The Dual Role of Nanomaterials in Ovarian Cancer and Female Fertility as Anti- and Prooxidants
Abstract
1. Introduction
1.1. Nanomaterials Generalities
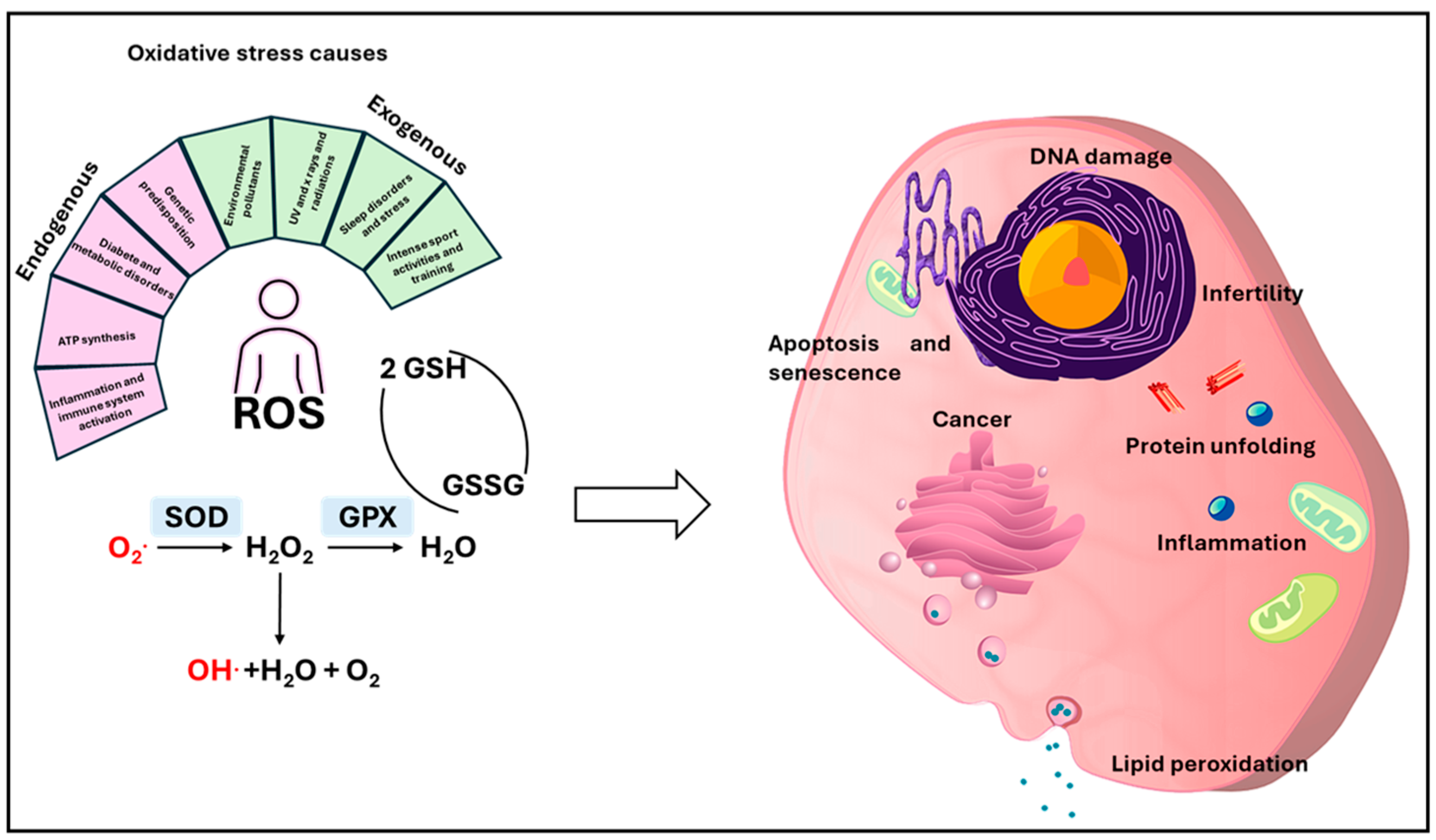
1.2. Oxidative Stress
1.3. Female Reproductive Health and Oxidative Stress
1.4. Methodologies
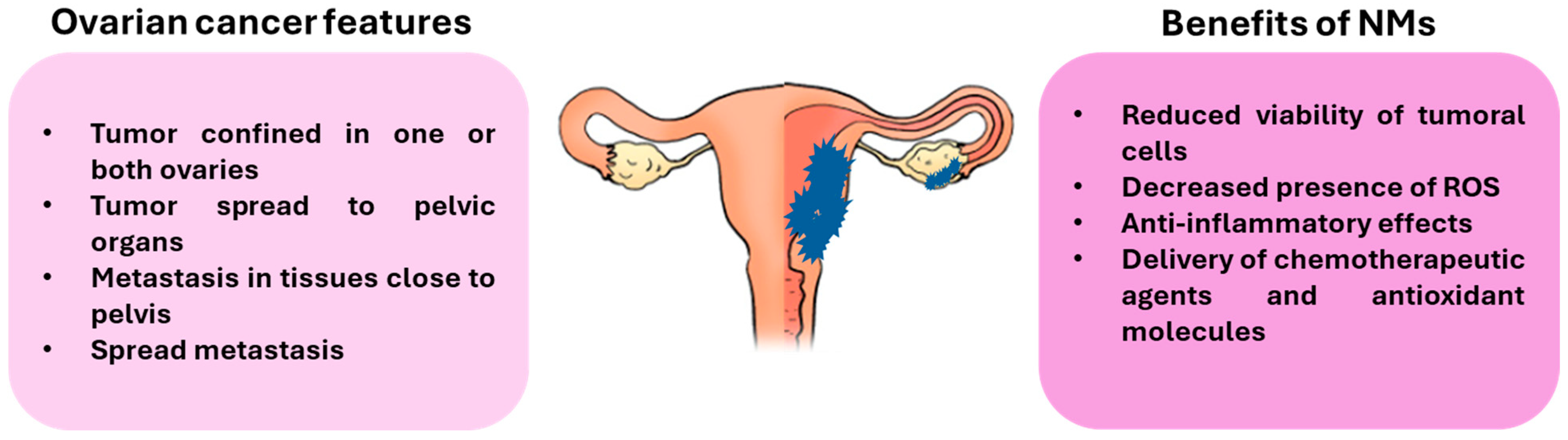
2. NMs and Ovarian Cancer (OC)
3. NMs and Female Fertility
3.1. Antioxidant Effects
3.2. Prooxidant Effects
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Ema, M.; Okuda, H.; Gamo, M.; Honda, K. A review of reproductive and developmental toxicity of silver nanoparticles in laboratory animals. Reprod. Toxicol. 2017, 67, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef]
- Boyes, W.K.; van Thriel, C. Neurotoxicology of nanomaterials. Chem. Res. Toxicol. 2020, 33, 1121–1144. [Google Scholar] [CrossRef] [PubMed]
- Tepe, N.; Bau, M. Importance of nanoparticles and colloids from volcanic ash for riverine transport of trace elements to the ocean: Evidence from glacial-fed rivers after the 2010 eruption of Eyjafjallajökull Volcano, Iceland. Sci. Total Environ. 2014, 488–489, 243–251. [Google Scholar] [CrossRef]
- Ermolin, M.S.; Fedotov, P.S.; Malik, N.A.; Karandashev, V.K. Nanoparticles of volcanic ash as a carrier for toxic elements on the global scale. Chemosphere 2018, 200, 16–22. [Google Scholar] [CrossRef]
- Churg, A.; Muller, N.L. Update on silicosis. Surg. Pathol. Clin. 2024, 17, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Colafarina, S.; Di Carlo, P.; Zarivi, O.; Aloisi, M.; Di Serafino, A.; Aruffo, E.; Arrizza, L.; Limongi, T.; Poma, A. Genotoxicity response of fibroblast cells and human epithelial adenocarcinoma in vitro model exposed to bare and ozone-treated silica microparticles. Cells 2022, 11, 226. [Google Scholar] [CrossRef]
- Katz, L.M.; Dewan, K.; Bronaugh, R.L. Nanotechnology in cosmetics. Food Chem. Toxicol. 2015, 85, 127–137. [Google Scholar] [CrossRef]
- Casiraghi, C.; Jurchescu, O.D.; Magdassi, S.; Su, W. Introduction to nanomaterials for printed electronics. Nanoscale 2023, 15, 10480–10483. [Google Scholar] [CrossRef]
- Hochella, M.F., Jr.; Mogk, D.W.; Ranville, J.; Allen, I.C.; Luther, G.W.; Marr, L.C.; McGrail, B.P.; Murayama, M.; Qafoku, N.P.; Rosso, K.M.; et al. Natural, incidental, and engineered nanomaterials and their impacts on the Earth system. Science 2019, 363, eaau8299. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef] [PubMed]
- Le, N.; Zhang, M.; Kim, K. Quantum dots and their interaction with biological systems. Int. J. Mol. Sci. 2022, 23, 10763. [Google Scholar] [CrossRef]
- Ota, S.; Li, T.; Li, Y.; Ye, Z.; Labno, A.; Yin, X.; Alam, M.R.; Zhang, X. Brownian motion of tethered nanowires. Phys. Rev. E 2014, 89, 053010. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, S.; Zhang, T.; Liu, Q.; Alvarez, P.J.J.; Chen, W. Current methods and prospects for analysis and characterization of nanomaterials in the environment. Environ. Sci. Technol. 2022, 56, 7426–7447. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.R.; Desu, P.K.; Nakkala, R.K.; Kondi, V.; Devi, S.; Alam, M.S.; Hamid, H.; Athawale, R.B.; Kesharwani, P. Nanotechnology-based approaches applied to nutraceuticals. Drug Deliv. Transl. Res. 2022, 12, 485–499. [Google Scholar] [CrossRef]
- Cong, Y.; Baimanov, D.; Zhou, Y.; Chen, C.; Wang, L. Penetration and translocation of functional inorganic nanomaterials into biological barriers. Adv. Drug Deliv. Rev. 2022, 191, 114615. [Google Scholar] [CrossRef]
- Parvez, S.; Karole, A.; Mudavath, S.L. Transport mechanism of hydroxy-propyl-beta-cyclodextrin modified solid lipid nanoparticles across human epithelial cells for the oral absorption of antileishmanial drugs. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130157. [Google Scholar] [CrossRef]
- Wang, W.D.; Guo, Y.Y.; Yang, Z.L.; Su, G.L.; Sun, Z.J. Sniping Cancer Stem Cells with Nanomaterials. ACS Nano 2023, 17, 23262–23298. [Google Scholar] [CrossRef] [PubMed]
- Oroojalian, F.; Beygi, M.; Baradaran, B.; Mokhtarzadeh, A.; Shahbazi, M.A. Immune Cell Membrane-Coated Biomimetic Nanoparticles for Targeted Cancer Therapy. Small 2021, 17, e2006484. [Google Scholar] [CrossRef]
- Deng, R.; Zhao, R.; Zhang, Z.; Chen, Y.; Yang, M.; Lin, Y.; Ye, J.; Li, N.; Qin, H.; Yan, X.; et al. Chondrocyte membrane-coated nanoparticles promote drug retention and halt cartilage damage in rat and canine osteoarthritis. Sci. Transl. Med. 2024, 16, eadh9751. [Google Scholar] [CrossRef]
- Kopac, T. Protein corona, understanding the nanoparticle-protein interactions and future perspectives: A critical review. Int. J. Biol. Macromol. 2021, 169, 290–301. [Google Scholar] [CrossRef]
- Zhou, F.; Liao, F.; Chen, L.; Liu, Y.; Wang, W.; Feng, S. The size-dependent genotoxicity and oxidative stress of silica nanoparticles on endothelial cells. Environ. Sci. Pollut. Res. Int. 2019, 26, 1911–1920. [Google Scholar] [CrossRef]
- Du, X.; Zhang, M.; Ma, Y.; Wang, X.; Liu, Y.; Huang, H.; Kang, Z. Size-dependent antibacterial of carbon dots by selective absorption and differential oxidative stress of bacteria. J. Colloid Interface Sci. 2023, 634, 44–53. [Google Scholar] [CrossRef]
- Crecente-Campo, J.; Guerra-Varela, J.; Peleteiro, M.; Gutiérrez-Lovera, C.; Fernández-Mariño, I.; Diéguez-Docampo, A.; González-Fernández, Á.; Sánchez, L.; Alonso, M.J. The size and composition of polymeric nanocapsules dictate their interaction with macrophages and biodistribution in zebrafish. J. Control. Release 2019, 308, 98–108. [Google Scholar] [CrossRef]
- Baroli, B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm. Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef] [PubMed]
- Furtado, D.; Björnmalm, M.; Ayton, S.; Bush, A.I.; Kempe, K.; Caruso, F. Overcoming the blood-brain barrier: The role of nanomaterials in treating neurological diseases. Adv. Mater. 2018, 30, e1801362. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, J.; Wang, H.; Chen, C.; Han, M.; Gao, L.; Tang, C.; Sun, P.; Zhao, X.; Guo, F.; et al. Trojan horse nanocapsule enabled in situ modulation of the phenotypic conversion of Th17 cells to treg cells for the treatment of multiple sclerosis in mice. Adv. Mater. 2023, 35, e2210262. [Google Scholar] [CrossRef]
- Dassonville-Klimpt, A.; Sonnet, P. Advances in ‘Trojan horse’ strategies in antibiotic delivery systems. Future Med. Chem. 2020, 12, 983–986. [Google Scholar] [CrossRef]
- Fu, F.; Crespy, D.; Landfester, K.; Jiang, S. In situ characterization techniques of protein corona around nanomaterials. Chem. Soc. Rev. 2024, 53, 10827–10851. [Google Scholar] [CrossRef] [PubMed]
- Kladko, D.V.; Falchevskaya, A.S.; Serov, N.S.; Prilepskii, A.Y. Nanomaterial shape influence on cell behavior. Int. J. Mol. Sci. 2021, 22, 5266. [Google Scholar] [CrossRef]
- Enea, M.; Pereira, E.; de Almeida, M.P.; Araújo, A.M.; de Lourdes Bastos, M.; Carmo, H. Gold nanoparticles induce oxidative stress and apoptosis in human kidney cells. Nanomaterials 2020, 10, 995. [Google Scholar] [CrossRef] [PubMed]
- Sultana, S.; Djaker, N.; Boca-Farcau, S.; Salerno, M.; Charnaux, N.; Astilean, S.; Hlawaty, H.; De La Chapelle, M.L. Comparative toxicity evaluation of flower-shaped and spherical gold nanoparticles on human endothelial cells. Nanotechnology 2015, 26, 055101. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Santra, C.R.; Ghosh, A.N.; Karmakar, P. Differential toxicity of rod and spherical zinc oxide nanoparticles on human peripheral blood mononuclear cells. J. Biomed. Nanotechnol. 2014, 10, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Niu, M.; Zhong, H.; Shao, H.; Hong, D.; Ma, T.; Xu, K.; Chen, X.; Han, J.; Sun, J. Shape-dependent genotoxicity of mesoporous silica nanoparticles and cellular mechanisms. J. Nanosci. Nanotechnol. 2016, 16, 2313–2318. [Google Scholar] [CrossRef]
- Arnida Malugin, A.; Ghandehari, H. Cellular uptake and toxicity of gold nanoparticles in prostate cancer cells: A comparative study of rods and spheres. J. Appl. Toxicol. 2010, 30, 212–217. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Li, J.; Liu, Z.; Cheng, L. Inorganic nanomaterials with rapid clearance for biomedical applications. Chem. Soc. Rev. 2021, 50, 8669–8742. [Google Scholar] [CrossRef] [PubMed]
- Sowmiya, P.; Dhas, T.S.; Inbakandan, D.; Anandakumar, N.; Nalini, S.; Suganya, K.S.U.; Remya, R.R.; Karthick, V.; Kumar, C.M.V. Optically active organic and inorganic nanomaterials for biological imaging applications: A review. Micron 2023, 172, 103486. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Mokkapati, V.R.S.S.; Garg, A.; Ravikumar, V.; Mijakovic, I. Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 2018, 19, 1979. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Rahdar, A. Functional nanomaterials in biomedicine: Current uses and potential applications. Chem. Med. Chem. 2022, 17, e202200142. [Google Scholar] [CrossRef]
- Cavallaro, P.A.; De Santo, M.; Belsito, E.L.; Longobucco, C.; Curcio, M.; Morelli, C.; Pasqua, L.; Leggio, A. Peptides targeting HER2-positive breast cancer cells and applications in tumor imaging and delivery of chemotherapeutics. Nanomaterials 2023, 13, 2476. [Google Scholar] [CrossRef]
- Stefanick, J.F.; Kiziltepe, T.; Bilgicer, B. Improved peptide-targeted liposome design through optimized peptide hydrophilicity, ethylene glycol linker length, and peptide density. J. Biomed. Nanotechnol. 2015, 11, 1418–1430. [Google Scholar] [CrossRef]
- Landsiedel, R.; Fabian, E.; Ma-Hock, L.; van Ravenzwaay, B.; Wohlleben, W.; Wiench, K.; Oesch, F. Toxico-/biokinetics of nanomaterials. Arch. Toxicol. 2012, 86, 1021–1060, Erratum in Arch. Toxicol. 2012, 86, 1061. [Google Scholar] [CrossRef]
- Nam, M.; Lee, J.; Lee, K.Y.; Kim, J. Sequential targeted delivery of liposomes to ischemic tissues by controlling blood vessel permeability. ACS Biomater. Sci. Eng. 2018, 4, 532–538. [Google Scholar] [CrossRef]
- Wang, N.; Chen, M.; Wang, T. Liposomes used as a vaccine adjuvant-delivery system: From basics to clinical immunization. J. Control Release 2019, 303, 130–150. [Google Scholar] [CrossRef]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef]
- Adler-Moore, J.; Lewis, R.E.; Brüggemann, R.J.M.; Rijnders, B.J.A.; Groll, A.H.; Walsh, T.J. Preclinical safety, tolerability, pharmacokinetics, pharmacodynamics, and antifungal activity of liposomal amphotericin B. Clin. Infect. Dis. 2019, 68, S244–S259. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Meyers, S.R.; Juhn, F.S.; Griset, A.P.; Luman, N.R.; Grinstaff, M.W. Anionic amphiphilic dendrimers as antibacterial agents. J. Am. Chem. Soc. 2008, 130, 14444–14445. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Anes, A.; Spataro, G.; Coppel, Y.; Moog, C.; Blanzat, M.; Turrin, C.O.; Caminade, A.M.; Rico-Lattes, I.; Majoral, J.P. Phosphonate terminated PPH dendrimers: Influence of pendant alkyl chains on the in vitro anti-HIV-1 properties. Org. Biomol. Chem. 2009, 7, 3491–3498. [Google Scholar] [CrossRef]
- Dhanikula, R.S.; Argaw, A.; Bouchard, J.F.; Hildgen, P. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: Enhanced efficacy and intratumoral transport capability. Mol. Pharm. 2008, 5, 105–116. [Google Scholar] [CrossRef]
- Lee, C.C.; Gillies, E.R.; Fox, M.E.; Guillaudeu, S.J.; Fréchet, J.M.; Dy, E.E.; Szoka, F.C. A single dose of doxorubicin-functionalized bow-tie dendrimer cures mice bearing C-26 colon carcinomas. Proc. Natl. Acad. Sci. USA 2006, 103, 16649–16654. [Google Scholar] [CrossRef]
- Zhong, S.; Yung, L.Y. Enhanced biological stability of collagen with incorporation of PAMAM dendrimer. J. Biomed. Mater. Res. A 2009, 91, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Rudovský, J.; Botta, M.; Hermann, P.; Hardcastle, K.I.; Lukes, I.; Aime, S. PAMAM dendrimeric conjugates with a Gd-DOTA phosphinate derivative and their adducts with polyaminoacids: The interplay of global motion, internal rotation, and fast water exchange. Bioconjug. Chem. 2006, 17, 975–987. [Google Scholar] [CrossRef]
- Han, X.M.; Zheng, K.W.; Wang, R.L.; Yue, S.F.; Chen, J.; Zhao, Z.W.; Song, F.; Su, Y.; Ma, Q. Functionalization and optimization-strategy of graphene oxide-based nanomaterials for gene and drug delivery. Am. J. Transl. Res. 2020, 12, 1515–1534. [Google Scholar]
- Xu, J.; Xu, K.; Han, Y.; Wang, D.; Li, X.; Hu, T.; Yi, H.; Ni, Z. A 3D porous graphene aerogel@ GOx based microfluidic biosensor for electrochemical glucose detection. Analyst 2020, 145, 5141–5147. [Google Scholar] [CrossRef]
- Sheng, Z.; Song, L.; Zheng, J.; Hu, D.; He, M.; Zheng, M.; Gao, G.; Gong, P.; Zhang, P.; Ma, Y.; et al. Protein-assisted fabrication of nano-reduced graphene oxide for combined in vivo photoacoustic imaging and photothermal therapy. Biomaterials 2013, 34, 5236–5243. [Google Scholar] [CrossRef]
- Shin, S.R.; Zihlmann, C.; Akbari, M.; Assawes, P.; Cheung, L.; Zhang, K.; Manoharan, V.; Zhang, Y.S.; Yüksekkaya, M.; Wan, K.T.; et al. Reduced graphene oxide-GelMA hybrid hydrogels as scaffolds for cardiac tissue engineering. Small 2016, 12, 3677–3689. [Google Scholar] [CrossRef]
- Araya-Hermosilla, R.; Dervillé, F.; Cohn-Inostroza, N.; Picchioni, F.; Pescarmona, P.P.; Poortinga, A. Pickering Emulsions and Antibubbles Stabilized by PLA/PLGA Nanoparticles. Langmuir 2022, 38, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sung, J.; Alghoul, Z.; Merlin, D. Preparation, Characterization, and Cell Uptake of PLGA/PLA-PEG-FA Nanoparticles. Bio Protoc. 2022, 12, e4373. [Google Scholar] [CrossRef] [PubMed]
- Banappagari, S.; Ronald, S.; Satyanarayanajois, S.D. A conformationally constrained peptidomimetic binds to the extracellular region of HER2 protein. J. Biomol. Struct. Dyn. 2010, 28, 289–308. [Google Scholar] [CrossRef]
- Stefanick, J.F.; Ashley, J.D.; Bilgicer, B. Enhanced cellular uptake of peptide-targeted nanoparticles through increased peptide hydrophilicity and optimized ethylene glycol peptide-linker length. ACS Nano 2013, 7, 8115–8127. [Google Scholar] [CrossRef]
- Nakajima, H.; Mizuta, N.; Sakaguchi, K.; Fujiwara, I.; Yoshimori, A.; Takahashi, S.; Takasawa, R.; Tanuma, S. Development of HER2-antagonistic peptides as novel anti-breast cancer drugs by in silico methods. Breast Cancer 2008, 15, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, G.; Xu, J.; Li, C.; Han, S.; Zhang, C.; Wu, P.; Lin, Y.; Wang, C.; Zhang, J.; et al. Hydrogel Transformed from Nanoparticles for Prevention of Tissue Injury and Treatment of Inflammatory Diseases. Adv. Mater. 2022, 34, 2109178. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Lu, F.; Chang, Q. The diversified hydrogels for biomedical applications and their imperative roles in tissue regeneration. Biomater. Sci. 2023, 11, 2639–2660. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Fu, Z.; Li, H.; Wei, R.; Guo, J.; Wang, H.; Qi, J. Smart hydrogel: A new platform for cancer therapy. Adv. Colloid. Interface Sci. 2025, 340, 103470. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Gericke, M.; Schulze, P.; Heinze, T. Nanoparticles Based on Hydrophobic Polysaccharide Derivatives-Formation Principles, Characterization Techniques, and Biomedical Applications. Macromol. Biosci. 2020, 20, e1900415. [Google Scholar] [CrossRef]
- Allawadhi, P.; Singh, V.; Govindaraj, K.; Khurana, I.; Sarode, L.P.; Navik, U.; Banothu, A.K.; Weiskirchen, R.; Bharani, K.K.; Khurana, A. Biomedical applications of polysaccharide nanoparticles for chronic inflammatory disorders: Focus on rheumatoid arthritis, diabetes and organ fibrosis. Carbohydr. Polym. 2022, 281, 118923. [Google Scholar] [CrossRef]
- Saravanakumar, G.; Jo, D.G.; Park, J.H. Polysaccharide-based nanoparticles: A versatile platform for drug delivery and biomedical imaging. Curr. Med. Chem. 2012, 19, 3212–3229. [Google Scholar] [CrossRef]
- Swierczewska, M.; Han, H.S.; Kim, K.; Park, J.H.; Lee, S. Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv. Drug Deliv. Rev. 2016, 99 Pt A, 70–84. [Google Scholar] [CrossRef]
- Bakshi, M.S. Colloidal micelles of block copolymers as nanoreactors, templates for gold nanoparticles, and vehicles for biomedical applications. Adv. Colloid Interface Sci. 2014, 213, 1–20. [Google Scholar] [CrossRef]
- Cao, Z.; Zuo, X.; Liu, X.; Xu, G.; Yong, K.T. Recent progress in stimuli-responsive polymeric micelles for targeted delivery of functional nanoparticles. Adv. Colloid Interface Sci. 2024, 330, 103206. [Google Scholar] [CrossRef] [PubMed]
- Hallan, S.S.; Ferrara, F.; Sguizzato, M.; Cortesi, R. Cyclodextrin-Based Nanotransporters as a Versatile Tool to Manage Oxidative Stress-Induced Lung Diseases. Antioxidants 2025, 14, 1007. [Google Scholar] [CrossRef] [PubMed]
- Jana, M.; Biswas, U.K.; Patro, C.S.; Debnath, B.; Sharma, S.; Naskar, S. Solid Lipid Nanoparticles: A Review of their Biomedical Applications and Preparation. Pharm. Nanotechnol. 2024, 13, 758–774. [Google Scholar] [CrossRef]
- Eygeris, Y.; Gupta, M.; Kim, J.; Sahay, G. Chemistry of Lipid Nanoparticles for RNA Delivery. Acc. Chem. Res. 2022, 55, 2–12. [Google Scholar] [CrossRef]
- Li, L.; Xing, H.; Zhang, J.; Lu, Y. Functional DNA Molecules Enable Selective and Stimuli-Responsive Nanoparticles for Biomedical Applications. Acc. Chem Res. 2019, 52, 2415–2426. [Google Scholar] [CrossRef]
- Bhumkar, D.R.; Joshi, H.M.; Sastry, M.; Pokharkar, V.B. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm. Res. 2007, 24, 1415–1426. [Google Scholar] [CrossRef]
- Tong, L.; Zhao, Y.; Huff, T.B.; Hansen, M.N.; Wei, A.; Cheng, J.X. Gold nanorods mediate tumor cell death by compromising membrane integrity. Adv. Mater. 2007, 19, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Medley, C.D.; Smith, J.E.; Tang, Z.; Wu, Y.; Bamrungsap, S.; Tan, W. Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells. Anal. Chem. 2008, 80, 1067–1072. [Google Scholar] [CrossRef]
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnology 2010, 8, 19. [Google Scholar] [CrossRef]
- Gopinath, V.; Velusamy, P. Extracellular biosynthesis of silver nanoparticles using Bacillus sp. GP-23 and evaluation of their antifungal activity towards Fusarium oxysporum. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2013, 106, 170–174. [Google Scholar] [CrossRef]
- Saad, H.A.; Soliman, M.I.; Azzam, A.M.; Mostafa, B. Antiparasitic activity of silver and copper oxide nanoparticles against entamoeba histolytica and cryptosporidium parvum cysts. J. Egypt Soc. Parasitol. 2015, 45, 593–602. [Google Scholar]
- Zhang, M.; Zhang, K.; De Gusseme, B.; Verstraete, W.; Field, R. The antibacterial and anti-biofouling performance of biogenic silver nanoparticles by Lactobacillus fermentum. Biofouling 2014, 30, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.N.; Newall, N.; Kapp, S.E.; Lewin, G.; Karimi, L.; Carville, K.; Gliddon, T.; Santamaria, N.M. A randomized-controlled trial comparing cadexomer iodine and nanocrystalline silver on the healing of leg ulcers. Wound Repair Regen. 2010, 18, 359–367. [Google Scholar] [CrossRef]
- Sriram, M.I.; Kanth, S.B.; Kalishwaralal, K.; Gurunathan, S. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int. J. Nanomedicine 2010, 5, 753–762. [Google Scholar] [PubMed]
- Benyettou, F.; Rezgui, R.; Ravaux, F.; Jaber, T.; Blumer, K.; Jouiad, M.; Motte, L.; Olsen, J.C.; Platas-Iglesias, C.; Magzoub, M.; et al. Synthesis of silver nanoparticles for the dual delivery of doxorubicin and alendronate to cancer cells. J. Mater. Chem. B 2015, 3, 7237–7245. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.A.; Boegli, L.; James, G.; Velasquillo, C.; Sánchez-Sánchez, R.; Martínez-Martínez, R.E.; Martínez-Castañón, G.A.; Martinez-Gutierrez, F. Silver nanoparticles with antimicrobial activities against Streptococcus mutans and their cytotoxic effect. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 55, 360–366. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, B.; Jiang, H.; Wang, C.; Wang, H.; Wang, X. A strategy for ZnO nanorod mediated multi-mode cancer treatment. Biomaterials 2011, 32, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wu, C.; Jiang, H.; Li, Q.; Wang, X.; Chen, B. Synergistic cytotoxic effect of different sized ZnO nanoparticles and daunorubicin against leukemia cancer cells under UV irradiation. J. Photochem. Photobiol. B 2008, 93, 119–126. [Google Scholar] [CrossRef]
- Zhao, J.; Wu, D.; Zhi, J. A novel tyrosinase biosensor based on biofunctional ZnO nanorod microarrays on the nanocrystalline diamond electrode for detection of phenolic compounds. Bioelectrochemistry 2009, 75, 44–49. [Google Scholar] [CrossRef]
- Fu, S.; Chen, H.; Yang, W.; Xia, X.; Zhao, S.; Xu, X.; Ai, P.; Cai, Q.; Li, X.; Wang, Y.; et al. ROS-targeted depression therapy via BSA-incubated ceria nanoclusters. Nano Lett. 2022, 22, 4519–4527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Q.; Xu, Q.; Li, B.; Dong, H.; Mou, Y. Polydopamine modified ceria nanorods alleviate inflammation in colitis by scavenging ROS and regulating macrophage M2 polarization. Int. J. Nanomed. 2023, 18, 4601–4616. [Google Scholar] [CrossRef]
- Fox, C.R.; Kedarinath, K.; Neal, C.J.; Sheiber, J.; Kolanthai, E.; Kumar, U.; Drake, C.; Seal, S.; Parks, G.D. Broad-spectrum, potent, and durable ceria nanoparticles inactivate RNA virus infectivity by targeting virion surfaces and disrupting virus-receptor interactions. Molecules 2023, 28, 5190. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Tang, L.; Zhu, H. Titanium dioxide nanoparticles enhance photocurrent generation of cyanobacteria. Biochem. Biophys. Res. Commun. 2023, 672, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Landsiedel, R.; Ma-Hock, L.; Van Ravenzwaay, B.; Schulz, M.; Wiench, K.; Champ, S.; Schulte, S.; Wohlleben, W.; Oesch, F. Gene toxicity studies on titanium dioxide and zinc oxide nanomaterials used for UV-protection in cosmetic formulations. Nanotoxicology 2010, 4, 364–381. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, N.; Sun, Y.; Zhao, W.W.; Ye, D.; Xu, J.J.; Chen, H.Y. Activatable QD-based near-infrared fluorescence probe for sensitive detection and imaging of DNA. ACS Appl. Mater. Interfaces 2017, 9, 25107–25113. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiu, W.; Sun, Y.; Zhu, D.; Zhang, Q.; Yuwen, L.; Weng, L.; Teng, Z.; Wang, L. RGD-QD-MoS2 nanosheets for targeted fluorescent imaging and photothermal therapy of cancer. Nanoscale 2017, 9, 15835–15845. [Google Scholar] [CrossRef]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Oxidative stress. In Encyclopedia of Stress, 2nd ed.; Fink, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 3, pp. 45–48. [Google Scholar]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.I.; Radu, C.I.; Vladâcenco, O.; Roza, E.; Costăchescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Ho, H.J.; Shirakawa, H. Oxidative stress and mitochondrial dysfunction in chronic kidney disease. Cells 2022, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Hu, Y.; Jiang, L.; Lei, L.; Fu, C.; Wu, S.; Zhang, X.; Zhu, L.; Zhang, F.; Chen, J.; et al. Decreased oxidative stress response and oxidant detoxification of skin during aging. Mech. Ageing Dev. 2023, 216, 111878. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Aguayo, F.; Sergi, C.M.; Juarranz, A.; Roy, D. Antioxidants and cancer: Theories, techniques, and trials in preventing cancer. Oxid. Med. Cell. Longev. 2018, 2018, 5363064. [Google Scholar] [CrossRef]
- Vecchiotti, G.; Colafarina, S.; Aloisi, M.; Zarivi, O.; Di Carlo, P.; Poma, A. Genotoxicity and oxidative stress induction by polystyrene nanoparticles in the colorectal cancer cell line HCT116. PLoS ONE 2021, 16, e0255120. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709, Erratum in Nat. Rev. Drug Discov. 2021, 20, 652. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Behrman, H.R.; Kodaman, P.H.; Preston, S.L.; Gao, S. Oxidative stress and the ovary. J. Soc. Gynecol. Investig. 2001, 8, S40–S42. [Google Scholar] [CrossRef] [PubMed]
- Shkolnik, K.; Tadmor, A.; Ben-Dor, S.; Nevo, N.; Galiani, D.; Dekel, N. Reactive oxygen species are indispensable in ovulation. Proc. Natl. Acad. Sci. USA 2011, 108, 1462–1467. [Google Scholar] [CrossRef]
- Tropea, A.; Miceli, F.; Minici, F.; Tiberi, F.; Orlando, M.; Gangale, M.F.; Romani, F.; Catino, S.; Mancuso, S.; Navarra, P.; et al. Regulation of vascular endothelial growth factor synthesis and release by human luteal cells in vitro. J. Clin. Endocrinol. Metab. 2006, 91, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Hamadneh, S.; Hamadneh, J. Active and passive maternal smoking during pregnancy and birth outcomes: A study from a developing country. Ann. Glob. Health 2021, 87, 122. [Google Scholar] [CrossRef]
- Ujhelyi Gomez, K.; Goodwin, L.; Chisholm, A.; Rose, A.K. Alcohol use during pregnancy and motherhood: Attitudes and experiences of pregnant women, mothers, and healthcare professionals. PLoS ONE 2022, 17, 0275609. [Google Scholar] [CrossRef]
- Hayer, S.; Mandelbaum, A.D.; Watch, L.; Ryan, K.S.; Hedges, M.A.; Manuzak, J.A.; Easley, C.A.; Schust, D.J.; Lo, J.O. Cannabis and pregnancy: A Review. Obstet. Gynecol. Surv. 2023, 78, 411–428. [Google Scholar] [CrossRef]
- Dutta, S.; Gorain, B.; Choudhury, H.; Roychoudhury, S.; Sengupta, P. Environmental and occupational exposure of metals and female reproductive health. Environ. Sci. Pollut. Res. Int. 2022, 29, 62067–62092. [Google Scholar] [CrossRef] [PubMed]
- Ovarian Cancer Research Alliance. Statistics. Available online: https://ocrahope.org/for-patients/gynecologic-cancers/ovarian-cancer/ovarian-cancer-statistics/ (accessed on 29 August 2025).
- Vargas, A.N. Natural history of ovarian cancer. Ecancermedicalscience 2014, 8, 465. [Google Scholar]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian Cancer: An Integrated Review. Semin. Oncol. Nurs. 2019, 35, 151–156. [Google Scholar] [CrossRef]
- La Vecchia, C. Ovarian cancer: Epidemiology and risk factors. Eur. J. Cancer Prev. 2017, 26, 55–62. [Google Scholar] [CrossRef]
- Coelho, R.; Tozzi, A.; Disler, M.; Lombardo, F.; Fedier, A.; Lopez, M.N.; Freuler, F.; Jacob, F.; Heinzelmann-Schwarz, V. Overlapping gene dependencies for PARP inhibitors and carboplatin response identified by functional CRISPR-Cas9 screening in ovarian cancer. Cell Death Dis. 2022, 13, 909. [Google Scholar] [CrossRef] [PubMed]
- Muggia, F. Weekly Carboplatin and Paclitaxel for Ovarian Cancer: The “Finer Points”. Oncologist 2021, 26, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xie, H.J.; Li, Y.Y.; Wang, X.; Liu, X.X.; Mai, J. Molecular mechanisms of platinum-based chemotherapy resistance in ovarian cancer (Review). Oncol. Rep. 2022, 47, 82. [Google Scholar] [CrossRef]
- Morand, S.; Devanaboyina, M.; Staats, H.; Stanbery, L.; Nemunaitis, J. Ovarian cancer immunotherapy and personalized medicine. Int. J. Mol. Sci. 2021, 22, 6532. [Google Scholar] [CrossRef]
- Jain, N.K.; Tare, M.S.; Mishra, V.; Tripathi, P.K. The development, characterization and in vivo anti-ovarian cancer activity of poly(propylene imine) (PPI)-antibody conjugates containing encapsulated paclitaxel. Nanomedicine 2015, 11, 207–218. [Google Scholar] [CrossRef]
- Pandey, S.; Rai, L.C.; Dubey, S.K. Cyanobacteria: Miniature factories for green synthesis of metallic nanomaterials: A review. Biometals 2022, 35, 653–674. [Google Scholar] [CrossRef]
- Irannejad, F.; Shahbazi, S.; Reiisi, S.; Heidari, R. Study of the effect of zinc oxide, selenium, and silver nanoparticles on the expression level of oxidative stress-associated genes in ovarian cancer. Med. Oncol. 2025, 42, 39. [Google Scholar] [CrossRef]
- Scicchitano, S.; Gagliardi, A.; Ambrosio, N.; Vecchio, E.; Garofalo, C.; Battaglia, A.M.; Costanzo, F.S.; Faniello, M.C.; Cosco, D. Exploring the effects of paclitaxel-loaded zein nanoparticles on human ovarian carcinoma cells. Sci. Rep. 2025, 15, 10553. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Fang, J.; Cao, Z.; Shang, Y.; Alfarraj, S.; Ali Alharbi, S.; Duan, X.; Yang, S.; Li, J. Green synthesis, characterization, cytotoxicity, antioxidant, and anti-human ovarian cancer activities of Curcumae kwangsiensis leaf aqueous extract green-synthesized gold nanoparticles. Arabian J. Chem. 2021, 14, 103000. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Verma, R.; Salgotra, T.; Rahman, M.H.; Shah, M.; Akter, R.; Murad, W.; Mubin, S.; Bibi, P.; Qusti, S.; et al. Impacting the remedial potential of nano delivery-based flavonoids for breast cancer treatment. Molecules 2021, 26, 5163. [Google Scholar] [CrossRef]
- Bahrami, A.; Makiabadi, E.; Jalali, S.; Heidari, Z.; Assadi, M.; Rashidkhani, B. Dietary intake of polyphenols and the risk of breast cancer: A case-control study. Clin. Nutr. Res. 2021, 10, 330–340. [Google Scholar] [CrossRef]
- Park, M.Y.; Kim, Y.; Ha, S.E.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Jeong, S.H.; Kim, G.S. Function and application of flavonoids in the breast cancer. Int. J. Mol. Sci. 2022, 23, 7732. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yin, Q.; Shen, J.; Chen, L.; Yu, H.; Zhang, Z.; Li, Y. Synergistic inhibition of breast cancer metastasis by silibinin-loaded lipid nanoparticles containing TPGS. Int. J. Pharm. 2013, 454, 21–30. [Google Scholar] [CrossRef]
- Ding, S.M.; Zhang, Z.H.; Song, J.; Cheng, X.D.; Jiang, J.; Jia, X.B. Enhanced bioavailability of apigenin via preparation of a carbon nano powder solid dispersion. Int. J. Nanomed. 2014, 9, 2327. [Google Scholar] [CrossRef] [PubMed]
- Shindikar, A.; Singh, A.; Nobre, M.; Kirolikar, S. Curcumin and Resveratrol as Promising Natural Remedies with Nanomedicine Approach for the Effective Treatment of Triple Negative Breast Cancer. J. Oncol. 2016, 2016, 9750785. [Google Scholar] [CrossRef]
- Niazaand, F.; Razizadeh, M.; Khorsandi, L.; Abbaspour, M.; Mansouri, E.; Khodadadi, A. Effects of quercetin-loaded nanoparticles on MCF-7 human breast cancer cells. Medicina 2019, 55, 114. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.; Hou, Z.; Shen, J.; Shi, J.; Chen, H.; He, Y.; Wang, Z.; Feng, N. Functional oral nanoparticles for delivering silibinin and cryptotanshinone against breast cancer lung metastasis. J. Nanobiotechnol. 2020, 18, 83. [Google Scholar] [CrossRef]
- Kim, Y.G.; Lee, Y.; Lee, N.; Soh, M.; Kim, D.; Hyeon, T. Ceria-based therapeutic antioxidants for biomedical applications. Adv. Mater. 2024, 36, e2210819. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Zhang, L.; Liu, X.; Ye, C.; Zhu, P.; Tan, T.; Wang, D.; Wang, Y. Tuning oxidant and antioxidant activities of ceria by anchoring copper single-site for antibacterial application. Nat. Commun. 2024, 15, 1010. [Google Scholar] [CrossRef]
- Varukattu, N.B.; Lin, W.; Vivek, R.; Rejeeth, C.; Sabarathinam, S.; Yao, Z.; Zhang, H. Targeted and intrinsic activity of HA-functionalized PEI-nanoceria as a nano reactor in potential triple-negative breast cancer treatment. ACS Appl. Bio Mater. 2020, 3, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, O.A.; Akinloye, O.; Adaramoye, O.A. Cerium oxide nanoparticles elicit antitumourigenic effect in experimental breast cancer induced by N-methyl-N-nitrosourea and benzo(a)pyrene in female Wistar rats. J. Biochem. Mol. Toxicol. 2021, 35, e22687. [Google Scholar] [CrossRef]
- Bala, R.; Singh, V.; Rajender, S.; Singh, K. Environment, lifestyle, and female infertility. Reprod. Sci. 2021, 28, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Canipari, R.; De Santis, L.; Cecconi, S. Female fertility and environmental pollution. Int. J. Environ. Res. Public Health 2020, 17, 8802. [Google Scholar] [CrossRef]
- Aloisi, M.; Rossi, G.; Colafarina, S.; Guido, M.; Cecconi, S.; Poma, A.M.G. The impact of metal nanoparticles on female reproductive system: Risks and opportunities. Int. J. Environ. Res. Public Health 2022, 19, 13748. [Google Scholar] [CrossRef]
- Dinsdale, N.L.; Crespi, B.J. Endometriosis and polycystic ovary syndrome are diametric disorders. Evol. Appl. 2021, 14, 1693–1715. [Google Scholar] [CrossRef]
- Chaudhury, K.; Babu, K.N.; Singh, A.K.; Das, S.; Kumar, A.; Seal, S. Mitigation of endometriosis using regenerative cerium oxide nanoparticles. Nanomedicine 2013, 9, 439–448. [Google Scholar] [CrossRef]
- Spivak, N.Y.A.; Shepel, E.A.; Zholobak, N.M.; Shcherbakov, A.B.; Antonovitch, G.V.; Yanchiy, R.I.; Ivanov, V.K.; Tretyakov, Y.D. Ceria nanoparticles boost activity of aged murine oocytes. Nano Biomed. Eng. 2012, 4, 188–194. [Google Scholar] [CrossRef][Green Version]
- Ariu, F.; Bogliolo, L.; Pinna, A.; Malfatti, L.; Innocenzi, P.; Falchi, L.; Bebbere, D.; Ledda, S. Cerium oxide nanoparticles (CeO2 NPs) improve the developmental competence of in vitro-matured prepubertal ovine oocytes. Reprod. Fertil. Dev. 2017, 29, 1046–1056. [Google Scholar] [CrossRef]
- El-Beltagy, A.E.B.M.; Bakr, S.M.; Mekhaimer, S.S.G.; Ghanem, N.F.; Attaallah, A. Zinc nanoparticles alleviate the ovarian damage induced by bacterial lipopolysaccharide (LPS) in pregnant rats and their fetuses. Histochem. Cell Biol. 2023, 160, 453–475. [Google Scholar] [CrossRef] [PubMed]
- Nejadali Chaleshtari, S.; Amini, E.; Baniasadi, F.; Tavana, S.; Ghalamboran, M. Oocyte maturation, fertilization, and embryo development in vitro by green and chemical iron oxide nanoparticles: A comparative study. Sci. Rep. 2024, 14, 14157, Erratum in Sci. Rep. 2024, 14, 24494. [Google Scholar]
- Xi, H.; Huang, L.; Qiu, L.; Li, S.; Yan, Y.; Ding, Y.; Zhu, Y.; Wu, F.; Shi, X.; Zhao, J.; et al. Enhancing oocyte in vitro maturation and quality by melatonin/bilirubin cationic nanoparticles: A promising strategy for assisted reproduction techniques. Int. J. Pharm. X 2024, 8, 100268. [Google Scholar] [CrossRef]
- Li, W.J.; Zhou, X.L.; Liu, B.L.; Dai, J.J.; Song, P.; Teng, Y. Effect of nanoparticles on the survival and development of vitrified porcine GV oocytes. Cryo Lett. 2017, 37, 401–405. [Google Scholar]
- Hai, G.; Bai, J.; Liu, Y.; Li, J.; Liu, A.; Wang, J.; Liu, Q.; Liu, W.; Wan, P.; Fu, X. Superior performance of biocomposite nanoparticles PLGA-RES in protecting oocytes against vitrification stimuli. Front. Bioeng. Biotechnol. 2024, 12, 1376205. [Google Scholar] [CrossRef]
- Hou, C.C.; Zhu, J.Q. Nanoparticles and female reproductive system: How do nanoparticles affect oogenesis and embryonic development. Oncotarget 2017, 8, 109799–109817. [Google Scholar] [CrossRef]
- Preaubert, L.; Courbiere, B.; Achard, V.; Tassistro, V.; Greco, F.; Orsiere, T.; Bottero, J.Y.; Rose, J.; Auffan, M.; Perrin, J. Cerium dioxide nanoparticles affect in vitro fertilization in mice. Nanotoxicology 2016, 10, 111–117. [Google Scholar]
- Zhang, C.; Zhai, S.; Wu, L.; Bai, Y.; Jia, J.; Zhang, Y.; Zhang, B.; Yan, B. Induction of size-dependent breakdown of blood-milk barrier in lactating mice by TiO2 nanoparticles. PLoS ONE 2015, 10, e0122591. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Ze, Y.; Li, B.; Zhao, X.; Zhang, T.; Sheng, L.; Hu, R.; Gui, S.; Sang, X.; Sun, Q.; et al. Ovarian dysfunction and gene-expressed characteristics of female mice caused by long-term exposure to titanium dioxide nanoparticles. J. Hazard. Mater. 2012, 243, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Karimipour, M.; Zirak Javanmard, M.; Ahmadi, A.; Jafari, A. Oral administration of titanium dioxide nanoparticle through ovarian tissue alterations impairs mice embryonic development. Int. J. Reprod. Biomed. 2018, 16, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a potential oxidative stress marker for allergy-oriented diseases: An update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef]
- Liu, W.; Liu, H.; Zhang, S.; Hao, H.; Meng, F.; Ma, W.; Guo, Z.; Jiang, S.; Shang, X. Silica nanoparticles cause ovarian dysfunction and fertility decrease in mice via oxidative stress-activated autophagy and apoptosis. Ecotoxicol. Environ. Saf. 2024, 285, 117049. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.; Zhang, D.; Zhang, Y.; Yang, Z.; Li, Y.; Fang, F.; Xue, Y.; Zhang, Y. Copper oxide nanoparticles impairs oocyte meiosis maturation by inducing mitochondrial dysfunction and oxidative stress. Food Chem. Toxicol. 2024, 185, 114441. [Google Scholar] [CrossRef]
- Miu, B.A.; Dinischiotu, A. New green approaches in nanoparticles synthesis: An overview. Molecules 2022, 27, 6472. [Google Scholar] [CrossRef]
- Zhu, Q.; Sun, J.; An, C.; Li, X.; Xu, S.; He, Y.; Zhang, X.; Liu, L.; Hu, K.; Liang, M. Mechanism of LncRNA Gm2044 in germ cell development. Front. Cell Dev. Biol. 2024, 12, 1410914. [Google Scholar]
- Han, L.; Xu, S.; Zhou, D.; Chen, R.; Ding, Y.; Zhang, M.; Bao, M.; He, B.; Li, S. Unveiling the causal link between metabolic factors and ovarian cancer risk using Mendelian randomization analysis. Front. Endocrinol. 2024, 15, 1401648. [Google Scholar] [CrossRef]
- Pang, J.; Ding, N.; Liu, X.; He, X.; Zhou, W.; Xie, H.; Feng, J.; Li, Y.; He, Y.; Wang, S. Prognostic value of the baseline systemic immune-inflammation index in HER2-positive metastatic breast cancer: Exploratory analysis of two prospective trials. Ann. Surg. Oncol. 2025, 32, 750–759. [Google Scholar] [CrossRef]
- Zhou, J.; Guo, Z.; Peng, X.; Wu, B.; Meng, Q.; Lu, X.; Feng, L.; Guo, T. Chrysotoxine regulates ferroptosis and the PI3K/AKT/mTOR pathway to prevent cervical cancer. J. Ethnopharmacol. 2025, 338, 119126. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Ma, W.; Xu, M.; Xu, Z.; Wang, J.; Liang, Z.; Zhu, L.; Wu, M.; Luo, J.; Liu, H.; et al. A clinical prognostic model related to T cells based on machine learning for predicting the prognosis and immune response of ovarian cancer. Heliyon 2024, 10, 36898. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zang, W.; Mi, Z.; Li, J.; Wang, L.; Xie, D.; Zhao, L.; Wang, D. Tailoring carrier-free nanocombo of small-molecule prodrug for combinational cancer therapy. J. Control Release 2022, 352, 256–275. [Google Scholar] [CrossRef] [PubMed]

| Nanomaterial | Biomedical Applications | Ref. | |
|---|---|---|---|
| Organic | Liposomes | Drug carrier, fungal and bacterial infections, vaccine formulations, ischemia treatment | [43,44,45,46,47] |
| Dendrimers | Antimicrobial and antiviral effects, encapsulation and covalent binding of compounds, tissue engineering, imaging | [48,49,50,51,52,53] | |
| Carbon-Based | Gene therapy, biosensor, imaging, tissue engineering | [54,55,56,57] | |
| PLGA/PLA | Imaging, drug delivery | [58,59] | |
| Protein/Peptide-Based | Target therapy, cancer therapy | [60,61,62] | |
| Hydrogels | Anti-inflammatory, tissue regeneration, anti-infection | [63,64,65] | |
| PEG | Drug and Gene delivery | [66] | |
| Polysaccharide | Drug and Gene delivery, anti-inflammatory, imaging, theranostic medicine | [67,68,69,70] | |
| Micelles | Cosmetics, drug delivery | [71,72] | |
| Cyclodextrin-Based | Imaging, anti-cancer drug delivery | [73] | |
| Solid Lipid/Nanostructured Lipid | Drug loading, diagnostics, RNA delivery, cancer therapies | [74,75] | |
| DNA Nanoparticles | Imaging, drug delivery | [76] | |
| Inorganic | Gold (Au) | Biosensor, phototherapy, drug delivery | [77,78,79] |
| Silver (Ag) | Antiviral, antifungal, antiparasitic, antifouling, wound healing, cancer treatment, drug delivery, dentistry | [80,81,82,83,84,85,86,87] | |
| Zinc (Zn) | Imaging, drug delivery, diagnostics | [88,89,90] | |
| Ceria (Ce) | Antioxidant, anti-inflammatory, antiviral | [91,92,93] | |
| Titanium (Ti) | Antibacterial, cosmetics | [94,95] | |
| Quantum Dots (QD) | Imaging, drug carrier | [96,97] | |
| Mesoporous Silica NMs | Drug delivery, imaging, theranostics, tissue engineering, gene therapy | [98] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloisi, M.; Rossi, G.; Cecconi, S. The Dual Role of Nanomaterials in Ovarian Cancer and Female Fertility as Anti- and Prooxidants. Antioxidants 2025, 14, 1066. https://doi.org/10.3390/antiox14091066
Aloisi M, Rossi G, Cecconi S. The Dual Role of Nanomaterials in Ovarian Cancer and Female Fertility as Anti- and Prooxidants. Antioxidants. 2025; 14(9):1066. https://doi.org/10.3390/antiox14091066
Chicago/Turabian StyleAloisi, Massimo, Gianna Rossi, and Sandra Cecconi. 2025. "The Dual Role of Nanomaterials in Ovarian Cancer and Female Fertility as Anti- and Prooxidants" Antioxidants 14, no. 9: 1066. https://doi.org/10.3390/antiox14091066
APA StyleAloisi, M., Rossi, G., & Cecconi, S. (2025). The Dual Role of Nanomaterials in Ovarian Cancer and Female Fertility as Anti- and Prooxidants. Antioxidants, 14(9), 1066. https://doi.org/10.3390/antiox14091066







