Evaluation of Antioxidant Effects of Pumpkin (Cucurbita pepo L.) Seed Extract on Aging- and Menopause-Related Diseases Using Saos-2 Cells and Ovariectomized Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pumpkin Seed Extract
2.3. Antioxidant Content Analysis
2.3.1. Quantitation of Total Polyphenol Content
2.3.2. Quantitation of Total Flavonoid Content
2.4. Analysis of the Radical-Scavenging Capacity
2.5. Gas Chromatograph–Mass Spectrometer (GC-MS) Analysis
2.6. Cell Culture
2.7. Cell Proliferation Assay
2.8. Alkaline Phosphatase (ALP) Activity
2.9. Animals and Housing Conditions
2.10. Experimental Design of Animal Groups
2.11. Measurement of b.w. and Food Intake
2.12. Sample Preparation
2.13. Lipid Profile
2.14. Atherogenic Index (AI) and Cardiac Risk Indices (CRI)-I and -II
2.15. Hepatic Function
2.16. Measurement of an Oxidative Stress Marker in the Serum
2.17. Histological Analysis
2.18. Femur Function Analysis
2.19. mRNA Expression Analysis
2.20. Statistical Analysis
3. Results
3.1. Total Polyphenol and Flavonoid Content of the Pumpkin Seed Extract
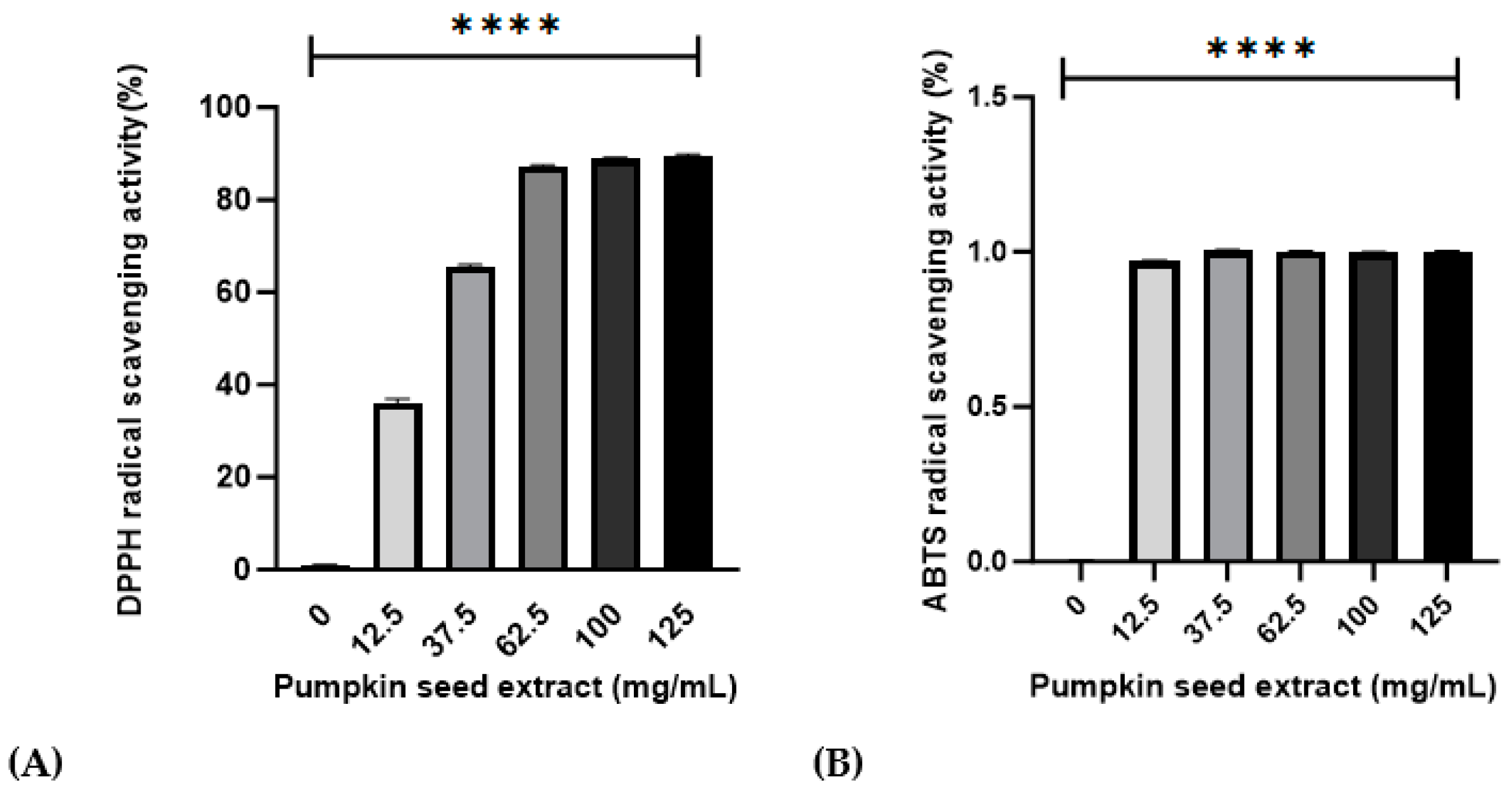
3.2. Effects of Pumpkin Seed Extract on DPPH and ABTS Radical-Scavenging Activities
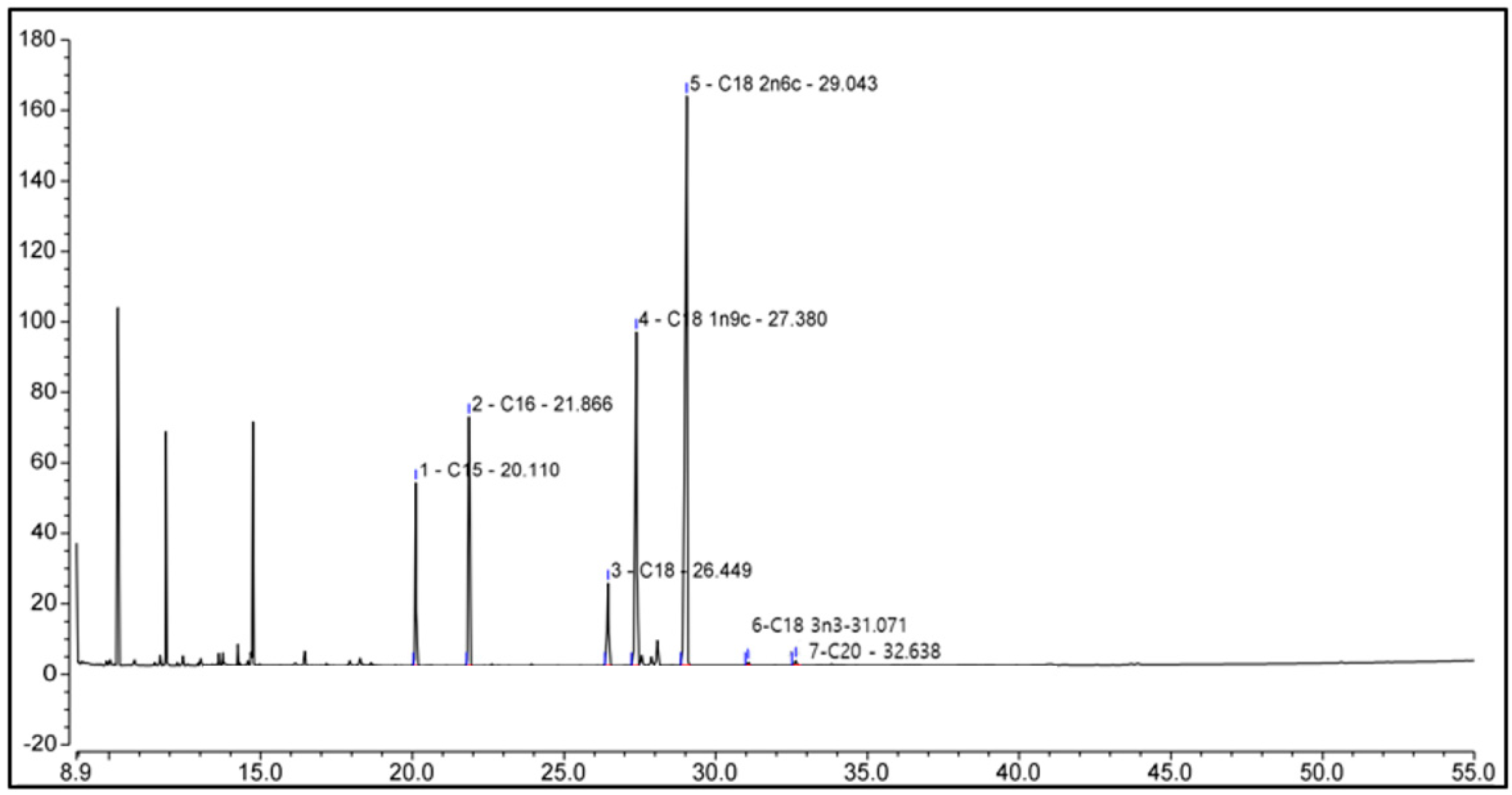
3.3. Characterization of Chemicals in Pumpkin Seed Extract by Gas Chromatograph–Mass Spectorometer (GC-MS)
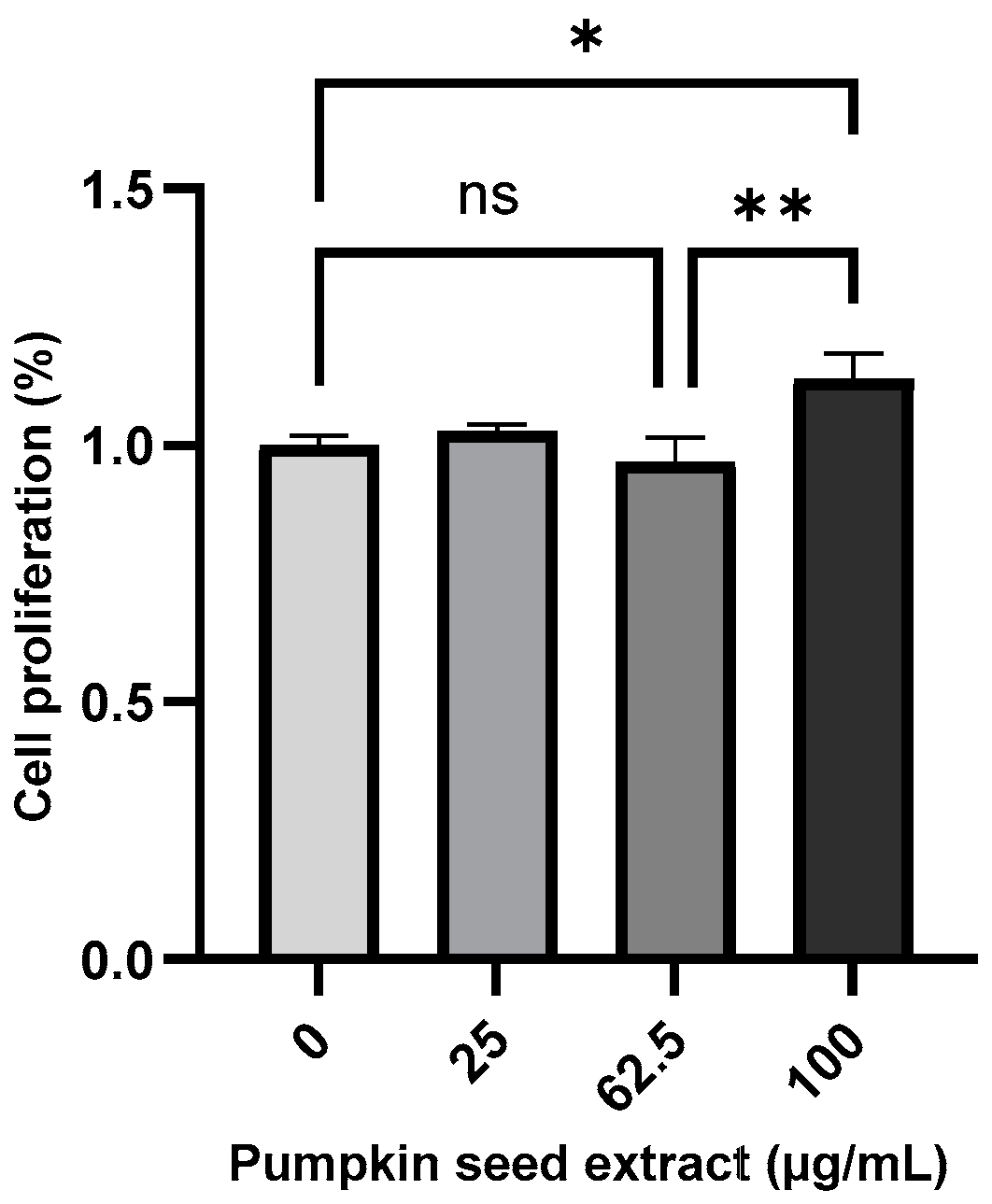
3.4. Effects of Pumpkin Seed Extract on Cell Proliferation
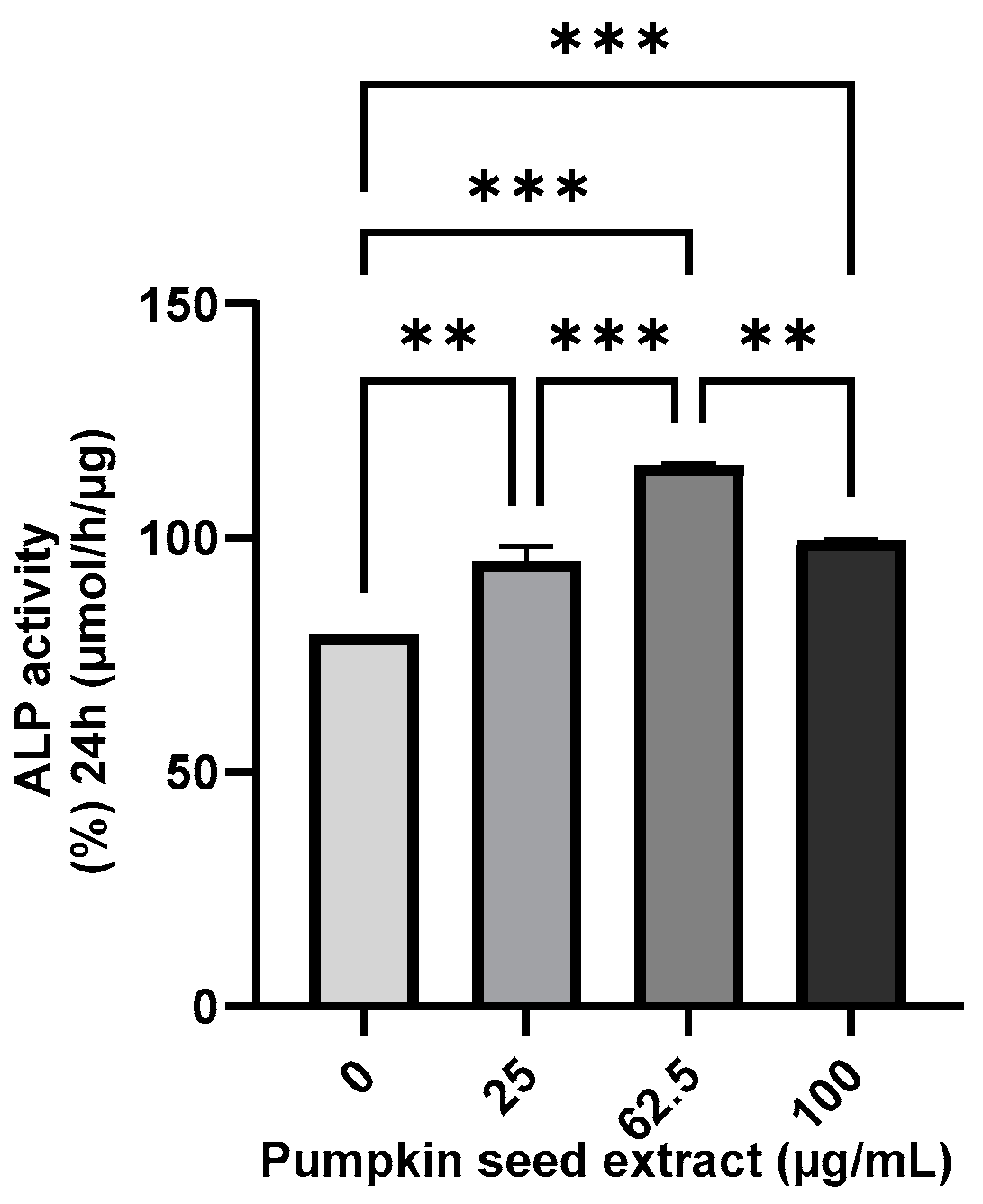
3.5. Effect of Pumpkin Seed Extract on the ALP Activity
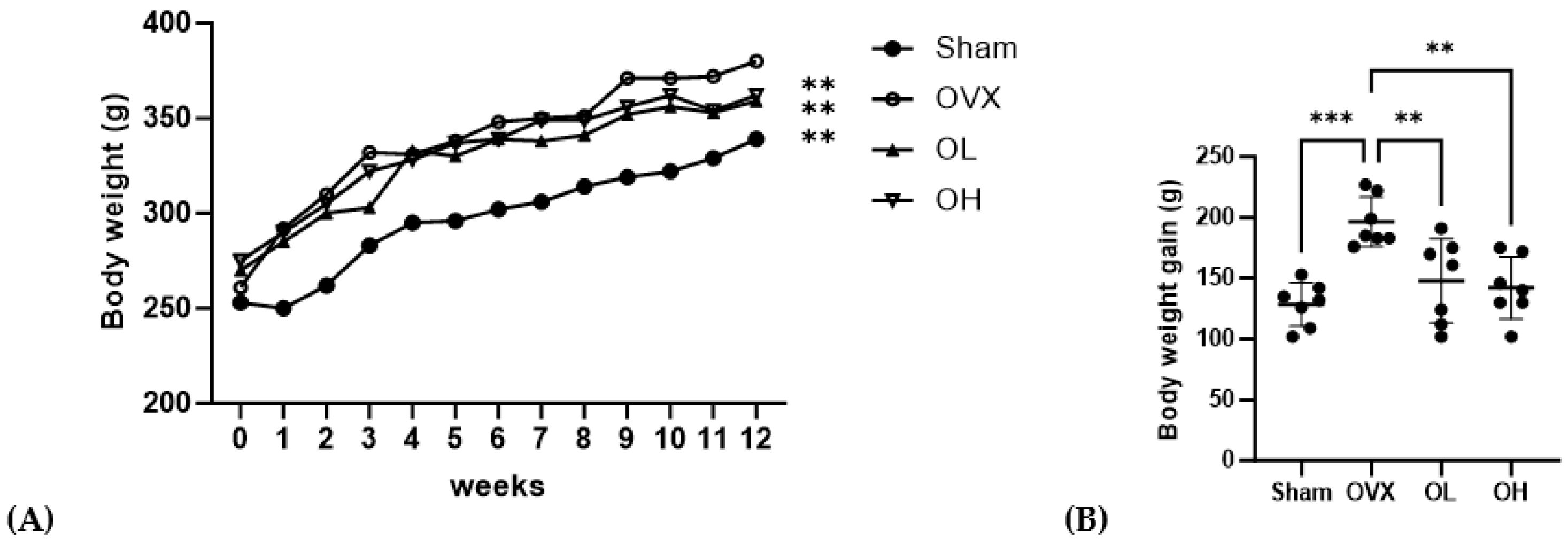
3.6. Effects of Pumpkin Seed Extract on Body Weight, Body Weight Gain, Food Intake, and Food Efficiency Ratio
3.7. Effects of Pumpkin Seed Extract on ABF, UF, BAT, and SCF Weight
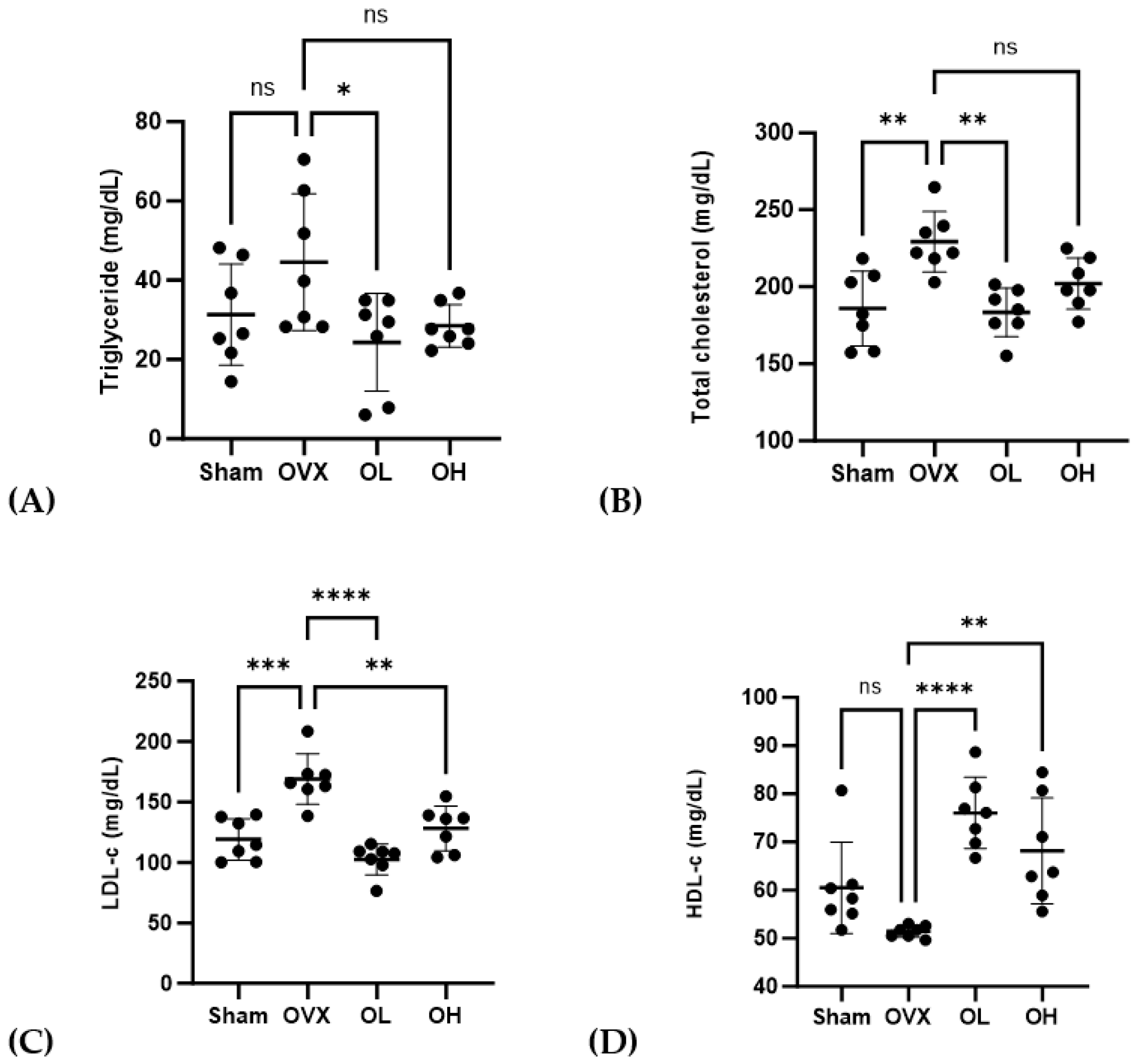
3.8. Effects of Pumpkin Seed Extract on Serum Lipid Profile
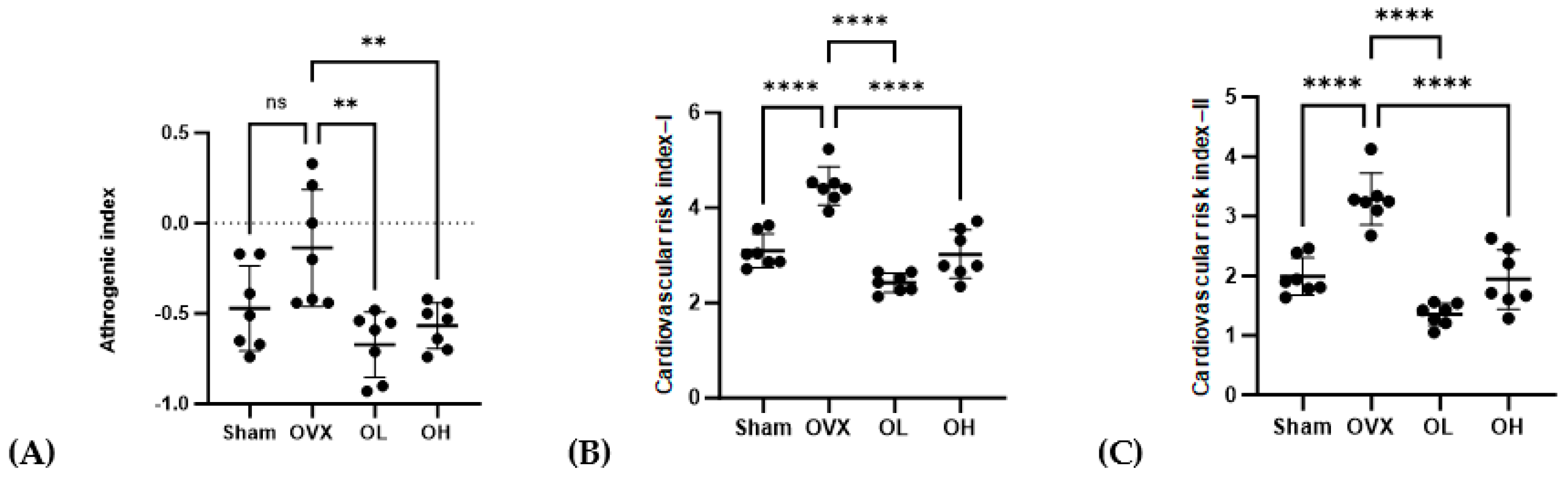
3.9. Effects of Pumpkin Seed Extract on Atherogenic Index (AI) and Cardiac Risk Indices (CRI-I and CRI-II)
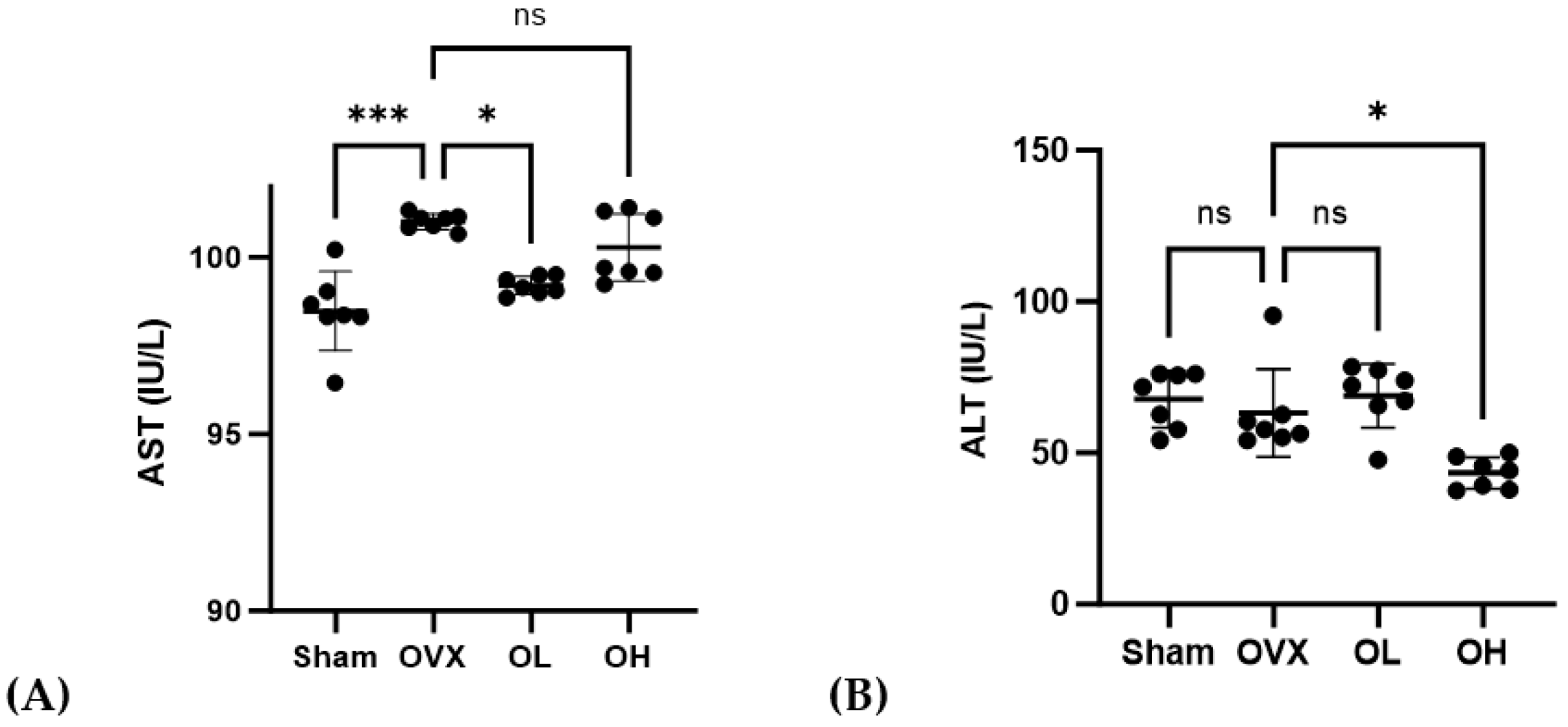
3.10. Effects of Pumpkin Seed Extract on Hepatic Function Levels
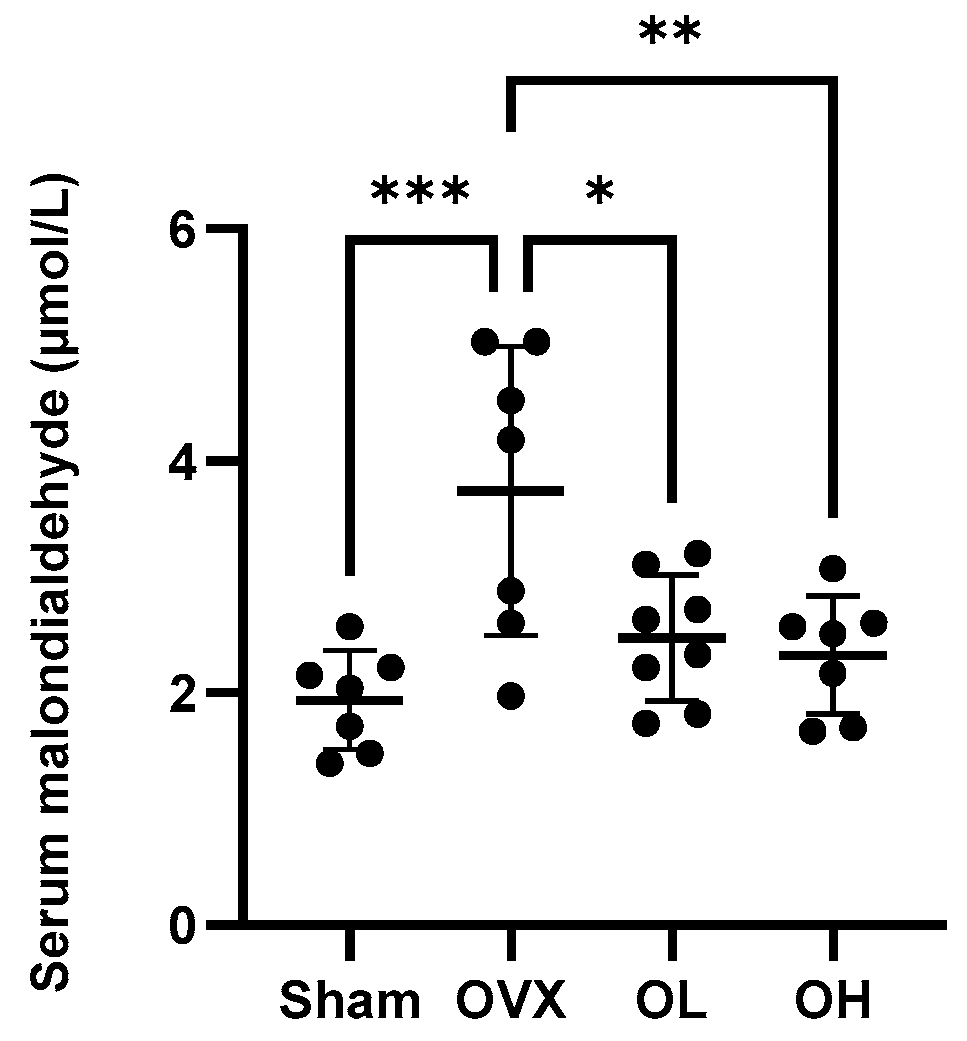
3.11. Effects of Pumpkin Seed Extract on an Oxidative Stress Marker in Serum
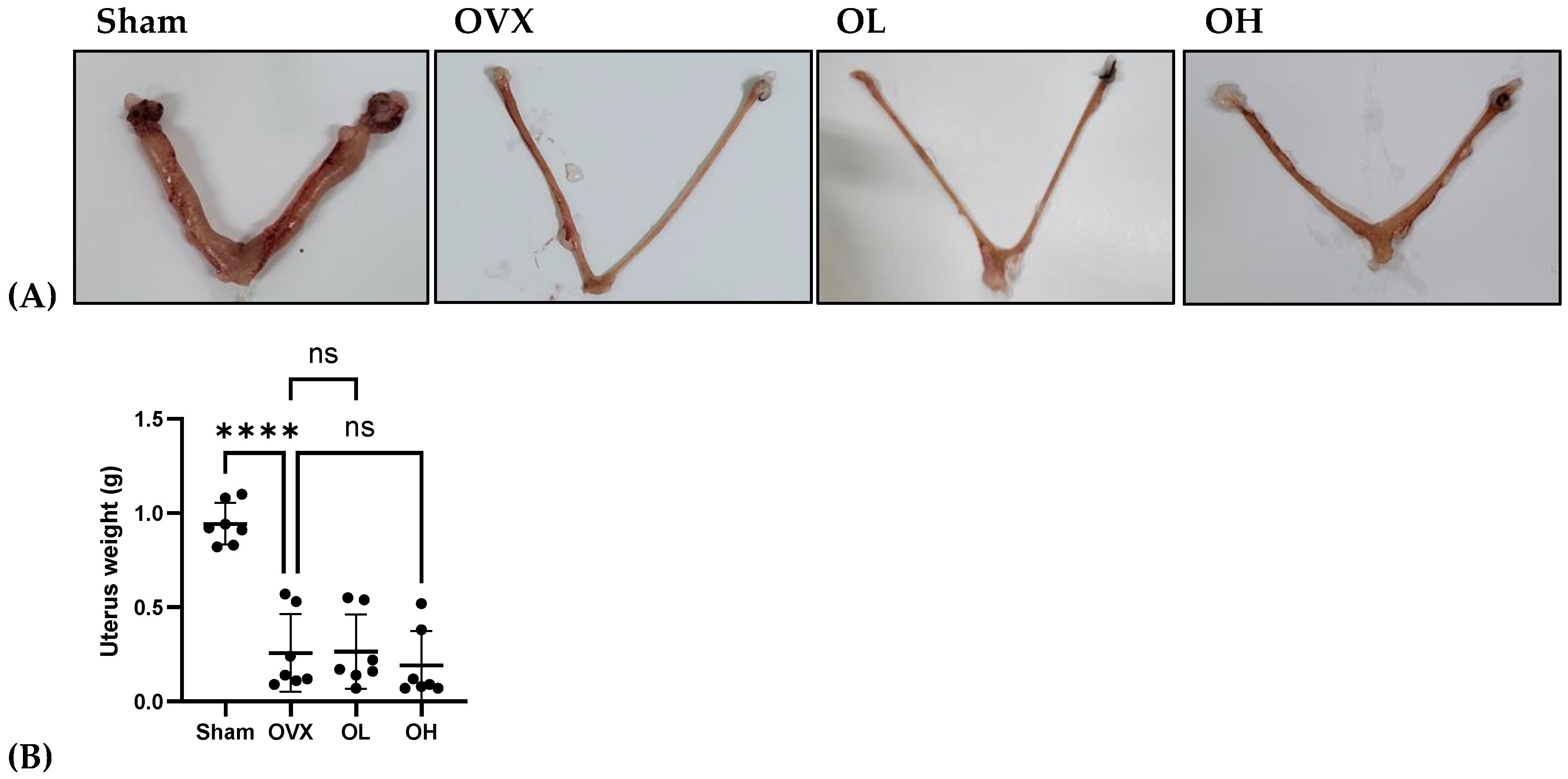
3.12. Effects of Pumpkin Seed Extract on Estrogenic Safety Function in the Uterus
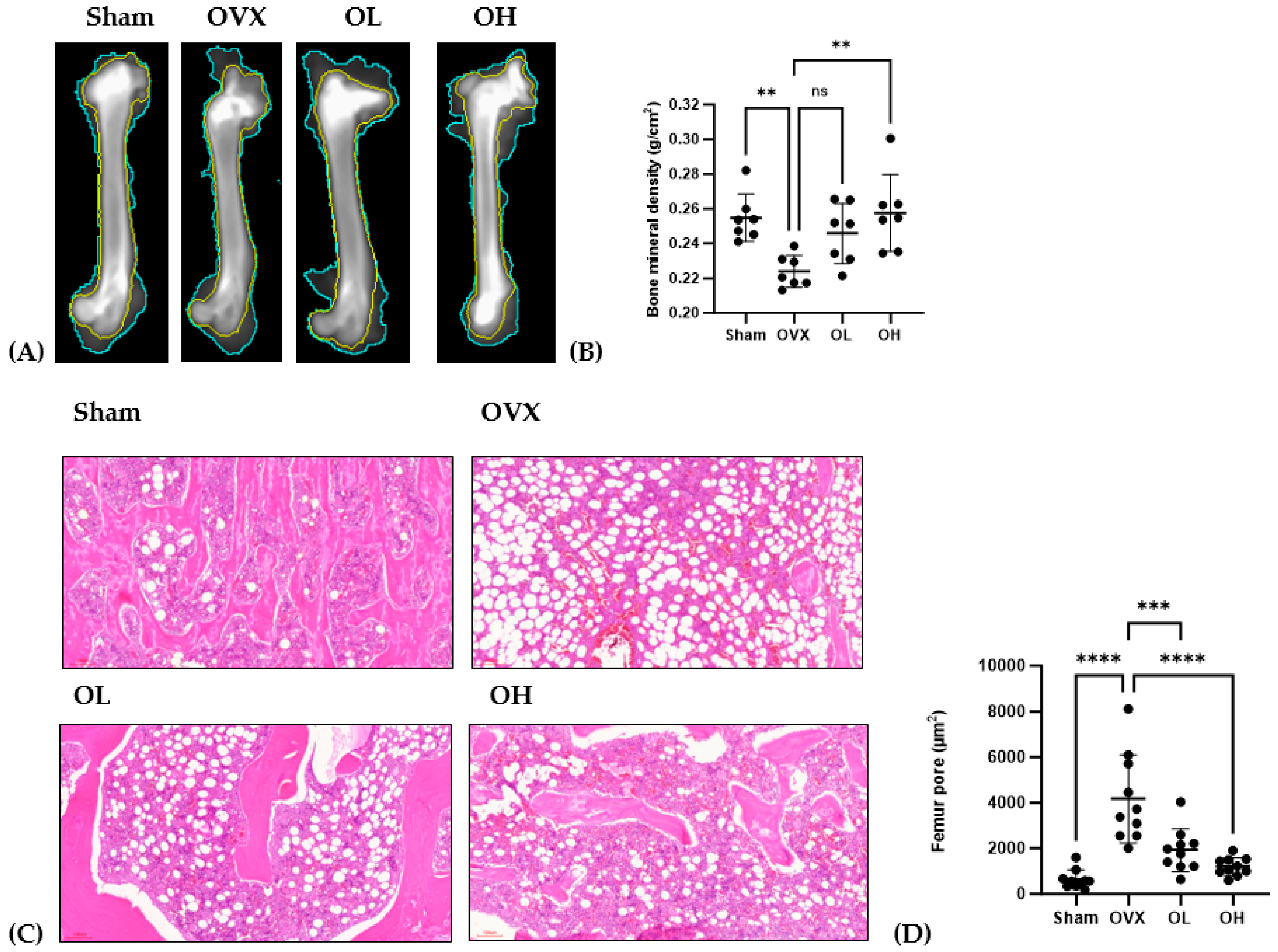
3.13. Antiosteoporotic Effect of Pumpkin Seed Extract in the Femur
3.14. Effects of Pumpkin Seed Extract on the mRNA Levels of Antiaging Genes in the Liver
3.15. Effects of Pumpkin Seed Extract on the mRNA Expression of Cardiovascular Protective Genes in the Liver
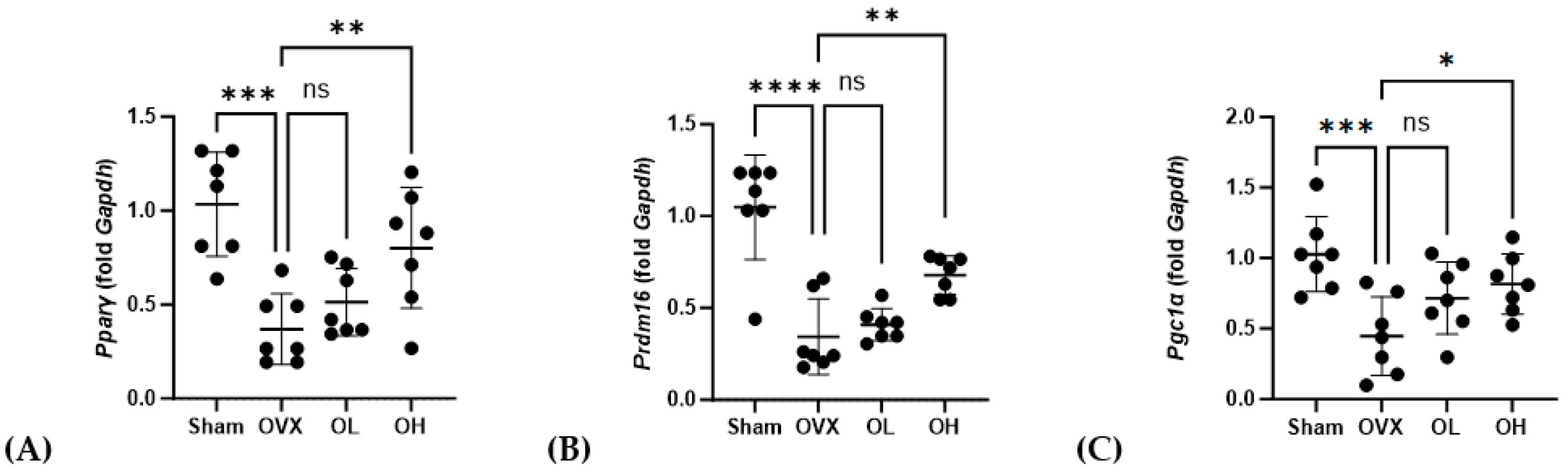
3.16. Effects of Pumpkin Seed Extract on mRNA Expression of Brown Adipogenesis Genes in BAT
3.17. Effects of Pumpkin Seed Extract on Thermogenesis in BAT
3.18. Effects of Pumpkin Seed Extract on the mRNA Levels of Thermogenesis Genes in SCF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, F.; Zhao, Q.; Li, Y.; Zheng, Z.; Kong, X.; Shu, C.; Liu, Y.; Shi, Y. The role of oxidative stress in ovarian aging: A review. J. Ovarian Res. 2022, 15, 100. [Google Scholar] [CrossRef]
- Gilbert, S.F. Aging: The Biology of Senescence. In Developmental Biology, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK10041/ (accessed on 9 September 2023).
- Chen, R.; Xu, P.; Song, P.; Wang, M.; He, J. China has faster pace than Japan in population aging in next 25 years. Biosci. Trends 2019, 13, 287–291. [Google Scholar] [CrossRef]
- Finch, C.E. The menopause and aging, a comparative perspective. J. Steroid Biochem. Mol. Biol. 2014, 142, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Vitale, C.; Marazzi, G.; Volterrani, M. Menopause and cardiovascular disease: The evidence. Climacteric 2007, 10, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, S.S.; Neri, S.; Sciacchitano, S.; Di Pino, L.; Costa, M.P.; Marchese, G.; Celotta, G.; Cassibba, N.; Pennisi, G.; Caschetto, S. Behaviour of some indicators of oxidative stress in postmenopausal and fertile women. Maturitas 2006, 53, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.R.; Jablonski, K.L.; Donato, A.J. Aging and vascular endothelial function in humans. Clin. Sci. 2011, 120, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Sie, Y.-Y.; Chen, L.-C.; Li, C.-W.; Wang, C.-C.; Li, C.-J.; Liu, D.-Z.; Lee, M.-H.; Chen, L.-G.; Hou, W.-C. Extracts and Scirpusin B from Recycled Seeds and Rinds of Passion Fruits (Passiflora edulis var. Tainung No. 1) Exhibit Improved Functions in Scopolamine-Induced Impaired-Memory ICR Mice. Antioxidants 2023, 12, 2058. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, M.A.; Ruiz-Ramos, M.; Correa-Muñoz, E.; Mendoza-Núñez, V.M. Oxidative stress as a risk factor for osteoporosis in elderly Mexicans as characterized by antioxidant enzymes. BMC Musculoskelet. Disord. 2007, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Lee, H.Y.; Bressan, R.A.; Yun, D.J.; Kim, M.O. Novel osmotin attenuates glutamate-induced synaptic dysfunction and neurodegeneration via the JNK/PI3K/Akt pathway in postnatal rat brain. Cell Death Dis. 2014, 5, e1026. [Google Scholar] [CrossRef] [PubMed]
- Kitagishi, Y.; Nakanishi, A.; Ogura, Y.; Matsuda, S. Dietary regulation of PI3K/AKT/GSK-3beta pathway in Alzheimer’s disease. Alzheimers Res. Ther. 2014, 6, 35. [Google Scholar] [CrossRef] [PubMed]
- Lestari, B.; Meiyanto, E. A review: The emerging nutraceutical potential of pumpkin seeds. Indones. J. Cancer Chemoprevent. 2018, 9, 92–101. [Google Scholar] [CrossRef]
- Šamec, D.; Loizzo, M.R.; Gortzi, O.; Çankaya, İ.T.; Tundis, R.; Suntar, İ.; Shirooie, S.; Zengin, G.; Devkota, H.P.; Reboredo-Rodríguez, P.; et al. The potential of pumpkin seed oil as a functional food-A comprehensive review of chemical composition, health benefits, and safety. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4422–4446. [Google Scholar] [CrossRef]
- Kostelac, D.; Rechkemmer, G.; Briviba, K. Phytoestrogens modulate binding response of estrogen receptors α and β to the estrogen response element. J. Agric. Food Chem. 2003, 51, 7632–7635. [Google Scholar] [CrossRef]
- Lestari, B.; Walidah, Z.; Utomo, R.Y.; Murwanti, R.; Meiyanto, E. Supplementation with extract of pumpkin seeds exerts estrogenic effects upon the uterine, serum lipids, mammary glands, and bone density in ovariectomized rats. Phytother. Res. 2019, 33, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Phillips, K.M.; Ruggio, D.M.; Ashraf-Khorassani, M. Phytosterol composition of nuts and seeds commonly consumed in the United States. J. Agric. Food Chem. 2005, 53, 9436–9445. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.Z.; Islam, T.; Mostofa, F.; Uddin, M.J.; Rahman, M.M.; Satter, M.A. Comparative assessment of the physicochemical and biochemical properties of native and hybrid varieties of pumpkin seed and seed oil (Cucurbita maxima Linn.). Heliyon 2019, 5, e02994. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.Z.; Wu, S.-K.; Zailani, H.; Chiu, W.-C.; Liu, W.-C.; Su, K.-P.; Lee, S.-D. Effects of Omega-3 Polyunsaturated Fatty Acids Intake on Vasomotor Symptoms, Sleep Quality and Depression in Postmenopausal Women: A Systematic Review. Nutrients 2023, 15, 4231. [Google Scholar] [CrossRef] [PubMed]
- Berai, V. Million Women Collaborators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 2003, 362, 419–427. [Google Scholar] [CrossRef]
- Bedigian, D. Phytoestrogens in functional foods. Econ. Bot. 2007, 61, 107. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic Content, Flavonoid Content and Antioxidant Potential of Wild Vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef]
- Chae, S.K.; Kang, G.S.; Ma, S.J.; Bang, K.M.; Oh, M.W.; Oh, S.H. Standard Food Analysis; Jigu-moonwha Sa: Seoul, Korea, 2002; pp. 381–382. [Google Scholar]
- Kim, Y.; Lee, S.-b.; Cho, M.; Choe, S.; Jang, M. Indian Almond (Terminalia catappa Linn.) Leaf Extract Extends Lifespan by Improving Lipid Metabolism and Antioxidant Activity Dependent on AMPK Signaling Pathway in Caenorhabditis elegans under High-Glucose-Diet Conditions. Antioxidants 2024, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.T.; Yang, C.R.; Xu, M. Phenolic antioxidants from green tea produced from Camellia crassicolumna Var. multiplex. J. Agric. Food Chem. 2009, 57, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Ulewicz-Magulska, B.; Wesolowski, M. Antioxidant Activity of Medicinal Herbs and Spices from Plants of the Lamiaceae, Apiaceae and Asteraceae Families: Chemometric Interpretation of the Data. Antioxidants 2023, 12, 2039. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-M.; Yoon, H.; Shin, M.-J.; Lee, S.; Yi, J.; Jeon, Y.-a.; Wang, X.; Desta, K.T. Nutrient Levels, Bioactive Metabolite Contents, and Antioxidant Capacities of Faba Beans as Affected by Dehulling. Foods 2023, 12, 4063. [Google Scholar] [CrossRef] [PubMed]
- Galea, G.L.; Meakin, L.B.; Sugiyama, T.; Zebda, N.; Sunters, A.; Taipaleenmaki, H.; Stein, G.S.; van Wijnen, A.J.; Lanyon, L.E.; Price, J.S. Estrogen receptor α mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor β. J. Biol. Chem. 2013, 288, 9035–9048. [Google Scholar] [CrossRef] [PubMed]
- Quah, Y.; Lee, E.-B.; Chan, J.Y.-L.; Jang, S.-H.; Park, S.-C. Optimal red clover ethanolic extract by relative aggregated metric increases osteoblastic activity and nuclear factor kappa-B ligand gene expression in SaOS-2 cells. All Life 2020, 13, 321–327. [Google Scholar] [CrossRef]
- Yin, X.; Chen, Z.; Liu, Z.; Song, C. Tissue transglutaminase (TG2) activity regulates osteoblast differentiation and mineralization in the SAOS-2 cell line. Braz. J. Med. Biol. Res. 2012, 45, 693–700. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Knopfholz, J.; Disserol, C.C.; Pierin, A.J.; Schirr, F.L.; Streisky, L.; Takito, L.L.; Massucheto Ledesma, P.; Faria-Neto, J.R.; Olandoski, M.; da Cunha, C.L.; et al. Validation of the friedewald formula in patients with metabolic syndrome. Cholesterol 2014, 2014, 261878. [Google Scholar] [CrossRef]
- Ghoneim, M.A.M.; Hassan, A.I.; Mahmoud, M.G.; Asker, M.S. Effect of polysaccharide from Bacillus subtilis sp. on cardiovascular diseases and atherogenic indices in diabetic rats. BMC Complement. Altern. Med. 2016, 16, 112. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; Lu, D.; Liu, J.; Jiang, B.; Chen, J. Effect of roasting on the antioxidant activity, phenolic composition, and nutritional quality of pumpkin (Cucurbita pepo L.) Seeds. Front. Nutr. 2021, 8, 647354. [Google Scholar] [CrossRef] [PubMed]
- Hajimehdipoor, H.; Shahrestani, R.; Shekarchi, M. Investigating the synergistic antioxidant effects of some flavonoid and phenolic compounds. Res. J. Pharmacogn. 2014, 1, 35–40. [Google Scholar]
- Fattahi, S.; Zabihi, E.; Abedian, Z.; Pourbagher, R.; Motevalizadeh Ardekani, A.; Mostafazadeh, A.; Akhavan-Niaki, H. Total phenolic and flavonoid contents of aqueous extract of stinging nettle and in vitro antiproliferative effect on Hela and BT-474 cell lines. Int. J. Mol. Cell Med. 2014, 3, 102–107. [Google Scholar]
- Olszowy, M.; Dawidowicz, A.L. Is it possible to use the DPPH and ABTS methods for reliable estimation of antioxidant power of colored compounds? Chem. Pap. 2018, 72, 393–400. [Google Scholar] [CrossRef]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-H.; Kim, H.-S. Menopause-Associated Lipid Metabolic Disorders and Foods Beneficial for Postmenopausal Women. Nutrients 2020, 12, 202. [Google Scholar] [CrossRef]
- Kuryłowicz, A.; Cąkała-Jakimowicz, M.; Puzianowska-Kuźnicka, M. Targeting Abdominal Obesity and Its Complications with Dietary Phytoestrogens. Nutrients 2020, 12, 582. [Google Scholar] [CrossRef]
- Jin, H.J.; Park, S.M.; Kim, E.O.; Kim, S.C. Hepatoprotective effect of Ikwiseungyang-tang via Nrf2 activation. Herb. Formula Sci. 2021, 29, 167–179. [Google Scholar] [CrossRef]
- Chang, B.Y.; Kim, D.S.; Kim, S.Y. Improvement in menopause-associated hepatic lipid metabolic disorders by herbal formula HPC03 on ovariectomized rats. Evid. Based Complement. Alternat. Med. 2020, 2020, 1409376. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kang, M.I.; Watai, Y.; Tong, K.I.; Shibata, T.; Uchida, K.; Yamamoto, M. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol. Cell. Biol. 2006, 26, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, M.A.; Zacarías-Flores, M.; Arronte-Rosales, A.; Correa-Muñoz, E.; Mendoza-Núñez, V.M. Menopause as risk factor for oxidative stress. Menopause 2012, 19, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, C.J.; Dodson, M.B.; Madhavan, L.; Zhang, D.D. Redox regulation by NRF2 in aging and disease. Free Radic. Biol. Med. 2019, 134, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Jozkowicz, A.; Was, H.; Dulak, J. Heme oxygenase-1 in tumors: Is it a false friend? Antioxid. Redox Signal. 2007, 9, 2099–2117. [Google Scholar] [CrossRef] [PubMed]
- Pósa, A.; Szabó, R.; Csonka, A.; Veszelka, M.; Magyariné Berkó, A.; Baráth, Z.; Ménesi, R.; Pávó, I.; Gyöngyösi, M.; László, F.; et al. Endogenous estrogen-mediated heme oxygenase regulation in experimental menopause. Oxid. Med. Cell. Longev. 2015, 2015, 429713. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Paneni, F.; Diaz Cañestro, C.; Libby, P.; Lüscher, T.F.; Camici, G.G. The aging cardiovascular system: Understanding it at the cellular and clinical levels. J. Am. Coll. Cardiol. 2017, 69, 1952–1967. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef] [PubMed]
- North, B.J.; Sinclair, D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-H.; Li, J.-J. Ageing and dyslipidemia: A review of potential mechanisms. Ageing Res. Rev. 2014, 19, 43–52. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.W.; Whelton Seamus, P.; Blumenthal, R.S. 38—Dyslipidemia. In Hypertension: A Companion to Braunwald’s Heart Disease, 3rd ed.; Bakris, G.L., Sorrentino, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 353–360. [Google Scholar] [CrossRef]
- Baber, R.J.; Panay, N.; Fenton, A.; IMS Writing Group. 2016 IMS recommendations on women’s midlife health and menopause hormone therapy. Climacteric 2016, 19, 109–150. [Google Scholar] [CrossRef] [PubMed]
- Nie, G.; Yang, X.; Wang, Y.; Liang, W.; Li, X.; Luo, Q.; Yang, H.; Liu, J.; Wang, J.; Guo, Q.; et al. The effects of menopause hormone therapy on lipid profile in postmenopausal women: A systematic review and meta-Analysis. Front. Pharmacol. 2022, 13, 850815. [Google Scholar] [CrossRef] [PubMed]
- Creatsas, G.; Christodoulakos, G.; Lambrinoudaki, I. Cardiovascular disease: Screening and management of the a-symptomatic high-risk post-menopausal woman. Maturitas 2005, 52, S32–S37. [Google Scholar] [CrossRef] [PubMed]
- Kypreos, K.E.; Zafirovic, S.; Petropoulou, P.-I.; Bjelogrlic, P.; Resanovic, I.; Traish, A.; Isenovic, E.R. Regulation of endothelial nitric oxide synthase and high-density lipoprotein quality by estradiol in cardiovascular pathology. J. Cardiovasc. Pharmacol. Therap. 2014, 19, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.; Tzima, E. Hemodynamic forces in endothelial dysfunction and vascular aging. Exp. Gerontol. 2011, 46, 185–188. [Google Scholar] [CrossRef] [PubMed]
- Lind, M.; Hayes, A.; Caprnda, M.; Petrovic, D.; Rodrigo, L.; Kruzliak, P.; Zulli, A. Inducible nitric oxide synthase: Good or bad? Biomed. Pharmacother. 2017, 93, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Song, Y.M.; Lee, H.J.; Min, S.K.; Park, Y.H.; Oh, J.K.; Kim, J.Y.; Park, J.B. Effects of noni on cellular viability and osteogenic differentiation of gingiva-derived stem cells demonstrated by RNA sequencing and quantitative PCR. Exp. Ther. Med. 2022, 23, 32. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S. Current perspectives on the beneficial effects of soybean isoflavones and their metabolites for humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef] [PubMed]
- Salvio, G.; Ciarloni, A.; Gianfelice, C.; Lacchè, F.; Sabatelli, S.; Giacchetti, G.; Balercia, G. The effects of polyphenols on bone metabolism in postmenopausal women: Systematic review and meta-analysis of randomized control trials. Antioxidants 2023, 12, 1830. [Google Scholar] [CrossRef]
- Aldiss, P.; Budge, H.; Symonds, M.E. Is a reduction in brown adipose thermogenesis responsible for the change in core body temperature at menopause? Cardiovas. Endocrinol. 2016, 5, 155–156. [Google Scholar] [CrossRef]
- Yau, W.W.; Yen, P.M. Thermogenesis in adipose tissue activated by thyroid hormone. Int. J. Mol. Sci. 2020, 21, 3020. [Google Scholar] [CrossRef] [PubMed]
- Tabuchi, C.; Sul, H.S. Signaling pathways regulating thermogenesis. Front. Endocrinol. 2021, 12, 595020. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, I.J.; Dean, J.M.; He, A.; Park, H.; Tan, M.; Feng, C.; Song, H.; Hsu, F.-F.; Semenkovich, C.F. PexRAP inhibits PRDM16-mediated thermogenic gene expression. Cell Rep. 2017, 20, 2766–2774. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hondares, E.; Rosell, M.; Díaz-Delfín, J.; Olmos, Y.; Monsalve, M.; Iglesias, R.; Villarroya, F.; Giralt, M. Peroxisome proliferator-activated receptor α (PPARα) induces PPARγ coactivator 1α (PGC-1α) gene expression and contributes to thermogenic activation of brown fat: Involvement of PRDM16. J. Biol. Chem. 2011, 286, 43112–43122. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yang, M.; Han, Y.; Zhao, H.; Sun, L. PRDM16 regulating adipocyte transformation and thermogenesis: A promising therapeutic target for obesity and diabetes. Front. Pharmacol. 2022, 13, 870250. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Ko, S.-H. Common Regulators of Lipid Metabolism and Bone Marrow Adiposity in Postmenopausal Women. Pharmaceuticals 2023, 16, 322. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Gunta, S.; Oliveira, E.A.; Cheung, W.W. Growth hormone improves adipose tissue browning and muscle wasting in mice with chronic kidney disease-associated cachexia. Int. J. Mol. Sci. 2022, 23, 15310. [Google Scholar] [CrossRef]
- Badkoobeh Hezaveh, M.; Abedi, B.; Rahmati-Ahmadabad, S. Effects of aerobic training and pumpkin seed extract consumption on the heart and aorta oxidative stress biomarkers: A case of rats exposed with arsenic. J. Cardiothorac. Med. 2021, 9, 747–754. [Google Scholar] [CrossRef]
- Ghahremanloo, A.; Hajipour, R.; Hemmati, M.; Moossavi, M.; Mohaqiq, Z. The beneficial effects of pumpkin extract on atherogenic lipid, insulin resistance and oxidative stress status in high-fat diet-induced obese rats. J. Complement. Integr. Med. 2017, 15, 20170051. [Google Scholar] [CrossRef] [PubMed]

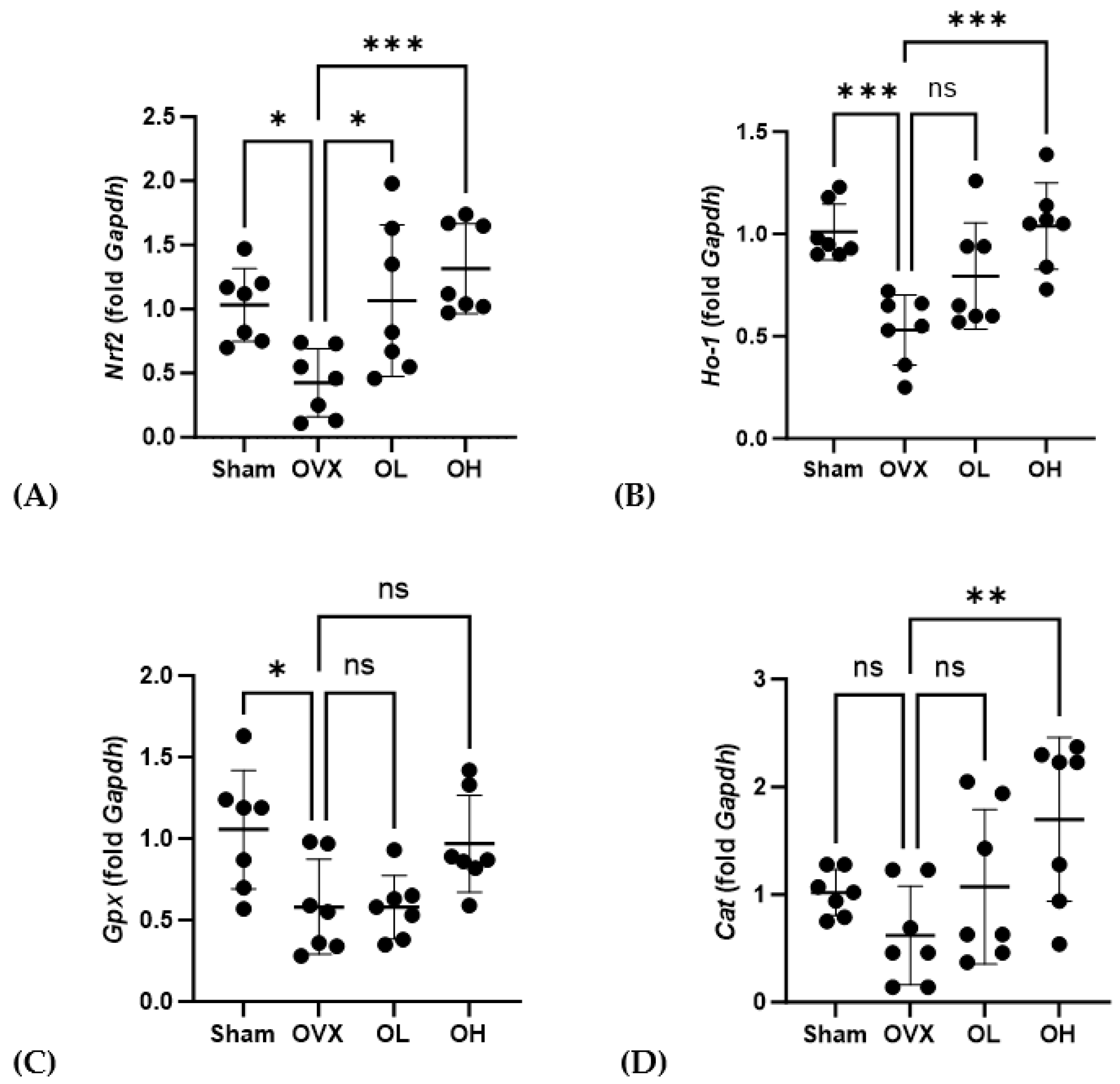
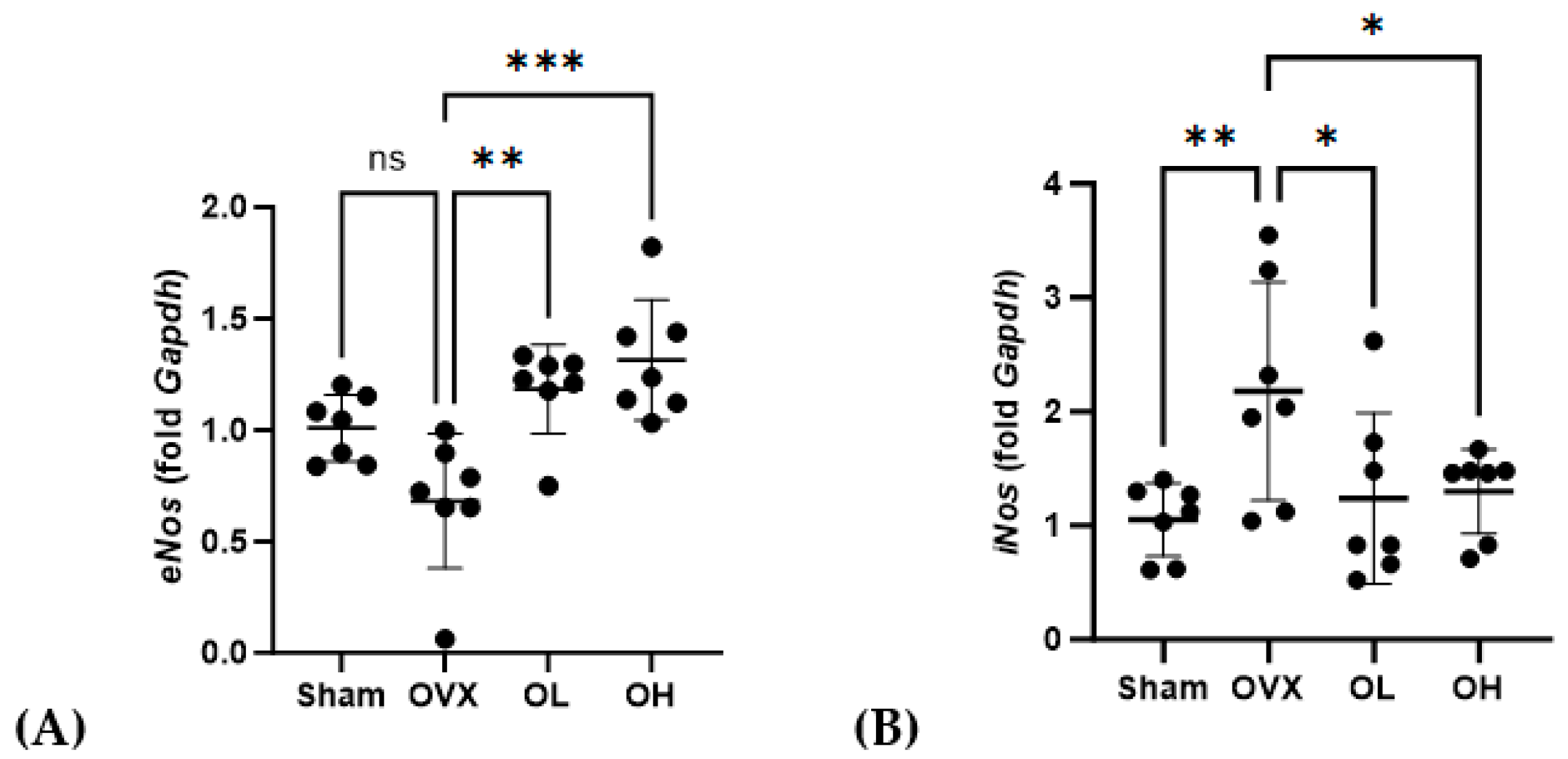
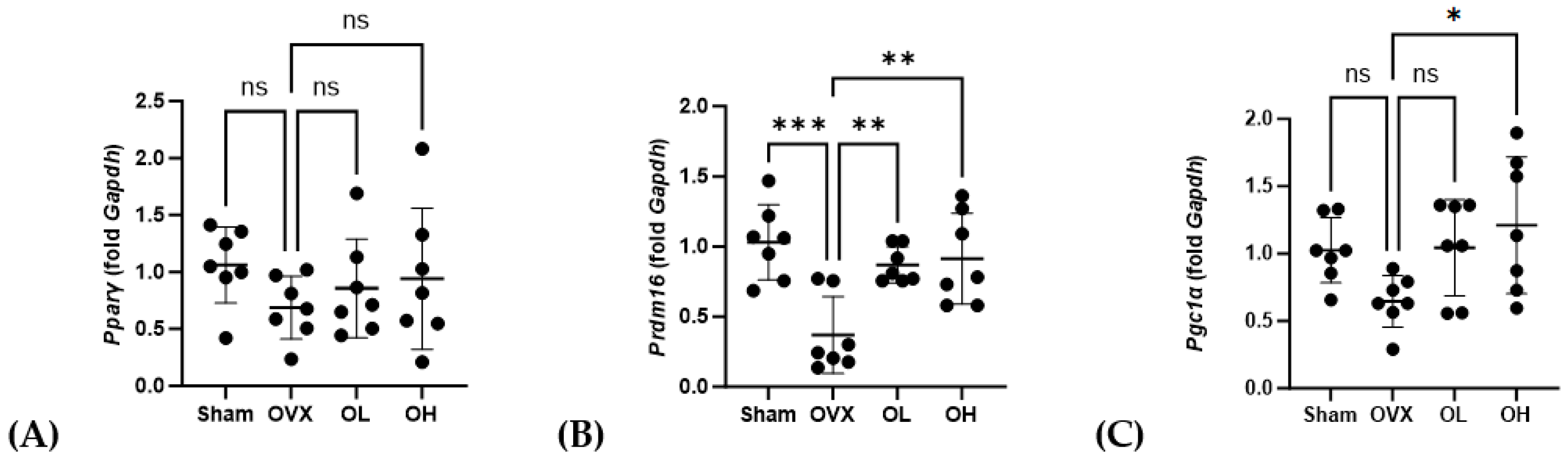
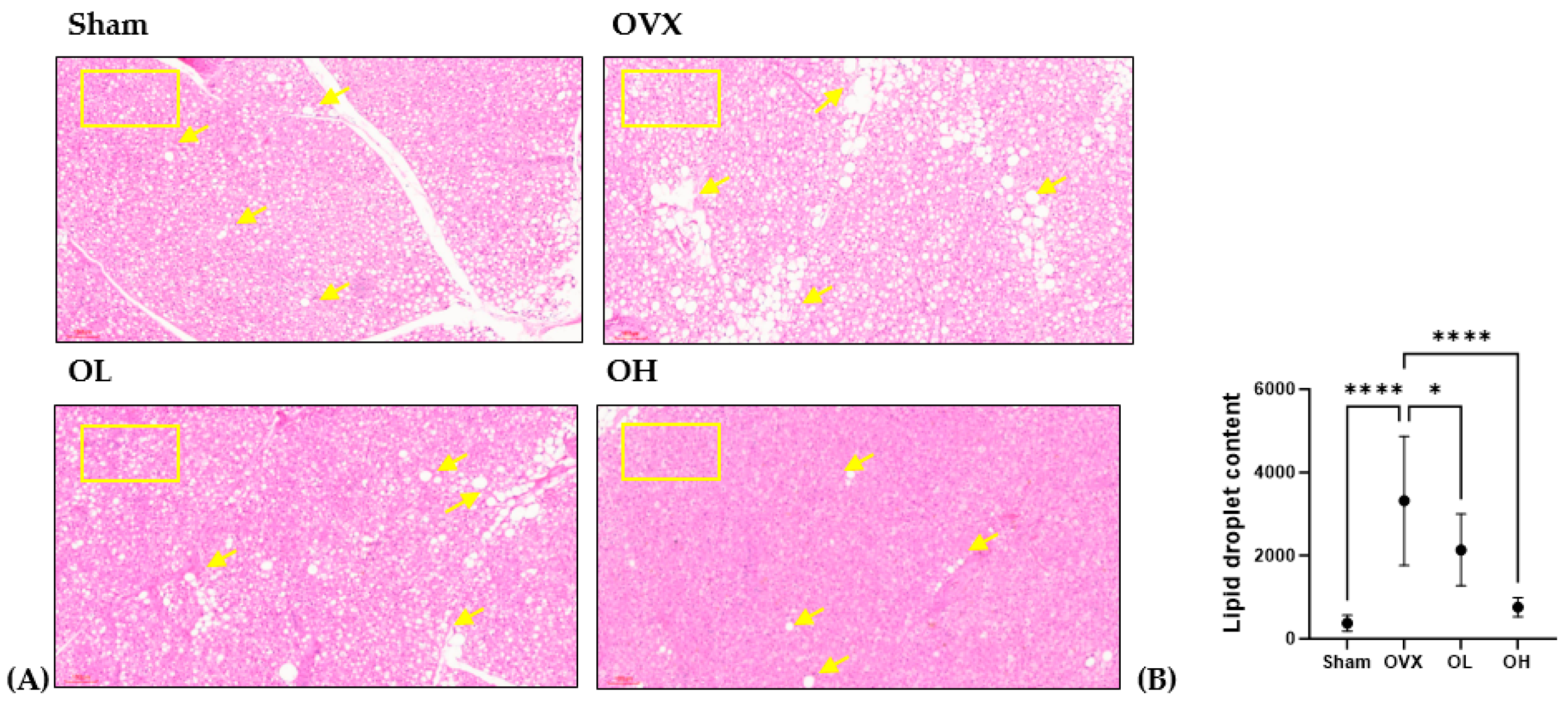
| Total Polyphenols (mg GAE/g) | Total Flavonoids (mg QE/g) |
|---|---|
| 784.61 ± 7.94 | 0.70 ± 0.18 |
| Peak Name | Retention Time (min) | Compound Name | Relative Area% | |
|---|---|---|---|---|
| 1 | C15 | 20.110 | cis-10-Pentadecenoic acid | 12.83 |
| 2 | C16 | 21.866 | Palmitic acid (hexadecanoic acid) | 17.47 |
| 3 | C18 | 26.449 | Stearic acid (octadecanoic acid) | 5.79 |
| 4 | C18 1n9c | 27.380 | Oleic acid | 23.40 |
| 5 | C18 2n6c | 29.043 | Linoleic acid | 39.94 |
| 6 | C183n3 | 31.071 | Alpha-linolenic acid | 0.23 |
| 7 | C20 | 32.638 | Arachidic acid (icosanoic acid) | 0.34 |
| Group | Sham | OVX | OL | OH |
|---|---|---|---|---|
| Initial body weight (g) | 253.33 ± 14.01 ns | 261.14 ± 33.12 | 270.82 ± 24.95 | 275.36 ± 21.00 |
| Final body weight (g) | 339.17 ± 29.39 ** | 380.83 ± 28.49 | 359.09 ± 47.36 ** | 362.55 ± 39.97 ** |
| Body weight gain (g) | 128.43 ± 17.92 *** | 196.43 ± 20.43 | 147.86 ± 34.69 ** | 142.14 ± 25.48 ** |
| Food intake (g/day) | 17.27 ± 0.52 ns | 18.83 ± 0.48 | 17.64 ± 1.27 | 18.81 ± 0.38 |
| FER (%) | 9.23 ± 0.39 ns | 10.99 ± 1.24 | 7.77 ± 0.22 | 11.64 ± 2.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.; Hong, S.; Ko, S.-H.; Kim, H.-S. Evaluation of Antioxidant Effects of Pumpkin (Cucurbita pepo L.) Seed Extract on Aging- and Menopause-Related Diseases Using Saos-2 Cells and Ovariectomized Rats. Antioxidants 2024, 13, 241. https://doi.org/10.3390/antiox13020241
Oh J, Hong S, Ko S-H, Kim H-S. Evaluation of Antioxidant Effects of Pumpkin (Cucurbita pepo L.) Seed Extract on Aging- and Menopause-Related Diseases Using Saos-2 Cells and Ovariectomized Rats. Antioxidants. 2024; 13(2):241. https://doi.org/10.3390/antiox13020241
Chicago/Turabian StyleOh, Joohee, Sookyeong Hong, Seong-Hee Ko, and Hyun-Sook Kim. 2024. "Evaluation of Antioxidant Effects of Pumpkin (Cucurbita pepo L.) Seed Extract on Aging- and Menopause-Related Diseases Using Saos-2 Cells and Ovariectomized Rats" Antioxidants 13, no. 2: 241. https://doi.org/10.3390/antiox13020241
APA StyleOh, J., Hong, S., Ko, S.-H., & Kim, H.-S. (2024). Evaluation of Antioxidant Effects of Pumpkin (Cucurbita pepo L.) Seed Extract on Aging- and Menopause-Related Diseases Using Saos-2 Cells and Ovariectomized Rats. Antioxidants, 13(2), 241. https://doi.org/10.3390/antiox13020241










