Abstract
Phospholipases (PL) A2 catalyzes the hydrolysis of membrane phospholipids and mostly generates arachidonic acid (AA). The enzyme 5-lipoxygenase (5-LOX) can metabolize AA to obtain inflammatory leukotrienes, whose biosynthesis highly depends on cPLA2 and 5-LOX activities. Formyl Peptide Receptor 2 (FPR2) belongs to a subfamily of class A GPCRs and is considered the most versatile FPRs isoform. Signaling triggered by FPR2 includes the activation of several downstream kinases and NADPH oxidase (NOX)-dependent ROS generation. In a metabolomic analysis we observed a significant increase in AA concentration in FPR2-stimulated lung cancer cell line CaLu-6. We analyzed cPLA2 phosphorylation and observed a time-dependent increase in cPLA2 Ser505 phosphorylation in FPR2-stimulated cells, which was prevented by the MEK inhibitor (PD098059) and the p38MAPK inhibitor (SB203580) and by blocking NOX function. Similarly, we demonstrated that phosphorylation of 5-LOX at Ser271 and Ser663 residues requires FPR2-dependent p38MAPK and ERKs activation. Moreover, we showed that 5-LOX Ser271 phosphorylation depends on a functional NOX expression. Our overall data demonstrate for the first time that FPR2-induced ERK- and p38MAPK-dependent phosphorylation/activation of cPLA2 and 5-LOX requires a functional NADPH oxidase. These findings represent an important step towards future novel therapeutic possibilities aimed at resolving the inflammatory processes underlying many human diseases.
Keywords:
arachidonic acid; cell metabolism; Formyl Peptide Receptor; FPR2; NADPH oxidase; ROS; 5-LOX; cPLA2; redox pathway 1. Introduction
Arachidonic acid (AA) is a ω-6 polyunsaturated fatty acid present in membrane phospholipids that participates in many cellular physiological processes, including inflammation and immunity [1]. Phospholipases (PL) (EC 3.1) are hydrolase enzymes that share the ability to catalyze the hydrolysis of membrane phospholipids [2] and are classified based on the site cleaved in the phospholipid molecule [3]. The hydrolytic cleavage catalyzed by PLA2 generates polyunsaturated fatty acids, mostly AA and lysophospholipids, thus determining the phospholipid composition of membranes [4]. The PLA2 (PLA2 EC 3.1.1.4) superfamily includes cytosolic (cPLA2), secreted (sPLA2), calcium-independent (iPLA2), platelet activator acetyl-hydrolase (PAF-AH), lysosomal (LPLA2), and adipose tissue-specific PLA2s (AdPLA) phospholipase. The first two classes of these enzymes are highly expressed in tumor cells [5].
Eicosanoids are a family of bioactive compounds derived from AA that play crucial roles in pathophysiology, including inflammatory conditions of multiple organ systems. They include prostaglandins (PG), thromboxanes (TX), leukotrienes (LT), and lipoxins (LX). Once released by the catalytic action of cPLA2, AA can be metabolized by cyclooxygenases (COXs) that catalyze the transformation of AA in PGH2 [6,7]. The aberrant AA metabolism observed in cancer cells results in a high concentration of PGs, in particular PGE2 [8,9]. Alternatively, AA can be metabolized by the enzyme 5-lipoxygenase (5-LOX) to generate LTs. 5-LOX interacts with 5-lipoxygenase activating protein (FLAP) and converts AA to LTA4 [10]. Therefore, LTs biosynthesis is highly dependent on the activities of cPLA2 and 5-LOX. In addition, AA can be metabolized by cytochrome P450, generating epoxides and a wide spectrum of biologically active fatty acid mediators [9].
The biosynthesis of eicosanoids requires several catalytic steps regulated by inflammatory and stress signals via different molecular mechanisms. In fact, phosphorylation at Ser505 residue of cPLA2 by extracellular response kinases (ERKs) and the stress-regulated p38MAPK regulates the activity of this enzyme [11,12], whereas 5-LOX is phosphorylated at Ser271 and Ser663 residues by p38MAPK-regulated MAPKAPK-2/3, ERKs, and CaMKII. Conversely, phosphorylation at Ser523 residue by PKA suppresses 5-LOX activity [13]. The G-protein coupled receptors (GPCRs) can sense extracellular metabolites and thus regulate inflammatory responses, including eicosanoid production [10]. Signaling triggered by GPCRs includes calcium mobilization and the activation of several downstream kinases, such as ERKs, p38MAPK, and p38MAPK-regulated MAPKAPK, which in turn can regulate cPLA2 and 5-LOX activity.
The Formyl Peptide Receptors (FPRs) are a subfamily of class A GPCRs that, in humans, includes three different isoforms (FPR1, FPR2, and FPR3) [14]. The distinct members of this family are functionally expressed on the cellular and nuclear membrane of several cell types [15,16,17]. FPRs can recognize a plethora of ligands, which include both pathogen-associated molecular patterns (PAMPs) and damage-associated molecular pattern (DAMPs) [18,19], modulating several biological functions, such as angiogenesis, metabolism, cell proliferation, and cell death [14,20,21,22]. FPRs are also able to modulate inflammatory responses in many physio-pathological processes, such as cancer [16,23,24,25,26], neurodegeneration [27,28,29], and cardiovascular diseases [30,31].
FPR2 is considered the most versatile FPR isoform, being able to recognize an array of structurally and chemically unrelated ligands [32,33]. FPR2 agonists include both endogenous ligands and exogenous ligands [32]. Depending on the nature of its ligands and/or FPR2 rearrangement with other FPR isoforms or with the scavenger macrophage receptor with collagenous structure (MARCO) [34], FPR2 can modulate pro- or anti-inflammatory responses [14,35].
Intracellular cascades triggered by FPR2 include the activation of several kinases [36,37,38] and of signaling and non-signaling proteins [14,22], as well as the NADPH oxidase (NOX)-dependent release of reactive oxygen species (ROS) [15,39,40]. Notably, low levels of ROS can act as second messengers, and FPR-mediated NOX-dependent ROS generation is crucially involved in transactivation of various Tyrosine Kinase Receptors (RTKs) [41], such as EGFR [38], TrkA [42], c-Met [43], VEGFR [20], and IGFR [44].
We recently demonstrated that the FPR2 agonists WKYMVm and ANXA1 elicit intracellular redox signaling pathways involved in glucose uptake and aerobic metabolism of glucose typical of the Warburg effect in the human lung adenocarcinoma cell line CaLu-6 [44,45]. Moreover, we proved that exposure to FPR2 agonists enhances the non-oxidative phase of pentose phosphate pathway (PPP), improves the expression of the glutamine transporter ASCT2, and induces the de novo synthesis of pyrimidine nucleotides [46].
We herein report our metabolomic data showing a significant increase in AA in FPR2-stimulated CaLu-6 cells. Therefore, we dissect the molecular mechanisms involved in FPR2-dependent cPLA2 and 5-LOX activation that trigger AA release and LTs synthesis, respectively. We also evaluated the role of redox signaling in the cell metabolism of AA, disclosing that FPR2-mediated NADPH oxidase-dependent ROS generation plays a key role in both cPLA2 and 5-LOX phosphorylation and, in turn, in AA metabolism.
2. Materials and Methods
2.1. Cell Culture and Reagents
Epithelial anaplastic human lung cancer CaLu-6 and p22phoxCrispr/Cas9 CaLu-6 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplied with 10% fetal bovine serum (FBS) (Invitrogen Corp., Carlsbad, CA, USA) at 37 °C and 5% CO2. Cells at 70% of confluence were serum deprived for 24 h and stimulated or not with 10 μM WKYMVm (Primm, Milan, Italy). Unstimulated CaLu-6 cells were used as negative control. In other experiments, CaLu-6 cells were preincubated with the selective FPR2 antagonist WRWWWW (WRW4) (Primm, Milan, Italy) for 15 min at a final concentration of 10 μM, or with 100 μM apocynin, the selective inhibitor of NADPH oxidase (Sigma Chemical, St. Louis, MO, USA), for 2 h, or with 50 μM PD09805, a selective inhibitor of MEK (Sigma), for 90 min, or with 10 μM SB203580, a selective inhibitor of p38MAPK (Sigma), for 1 h. FPR2-unstimulated CaLu-6 cells only preincubated with the appropriate amount of the above-mentioned selective inhibitor were used as a negative control of pretreatments.
2.2. p22phoxCrispr/Cas9 Double-Nickase CaLu-6 Cells
p22phoxCrispr/Cas9 cells were generated by transfecting CaLu-6 cells with Double Nickase Plasmid or with a Double Nickase Plasmid control (Santa Cruz Biotechnology, Irvine, CA, USA) following the manufacturer’s instructions, as previously described. The expression of p22phox was analyzed by Western blotting [40].
2.3. Metabolomic Analysis by LC-MS
Metabolomic analysis by LC-MS was performed in growing and in 24 h serum-starved CaLu-6 cells stimulated or not with WKYMVm in the presence or absence of WRW4 as previously described [46]. Briefly, 24 h serum-starved cells were treated as above mentioned and lysed in 400 μL of a 1:1 prechilled MetOH:H2O solution. The samples were centrifuged at 10,000× g at 4 °C for 10 min. Supernatants were dried and then reconstituted with 125 μL of methanol/acetonitrile/water (50:25:25). Extracted metabolites were analyzed using an ACQUITY UPLC system online coupled to a Synapt G2-Si QTOF-MS (Waters Corporation, Milford, MA, USA) in positive and negative modes in the following settings: reverse-phase ACQUITY UPLC CSH C18 (1.7 μm, 100 × 2.1 mm2) column (Waters), 0.3 mL/min flow rate, mobile phases composed of acetonitrile/H2O (60:40) containing 0.1% formic acid and 10 mM ammonium formate (Phase A), and isopropanol/acetonitrile (90:10) containing 0.1% formic acid and 10 mM ammonium formate (Phase B). Peak detection, metabolite identification, and quantitation were performed as previously described [44], fitting experimental data with internal standard and calibration curves. Data analysis and heatmap were generated with the online software MetaboAnalyst 5.0 (https://www.metaboanalyst.ca, accessed on 1 June 2021).
2.4. Protein Extraction and Western Blot
Whole lysates were obtained as previously described [47]. Briefly, 24 h serum-starved CaLu-6 or p22phoxCrispr/Cas9 CaLu-6 cells were stimulated or not with 10 μM WKYMVm, in the presence or absence of the above-mentioned selective inhibitors, and lysed with RIPA lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM EDTA, 0.25% sodium deoxycholate, 1 mM NaF, 10 μM Na3VO4, 1 mM phenyl-methyl-sulfonyl-fluoride, 10 μg/mL aprotinin, 10 μg/mL pepstatin, and 10 μg/mL leupeptin). Bio-Rad protein assay was used to determine proteins concentration (BioRAD, Hercules, CA, USA). Western blot analysis on whole lysates was performed as previously described (PMID: 36168728). Anti-GAPDH (SC-47724) antibody was purchased from Santa Cruz Biotechnology (Irvine, CA, USA). Anti-phospho-cPLA2 S505, anti-phospho-5-LOX S271, and anti-phospho-5-LOX S663 were from Cell Signalling Technology (Denvers, MA, USA). Goat-anti-mouse (bs-0296G-HRP) and goat-anti-rabbit (bs-0295G-HRP) were from Bioss Antibodies (Woburn, MA, USA). Proteins were visualized by enhanced chemiluminescence reagent (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK) and were quantified using densitometry (Chemidoc, BioRAD). Each experiment with the relative densitometric quantification was separately repeated at least three times.
2.5. Statistical Analysis
All data reported are expressed as means ± standard error mean (SEM) and are representative of at least three or more independent experiments. Statistical analyses were performed with unpaired t-test to compare the mean of two independent groups of experiments or by one-way analysis of variance (ANOVA). GraphPad Prism 7 (GraphPad Software Inc., San Diego, CA, USA) was used to compare more than two experiments. A p value of less than 0.05 was considered to be statistically significant.
3. Results and Discussion
3.1. FPR2 Stimulation Induces Arachidonic Acid Release by Activating cPLA2
We previously demonstrated that FPR2 stimulation triggers the metabolic reprogramming of CaLu-6 cells by modulating aerobic glycolysis, PPP, glutamine transport, and the de novo synthesis of pyrimidine nucleotides [44,45,46]. Besides glycolysis and glutamine metabolism, an emerging role of lipids in metabolic reprogramming of many types of cancers cells has been observed [48].
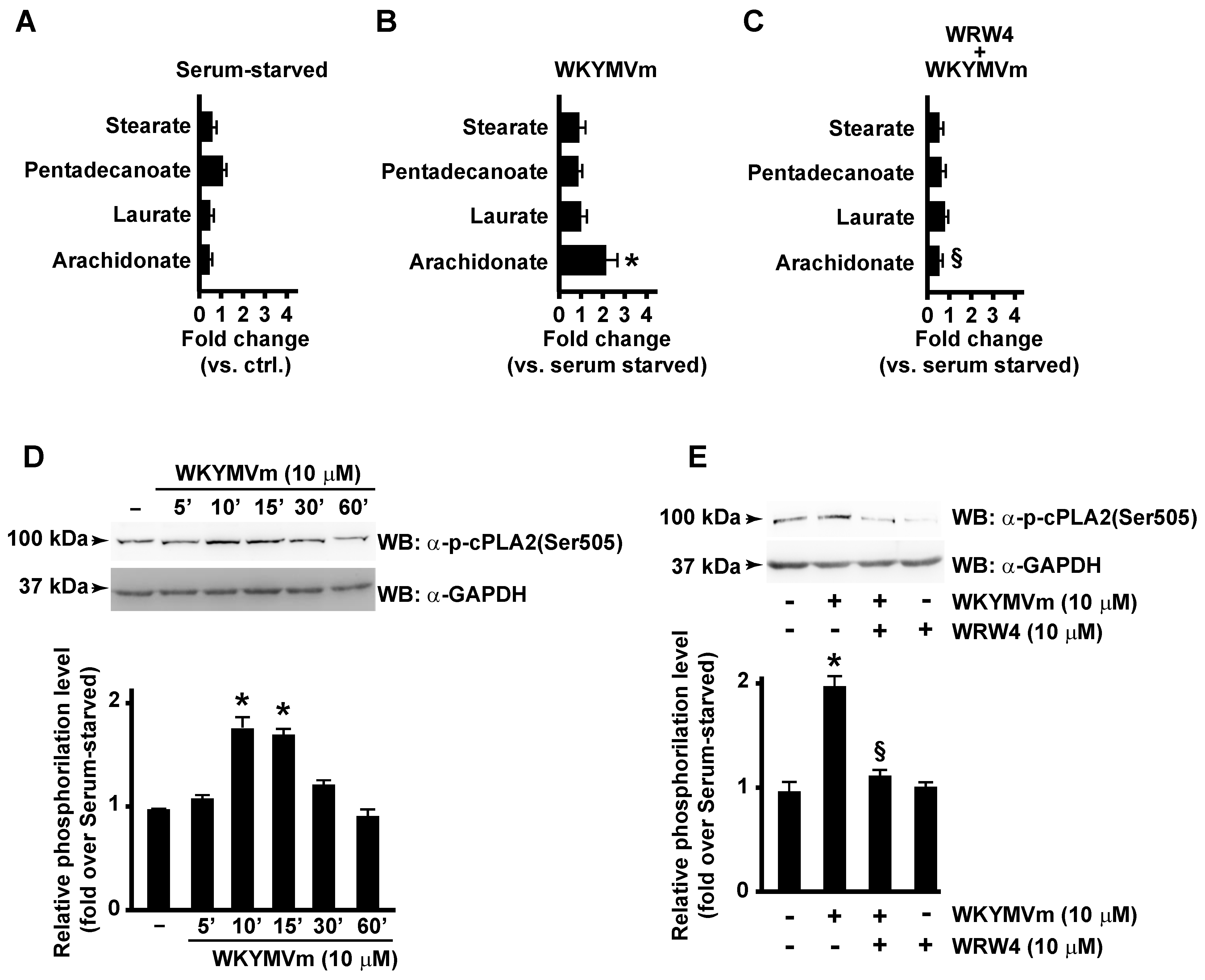
By metabolomic analysis, we observed that AA concentration was significantly enhanced in WKYMVm-stimulated CaLu-6 cells (Figure 1B) compared to unstimulated cells (Figure 1A), whereas concentrations of other fatty acids, such as stearate, pentadecanoate, and laurate, were unaffected (Figure 1A,B). AA levels remained unchanged when cells were preincubated with the FPR2 antagonist WRWWWW (WRW4) (Figure 1C), thus indicating that it depends on FPR2 stimulation.
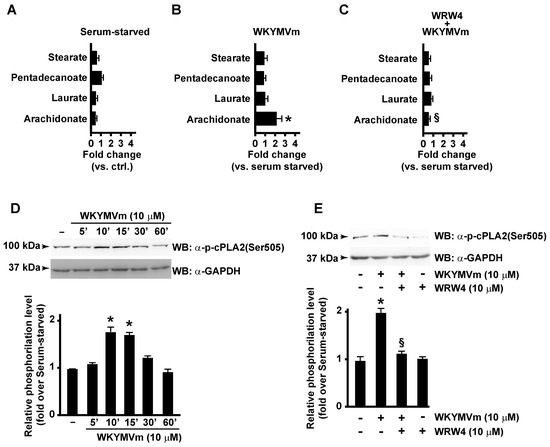
Figure 1.
FPR2 promotes arachidonic acid metabolism by cPLA2 Serine 505 phosphorylation. FPR2 stimulation induces arachidonic acid metabolism in CaLu-6 cells (A–C). Bar graphs represent the metabolomic analysis of stearate, pentadecanoate, laurate, and arachidonate levels detected in 24 h serum-starved CaLu-6 cells (A), 10 μM WKYMVm-stimulated (WKYMVm) (B) and in 10 μM WRWWWW (WRW4)-pre-treated and WKYMVm-stimulated (WKYMVm + WRW4) (C) CaLu-6 cells. Growing cells (ctrl) were serum-starved for 24 h and then stimulated or not with 10 μM WKYMVm for 1 h in presence or absence of 10 μM WRW4. Metabolomic analysis was performed as described in Materials and Methods. cPLA2 Serine 505 (Ser505) phosphorylation is promoted by FPR2 stimulation with WKYMVm (D,E). Serum-starved CaLu-6 cells were exposed to 10 μM WKYMVm for 5, 10, 30, or 60 min (D), or pre-treated for 15 min with WRW4 before the stimulation with WKYMVm (E). Sixty micrograms of whole lysates were resolved on 10% SDS-PAGE and incubated with an anti-phospho-cPLA2 (Ser505) (α-p-cPLA2(Ser505)) antibody. An anti-GAPDH (α-GAPDH) antibody was used as control for protein loading. Data are representative of three independent experiments. * p < 0.05 compared to unstimulated cells. § p < 0.05 compared to stimulated cells.
Three main mechanisms allow cells to obtain AA: (i) direct exogenous uptake via specific transporters, such as CD36, and TWIK-related AA-stimulated K+ (TRAAK) channels; (ii) de novo synthesis from linoleic acid; (iii) cleavage of the sn-2 position of existing membrane phospholipids through the enzymatic activity of cPLA2 [49,50,51].
cPLA2 activity is tightly regulated in cells by at least three mechanisms. The increase in the intracellular Ca2+ concentration represents a key regulator of cPLA2 activity in several cell types [52,53]. In addition to Ca2+, cPLA2 is also regulated by intracellular lipids, which allosterically activate the enzyme and increase its residence time in membranes [54]. cPLA2 is also regulated by phosphorylation at Ser505, Ser515, and Ser727 residues, which controls agonist-induced AA mobilization [55]. Ser515 and Ser727 phosphorylation depends on cell type and stimulation conditions, whereas only Ser505 phosphorylation is required for cPLA2 full activation and cell membrane translocation [55].
In WKYMVm-stimulated cells, we observed a time-dependent increase in cPLA2 Ser505 phosphorylation (Figure 1D), which was prevented by preincubation with the FPR2 antagonist (Figure 1E).
FPR2 ability to induce an increase in PLA2 expression was previously observed in SAA-stimulated cells [56], as well as in conjunctival goblet cells and in neutrophils incubated with ANXA1 or WKYMVm, respectively [57,58].
Our results demonstrate, for the first time, that FPR2 stimulation triggers cPLA2 phosphorylation at Ser505 residue, thereby suggesting that cPLA2 activation can contribute to the concomitant increase in AA concentration.
3.2. FPR2-Mediated cPLA2 Ser505 Phosphorylation Depends on NOX Activation
Ser505 residue of cPLA2 is phosphorylated by different kinases, such as ERKs [11] and p38MAPK [12]. Furthermore, there is increasing evidence that NOX-dependent ROS generation can activate cPLA2 [59] and that hydrolytic products of cPLA2, including AA, could enhance NOX activity [60,61,62]. The assembled NOX complex serves as a target for anchoring cPLA2 to the plasma membranes [63]. Therefore, it is conceivable that these two enzymes share a common mechanism for activation by intracellular kinases. ERKs and p38MAPK trigger both cPLA2 phosphorylation and ROS production, and both events are prevented by NADPH oxidase inhibitors [64].
Several GPCR agonists elicit an increase in NOX-dependent ROS concentration and trigger the activity of several kinases, such as p38MAPK, ERKs, and JNK, which are able to phosphorylate cPLA2 [65,66]. These kinases can be also activated by the canonical pathway of TKRs elicited by GPCR-dependent TKRs transactivation [67].
Previously, we demonstrated that intracellular domains of activated FPR2 mediate signaling to G-proteins, which trigger several agonist-dependent signaling cascades, including activation of PLC, PKC isoforms, p38MAPK, PI3K/Akt, and MAPK pathway. Phosphorylation of cytosolic tyrosine kinases, TKRs transactivation, phosphorylation and nuclear translocation of regulatory transcriptional factors, and release of calcium and production of oxidants also belong to the distinct intracellular pathways elicited by FPR2 [32].
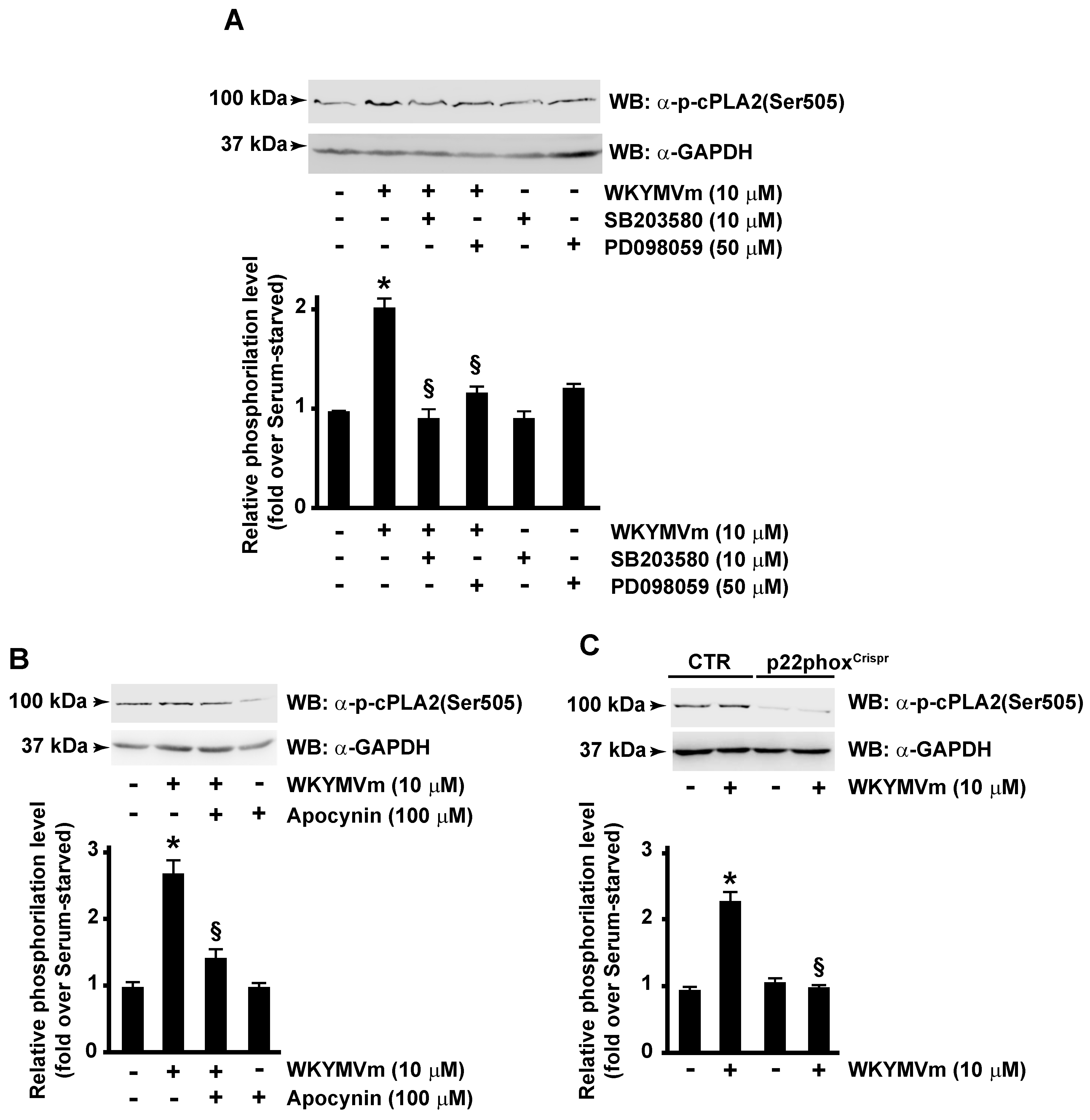
Therefore, we analyzed molecular mechanisms involved in FPR2-induced cPLA2 phosphorylation by pretreating cells with selective inhibitors of MEK and p38MAPK. Cells were preincubated with PD098059 or SB203580 before WKYMVm stimulation, and in Western blot experiments, we observed that both inhibitors prevent cPLA2 phosphorylation at Ser505 residue (Figure 2A). Previously, we demonstrated that, in CaLu-6 cells, WKYMVm stimulation induces ERK-dependent p47phox phosphorylation and membrane translocation and, in turn, NOX-dependent superoxide generation [38]. Therefore, we investigated the possible involvement of NOX in cPLA2 Ser505 phosphorylation by treating cells with apocynin, a selective inhibitor of NOX activity, before agonist stimulation. Obtained results demonstrated that specific block of NOX function prevented cPLA2 Ser505 phosphorylation (Figure 2B). Consistently, cPLA2 Ser505 phosphorylation was not observed following FPR2 stimulation with WKYMVm of the p22phoxCaLu-6Crispr/Cas9 cells, which lack the p22phox gene encoding a critical component of the superoxide-generating NOX [40] (Figure 2C). Taken together, these results prove that FPR2 modulates cPLA2 activation in an ERK-, p38MAPK-, and NOX-dependent manner.
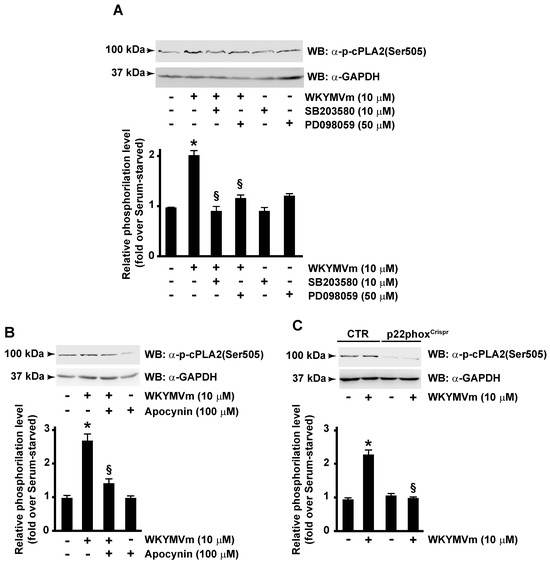
Figure 2.
cPLA2 serine 505 phosphorylation requires both p38MAPK, ERKs and NADPH oxidase activation. FPR2-WKYMVm-mediated cPLA2 serine 505 phosphorylation depends on p38MAPK, ERKs (A), and NADPH oxidase activation (B,C). Serum-starved CaLu-6 cells were stimulated or not for 10 min with 10 μM WKYMVm in presence or absence of 10 μM SB203580 or 50 μM PD098059 (A), or 100 μM apocynin (B). CaLu-6-controlCrispr/Cas9 cells (CTR) and p22phoxCrispr/Cas9 (p22phoxCrispr) cells were serum starved for 24 h and then stimulated with WKYMVm (C). Sixty micrograms of whole lysates was resolved on 10% SDS-PAGE and incubated with an anti-phospho-cPLA2 (Ser505) (α-p-cPLA2(Ser505)) antibody. An anti-GAPDH (α-GAPDH) antibody was used as control for protein loading. Data are representative of four independent experiments. * p < 0.05 compared to unstimulated cells. § p < 0.05 compared to stimulated cells.
Notably, ROS play an important role in inflammation processes and in signal transduction. According with our findings, NOX activation increases ROS levels that in turn inactivate the function of phosphothyrosine and serine/threonine phosphatases containing cysteine moieties within their active centers, which are susceptible to oxidation by ROS. Therefore, inactivation of phosphatases results in activation of MAPKs, p38MAPK, and ERKs which, in turn, activate cPLA2 [68]. Very interestingly, protein phosphatase inhibition also induces activation of non-receptor tyrosine kinase family Src, which, in turn, leads to EGFR phosphorylation [38] and thus to the transduction of this signal into MAPK cascades [69], further improving cPLA2 activation.
3.3. FPR2 Stimulation Induces 5-LOX Activation
Eicosanoids, including PGs, TXs, LTs, and lipoxins, are generated during the various phases of AA metabolism along the COX and LOX pathways [70]. Release of AA and activation of 5-LOX, in response to several stimuli, initiates the biosynthesis of proinflammatory LTs, a family of lipid mediators with pivotal roles in inflammatory disorders [71].
In addition to the normal expression in the various leukocyte types, aberrant expression of 5-LOX has been detected in many tumor cells [72,73,74] and, besides inflammatory processes, 5-LOX is involved in cell differentiation, oxidative stress, and in the progression of different diseases [75,76,77].
In the resting cell, 5-LOX is localized in either the cytosol or a soluble compartment inside the nucleus. Activation of cellular 5-LOX involves enzyme translocation to the nuclear envelope, where it colocalizes with cPLA2 and FLAP [78,79,80].
5-LOX is a substrate for several protein kinases, and phosphorylation of different residues has divergent consequences for 5-LOX subcellular localization and activity. Phosphorylation at Ser271 and Ser663 residues increases 5-LOX expression and is strongly promoted by unsaturated fatty acids, including AA. Enhanced 5-LOX activity following dual phosphorylation at Ser271 and Ser663 residues is observed when intracellular Ca2+ levels are low and, thus, insufficient to activate 5-LOX alone. In addition, Ser271 phosphorylation promotes nuclear localization of 5-LOX [81]. An increase in cAMP levels activates PKA, which represses 5-LOX activity through phosphorylation at Ser523 residue [82]. This phosphorylation prevents nuclear import of the enzyme and, in turn, its cytoplasmic enrichment [83].
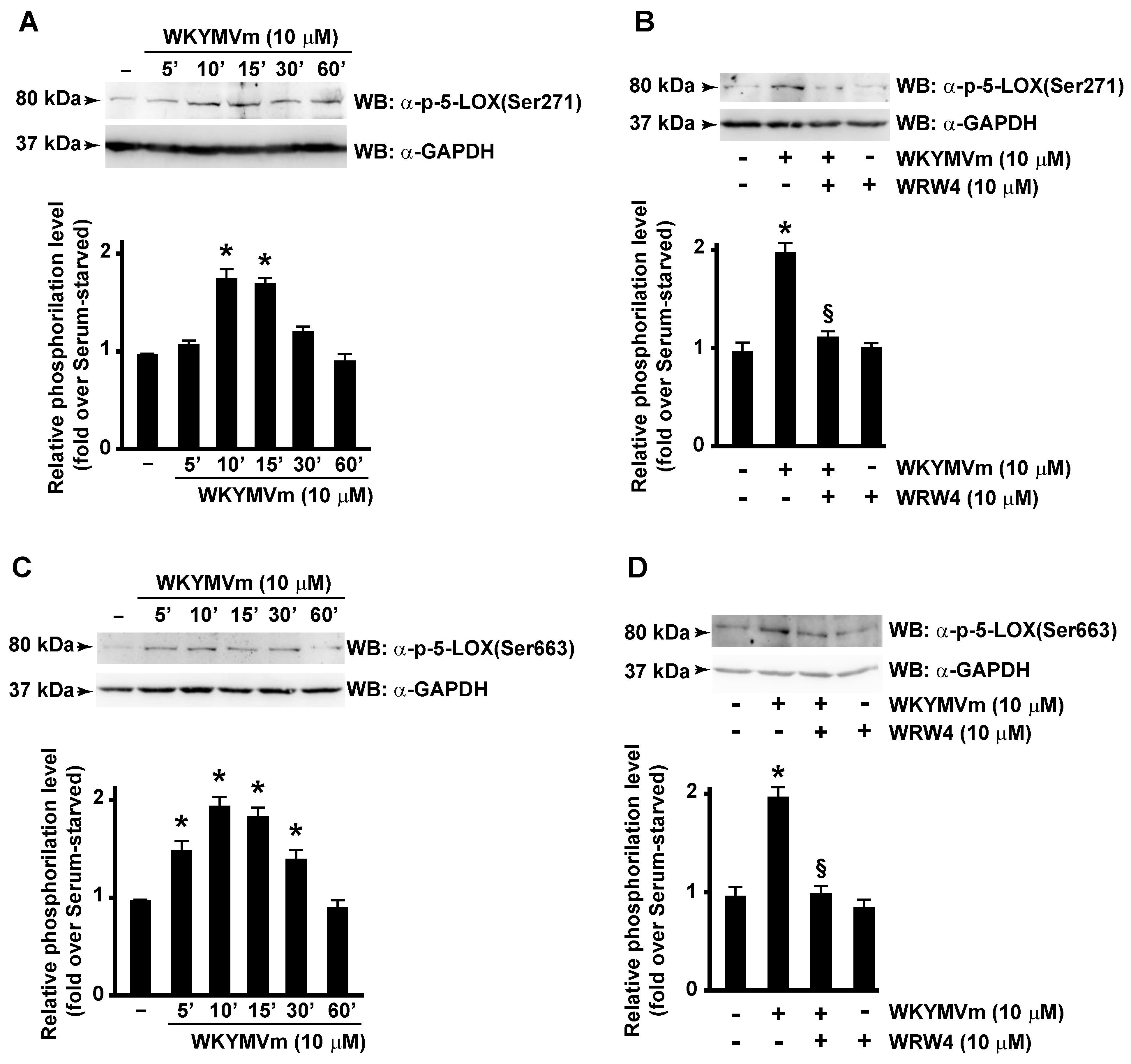
In this scenario, we investigated the ability of FPR2 stimulation to trigger 5-LOX activation. By analyzing WKYMVm-treated CaLu-6 cells, we demonstrated that FPR2 stimulation induces Ser271 (Figure 3A) and Ser663 (Figure 3C), but not Ser523, phosphorylation of 5-LOX, whereas preincubation with WRW4, a selective FPR2 antagonist, prevented both Ser271 (Figure 3A) and Ser663 phosphorylation (Figure 3B,D). These findings, for the first time, demonstrate that 5-LOX is specifically activated by FPR2 stimulation.
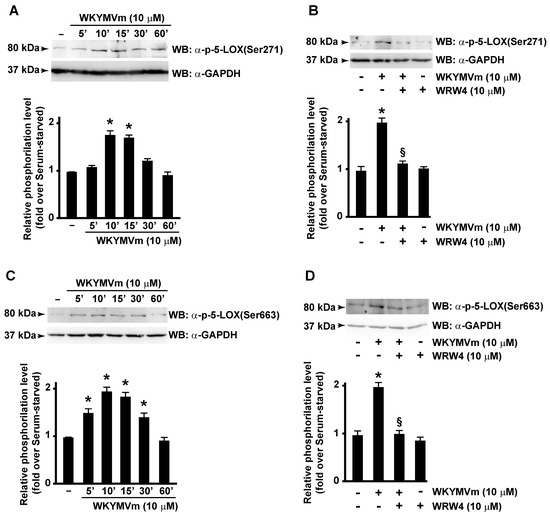
Figure 3.
FPR2 stimulation induces 5-LOX activation by enhancing Serine 271 and Serine 663 phosphorylation. FPR2 exposure to WKYMVm enhances 5-LOX phosphorylation on Serine 271 and Serine 663. Serum-starved CaLu-6 cells were stimulated with WKYMVm for 5, 10, 15, 30, and 60 min (A,C) in presence or absence of WRW4 (B,D). Fifty micrograms of whole lysates was resolved on 10% SDS-PAGE and incubated with an anti phospho-5-LOX (Ser271) (α-p-5-LOX(Ser271)) or anti phospho-5-LOX (Ser663) (α-p-5-LOX(Ser663)) antibodies. An anti-GAPDH (α-GAPDH) antibody was used as control for protein loading. Data are representative of three independent experiments. * p < 0.05 compared to unstimulated cells. § p < 0.05 compared to stimulated cells.
3.4. p38MAPK, ERKs and NOX Are Required for FPR2-Dependent 5-LOX Phosphorylation
p38MAPK-regulated MAPK-activated protein kinases (MAPKAPKs) have been identified as the kinases that phosphorylate 5-LOX at Ser271 residue in vitro [84,85].
In PMN, AA and fMLP, an FPR1 agonist, activate p38MAPK, leading to 5-LOX activation [84]. ERKs have been described as kinases that phosphorylate 5-LOX at Ser663 residue, and dual phosphorylation by ERK2 and p38MAPKAPKs at different sites is necessary for AA-induced 5-LOX activation [86]. AA plays a central role in the convergence of MAPK signaling cascades, leading to phosphorylation and activation of 5-LOX. Synergistic actions of MAPK pathways were also observed for the activation of cPLA2 [13,87].
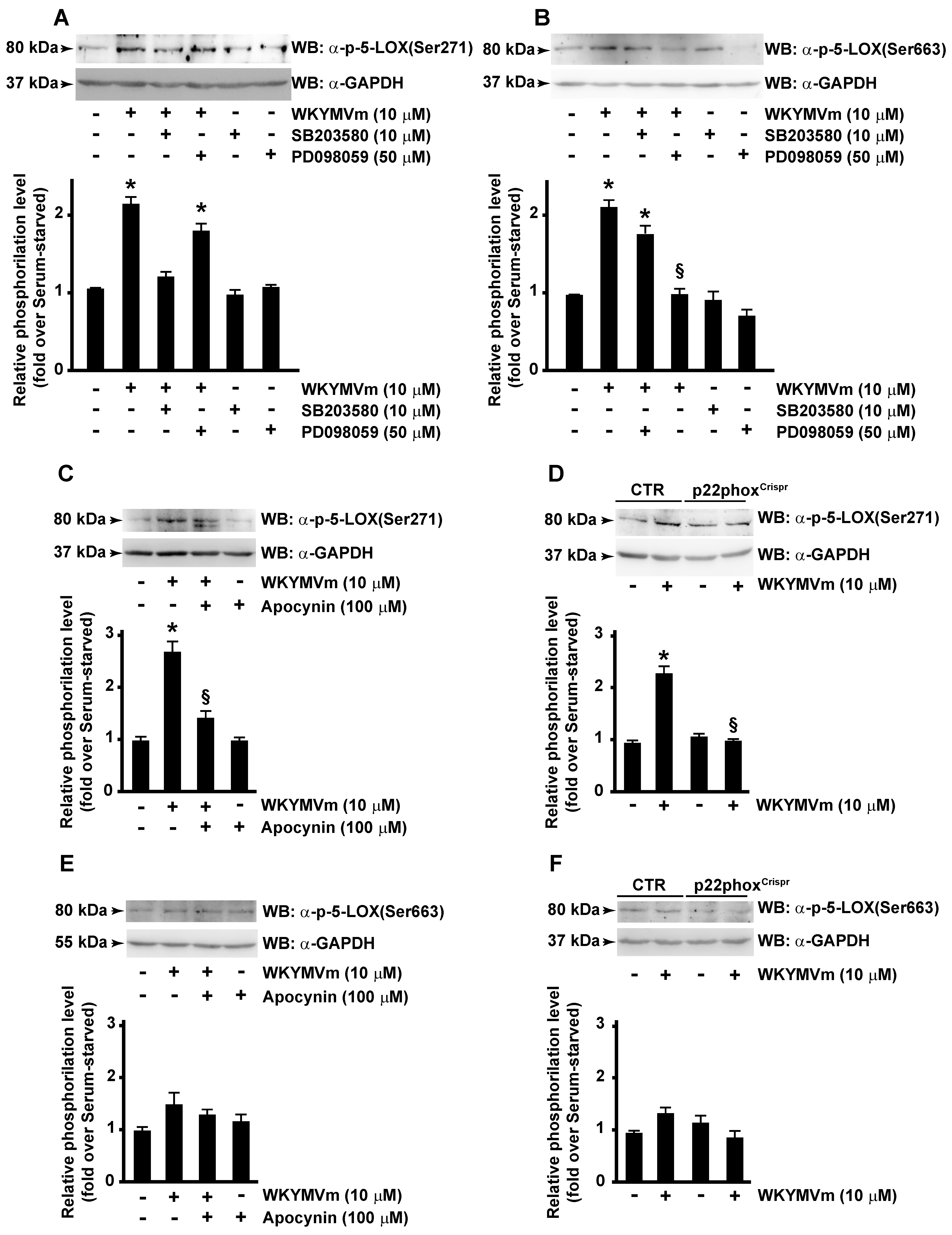
ERKs are typically activated by growth-related stimuli through the Raf-1/MEK1/2 protein kinase cascade. However, upon activation, FPR2 triggers several agonist-dependent signal transduction cascades, including Ras-MAPK pathway, PLC, and p38MAPK activation [32]. Consistently, we observed that, upon WKYMVm stimulation, phosphorylation of 5-LOX at Ser271 residue was prevented in CaLu-6 cells pretreated with SB203580, an inhibitor of p38MAPK signaling pathway (Figure 4A); similarly, Ser663 phosphorylation failed when cells were preincubated with the MAPK inhibitor PD098059 (Figure 4B). These results prove that FPR2 stimulation induces 5-LOX phosphorylation through FPR2-dependent p38MAPK and ERKs activation.
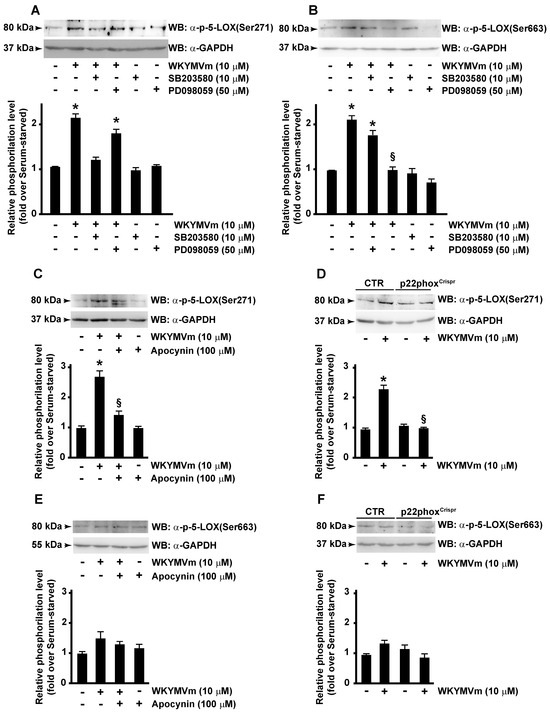
Figure 4.
FPR2-mediated 5-LOX phosphorylation depends on p38MAPK, ERKs and NADPH oxidase activation. Serum-deprived CaLu-6 cells were pre-incubated with SB203580 or PD098059 (A,B) or apocynin (C,E) and stimulated or not with WKYMVm for 10 min. CaLu-6-controlCrispr/Cas9 cells (CTR) and p22phoxCrispr/Cas9 (p22phoxCrispr) cells were serum-starved for 24 h and then stimulated with WKYMVm (D,F). Fifty micrograms of whole lysates was resolved on 10% SDS-PAGE and incubated with an anti phospho-5-LOX (Ser271) (α-p-5-LOX(Ser271)) or anti phospho-5-LOX (Ser663) (α-p-5-LOX(Ser663)) antibodies. An anti-GAPDH (α-GAPDH) antibody was used as control for protein loading. Data are representative of three independent experiments. * p < 0.05 compared to unstimulated cells. § p < 0.05 compared to stimulated cells.
Because catalysis by 5-LOX requires oxidation of Fe2+ to Fe3+ in the active site of the enzyme, cellular redox conditions represent a crucial factor that modulates 5-LOX activity. Conditions that promote the formation of ROS upregulate 5-LOX, whereas reducing conditions, as well as the presence of suitable thiols (GSH or DTT), prevent 5-LOX activity [81,88].
In mammalian cells, ROS are generated via a variety of cellular oxidative processes, including the activities of NOX, xanthine oxidases, and the mitochondrial respiratory chain. NOX-generated ROS are the best characterized examples of ROS, although they are also generated by the oxidative metabolism of AA, released from the membrane phospholipids via cPLA2 activity. In fact, LOX- and COX-generated AA metabolites can induce ROS generation by stimulating NOX, highlighting a potential signaling connection between LOX/COX metabolites and NOX [89].
We analyzed the role of NOX in FPR2-dependent phosphorylation of Ser271 and Ser663 of 5-LOX. CaLu-6 cells were stimulated with WYKMVm or preincubated with apocynin, a NOX inhibitor, before stimulation. Results showed that Ser271 phosphorylation of 5-LOX was prevented by apocynin (Figure 4C), whereas Ser663 phosphorylation was not affected by NOX-dependent modulations of redox state (Figure 4E). Accordingly, in p22phoxCrispr/Cas9 cells, Ser271 phosphorylation (Figure 4D), but not Ser663 phosphorylation (Figure 4E), required functional expression of NOX.
Ser271 is phosphorylated by p38MAPK-regulated MAPKAPKs, and among MAPK families, p38MAPK is also activated when cells are exposed to various cellular stress. We previously proved that in CaLu-6 cells FPR2 stimulation with WKYMVm or ANXA1 induced NOX-dependent activation of p38MAPK [40]. Thus, an increase in ROS in these cells may activate p38MAPK and, consequently, 5-LOX by promoting Ser271 phosphorylation [90].
This latter evidence and the results herein presented strongly demonstrate that FPR2 signaling induces 5-LOX phosphorylation at Ser271 and Ser663, which requires p38MAPK and ERKs activation, respectively. Furthermore, since NOX inhibition prevents Ser271 phosphorylation, we can hypothesize that FPR2-dependent NOX activation might contribute to 5-LOX nuclear translocation.
4. Conclusions
In the last decade, much progress has been made in understanding the effects of ROS on inflammatory processes that are associated with many human diseases. In many cases, the disease, or its progression, is associated with NOX activation, which generates ROS that trigger inflammatory reactions, representing one of the risk factors for cancer.
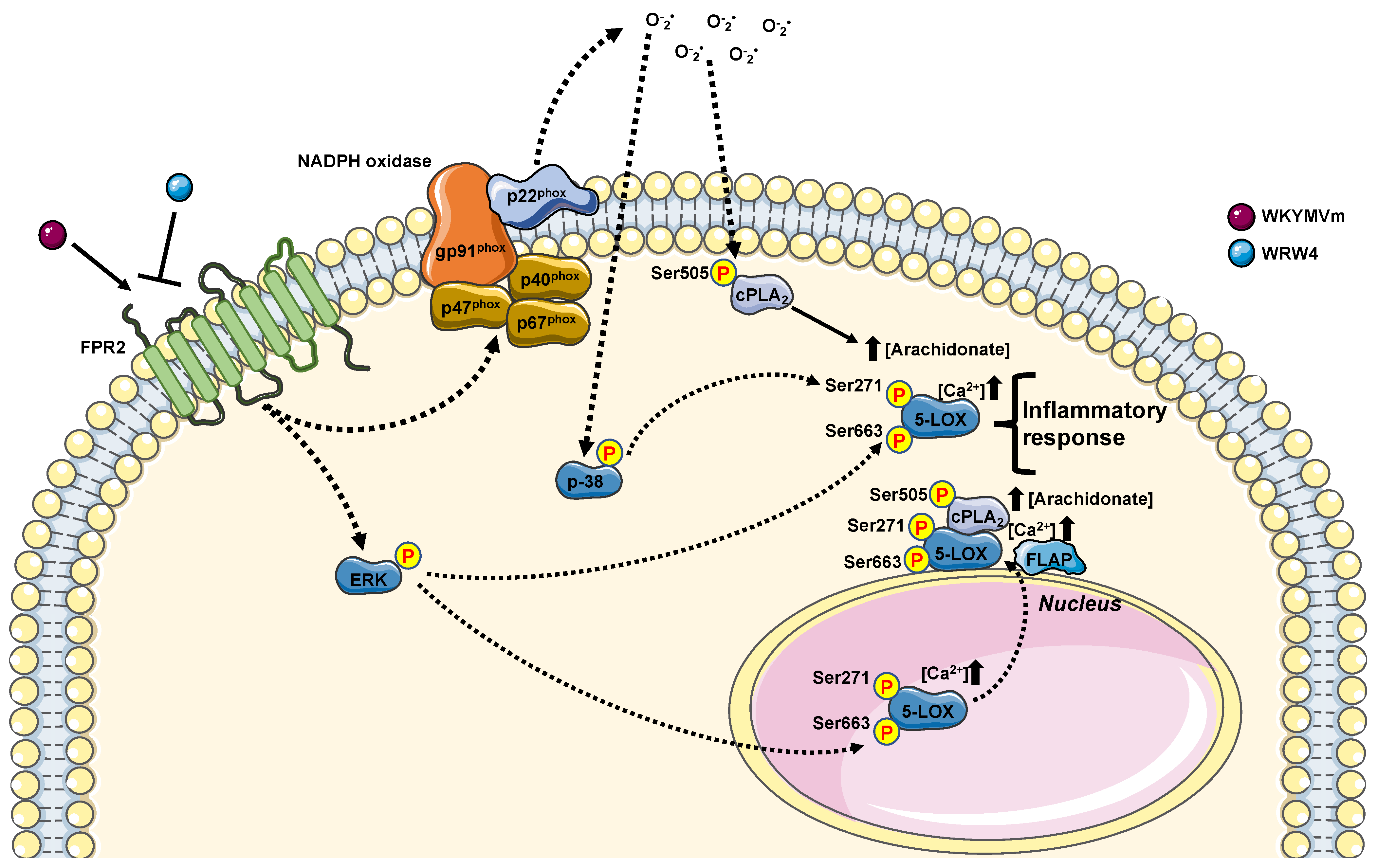
Although 5-LOX catalyzes the first step of LTs molecules with proinflammatory functions, it is also involved in the biosynthesis of lipid mediators with anti-inflammatory properties, such as lipoxins. In many diseases, an imbalance between proinflammatory LTs and anti-inflammatory lipoxins is observed. However, some questions remain unresolved. How does intranuclear localization of 5-LOX confer a higher activity? Does 5-LOX have other roles inside the nucleus? Since inhibition of LTs biosynthesis shows beneficial effects in various inflammatory diseases, new findings on 5-LOX activation/translocation may lead to future novel therapeutic possibilities. In this context, the elucidation of the molecular mechanisms underlying inflammatory reactions triggered by NOX-dependent ROS generation (Figure 5) can provide new strategies to inhibit 5-LOX activation, whereas the control of FPR2-mediated nuclear import of 5-LOX might represent an important step in resolving the inflammatory process in many human diseases.
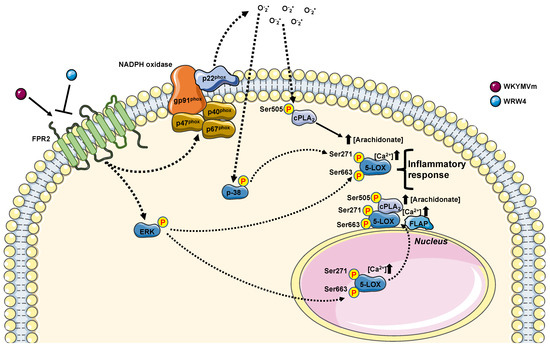
Figure 5.
FPR2 stimulation contribute to regulate arachidonic acid metabolism. Upon WKYMVm incubation, FPR2 increases the concentration of arachidonate. FPR2, once stimulated, can contribute to arachidonic acid metabolism by phosphorylating cPLA2 on its Serine 505 (Ser505) in a p38MAPK-, ERKs-, and NADPH oxidase-dependent fashion. FPR2-cPLA2-mediated increase in arachidonic acid can improve 5-LOX activation by phosphorylating its Serine 271 (Ser271) and Serine 663 (Ser663). 5-LOX activation can be also promoted by an increase in intracellular Ca2+ concentration ([Ca2+]). Both 5-LOX phosphorylation required FPR2 stimulation. However, Ser271 required p38MAPK and NADPH oxidase activation, whereas Ser663 ERKs activation. 5-LOX, once phosphorylated, can migrate to the nuclear envelope, where it colocalizes with PLA2 and 5-lipoxygenase activating protein (FLAP), and in turn, it can contribute to generating anti-inflammatory mediators. Furthermore, 5-LOX phosphorylation at Ser271 promotes its nuclear localization, where it can favor biosynthesis of pro-inflammatory mediators.
Author Contributions
T.P.C. designed and performed experiments. I.P. and S.S. performed experiments. M.S. was involved in development of the methodology and in the data analysis. G.E. revised critically the article. R.A. evaluated and analyzed data and wrote the first draft of the manuscript. F.C. supervised experiments, edited the manuscript and obtained funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Unione Europea–Next Generation EU–PRIN 2022 PNRR grant number P2022E45MP and by Università degli Studi di Napoli Federico II, Italy, Finanziamento Ricerca di Ateneo 2020 SDPFOSAA.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article. Other data that support the findings of this study are available upon request to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Murakami, M.; Kudo, I. Phospholipase A2. J. Biochem. 2002, 131, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, L.; Araujo, T.G.; Azevedo, F.; Mota, S.T.S.; Avila, V.M.R.; Ribeiro, M.A.; Goulart, L.R. Phospholipase A2 Drives Tumorigenesis and Cancer Aggressiveness through Its Interaction with Annexin A1. Cells 2021, 10, 1472. [Google Scholar] [CrossRef]
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed]
- Ilic, D.; Bollinger, J.M.; Gelb, M.; Mauro, T.M. sPLA2 and the epidermal barrier. Biochim. Biophys. Acta 2014, 1841, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Schaloske, R.H.; Dennis, E.A. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta 2006, 1761, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.L.; Urade, Y.; Jakobsson, P.J. Enzymes of the cyclooxygenase pathways of prostanoid biosynthesis. Chem. Rev. 2011, 111, 5821–5865. [Google Scholar] [CrossRef]
- Karpisheh, V.; Nikkhoo, A.; Hojjat-Farsangi, M.; Namdar, A.; Azizi, G.; Ghalamfarsa, G.; Sabz, G.; Yousefi, M.; Yousefi, B.; Jadidi-Niaragh, F. Prostaglandin E2 as a potent therapeutic target for treatment of colon cancer. Prostaglandins Other Lipid Mediat. 2019, 144, 106338. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism pathways of arachidonic acids: Mechanisms and potential therapeutic targets. Signal Transduct. Target. Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- Tang, X.; Hou, Y.; Schwartz, T.W.; Haeggstrom, J.Z. Metabolite G-protein coupled receptor signaling: Potential regulation of eicosanoids. Biochem. Pharmacol. 2022, 204, 115208. [Google Scholar] [CrossRef]
- Lin, L.L.; Wartmann, M.; Lin, A.Y.; Knopf, J.L.; Seth, A.; Davis, R.J. cPLA2 is phosphorylated and activated by MAP kinase. Cell 1993, 72, 269–278. [Google Scholar] [CrossRef]
- Nito, C.; Kamada, H.; Endo, H.; Niizuma, K.; Myer, D.J.; Chan, P.H. Role of the p38 mitogen-activated protein kinase/cytosolic phospholipase A2 signaling pathway in blood-brain barrier disruption after focal cerebral ischemia and reperfusion. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2008, 28, 1686–1696. [Google Scholar] [CrossRef] [PubMed]
- Gijon, M.A.; Spencer, D.M.; Siddiqi, A.R.; Bonventre, J.V.; Leslie, C.C. Cytosolic phospholipase A2 is required for macrophage arachidonic acid release by agonists that Do and Do not mobilize calcium. Novel role of mitogen-activated protein kinase pathways in cytosolic phospholipase A2 regulation. J. Biol. Chem. 2000, 275, 20146–20156. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.C.; Parisi, M.; Esposito, G.; Fabbrocini, G.; Ammendola, R.; Cattaneo, F. Phosphorylation Sites in Protein Kinases and Phosphatases Regulated by Formyl Peptide Receptor 2 Signaling. Int. J. Mol. Sci. 2020, 21, 3818. [Google Scholar] [CrossRef]
- Iaccio, A.; Cattaneo, F.; Mauro, M.; Ammendola, R. FPRL1-mediated induction of superoxide in LL-37-stimulated IMR90 human fibroblast. Arch. Biochem. Biophys. 2009, 481, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.; Kretschmer, D. Formyl-Peptide Receptors in Infection, Inflammation, and Cancer. Trends Immunol. 2018, 39, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, F.; Parisi, M.; Fioretti, T.; Sarnataro, D.; Esposito, G.; Ammendola, R. Nuclear localization of Formyl-Peptide Receptor 2 in human cancer cells. Arch. Biochem. Biophys. 2016, 603, 10–19. [Google Scholar] [CrossRef] [PubMed]
- McDonald, B.; Pittman, K.; Menezes, G.B.; Hirota, S.A.; Slaba, I.; Waterhouse, C.C.; Beck, P.L.; Muruve, D.A.; Kubes, P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010, 330, 362–366. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Cattaneo, F.; Castaldo, M.; Parisi, M.; Faraonio, R.; Esposito, G.; Ammendola, R. Formyl Peptide Receptor 1 Modulates Endothelial Cell Functions by NADPH Oxidase-Dependent VEGFR2 Transactivation. Oxidative Med. Cell. Longev. 2018, 2018, 2609847. [Google Scholar] [CrossRef]
- Jang, I.H.; Heo, S.C.; Kwon, Y.W.; Choi, E.J.; Kim, J.H. Role of formyl peptide receptor 2 in homing of endothelial progenitor cells and therapeutic angiogenesis. Adv. Biol. Regul. 2015, 57, 162–172. [Google Scholar] [CrossRef]
- Cattaneo, F.; Russo, R.; Castaldo, M.; Chambery, A.; Zollo, C.; Esposito, G.; Pedone, P.V.; Ammendola, R. Phosphoproteomic analysis sheds light on intracellular signaling cascades triggered by Formyl-Peptide Receptor 2. Sci. Rep. 2019, 9, 17894. [Google Scholar] [CrossRef]
- Li, Y.; Ye, D. Molecular biology for formyl peptide receptors in human diseases. J. Mol. Med. 2013, 91, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Prevete, N.; Liotti, F.; Marone, G.; Melillo, R.M.; de Paulis, A. Formyl peptide receptors at the interface of inflammation, angiogenesis and tumor growth. Pharmacol. Res. 2015, 102, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, F.; Guerra, G.; Parisi, M.; Lucariello, A.; De Luca, A.; De Rosa, N.; Mazzarella, G.; Bianco, A.; Ammendola, R. Expression of Formyl-peptide Receptors in Human Lung Carcinoma. Anticancer. Res. 2015, 35, 2769–2774. [Google Scholar] [PubMed]
- Liang, W.; Chen, K.; Gong, W.; Yoshimura, T.; Le, Y.; Wang, Y.; Wang, J.M. The Contribution of Chemoattractant GPCRs, Formylpeptide Receptors, to Inflammation and Cancer. Front. Endocrinol. 2020, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.; Cattaneo, F.; Lippiello, P.; Cristiano, C.; Zurlo, F.; Castaldo, M.; Irace, C.; Borsello, T.; Santamaria, R.; Ammendola, R.; et al. Motor coordination and synaptic plasticity deficits are associated with increased cerebellar activity of NADPH oxidase, CAMKII, and PKC at preplaque stage in the TgCRND8 mouse model of Alzheimer’s disease. Neurobiol. Aging 2018, 68, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, F.; Guerra, G.; Ammendola, R. Expression and signaling of formyl-peptide receptors in the brain. Neurochem. Res. 2010, 35, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Busch, L.; Vieten, S.; Brodel, S.; Endres, K.; Bufe, B. Emerging contributions of formyl peptide receptors to neurodegenerative diseases. Biol. Chem. 2022, 403, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Caso, V.M.; Manzo, V.; Pecchillo Cimmino, T.; Conti, V.; Caso, P.; Esposito, G.; Russo, V.; Filippelli, A.; Ammendola, R.; Cattaneo, F. Regulation of Inflammation and Oxidative Stress by Formyl Peptide Receptors in Cardiovascular Disease Progression. Life 2021, 11, 243. [Google Scholar] [CrossRef]
- Lupisella, J.A.; Shirude, P.S.; Wurtz, N.R.; Garcia, R.A. Formyl peptide receptor 2 and heart disease. Semin. Immunol. 2022, 59, 101602. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, F.; Parisi, M.; Ammendola, R. Distinct signaling cascades elicited by different formyl peptide receptor 2 (FPR2) agonists. Int. J. Mol. Sci. 2013, 14, 7193–7230. [Google Scholar] [CrossRef] [PubMed]
- Raabe, C.A.; Groper, J.; Rescher, U. Biased perspectives on formyl peptide receptors. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 305–316. [Google Scholar] [CrossRef]
- Cooray, S.N.; Gobbetti, T.; Montero-Melendez, T.; McArthur, S.; Thompson, D.; Clark, A.J.; Flower, R.J.; Perretti, M. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc. Natl. Acad. Sci. USA 2013, 110, 18232–18237. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Wang, L.; Guo, J.; Sun, D.; Wang, Y.; Liu, W.; Xu, H.E.; Zhang, C. Molecular recognition of formylpeptides and diverse agonists by the formylpeptide receptors FPR1 and FPR2. Nat. Commun. 2022, 13, 1054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, G.; Chen, X.; Xue, X.; Guo, Q.; Liu, M.; Zhao, J. Formyl peptide receptors promotes neural differentiation in mouse neural stem cells by ROS generation and regulation of PI3K-AKT signaling. Sci. Rep. 2017, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Mottola, G.; Chatterjee, A.; Wu, B.; Chen, M.; Conte, M.S. Aspirin-triggered resolvin D1 attenuates PDGF-induced vascular smooth muscle cell migration via the cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) pathway. PLoS ONE 2017, 12, e0174936. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, F.; Iaccio, A.; Guerra, G.; Montagnani, S.; Ammendola, R. NADPH-oxidase-dependent reactive oxygen species mediate EGFR transactivation by FPRL1 in WKYMVm-stimulated human lung cancer cells. Free Radic. Biol. Med. 2011, 51, 1126–1136. [Google Scholar] [CrossRef]
- Filina, Y.; Gabdoulkhakova, A.; Rizvanov, A.; Safronova, V. MAP kinases in regulation of NOX activity stimulated through two types of formyl peptide receptors in murine bone marrow granulocytes. Cell Signal. 2022, 90, 110205. [Google Scholar] [CrossRef]
- Ammendola, R.; Parisi, M.; Esposito, G.; Cattaneo, F. Pro-Resolving FPR2 Agonists Regulate NADPH Oxidase-Dependent Phosphorylation of HSP27, OSR1, and MARCKS and Activation of the Respective Upstream Kinases. Antioxidants 2021, 10, 134. [Google Scholar] [CrossRef]
- Cattaneo, F.; Guerra, G.; Parisi, M.; De Marinis, M.; Tafuri, D.; Cinelli, M.; Ammendola, R. Cell-surface receptors transactivation mediated by g protein-coupled receptors. Int. J. Mol. Sci. 2014, 15, 19700–19728. [Google Scholar] [CrossRef]
- Castaldo, M.; Zollo, C.; Esposito, G.; Ammendola, R.; Cattaneo, F. NOX2-Dependent Reactive Oxygen Species Regulate Formyl-Peptide Receptor 1-Mediated TrkA Transactivation in SH-SY5Y Cells. Oxidative Med. Cell. Longev. 2019, 2019, 2051235. [Google Scholar] [CrossRef]
- Cattaneo, F.; Parisi, M.; Ammendola, R. WKYMVm-induced cross-talk between FPR2 and HGF receptor in human prostate epithelial cell line PNT1A. FEBS Lett. 2013, 587, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, T.P.; Pagano, E.; Stornaiuolo, M.; Esposito, G.; Ammendola, R.; Cattaneo, F. Formyl-peptide receptor 2 signalling triggers aerobic metabolism of glucose through Nox2-dependent modulation of pyruvate dehydrogenase activity. Open Biol. 2023, 13, 230336. [Google Scholar] [CrossRef] [PubMed]
- Pecchillo Cimmino, T.; Ammendola, R.; Cattaneo, F.; Esposito, G. NOX Dependent ROS Generation and Cell Metabolism. Int. J. Mol. Sci. 2023, 24, 2086. [Google Scholar] [CrossRef] [PubMed]
- Pecchillo Cimmino, T.; Pagano, E.; Stornaiuolo, M.; Esposito, G.; Ammendola, R.; Cattaneo, F. Formyl-Peptide Receptor 2 Signaling Redirects Glucose and Glutamine into Anabolic Pathways in Metabolic Reprogramming of Lung Cancer Cells. Antioxidants 2022, 11, 1692. [Google Scholar] [CrossRef] [PubMed]
- Pavone, L.M.; Cattaneo, F.; Rea, S.; De Pasquale, V.; Spina, A.; Sauchelli, E.; Mastellone, V.; Ammendola, R. Intracellular signaling cascades triggered by the NK1 fragment of hepatocyte growth factor in human prostate epithelial cell line PNT1A. Cell Signal. 2011, 23, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Lin, Y.; Zhang, H.; Liu, C.; Cheng, Z.; Yang, X.; Zhang, J.; Xiao, Y.; Sang, N.; Qian, X.; et al. Reprogramming of lipid metabolism in cancer-associated fibroblasts potentiates migration of colorectal cancer cells. Cell Death Dis. 2020, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Hanna, V.S.; Hafez, E.A.A. Synopsis of arachidonic acid metabolism: A review. J. Adv. Res. 2018, 11, 23–32. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. Br. J. Cancer 2020, 122, 4–22. [Google Scholar] [CrossRef]
- Casas, J.; Balsinde, J.; Balboa, M.A. Phosphorylation of cPLA2α at Ser505 Is Necessary for Its Translocation to PtdInsP2-Enriched Membranes. Molecules 2022, 27, 2347. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Shindou, H.; Shimizu, T. Cytosolic phospholipase A2 and lysophospholipid acyltransferases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Leslie, C.C. Cytosolic phospholipase A2: Physiological function and role in disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef] [PubMed]
- Stahelin, R.V.; Subramanian, P.; Vora, M.; Cho, W.; Chalfant, C.E. Ceramide-1-phosphate binds group IVA cytosolic phospholipase a2 via a novel site in the C2 domain. J. Biol. Chem. 2007, 282, 20467–20474. [Google Scholar] [CrossRef] [PubMed]
- Perez-Chacon, G.; Astudillo, A.M.; Balgoma, D.; Balboa, M.A.; Balsinde, J. Control of free arachidonic acid levels by phospholipases A2 and lysophospholipid acyltransferases. Biochim. Biophys. Acta 2009, 1791, 1103–1113. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, Z.; Liu, H.; Xia, Y.F.; Liu, X.M.; Luo, B.B.; Wang, W.K.; Li, B.; Gao, F.; Zhang, C.; et al. Serum amyloid A stimulates lipoprotein-associated phospholipase A2 expression in vitro and in vivo. Atherosclerosis 2013, 228, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Lyngstadaas, A.V.; Olsen, M.V.; Bair, J.A.; Hodges, R.R.; Utheim, T.P.; Serhan, C.N.; Dartt, D.A. Pro-Resolving Mediator Annexin A1 Regulates Intracellular Ca2+ and Mucin Secretion in Cultured Goblet Cells Suggesting a New Use in Inflammatory Conjunctival Diseases. Front. Immunol. 2021, 12, 618653. [Google Scholar] [CrossRef]
- Bae, Y.S.; Park, J.C.; He, R.; Ye, R.D.; Kwak, J.Y.; Suh, P.G.; Ho Ryu, S. Differential signaling of formyl peptide receptor-like 1 by Trp-Lys-Tyr-Met-Val-Met-CONH2 or lipoxin A4 in human neutrophils. Mol. Pharmacol. 2003, 64, 721–730. [Google Scholar] [CrossRef]
- Zhu, D.; Lai, Y.; Shelat, P.B.; Hu, C.; Sun, G.Y.; Lee, J.C. Phospholipases A2 mediate amyloid-β peptide-induced mitochondrial dysfunction. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 11111–11119. [Google Scholar] [CrossRef]
- Pessach, I.; Leto, T.L.; Malech, H.L.; Levy, R. Essential requirement of cytosolic phospholipase A2 for stimulation of NADPH oxidase-associated diaphorase activity in granulocyte-like cells. J. Biol. Chem. 2001, 276, 33495–33503. [Google Scholar] [CrossRef]
- Cherny, V.V.; Henderson, L.M.; Xu, W.; Thomas, L.L.; DeCoursey, T.E. Activation of NADPH oxidase-related proton and electron currents in human eosinophils by arachidonic acid. J. Physiol. 2001, 535, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Bey, E.A.; Wientjes, F.B.; Cathcart, M.K. Cytosolic phospholipase A2 (cPLA2) regulation of human monocyte NADPH oxidase activity. cPLA2 affects translocation but not phosphorylation of p67phox and p47phox. J. Biol. Chem. 2002, 277, 25385–25392. [Google Scholar] [CrossRef] [PubMed]
- Shmelzer, Z.; Haddad, N.; Admon, E.; Pessach, I.; Leto, T.L.; Eitan-Hazan, Z.; Hershfinkel, M.; Levy, R. Unique targeting of cytosolic phospholipase A2 to plasma membranes mediated by the NADPH oxidase in phagocytes. J. Cell Biol. 2003, 162, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Horrocks, L.A.; Farooqui, A.A. The roles of NADPH oxidase and phospholipases A2 in oxidative and inflammatory responses in neurodegenerative diseases. J. Neurochem. 2007, 103, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Lin, C.C.; Lee, I.T.; Lee, H.C.; Yang, C.M. Activation and induction of cytosolic phospholipase A2 by TNF-α mediated through Nox2, MAPKs, NF-κB, and p300 in human tracheal smooth muscle cells. J. Cell. Physiol. 2011, 226, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Chakraborti, S.; Roy, S.; Mandal, A.; Dey, K.; Chowdhury, A.; Shaikh, S.; Chakraborti, T. Role of PKCα-p38MAPK-Giα axis in NADPH oxidase derived O2·−-mediated activation of cPLA2 under U46619 stimulation in pulmonary artery smooth muscle cells. Arch. Biochem. Biophys. 2012, 523, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Daub, H.; Weiss, F.U.; Wallasch, C.; Ullrich, A. Role of transactivation of the EGF receptor in signalling by G-protein-coupled receptors. Nature 1996, 379, 557–560. [Google Scholar] [CrossRef]
- Korbecki, J.; Baranowska-Bosiacka, I.; Gutowska, I.; Chlubek, D. The effect of reactive oxygen species on the synthesis of prostanoids from arachidonic acid. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2013, 64, 409–421. [Google Scholar]
- Kefaloyianni, E.; Gaitanaki, C.; Beis, I. ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-κB transactivation during oxidative stress in skeletal myoblasts. Cell Signal. 2006, 18, 2238–2251. [Google Scholar] [CrossRef]
- Werz, O.; Burkert, E.; Fischer, L.; Szellas, D.; Dishart, D.; Samuelsson, B.; Radmark, O.; Steinhilber, D. 5-Lipoxygenase activation by MAPKAPK-2 and ERKs. Adv. Exp. Med. Biol. 2003, 525, 129–132. [Google Scholar] [CrossRef]
- Wan, M.; Tang, X.; Stsiapanava, A.; Haeggstrom, J.Z. Biosynthesis of leukotriene B4. Semin. Immunol. 2017, 33, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Srivastava, M.; Ahmad, N.; Sakamoto, K.; Bostwick, D.G.; Mukhtar, H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer 2001, 91, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Hennig, R.; Grippo, P.; Ding, X.Z.; Rao, S.M.; Buchler, M.W.; Friess, H.; Talamonti, M.S.; Bell, R.H.; Adrian, T.E. 5-Lipoxygenase, a marker for early pancreatic intraepithelial neoplastic lesions. Cancer Res. 2005, 65, 6011–6016. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dubois, R.N. Eicosanoids and cancer. Nat. Rev. Cancer 2010, 10, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Skibbe, J.R.; Hu, C.; Dong, L.; Ferchen, K.; Su, R.; Li, C.; Huang, H.; Weng, H.; Huang, H.; et al. ALOX5 exhibits anti-tumor and drug-sensitizing effects in MLL-rearranged leukemia. Sci. Rep. 2017, 7, 1853. [Google Scholar] [CrossRef]
- Mashima, R.; Okuyama, T. The role of lipoxygenases in pathophysiology; new insights and future perspectives. Redox Biol. 2015, 6, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xie, H.; Liu, Y.; Ou, Q.; Deng, S. Interference of ALOX5 alleviates inflammation and fibrosis in high glucose-induced renal mesangial cells. Exp. Ther. Med. 2023, 25, 34. [Google Scholar] [CrossRef] [PubMed]
- Peters-Golden, M.; Brock, T.G. Intracellular compartmentalization of leukotriene biosynthesis. Am. J. Respir. Crit. Care Med. 2000, 161, S36–S40. [Google Scholar] [CrossRef]
- Radmark, O.P. The molecular biology and regulation of 5-lipoxygenase. Am. J. Respir. Crit. Care Med. 2000, 161, S11–S15. [Google Scholar] [CrossRef]
- Hammarberg, T.; Provost, P.; Persson, B.; Radmark, O. The N-terminal domain of 5-lipoxygenase binds calcium and mediates calcium stimulation of enzyme activity. J. Biol. Chem. 2000, 275, 38787–38793. [Google Scholar] [CrossRef]
- Radmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase, a key enzyme for leukotriene biosynthesis in health and disease. Biochim. Biophys. Acta 2015, 1851, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Jones, S.M.; Phare, S.M.; Coffey, M.J.; Peters-Golden, M.; Brock, T.G. Protein kinase A inhibits leukotriene synthesis by phosphorylation of 5-lipoxygenase on serine 523. J. Biol. Chem. 2004, 279, 41512–41520. [Google Scholar] [CrossRef]
- Luo, M.; Jones, S.M.; Flamand, N.; Aronoff, D.M.; Peters-Golden, M.; Brock, T.G. Phosphorylation by protein kinase a inhibits nuclear import of 5-lipoxygenase. J. Biol. Chem. 2005, 280, 40609–40616. [Google Scholar] [CrossRef] [PubMed]
- Werz, O.; Klemm, J.; Samuelsson, B.; Radmark, O. 5-lipoxygenase is phosphorylated by p38 kinase-dependent MAPKAP kinases. Proc. Natl. Acad. Sci. USA 2000, 97, 5261–5266. [Google Scholar] [CrossRef] [PubMed]
- Werz, O.; Szellas, D.; Steinhilber, D.; Radmark, O. Arachidonic acid promotes phosphorylation of 5-lipoxygenase at Ser-271 by MAPK-activated protein kinase 2 (MK2). J. Biol. Chem. 2002, 277, 14793–14800. [Google Scholar] [CrossRef] [PubMed]
- Werz, O.; Burkert, E.; Fischer, L.; Szellas, D.; Dishart, D.; Samuelsson, B.; Radmark, O.; Steinhilber, D. Extracellular signal-regulated kinases phosphorylate 5-lipoxygenase and stimulate 5-lipoxygenase product formation in leukocytes. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2002, 16, 1441–1443. [Google Scholar] [CrossRef] [PubMed]
- Hazan-Halevy, I.; Seger, R.; Levy, R. The requirement of both extracellular regulated kinase and p38 mitogen-activated protein kinase for stimulation of cytosolic phospholipase A2 activity by either FcgammaRIIA or FcgammaRIIIB in human neutrophils. A possible role for Pyk2 but not for the Grb2-Sos-Shc complex. J. Biol. Chem. 2000, 275, 12416–12423. [Google Scholar] [CrossRef]
- Radmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-Lipoxygenase: Regulation of expression and enzyme activity. Trends Biochem. Sci. 2007, 32, 332–341. [Google Scholar] [CrossRef]
- Cho, K.J.; Seo, J.M.; Kim, J.H. Bioactive lipoxygenase metabolites stimulation of NADPH oxidases and reactive oxygen species. Mol. Cells 2011, 32, 1–5. [Google Scholar] [CrossRef]
- Werz, O.; Burkert, E.; Samuelsson, B.; Radmark, O.; Steinhilber, D. Activation of 5-lipoxygenase by cell stress is calcium independent in human polymorphonuclear leukocytes. Blood 2002, 99, 1044–1052. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).