Key Drivers in Neurodegenerative Disorders. Ageing Res. Rev. 2025, 104, 102667. [Google Scholar] [CrossRef]
- San-Millán, I. The Key Role of Mitochondrial Function in Health and Disease. Antioxidants 2023, 12, 782. [Google Scholar] [CrossRef]
- Skawratananond, S.; Xiong, D.X.; Zhang, C.; Tonk, S.; Pinili, A.; Delacruz, B.; Pham, P.; Smith, S.C.; Navab, R.; Reddy, P.H. Mitophagy in Alzheimer’s Disease and Other Metabolic Disorders: A Focus on Mitochondrial-Targeted Therapeutics. Ageing Res. Rev. 2025, 108, 102732. [Google Scholar] [CrossRef] [PubMed]
- Pradeepkiran, J.A.; Hemachandra Reddy, P. Defective Mitophagy in Alzheimer’s Disease. Ageing Res. Rev. 2020, 64, 101191. [Google Scholar] [CrossRef]
- Clemente-Suárez, V.J.; Redondo-Flórez, L.; Beltrán-Velasco, A.I.; Ramos-Campo, D.J.; Belinchón-deMiguel, P.; Martinez-Guardado, I.; Dalamitros, A.A.; Yáñez-Sepúlveda, R.; Martín-Rodríguez, A.; Tornero-Aguilera, J.F. Mitochondria and Brain Disease: A Comprehensive Review of Pathological Mechanisms and Therapeutic Opportunities. Biomedicines 2023, 11, 2488. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial Dynamics in Health and Disease: Mechanisms and Potential Targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Wesselink, E.; Koekkoek, W.A.C.; Grefte, S.; Witkamp, R.F.; van Zanten, A.R.H. Feeding Mitochondria: Potential Role of Nutritional Components to Improve Critical Illness Convalescence. Clin. Nutr. 2019, 38, 982–995. [Google Scholar] [CrossRef] [PubMed]
- A Map of Mitochondrial Biology Reveals the Energy Landscape of the Human Brain. Nature 2025. [CrossRef]
- Meyer, J.H.; Cervenka, S.; Kim, M.J.; Kreisl, W.C.; Henter, I.D.; Innis, R.B. Neuroinflammation in Psychiatric Disorders: PET Imaging and Promising New Targets. Lancet Psychiatry 2020, 7, 1064–1074. [Google Scholar] [CrossRef]
- Ganjam, G.K.; Bolte, K.; Matschke, L.A.; Neitemeier, S.; Dolga, A.M.; Höllerhage, M.; Höglinger, G.U.; Adamczyk, A.; Decher, N.; Oertel, W.H.; et al. Mitochondrial Damage by α-Synuclein Causes Cell Death in Human Dopaminergic Neurons. Cell Death Dis. 2019, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Norat, P.; Soldozy, S.; Sokolowski, J.D.; Gorick, C.M.; Kumar, J.S.; Chae, Y.; Yağmurlu, K.; Prada, F.; Walker, M.; Levitt, M.R.; et al. Mitochondrial Dysfunction in Neurological Disorders: Exploring Mitochondrial Transplantation. NPJ Regen. Med. 2020, 5, 22. [Google Scholar] [CrossRef]
- Jia, K.; Du, H. Mitochondrial Permeability Transition: A Pore Intertwines Brain Aging and Alzheimer’s Disease. Cells 2021, 10, 649. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, H.; Qu, S. Phytochemicals Targeting Mitophagy: Therapeutic Opportunities and Prospects for Treating Alzheimer’s Disease. Biomed. Pharmacother. 2024, 177, 117144. [Google Scholar] [CrossRef]
- Mitchell, C.S.; Lafleur, B.; Parr-Brownlie, L.C.; Kip, E. Healthy Lifestyles and Wellbeing Reduce Neuroinflammation and Prevent Neurodegenerative and Psychiatric Disorders. Front. Neurosci. 2023, 17, 1092537. [Google Scholar] [CrossRef]
- Ghosh, D.; Kumar, A. Harnessing Mitophagy for Therapeutic Advances in Aging and Chronic Neurodegenerative Diseases. Neuroglia 2024, 5, 391–409. [Google Scholar] [CrossRef]
- Wang, S.; Long, H.; Hou, L.; Feng, B.; Ma, Z.; Wu, Y.; Zeng, Y.; Cai, J.; Zhang, D.W.; Zhao, G. The Mitophagy Pathway and Its Implications in Human Diseases. Signal Transduct. Target. Ther. 2023, 8, 304. [Google Scholar] [CrossRef]
- Li, S.; Cai, Y.; Guan, T.; Zhang, Y.; Huang, K.; Zhang, Z.; Cao, W.; Guan, X. Quinic Acid Alleviates High-Fat Diet-Induced Neuroinflammation by Inhibiting DR3/IKK/NF-ΚB Signaling via Gut Microbial Tryptophan Metabolites. Gut Microbes 2024, 16, 2374608. [Google Scholar] [CrossRef]
- Chen, D.; Li, X.; Zhang, L.T.; Zhu, M.; Gao, L. A High-fat Diet Impairs Mitochondrial Biogenesis, Mitochondrial Dynamics, and the Respiratory Chain Complex in Rat Myocardial Tissues. J. Cell Biochem. 2018, 119, 9602. [Google Scholar] [CrossRef]
- Pradeepkiran, J.A.; Islam, M.A.; Sehar, U.; Reddy, A.P.; Vijayan, M.; Reddy, P.H. Impact of Diet and Exercise on Mitochondrial Quality and Mitophagy in Alzheimer’s Disease. Ageing Res. Rev. 2025, 108, 102734. [Google Scholar] [CrossRef]
- Beveridge, J.; Montgomery, A.; Grossberg, G. Intermittent Fasting and Neurocognitive Disorders: What the Evidence Shows. J. Nutr. Health Aging 2025, 29, 100480. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Chu, X.; Park, J.H.; Zhu, Q.; Hussain, M.; Li, Z.; Madsen, H.B.; Yang, B.; Wei, Y.; Wang, Y.; et al. Urolithin A Improves Alzheimer’s Disease Cognition and Restores Mitophagy and Lysosomal Functions. Alzheimer’s Dement. 2024, 20, 4212. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, T.; Li, W.; Gao, N.; Zhang, T. Resveratrol Attenuates Oxidative Damage through Activating Mitophagy in an in Vitro Model of Alzheimer’s Disease. Toxicol. Lett. 2018, 282, 100–108. [Google Scholar] [CrossRef]
- Lee, D.Y.; Lee, K.M.; Um, J.H.; Kim, Y.Y.; Kim, D.H.; Yun, J. The Natural Alkaloid Palmatine Selectively Induces Mitophagy and Restores Mitochondrial Function in an Alzheimer’s Disease Mouse Model. Int. J. Mol. Sci. 2023, 24, 16542. [Google Scholar] [CrossRef]
- Ramirez, A.; Old, W.; Selwood, D.L.; Liu, X. Cannabidiol Activates PINK1-Parkin-Dependent Mitophagy and Mitochondrial-Derived Vesicles. Eur. J. Cell Biol. 2021, 101, 151185. [Google Scholar] [CrossRef]
- Bénard, G.; Massa, F.; Puente, N.; Lourenço, J.; Bellocchio, L.; Soria-Gómez, E.; Matias, I.; Delamarre, A.; Metna-Laurent, M.; Cannich, A.; et al. Mitochondrial CB1 Receptors Regulate Neuronal Energy Metabolism. Nat. Neurosci. 2012, 15, 558–564. [Google Scholar] [CrossRef]
- Um, J.H.; Lee, K.M.; Kim, Y.Y.; Lee, D.Y.; Kim, E.; Kim, D.H.; Yun, J. Berberine Induces Mitophagy through Adenosine Monophosphate-Activated Protein Kinase and Ameliorates Mitochondrial Dysfunction in PINK1 Knockout Mouse Embryonic Fibroblasts. Int. J. Mol. Sci. 2023, 25, 219. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Gao, X.; Sha, S.; Wang, S.; Shan, Y.; Li, L.; Xing, C.; Guan, H.; Du, H. Berberine Alleviates Alzheimer’s Disease by Activating Autophagy and Inhibiting Ferroptosis through the JNK-P38MAPK Signaling Pathway. Int. Immunopharmacol. 2025, 155, 114550. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Li, B.; Shi, J.; Xu, J.; Yuan, M. Targeting Mitochondrial Dysfunction in Neurodegenerative Diseases: Expanding the Therapeutic Approaches by Plant-Derived Natural Products. Pharmaceuticals 2023, 16, 277. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Bernatoniene, J.; Jakstas, V.; Morkuniene, R. Catechins, Neuroprotection, and Brain Mitochondria. In Mitochondrial Physiology and Vegetal Molecules: Therapeutic Potential of Natural Compounds on Mitochondrial Health; Academic Press: Cambridge, MA, USA, 2021; pp. 455–470. [Google Scholar] [CrossRef]
- Varghese, N.; Werner, S.; Grimm, A.; Eckert, A. Dietary Mitophagy Enhancer: A Strategy for Healthy Brain Aging? Antioxidants 2020, 9, 932. [Google Scholar] [CrossRef] [PubMed]
- Moselhy, O.A.; Abdel-Aziz, N.; El-Bahkery, A.; Moselhy, S.S.; Ibrahim, E.A. Curcumin Nanoparticles Alleviate Brain Mitochondrial Dysfunction and Cellular Senescence in γ-Irradiated Rats. Sci. Rep. 2025, 15, 3857. [Google Scholar] [CrossRef]
- Babalola, J.A.; Lang, M.; George, M.; Stracke, A.; Tam-Amersdorfer, C.; Itxaso, I.; Lucija, D.; Tadic, J.; Schilcher, I.; Loeffler, T.; et al. Astaxanthin Enhances Autophagy, Amyloid Beta Clearance and Exerts Anti-Inflammatory Effects in in Vitro Models of Alzheimer’s Disease-Related Blood Brain Barrier Dysfunction and Inflammation. Brain Res. 2023, 1819, 148518. [Google Scholar] [CrossRef] [PubMed]
- Fairley, L.H.; Lejri, I.; Grimm, A.; Eckert, A. Spermidine Rescues Bioenergetic and Mitophagy Deficits Induced by Disease-Associated Tau Protein. Int. J. Mol. Sci. 2023, 24, 5297. [Google Scholar] [CrossRef]
- He, X.F.; Liu, D.X.; Zhang, Q.; Liang, F.Y.; Dai, G.Y.; Zeng, J.S.; Pei, Z.; Xu, G.Q.; Lan, Y. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front. Mol. Neurosci. 2017, 10, 144. [Google Scholar] [CrossRef]
- Kylkilahti, T.M.; Berends, E.; Ramos, M.; Shanbhag, N.C.; Töger, J.; Markenroth Bloch, K.; Lundgaard, I. Achieving Brain Clearance and Preventing Neurodegenerative Diseases—A Glymphatic Perspective. J. Cereb. Blood Flow Metab. 2021, 41, 2137–2149. [Google Scholar] [CrossRef]
- Huang, H.; Lin, L.; Wu, T.; Wu, C.; Zhou, L.; Li, G.; Su, F.; Liang, F.; Guo, W.; Chen, W.; et al. Phosphorylation of AQP4 by LRRK2 R1441G Impairs Glymphatic Clearance of IFNγ and Aggravates Dopaminergic Neurodegeneration. NPJ Park. Dis. 2024, 10, 31. [Google Scholar] [CrossRef]
- Delle, C.; Cankar, N.; Digebjerg Holgersson, C.; Hvorup Knudsen, H.; Schiøler Nielsen, E.; Kjaerby, C.; Mori, Y.; Nedergaard, M.; Weikop, P. Long-Term High-Fat Diet Increases Glymphatic Activity in the Hypothalamus in Mice. Sci. Rep. 2023, 13, 4137. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Yuan, Y.; Lei, J.; Zhao, Y.; Li, Y.; Qu, Q.; Wang, J. Long-Term High-Fat Diet Impairs AQP4-Mediated Glymphatic Clearance of Amyloid Beta. Mol. Neurobiol. 2024, 62, 1079–1093. [Google Scholar] [CrossRef]
- Jovanovic Macura, I.; Milanovic, D.; Tesic, V.; Major, T.; Perovic, M.; Adzic, M.; Ivkovic, S. The Impact of High-Dose Fish Oil Supplementation on Mfsd2a, Aqp4, and Amyloid-β Expression in Retinal Blood Vessels of 5xFAD Alzheimer’s Mouse Model. Int. J. Mol. Sci. 2024, 25, 9400. [Google Scholar] [CrossRef]
- Tan, X.; Li, X.; Li, R.; Meng, W.; Xie, Z.; Li, J.; Pang, Y.; Huang, G.; Li, L.; Li, H. Β-Hydroxybutyrate Alleviates Neurological Deficits By Restoring Glymphatic and Inflammation After Subarachnoid Hemorrhage in Mice. Exp. Neurol. 2024, 378, 114819. [Google Scholar] [CrossRef]
- Yao, D.; Li, R.; Hao, J.; Huang, H.; Wang, X.; Ran, L.; Fang, Y.; He, Y.; Wang, W.; Liu, X.; et al. Melatonin Alleviates Depression-like Behaviors and Cognitive Dysfunction in Mice by Regulating the Circadian Rhythm of AQP4 Polarization. Transl. Psychiatry 2023, 13, 310. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Ghezzi, L.; Cross, A.H.; Piccio, L. Effects of Dietary Restriction on Neuroinflammation in Neurodegenerative Diseases. J. Exp. Med. 2021, 218, e20190086. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Jadhav, P.A.; Thomas, A.B.; Chopada, V.M.; Bokaria, P.V.; Deokate, S.B.; Chougule, P.S.; Chavan, P.N.; Chitlange, S.S. Correlation of Non-Alcoholic Fatty Liver Disease and Neurodegenerative Disorders. Egypt. Liver J. 2024, 14, 79. [Google Scholar] [CrossRef]
- Lobo, F.; Haase, J.; Brandhorst, S. The Effects of Dietary Interventions on Brain Aging and Neurological Diseases. Nutrients 2022, 14, 5086. [Google Scholar] [CrossRef]
- Park, K.J.; Gao, Y. Gut-Brain Axis and Neurodegeneration: Mechanisms and Therapeutic Potentials. Front. Neurosci. 2024, 18, 1481390. [Google Scholar] [CrossRef]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The Brain-Gut-Microbiome Axis. Cell Mol. Gastroenterol. Hepatol. 2018, 6, 133. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus Strain Regulates Emotional Behavior and Central GABA Receptor Expression in a Mouse via the Vagus Nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Chianese, R.; Coccurello, R.; Viggiano, A.; Scafuro, M.; Fiore, M.; Coppola, G.; Operto, F.F.; Fasano, S.; Laye, S.; Pierantoni, R.; et al. Impact of Dietary Fats on Brain Functions. Curr. Neuropharmacol. 2017, 16, 1059–1085. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203. [Google Scholar]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal Barrier and Gut Microbiota: Shaping Our Immune Responses throughout Life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Liu, W.W.; Reicher, N.; Alway, E.; Rupprecht, L.E.; Weng, P.; Schaefgen, C.; Klein, M.E.; Villalobos, J.A.; Puerto-Hernandez, C.; Kiesling Altún, Y.G.; et al. A Gut Sense for a Microbial Pattern Regulates Feeding. Nature 2025, 7, 1–8. [Google Scholar] [CrossRef]
- Mishra, S.P.; Jain, S.; Wang, B.; Wang, S.; Miller, B.C.; Lee, J.Y.; Borlongan, C.V.; Jiang, L.; Pollak, J.; Taraphder, S.; et al. Abnormalities in Microbiota/Butyrate/FFAR3 Signaling in Aging Gut Impair Brain Function. JCI Insight 2024, 9, e168443. [Google Scholar] [CrossRef]
- Needham, B.D.; Kaddurah-Daouk, R.; Mazmanian, S.K. Gut Microbial Molecules in Behavioural and Neurodegenerative Conditions. Nat. Rev. Neurosci. 2020, 21, 717–731. [Google Scholar] [CrossRef]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut–Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef]
- Wilkins, L.J.; Monga, M.; Miller, A.W. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci. Rep. 2019, 9, 12918. [Google Scholar] [CrossRef] [PubMed]
- Ritz, N.L.; Draper, L.A.; Bastiaanssen, T.F.S.; Turkington, C.J.R.; Peterson, V.L.; van de Wouw, M.; Vlckova, K.; Fülling, C.; Guzzetta, K.E.; Burokas, A.; et al. The Gut Virome Is Associated with Stress-Induced Changes in Behaviour and Immune Responses in Mice. Nat. Microbiol. 2024, 9, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, A. Role of Gut Virome and Mycobiome in Neurological Disorders. In Microbiota-Gut-Brain Axis and CNS Disorders: Recent Progress and Perspectives; Academic Press: Cambridge, MA, USA, 2025; pp. 383–408. [Google Scholar] [CrossRef]
- Jinato, T.; Sikaroodi, M.; Fagan, A.; Sterling, R.K.; Lee, H.; Puri, P.; Davis, B.C.; Fuchs, M.; Gavis, E.; Gillevet, P.M.; et al. Alterations in Gut Virome Are Associated with Cognitive Function and Minimal Hepatic Encephalopathy Cross-Sectionally and Longitudinally in Cirrhosis. Gut Microbes 2023, 15, 2288168. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Guo, R.; Xing, G.; Xu, D.; Ma, X.; Chen, Q.; Li, S.; Qin, Y.; Liu, J.; et al. Alterations of the Gut Virome in Patients with Parkinson’s Disease. Res. Sq. 2024. [Google Scholar] [CrossRef]
- Deng, L.; Fu, P.; Ding, L.; Duan, X.; Feng, S.; Peng, Y. Virome Analysis Provides New Insights into the Association between Viruses and Parkinson’s Disease. J. Med. Virol. 2023, 95, e28111. [Google Scholar] [CrossRef]
- de Ora, L.O.; Balsamo, J.M.; Uyeda, K.S.; Bess, E.N. Discovery of a Gut Bacterial Metabolic Pathway That Drives α-Synuclein Aggregation. ACS Chem. Biol. 2024, 19, 1011–1021. [Google Scholar] [CrossRef]
- Stockdale, S.R.; Draper, L.A.; O’Donovan, S.M.; Barton, W.; O’Sullivan, O.; Volpicelli-Daley, L.A.; Sullivan, A.M.; O’Neill, C.; Hill, C. Alpha-Synuclein Alters the Faecal Viromes of Rats in a Gut-Initiated Model of Parkinson’s Disease. Commun. Biol. 2021, 4, 1140. [Google Scholar] [CrossRef]
- Nakahara, K.; Nakane, S.; Ishii, K.; Ikeda, T.; Ando, Y. Gut Microbiota of Parkinson’s Disease in an Appendectomy Cohort: A Preliminary Study. Sci. Rep. 2023, 13, 2210. [Google Scholar] [CrossRef] [PubMed]
- Sagor, M.S.; Islam, T.; Tamanna, N.T.; Bappy, M.K.I.; Danishuddin; Haque, M.A.; Lackner, M. The Functional Landscape of the Appendix Microbiome under Conditions of Health and Disease. Gut Pathog. 2025, 17, 38. [Google Scholar] [CrossRef]
- Yap, D.R.Y.; Lui, R.N.; Samol, J.; Ngeow, J.; Sung, J.J.Y.; Wong, S.H. Beyond a Vestigial Organ: Effects of the Appendix on Gut Microbiome and Colorectal Cancer. J. Gastroenterol. Hepatol. 2024, 39, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Liu, G.; Lin, Y.; Guo, C.L.; Han, J.; Chu, E.S.H.; Shi, C.; Li, Y.; Zhang, H.; Hu, C.; et al. Altered Gut Microbiome Composition by Appendectomy Contributes to Colorectal Cancer. Oncogene 2023, 42, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the Barriers: The Gut Microbiome, Intestinal Permeability and Stress-Related Psychiatric Disorders. Front. Cell Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
- Attaye, I.; van Oppenraaij, S.; Warmbrunn, M.V.; Nieuwdorp, M. The Role of the Gut Microbiota on the Beneficial Effects of Ketogenic Diets. Nutrients 2022, 14, 191. [Google Scholar] [CrossRef]
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef]
- Krakovski, M.A.; Arora, N.; Jain, S.; Glover, J.; Dombrowski, K.; Hernandez, B.; Yadav, H.; Sarma, A.K. Diet-Microbiome-Gut-Brain Nexus in Acute and Chronic Brain Injury. Front. Neurosci. 2022, 16, 1586. [Google Scholar] [CrossRef]
- Pröbstel, A.K.; Zhou, X.; Baumann, R.; Wischnewski, S.; Kutza, M.; Rojas, O.L.; Sellrie, K.; Bischof, A.; Kim, K.; Ramesh, A.; et al. Gut Microbiota-Specific IgA+ B Cells Traffic to the CNS in Active Multiple Sclerosis. Sci. Immunol. 2020, 5, eabc7191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ning, J.; Bao, X.Q.; Shang, M.; Ma, J.; Li, G.; Zhang, D. Fecal Microbiota Transplantation Protects Rotenone-Induced Parkinson’s Disease Mice via Suppressing Inflammation Mediated by the Lipopolysaccharide-TLR4 Signaling Pathway through the Microbiota-Gut-Brain Axis. Microbiome 2021, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Kuai, X.Y.; Yao, X.H.; Xu, L.J.; Zhou, Y.Q.; Zhang, L.P.; Liu, Y.; Pei, S.F.; Zhou, C.L. Evaluation of Fecal Microbiota Transplantation in Parkinson’s Disease Patients with Constipation. Microb. Cell Fact. 2021, 20, 98. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Yim, Y.S.; Ha, S.; Atarashi, K.; Tan, T.G.; Longman, R.S.; Honda, K.; Littman, D.R.; Choi, G.B.; et al. Maternal Gut Bacteria Promote Neurodevelopmental Abnormalities in Mouse Offspring. Nature 2017, 549, 528–532. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, X.; Ye, Y.; Yan, X.; Cheng, Y.; Zhao, L.; Chen, F.; Ling, Z. Gut Microbiota: A Novel Therapeutic Target for Parkinson’s Disease. Front. Immunol. 2022, 13, 937555. [Google Scholar] [CrossRef] [PubMed]
- Perrone, L.; Valente, M. The Emerging Role of Metabolism in Brain-Heart Axis: New Challenge for the Therapy and Prevention of Alzheimer Disease: May Thioredoxin Interacting Protein (Txnip) Play a Role? Biomolecules 2021, 11, 1652. [Google Scholar] [CrossRef]
- van der Wall, E.E.; van Gilst, W.H. Neurocardiology: Close Interaction between Heart and Brain. Neth. Heart J. 2013, 21, 51–52. [Google Scholar] [CrossRef]
- Devinsky, O.; Hesdorffer, D.C.; Thurman, D.J.; Lhatoo, S.; Richerson, G. Sudden Unexpected Death in Epilepsy: Epidemiology, Mechanisms, and Prevention. Lancet Neurol. 2016, 15, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Ritz, K.; van Buchem, M.A.; Daemen, M.J. The Heart-Brain Connection: Mechanistic Insights and Models. Neth. Heart J. 2013, 21, 55–57. [Google Scholar] [CrossRef]
- Fioranelli, M.; Garo, M.L.; Roccia, M.G.; Prizbelek, B.; Sconci, F.R. Brain–Heart Axis: Brain-Derived Neurotrophic Factor and Cardiovascular Disease—A Review of Systematic Reviews. Life 2023, 13, 2252. [Google Scholar] [CrossRef]
- Lee, S.S.; Yoo, Y.C. NOX-NOS Crosstalk in the Liver-Brain Axis: Novel Insights for Redox Regulation and Neurodegenerative Diseases. Redox Biol. 2025, 86, 103807. [Google Scholar] [CrossRef]
- Peng, Z.; Duggan, M.R.; Dark, H.E.; Daya, G.N.; An, Y.; Davatzikos, C.; Erus, G.; Lewis, A.; Moghekar, A.R.; Walker, K.A. Association of Liver Disease with Brain Volume Loss, Cognitive Decline, and Plasma Neurodegenerative Disease Biomarkers. Neurobiol. Aging 2022, 120, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Rivest, S. Lipid Metabolism and Neuroinflammation in Alzheimer’s Disease: A Role for Liver X Receptors. Endocr. Rev. 2012, 33, 715–746. [Google Scholar] [CrossRef]
- Kong, Y.; Zhao, K.; Zeng, D.; Lu, F.; Li, X.; Wu, Y.; Jiang, Z.; Wen, W. Effects of Vagus Nerve Stimulation on Cognitive Function in Patients with Epilepsy: A Systematic Review and Meta-Analysis. Front. Neurol. 2024, 15, 1332882. [Google Scholar] [CrossRef]
- Jin, Z.; Dong, J.; Wang, Y.; Liu, Y. Exploring the Potential of Vagus Nerve Stimulation in Treating Brain Diseases: A Review of Immunologic Benefits and Neuroprotective Efficacy. Eur. J. Med. Res. 2023, 28, 444. [Google Scholar] [CrossRef]
- Bodenlos, J.S.; Kose, S.; Borckardt, J.J.; Nahas, Z.; Shaw, D.; O’Neil, P.M.; George, M.S. Vagus Nerve Stimulation Acutely Alters Food Craving in Adults with Depression. Appetite 2007, 48, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Altuncevahir, İ.Ö. The Effects of Diet Content on the Vagus Nerve. Turk. Klin. Tradit. Complement. Med.—Spec. Top. 2024, 5, 41–44. [Google Scholar]
- Yan, H.f.; Zou, T.; Tuo, Q.z.; Xu, S.; Li, H.; Belaidi, A.A.; Lei, P. Ferroptosis: Mechanisms and Links with Diseases. Signal Transduct. Target. Ther. 2021, 6, 49. [Google Scholar] [CrossRef]
- Mohan, S.; Alhazmi, H.A.; Hassani, R.; Khuwaja, G.; Maheshkumar, V.P.; Aldahish, A.; Chidambaram, K. Role of Ferroptosis Pathways in Neuroinflammation and Neurological Disorders: From Pathogenesis to Treatment. Heliyon 2024, 10, e24786. [Google Scholar] [CrossRef]
- Cheng, Y.; Song, Y.; Chen, H.; Li, Q.; Gao, Y.; Lu, G.; Luo, C. Ferroptosis Mediated by Lipid Reactive Oxygen Species: A Possible Causal Link of Neuroinflammation to Neurological Disorders. Oxidative Med. Cell. Longev. 2021, 2021, 5005136. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhao, S.; Han, K.; Fan, L.; Zhao, C.; Yin, S.; Hu, H. Managing Ferroptosis-Related Diseases with Indirect Dietary Modulators of Ferroptosis. J. Nutr. Biochem. 2023, 120, 109427. [Google Scholar] [CrossRef] [PubMed]
- Weiland, A.; Wang, Y.; Wu, W.; Lan, X.; Han, X.; Li, Q.; Wang, J. Ferroptosis and Its Role in Diverse Brain Diseases. Mol. Neurobiol. 2018, 56, 4880. [Google Scholar] [CrossRef]
- Qin, Y.; Bai, D.; Tang, M.; Zhang, M.; Zhao, L.; Li, J.; Yang, R.; Jiang, G. Ketogenic Diet Alleviates Brain Iron Deposition and Cognitive Dysfunction via Nrf2-Mediated Ferroptosis Pathway in APP/PS1 Mouse. Brain Res. 2023, 1812, 148404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhan, Z.; Li, X.; Xing, A.; Jiang, C.; Chen, Y.; Shi, W.; An, L. Intermittent Fasting Protects against Alzheimer’s Disease Possible through Restoring Aquaporin-4 Polarity. Front. Mol. Neurosci. 2017, 10, 395. [Google Scholar] [CrossRef]
- Elias, A.; Padinjakara, N.; Lautenschlager, N.T. Effects of Intermittent Fasting on Cognitive Health and Alzheimer’s Disease. Nutr. Rev. 2023, 81, 1225–1233. [Google Scholar] [CrossRef]
- De Carvalho, T. Calorie Restriction or Dietary Restriction: How Far They Can Protect the Brain against Neurodegenerative Diseases? Neural Regen. Res. 2022, 17, 1640–1644. [Google Scholar] [CrossRef]
- Bronzini, M.; Maglione, A.; Rosso, R.; Matta, M.; Masuzzo, F.; Rolla, S.; Clerico, M. Feeding the Gut Microbiome: Impact on Multiple Sclerosis. Front. Immunol. 2023, 14, 1176016. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Molecular Mechanism of Caloric Restriction Mimetics-Mediated Neuroprotection of Age-Related Neurodegenerative Diseases: An Emerging Therapeutic Approach. Biogerontology 2023, 24, 679–708. [Google Scholar] [CrossRef] [PubMed]
- Martel, J.; Chang, S.H.; Wu, C.Y.; Peng, H.H.; Hwang, T.L.; Ko, Y.F.; Young, J.D.; Ojcius, D.M. Recent Advances in the Field of Caloric Restriction Mimetics and Anti-Aging Molecules. Ageing Res. Rev. 2021, 66, 101240. [Google Scholar] [CrossRef]
- Włodarek, D. Role of Ketogenic Diets in Neurodegenerative Diseases (Alzheimer’s Disease and Parkinson’s Disease). Nutrients 2019, 11, 169. [Google Scholar] [CrossRef] [PubMed]
- Richard, E.L.; Laughlin, G.A.; Kritz-Silverstein, D.; Reas, E.T.; Barrett-Connor, E.; McEvoy, L.K. Dietary Patterns and Cognitive Function among Older Community-Dwelling Adults. Nutrients 2018, 10, 1088. [Google Scholar] [CrossRef]
- Marchand, N.E.; Jensen, M.K. The Role of Dietary and Lifestyle Factors in Maintaining Cognitive. Am. J. Lifestyle Med. 2018, 12, 268–285. [Google Scholar] [CrossRef]
- Campdelacreu, J. Parkinson Disease and Alzheimer Disease: Environmental Risk Factors. Neurologia 2014, 29, 541–549. [Google Scholar] [CrossRef]
- Roberts, R.O.; Roberts, L.A.; Geda, Y.E.; Cha, R.H.; Pankratz, V.S.; O’Connor, H.M.; Knopman, D.S.; Petersen, R.C. Relative Intake of Macronutrients Impacts Risk of Mild Cognitive Impairment or Dementia. J. Alzheimer’s Dis. 2012, 32, 329–339. [Google Scholar] [CrossRef]
- Carneiro, L.; Pellerin, L. Nutritional Impact on Metabolic Homeostasis and Brain Health. Front. Neurosci. 2022, 15, 767405. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Trushina, E.; Morland, C.; Prigione, A.; Casadesus, G.; Andrews, Z.B.; Beal, M.F.; Bergersen, L.H.; Brinton, R.D.; de la Monte, S.; et al. Brain Energy Rescue: An Emerging Therapeutic Concept for Neurodegenerative Disorders of Ageing. Nat. Rev. Drug Discov. 2020, 19, 609–633. [Google Scholar] [CrossRef]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Bánáti, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E.; et al. Nutrition for the Ageing Brain: Towards Evidence for an Optimal Diet. Ageing Res. Rev. 2017, 35, 222–240. [Google Scholar] [CrossRef]
- Gabbianelli, R.; Damiani, E. Epigenetics and Neurodegeneration: Role of Early-Life Nutrition. J. Nutr. Biochem. 2018, 57, 1–13. [Google Scholar] [CrossRef]
- Daliry, A.; Pereira, E.N.G.d.S. Role of Maternal Microbiota and Nutrition in Early-Life Neurodevelopmental Disorders. Nutrients 2021, 13, 3533. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, L.W.J.; Garssen, J.; Burcelin, R.; Verhasselt, V. Shaping the Gut Microbiota by Breastfeeding: The Gateway to Allergy Prevention? Front. Pediatr. 2019, 7, 47. [Google Scholar] [CrossRef]
- Dalile, B.; Kim, C.; Challinor, A.; Geurts, L.; Gibney, E.R.; Galdos, M.V.; La Fata, G.; Layé, S.; Mathers, J.C.; Vauzour, D.; et al. The EAT–Lancet Reference Diet and Cognitive Function across the Life Course. Lancet Planet. Health 2022, 6, e749–e759. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, J.C.; Björck, I.M.E.; Nilsson, A.C. Impact of Rye-Based Evening Meals on Cognitive Functions, Mood and Cardiometabolic Risk Factors: A Randomized Controlled Study in Healthy Middle-Aged Subjects. Nutr. J. 2018, 17, 102. [Google Scholar] [CrossRef]
- Nyaradi, A.; Li, J.; Hickling, S.; Whitehouse, A.J.O.; Foster, J.K.; Oddy, W.H. Diet in the Early Years of Life Influences Cognitive Outcomes at 10 Years: A Prospective Cohort Study. Acta Paediatr. Int. J. Paediatr. 2013, 102, 1165–1173. [Google Scholar] [CrossRef]
- Kesse-Guyot, E.; Assmann, K.E.; Andreeva, V.A.; Ferry, M.; Hercberg, S.; Galan, P. Consumption of Dairy Products and Cognitive Functioning: Findings from the SU.VI.MAX 2 Study. J. Nutr. Health Aging 2016, 20, 128–137. [Google Scholar] [CrossRef]
- Liu, B.; Qiao, W.; Zhang, M.; Liu, Y.; Zhao, J.; Chen, L. Bovine Milk with Variant β-Casein Types on Immunological Mediated Intestinal Changes and Gut Health of Mice. Front. Nutr. 2022, 9, 970685. [Google Scholar] [CrossRef]
- Behera, R.; Sahu, A.; Mandal, A.; Rai, S.; Karunakaran, M.; Dutta, T. A1 versus A2 Milk—Impact on Human Health. Int. J. Livest. Res. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Jianqin, S.; Leiming, X.; Lu, X.; Yelland, G.W.; Ni, J.; Clarke, A.J. Effects of Milk Containing Only A2 Beta Casein versus Milk Containing Both A1 and A2 Beta Casein Proteins on Gastrointestinal Physiology, Symptoms of Discomfort, and Cognitive Behavior of People with Self-Reported Intolerance to Traditional Cows’ Milk. Nutr. J. 2016, 15, 35. [Google Scholar] [CrossRef]
- Wei, M.; Brandhorst, S.; Shelehchi, M.; Mirzaei, H.; Cheng, C.W.; Budniak, J.; Groshen, S.; Mack, W.J.; Guen, E.; Di Biase, S.; et al. Fasting-Mimicking Diet and Markers/Risk Factors for Aging, Diabetes, Cancer, and Cardiovascular Disease. Sci. Transl. Med. 2017, 9, eaai8700. [Google Scholar] [CrossRef]
- Khan, N.A.; Raine, L.B.; Drollette, E.S.; Scudder, M.R.; Hillman, C.H. The Relation of Saturated Fats and Dietary Cholesterol to Childhood Cognitive Flexibility. Appetite 2015, 93, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Martínez Leo, E.E.; Segura Campos, M.R. Effect of Ultra-Processed Diet on Gut Microbiota and Thus Its Role in Neurodegenerative Diseases. Nutrition 2020, 71, 110609. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.N.; Ipsen, D.H.; Schou-Pedersen, A.M.; Lykkesfeldt, J.; Tveden-Nyborg, P. Long Term Westernized Diet Leads to Region-Specific Changes in Brain Signaling Mechanisms. Neurosci. Lett. 2018, 676, 85–91. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, M.; Yuan, S.; Larsson, S.C.; Liu, X. Genetically Predicted Milk Intake and Risk of Neurodegenerative Diseases. Nutrients 2021, 13, 2893. [Google Scholar] [CrossRef]
- Gao, Q.; Luan, D.; Wang, X.; Xin, S.; Liu, Y.; Li, J. Effect of Sun Exposure on Cognitive Function among Elderly Individuals in Northeast China. Clin. Interv. Aging 2018, 13, 2075–2082. [Google Scholar] [CrossRef] [PubMed]
- Ylilauri, M.P.T.; Hantunen, S.; Lönnroos, E.; Salonen, J.T.; Tuomainen, T.P.; Virtanen, J.K. Associations of Dairy, Meat, and Fish Intakes with Risk of Incident Dementia and with Cognitive Performance: The Kuopio Ischaemic Heart Disease Risk Factor Study (KIHD). Eur. J. Nutr. 2022, 61, 2531–2542. [Google Scholar] [CrossRef]
- Cleret de Langavant, L.; Roze, E.; Petit, A.; Tressières, B.; Gharbi-Meliani, A.; Chaumont, H.; Michel, P.P.; Bachoud-Lévi, A.C.; Remy, P.; Edragas, R.; et al. Annonaceae Consumption Worsens Disease Severity and Cognitive Deficits in Degenerative Parkinsonism. Mov. Disord. 2022, 37, 2355–2366. [Google Scholar] [CrossRef]
- Krishnan, A.; Katchur, N. Food for Memory: A Review of the Effects of Diet on Alzheimer’s Disease Progression. J. Stud. Res. 2021, 10. [Google Scholar] [CrossRef]
- de la O, V.; Zazpe, I.; Goni, L.; Santiago, S.; Martín-Calvo, N.; Bes-Rastrollo, M.; Martínez, J.A.; Martínez-González, M.; Ruiz-Canela, M. A Score Appraising Paleolithic Diet and the Risk of Cardiovascular Disease in a Mediterranean Prospective Cohort. Eur. J. Nutr. 2022, 61, 957–971. [Google Scholar] [CrossRef]
- Titcomb, T.J.; Bisht, B.; Moore, D.D.; Chhonker, Y.S.; Murry, D.J.; Snetselaar, L.G.; Wahls, T.L. Eating Pattern and Nutritional Risks among People with Multiple Sclerosis Following a Modified Paleolithic Diet. Nutrients 2020, 12, 1844. [Google Scholar] [CrossRef]
- Genoni, A.; Lo, J.; Lyons-Wall, P.; Boyce, M.C.; Christophersen, C.T.; Bird, A.; Devine, A. A Paleolithic Diet Lowers Resistant Starch Intake but Does Not Affect Serum Trimethylamine-N-Oxide Concentrations in Healthy Women. Br. J. Nutr. 2019, 121, 322–329. [Google Scholar] [CrossRef]
- Challa, H.J.; Bandlamudi, M.; Uppaluri, K.R. Paleolithic Diets. In Nutrition and Cardiometabolic Health; CRC Press: Boca Raton, FL, USA, 2019; pp. 493–516. [Google Scholar] [CrossRef]
- Klonoff, D.C. The Beneficial Effects of a Paleolithic Diet on Type 2 Diabetes and Other Risk Factors for Cardiovascular Disease. J. Diabetes Sci. Technol. 2009, 3, 1229–1232. [Google Scholar] [CrossRef]
- Stomby, A.; Otten, J.; Ryberg, M.; Nyberg, L.; Olsson, T.; Boraxbekk, C.J. A Paleolithic Diet with and without Combined Aerobic and Resistance Exercise Increases Functional Brain Responses and Hippocampal Volume in Subjects with Type 2 Diabetes. Front. Aging Neurosci. 2017, 9, 391. [Google Scholar] [CrossRef]
- Marosi, K.; Mattson, M.P. BDNF Mediates Adaptive Brain and Body Responses to Energetic Challenges. Trends Endocrinol. Metab. 2014, 25, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, L.M.; Bujko, J. Evaluation of Biological and Clinical Potential of Paleolithic Diet. Rocz. Panstw. Zakl. Hig. 2012, 63, 9–15. [Google Scholar]
- Chenard, C.A.; Rubenstein, L.M.; Snetselaar, L.G.; Wahls, T.L. Nutrient Composition Comparison between a Modified Paleolithic Diet for Multiple Sclerosis and the Recommended Healthy U.S.-Style Eating Pattern. Nutrients 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Gardener, H.; Caunca, M.R. Mediterranean Diet in Preventing Neurodegenerative Diseases. Curr. Nutr. Rep. 2018, 7, 10–20. [Google Scholar] [CrossRef]
- Cole, G.M.; Lim, G.P.; Yang, F.; Teter, B.; Begum, A.; Ma, Q.; Harris-White, M.E.; Frautschy, S.A. Prevention of Alzheimer’s Disease: Omega-3 Fatty Acid and Phenolic Anti-Oxidant Interventions. Neurobiol. Aging 2005, 26, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Stefani, M. Nutraceutical Properties of Olive Oil Polyphenols. An Itinerary from Cultured Cells through Animal Models to Humans. Int. J. Mol. Sci. 2016, 17, 843. [Google Scholar] [CrossRef]
- Gardener, H.; Wright, C.B.; Rundek, T.; Sacco, R.L. Brain Health and Shared Risk Factors for Dementia and Stroke. Nat. Rev. Neurol. 2015, 11, 651–657. [Google Scholar] [CrossRef]
- Appelman, A.P.A.; Exalto, L.G.; Van Der Graaf, Y.; Biessels, G.J.; Mali, W.P.T.M.; Geerlings, M.I. White Matter Lesions and Brain Atrophy: More than Shared Risk Factors? A Systematic Review. Cerebrovasc. Dis. 2009, 28, 227–242. [Google Scholar] [CrossRef]
- Qosa, H.; Mohamed, L.A.; Batarseh, Y.S.; Alqahtani, S.; Ibrahim, B.; LeVine, H.; Keller, J.N.; Kaddoumi, A. Extra-Virgin Olive Oil Attenuates Amyloid-β and Tau Pathologies in the Brains of TgSwDI Mice. J. Nutr. Biochem. 2015, 26, 1479–1490. [Google Scholar] [CrossRef]
- Challa, H.J.; Ameer, M.A.; Uppaluri, K.R. DASH Diet To Stop Hypertension. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482514/ (accessed on 27 August 2025).
- Cremonini, A.L.; Caffa, I.; Cea, M.; Nencioni, A.; Odetti, P.; Monacelli, F. Nutrients in the Prevention of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2019, 2019, 9874159. [Google Scholar] [CrossRef]
- Daniel, G.D.; Chen, H.; Bertoni, A.G.; Rapp, S.R.; Fitzpatrick, A.L.; Luchsinger, J.A.; Wood, A.C.; Hughes, T.M.; Burke, G.L.; Hayden, K.M. DASH Diet Adherence and Cognitive Function: Multi-Ethnic Study of Atherosclerosis. Clin. Nutr. ESPEN 2021, 46, 223–231. [Google Scholar] [CrossRef]
- Nishi, S.K.; Babio, N.; Gómez-Martínez, C.; Martínez-González, M.Á.; Ros, E.; Corella, D.; Castañer, O.; Martínez, J.A.; Alonso-Gómez, Á.M.; Wärnberg, J.; et al. Mediterranean, DASH, and MIND Dietary Patterns and Cognitive Function: The 2-Year Longitudinal Changes in an Older Spanish Cohort. Front. Aging Neurosci. 2021, 13, 847. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, G.; Abbas-Zadeh, M.; Eftekhari, M.H. Effect of MIND Diet Intervention on Cognitive Performance and Brain Structure in Healthy Obese Women: A Randomized Controlled Trial. Sci. Rep. 2022, 12, 2871. [Google Scholar] [CrossRef]
- Levitan, E.B.; Wolk, A.; Mittleman, M.A. Relation of Consistency With the Dietary Approaches to Stop Hypertension Diet and Incidence of Heart Failure in Men Aged 45 to 79 Years. Am. J. Cardiol. 2009, 104, 1416–1420. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Shafie, D.; Moghaddam, R.H.; Sadeghi, M.; Safavi, S.M. Investigation of Adherence to DASH Diet Components and Reduction of Heart Failure Risk in Adults: A Case-Control Study. ARYA Atheroscler. 2024, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Cherian, L.; Wang, Y.; Fakuda, K.; Leurgans, S.; Aggarwal, N.; Morris, M. Mediterranean-Dash Intervention for Neurodegenerative Delay (MIND) Diet Slows Cognitive Decline After Stroke. J. Prev. Alzheimer’s Dis. 2019, 6, 267. [Google Scholar] [CrossRef] [PubMed]
- Mietelska-Porowska, A.; Domańska, J.; Want, A.; Więckowska-Gacek, A.; Chutorański, D.; Koperski, M.; Wojda, U. Induction of Brain Insulin Resistance and Alzheimer’s Molecular Changes by Western Diet. Int. J. Mol. Sci. 2022, 23, 4744. [Google Scholar] [CrossRef] [PubMed]
- The MIND Diet for Alzheimer’s Prevention|Everyday Health. Available online: https://www.everydayhealth.com/diet-and-nutrition/diet/mind-diet-can-this-diet-plan-help-reverse-alzheimers-disease/ (accessed on 27 August 2025).
- Pearson, K. The MIND Diet: A Detailed Guide for Beginners. Available online: https://www.healthline.com/nutrition/mind-diet#foods-to-eat (accessed on 27 August 2025).
- Kashiwaya, Y.; Takeshima, T.; Mori, N.; Nakashima, K.; Clarke, K.; Veech, R.L. D-β-Hydroxybutyrate Protects Neurons in Models of Alzheimer’s and Parkinson’s Disease. Proc. Natl. Acad. Sci. USA 2000, 97, 5440–5444. [Google Scholar] [CrossRef]
- Prins, M.L.; Matsumoto, J.H. The Collective Therapeutic Potential of Cerebral Ketone Metabolism in Traumatic Brain Injury. J. Lipid Res. 2014, 55, 2450–2457. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Bi, D.; Zhang, Y.; Kong, C.; Du, J.; Wu, X.; Wei, Q.; Qin, H. Ketogenic Diet for Human Diseases: The Underlying Mechanisms and Potential for Clinical Implementations. Signal Transduct. Target. Ther. 2022, 7, 11. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Jabir, M.S.; Albuhadily, A.K.; Al-Gareeb, A.I.; Jawad, S.F.; Swelum, A.A.; Hadi, N.R. Role of Ketogenic Diet in Neurodegenerative Diseases Focusing on Alzheimer Diseases: The Guardian Angle. Ageing Res. Rev. 2024, 95, 102233. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Murtagh, D.K.J.; Gilbertson, L.J.; Asztely, F.J.S.; Lynch, C.D.P. Low-Fat versus Ketogenic Diet in Parkinson’s Disease: A Pilot Randomized Controlled Trial. Mov. Disord. 2018, 33, 1306–1314. [Google Scholar] [CrossRef]
- Helms, E.R.; Zinn, C.; Rowlands, D.S.; Brown, S.R. A Systematic Review of Dietary Protein during Caloric Restriction in Resistance Trained Lean Athletes: A Case for Higher Intakes. Int. J. Sport. Nutr. Exerc. Metab. 2014, 24, 127–138. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, P. The Modified Atkins Diet in Refractory Epilepsy. Epilepsy Res. Treat. 2014, 2014, 404202. [Google Scholar] [CrossRef]
- Kang, H.C.; Lee, H.S.; You, S.J.; Kang, D.C.; Ko, T.S.; Kim, H.D. Use of a Modified Atkins Diet in Intractable Childhood Epilepsy. Epilepsia 2007, 48, 182–186. [Google Scholar] [CrossRef]
- Wahls, T.L.; Titcomb, T.J.; Bisht, B.; Eyck, P.T.; Rubenstein, L.M.; Carr, L.J.; Darling, W.G.; Hoth, K.F.; Kamholz, J.; Snetselaar, L.G. Impact of the Swank and Wahls Elimination Dietary Interventions on Fatigue and Quality of Life in Relapsing-Remitting Multiple Sclerosis: The WAVES Randomized Parallel-Arm Clinical Trial. Mult. Scler. J. Exp. Transl. Clin. 2021, 7, 20552173211035400. [Google Scholar] [CrossRef]
- Chenard, C.A.; Rubenstein, L.M.; Snetselaar, L.G.; Wahls, T.L. Nutrient Composition Comparison between the Low Saturated Fat Swank Diet for Multiple Sclerosis and Healthy U.S.-Style Eating Pattern. Nutrients 2019, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- SWANK, R.L. Treatment of Multiple Sclerosis with Low-Fat Diet: Result of Seven Years’ Experience. Ann. Intern. Med. 1956, 45, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Katz Sand, I. The Role of Diet in Multiple Sclerosis: Mechanistic Connections and Current Evidence. Curr. Nutr. Rep. 2018, 7, 150–160. [Google Scholar] [CrossRef]
- NIH National Institute on Aging. Parkinson’s Disease: Causes, Symptoms, and Treatments|National Institute on Aging. Available online: https://www.nia.nih.gov/health/parkinsons-disease/parkinsons-disease-causes-symptoms-and-treatments (accessed on 27 August 2025).
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Primers 2017, 3, 17013. [Google Scholar] [CrossRef]
- DeMaagd, G.; Philip, A. Parkinson’s Disease and Its Management Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. Pharm. Ther. 2015, 40, 504–532. [Google Scholar]
- Knight, E.; Geetha, T.; Burnett, D.; Babu, J.R. The Role of Diet and Dietary Patterns in Parkinson’s Disease. Nutrients 2022, 14, 4472. [Google Scholar] [CrossRef] [PubMed]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D. Role of Diet and Nutritional Supplements in Parkinson’s Disease Progression. Oxidative Med. Cell. Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef]
- Paknahad, Z.; Sheklabadi, E.; Moravejolahkami, A.R.; Chitsaz, A.; Hassanzadeh, A. The Effects of Mediterranean Diet on Severity of Disease and Serum Total Antioxidant Capacity (TAC) in Patients with Parkinson’s Disease: A Single Center, Randomized Controlled Trial. Nutr. Neurosci. 2022, 25, 313–320. [Google Scholar] [CrossRef]
- Yang, F.; Wolk, A.; Håkansson, N.; Pedersen, N.L.; Wirdefeldt, K. Dietary Antioxidants and Risk of Parkinson’s Disease in Two Population-Based Cohorts. Mov. Disord. 2017, 32, 1631–1636. [Google Scholar] [CrossRef]
- Zuccari, G.; Baldassari, S.; Ailuno, G.; Turrini, F.; Alfei, S.; Caviglioli, G. Formulation Strategies to Improve Oral Bioavailability of Ellagic Acid. Appl. Sci. 2020, 10, 3353. [Google Scholar] [CrossRef]
- Nagpal, D.; Verma, R.; Mittal, V.; Jeandet, P.; Kaushik, D. Targeted Therapies against Breast Cancer: Clinical Perspectives, Obstacles and New Opportunities. J. Drug Deliv. Sci. Technol. 2023, 89, 105049. [Google Scholar] [CrossRef]
- Petrou, B.; Ginzberg, A.; Binyamin, O.; Karussis, D. Beneficial Effects of a Nano Formulation of Pomegranate Seed Oil, GranaGard, on the Cognitive Function of Multiple Sclerosis Patients. Mult. Scler. Relat. Disord. 2021, 54, 103103. [Google Scholar] [CrossRef]
- Rusch, C.; Beke, M.; Tucciarone, L.; Nieves, C.; Ukhanova, M.; Tagliamonte, M.S.; Mai, V.; Suh, J.H.; Wang, Y.; Chiu, S.; et al. Mediterranean Diet Adherence in People With Parkinson’s Disease Reduces Constipation Symptoms and Changes Fecal Microbiota After a 5-Week Single-Arm Pilot Study. Front. Neurol. 2021, 12, 2307. [Google Scholar] [CrossRef] [PubMed]
- Maraki, M.I.; Yannakoulia, M.; Stamelou, M.; Stefanis, L.; Xiromerisiou, G.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Anastasiou, C.A.; et al. Mediterranean Diet Adherence Is Related to Reduced Probability of Prodromal Parkinson’s Disease. Mov. Disord. 2019, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, S.; Huang, X.; Tong, H.; Niu, H.; Lu, L. Neuroprotective Effect of a Medium-Chain Triglyceride Ketogenic Diet on MPTP-Induced Parkinson’s Disease Mice: A Combination of Transcriptomics and Metabolomics in the Substantia Nigra and Fecal Microbiome. Cell Death Discov. 2023, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.T.; Chen, J.H.; Huang, T.W. Probiotics Treatment for Parkinson Disease: A Systematic Review and Meta-Analysis of Clinical Trials. Aging 2022, 14, 7014–7025. [Google Scholar] [CrossRef]
- Jin, X.; Dong, W.; Chang, K.; Yan, Y.; Liu, X. Efficacy of Probiotic Supplements on Parkinson’s Disease: A Systematic Review and Meta-Analysis. Complement. Ther. Med. 2024, 82, 103045. [Google Scholar] [CrossRef]
- Hegelmaier, T.; Lebbing, M.; Duscha, A.; Tomaske, L.; Tönges, L.; Holm, J.B.; Bjørn Nielsen, H.; Gatermann, S.G.; Przuntek, H.; Haghikia, A. Interventional Influence of the Intestinal Microbiome Through Dietary Intervention and Bowel Cleansing Might Improve Motor Symptoms in Parkinson’s Disease. Cells 2020, 9, 376. [Google Scholar] [CrossRef]
- Barichella, M.; Cereda, E.; Pinelli, G.; Iorio, L.; Caroli, D.; Masiero, I.; Ferri, V.; Cassani, E.; Bolliri, C.; Caronni, S.; et al. Muscle-Targeted Nutritional Support for Rehabilitation in Patients with Parkinsonian Syndrome. Neurology 2019, 93, E485–E496. [Google Scholar] [CrossRef]
- Adhihetty, P.J.; Beal, M.F. Creatine and Its Potential Therapeutic Value for Targeting Cellular Energy Impairment in Neurodegenerative Diseases. Neuromol. Med. 2008, 10, 275–290. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Xu, W.; Gaglioti, A.H.; Holt, J.B.; Croft, J.B.; Mack, D.; McGuire, L.C. Racial and Ethnic Estimates of Alzheimer’s Disease and Related Dementias in the United States (2015–2060) in Adults Aged ≥65 Years. Alzheimer’s Dement. 2019, 15, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Golde, T.E. Alzheimer’s Disease—The Journey of a Healthy Brain into Organ Failure. Mol. Neurodegener. 2022, 17, 18. [Google Scholar] [CrossRef]
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Śliwińska, S.; Jeziorek, M. The Role of Nutrition in Alzheimer’s Disease. Rocz. Panstw. Zakl. Hig. 2021, 72, 29–39. [Google Scholar] [CrossRef]
- Lee, J.E.; Titcomb, T.J.; Bisht, B.; Rubenstein, L.M.; Louison, R.; Wahls, T.L. A Modified MCT-Based Ketogenic Diet Increases Plasma β-Hydroxybutyrate but Has Less Effect on Fatigue and Quality of Life in People with Multiple Sclerosis Compared to a Modified Paleolithic Diet: A Waitlist-Controlled, Randomized Pilot Study. J. Am. Coll. Nutr. 2021, 40, 13–25. [Google Scholar] [CrossRef]
- Brandt, J.; Buchholz, A.; Henry-Barron, B.; Vizthum, D.; Avramopoulos, D.; Cervenka, M.C. Preliminary Report on the Feasibility and Efficacy of the Modified Atkins Diet for Treatment of Mild Cognitive Impairment and Early Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 68, 969–981. [Google Scholar] [CrossRef]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized Crossover Trial of a Modified Ketogenic Diet in Alzheimer’s Disease. Alzheimer’s Res. Ther. 2021, 13, 51. [Google Scholar] [CrossRef]
- Esselun, C.; Dieter, F.; Sus, N.; Frank, J.; Eckert, G.P. Walnut Oil Reduces Aβ Levels and Increases Neurite Length in a Cellular Model of Early Alzheimer Disease. Nutrients 2022, 14, 1694. [Google Scholar] [CrossRef]
- Dhana, K.; James, B.D.; Agarwal, P.; Aggarwal, N.T.; Cherian, L.J.; Leurgans, S.E.; Barnes, L.L.; Bennett, D.A.; Schneider, J.A.; Gardener, H. MIND Diet, Common Brain Pathologies, and Cognition in Community-Dwelling Older Adults. J. Alzheimer’s Dis. 2021, 83, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.; Agrón, E.; Mares, J.A.; Clemons, T.E.; van Asten, F.; Swaroop, A.; Chew, E.Y. Adherence to a Mediterranean Diet and Cognitive Function in the Age-Related Eye Disease Studies 1 & 2. Alzheimer’s Dement. 2020, 16, 831–842. [Google Scholar] [CrossRef]
- Bello-Corral, L.; Sánchez-Valdeón, L.; Casado-Verdejo, I.; Seco-Calvo, J.Á.; Antonio Fernández-Fernández, J.; Nélida Fernández-Martínez, M. The Influence of Nutrition in Alzheimer’s Disease: Neuroinflammation and the Microbiome vs. Transmissible Prion. Front. Neurosci. 2021, 15, 1058. [Google Scholar] [CrossRef]
- Ashley, S.; Bradburn, S.; Murgatroyd, C. A Meta-Analysis of Peripheral Tocopherol Levels in Age-Related Cognitive Decline and Alzheimer’s Disease. Nutr. Neurosci. 2021, 24, 795–809. [Google Scholar] [CrossRef] [PubMed]
- Soininen, H.; Solomon, A.; Visser, P.J.; Hendrix, S.B.; Blennow, K.; Kivipelto, M.; Hartmann, T. 36-Month LipiDiDiet Multinutrient Clinical Trial in Prodromal Alzheimer’s Disease. Alzheimer’s Dement. 2021, 17, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Noel, K.; Hoffman, J.; Ellis, L.; Yurko-Mauro, K.; Cella, C.; Sercus, B.; Nalysnyk, L.; Fornazzari, L.; Castle, T.; Nadkarni, S.; et al. [P-167]: The Development of NIC5-15, a Natural Anti-Diabetic Agent, in the Treatment of Alzheimer’s Disease. Alzheimer’s Dement. 2005, 1, S62. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-Ketogenic Diet Modulates Gut Microbiome and Short-Chain Fatty Acids in Association with Alzheimer’s Disease Markers in Subjects with Mild Cognitive Impairment. eBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- Amelianchik, A.; Merkel, J.; Palanisamy, P.; Kaneki, S.; Hyatt, E.; Norris, E.H. The Protective Effect of Early Dietary Fat Consumption on Alzheimer’s Disease-Related Pathology and Cognitive Function in Mice. Alzheimer’s Dement. 2021, 7, e12173. [Google Scholar] [CrossRef]
- de la Rubia Ortí, J.E.; García-Pardo, M.P.; Drehmer, E.; Sancho Cantus, D.; Julián Rochina, M.; Aguilar, M.A.; Hu Yang, I. Improvement of Main Cognitive Functions in Patients with Alzheimer’s Disease after Treatment with Coconut Oil Enriched Mediterranean Diet: A Pilot Study. J. Alzheimer’s Dis. 2018, 65, 577–587. [Google Scholar] [CrossRef]
- Taylor, M.K.; Sullivan, D.K.; Swerdlow, R.H.; Vidoni, E.D.; Morris, J.K.; Mahnken, J.D.; Burns, J.M. A High-Glycemic Diet Is Associated with Cerebral Amyloid Burden in Cognitively Normal Older Adults. Am. J. Clin. Nutr. 2017, 106, 1463–1470. [Google Scholar] [CrossRef]
- Mazza, E.; Fava, A.; Ferro, Y.; Rotundo, S.; Romeo, S.; Bosco, D.; Pujia, A.; Montalcini, T. Effect of the Replacement of Dietary Vegetable Oils with a Low Dose of Extravirgin Olive Oil in the Mediterranean Diet on Cognitive Functions in the Elderly. J. Transl. Med. 2018, 16, 10. [Google Scholar] [CrossRef]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple Sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar] [CrossRef]
- Hauser, S.L.; Cree, B.A.C. Treatment of Multiple Sclerosis: A Review. Am. J. Med. 2020, 133, 1380–1390.e2. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke. Available online: https://www.ninds.nih.gov/health-information/disorders/multiple-sclerosis (accessed on 27 August 2025).
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between Genetic, Lifestyle and Environmental Risk Factors for Multiple Sclerosis. Nat. Rev. Neurol. 2017, 13, 26–36. [Google Scholar] [CrossRef]
- Munger, K.L.; Levin, L.I.; Hollis, B.W.; Howard, N.S.; Ascherio, A. Serum 25-Hydroxyvitamin D Levels and Risk of Multiple Sclerosis. JAMA 2006, 296, 2832–2838. [Google Scholar] [CrossRef]
- Hedström, A.K.; Olsson, T.; Alfredsson, L. Smoking Is a Major Preventable Risk Factor for Multiple Sclerosis. Mult. Scler. 2016, 22, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Van Der Mei, I.A.F.; Dwyer, T.; Blizzard, l.; Ponson, A.L.; Simmons, R.; Taylor, B.V.; Butzkueven, H.; Kilpatrick, T. Past Exposure to Sun, Skin Phenotype, and Risk of Multiple Sclerosis: Case-Control Study. BMJ 2003, 327, 316. [Google Scholar] [CrossRef]
- Gajofatto, A.; Benedetti, M.D. Treatment Strategies for Multiple Sclerosis: When to Start, When to Change, When to Stop? World J. Clin. Cases 2015, 3, 545. [Google Scholar] [CrossRef] [PubMed]
- Münzel, E.J.; Williams, A. Promoting Remyelination in Multiple Sclerosis-Recent Advances. Drugs 2013, 73, 2017–2029. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, T.; Antel, J.; König, F.B.; Brück, W.; Kuhlmann, T. Remyelination Capacity of the MS Brain Decreases with Disease Chronicity. Neurology 2009, 72, 1914–1921. [Google Scholar] [CrossRef]
- Langer-Gould, A.; Brara, S.M.; Beaber, B.E.; Koebnick, C. Childhood Obesity and Risk of Pediatric Multiple Sclerosis and Clinically Isolated Syndrome. Neurology 2013, 80, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Hedström, A.K.; Bomfim, I.L.; Barcellos, L.; Gianfrancesco, M.; Schaefer, C.; Kockum, I.; Olsson, T.; Alfredsson, L. Interaction between Adolescent Obesity and HLA Risk Genes in the Etiology of Multiple Sclerosis. Neurology 2014, 82, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Platero, J.L.; Cuerda-Ballester, M.; Ibáñez, V.; Sancho, D.; Lopez-Rodríguez, M.M.; Drehmer, E.; Ortí, J.E.d.l.R. The Impact of Coconut Oil and Epigallocatechin Gallate on the Levels of Il-6, Anxiety and Disability in Multiple Sclerosis Patients. Nutrients 2020, 12, 305. [Google Scholar] [CrossRef]
- Mokra, D.; Adamcakova, J.; Mokry, J. Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate (EGCG): A Time for a New Player in the Treatment of Respiratory Diseases? Antioxidants 2022, 11, 1566. [Google Scholar] [CrossRef]
- Von Geldern, G.; Mowry, E.M. The Influence of Nutritional Factors on the Prognosis of Multiple Sclerosis. Nat. Rev. Neurol. 2012, 8, 678–689. [Google Scholar] [CrossRef]
- Ramberg, J.E.; Nelson, E.D.; Sinnott, R.A. Immunomodulatory Dietary Polysaccharides: A Systematic Review of the Literature. Nutr. J. 2010, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Reginald McDaniel, H.; LaGanke, C.; Bloom, L.; Goldberg, S.; Lages, L.C.; Lantigua, L.A.; Atlas, S.E.; Woolger, J.M.; Lewis, J.E. The Effect of a Polysaccharide-Based Multinutrient Dietary Supplementation Regimen on Infections and Immune Functioning in Multiple Sclerosis. J. Diet. Suppl. 2020, 17, 184–199. [Google Scholar] [CrossRef]
- Shahi, S.K.; Ghimire, S.; Lehman, P.; Mangalam, A.K. Obesity Induced Gut Dysbiosis Contributes to Disease Severity in an Animal Model of Multiple Sclerosis. Front. Immunol. 2022, 13, 4874. [Google Scholar] [CrossRef]
- Katz Sand, I.B.; Fitzgerald, K.C.; Gu, Y.; Brandstadter, R.; Riley, C.S.; Buyukturkoglu, K.; Leavitt, V.M.; Krieger, S.; Miller, A.; Lublin, F.; et al. Dietary Factors and MRI Metrics in Early Multiple Sclerosis. Mult. Scler. Relat. Disord. 2021, 53, 103031. [Google Scholar] [CrossRef]
- Azary, S.; Schreiner, T.; Graves, J.; Waldman, A.; Belman, A.; Guttman, B.W.; Aaen, G.; Tillema, J.M.; Mar, S.; Hart, J.; et al. Contribution of Dietary Intake to Relapse Rate in Early Paediatric Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 28. [Google Scholar] [CrossRef]
- Thorburn, A.N.; Macia, L.; Mackay, C.R. Diet, Metabolites, and “Western-Lifestyle” Inflammatory Diseases. Immunity 2014, 40, 833–842. [Google Scholar] [CrossRef]
- Ruiz-Núñez, B.; Dijck-Brouwer, D.A.J.; Muskiet, F.A.J. The Relation of Saturated Fatty Acids with Low-Grade Inflammation and Cardiovascular Disease. J. Nutr. Biochem. 2016, 36, 1–20. [Google Scholar] [CrossRef]
- Huang, S.; Rutkowsky, J.M.; Snodgrass, R.G.; Ono-Moore, K.D.; Schneider, D.A.; Newman, J.W.; Adams, S.H.; Hwang, D.H. Saturated Fatty Acids Activate TLR-Mediated Proinflammatory Signaling Pathways. J. Lipid Res. 2012, 53, 2002–2013. [Google Scholar] [CrossRef]
- Augustin, K.; Khabbush, A.; Williams, S.; Eaton, S.; Orford, M.; Cross, J.H.; Heales, S.J.R.; Walker, M.C.; Williams, R.S.B. Mechanisms of Action for the Medium-Chain Triglyceride Ketogenic Diet in Neurological and Metabolic Disorders. Lancet Neurol. 2018, 17, 84–93. [Google Scholar] [CrossRef]
- Chatterjee, P.; Fernando, M.; Fernando, B.; Dias, C.B.; Shah, T.; Silva, R.; Williams, S.; Pedrini, S.; Hillebrandt, H.; Goozee, K.; et al. Potential of Coconut Oil and Medium Chain Triglycerides in the Prevention and Treatment of Alzheimer’s Disease. Mech. Ageing Dev. 2020, 186, 111209. [Google Scholar] [CrossRef]
- Balomenos, V.; Bounou, L.; Charisis, S.; Stamelou, M.; Ntanasi, E.; Georgiadi, K.; Mourtzinos, I.; Tzima, K.; Anastasiou, C.A.; Xiromerisiou, G.; et al. Dietary Inflammatory Index Score and Prodromal Parkinson’s Disease Incidence: The HELIAD Study. J. Nutr. Biochem. 2022, 105, 108994. [Google Scholar] [CrossRef]
- McMaster, M.; Kim, S.; Clare, L.; Torres, S.J.; Cherbuin, N.; D’Este, C.; Anstey, K.J. Lifestyle Risk Factors and Cognitive Outcomes from the Multidomain Dementia Risk Reduction Randomized Controlled Trial, Body Brain Life for Cognitive Decline (BBL-CD). J. Am. Geriatr. Soc. 2020, 68, 2629–2637. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Bhargava, P.; Smith, M.D.; Vizthum, D.; Henry-Barron, B.; Kornberg, M.D.; Cassard, S.D.; Kapogiannis, D.; Sullivan, P.; Baer, D.J.; et al. Intermittent Calorie Restriction Alters T Cell Subsets and Metabolic Markers in People with Multiple Sclerosis. eBioMedicine 2022, 82, 104124. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.C.; Vizthum, D.; Henry-Barron, B.; Schweitzer, A.; Cassard, S.D.; Kossoff, E.; Hartman, A.L.; Kapogiannis, D.; Sullivan, P.; Baer, D.J.; et al. Effect of Intermittent vs. Daily Calorie Restriction on Changes in Weight and Patient-Reported Outcomes in People with Multiple Sclerosis. Mult. Scler. Relat. Disord. 2018, 23, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; Stangl, G.I.; Dawin, E.; Bader, V.; Haase, S.; Kaisler, J.; David, C.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067–1080.e16. [Google Scholar] [CrossRef]
- Bronzini, M.; Maglione, A.; Rosso, R.; Masuzzo, F.; Matta, M.; Meroni, R.; Rolla, S.; Clerico, M. Lower Multiple Sclerosis Severity Score Is Associated with Higher Adherence to Mediterranean Diet in Subjects with Multiple Sclerosis from Northwestern Italy. Nutrients 2024, 16, 880. [Google Scholar] [CrossRef]
- Wijesekera, L.C.; Leigh, P.N. Amyotrophic Lateral Sclerosis. Orphanet J. Rare Dis. 2009, 4, 3. [Google Scholar] [CrossRef]
- Ragagnin, A.M.G.; Shadfar, S.; Vidal, M.; Jamali, M.S.; Atkin, J.D. Motor Neuron Susceptibility in ALS/FTD. Front. Neurosci. 2019, 13, 532. [Google Scholar] [CrossRef]
- Masrori, P.; Van Damme, P. Amyotrophic Lateral Sclerosis: A Clinical Review. Eur. J. Neurol. 2020, 27, 1918–1929. [Google Scholar] [CrossRef]
- Hardiman, O.; Al-Chalabi, A.; Chio, A.; Corr, E.M.; Logroscino, G.; Robberecht, W.; Shaw, P.J.; Simmons, Z.; Van Den Berg, L.H. Amyotrophic Lateral Sclerosis. Nat. Rev. Dis. Primers 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed]
- Caga, J.; Devenney, E.; Huynh, W.; Zoing, M.C.; Ahmed, R.M.; Kiernan, M.C. Illness Cognitions in ALS: New Insights Into Clinical Management of Behavioural Symptoms. Front. Neurol. 2021, 12, 1698. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, C.C.; Zamba-Papanicolaou, E.; Demetriou, C.A. Dietary Intake, Mediterranean Diet Adherence and Caloric Intake in Huntington’s Disease: A Review. Nutrients 2020, 12, 2946. [Google Scholar] [CrossRef]
- Motataianu, A.; Serban, G.; Barcutean, L.; Balasa, R. Oxidative Stress in Amyotrophic Lateral Sclerosis: Synergy of Genetic and Environmental Factors. Int. J. Mol. Sci. 2022, 23, 9339. [Google Scholar] [CrossRef]
- D’antona, S.; Caramenti, M.; Porro, D.; Castiglioni, I.; Cava, C. Amyotrophic Lateral Sclerosis: A Diet Review. Foods 2021, 10, 3128. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Wang, Y.; Zhang, L.; Tang, L.; Zhang, G.; Huang, T.; Huang, N.; Fan, D. Dietary-Derived Essential Nutrients and Amyotrophic Lateral Sclerosis: A Two-Sample Mendelian Randomization Study. Nutrients 2022, 14, 920. [Google Scholar] [CrossRef]
- Wei, T.; Guo, Z.; Wang, Z.; Li, X.; Zheng, Y.; Hou, H.; Tang, Y. Exploring the Causal Relationship between Dietary Macronutrients and Neurodegenerative Diseases: A Bi-Directional Two-Sample Mendelian Randomization Study. Ageing Neurodegener. Dis. 2022, 2, 14. [Google Scholar] [CrossRef]
- Weerasekera, A.; Sima, D.M.; Dresselaers, T.; Van Huffel, S.; Van Damme, P.; Himmelreich, U. Non-Invasive Assessment of Disease Progression and Neuroprotective Effects of Dietary Coconut Oil Supplementation in the ALS SOD1G93A Mouse Model: A 1H-Magnetic Resonance Spectroscopic Study. Neuroimage Clin. 2018, 20, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Ludolph, A.C.; Dorst, J.; Dreyhaupt, J.; Weishaupt, J.H.; Kassubek, J.; Weiland, U.; Meyer, T.; Petri, S.; Hermann, A.; Emmer, A.; et al. Effect of High-Caloric Nutrition on Survival in Amyotrophic Lateral Sclerosis. Ann. Neurol. 2020, 87, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Roos, R.A.C. Huntington’s Disease: A Clinical Review. Orphanet J. Rare Dis. 2010, 5, 40. [Google Scholar] [CrossRef]
- Chen, C.M.; Lin, Y.S.; Wu, Y.R.; Chen, P.; Tsai, F.J.; Yang, C.L.; Tsao, Y.T.; Chang, W.; Hsieh, I.S.; Chern, Y.; et al. High Protein Diet and Huntington’s Disease. PLoS ONE 2015, 10, e0127654. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, S.J.; Flower, M.D.; Ross, C.A.; Wild, E.J. Huntington Disease: New Insights into Molecular Pathogenesis and Therapeutic Opportunities. Nat. Rev. Neurol. 2020, 16, 529–546. [Google Scholar] [CrossRef]
- Pizzorni, N.; Pirola, F.; Ciammola, A.; Schindler, A. Management of Dysphagia in Huntington’s Disease: A Descriptive Review. Neurol. Sci. 2020, 41, 1405–1417. [Google Scholar] [CrossRef]
- Suganya, S.N.; Sumathi, T. Effect of Rutin against a Mitochondrial Toxin, 3-Nitropropionicacid Induced Biochemical, Behavioral and Histological Alterations-a Pilot Study on Huntington’s Disease Model in Rats. Metab. Brain Dis. 2017, 32, 471–481. [Google Scholar] [CrossRef]
- Elifani, F.; Amico, E.; Pepe, G.; Capocci, L.; Castaldo, S.; Rosa, P.; Montano, E.; Pollice, A.; Madonna, M.; Filosa, S.; et al. Curcumin Dietary Supplementation Ameliorates Disease Phenotype in an Animal Model of Huntington’s Disease. Hum. Mol. Genet. 2019, 28, 4012–4021. [Google Scholar] [CrossRef]
- Aganzo, M.; Montojo, M.T.; López de Las Hazas, M.C.; Martínez-Descals, A.; Ricote-Vila, M.; Sanz, R.; González-Peralta, I.; Martín-Hernández, R.; de Dios, O.; Garcés, C.; et al. Customized Dietary Intervention Avoids Unintentional Weight Loss and Modulates Circulating MiRNAs Footprint in Huntington’s Disease. Mol. Nutr. Food Res. 2018, 62, e1800619. [Google Scholar] [CrossRef]
- Doyen, M.; Lambert, C.; Roeder, E.; Boutley, H.; Chen, B.; Pierson, J.; Verger, A.; Raffo, E.; Karcher, G.; Marie, P.Y.; et al. Assessment of a One-Week Ketogenic Diet on Brain Glycolytic Metabolism and on the Status Epilepticus Stage of a Lithium–Pilocarpine Rat Model. Sci. Rep. 2024, 14, 5063. [Google Scholar] [CrossRef]
- Gzieło, K.; Janeczko, K.; Węglarz, W.; Jasiński, K.; Kłodowski, K.; Setkowicz, Z. MRI Spectroscopic and Tractography Studies Indicate Consequences of Long-Term Ketogenic Diet. Brain Struct. Funct. 2020, 225, 2077–2089. [Google Scholar] [CrossRef]
- Lefebvre, S.; Bürglen, L.; Reboullet, S.; Clermont, O.; Burlet, P.; Viollet, L.; Benichou, B.; Cruaud, C.; Millasseau, P.; Zeviani, M.; et al. Identification and Characterization of a Spinal Muscular Atrophy-Determining Gene. Cell 1995, 80, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Subramony, S.H. Degenerative Ataxias: Challenges in Clinical Research. Ann. Clin. Transl. Neurol. 2017, 4, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Schulz, J.B.; Boesch, S.; Bürk, K.; Dürr, A.; Giunti, P.; Mariotti, C.; Pousset, F.; Schöls, L.; Vankan, P.; Pandolfo, M. Diagnosis and Treatment of Friedreich Ataxia: A European Perspective. Nat. Rev. Neurol. 2009, 5, 222–234. [Google Scholar] [CrossRef]
- Punga, T.; Bühler, M. Long Intronic GAA Repeats Causing Friedreich Ataxia Impede Transcription Elongation. EMBO Mol. Med. 2010, 2, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.K.; DiDonato, C.J.; Larsen, P.D. Friedreich’s Ataxia: Clinical Presentation of a Compound Heterozygote Child with a Rare Nonsense Mutation and Comparison with Previously Published Cases. Case Rep. Neurol. Med. 2018, 2018, 8587203. [Google Scholar] [CrossRef]
- Wagner, G.R.; Melanie Pride, P.; Babbey, C.M.; Mark Payne, R. Friedreich’s Ataxia Reveals a Mechanism for Coordinate Regulation of Oxidative Metabolism via Feedback Inhibition of the SIRT3 Deacetylase. Hum. Mol. Genet. 2012, 21, 2688–2697. [Google Scholar] [CrossRef]
- Soragni, E.; Gottesfeld, J.M. Translating HDAC Inhibitors in Friedreich’s Ataxia. Expert Opin. Orphan Drugs 2016, 4, 961–970. [Google Scholar] [CrossRef]
- Davis, R.H.; Godshall, B.J.; Seffrood, E.; Marcus, M.; Lasalle, B.A.; Wong, B.; Schroth, M.K.; Swoboda, K.J. Nutritional Practices at a Glance: Spinal Muscular Atrophy Type I Nutrition Survey Findings. J. Child. Neurol. 2014, 29, 1467. [Google Scholar] [CrossRef]
- Taylor, J.P.; McKeith, I.G.; Burn, D.J.; Boeve, B.F.; Weintraub, D.; Bamford, C.; Allan, L.M.; Thomas, A.J.; O’Brien, J.T. New Evidence on the Management of Lewy Body Dementia. Lancet Neurol. 2020, 19, 157. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, S.T. Modern Management of Spinal Muscular Atrophy. J. Child. Neurol. 2007, 22, 974–978. [Google Scholar] [CrossRef]
- La Pean, A.; Jeffries, N.; Grow, C.; Ravina, B.; di Prospero, N.A. Predictors of Progression in Patients with Friedreich Ataxia. Mov. Disord. 2008, 23, 2026–2032. [Google Scholar] [CrossRef]
- Myers, L.; Farmer, J.M.; Wilson, R.B.; Friedman, L.; Tsou, A.; Perlman, S.L.; Subramony, S.H.; Gomez, C.M.; Ashizawa, T.; Wilmot, G.R.; et al. Antioxidant Use in Friedreich Ataxia. J. Neurol. Sci. 2008, 267, 174–176. [Google Scholar] [CrossRef] [PubMed]
- Seto, M.; Weiner, R.L.; Dumitrescu, L.; Hohman, T.J. Protective Genes and Pathways in Alzheimer’s Disease: Moving towards Precision Interventions. Mol. Neurodegener. 2021, 16, 29. [Google Scholar] [CrossRef] [PubMed]

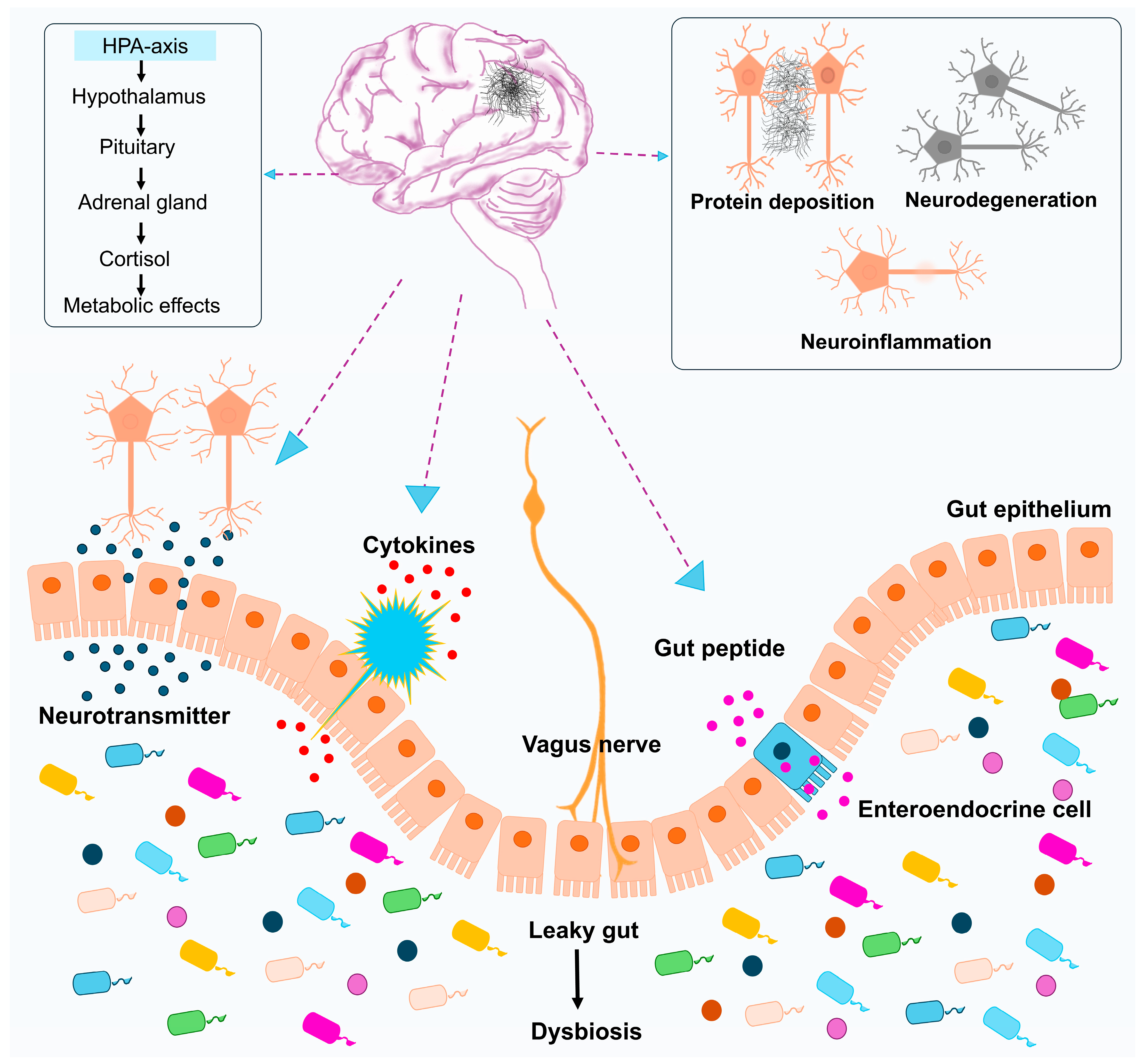
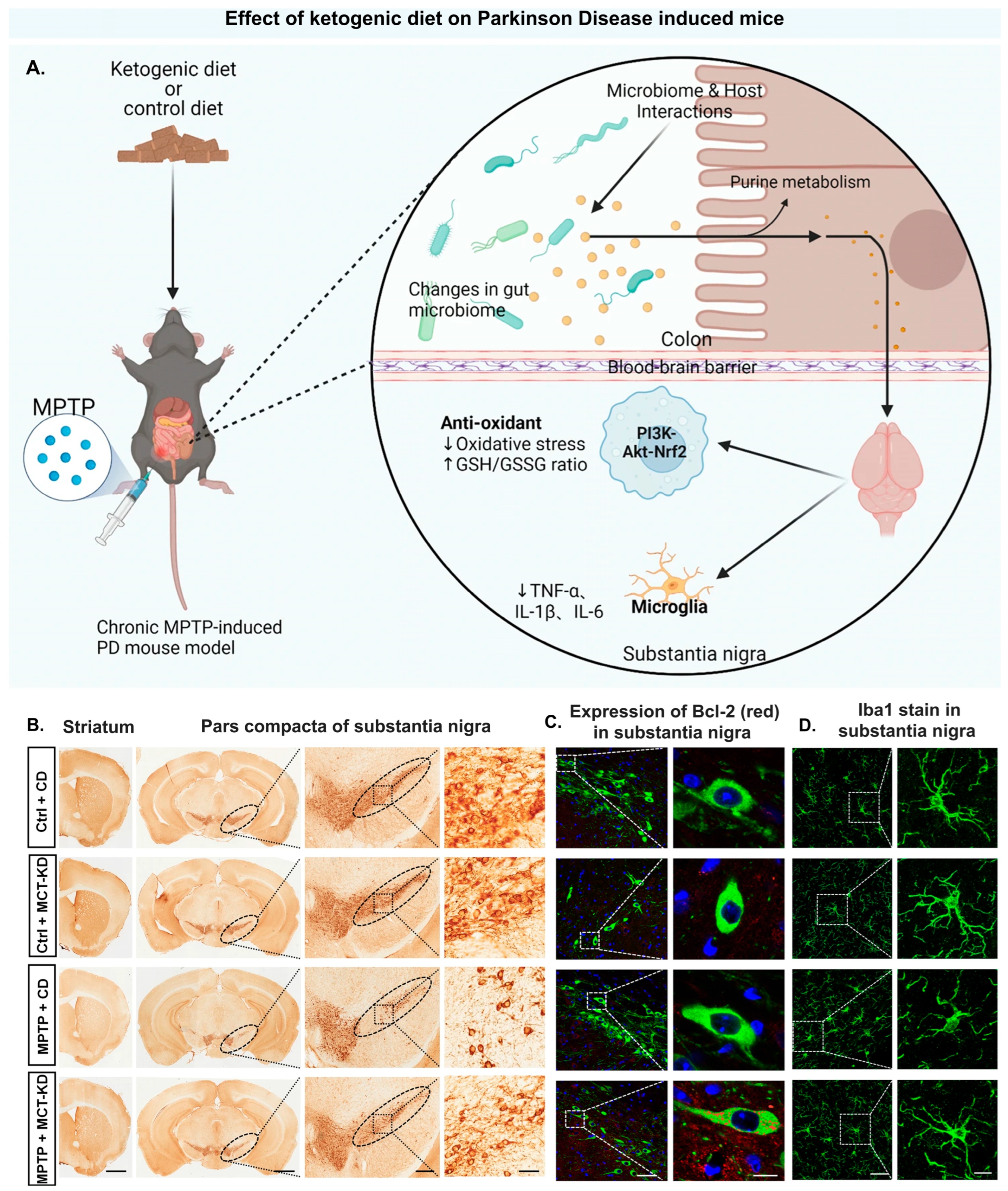
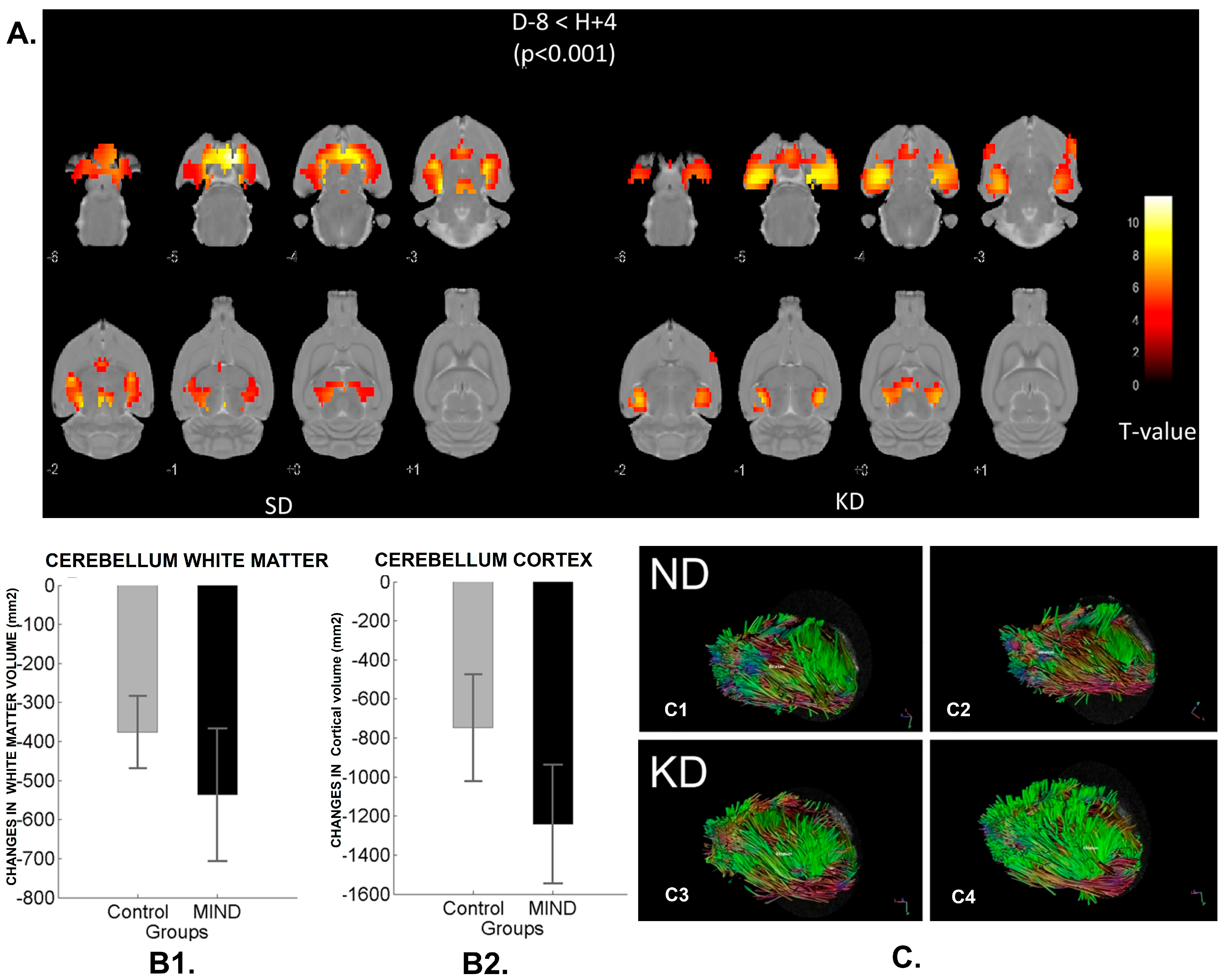
| Mitophagy Inducer | Source | Impact on Mitochondrial Function | Impact on Brain Function |
|---|---|---|---|
| Urolithin A | Gut microbiota–derived metabolite (natural polyphenol ellagic acid) | Activation of mitophagy [71] | Reduces neuroinflammation and improves cognition [89] |
| Resveratrol | Non-flavonoid polyphenol | Relieves the oxidative stress, reduces mitochondrial damage, and prevents apoptosis [81] | Mitophagy imparts neuroprotective effects [90] |
| Palmatine | Alkaloid from Coptis chinensis and Berberis aristata | Enhances mitochondrial membrane potential and reduces mitochondrial ROS. [81] | Improves cognitive function in an AD mouse model [91] |
| Tetrahydro-cannabinol | Cannabinoid from Cannabis sativa | Activates mitophagy via the PINK1/Parkin pathway [92] | Modulates neuronal energy metabolism [93] |
| Berberine | Alkaloid from Coptis chinensis and Berberis aristata | Activation of the AMPK pathway that induced mitophagy [94] | Alleviates cognitive dysfunction, imparts neuroprotection, and inhibits Aβ production [95] |
| Quinic acid | Polyphenol from millet | Activates mitochondrial ATP synthase–dependent respiration [96] | Reduces high-fat diet–induced brain oxidative stress [85] |
| Catechin | Flavonoid from green tea | Promotes mitochondrial biogenesis [96] | Prevents neurodegeneration [97] |
| Curcumin | Polyphenol from Curcuma longa | Induction of mitophagy [98] | Alleviates mitochondrial dysfunction in the brain [99] |
| Astaxanthin | Carotenoid from microglia | Induction of mitophagy through PINK1/PARKIN pathway activation [98] | Reduces Aβ production [100] |
| Spermidine | Polyamine obtained from putrescine | Induction of mitophagy through PINK1/PARKIN pathway and improvement of mitochondrial respiration [101] | Reduces Aβ levels and improves cognitive function [101] |
| Food as Medicine | Mechanism of Action | Bioactive Compounds/Chemical Constitutes | Outcome | Ref. |
|---|---|---|---|---|
| Curcumin. | Prohibits gastrointestinal intestinal dysfunction, conserves homeostasis of the intestine, and decreases malabsorption. | Polyphenols. | Normal motor function, preserved neuropathology, GI upset, GI emptying, and intestinal contractility. | [323] |
| Rutin (25 and 50 mg/kg). | Antioxidant activity. | Flavonoids. | For Huntington’s treatment, rutin may be a drug of choice. | [322] |
| Green tea. | Decreases mutant Huntingtin-associated neurodegeneration in Drosophila. | (−)-Epigallocatechin-3-gallate. | Green tea consumption indicates positive effects on symptoms of Huntington’s disease. | [47] |
| Diet containing olive oil and fish oil. | Increases GABA levels. | Polyunsaturated fatty acid (PUFA) and monounsaturated fatty acid (MUFA). | Maintains muscle strength and decreases unwanted weight loss associated with Huntington’s disease. | [324] |
| Diet containing fruits and vegetables | Increases antioxidant levels and promotes neurogenesis. | Polyphenols and flavonoids. | Improves anxiety and decreases depression. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagpal, D.; Nema, S.; Nagpal, S.; Pandey, M.M.; Kaushik, D.; Kathuria, H. Management and Prevention of Neurodegenerative Disorders: Can Antioxidant-Rich Dietary Interventions Help? Antioxidants 2025, 14, 1078. https://doi.org/10.3390/antiox14091078
Nagpal D, Nema S, Nagpal S, Pandey MM, Kaushik D, Kathuria H. Management and Prevention of Neurodegenerative Disorders: Can Antioxidant-Rich Dietary Interventions Help? Antioxidants. 2025; 14(9):1078. https://doi.org/10.3390/antiox14091078
Chicago/Turabian StyleNagpal, Diksha, Shivangi Nema, Shakti Nagpal, Murali Monohar Pandey, Deepak Kaushik, and Himanshu Kathuria. 2025. "Management and Prevention of Neurodegenerative Disorders: Can Antioxidant-Rich Dietary Interventions Help?" Antioxidants 14, no. 9: 1078. https://doi.org/10.3390/antiox14091078
APA StyleNagpal, D., Nema, S., Nagpal, S., Pandey, M. M., Kaushik, D., & Kathuria, H. (2025). Management and Prevention of Neurodegenerative Disorders: Can Antioxidant-Rich Dietary Interventions Help? Antioxidants, 14(9), 1078. https://doi.org/10.3390/antiox14091078






