Gas Plasma Combination Therapies—Promises from Preclinical Oncology Research
Abstract
1. Intoduction
2. Gas Plasma Technology
3. Combination Treatment with Gas Plasmas in Experimental Models
| Combined Anticancer Treatment | Type of Cancer | Agent/Clinically Relevant | Gas Plasma Source | Study Model | Additive/Synergistic Effect | Ref |
|---|---|---|---|---|---|---|
| BT | Melanoma | Cell starvation/yes | kINPen (Ar) | In vivo: Syngeneic | Yes | [57] |
| BT | Breast | Melittin/yes | kINPen (Ar) | In ovo: TUM-CAM | Yes | [58] |
| CT | Melanoma | Cyclophosphamide/no | Jet (He) | In vivo: Syngeneic | Yes | [59] |
| CT | Melanoma | Dacarbazine/yes | Jet (Ar) | In vivo: Syngeneic | Yes | [60] |
| CT | Melanoma | Bleomycin/yes Dacarbazine/yes Paclitaxel/yes | Jet (Ar) | In vitro | Yes | [61] |
| CT | Melanoma | Doxorubicin/yes Epirubicin/yes Oxaliplatin/yes | kINPen (Ar) | In vitro | Yes | [8] |
| CT | Melanoma | Doxorubicin/yes | DBD | In vitro | Yes | [62] |
| CT | OSCC | Cisplatin/yes | P500-SM (Ar) | In vitro | Yes | [63] |
| CT | HNSCC | Cisplatin/yes | SMD | In vitro | Yes | [64] |
| CT | Breast | Doxorubicin/yes | Jet (He) | In vitro | Yes | [65] |
| CT | Breast | Doxorubicin | Jet (Ar) | In vitro | Yes | [66] |
| CT | Breast | Paclitaxel/yes | DBD | In vitro | Yes | [67] |
| CT | Glioma | Temozolomide/yes | Jet (He) | In vitro | Yes | [68] |
| CT | Glioma | Temozolomide/yes | kINPen (Ar) | In vitro | Yes | [69] |
| CT | Glioma | Temozolomide/yes | DBD | In vitro | Yes | [70] |
| CT | Glioma | Temozolomide/yes | SMD | In vitro | Yes | [71] |
| CT | Glioma | Topotecan/no | Glow charge/spark discharge (Air) | In vitro | Yes | [72] |
| CT | Endometrial, Gastric | Cisplatin/yes | Unknown (Ar) | In vivo: Xenograft | Yes Yes | [73] |
| CT | Liver | Cisplatin/yes | DBD | In vitro | Yes | [74] |
| CT | Pancreas | Gemcitabine/yes Cisplatin/yes | kINPen (Ar) | In ovo: TUM-CAM | Yes Yes | [75] |
| CT | Pancreas | Gemcitabine/yes | Plasma gun (He) | In vitro | Yes | [76] |
| CT | Bladder | Cisplatin/yes Methotrexate/yes Adriamycin/yes paclitaxel/yes | Jet (He) | In vitro | Yes | [77] |
| CT | Ovary | Cisplatin/yes | Jet (Ar) | In vitro | Yes | [78] |
| CT | Prostate (Bone Metastasis) | Doxorubicin/yes | kINPen (Ar) | In vitro | Yes | [79] |
| CT | Ewing Sarcoma | Doxorubicin/yes Vincristine/yes | kINPen (Ar) | In vitro | Yes Yes | [80] |
| CT | Ewing Sarcoma | Methotrexate/yes Cisplatin/yes | kINPen (Ar) | In vitro | Yes | [37] |
| CT | Osteosarcoma | Salinomycin/no | Jet (He) | In vivo: Syngeneic | Yes | [81] |
| EA | Melanoma, SCC | Sm837/no, IS112/no | kINPen (Ar) | In vivo: Xenograft | Yes | [82] |
| EA | Melanoma | ADDA 5/no | kINPen (Ar) | In vitro | Yes | [83] |
| EA | Melanoma | Curcumin/no | Jet (Ar) | In vitro | No | [84] |
| EA | Melanoma | Salinomycin/no | Jet (Ar) | In vitro | Yes | [81] |
| EA | Breast | Pluronic F127/no | Jet (He) | In vivo: Syngeneic | Yes | [85] |
| EA | Non-Small Cell Lung Cancer | Chloroquine/no | Jet (He) | In vitro | Yes | [86] |
| EA | Glioma | Auranofin/no | kINPen (Ar) | In vivo: Syngeneic | Yes | [87] |
| EA | Glioma | Vitamin C/no | kINPen (Ar) | In vivo: Xenograft | Yes | [88] |
| EA | Glioma | Pyrazolopyrimidinone/no | DBD | In vitro | Yes | [89] |
| EA | Acute Lymphoid Leukemia | Sulfasalazine/no | PN-120TPG (Ar) | In vitro | Yes | [90] |
| ECT | Melanoma | ECT bleomycin/yes | Jet (Ar), DBD | In vivo: Syngeneic | Yes | [91] |
| ECT | Fibrosarcoma | ECT bleomycin/yes | PMJ (He) | In vivo: Syngeneic | Yes | [92] |
| HT | Non-Small Cell Lung Cancer | Hyperthermia/yes | Unknown (Ar) | In vitro | Yes | [93] |
| IT | Melanoma | Anti-PD-L1/no | Jet (He) | In vivo: Syngeneic | Yes | [94] |
| IT and EA | Melanoma | Pembrolizumab/yes, A-1210477/no, Carvedilol/no, Cozymasei/no, SBI-0206965/no, Navitoclax/no | kINPen (Ar) | In vivo: Syngeneic | Yes | [95] |
| NP | Melanoma | Nanoparticle/no (Si, Ag, FeO, CeO2, TiO2, FeTiO2) | kINPen (Ar) | In vitro | Yes | [96] |
| NP | Melanoma | Anti-NEU gold nanoparticle/no | DBD | In vitro | Yes | [97] |
| NP | Melanoma | p-FAK gold nanoparticle/no | DBD | In vitro | Yes | [98] |
| NP | Melanoma | Anti-FAK gold nanoparticle/no | Unknown (Air) | In vitro | Yes | [99] |
| NP | Melanoma | Silver nanoparticle/no | pm-rf-APGD | In vitro | Yes | [100] |
| NP | Breast | Iron particle/no | Jet (He) | In vitro | Yes | [101] |
| NP | Breast | Nanoparticle/no | Unknown | In vitro | Yes | [102] |
| NP | Colon | Gold nanoparticle/no | Jet (He) | In vitro | Yes | [103] |
| NP | Glioma | Gold nanoparticle/no | DBD | In vitro | Yes | [104] |
| NP | Glioma | Silver nanoparticle/no | DBD | In vitro | Yes | [105] |
| NP | Glioma | Gold nanoparticle/no | Jet (He) | In vitro | Yes | [106] |
| NP | Glioma | Gold quantum dots/no | Jet (Air) | In vitro | Yes | [107] |
| NP | Non-Small Cell Lung Cancer | Iron oxide-based magnetic nanoparticle/no | Jet (He) | In vitro | Yes | [108] |
| NP and BT | Melanoma | Silymarin nanoemulsion/no | DBD | In vitro | Yes | [109] |
| NP and BT | Melanoma | Silymarin nanoemulsion/no | DBD | In vivo: Xenograft | Yes | [110] |
| NP and CT | Non-Small Cell Lung Cancer | Paclitaxel-loaded magnetic nanoparticles | Jet (He) | In vitro | Yes | [111] |
| NP and PDT | Melanoma | Photodynamic therapy (nanoparticle)/yes | Unknown | In vitro | Yes | [112] |
| PDT | Colon | Photodynamic therapy/yes | Jet (He) | In vitro | Yes | [113] |
| PDT | Glioma | Photodynamic therapy/yes | Jet (He) | In vitro | Yes | [113] |
| PDT | Non-Small Cell Lung Cancer | Photodynamic therapy/yes | Jet (He) | In vitro | Yes | [114] |
| PEF | Pancreas | Pulsed electric field/no | Jet (He) | In vitro | Yes | [115] |
| RT | Melanoma | Radiation/yes | kINPen (Ar) | In vitro | Yes | [116] |
| RT | Hepatoblastoma | Radiation/yes | Jet (Ar + O2) | In vivo: Xenograft | No | [6] |
| RT and TT | Breast | Radiation therapy/yes Olaparib/yes | Jet (He) | In vitro | Yes | [117] |
3.1. Systemic Agents
3.1.1. Chemotherapy
3.1.2. Targeted Therapy
3.1.3. Immunotherapy
3.1.4. Nanoparticles
3.2. Local Treatment Modalities
3.2.1. Radiotherapy
3.2.2. Pulsed Electric Fields (PEFs) and Electrochemotherapy (ECT)
3.2.3. Photodynamic Therapy
3.2.4. Hyperthermia
3.2.5. Hydrosurgery
4. Bridging the Gap from Gas Plasma Medicine to Oncology in Patients
4.1. Melanoma
4.2. Lung Cancer and Bronchial Carcinoma
4.3. Colorectal Cancer
4.4. Pancreatic Cancer
4.5. Breast Cancer
4.6. Prostate Cancer
4.7. Urothelial (Bladder) Cancer
4.8. Liver Cancer and Malignancies of Intrahepatic Bile Duct
4.9. Leukemia
4.10. Non-Hodgkin Lymphoma
4.11. High-Grade Glioma
4.12. Esophagus Cancer
4.13. Other Cancers
5. Challenges in Gas Plasma Combination Therapy
6. Conclusions and Outlook
Funding
Conflicts of Interest
References
- Apalla, Z.; Lallas, A.; Sotiriou, E.; Lazaridou, E.; Ioannides, D. Epidemiological trends in skin cancer. Dermatol. Pract. Concept. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Winther, S.B.; Liposits, G.; Skuladottir, H.; Hofsli, E.; Shah, C.H.; Poulsen, L.O.; Ryg, J.; Osterlund, P.; Berglund, A.; Qvortrup, C.; et al. Reduced-dose combination chemotherapy (S-1 plus oxaliplatin) versus full-dose monotherapy (S-1) in older vulnerable patients with metastatic colorectal cancer (NORDIC9): A randomised, open-label phase 2 trial. Lancet Gastroenterol. Hepatol. 2019, 4, 376–388. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Panahi, Y.; Saadat, A.; Beiraghdar, F.; Hosseini Nouzari, S.M.; Jalalian, H.R.; Sahebkar, A. Antioxidant effects of bioavailability-enhanced curcuminoids in patients with solid tumors: A randomized double-blind placebo-controlled trial. J. Funct. Foods 2014, 6, 615–622. [Google Scholar] [CrossRef]
- Bekeschus, S. Medical gas plasma technology: Roadmap on cancer treatment and immunotherapy. Redox Biol. 2023, 65, 102798. [Google Scholar] [CrossRef]
- Lin, L.; Wang, L.; Liu, Y.; Xu, C.; Tu, Y.; Zhou, J. Non-thermal plasma inhibits tumor growth and proliferation and enhances the sensitivity to radiation in vitro and in vivo. Oncol. Rep. 2018, 40, 3405–3415. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Kim, Y.J. Photodynamic and Cold Atmospheric Plasma Combination Therapy Using Polymeric Nanoparticles for the Synergistic Treatment of Cervical Cancer. Int. J. Mol. Sci. 2021, 22, 1172. [Google Scholar] [CrossRef] [PubMed]
- Sagwal, S.K.; Pasqual-Melo, G.; Bodnar, Y.; Gandhirajan, R.K.; Bekeschus, S. Combination of chemotherapy and physical plasma elicits melanoma cell death via upregulation of SLC22A16. Cell Death Dis. 2018, 9, 1179. [Google Scholar] [CrossRef] [PubMed]
- Mahdikia, H.; Saadati, F.; Freund, E.; Gaipl, U.S.; Majidzadeh, A.K.; Shokri, B.; Bekeschus, S. Gas plasma irradiation of breast cancers promotes immunogenicity, tumor reduction, and an abscopal effect in vivo. Oncoimmunology 2020, 10, 1859731. [Google Scholar] [CrossRef]
- Conrads, H.; Schmidt, M. Plasma generation and plasma sources. Plasma Sources Sci. T 2000, 9, 441–454. [Google Scholar] [CrossRef]
- Kramer, A.; Lindequist, U.; Weltmann, K.D.; Wilke, C.; von Woedtke, T. Plasma Medicine—Its perspective for wound therapy. GMS Krankenhaushygiene Interdiszip. 2008, 3, Doc16. [Google Scholar]
- Weltmann, K.D.; Brandenburg, R.; von Woedtke, T.; Ehlbeck, J.; Foest, R.; Stieber, M.; Kindel, E. Antimicrobial treatment of heat sensitive products by miniaturized atmospheric pressure plasma jets (APPJs). J. Phys. D Appl. Phys. 2008, 41, 194008. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Brandenburg, R.; Ehlbeck, J.; Foest, R.; Kindel, E.; Stieber, M.; von Woedtke, T. Plasma decontamination at atmospheric pressure—Basics and applications. In Proceedings of the 35th IEEE International Conference on Plasma Science (ICOPS 2008), Karlsruhe, Germany, 15–19 June 2008. [Google Scholar]
- O’Meara, S.M.; Cullum, N.A.; Majid, M.; Sheldon, T.A. Systematic review of antimicrobial agents used for chronic wounds. Br. J. Surg. 2001, 88, 4–21. [Google Scholar] [CrossRef]
- Filius, P.M.; Gyssens, I.C. Impact of increasing antimicrobial resistance on wound management. Am. J. Clin. Dermatol. 2002, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Morfill, G.; Schmidt, H.U.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.; Kluschke, F.; Patzelt, A.; Vandersee, S.; Czaika, V.A.; Richter, H.; Bob, A.; von Hutten, J.; Painsi, C.; Hugel, R.; et al. Clinical use of cold atmospheric pressure argon plasma in chronic leg ulcers: A pilot study. J. Wound Care 2015, 24, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Diedrich, S.; Pati, O.; Freund, E.; Flieger, R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Cold Physical Plasma Selectively Elicits Apoptosis in Murine Pancreatic Cancer Cells In Vitro and In Ovo. Anticancer Res. 2018, 38, 5655–5663. [Google Scholar] [CrossRef]
- Pasqual-Melo, G.; Nascimento, T.; Sanches, L.J.; Blegniski, F.P.; Bianchi, J.K.; Sagwal, S.K.; Berner, J.; Schmidt, A.; Emmert, S.; Weltmann, K.D.; et al. Plasma Treatment Limits Cutaneous Squamous Cell Carcinoma Development In Vitro and In Vivo. Cancers 2020, 12, 1993. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Ogawa, T.; Uemura, M.; Shumulinsky, G.; Valle, B.L.; Pirini, F.; Ravi, R.; Sidransky, D.; Keidar, M.; Trink, B. Cold atmospheric plasma treatment selectively targets head and neck squamous cell carcinoma cells. Int. J. Mol. Med. 2014, 34, 941–946. [Google Scholar] [CrossRef]
- Wende, K.; Bekeschus, S.; Schmidt, A.; Jatsch, L.; Hasse, S.; Weltmann, K.D.; Masur, K.; von Woedtke, T. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 798–799, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Woedtke, T.V.; Stenzel, J.; Lindner, T.; Polei, S.; Vollmar, B.; Bekeschus, S. One Year Follow-Up Risk Assessment in SKH-1 Mice and Wounds Treated with an Argon Plasma Jet. Int. J. Mol. Sci. 2017, 18, 868. [Google Scholar] [CrossRef] [PubMed]
- Berner, J.; Herold, L.; Martinet, A.; Miebach, L.; von Woedke, T.; Weltmann, K.D.; Emmert, S.; Boeckmann, L.; Bekeschus, S. Reactive Species Risk Assessment Using Optimized HET-CAM Safety Evaluation of Feed Gas-Modified Gas Plasma Technology and Anticancer Drugs. ACS Appl. Mater. Interfaces 2024, 16, 34480–34495. [Google Scholar] [CrossRef]
- Kumar Dubey, S.; Dabholkar, N.; Narayan Pal, U.; Singhvi, G.; Kumar Sharma, N.; Puri, A.; Kesharwani, P. Emerging innovations in cold plasma therapy against cancer: A paradigm shift. Drug Discov. Today 2022, 27, 2425–2439. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kindel, E.; von Woedtke, T.; Hähnel, M.; Stieber, M.; Brandenburg, R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure Appl. Chem. 2010, 82, 1223–1237. [Google Scholar] [CrossRef]
- Mitra, S.; Nguyen, L.N.; Akter, M.; Park, G.; Choi, E.H.; Kaushik, N.K. Impact of ROS Generated by Chemical, Physical, and Plasma Techniques on Cancer Attenuation. Cancers 2019, 11, 1030. [Google Scholar] [CrossRef]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.D.; Wende, K.; Bogaerts, A.; Bekeschus, S. ROS from Physical Plasmas: Redox Chemistry for Biomedical Therapy. Oxidative Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Bio 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: Oxidative eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Oxidative stress in apoptosis and cancer: An update. Arch. Toxicol. 2012, 86, 1649–1665. [Google Scholar] [CrossRef]
- Bekeschus, S.; Saadati, F.; Emmert, S. The potential of gas plasma technology for targeting breast cancer. Clin. Transl. Med. 2022, 12, e1022. [Google Scholar] [CrossRef]
- Xu, D.; Xu, Y.; Cui, Q.; Liu, D.; Liu, Z.; Wang, X.; Yang, Y.; Feng, M.; Liang, R.; Chen, H.; et al. Cold atmospheric plasma as a potential tool for multiple myeloma treatment. Oncotarget 2018, 9, 18002–18017. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.S. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS ONE 2011, 6, e28154. [Google Scholar] [CrossRef]
- Chang, J.W.; Kang, S.U.; Shin, Y.S.; Kim, K.I.; Seo, S.J.; Yang, S.S.; Lee, J.S.; Moon, E.; Baek, S.J.; Lee, K.; et al. Non-thermal atmospheric pressure plasma induces apoptosis in oral cavity squamous cell carcinoma: Involvement of DNA-damage-triggering sub-G(1) arrest via the ATM/p53 pathway. Arch. Biochem. Biophys. 2014, 545, 133–140. [Google Scholar] [CrossRef]
- Nitsch, A.; Qarqash, S.; Römer, S.; Schoon, J.; Singer, D.; Bekeschus, S.; Ekkernkamp, A.; Wassilew, G.I.; Tzvetkov, M.V.; Haralambiev, L. Effective combination of cold physical plasma and chemotherapy against Ewing sarcoma cells in vitro. Sci. Rep. 2024, 14, 6505. [Google Scholar] [CrossRef]
- Furuta, T.; Shi, L.; Toyokuni, S. Non-thermal plasma as a simple ferroptosis inducer in cancer cells: A possible role of ferritin. Pathol. Int. 2018, 68, 442–443. [Google Scholar] [CrossRef]
- Okazaki, Y.; Toyokuni, S. Induction of cancer cell-specific ferroptosis by non-thermal plasma exposure. Jpn. J. Appl. Phys. 2020, 59, 110501. [Google Scholar] [CrossRef]
- Yang, X.; Chen, G.; Yu, K.N.; Yang, M.; Peng, S.; Ma, J.; Qin, F.; Cao, W.; Cui, S.; Nie, L.; et al. Cold atmospheric plasma induces GSDME-dependent pyroptotic signaling pathway via ROS generation in tumor cells. Cell Death Dis. 2020, 11, 295. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, N.; Liu, W.; Nakamura, K.; Yoshida, K.; Ikeda, Y.; Tanaka, H.; Mizuno, M.; Toyokuni, S.; Hori, M.; Kikkawa, F.; et al. Plasma-activated medium promotes autophagic cell death along with alteration of the mTOR pathway. Sci. Rep. 2020, 10, 1614. [Google Scholar] [CrossRef]
- Lin, A.G.; Xiang, B.; Merlino, D.J.; Baybutt, T.R.; Sahu, J.; Fridman, A.; Snook, A.E.; Miller, V. Non-thermal plasma induces immunogenic cell death in vivo in murine CT26 colorectal tumors. Oncoimmunology 2018, 7, e1484978. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; van der Linde, J.; Metelmann, H.R.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Physical plasma-treated saline promotes an immunogenic phenotype in CT26 colon cancer cells in vitro and in vivo. Sci. Rep. 2019, 9, 634. [Google Scholar] [CrossRef]
- Troitskaya, O.; Golubitskaya, E.; Biryukov, M.; Varlamov, M.; Gugin, P.; Milakhina, E.; Richter, V.; Schweigert, I.; Zakrevsky, D.; Koval, O. Non-Thermal Plasma Application in Tumor-Bearing Mice Induces Increase of Serum HMGB1. Int. J. Mol. Sci. 2020, 21, 5128. [Google Scholar] [CrossRef]
- Weiss, M.; Arnholdt, M.; Hissnauer, A.; Fischer, I.; Schonfisch, B.; Andress, J.; Gerstner, S.; Dannehl, D.; Bosmuller, H.; Staebler, A.; et al. Tissue-preserving treatment with non-invasive physical plasma of cervical intraepithelial neoplasia-a prospective controlled clinical trial. Front. Med. 2023, 10, 1242732. [Google Scholar] [CrossRef]
- de Pokomandy, A.; Rouleau, D.; Lalonde, R.; Beauvais, C.; de Castro, C.; Coutlee, F. Human Immunodeficiency and Papilloma Virus Research Group (HIPVIRG) Study Group Argon plasma coagulation treatment of anal high-grade squamous intraepithelial lesions in men who have sex with men living with HIV: Results of a 2-year prospective pilot study. HIV Med. 2018, 19, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Friedman, P.C.; Miller, V.; Fridman, G.; Lin, A.; Fridman, A. Successful treatment of actinic keratoses using nonthermal atmospheric pressure plasma: A case series. J. Am. Acad. Dermatol. 2017, 76, 349–350. [Google Scholar] [CrossRef]
- Canady, J.; Murthy, S.R.K.; Zhuang, T.; Gitelis, S.; Nissan, A.; Ly, L.; Jones, O.Z.; Cheng, X.; Adileh, M.; Blank, A.T.; et al. The First Cold Atmospheric Plasma Phase I Clinical Trial for the Treatment of Advanced Solid Tumors: A Novel Treatment Arm for Cancer. Cancers 2023, 15, 3688. [Google Scholar] [CrossRef] [PubMed]
- Freund, E.; Bekeschus, S. Gas Plasma-Oxidized Liquids for Cancer Treatment: Preclinical Relevance, Immuno-Oncology, and Clinical Obstacles. IEEE Trans. Radiat. Plasma Med. Sci. 2021, 5, 761–774. [Google Scholar] [CrossRef]
- Lango, M.N. Multimodal treatment for head and neck cancer. Surg. Clin. N. Am. 2009, 89, 43–52, viii. [Google Scholar] [CrossRef]
- Terlizzi, M.; Bossi, A. High-risk Locally Advanced Prostate Cancer: Multimodal Treatment Is the Key. Eur. Urol. Open Sci. 2022, 38, 14–16. [Google Scholar] [CrossRef]
- Smith, P.; Lavery, A.; Turkington, R.C. An overview of acute gastrointestinal side effects of systemic anti-cancer therapy and their management. Best. Pr. Res. Clin. Gastroenterol. 2020, 48–49, 101691. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Chen, X. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Sander, C.; Nitsch, A.; Erb, H.H.H.; Egger, E.K.; Haralambiev, L.; Eggers, B.; Kramer, F.-J.; Weiss, M.; Mustea, A.; Stope, M.B. Non-Invasive Physical Plasma Enhances the Membrane Permeability to Low Molecular Weight Compounds and Subsequently Leads to the Loss of Cellular ATP and the Devitalization of Epithelial Cancer Cells. Appl. Sci. 2021, 11, 9801. [Google Scholar] [CrossRef]
- Jacoby, J.M.; Strakeljahn, S.; Nitsch, A.; Bekeschus, S.; Hinz, P.; Mustea, A.; Ekkernkamp, A.; Tzvetkov, M.V.; Haralambiev, L.; Stope, M.B. An Innovative Therapeutic Option for the Treatment of Skeletal Sarcomas: Elimination of Osteo- and Ewing’s Sarcoma Cells Using Physical Gas Plasma. Int. J. Mol. Sci. 2020, 21, 4460. [Google Scholar] [CrossRef]
- Vernier, P.T.; Levine, Z.A.; Wu, Y.H.; Joubert, V.; Ziegler, M.J.; Mir, L.M.; Tieleman, D.P. Electroporating fields target oxidatively damaged areas in the cell membrane. PLoS ONE 2009, 4, e7966. [Google Scholar] [CrossRef]
- Golpour, M.; Alimohammadi, M.; Sohbatzadeh, F.; Fattahi, S.; Bekeschus, S.; Rafiei, A. Cold atmospheric pressure plasma treatment combined with starvation increases autophagy and apoptosis in melanoma in vitro and in vivo. Exp. Dermatol. 2022, 31, 1016–1028. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Hammerschmid, D.; Privat-Maldonado, A.; Dewilde, S.; Bogaerts, A. Synergistic Effects of Melittin and Plasma Treatment: A Promising Approach for Cancer Therapy. Cancers 2019, 11, 1109. [Google Scholar] [CrossRef]
- Saadati, F.; Mahdikia, H.; Abbaszadeh, H.A.; Abdollahifar, M.A.; Khoramgah, M.S.; Shokri, B. Comparison of Direct and Indirect cold atmospheric-pressure plasma methods in the B(16)F(10) melanoma cancer cells treatment. Sci. Rep. 2018, 8, 7689. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Golpur, M.; Sohbatzadeh, F.; Hadavi, S.; Bekeschus, S.; Niaki, H.A.; Valadan, R.; Rafiei, A. Cold Atmospheric Plasma Is a Potent Tool to Improve Chemotherapy in Melanoma In Vitro and In Vivo. Biomolecules 2020, 10, 1011. [Google Scholar] [CrossRef]
- Daeschlein, G.; Hillmann, A.; Gumbel, D.; Sicher, C.; von Podewils, S.; Stope, M.B.; Junger, M. Enhanced Anticancer Efficacy by Drug Chemotherapy and Cold Atmospheric Plasma Against Melanoma and Glioblastoma Cell Lines In Vitro. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 153–159. [Google Scholar] [CrossRef]
- Pefani-Antimisiari, K.; Athanasopoulos, D.K.; Marazioti, A.; Sklias, K.; Rodi, M.; de Lastic, A.L.; Mouzaki, A.; Svarnas, P.; Antimisiaris, S.G. Synergistic effect of cold atmospheric pressure plasma and free or liposomal doxorubicin on melanoma cells. Sci. Rep. 2021, 11, 14788. [Google Scholar] [CrossRef]
- Lee, C.M.; Jeong, Y.I.; Kook, M.S.; Kim, B.H. Combinatorial Effect of Cold Atmosphere Plasma (CAP) and the Anticancer Drug Cisplatin on Oral Squamous Cell Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 7646. [Google Scholar] [CrossRef]
- Brunner, T.F.; Probst, F.A.; Troeltzsch, M.; Schwenk-Zieger, S.; Zimmermann, J.L.; Morfill, G.; Becker, S.; Harreus, U.; Welz, C. Primary cold atmospheric plasma combined with low dose cisplatin as a possible adjuvant combination therapy for HNSCC cells-an in-vitro study. Head. Face Med. 2022, 18, 21. [Google Scholar] [CrossRef]
- Mirpour, S.; Ghomi, H.; Piroozmand, S.; Nikkhah, M.; Tavassoli, S.H.; Azad, S.Z. The Selective Characterization of Nonthermal Atmospheric Pressure Plasma Jet on Treatment of Human Breast Cancer and Normal Cells. IEEE Trans. Plasma Sci. 2014, 42, 315–322. [Google Scholar] [CrossRef]
- Zahedian, S.; Hekmat, A.; Tackallou, S.H.; Ghoranneviss, M. The Impacts of Prepared Plasma-Activated Medium (PAM) Combined with Doxorubicin on the Viability of MCF-7 Breast Cancer Cells: A New Cancer Treatment Strategy. Rep. Biochem. Mol. Biol. 2022, 10, 640–652. [Google Scholar] [CrossRef]
- Mihai, C.T.; Mihaila, I.; Pasare, M.A.; Pintilie, R.M.; Ciorpac, M.; Topala, I. Cold Atmospheric Plasma-Activated Media Improve Paclitaxel Efficacy on Breast Cancer Cells in a Combined Treatment Model. Curr. Issues Mol. Biol. 2022, 44, 1995–2014. [Google Scholar] [CrossRef]
- Gjika, E.; Pal-Ghosh, S.; Kirschner, M.E.; Lin, L.; Sherman, J.H.; Stepp, M.A.; Keidar, M. Combination therapy of cold atmospheric plasma (CAP) with temozolomide in the treatment of U87MG glioblastoma cells. Sci. Rep. 2020, 10, 16495. [Google Scholar] [CrossRef]
- Shaw, P.; Kumar, N.; Privat-Maldonado, A.; Smits, E.; Bogaerts, A. Cold Atmospheric Plasma Increases Temozolomide Sensitivity of Three-Dimensional Glioblastoma Spheroids via Oxidative Stress-Mediated DNA Damage. Cancers 2021, 13, 1780. [Google Scholar] [CrossRef]
- Conway, G.E.; Casey, A.; Milosavljevic, V.; Liu, Y.; Howe, O.; Cullen, P.J.; Curtin, J.F. Non-thermal atmospheric plasma induces ROS-independent cell death in U373MG glioma cells and augments the cytotoxicity of temozolomide. Brit J. Cancer 2016, 114, 435–443. [Google Scholar] [CrossRef]
- Koritzer, J.; Boxhammer, V.; Schafer, A.; Shimizu, T.; Klampfl, T.G.; Li, Y.F.; Welz, C.; Schwenk-Zieger, S.; Morfill, G.E.; Zimmermann, J.L.; et al. Restoration of sensitivity in chemo-resistant glioma cells by cold atmospheric plasma. PLoS ONE 2013, 8, e64498. [Google Scholar] [CrossRef]
- Pinheiro Lopes, B.; O’Neill, L.; Bourke, P.; Boehm, D. Combined Effect of Plasma-Activated Water and Topotecan in Glioblastoma Cells. Cancers 2023, 15, 4858. [Google Scholar] [CrossRef]
- Ikeda, J.I.; Tanaka, H.; Ishikawa, K.; Sakakita, H.; Ikehara, Y.; Hori, M. Plasma-activated medium (PAM) kills human cancer-initiating cells. Pathol. Int. 2018, 68, 23–30. [Google Scholar] [CrossRef]
- Li, Y.; Tang, T.; Lee, H.J.; Song, K. Selective Anti-Cancer Effects of Plasma-Activated Medium and Its High Efficacy with Cisplatin on Hepatocellular Carcinoma with Cancer Stem Cell Characteristics. Int. J. Mol. Sci. 2021, 22, 3956. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Freund, E.; Hermes, M.; Oswald, S.; Heidecke, C.D.; Partecke, L.I.; Bekeschus, S. Gas Plasma-Conditioned Ringer’s Lactate Enhances the Cytotoxic Activity of Cisplatin and Gemcitabine in Pancreatic Cancer In Vitro and In Ovo. Cancers 2020, 12, 123. [Google Scholar] [CrossRef]
- Brulle, L.; Vandamme, M.; Ries, D.; Martel, E.; Robert, E.; Lerondel, S.; Trichet, V.; Richard, S.; Pouvesle, J.M.; Le Pape, A. Effects of a non thermal plasma treatment alone or in combination with gemcitabine in a MIA PaCa2-luc orthotopic pancreatic carcinoma model. PLoS ONE 2012, 7, e52653. [Google Scholar] [CrossRef]
- Zhang, J.S.; Li, B.; Xu, S.D.; Liu, D.X.; Zhang, H.; Xu, D.H.; Guo, L.; Kong, M.G. Study of the anticancer effects of a helium plasma jet combined with four anticancer drugs on 3D bladder tumour spheroids. Plasma Process Polym. 2021, 18, 2000226. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.W.; Jung, M.H.; Kim, Y.S.; Kim, K.S.; Suh, D.S.; Kim, K.H.; Choi, E.H.; Kim, J.; Kwon, B.S. Plasma-activated medium inhibits cancer stem cell-like properties and exhibits a synergistic effect in combination with cisplatin in ovarian cancer. Free Radic. Biol. Med. 2022, 182, 276–288. [Google Scholar] [CrossRef]
- Mateu-Sanz, M.; Ginebra, M.P.; Tornin, J.; Canal, C. Cold atmospheric plasma enhances doxorubicin selectivity in metastasic bone cancer. Free Radic. Biol. Med. 2022, 189, 32–41. [Google Scholar] [CrossRef]
- Nitsch, A.; Qarqash, S.; Romer, S.; Schoon, J.; Ekkernkamp, A.; Niethard, M.; Reichert, J.C.; Wassilew, G.I.; Tzvetkov, M.V.; Haralambiev, L. Enhancing the Impact of Chemotherapy on Ewing Sarcoma Cells through Combination with Cold Physical Plasma. Int. J. Mol. Sci. 2023, 24, 8669. [Google Scholar] [CrossRef]
- Ando, T.; Suzuki-Karasaki, M.; Suzuki-Karasaki, M.; Ichikawa, J.; Ochiai, T.; Yoshida, Y.; Haro, H.; Suzuki-Karasaki, Y. Combined Anticancer Effect of Plasma-Activated Infusion and Salinomycin by Targeting Autophagy and Mitochondrial Morphology. Front. Oncol. 2021, 11, 593127. [Google Scholar] [CrossRef]
- Boeckmann, L.; Berner, J.; Kordt, M.; Lenz, E.; Schafer, M.; Semmler, M.L.; Frey, A.; Sagwal, S.K.; Rebl, H.; Miebach, L.; et al. Synergistic effect of cold gas plasma and experimental drug exposure exhibits skin cancer toxicity in vitro and in vivo. J. Adv. Res. 2024, 57, 181–196. [Google Scholar] [CrossRef]
- Gandhirajan, R.K.; Rodder, K.; Bodnar, Y.; Pasqual-Melo, G.; Emmert, S.; Griguer, C.E.; Weltmann, K.D.; Bekeschus, S. Cytochrome C oxidase Inhibition and Cold Plasma-derived Oxidants Synergize in Melanoma Cell Death Induction. Sci. Rep. 2018, 8, 12734. [Google Scholar] [CrossRef]
- Yazdani, Z.; Mehrabanjoubani, P.; Rafiei, A.; Biparva, P.; Kardan, M. Combined Effect of Cold Atmospheric Plasma and Curcumin in Melanoma Cancer. BioMed Res. Int. 2021, 2021, 1969863. [Google Scholar] [CrossRef]
- Fang, T.; Cao, X.; Shen, B.; Chen, Z.; Chen, G. Injectable cold atmospheric plasma-activated immunotherapeutic hydrogel for enhanced cancer treatment. Biomaterials 2023, 300, 122189. [Google Scholar] [CrossRef]
- Patrakova, E.; Biryukov, M.; Troitskaya, O.; Gugin, P.; Milakhina, E.; Semenov, D.; Poletaeva, J.; Ryabchikova, E.; Novak, D.; Kryachkova, N.; et al. Chloroquine Enhances Death in Lung Adenocarcinoma A549 Cells Exposed to Cold Atmospheric Plasma Jet. Cells 2023, 12, 290. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Freire Boullosa, L.; Quatannens, D.; De Waele, J.; Merlin, C.; Lambrechts, H.; Lau, H.W.; Hermans, C.; Lin, A.; Lardon, F.; et al. Auranofin and Cold Atmospheric Plasma Synergize to Trigger Distinct Cell Death Mechanisms and Immunogenic Responses in Glioblastoma. Cells 2021, 10, 2936. [Google Scholar] [CrossRef]
- Yu, H.; Song, X.; Yang, F.; Wang, J.; Sun, M.; Liu, G.; Ahmad, N.; Zhou, Y.; Zhang, Y.; Shi, G.; et al. Combined effects of vitamin C and cold atmospheric plasma-conditioned media against glioblastoma via hydrogen peroxide. Free Radic. Biol. Med. 2023, 194, 1–11. [Google Scholar] [CrossRef]
- He, Z.; Charleton, C.; Devine, R.W.; Kelada, M.; Walsh, J.M.D.; Conway, G.E.; Gunes, S.; Mondala, J.R.M.; Tian, F.; Tiwari, B.; et al. Enhanced pyrazolopyrimidinones cytotoxicity against glioblastoma cells activated by ROS-Generating cold atmospheric plasma. Eur. J. Med. Chem. 2021, 224, 113736. [Google Scholar] [CrossRef]
- Moniruzzaman, R.; Rehman, M.U.; Zhao, Q.L.; Jawaid, P.; Mitsuhashi, Y.; Imaue, S.; Fujiwara, K.; Ogawa, R.; Tomihara, K.; Saitoh, J.I.; et al. Roles of intracellular and extracellular ROS formation in apoptosis induced by cold atmospheric helium plasma and X-irradiation in the presence of sulfasalazine. Free Radic. Biol. Med. 2018, 129, 537–547. [Google Scholar] [CrossRef]
- Daeschlein, G.; Scholz, S.; Lutze, S.; Arnold, A.; von Podewils, S.; Kiefer, T.; Tueting, T.; Hardt, O.; Haase, H.; Grisk, O.; et al. Comparison between cold plasma, electrochemotherapy and combined therapy in a melanoma mouse model. Exp. Dermatol. 2013, 22, 582–586. [Google Scholar] [CrossRef]
- Chung, T.-H. Enhancement of Electrochemotherapy by Non-Thermal Plasma-Treated Liquids: Protocol Design and Proof of Concept. Doctoral Dissertation, Université Paris-Saclay, Gif-sur-Yvette, France, 2021. [Google Scholar]
- Ishii, R.; Kamiya, T.; Hara, H.; Adachi, T. Hyperthermia synergistically enhances cancer cell death by plasma-activated acetated Ringer’s solution. Arch. Biochem. Biophys. 2020, 693, 108565. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.; Wen, D.; Wang, Z.; Li, H.; Zeng, Y.; Dotti, G.; Wirz, R.E.; Gu, Z. Transdermal cold atmospheric plasma-mediated immune checkpoint blockade therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 3687–3692. [Google Scholar] [CrossRef]
- Miebach, L.; Melo-Zainzinger, G.; Freund, E.; Clemen, R.; Cecchini, A.L.; Bekeschus, S. Medical Gas Plasma Technology Combines with Antimelanoma Therapies and Promotes Immune-Checkpoint Therapy Responses. Adv. Sci. 2023, 10, e2303183. [Google Scholar] [CrossRef]
- Bekeschus, S. Combined Toxicity of Gas Plasma Treatment and Nanoparticles Exposure in Melanoma Cells In Vitro. Nanomaterials 2021, 11, 806. [Google Scholar] [CrossRef]
- Choi, B.B.; Kim, M.S.; Kim, U.K.; Hong, J.W.; Lee, H.J.; Kim, G.C. Targeting NEU Protein in Melanoma Cells with Non-Thermal Atmospheric Pressure Plasma and Gold Nanoparticles. J. Biomed. Nanotechnol. 2015, 11, 900–905. [Google Scholar] [CrossRef]
- Choi, B.B.R.; Choi, J.H.; Hong, J.W.; Song, K.W.; Lee, H.J.; Kim, U.K.; Kim, G.C. Selective Killing of Melanoma Cells With Non-Thermal Atmospheric Pressure Plasma and p-FAK Antibody Conjugated Gold Nanoparticles. Int. J. Med. Sci. 2017, 14, 1101–1109. [Google Scholar] [CrossRef]
- Kim, G.C.; Kim, G.J.; Park, S.R.; Jeon, S.M.; Seo, H.J.; Iza, F.; Lee, J.K. Air plasma coupled with antibody-conjugated nanoparticles: A new weapon against cancer. J. Phys. D Appl. Phys. 2009, 42, 032005. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Bielawska-Pohl, A.; diCenzo, G.C.; Jamroz, P.; Macioszczyk, J.; Klimczak, A.; Pohl, P. Pulse-Modulated Radio-Frequency Alternating-Current-Driven Atmospheric-Pressure Glow Discharge for Continuous-Flow Synthesis of Silver Nanoparticles and Evaluation of Their Cytotoxicity toward Human Melanoma Cells. Nanomaterials 2018, 8, 398. [Google Scholar] [CrossRef]
- Jalili, A.; Irani, S.; Mirfakhraie, R. Combination of cold atmospheric plasma and iron nanoparticles in breast cancer: Gene expression and apoptosis study. Onco Targets Ther. 2016, 9, 5911–5917. [Google Scholar] [CrossRef]
- Zhu, W.; Lee, S.J.; Castro, N.J.; Yan, D.; Keidar, M.; Zhang, L.G. Synergistic Effect of Cold Atmospheric Plasma and Drug Loaded Core-shell Nanoparticles on Inhibiting Breast Cancer Cell Growth. Sci. Rep. 2016, 6, 21974. [Google Scholar] [CrossRef]
- Irani, S.; Shahmirani, Z.; Atyabi, S.M.; Mirpoor, S. Induction of growth arrest in colorectal cancer cells by cold plasma and gold nanoparticles. Arch. Med. Sci. 2015, 11, 1286–1295. [Google Scholar] [CrossRef]
- He, Z.; Liu, K.; Manaloto, E.; Casey, A.; Cribaro, G.P.; Byrne, H.J.; Tian, F.; Barcia, C.; Conway, G.E.; Cullen, P.J.; et al. Cold Atmospheric Plasma Induces ATP-Dependent Endocytosis of Nanoparticles and Synergistic U373MG Cancer Cell Death. Sci. Rep. 2018, 8, 5298. [Google Scholar] [CrossRef]
- Manaloto, E.; Gowen, A.A.; Lesniak, A.; He, Z.; Casey, A.; Cullen, P.J.; Curtin, J.F. Cold atmospheric plasma induces silver nanoparticle uptake, oxidative dissolution and enhanced cytotoxicity in glioblastoma multiforme cells. Arch. Biochem. Biophys. 2020, 689, 108462. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Rajjoub, K.; Sherman, J.; Canady, J.; Recek, N.; Yan, D.Y.; Bian, K.; Murad, F.; Keidar, M. Cold Plasma Accelerates the Uptake of Gold Nanoparticles Into Glioblastoma Cells. Plasma Process Polym. 2015, 12, 1364–1369. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kaushik, N.; Wahab, R.; Bhartiya, P.; Linh, N.N.; Khan, F.; Al-Khedhairy, A.A.; Choi, E.H. Cold Atmospheric Plasma and Gold Quantum Dots Exert Dual Cytotoxicity Mediated by the Cell Receptor-Activated Apoptotic Pathway in Glioblastoma Cells. Cancers 2020, 12, 457. [Google Scholar] [CrossRef]
- Li, W.; Yu, H.; Ding, D.; Chen, Z.; Wang, Y.; Wang, S.; Li, X.; Keidar, M.; Zhang, W. Cold atmospheric plasma and iron oxide-based magnetic nanoparticles for synergetic lung cancer therapy. Free Radic. Biol. Med. 2019, 130, 71–81. [Google Scholar] [CrossRef]
- Adhikari, M.; Adhikari, B.; Ghimire, B.; Baboota, S.; Choi, E.H. Cold Atmospheric Plasma and Silymarin Nanoemulsion Activate Autophagy in Human Melanoma Cells. Int. J. Mol. Sci. 2020, 21, 1939. [Google Scholar] [CrossRef]
- Adhikari, M.; Kaushik, N.; Ghimire, B.; Adhikari, B.; Baboota, S.; Al-Khedhairy, A.A.; Wahab, R.; Lee, S.J.; Kaushik, N.K.; Choi, E.H. Cold atmospheric plasma and silymarin nanoemulsion synergistically inhibits human melanoma tumorigenesis via targeting HGF/c-MET downstream pathway. Cell Commun. Signal 2019, 17, 52. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Wang, S.; Li, X.; Li, W.; Ding, D.; Gong, X.; Keidar, M.; Zhang, W. Paclitaxel-Loaded Core-Shell Magnetic Nanoparticles and Cold Atmospheric Plasma Inhibit Non-Small Cell Lung Cancer Growth. ACS Appl. Mater. Interfaces 2018, 10, 43462–43471. [Google Scholar] [CrossRef]
- Wang, M.; Geilich, B.M.; Keidar, M.; Webster, T.J. Killing malignant melanoma cells with protoporphyrin IX-loaded polymersome-mediated photodynamic therapy and cold atmospheric plasma. Int. J. Nanomed. 2017, 12, 4117–4127. [Google Scholar] [CrossRef]
- Ara, E.S.; Noghreiyan, A.V.; Sazgarnia, A. Evaluation of photodynamic effect of Indocyanine green (ICG) on the colon and glioblastoma cancer cell lines pretreated by cold atmospheric plasma. Photodiagnosis Photodyn. Ther. 2021, 35, 102408. [Google Scholar] [CrossRef]
- Karami-Gadallo, L.; Ghoranneviss, M.; Ataie-Fashtami, L.; Pouladian, M.; Sardari, D. Enhancement of cancerous cells treatment by applying cold atmospheric plasma and photo dynamic therapy simultaneously. Clin. Plasma Med. 2017, 7–8, 46–51. [Google Scholar] [CrossRef]
- Jiang, C.; Oshin, E.A.; Guo, S.; Scott, M.; Li, X.; Mangiamele, C.; Heller, R. Synergistic effects of an atmospheric pressure plasma jet and pulsed electric field on cells and skin. IEEE Trans. Plasma Sci. IEEE Nucl. Plasma Sci. Soc. 2021, 49, 3317–3324. [Google Scholar] [CrossRef]
- Pasqual-Melo, G.; Sagwal, S.K.; Freund, E.; Gandhirajan, R.K.; Frey, B.; von Woedtke, T.; Gaipl, U.; Bekeschus, S. Combination of Gas Plasma and Radiotherapy Has Immunostimulatory Potential and Additive Toxicity in Murine Melanoma Cells in Vitro. Int. J. Mol. Sci. 2020, 21, 1379. [Google Scholar] [CrossRef]
- Lafontaine, J.; Boisvert, J.S.; Glory, A.; Coulombe, S.; Wong, P. Synergy between Non-Thermal Plasma with Radiation Therapy and Olaparib in a Panel of Breast Cancer Cell Lines. Cancers 2020, 12, 348. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. Antineoplastic Agents. In LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Mollaei, M.; Hassan, Z.M.; Khorshidi, F.; Langroudi, L. Chemotherapeutic drugs: Cell death- and resistance-related signaling pathways. Are they really as smart as the tumor cells? Transl. Oncol. 2021, 14, 101056. [Google Scholar] [CrossRef]
- Gerber, D.E. Targeted therapies: A new generation of cancer treatments. Am. Fam. Physician 2008, 77, 311–319. [Google Scholar]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef]
- Hughes, P.E.; Caenepeel, S.; Wu, L.C. Targeted Therapy and Checkpoint Immunotherapy Combinations for the Treatment of Cancer. Trends Immunol. 2016, 37, 462–476. [Google Scholar] [CrossRef]
- Seebacher, N.A.; Stacy, A.E.; Porter, G.M.; Merlot, A.M. Clinical development of targeted and immune based anti-cancer therapies. J. Exp. Clin. Cancer Res. 2019, 38, 156. [Google Scholar] [CrossRef]
- Gajria, D.; Chandarlapaty, S. HER2-amplified breast cancer: Mechanisms of trastuzumab resistance and novel targeted therapies. Expert. Rev. Anticancer. Ther. 2011, 11, 263–275. [Google Scholar] [CrossRef]
- Yamaoka, T.; Ohba, M.; Ohmori, T. Molecular-Targeted Therapies for Epidermal Growth Factor Receptor and Its Resistance Mechanisms. Int. J. Mol. Sci. 2017, 18, 2420. [Google Scholar] [CrossRef]
- Ellis, L.M.; Hicklin, D.J. VEGF-targeted therapy: Mechanisms of anti-tumour activity. Nat. Rev. Cancer 2008, 8, 579–591. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Y.; Bao, J.; Chen, J.; Song, W. Low Temperature Plasma Suppresses Lung Cancer Cells Growth via VEGF/VEGFR2/RAS/ERK Axis. Molecules 2022, 27, 5934. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, J.; Cai, L.; Dai, X. Cold Atmospheric Plasma Conveys Selectivity Against Hepatocellular Carcinoma Cells via Triggering EGFR(Tyr1068)-Mediated Autophagy. Front. Oncol. 2022, 12, 895106. [Google Scholar] [CrossRef]
- Chang, J.W.; Kang, S.U.; Shin, Y.S.; Seo, S.J.; Kim, Y.S.; Yang, S.S.; Lee, J.S.; Moon, E.; Lee, K.; Kim, C.H. Combination of NTP with cetuximab inhibited invasion/migration of cetuximab-resistant OSCC cells: Involvement of NF-kappaB signaling. Sci. Rep. 2015, 5, 18208. [Google Scholar] [CrossRef]
- Freund, E.; Liedtke, K.R.; Miebach, L.; Wende, K.; Heidecke, A.; Kaushik, N.K.; Choi, E.H.; Partecke, L.I.; Bekeschus, S. Identification of Two Kinase Inhibitors with Synergistic Toxicity with Low-Dose Hydrogen Peroxide in Colorectal Cancer Cells in vitro. Cancers 2020, 12, 122. [Google Scholar] [CrossRef]
- Schulan, P.; Wende, K.; von Woedtke, T.; Weltmann, K.D.; Bekeschus, S.; Clemen, R. Oxidative modifications control aberrant tyrosine kinase activity. Biointerphases 2024, 19, 061004. [Google Scholar] [CrossRef]
- Rebl, H.; Sawade, M.; Hein, M.; Bergemann, C.; Wende, M.; Lalk, M.; Langer, P.; Emmert, S.; Nebe, B. Synergistic effect of plasma-activated medium and novel indirubin derivatives on human skin cancer cells by activation of the AhR pathway. Sci. Rep. 2022, 12, 2528. [Google Scholar] [CrossRef]
- Xu, D.; Luo, X.; Xu, Y.; Cui, Q.; Yang, Y.; Liu, D.; Chen, H.; Kong, M.G. The effects of cold atmospheric plasma on cell adhesion, differentiation, migration, apoptosis and drug sensitivity of multiple myeloma. Biochem. Biophys. Res. Commun. 2016, 473, 1125–1132. [Google Scholar] [CrossRef]
- Schulan, P.; Wende, M.; Bekeschus, S.; Lalk, M.; Wende, K. Activity Alterations of 37 Tyrosine Kinase Inhibitors Upon kINPen Plasma Exposure in A549 Lung Cancer Cells. IEEE Trans. Radiat. Plasma Med. Sci. 2024, 8, 700–707. [Google Scholar] [CrossRef]
- Ahmadi, M.; Singer, D.; Potlitz, F.; Nasri, Z.; von Woedtke, T.; Link, A.; Bekeschus, S.; Wende, K. Cold Physical Plasma-Mediated Fenretinide Prodrug Activation Confers Additive Cytotoxicity in Epithelial Cells. Antioxidants 2023, 12, 1271. [Google Scholar] [CrossRef]
- Metelmann, H.R.; Seebauer, C.; Rutkowski, R.; Schuster, M.; Bekeschus, S.; Metelmann, P. Treating cancer with cold physical plasma: On the way to evidence-based medicine. Contrib. Plasm. Phys. 2018, 58, 415–419. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.M.; Buque, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.P.; Agostinis, P.; et al. Classification of current anticancer immunotherapies. Oncotarget 2014, 5, 12472–12508. [Google Scholar] [CrossRef]
- Butterfield, L.H.; Najjar, Y.G. Immunotherapy combination approaches: Mechanisms, biomarkers and clinical observations. Nat. Rev. Immunol. 2024, 24, 399–416. [Google Scholar] [CrossRef]
- Khalili, M.; Daniels, L.; Lin, A.; Krebs, F.C.; Snook, A.E.; Bekeschus, S.; Bowne, W.B.; Miller, V. Non-Thermal Plasma-Induced Immunogenic Cell Death in Cancer: A Topical Review. J. Phys. D Appl. Phys. 2019, 52, 423001. [Google Scholar] [CrossRef]
- Bekeschus, S.; Clemen, R. Plasma, cancer, immunity. J. Phys. D: Appl. Phys. 2022, 55, 473003. [Google Scholar] [CrossRef]
- Clemen, R.; Heirman, P.; Lin, A.; Bogaerts, A.; Bekeschus, S. Physical Plasma-Treated Skin Cancer Cells Amplify Tumor Cytotoxicity of Human Natural Killer (NK) Cells. Cancers 2020, 12, 3575. [Google Scholar] [CrossRef]
- Miebach, L.; Freund, E.; Horn, S.; Niessner, F.; Sagwal, S.K.; von Woedtke, T.; Emmert, S.; Weltmann, K.D.; Clemen, R.; Schmidt, A.; et al. Tumor cytotoxicity and immunogenicity of a novel V-jet neon plasma source compared to the kINPen. Sci. Rep. 2021, 11, 136. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Singh, R.P.; Rab, S.; Suman, R. Applications of nanotechnology in medical field: A brief review. Glob. Health J. 2023, 7, 70–77. [Google Scholar] [CrossRef]
- Austria, E.; Bilek, M.; Varamini, P.; Akhavan, B. Breaking biological barriers: Engineering polymeric nanoparticles for cancer therapy. Nano Today 2025, 60, 102552. [Google Scholar] [CrossRef]
- Hui, Y.; Yi, X.; Wibowo, D.; Yang, G.; Middelberg, A.P.J.; Gao, H.; Zhao, C.X. Nanoparticle elasticity regulates phagocytosis and cancer cell uptake. Sci. Adv. 2020, 6, eaaz4316. [Google Scholar] [CrossRef]
- Salatin, S.; Maleki Dizaj, S.; Yari Khosroushahi, A. Effect of the surface modification, size, and shape on cellular uptake of nanoparticles. Cell Biol. Int. 2015, 39, 881–890. [Google Scholar] [CrossRef]
- de la Fuente, J.M.; Grazu, V. Nanobiotechnology: Inorganic Nanoparticles vs. Organic Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2012; Volume 4. [Google Scholar]
- Gavas, S.; Quazi, S.; Karpinski, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Wolfram, J.; Zhu, M.; Yang, Y.; Shen, J.; Gentile, E.; Paolino, D.; Fresta, M.; Nie, G.; Chen, C.; Shen, H.; et al. Safety of Nanoparticles in Medicine. Curr. Drug Targets 2015, 16, 1671–1681. [Google Scholar] [CrossRef]
- Abdal Dayem, A.; Hossain, M.K.; Lee, S.B.; Kim, K.; Saha, S.K.; Yang, G.M.; Choi, H.Y.; Cho, S.G. The Role of Reactive Oxygen Species (ROS) in the Biological Activities of Metallic Nanoparticles. Int. J. Mol. Sci. 2017, 18, 120. [Google Scholar] [CrossRef]
- Chen, G.L.; Chen, S.H.; Feng, W.R.; Chen, W.X.; Yang, S.Z. Surface modification of the nanoparticles by an atmospheric room-temperature plasma fluidized bed. Appl. Surf. Sci. 2008, 254, 3915–3920. [Google Scholar] [CrossRef]
- Hasirci, N.; Endogan, T.; Vardar, E.; Kiziltay, A.; Hasirci, V. Effect of oxygen plasma on surface properties and biocompatibility of PLGA films. Surf. Interface Anal. 2010, 42, 486–491. [Google Scholar] [CrossRef]
- He, Z.; Liu, K.; Scally, L.; Manaloto, E.; Gunes, S.; Ng, S.W.; Maher, M.; Tiwari, B.; Byrne, H.J.; Bourke, P.; et al. Cold Atmospheric Plasma Stimulates Clathrin-Dependent Endocytosis to Repair Oxidised Membrane and Enhance Uptake of Nanomaterial in Glioblastoma Multiforme Cells. Sci. Rep. 2020, 10, 6985. [Google Scholar] [CrossRef]
- Shahmirani, Z.; Irani, S.; Atyabi, S.M.; Mirpour, S.; Shadpour, S.; Ghorannevis, M.; Joupari, M.D. Effect of cold atmospheric pressure plasma and gold nanoparticles on cell viability. Annu. Res. Rev. Biol. 2014, 4, 3108–3118. [Google Scholar] [CrossRef]
- Xie, J.; Lim, L.K.; Phua, Y.; Hua, J.; Wang, C.H. Electrohydrodynamic atomization for biodegradable polymeric particle production. J. Colloid. Interface Sci. 2006, 302, 103–112. [Google Scholar] [CrossRef]
- Agarwal, A.; Kasinathan, A.; Ganesan, R.; Balasubramanian, A.; Bhaskaran, J.; Suresh, S.; Srinivasan, R.; Aravind, K.B.; Sivalingam, N. Curcumin induces apoptosis and cell cycle arrest via the activation of reactive oxygen species-independent mitochondrial apoptotic pathway in Smad4 and p53 mutated colon adenocarcinoma HT29 cells. Nutr. Res. 2018, 51, 67–81. [Google Scholar] [CrossRef]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef]
- Murphy, J.M.; Rodriguez, Y.A.R.; Jeong, K.; Ahn, E.E.; Lim, S.S. Targeting focal adhesion kinase in cancer cells and the tumor microenvironment. Exp. Mol. Med. 2020, 52, 877–886. [Google Scholar] [CrossRef]
- Menard, S.; Casalini, P.; Campiglio, M.; Pupa, S.M.; Tagliabue, E. Role of HER2/neu in tumor progression and therapy. Cell. Mol. Life Sci. 2004, 61, 2965–2978. [Google Scholar] [CrossRef]
- Sagwal, S.K.; Bekeschus, S. ROS Pleiotropy in Melanoma and Local Therapy with Physical Modalities. Oxidative Med. Cell. Longev. 2021, 2021, 6816214. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef]
- Liu, Y.P.; Zheng, C.C.; Huang, Y.N.; He, M.L.; Xu, W.W.; Li, B. Molecular mechanisms of chemo- and radiotherapy resistance and the potential implications for cancer treatment. MedComm 2021, 2, 315–340. [Google Scholar] [CrossRef]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef]
- Wu, Y.; Song, Y.; Wang, R.; Wang, T. Molecular mechanisms of tumor resistance to radiotherapy. Mol. Cancer 2023, 22, 96. [Google Scholar] [CrossRef]
- Stone, H.B.; Coleman, C.N.; Anscher, M.S.; McBride, W.H. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003, 4, 529–536. [Google Scholar] [CrossRef]
- Harley, J.C.; Suchowerska, N.; Rogers, L.J.; Lo, H.; Choong, E.S.; McKenzie, D.R. Plasma activated liquid synergistically enhances response to radiation for improved cancer therapy. Plasma Process Polym. 2022, 19, 2200026. [Google Scholar] [CrossRef]
- Ji, W.O.; Lee, M.H.; Kim, G.H.; Kim, E.H. Quantitation of the ROS production in plasma and radiation treatments of biotargets. Sci. Rep. 2019, 9, 19837. [Google Scholar] [CrossRef]
- Kawamura, K.; Qi, F.; Kobayashi, J. Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production. J. Radiat. Res. 2018, 59, ii91–ii97. [Google Scholar] [CrossRef] [PubMed]
- Graham, W.G.; Schaper, L.; Muir, M.; Currell, F.J. The Effect of Electrical Discharges in the Cell Media on Their Viability and DNA Damage and Comparison with the Effect of X-Rays. Plasma Med. 2012, 2, 169–178. [Google Scholar] [CrossRef][Green Version]
- Dejonckheere, C.S.; Torres-Crigna, A.; Layer, J.P.; Layer, K.; Wiegreffe, S.; Sarria, G.R.; Scafa, D.; Koch, D.; Leitzen, C.; Koksal, M.A.; et al. Non-Invasive Physical Plasma for Preventing Radiation Dermatitis in Breast Cancer: A First-In-Human Feasibility Study. Pharmaceutics 2022, 14, 1767. [Google Scholar] [CrossRef]
- Goto, T. Radiation as an In Situ Auto-Vaccination: Current Perspectives and Challenges. Vaccines 2019, 7, 100. [Google Scholar] [CrossRef]
- Bekeschus, S.; Clemen, R.; Niessner, F.; Sagwal, S.K.; Freund, E.; Schmidt, A. Medical Gas Plasma Jet Technology Targets Murine Melanoma in an Immunogenic Fashion. Adv. Sci. 2020, 7, 1903438. [Google Scholar] [CrossRef]
- Clemen, R.; Bekeschus, S. ROS Cocktails as an Adjuvant for Personalized Antitumor Vaccination? Vaccines 2021, 9, 527. [Google Scholar] [CrossRef]
- Belehradek, M.; Domenge, C.; Luboinski, B.; Orlowski, S.; Belehradek, J., Jr.; Mir, L.M. Electrochemotherapy, a new antitumor treatment. First clinical phase I-II trial. Cancer 1993, 72, 3694–3700. [Google Scholar] [CrossRef] [PubMed]
- Domenge, C.; Orlowski, S.; Luboinski, B.; De Baere, T.; Schwaab, G.; Belehradek, J., Jr.; Mir, L.M. Antitumor electrochemotherapy: New advances in the clinical protocol. Cancer 1996, 77, 956–963. [Google Scholar] [CrossRef]
- Esmaeili, N.; Friebe, M. Electrochemotherapy: A Review of Current Status, Alternative IGP Approaches, and Future Perspectives. J. Healthc. Eng. 2019, 2019, 2784516. [Google Scholar] [CrossRef]
- Mir, L.M. Bases and rationale of the electrochemotherapy. Ejc Suppl. 2006, 4, 38–44. [Google Scholar] [CrossRef]
- Tsoneva, I.; Semkova, S.; Bakalova, R.; Zhelev, Z.; Nuss, P.; Staneva, G.; Nikolova, B. Electroporation, electrochemotherapy and electro-assisted drug delivery in cancer. A state-of-the-art review. Biophys. Chem. 2022, 286, 106819. [Google Scholar] [CrossRef]
- Wolff, C.M.; Steuer, A.; Stoffels, I.; von Woedtke, T.; Weltmann, K.-D.; Bekeschus, S.; Kolb, J.F. Combination of cold plasma and pulsed electric fields—A rationale for cancer patients in palliative care. Clin. Plasma Med. 2019, 16, 100096. [Google Scholar] [CrossRef]
- Pakhomova, O.N.; Khorokhorina, V.A.; Bowman, A.M.; Rodaite-Riseviciene, R.; Saulis, G.; Xiao, S.; Pakhomov, A.G. Oxidative effects of nanosecond pulsed electric field exposure in cells and cell-free media. Arch. Biochem. Biophys. 2012, 527, 55–64. [Google Scholar] [CrossRef]
- Rajagopalan, N.R.; Munawar, T.; Sheehan, M.C.; Fujimori, M.; Vista, W.R.; Wimmer, T.; Gutta, N.B.; Solomon, S.B.; Srimathveeravalli, G. Electrolysis products, reactive oxygen species and ATP loss contribute to cell death following irreversible electroporation with microsecond-long pulsed electric fields. Bioelectrochemistry 2024, 155, 108579. [Google Scholar] [CrossRef]
- Wolff, C.M.; Kolb, J.F.; Bekeschus, S. Combined In Vitro Toxicity and Immunogenicity of Cold Plasma and Pulsed Electric Fields. Biomedicines 2022, 10, 3084. [Google Scholar] [CrossRef]
- Sai, D.L.; Lee, J.; Nguyen, D.L.; Kim, Y.P. Tailoring photosensitive ROS for advanced photodynamic therapy. Exp. Mol. Med. 2021, 53, 495–504. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kedzierska, E.; Knap-Czop, K.; Kotlinska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Berlanda, J.; Kiesslich, T.; Engelhardt, V.; Krammer, B.; Plaetzer, K. Comparative in vitro study on the characteristics of different photosensitizers employed in PDT. J. Photochem. Photobiol. B 2010, 100, 173–180. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Huang, M.; Zeng, S.; Zheng, J.; Peng, S.; Wang, Y.; Cheng, H.; Li, S. Innovative strategies for photodynamic therapy against hypoxic tumor. Asian J. Pharm. Sci. 2023, 18, 100775. [Google Scholar] [CrossRef]
- Casas, A.; Di Venosa, G.; Hasan, T.; Al, B. Mechanisms of resistance to photodynamic therapy. Curr. Med. Chem. 2011, 18, 2486–2515. [Google Scholar] [CrossRef]
- Vejdani Noghreiyan, A.; Imanparast, A.; Shayesteh Ara, E.; Soudmand, S.; Vejdani Noghreiyan, V.; Sazgarnia, A. In-vitro investigation of cold atmospheric plasma induced photodynamic effect by Indocyanine green and Protoporphyrin IX. Photodiagnosis Photodyn. Ther. 2020, 31, 101822. [Google Scholar] [CrossRef]
- Collet, G.; Robert, E.; Lenoir, A.; Vandamme, M.; Darny, T.; Dozias, S.; Kieda, C.; Pouvesle, J.M. Plasma jet-induced tissue oxygenation: Potentialities for new therapeutic strategies. Plasma Sources Sci. Technol. 2014, 23, 012005. [Google Scholar] [CrossRef]
- Schmidt, A.; Liebelt, G.; Niessner, F.; von Woedtke, T.; Bekeschus, S. Gas plasma-spurred wound healing is accompanied by regulation of focal adhesion, matrix remodeling, and tissue oxygenation. Redox Biol. 2021, 38, 101809. [Google Scholar] [CrossRef]
- Habash, R.W.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal therapy, part 1: An introduction to thermal therapy. Crit. Rev. Biomed. Eng. 2006, 34, 459–489. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Weng, S.; Yu, L.; Zhu, N.; Yang, M.; Yuan, Y. The Role of Hyperthermia in the Multidisciplinary Treatment of Malignant Tumors. Integr. Cancer Ther. 2019, 18, 1534735419876345. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, R.; Rehman, M.U.; Zhao, Q.L.; Jawaid, P.; Takeda, K.; Ishikawa, K.; Hori, M.; Tomihara, K.; Noguchi, K.; Kondo, T.; et al. Cold atmospheric helium plasma causes synergistic enhancement in cell death with hyperthermia and an additive enhancement with radiation. Sci. Rep. 2017, 7, 11659. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, J.S.; Xu, S.D.; Wang, Z.F.; Xu, D.H.; Guo, L.; Liu, D.X.; Kong, M.G.; Rong, M.Z. Antitumor effects of hyperthermia with plasma-treated solutions on 3D bladder tumor spheroids. Plasma Process Polym. 2021, 18, 2100070. [Google Scholar] [CrossRef]
- Ferrer-Sola, M.; Sureda-Vidal, H.; Altimiras-Roset, J.; Fontsere-Candell, E.; Gonzalez-Martinez, V.; Espaulella-Panicot, J.; Falanga, V.; Otero-Vinas, M. Hydrosurgery as a safe and efficient debridement method in a clinical wound unit. J. Wound Care 2017, 26, 593–599. [Google Scholar] [CrossRef]
- Song, X.F.; Zhao, J.Q.; Yan, H.; Yu, W.L.; Yin, L. Waterjet machining of biological tissues in medical surgeries: From soft tissue dissection to bone cutting. J. Manuf. Process. 2023, 107, 529–548. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; von Woedtke, T.; Hasse, S. Cell migration and adhesion of a human melanoma cell line is decreased by cold plasma treatment. Clin. Plasma Med. 2015, 3, 24–31. [Google Scholar] [CrossRef]
- Holl, M.; Rasch, M.L.; Becker, L.; Keller, A.L.; Schultze-Rhonhof, L.; Ruoff, F.; Templin, M.; Keller, S.; Neis, F.; Kessler, F.; et al. Cell Type-Specific Anti-Adhesion Properties of Peritoneal Cell Treatment with Plasma-Activated Media (PAM). Biomedicines 2022, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Golz, A.C.; Bergemann, C.; Hildebrandt, F.; Emmert, S.; Nebe, B.; Rebl, H. Selective adhesion inhibition and hyaluronan envelope reduction of dermal tumor cells by cold plasma-activated medium. Cell Adhes. Migr. 2023, 17, 1–19. [Google Scholar] [CrossRef]
- Kusakci-Seker, B.; Ozdemır, H. Assessment of pain perception after conventional frenectomy with application of cold atmospheric plasma. East. J. Med. 2020, 25, 558–564. [Google Scholar] [CrossRef]
- Brandenburg, R. Dielectric barrier discharges: Progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci. Technol. 2017, 26, 053001. [Google Scholar] [CrossRef]
- Winter, J.; Brandenburg, R.; Weltmann, K.D. Atmospheric pressure plasma jets: An overview of devices and new directions. Plasma Sources Sci. Technol. 2015, 24, 064001. [Google Scholar] [CrossRef]
- Hofmann, S.; van Gessel, A.F.H.; Verreycken, T.; Bruggeman, P. Power dissipation, gas temperatures and electron densities of cold atmospheric pressure helium and argon RF plasma jets. Plasma Sources Sci. Technol. 2011, 20, 065010. [Google Scholar] [CrossRef]
- Partecke, L.I.; Evert, K.; Haugk, J.; Doering, F.; Normann, L.; Diedrich, S.; Weiss, F.U.; Evert, M.; Huebner, N.O.; Guenther, C.; et al. Tissue tolerable plasma (TTP) induces apoptosis in pancreatic cancer cells in vitro and in vivo. BMC Cancer 2012, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Binenbaum, Y.; Ben-David, G.; Gil, Z.; Slutsker, Y.Z.; Ryzhkov, M.A.; Felsteiner, J.; Krasik, Y.E.; Cohen, J.T. Cold Atmospheric Plasma, Created at the Tip of an Elongated Flexible Capillary Using Low Electric Current, Can Slow the Progression of Melanoma. PLoS ONE 2017, 12, e0169457. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S. Acquired cancer tyrosine kinase inhibitor resistance: ROS as critical determinants. Signal Transduct. Target. Ther. 2021, 6, 437. [Google Scholar] [CrossRef]
- Miller, C.J.; Shin, T.M.; Sobanko, J.F.; Sharkey, J.M.; Grunyk, J.W.; Elenitsas, R.; Chu, E.Y.; Capell, B.C.; Ming, M.E.; Etzkorn, J.R. Risk factors for positive or equivocal margins after wide local excision of 1345 cutaneous melanomas. J. Am. Acad. Dermatol. 2017, 77, 333–340 e331. [Google Scholar] [CrossRef] [PubMed]
- Al Hmada, Y.; Brodell, R.T.; Kharouf, N.; Flanagan, T.W.; Alamodi, A.A.; Hassan, S.Y.; Shalaby, H.; Hassan, S.L.; Haikel, Y.; Megahed, M.; et al. Mechanisms of Melanoma Progression and Treatment Resistance: Role of Cancer Stem-like Cells. Cancers 2024, 16, 470. [Google Scholar] [CrossRef]
- Garbe, C.; Amaral, T.; Peris, K.; Hauschild, A.; Arenberger, P.; Basset-Seguin, N.; Bastholt, L.; Bataille, V.; Brochez, L.; Del Marmol, V.; et al. European consensus-based interdisciplinary guideline for melanoma. Part 2: Treatment—Update 2024. Eur. J. Cancer 2025, 215, 115153. [Google Scholar] [CrossRef]
- Sundstrom, S.; Bremnes, R.M.; Kaasa, S.; Aasebo, U.; Hatlevoll, R.; Dahle, R.; Boye, N.; Wang, M.; Vigander, T.; Vilsvik, J.; et al. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: Results from a randomized phase III trial with 5 years’ follow-up. J. Clin. Oncol. 2002, 20, 4665–4672. [Google Scholar] [CrossRef]
- Azzoli, C.G.; Baker, S., Jr.; Temin, S.; Pao, W.; Aliff, T.; Brahmer, J.; Johnson, D.H.; Laskin, J.L.; Masters, G.; Milton, D.; et al. American Society of Clinical Oncology Clinical Practice Guideline update on chemotherapy for stage IV non-small-cell lung cancer. J. Clin. Oncol. 2009, 27, 6251–6266. [Google Scholar] [CrossRef]
- Song, C.H.; Attri, P.; Ku, S.K.; Han, I.; Bogaerts, A.; Choi, E.H. Cocktail of reactive species generated by cold atmospheric plasma: Oral administration induces non-small cell lung cancer cell death. J. Phys. D Appl. Phys. 2021, 54, 185202. [Google Scholar] [CrossRef]
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B., 3rd; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 678–700. [Google Scholar] [CrossRef]
- Ishaq, M.; Evans, M.D.; Ostrikov, K.K. Atmospheric pressure gas plasma-induced colorectal cancer cell death is mediated by Nox2-ASK1 apoptosis pathways and oxidative stress is mitigated by Srx-Nrf2 anti-oxidant system. Biochim. Biophys. Acta 2014, 1843, 2827–2837. [Google Scholar] [CrossRef]
- Ishaq, M.; Han, Z.J.; Kumar, S.; Evans, M.D.M.; Ostrikov, K. Atmospheric-Pressure Plasma- and TRAIL-Induced Apoptosis in TRAIL-Resistant Colorectal Cancer Cells. Plasma Process Polym. 2015, 12, 574–582. [Google Scholar] [CrossRef]
- Wang, Y.; Mang, X.; Li, X.; Cai, Z.; Tan, F. Cold atmospheric plasma induces apoptosis in human colon and lung cancer cells through modulating mitochondrial pathway. Front. Cell Dev. Biol. 2022, 10, 915785. [Google Scholar] [CrossRef] [PubMed]
- Miebach, L.; Freund, E.; Clemen, R.; Kersting, S.; Partecke, L.I.; Bekeschus, S. Gas plasma-oxidized sodium chloride acts via hydrogen peroxide in a model of peritoneal carcinomatosis. Proc. Natl. Acad. Sci. USA 2022, 119, e2200708119. [Google Scholar] [CrossRef] [PubMed]
- Miebach, L.; Freund, E.; Cecchini, A.L.; Bekeschus, S. Conductive Gas Plasma Treatment Augments Tumor Toxicity of Ringer’s Lactate Solutions in a Model of Peritoneal Carcinomatosis. Antioxidants 2022, 11, 1439. [Google Scholar] [CrossRef]
- Winter, J.; Nishime, T.M.C.; Glitsch, S.; Lühder, H.; Weltmann, K.D. On the development of a deployable cold plasma endoscope. Contrib. Plasm. Phys. 2018, 58, 404–414. [Google Scholar] [CrossRef]
- Esposito, I.; Kleeff, J.; Bergmann, F.; Reiser, C.; Herpel, E.; Friess, H.; Schirmacher, P.; Buchler, M.W. Most pancreatic cancer resections are R1 resections. Ann. Surg. Oncol. 2008, 15, 1651–1660. [Google Scholar] [CrossRef]
- Mizrahi, J.D.; Surana, R.; Valle, J.W.; Shroff, R.T. Pancreatic cancer. Lancet 2020, 395, 2008–2020. [Google Scholar] [CrossRef]
- De Dosso, S.; Siebenhuner, A.R.; Winder, T.; Meisel, A.; Fritsch, R.; Astaras, C.; Szturz, P.; Borner, M. Treatment landscape of metastatic pancreatic cancer. Cancer Treat. Rev. 2021, 96, 102180. [Google Scholar] [CrossRef]
- Martinez-Useros, J.; Li, W.; Cabeza-Morales, M.; Garcia-Foncillas, J. Oxidative Stress: A New Target for Pancreatic Cancer Prognosis and Treatment. J. Clin. Med. 2017, 6, 29. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Yamada, S.; Torii, K.; Takeda, S.; Nakamura, K.; Tanaka, H.; Kajiyama, H.; Kanda, M.; Fujii, T.; Nakayama, G.; et al. Effectiveness of plasma treatment on pancreatic cancer cells. Int. J. Oncol. 2015, 47, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Khabipov, A.; Freund, E.; Liedtke, K.R.; Kading, A.; Riese, J.; van der Linde, J.; Kersting, S.; Partecke, L.I.; Bekeschus, S. Murine Macrophages Modulate Their Inflammatory Profile in Response to Gas Plasma-Inactivated Pancreatic Cancer Cells. Cancers 2021, 13, 2525. [Google Scholar] [CrossRef] [PubMed]
- Nasir, I.; McGuinness, C.; Poh, A.R.; Ernst, M.; Darcy, P.K.; Britt, K.L. Tumor macrophage functional heterogeneity can inform the development of novel cancer therapies. Trends Immunol. 2023, 44, 971–985. [Google Scholar] [CrossRef]
- Van Loenhout, J.; Flieswasser, T.; Freire Boullosa, L.; De Waele, J.; Van Audenaerde, J.; Marcq, E.; Jacobs, J.; Lin, A.; Lion, E.; Dewitte, H.; et al. Cold Atmospheric Plasma-Treated PBS Eliminates Immunosuppressive Pancreatic Stellate Cells and Induces Immunogenic Cell Death of Pancreatic Cancer Cells. Cancers 2019, 11, 1597. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Bekeschus, S.; Kaeding, A.; Hackbarth, C.; Kuehn, J.P.; Heidecke, C.D.; von Bernstorff, W.; von Woedtke, T.; Partecke, L.I. Non-thermal plasma-treated solution demonstrates antitumor activity against pancreatic cancer cells in vitro and in vivo. Sci. Rep. 2017, 7, 8319. [Google Scholar] [CrossRef]
- Liedtke, K.R.; Freund, E.; Hackbarth, C.; Heidecke, C.-D.; Partecke, L.-I.; Bekeschus, S. A myeloid and lymphoid infiltrate in murine pancreatic tumors exposed to plasma-treated medium. Clin. Plasma Med. 2018, 11, 10–17. [Google Scholar] [CrossRef]
- Miebach, L.; Berner, J.; Bekeschus, S. In ovo model in cancer research and tumor immunology. Front. Immunol. 2022, 13, 1006064. [Google Scholar] [CrossRef]
- Bekeschus, S.; Freund, E.; Spadola, C.; Privat-Maldonado, A.; Hackbarth, C.; Bogaerts, A.; Schmidt, A.; Wende, K.; Weltmann, K.D.; von Woedtke, T.; et al. Risk Assessment of kINPen Plasma Treatment of Four Human Pancreatic Cancer Cell Lines with Respect to Metastasis. Cancers 2019, 11, 1237. [Google Scholar] [CrossRef]
- Davies, C.; Pan, H.; Godwin, J.; Gray, R.; Arriagada, R.; Raina, V.; Abraham, M.; Medeiros Alencar, V.H.; Badran, A.; Bonfill, X.; et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013, 381, 805–816. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blattler, W.A.; Lambert, J.M.; Chari, R.V.; Lutz, R.J.; et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Peart, O. Metastatic Breast Cancer. Radiol. Technol. 2017, 88, 519M–539M. [Google Scholar] [PubMed]
- Burguin, A.; Diorio, C.; Durocher, F. Breast Cancer Treatments: Updates and New Challenges. J. Pers. Med. 2021, 11, 808. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, Y.; Kiani, M.F.; Wang, B. Classification, Treatment Strategy, and Associated Drug Resistance in Breast Cancer. Clin. Breast Cancer 2016, 16, 335–343. [Google Scholar] [CrossRef]
- Kolberg, H.C.; Hoffmann, O.; Baumann, R. The Abscopal Effect: Could a Phenomenon Described Decades Ago Become Key to Enhancing the Response to Immune Therapies in Breast Cancer? Breast Care 2020, 15, 443–449. [Google Scholar] [CrossRef]
- Chen, G.; Chen, Z.; Wang, Z.; Obenchain, R.; Wen, D.; Li, H.; Wirz, R.E.; Gu, Z. Portable air-fed cold atmospheric plasma device for postsurgical cancer treatment. Sci. Adv. 2021, 7, eabg5686. [Google Scholar] [CrossRef]
- Xiang, L.; Xu, X.; Zhang, S.; Cai, D.; Dai, X. Cold atmospheric plasma conveys selectivity on triple negative breast cancer cells both in vitro and in vivo. Free Radic. Biol. Med. 2018, 124, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.R.K.; Cheng, X.; Zhuang, T.; Ly, L.; Jones, O.; Basadonna, G.; Keidar, M.; Canady, J. BCL2A1 regulates Canady Helios Cold Plasma-induced cell death in triple-negative breast cancer. Sci. Rep. 2022, 12, 4038. [Google Scholar] [CrossRef]
- Ly, L.; Cheng, X.; Murthy, S.R.K.; Zhuang, T.; Jones, O.Z.; Basadonna, G.; Keidar, M.; Canady, J. Canady cold plasma conversion system treatment: An effective inhibitor of cell viability in breast cancer molecular subtypes. Clin. Plasma Med. 2020, 19–20, 100109. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.; Ji, H.W.; Kim, H.W.; Yun, S.H.; Choi, E.H.; Kim, S.J. Cold Atmospheric Plasma Restores Paclitaxel Sensitivity to Paclitaxel-Resistant Breast Cancer Cells by Reversing Expression of Resistance-Related Genes. Cancers 2019, 11, 2011. [Google Scholar] [CrossRef]
- Vijayarangan, V.; Delalande, A.; Dozias, S.; Pouvesle, J.M.; Robert, E.; Pichon, C. New insights on molecular internalization and drug delivery following plasma jet exposures. Int. J. Pharm. 2020, 589, 119874. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Attri, P.; Choi, E.H.; Uhm, H.S. Influence of water vapour with non-thermal plasma jet on the apoptosis of SK-BR-3 breast cancer cells. RSC Adv. 2015, 5, 14670–14677. [Google Scholar] [CrossRef]
- Dejonckheere, C.S.; Layer, J.P.; Nour, Y.; Layer, K.; Glasmacher, A.; Wiegreffe, S.; Fuhrmann, A.; Caglayan, L.; Grau, F.; Sarria, G.R.; et al. Non-invasive physical plasma for preventing radiation dermatitis in breast cancer: Results from an intrapatient-randomised double-blind placebo-controlled trial. Clin. Transl. Radiat. Oncol. 2024, 44, 100699. [Google Scholar] [CrossRef] [PubMed]
- Akbari, Z.; Saadati, F.; Mahdikia, H.; Freund, E.; Abbasvandi, F.; Shokri, B.; Zali, H.; Bekeschus, S. Antitumor Effects in Gas Plasma-Treated Patient-Derived Microtissues—An Adjuvant Therapy for Ulcerating Breast Cancer? Appl. Sci. 2021, 11, 4527. [Google Scholar] [CrossRef]
- Saadati, F.; Jahanbakhshi, F.; Mahdikia, H.; Abbasvandi, F.; Ghomi, H.; Yazdani, N.; Aghazadeh, K.; Emmert, S.; Bekeschus, S. Cold Physical Plasma Toxicity in Breast and Oral Squamous Carcinoma In Vitro and in Patient-Derived Cancer Tissue Ex Vivo. Appl. Sci. 2023, 13, 6472. [Google Scholar] [CrossRef]
- Hussein, A.A.; Shabir, U.; Mahmood, A.W.; Harrington, G.; Khan, M.; Ahmad, A.; Howlader, M.; Colan, N.; Shah, A.A.; Ghadersohi, S.; et al. The impact of NCCN-compliant multidisciplinary conference on the uptake of active surveillance among eligible patients with localized prostate cancer. Urol. Oncol. 2023, 41, 483.e21–483.e26. [Google Scholar] [CrossRef]
- Malcolm, J.B.; Fabrizio, M.D.; Barone, B.B.; Given, R.W.; Lance, R.S.; Lynch, D.F.; Davis, J.W.; Shaves, M.E.; Schellhammer, P.F. Quality of life after open or robotic prostatectomy, cryoablation or brachytherapy for localized prostate cancer. J. Urol. 2010, 183, 1822–1828. [Google Scholar] [CrossRef]
- Poulsen, M.H.; Frost, M.; Abrahamsen, B.; Gerke, O.; Walter, S.; Lund, L. Osteoporosis and prostate cancer; a 24-month prospective observational study during androgen deprivation therapy. Scand. J. Urol. 2019, 53, 34–39. [Google Scholar] [CrossRef]
- Hirst, A.M.; Simms, M.S.; Mann, V.M.; Maitland, N.J.; O’Connell, D.; Frame, F.M. Low-temperature plasma treatment induces DNA damage leading to necrotic cell death in primary prostate epithelial cells. Br. J. Cancer 2015, 112, 1536–1545. [Google Scholar] [CrossRef]
- Fofana, M.; Buñay, J.; Judée, F.; Baron, S.; Menecier, S.; Nivoix, M.; Perisse, F.; Vacavant, A.; Balandraud, X. Selective treatments of prostate tumor cells with a cold atmospheric plasma jet. Clin. Plasma Med. 2020, 17–18, 100098. [Google Scholar] [CrossRef]
- Hua, D.; Cai, D.; Ning, M.; Yu, L.; Zhang, Z.; Han, P.; Dai, X. Cold atmospheric plasma selectively induces G(0)/G(1) cell cycle arrest and apoptosis in AR-independent prostate cancer cells. J. Cancer 2021, 12, 5977–5986. [Google Scholar] [CrossRef]
- Hirst, A.M.; Frame, F.M.; Maitland, N.J.; O’Connell, D. Low temperature plasma: A novel focal therapy for localized prostate cancer? BioMed Res. Int. 2014, 2014, 878319. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reynies, A.; Allory, Y.; Sjodahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Comperat, E.M.; Cowan, N.C.; Gakis, G.; Hernandez, V.; Linares Espinos, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2021, 79, 82–104. [Google Scholar] [CrossRef]
- Yu, E.M.; Belay, S.; Li, W.; Aragon-Ching, J.B. Non-urothelial and urothelial variants of bladder cancer. Cancer Treat. Res. Commun. 2022, 33, 100661. [Google Scholar] [CrossRef]
- Gelbrich, N.; Miebach, L.; Berner, J.; Freund, E.; Saadati, F.; Schmidt, A.; Stope, M.; Zimmermann, U.; Burchardt, M.; Bekeschus, S. Medical gas plasma augments bladder cancer cell toxicity in preclinical models and patient-derived tumor tissues. J. Adv. Res. 2023, 47, 209–223. [Google Scholar] [CrossRef]
- Jo, A.; Joh, H.M.; Bae, J.H.; Kim, S.J.; Chung, J.W.; Chung, T.H. Plasma-Activated Media Produced by a Microwave-Excited Atmospheric Pressure Plasma Jet Is Effective against Cisplatin-Resistant Human Bladder Cancer Cells In Vitro. Int. J. Mol. Sci. 2024, 25, 1249. [Google Scholar] [CrossRef]
- Fukuhara, H.; Szili, E.J.; Oh, J.-S.; Chiaki, K.; Yamamoto, S.; Kurabayashi, A.; Furihata, M.; Tsuda, M.; Furuta, H.; Lindsay, H.D.; et al. Oxidative Stress Pathways Linked to Apoptosis Induction by Low-Temperature Plasma Jet Activated Media in Bladder Cancer Cells: An In Vitro and In Vivo Study. Plasma 2022, 5, 233–246. [Google Scholar] [CrossRef]
- Tavares-da-Silva, E.; Pereira, E.; Pires, A.S.; Neves, A.R.; Braz-Guilherme, C.; Marques, I.A.; Abrantes, A.M.; Goncalves, A.C.; Caramelo, F.; Silva-Teixeira, R.; et al. Cold Atmospheric Plasma, a Novel Approach against Bladder Cancer, with Higher Sensitivity for the High-Grade Cell Line. Biol. 2021, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhou, R.; Thomas, P.; Zhao, L.; Zhou, R.; Mandal, S.; Jolly, M.K.; Richard, D.J.; Rehm, B.H.A.; Ostrikov, K.K.; et al. Epithelial-to-Mesenchymal Transition Enhances Cancer Cell Sensitivity to Cytotoxic Effects of Cold Atmospheric Plasmas in Breast and Bladder Cancer Systems. Cancers 2021, 13, 2889. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Guo, B.; Chen, H.; Xu, D.; Kong, M.G. The Antitumor Effects of Plasma-Activated Saline on Muscle-Invasive Bladder Cancer Cells In Vitro and In Vivo Demonstrate Its Feasibility as a Potential Therapeutic Approach. Cancers 2021, 13, 1042. [Google Scholar] [CrossRef]
- Winter, J.; Nishime, T.M.C.; Bansemer, R.; Balazinski, M.; Wende, K.; Weltmann, K.D. Enhanced atmospheric pressure plasma jet setup for endoscopic applications. J. Phys. D Appl. Phys. 2019, 52, 024005. [Google Scholar] [CrossRef]
- Gweon, B.; Kim, M.; Kim, D.B.; Kim, D.; Kim, H.; Jung, H.; Shin, J.H.; Choe, W. Differential responses of human liver cancer and normal cells to atmospheric pressure plasma. Appl. Phys. Lett. 2011, 99, 063701. [Google Scholar] [CrossRef]
- Smolkova, B.; Lunova, M.; Lynnyk, A.; Uzhytchak, M.; Churpita, O.; Jirsa, M.; Kubinova, S.; Lunov, O.; Dejneka, A. Non-Thermal Plasma, as a New Physicochemical Source, to Induce Redox Imbalance and Subsequent Cell Death in Liver Cancer Cell Lines. Cell Physiol. Biochem. 2019, 52, 119–140. [Google Scholar] [CrossRef]
- Duan, J.; Lu, X.; He, G. The selective effect of plasma activated medium in an in vitro co-culture of liver cancer and normal cells. J. Appl. Phys. 2017, 121, 013302. [Google Scholar] [CrossRef]
- Vaquero, J.; Judee, F.; Vallette, M.; Decauchy, H.; Arbelaiz, A.; Aoudjehane, L.; Scatton, O.; Gonzalez-Sanchez, E.; Merabtene, F.; Augustin, J.; et al. Cold-Atmospheric Plasma Induces Tumor Cell Death in Preclinical In Vivo and In Vitro Models of Human Cholangiocarcinoma. Cancers 2020, 12, 1280. [Google Scholar] [CrossRef]
- Yang, H.; Lu, R.; Xian, Y.; Gan, L.; Lu, X.; Yang, X. Effects of atmospheric pressure cold plasma on human hepatocarcinoma cell and its 5-fluorouracil resistant cell line. Phys. Plasmas 2015, 22, 122006. [Google Scholar] [CrossRef]
- Decauchy, H.; Pavy, A.; Camus, M.; Fouassier, L.; Dufour, T. Cold plasma endoscopy applied to biliary ducts: Feasibility risk assessment on human-like and porcine models for the treatment of cholangiocarcinoma. J. Phys. D Appl. Phys. 2022, 55, 455401. [Google Scholar] [CrossRef]
- Renzulli, M.; Brandi, N.; Pecorelli, A.; Pastore, L.V.; Granito, A.; Martinese, G.; Tovoli, F.; Simonetti, M.; Dajti, E.; Colecchia, A.; et al. Segmental Distribution of Hepatocellular Carcinoma in Cirrhotic Livers. Diagn. 2022, 12, 834. [Google Scholar] [CrossRef]
- Barekzi, N.; Laroussi, M. Dose-dependent killing of leukemia cells by low-temperature plasma. J. Phys. D Appl. Phys. 2012, 45, 422002. [Google Scholar] [CrossRef]
- Bundscherer, L.; Wende, K.; Ottmuller, K.; Barton, A.; Schmidt, A.; Bekeschus, S.; Hasse, S.; Weltmann, K.D.; Masur, K.; Lindequist, U. Impact of non-thermal plasma treatment on MAPK signaling pathways of human immune cell lines. Immunobiology 2013, 218, 1248–1255. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.X.; Xue, Z.X.; Yin, H.J.; Niu, Q.; Chen, H.L. The relation between doses or post-plasma time points and apoptosis of leukemia cells induced by dielectric barrier discharge plasma. AIP Adv. 2015, 5, 127220. [Google Scholar] [CrossRef]
- Montaser, S.A.; Ahmed, M.M.; El-Aragi, G.M.; Elhadry, A.A.; Said, Z.S. Cytogenetic and Biochemical Investigations of Cultured Leukemia Cells Exposed to Gliding Arc Discharges. Plasma Med. 2017, 7, 27–41. [Google Scholar] [CrossRef][Green Version]
- Xu, D.; Ning, N.; Xu, Y.; Wang, B.; Cui, Q.; Liu, Z.; Wang, X.; Liu, D.; Chen, H.; Kong, M.G. Effect of cold atmospheric plasma treatment on the metabolites of human leukemia cells. Cancer Cell Int. 2019, 19, 135. [Google Scholar] [CrossRef]
- Ahmed, M.M.; El-Aragi, G.M.; Elhadary, A.M.A.; Said, Z.S. Promising Trial for Treatment of Chronic Myelogenous Leukemia Using Plasma Technology. Plasma Med. 2013, 3, 243–265. [Google Scholar] [CrossRef][Green Version]
- Turrini, E.; Laurita, R.; Stancampiano, A.; Catanzaro, E.; Calcabrini, C.; Maffei, F.; Gherardi, M.; Colombo, V.; Fimognari, C. Cold Atmospheric Plasma Induces Apoptosis and Oxidative Stress Pathway Regulation in T-Lymphoblastoid Leukemia Cells. Oxidative Med. Cell. Longev. 2017, 2017, 4271065. [Google Scholar] [CrossRef]
- Golpour, M.; Alimohammadi, M.; Mohseni, A.; Zaboli, E.; Sohbatzadeh, F.; Bekeschus, S.; Rafiei, A. Lack of Adverse Effects of Cold Physical Plasma-Treated Blood from Leukemia Patients: A Proof-of-Concept Study. Appl. Sci. 2022, 12, 128. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Bethge, L.; Masur, K.; von Woedtke, T.; Hasse, S.; Wende, K. Redox Stimulation of Human THP-1 Monocytes in Response to Cold Physical Plasma. Oxidative Med. Cell. Longev. 2016, 2016, 5910695. [Google Scholar] [CrossRef]
- Schmidt, A.; Rödder, K.; Hasse, S.; Masur, K.; Toups, L.; Lillig, C.H.; von Woedtke, T.; Wende, K.; Bekeschus, S. Redox-regulation of activator protein 1 family members in blood cancer cell lines exposed to cold physical plasma-treated medium. Plasma Process Polym. 2016, 13, 1179–1188. [Google Scholar] [CrossRef]
- Guo, B.; Li, W.; Liu, Y.; Xu, D.; Liu, Z.; Huang, C. Aberrant Expressional Profiling of Small RNA by Cold Atmospheric Plasma Treatment in Human Chronic Myeloid Leukemia Cells. Front. Genet. 2021, 12, 809658. [Google Scholar] [CrossRef]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef]
- Bekeschus, S.; Seebauer, C.; Wende, K.; Schmidt, A. Physical plasma and leukocytes—Immune or reactive? Biol. Chem. 2018, 400, 63–75. [Google Scholar] [CrossRef]
- Bekeschus, S.; Kolata, J.; Muller, A.; Kramer, A.; Weltmann, K.-D.; Broker, B.; Masur, K. Differential Viability of Eight Human Blood Mononuclear Cell Subpopulations After Plasma Treatment. Plasma Med. 2013, 3, 1–13. [Google Scholar] [CrossRef]
- Bundscherer, L.; Bekeschus, S.; Tresp, H.; Hasse, S.; Reuter, S.; Weltmann, K.-D.; Lindequist, U.; Masur, K. Viability of Human Blood Leukocytes Compared with Their Respective Cell Lines after Plasma Treatment. Plasma Med. 2013, 3, 71–80. [Google Scholar] [CrossRef]
- Bekeschus, S.; Liebelt, G.; Menz, J.; Berner, J.; Sagwal, S.K.; Wende, K.; Weltmann, K.D.; Boeckmann, L.; von Woedtke, T.; Metelmann, H.R.; et al. Tumor cell metabolism correlates with resistance to gas plasma treatment: The evaluation of three dogmas. Free Radic. Bio Med. 2021, 167, 12–28. [Google Scholar] [CrossRef]
- Bekeschus, S.; Winterbourn, C.C.; Kolata, J.; Masur, K.; Hasse, S.; Broker, B.M.; Parker, H.A. Neutrophil extracellular trap formation is elicited in response to cold physical plasma. J. Leukoc. Biol. 2016, 100, 791–799. [Google Scholar] [CrossRef]
- Bekeschus, S.; Poschkamp, B.; van der Linde, J. Medical gas plasma promotes blood coagulation via platelet activation. Biomaterials 2021, 278, 120433. [Google Scholar] [CrossRef]
- Lin, A.; Biscop, E.; Breen, C.; Butler, S.J.; Smits, E.; Bogaerts, A.; Jakovljevic, V. Critical Evaluation of the Interaction of Reactive Oxygen and Nitrogen Species with Blood to Inform the Clinical Translation of Nonthermal Plasma Therapy. Oxidative Med. Cell. Longev. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Striesow, J.; Wesche, J.; McKitterick, N.; Busch, L.M.; von Woedtke, T.; Greinacher, A.; Bekeschus, S.; Wende, K. Gas plasma-induced platelet activation corresponds to reactive species profiles and lipid oxidation. Free Radic. Bio Med. 2023, 207, 212–225. [Google Scholar] [CrossRef]
- Bekeschus, S.; Brüggemeier, J.; Hackbarth, C.; von Woedtke, T.; Partecke, L.-I.; van der Linde, J. Platelets are key in cold physical plasma-facilitated blood coagulation in mice. Clin. Plasma Med. 2017, 7–8, 58–65. [Google Scholar] [CrossRef]
- Bekeschus, S.; Brüggemeier, J.; Hackbarth, C.; Weltmann, K.-D.; von Woedtke, T.; Partecke, L.-I.; van der Linde, J. The feed gas composition determines the degree of physical plasma-induced platelet activation for blood coagulation. Plasma Sources Sci. Technol. 2018, 27, 034001. [Google Scholar] [CrossRef]
- Miebach, L.; Poschkamp, B.; van der Linde, J.; Bekeschus, S. Medical Gas Plasma-A Potent ROS-Generating Technology for Managing Intraoperative Bleeding Complications. Appl. Sci. 2022, 12, 3800. [Google Scholar] [CrossRef]
- Poletto, S.; Novo, M.; Paruzzo, L.; Frascione, P.M.M.; Vitolo, U. Treatment strategies for patients with diffuse large B-cell lymphoma. Cancer Treat. Rev. 2022, 110, 102443. [Google Scholar] [CrossRef]
- Goldfinger, M.; Cooper, D.L. Refractory DLBCL: Challenges and Treatment. Clin. Lymphoma Myeloma Leuk. 2022, 22, 140–148. [Google Scholar] [CrossRef]
- Peroja, P.; Haapasaari, K.M.; Mannisto, S.; Miinalainen, I.; Koivunen, P.; Leppa, S.; Karjalainen-Lindsberg, M.L.; Kuusisto, M.E.; Turpeenniemi-Hujanen, T.; Kuittinen, O.; et al. Total peroxiredoxin expression is associated with survival in patients with follicular lymphoma. Virchows Arch. 2016, 468, 623–630. [Google Scholar] [CrossRef]
- Miura, Y.; Miwa, H.; Ohno, T.; Haneda, M.; Hosokawa, Y.; Nitta, M.; Daibata, M. The Role of Reactive Oxygen Species In Apoptosis Induction of Mantle Cell Lymphoma (MCL); Inhibition of NADPH Oxidase 2 Induces Apoptosis In MCL. Blood 2010, 116, 1489. [Google Scholar] [CrossRef]
- Li, C.; Thompson, M.A.; Tamayo, A.T.; Zuo, Z.; Lee, J.; Vega, F.; Ford, R.J.; Pham, L.V. Over-expression of Thioredoxin-1 mediates growth, survival, and chemoresistance and is a druggable target in diffuse large B-cell lymphoma. Oncotarget 2012, 3, 314–326. [Google Scholar] [CrossRef]
- Kinowaki, Y.; Kurata, M.; Ishibashi, S.; Ikeda, M.; Tatsuzawa, A.; Yamamoto, M.; Miura, O.; Kitagawa, M.; Yamamoto, K. Glutathione peroxidase 4 overexpression inhibits ROS-induced cell death in diffuse large B-cell lymphoma. Lab. Investig. 2018, 98, 609–619. [Google Scholar] [CrossRef]
- McNeill, K.A. Epidemiology of Brain Tumors. Neurol. Clin. 2016, 34, 981–998. [Google Scholar] [CrossRef]
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosenthal, M.; Wen, P.Y.; Stupp, R.; et al. Glioma. Nat. Rev. Dis. Primers 2015, 1, 15017. [Google Scholar] [CrossRef]
- Stummer, W.; van den Bent, M.J.; Westphal, M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: New arguments in an old discussion. Acta Neurochir. 2011, 153, 1211–1218. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, T. Understanding high grade glioma: Molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013, 331, 139–146. [Google Scholar] [CrossRef]
- van den Bent, M.J.; Carpentier, A.F.; Brandes, A.A.; Sanson, M.; Taphoorn, M.J.; Bernsen, H.J.; Frenay, M.; Tijssen, C.C.; Grisold, W.; Sipos, L.; et al. Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: A randomized European Organisation for Research and Treatment of Cancer phase III trial. J. Clin. Oncol. 2006, 24, 2715–2722. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Kim, Y.H.; Han, Y.G.; Choi, E.H. Effect of jet plasma on T98G human brain cancer cells. Curr. Appl. Phys. 2013, 13, 176–180. [Google Scholar] [CrossRef]
- Lehmann, S.; Bien-Moller, S.; Marx, S.; Bekeschus, S.; Schroeder, H.W.S.; Mustea, A.; Stope, M.B. Devitalization of Glioblastoma Cancer Cells by Non-invasive Physical Plasma: Modulation of Proliferative Signalling Cascades. Anticancer Res. 2023, 43, 7–18. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Sherman, J.; Murphy, W.; Ratovitski, E.; Canady, J.; Keidar, M. The Effect of Tuning Cold Plasma Composition on Glioblastoma Cell Viability. PLoS ONE 2014, 9, e98652. [Google Scholar] [CrossRef]
- Wanigasekara, J.; Barcia, C.; Cullen, P.J.; Tiwari, B.; Curtin, J.F. Plasma induced reactive oxygen species-dependent cytotoxicity in glioblastoma 3D tumourspheres. Plasma Process Polym. 2022, 19, 2100157. [Google Scholar] [CrossRef]
- Zhuang, J.; Yuan, Q.; Chen, C.; Liu, G.; Zhong, Z.; Zhu, K.; Guo, J. Nanosecond pulsed cold atmospheric plasma jet suppresses proliferation and migration of human glioblastoma cells via apoptosis promotion and EMT inhibition. Arch. Biochem. Biophys. 2023, 747, 109757. [Google Scholar] [CrossRef]
- Yao, X.; Lin, L.; Soni, V.; Gjika, E.; Sherman, J.H.; Yan, D.; Keidar, M. Sensitization of glioblastoma cells to temozolomide by a helium gas discharge tube. Phys. Plasmas 2020, 27, 114502. [Google Scholar] [CrossRef]
- Cheng, X.Q.; Murphy, W.; Recek, N.; Yan, D.Y.; Cvelbar, U.; Vesel, A.; Mozetic, M.; Canady, J.; Keidar, M.; Sherman, J.H. Synergistic effect of gold nanoparticles and cold plasma on glioblastoma cancer therapy. J. Phys. D Appl. Phys. 2014, 47, 335402. [Google Scholar] [CrossRef]
- Zolotukhin, D.B.; Horkowitz, A.; Keidar, M. Electromagnetic Nature of Distant Interaction of the Atmospheric Pressure Helium Plasma Discharge Tube with Glioblastoma Cancer Cells. ACS Appl. Mater. Interfaces 2024, 16, 13597–13610. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Ispirjan, M.; Freund, E.; Kinnen, F.; Moritz, J.; Saadati, F.; Eckroth, J.; Singer, D.; Stope, M.B.; Wende, K.; et al. Gas Plasma Exposure of Glioblastoma Is Cytotoxic and Immunomodulatory in Patient-Derived GBM Tissue. Cancers 2022, 14, 813. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Roessler, K.; Kepp, O.; Freund, E. Gas Plasma Technology and Immunogenic Cell Death: Implications for Chordoma Treatment. Cancers 2025, 17, 681. [Google Scholar] [CrossRef]
- Crowe, T.P.; Greenlee, M.H.W.; Kanthasamy, A.G.; Hsu, W.H. Mechanism of intranasal drug delivery directly to the brain. Life Sci. 2018, 195, 44–52. [Google Scholar] [CrossRef]
- Conway, G.E.; He, Z.; Hutanu, A.L.; Cribaro, G.P.; Manaloto, E.; Casey, A.; Traynor, D.; Milosavljevic, V.; Howe, O.; Barcia, C.; et al. Cold Atmospheric Plasma induces accumulation of lysosomes and caspase-independent cell death in U373MG glioblastoma multiforme cells. Sci. Rep. 2019, 9, 12891. [Google Scholar] [CrossRef]
- Kawada, K.; Kawano, T.; Momma, K.; Fujiwara, J.; Nagal, K.; Nishikage, T.; Nakajima, Y.; Ogiya, K.; Tanaka, K.; Haruki, S.; et al. New argon plasma coagulation method for superficial esophageal carcinomas: Argon plasma coagulation-subepithelial ablation. Dig. Endosc. 2007, 19, 147–152. [Google Scholar] [CrossRef]
- Estarabadi, H.; Atyabi, S.A.; Tavakkoli, S.; Noormohammadi, Z.; Gholami, M.R.; Ghiaseddin, A.; Irani, S. Cold atmospheric plasma induced genotoxicity and cytotoxicity in esophageal cancer cells. Mol. Biol. Rep. 2021, 48, 1323–1333. [Google Scholar] [CrossRef]
- Hu, Y.; Ye, X.; Wang, R.; Poon, K. Current research progress in the role of reactive oxygen species in esophageal adenocarcinoma. Transl. Cancer Res. 2021, 10, 1568–1577. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, T.; Wang, S.; Bahetjan, Y.; Li, X.; Yang, X. Dehydrocostus lactone inhibits the proliferation of esophageal cancer cells in vivo and in vitro through ROS-mediated apoptosis and autophagy. Food Chem. Toxicol. 2022, 170, 113453. [Google Scholar] [CrossRef] [PubMed]
- Berner, J.; Miebach, L.; Kordt, M.; Seebauer, C.; Schmidt, A.; Lalk, M.; Vollmar, B.; Metelmann, H.R.; Bekeschus, S. Chronic oxidative stress adaptation in head and neck cancer cells generates slow-cyclers with decreased tumour growth in vivo. Br. J. Cancer 2023, 129, 869–883. [Google Scholar] [CrossRef]
- Liu, J.; Hu, F.; Wu, M.; Tian, L.; Gong, F.; Zhong, X.; Chen, M.; Liu, Z.; Liu, B. Bioorthogonal Coordination Polymer Nanoparticles with Aggregation-Induced Emission for Deep Tumor-Penetrating Radio- and Radiodynamic Therapy. Adv. Mater. 2021, 33, e2007888. [Google Scholar] [CrossRef]
- Son, S.; Kim, J.H.; Wang, X.; Zhang, C.; Yoon, S.A.; Shin, J.; Sharma, A.; Lee, M.H.; Cheng, L.; Wu, J.; et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem. Soc. Rev. 2020, 49, 3244–3261. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Wang, Y.; Lu, Y.; Cheng, L.; Feng, L.; Zhang, H.; Li, X.; Han, G.; Liu, Z. Platinum Nanoparticles to Enable Electrodynamic Therapy for Effective Cancer Treatment. Adv. Mater. 2019, 31, e1806803. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, H.; Bu, W. A Forward Vision for Chemodynamic Therapy: Issues and Opportunities. Angew. Chem. 2023, 62, e202210415. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Lu, X.; He, G. On the penetration depth of reactive oxygen and nitrogen species generated by a plasma jet through real biological tissue. Phys. Plasmas 2017, 24, 073506. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kuroeda, G.; Sei, R.; Yamaguchi, M.; Yoshinaga, R.; Yamashita, R.; Tasaki, H.; Koga, K.; Shiratani, M. Transportation of reactive oxygen species in a tissue phantom after plasma irradiation. Jpn. J. Appl. Phys. 2018, 57, 01AG01. [Google Scholar] [CrossRef]
- Striesow, J.; Nasri, Z.; von Woedtke, T.; Bekeschus, S.; Wende, K. Epilipidomics reveals lipid fatty acid and headgroup modification in gas plasma-oxidized biomembranes. Redox Biol. 2024, 77, 103343. [Google Scholar] [CrossRef]
- Yusupov, M.; Wende, K.; Kupsch, S.; Neyts, E.C.; Reuter, S.; Bogaerts, A. Effect of head group and lipid tail oxidation in the cell membrane revealed through integrated simulations and experiments. Sci. Rep. 2017, 7, 5761. [Google Scholar] [CrossRef]
- Gorbanev, Y.; O’Connell, D.; Chechik, V. Non-Thermal Plasma in Contact with Water: The Origin of Species. Chemistry 2016, 22, 3496–3505. [Google Scholar] [CrossRef]
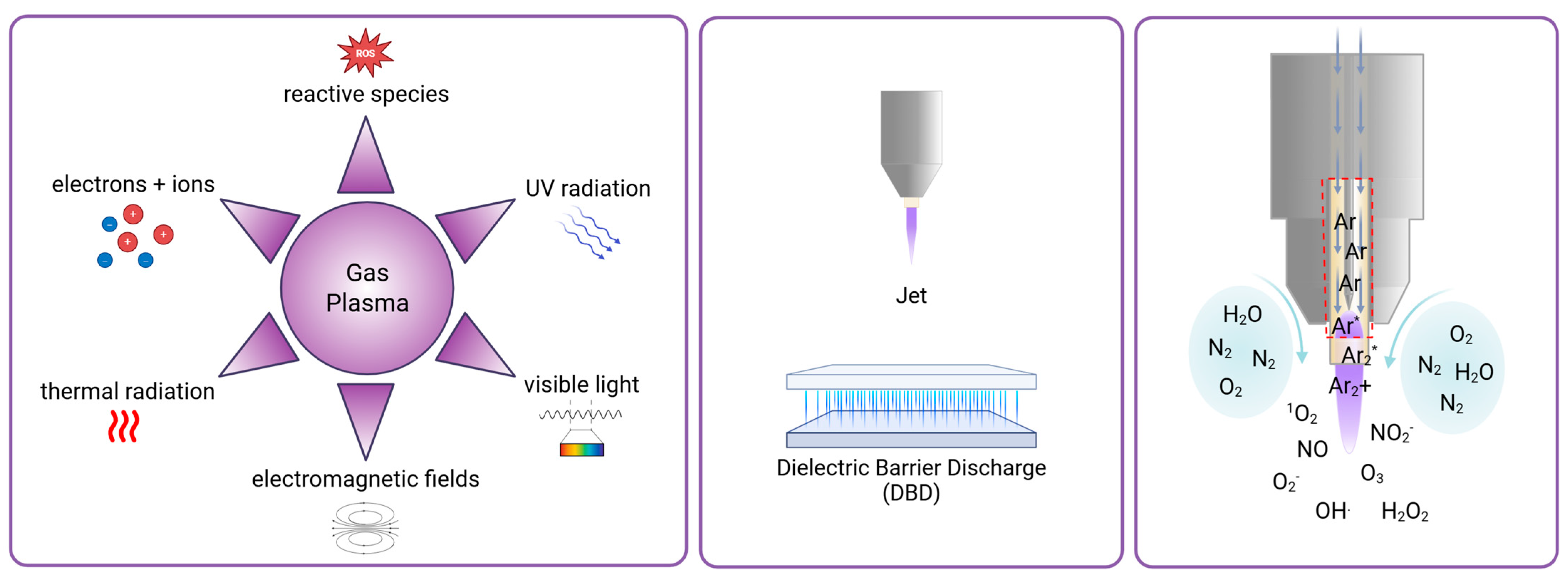
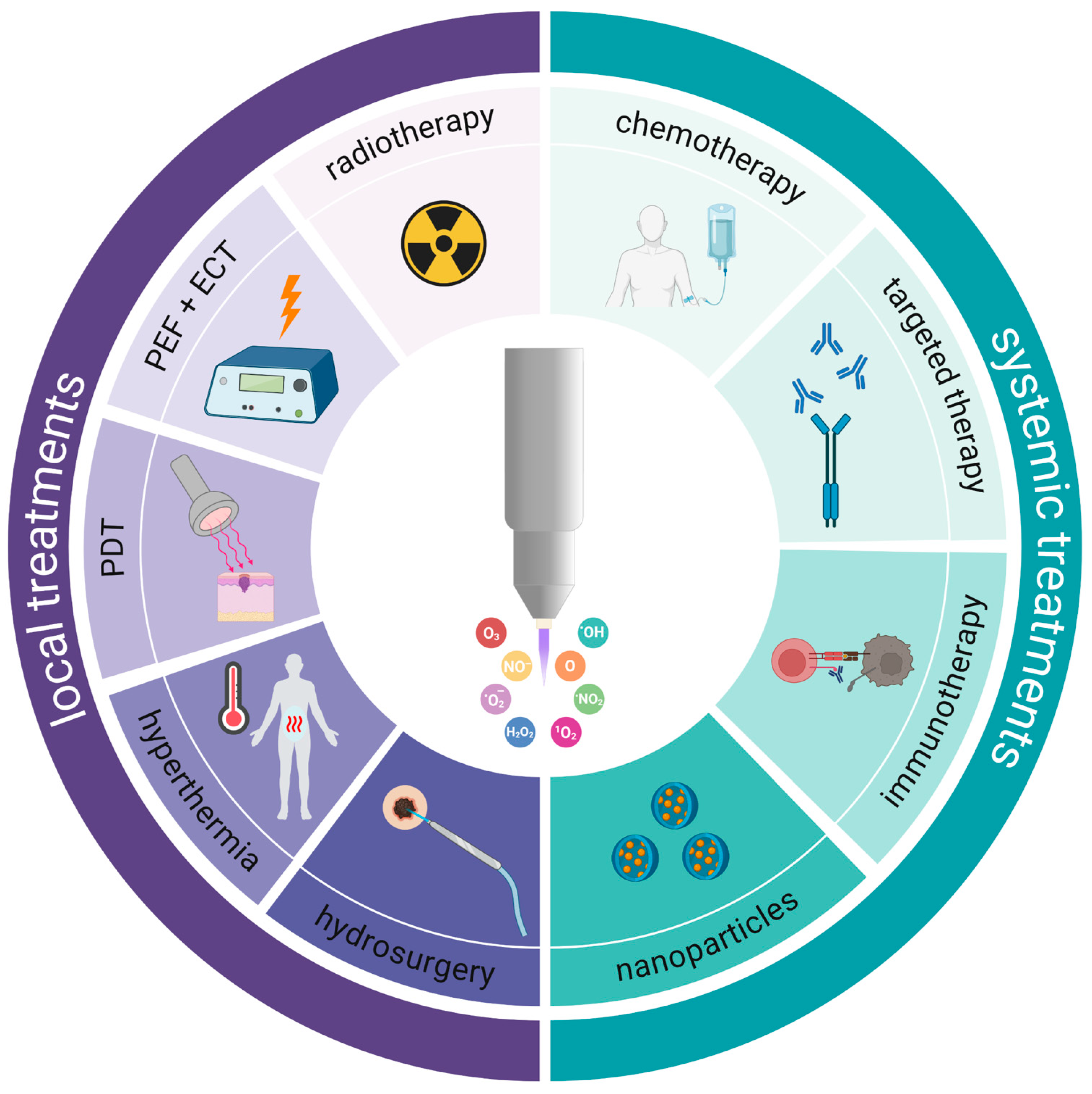
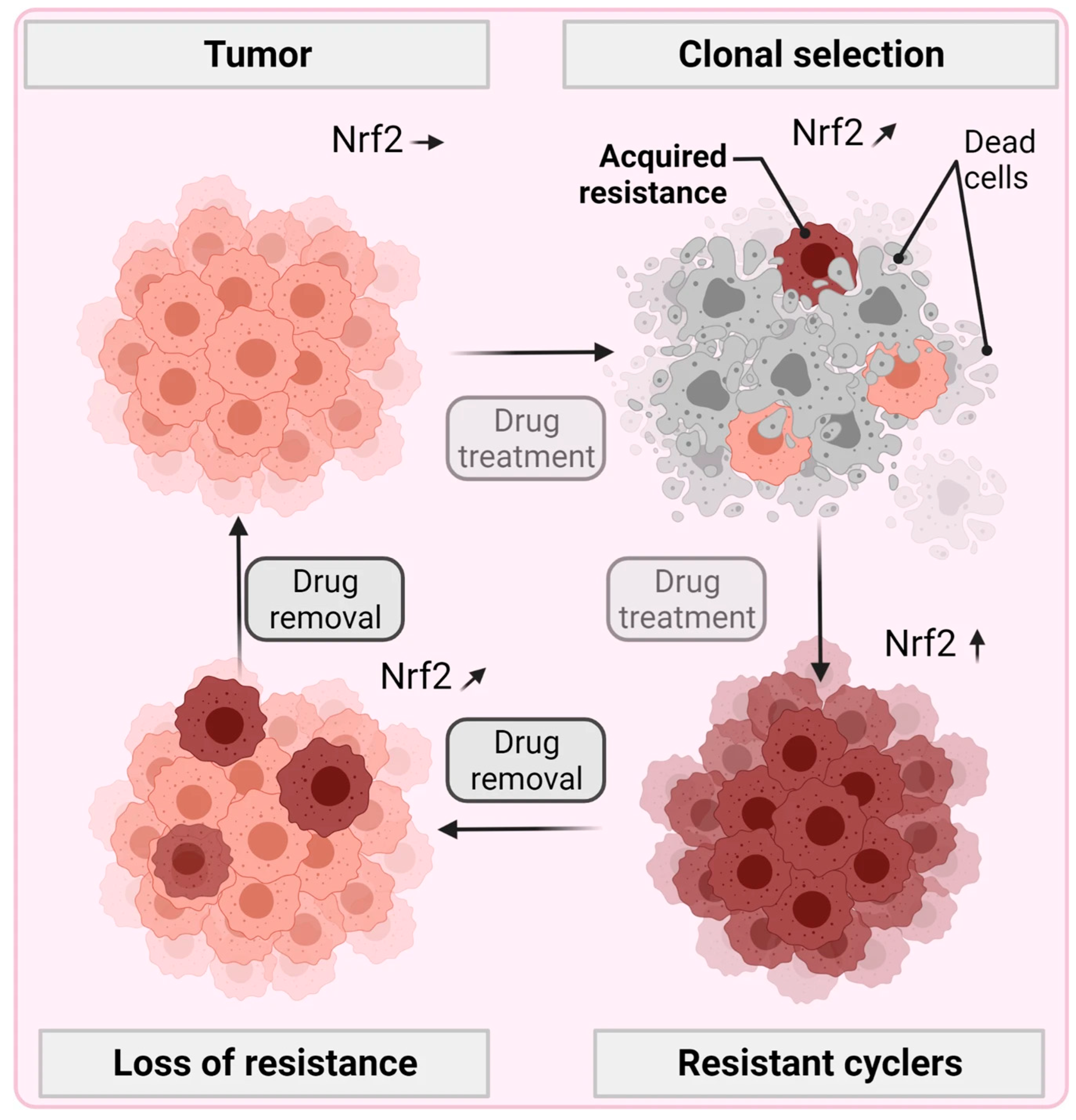
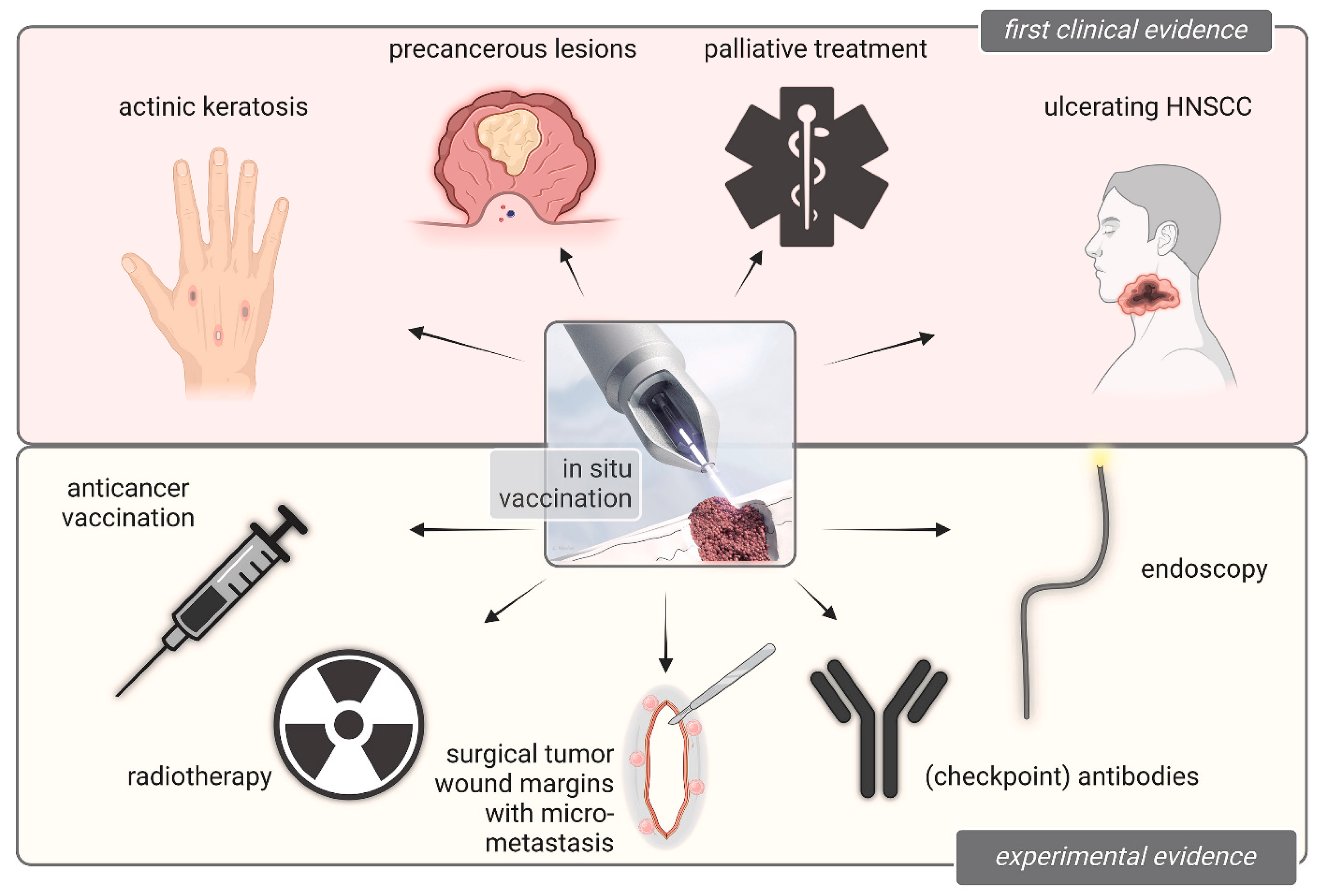
| Type of Cancer or Precancerous Lesion | Status | Main Outcome Indicators | Devices | Trials No. | Search Term | Outcome |
|---|---|---|---|---|---|---|
| Cervical Intraepithelial Neoplasia (CIN) Grade III | Completed | Rate of histological complete remission | Non-invasive physical plasma device | NCT04753073 | Cancer + Physical Plasma | - |
| Cervical Intraepithelial Neoplasia | Completed | Pathological remission of cervical intraepithelial neoplasia | Non-invasive physical plasma device | NCT03218436 | Cancer + Physical Plasma | 86.2% rate of full remission of CIN1/2 lesions [45] |
| * Surgical Margin and Macroscopic Tumor Sites | Completed | Number of participants with complications due to cold plasma application | Canady Helios cold plasma scalpel (CHCPS) | NCT04267575 | Cancer + Cold Plasma | CHCP combined with surgery significantly, is safe and reduces local recurrence after Stage IV tumor removal [48] |
| Familial Adenomatous Polyposis (FAP) | Recruiting | Polyp number and size | Argon plasma coagulation (APC) | NCT06435533 | Cancer + Cold Plasma | - |
| Skin Disorders (including Actinic Keratosis) | Completed | Clinical improvement upon reviewing photo-documentation | Custom-made nonthermal atmospheric plasma device | NCT02759900 | Cancer + Cold Plasma | Out of 17 treated lesions, 9 were removed and 3 significantly improved [47] |
| * Peritoneal Tumor Tissue | Recruiting | Histology of resected tumor nodules | J-plasma | NCT06796634 | Cancer + Cold Atmospheric Plasma (CAP) | - |
| Gastric Low-Grade Intramucosal Neoplasia | Recruiting | Complete ablation of gastric low-grade intraepithelial neoplasia | Hybrid argon plasma coagulation (APC) | NCT04197180 | Cancer + Gas Plasma | - |
| Non-small Lung Cancer with Endobronchial Obstruction | Terminated | Treatment time until failure | Argon plasma coagulation (APC) | NCT03564054 | Cancer + Gas Plasma | - |
| Anal Intraepithelial Neoplasia (AIN) | Completed | Absence of AIN at 24-month follow-up | Argon plasma coagulation (APC) | NCT00428285 | Cancer + Gas Plasma | 65% patients were clear of AINs; repeated APC treatment was needed; no serious adverse events [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, L.; Berner, J.; Martinet, A.; Freund, E.; Singer, D.; von Woedtke, T.; Weltmann, K.-D.; Emmert, S.; Clemen, R.; Bekeschus, S. Gas Plasma Combination Therapies—Promises from Preclinical Oncology Research. Antioxidants 2025, 14, 1055. https://doi.org/10.3390/antiox14091055
Yu L, Berner J, Martinet A, Freund E, Singer D, von Woedtke T, Weltmann K-D, Emmert S, Clemen R, Bekeschus S. Gas Plasma Combination Therapies—Promises from Preclinical Oncology Research. Antioxidants. 2025; 14(9):1055. https://doi.org/10.3390/antiox14091055
Chicago/Turabian StyleYu, Lingyun, Julia Berner, Alice Martinet, Eric Freund, Debora Singer, Thomas von Woedtke, Klaus-Dieter Weltmann, Steffen Emmert, Ramona Clemen, and Sander Bekeschus. 2025. "Gas Plasma Combination Therapies—Promises from Preclinical Oncology Research" Antioxidants 14, no. 9: 1055. https://doi.org/10.3390/antiox14091055
APA StyleYu, L., Berner, J., Martinet, A., Freund, E., Singer, D., von Woedtke, T., Weltmann, K.-D., Emmert, S., Clemen, R., & Bekeschus, S. (2025). Gas Plasma Combination Therapies—Promises from Preclinical Oncology Research. Antioxidants, 14(9), 1055. https://doi.org/10.3390/antiox14091055









