Micromolar Dihydroartemisinin Concentrations Elicit Lipoperoxidation in Plasmodium falciparum-Infected Erythrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Growth of Plasmodium falciparum and Life-Stage-Specific Enrichment of Parasitized Erythrocytes
2.2. DHA and N-Acetylcysteine (NAC) Treatment of Infected and Non-Infected Erythrocytes
2.3. Morphological Analysis of DHA-Treated Infected Erythrocytes by Light Microscopy
2.4. Parasite Growth and Damage Assessment by Flow Cytometry (FACS)
2.5. Hemoglobin Quantification
2.6. Quantification of Hemozoin Generation
2.7. Assay of ROS in Infected and Non-Infected Erythrocytes with DCF-DA and DHE by FACS
2.8. Assay of 4-HNE Conjugates in Infected and Non-Infected Erythrocytes by FACS
2.9. Assay of 4-HNE Conjugates in Whole Protein Lysate
2.10. Identification of 4-HNE-Modified Proteins and 4-HNE Binding Sites by Mass Spectrometry
2.11. Statistical Analysis
3. Results
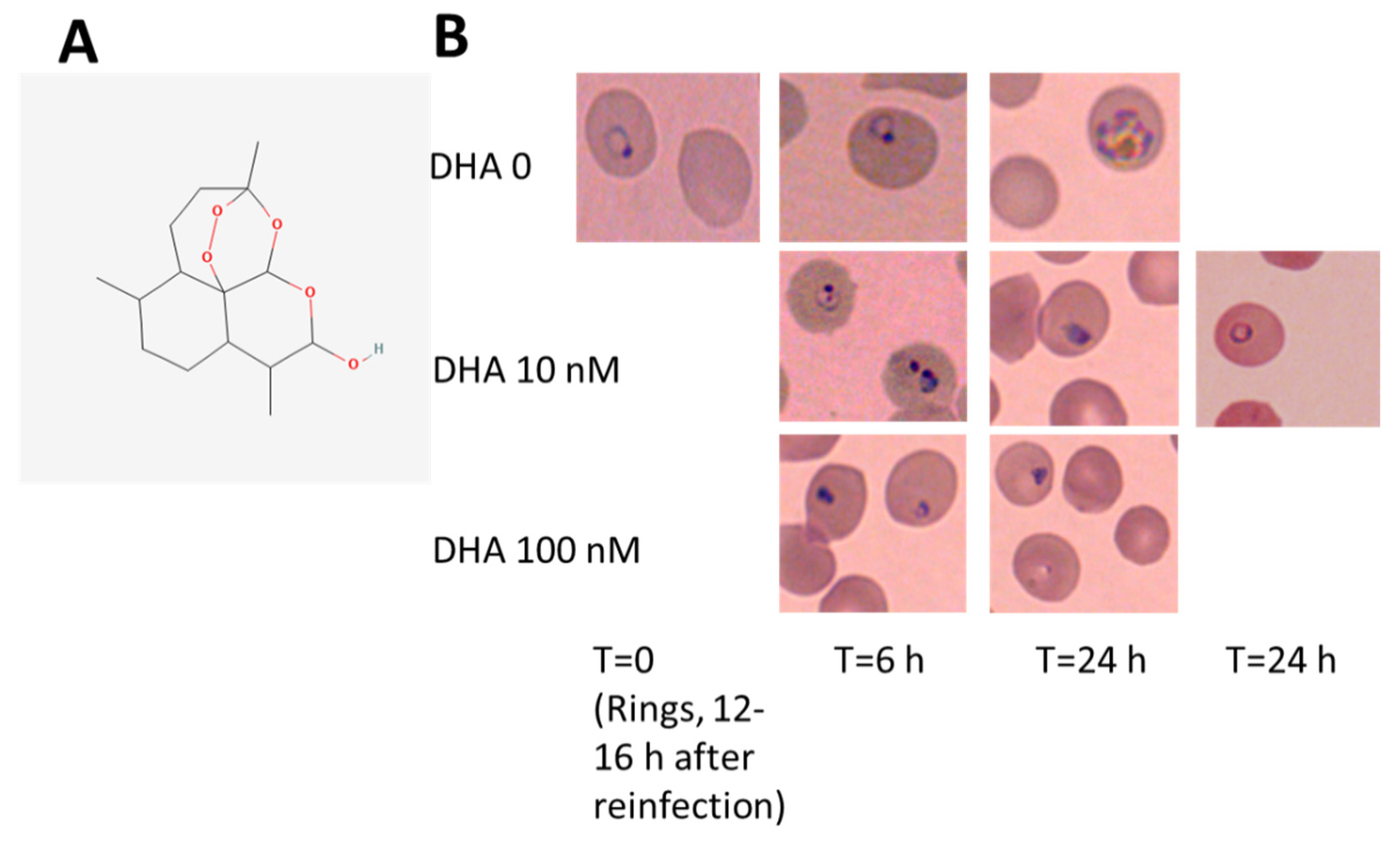
3.1. DHA Affects Ring-Stage P.f. Development and Impairs Reinfection
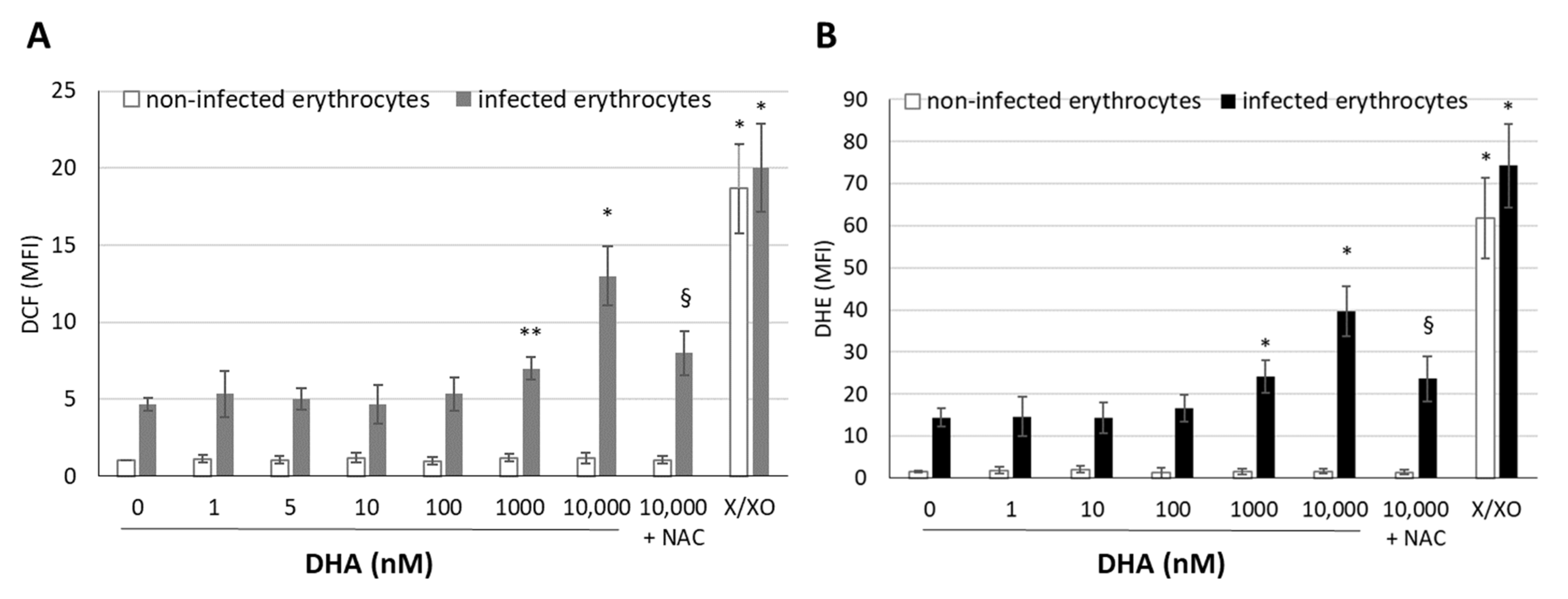
3.2. DHA Generates Free Radicals in Infected Erythrocytes
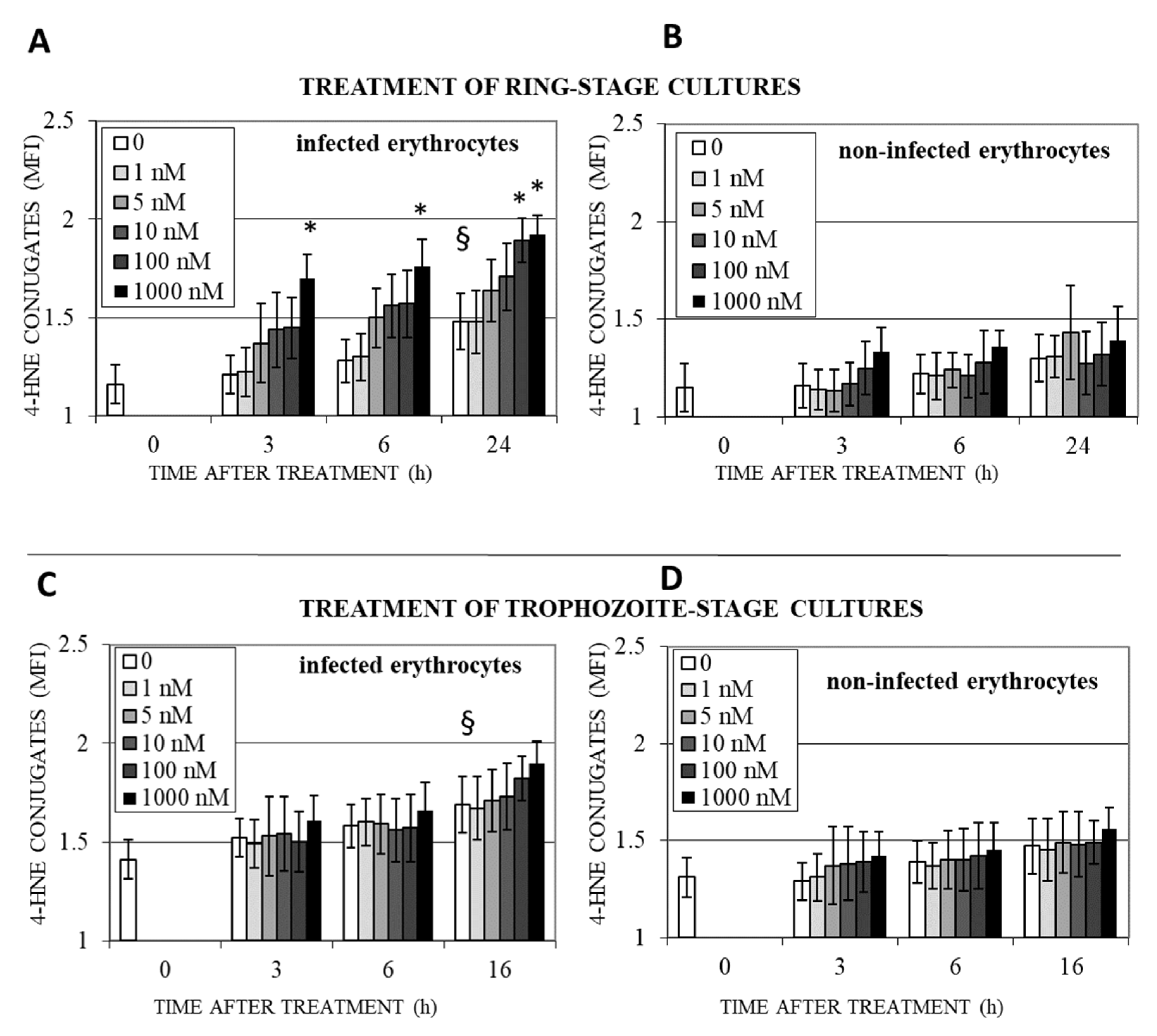
3.3. DHA Induces Lipoperoxidation and 4-HNE-Protein Conjugation in Infected Erythrocytes
3.4. Protein Targets of 4-HNE and Their Potential Functional Impact
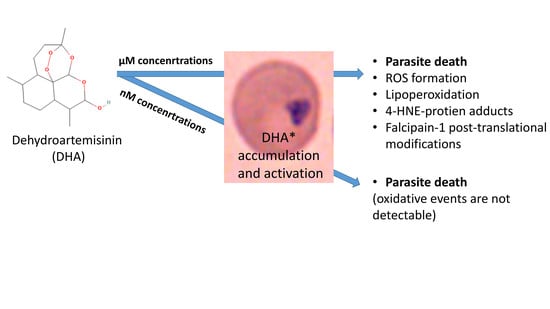
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meshnick, S.R.; Dobson, M.J. The History of Antimalarial Drugs. In Antimalarial Chemotherapy. Infectious Disease; Rosenthal, P.J., Ed.; Humana Press: Totowa, NJ, USA, 2001. [Google Scholar]
- Senok, A.; Nelson, E.; Li, K.; Oppenheimer, S. Thalassaemia trait, red blood cell age and oxidant stress: Effects on Plasmodium falciparum growth and sensitivity to artemisinin. Trans. R. Soc. Trop. Med. Hyg. 1997, 91, 585–589. [Google Scholar] [CrossRef]
- Bozdech, Z.; Ginsburg, H. Antioxidant defense in Plasmodium falciparum—Data mining of the transcriptome. Malar. J. 2004, 3, 23. [Google Scholar] [CrossRef] [PubMed]
- Müller, S. Redox and antioxidant systems of the malaria parasite Plasmodium falciparum. Mol. Microbiol. 2004, 53, 1291–1305. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Tilley, L.; Kenny, S.; Klonis, N. Dual labeling with a far red probe permits analysis of growth and oxidative stress in P. falciparum-infected erythrocytes. Cytom. Part A 2010, 77, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Buffinton, G.D.; Hunt, N.H.; Cowden, W.B.; Clark, I.A. Detection of short-chain carbonyl products of lipid peroxidation from malaria-parasite (Plasmodium vinckei)-infected red blood cells exposed to oxidative stress. Biochem. J. 1988, 249, 63–68. [Google Scholar] [CrossRef]
- Schwarzer, E.; Kühn, H.; Valente, E.; Arese, P. Malaria-parasitized erythrocytes and hemozoin nonenzymatically generate large amounts of hydroxy fatty acids that inhibit monocyte functions. Blood 2003, 101, 722–728. [Google Scholar] [CrossRef]
- Uyoga, S.; Skorokhod, O.A.; Opiyo, M.; Orori, E.N.; Williams, T.N.; Arese, P.; Schwarzer, E. Transfer of 4-hydroxynonenal from parasitized to non-parasitized erythrocytes in rosettes. Proposed role in severe malaria anemia. Br. J. Haematol. 2012, 157, 116–124. [Google Scholar] [CrossRef]
- Wagner, M.P.; Chitnis, C.E. Lipid peroxidation and its repair in malaria parasites. Trends Parasitol. 2023, 39, 200–211. [Google Scholar] [CrossRef]
- Crespo, M.d.P.; Avery, T.D.; Hanssen, E.; Fox, E.; Robinson, T.V.; Valente, P.; Taylor, D.K.; Tilley, L. Artemisinin and a Series of Novel Endoperoxide Antimalarials Exert Early Effects on Digestive Vacuole Morphology. Antimicrob. Agents Chemother. 2008, 52, 98–109. [Google Scholar] [CrossRef]
- Vennerstrom, J.L.; Eaton, J.W. Oxidants, oxidant drugs, and malaria. J. Med. Chem. 1988, 31, 1269–1277. [Google Scholar] [CrossRef]
- Armstrong, J.F.; Campo, B.; Alexander, S.P.H.; Arendse, L.B.; Cheng, X.; Davenport, A.P.; Faccenda, E.; Fidock, D.A.; Godinez-Macias, K.P.; Harding, S.D.; et al. Advances in Malaria Pharmacology and the online Guide to MALARIA PHARMACOLOGY: IUPHAR Review X. Br. J. Pharmacol. 2023, 180, 1899–1929. [Google Scholar] [CrossRef]
- Janse, C.; Waters, A.; Kos, J.; Lugt, C. Comparison of in vivo and in vitro antimalarial activity of artemisinin, dihydroartemisinin and sodium artesunate in the Plasmodium berghei-rodent model. Int. J. Parasitol. 1994, 24, 589–594. [Google Scholar] [CrossRef]
- Ringwald, P.; Basco, L.K.; Bickii, J. In vitro activity of dihydroartemisinin against clinical isolates of Plasmodium falciparum in Yaounde, Cameroon. Am. J. Trop. Med. Hyg. 1999, 61, 187–192. [Google Scholar] [CrossRef]
- Antoine, T.; Fisher, N.; Amewu, R.; O’Neill, P.M.; Ward, S.A.; Biagini, G.A. Rapid kill of malaria parasites by artemisinin and semi-synthetic endoperoxides involves ROS-dependent depolarization of the membrane potential. J. Antimicrob. Chemother. 2014, 69, 1005–1016. [Google Scholar] [CrossRef]
- Tilley, L.; Straimer, J.; Gnädig, N.F.; Ralph, S.A.; Fidock, D.A. Artemisinin Action and Resistance in Plasmodium falciparum. Trends Parasitol. 2016, 32, 682–696. [Google Scholar] [CrossRef]
- Talman, A.M.; Clain, J.; Duval, R.; Ménard, R.; Ariey, F. Artemisinin Bioactivity and Resistance in Malaria Parasites. Trends Parasitol. 2019, 35, 953–963. [Google Scholar] [CrossRef]
- Kavishe, R.A.; Koenderink, J.B.; Alifrangis, M. Oxidative stress in malaria and artemisinin combination therapy: Pros and Cons. FEBS J. 2017, 284, 2579–2591. [Google Scholar] [CrossRef]
- Haynes, R.; Cheu, K.-W.; N’Da, D.; Coghi, P.; Monti, D. Considerations on the Mechanism of Action of Artemisinin Antimalarials: Part 1-The ‘Carbon Radical’ and ‘Heme’ Hypotheses. Infect. Disord.-Drug Targets 2013, 13, 217–277. [Google Scholar] [CrossRef]
- Berman, P.A.; Adams, P.A. Artemisinin Enhances Heme-Catalysed Oxidation of Lipid Membranes. Free Radic. Biol. Med. 1997, 22, 1283–1288. [Google Scholar] [CrossRef]
- Hartwig, C.L.; Rosenthal, A.S.; D’angelo, J.; Griffin, C.E.; Posner, G.H.; Cooper, R.A. Accumulation of artemisinin trioxane derivatives within neutral lipids of Plasmodium falciparum malaria parasites is endoperoxide-dependent. Biochem. Pharmacol. 2009, 77, 322–336. [Google Scholar] [CrossRef]
- Parapini, S.; Basilico, N.; Mondani, M.; Olliaro, P.; Taramelli, D.; Monti, D. Evidence that haem iron in the malaria parasite is not needed for the antimalarial effects of artemisinin. FEBS Lett. 2004, 575, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.-J.; Ni Chia, W.; Loh, C.C.Y.; Li, Z.; Lee, Y.M.; He, Y.; Yuan, L.-X.; Lim, T.K.; Liu, M.; et al. Haem-activated promiscuous targeting of artemisinin in Plasmodium falciparum. Nat. Commun. 2015, 6, 10111. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.M.; Barton, V.; Phanchana, M.; Charoensutthivarakul, S.; Wong, M.H.L.; Hemingway, J.; Biagini, G.A.; O’neill, P.M.; Ward, S.A. Artemisinin activity-based probes identify multiple molecular targets within the asexual stage of the malaria parasites Plasmodium falciparum 3D7. Proc. Natl. Acad. Sci. USA 2016, 113, 2080–2085. [Google Scholar] [CrossRef] [PubMed]
- Eckstein-Ludwig, U.; Webb, R.J.; van Goethem, I.D.A.; East, J.M.; Lee, A.G.; Kimura, M.; O’Neill, P.M.; Bray, P.G.; Ward, S.A.; Krishna, S. Artemisinins target the SERCA of Plasmodium falciparum. Nature 2003, 424, 957–961. [Google Scholar] [CrossRef]
- Uhlemann, A.-C.; Cameron, A.; Eckstein-Ludwig, U.; Fischbarg, J.; Iserovich, P.; Zuniga, F.A.; East, M.; Lee, A.; Brady, L.; Haynes, R.K.; et al. A single amino acid residue can determine the sensitivity of SERCAs to artemisinins. Nat. Struct. Mol. Biol. 2005, 12, 628–629. [Google Scholar] [CrossRef]
- Grazzia, N.; Boaventura, S., Jr.; Garcia, V.L.; Gadelha, F.R.; Miguel, D.C. Dihydroartemisinin, an active metabolite of artemisinin, interferes with Leishmania braziliensis mitochondrial bioenergetics and survival. Parasitol. Res. 2021, 120, 705–713. [Google Scholar] [CrossRef]
- Yu, R.; Jin, G.; Fujimoto, M. Dihydroartemisinin: A Potential Drug for the Treatment of Malignancies and Inflammatory Diseases. Front. Oncol. 2021, 11, 722331. [Google Scholar] [CrossRef]
- Guan, L.; Wang, H.; Xu, X.; Fan, H. Therapeutical Utilization and Repurposing of Artemisinin and Its Derivatives: A Narrative Review. Adv. Biol. 2023, 13, e2300086. [Google Scholar] [CrossRef]
- Bader, S.; Wilmers, J.; Pelzer, M.; Jendrossek, V.; Rudner, J. Activation of anti-oxidant Keap1/Nrf2 pathway modulates efficacy of dihydroartemisinin-based monotherapy and combinatory therapy with ionizing radiation. Free Radic. Biol. Med. 2021, 168, 44–54. [Google Scholar] [CrossRef]
- Posadino, A.M.; Giordo, R.; Pintus, G.; Mohammed, S.A.; Orhan, I.E.; Fokou, P.V.T.; Sharopov, F.; Adetunji, C.O.; Gulsunoglu-Konuskan, Z.; Ydyrys, A.; et al. Medicinal and mechanistic overview of artemisinin in the treatment of human diseases. Biomed. Pharmacother. 2023, 163, 114866. [Google Scholar] [CrossRef]
- Skorokhod, O.A.; Davalos-Schafler, D.; Gallo, V.; Valente, E.; Ulliers, D.; Notarpietro, A.; Mandili, G.; Novelli, F.; Persico, M.; Taglialatela-Scafati, O.; et al. Oxidative stress-mediated antimalarial activity of plakortin, a natural endoperoxide from the tropical sponge Plakortis simplex. Free Radic. Biol. Med. 2015, 89, 624–637. [Google Scholar] [CrossRef]
- Gallo, V.; Skorokhod, O.A.; Schwarzer, E.; Arese, P. Simultaneous determination of phagocytosis of Plasmodium falciparum-parasitized and non-parasitized red blood cells by flow cytometry. Malar. J. 2012, 11, 428. [Google Scholar] [CrossRef]
- Schwarzer, E.; Turrini, F.; Arese, P. A luminescene method for the quantitative determination of phagocytosis of erythrocytes, of malaria-parasitized eryithrocytes and of malarial pigment. Br. J. Haematol. 1994, 88, 740–745. [Google Scholar] [CrossRef]
- Berneburg, I.; Peddibhotla, S.; Heimsch, K.C.; Haeussler, K.; Maloney, P.; Gosalia, P.; Preuss, J.; Rahbari, M.; Skorokhod, O.; Valente, E.; et al. An Optimized Dihydrodibenzothiazepine Lead Compound (SBI-0797750) as a Potent and Selective Inhibitor of Plasmodium falciparum and P. vivax Glucose 6-Phosphate Dehydrogenase 6-Phosphogluconolactonase. Antimicrob. Agents Chemother. 2022, 66, e0210921. [Google Scholar] [CrossRef]
- Pantaleo, A.; Kesely, K.R.; Pau, M.C.; Tsamesidis, I.; Schwarzer, E.; Skorokhod, O.A.; Chien, H.D.; Ponzi, M.; Bertuccini, L.; Low, P.S.; et al. Syk inhibitors interfere with erythrocyte membrane modification during P falciparum growth and suppress parasite egress. Blood 2017, 130, 1031–1040. [Google Scholar] [CrossRef]
- Amer, J.; Ghoti, H.; Rachmilewitz, E.; Koren, A.; Levin, C.; Fibach, E. Red blood cells, platelets and polymorphonuclear neutrophils of patients with sickle cell disease exhibit oxidative stress that can be ameliorated by antioxidants. Br. J. Haematol. 2006, 132, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.S.; Ballou, D.P.; Palmer, G.; Massey, V. The Mechanism of Action of Xanthine Oxidase. J. Biol. Chem. 1974, 249, 4363–4382. [Google Scholar] [CrossRef]
- Aguilar, R.; Marrocco, T.; Skorokhod, O.A.; Barbosa, A.; Nhabomba, A.; Manaca, M.N.; Guinovart, C.; Quintó, L.; Arese, P.; Alonso, P.L.; et al. Blood oxidative stress markers and Plasmodium falciparum malaria in non-immune African children. Br. J. Haematol. 2014, 164, 438–450. [Google Scholar] [CrossRef]
- Gallo, V.; Skorokhod, O.; Simula, L.F.; Marrocco, T.; Tambini, E.; Schwarzer, E.; Marget, P.; Duc, G.; Arese, P. No red blood cell damage and no hemolysis in G6PD-deficient subjects after ingestion of low vicine/convicine Vicia faba seeds. Blood 2018, 131, 1621–1625. [Google Scholar] [CrossRef]
- Skorokhod, O.; Barrera, V.; Mandili, G.; Costanza, F.; Valente, E.; Ulliers, D.; Schwarzer, E. Malaria Pigment Hemozoin Impairs GM-CSF Receptor Expression and Function by 4-Hydroxynonenal. Antioxidants 2021, 10, 1259. [Google Scholar] [CrossRef]
- Bergandi, L.; Skorokhod, O.A.; La Grotta, R.; Schwarzer, E.; Nuzzi, R. Oxidative Stress, Lipid Peroxidation, and Loss of Hyaluronic Acid in the Human Vitreous Affected by Synchysis Scintillans. J. Ophthalmol. 2019, 2019, 7231015. [Google Scholar] [CrossRef] [PubMed]
- Skorokhod, O.A.; Barrera, V.; Heller, R.; Carta, F.; Turrini, F.; Arese, P.; Schwarzer, E. Malarial pigment hemozoin impairs chemotactic motility and transendothelial migration of monocytes via 4-hydroxynonenal. Free Radic. Biol. Med. 2014, 75, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Skorokhod, A.; Schwarzer, E.; Gremo, G.; Arese, P. HNE produced by the malaria parasite Plasmodium falciparum generates HNE-protein adducts and decreases erythrocyte deformability. Redox Rep. 2007, 12, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Bridgford, J.L.; Xie, S.C.; Cobbold, S.A.; Pasaje, C.F.A.; Herrmann, S.; Yang, T.; Gillett, D.L.; Dick, L.R.; Ralph, S.A.; Dogovski, C.; et al. Artemisinin kills malaria parasites by damaging proteins and inhibiting the proteasome. Nat. Commun. 2018, 9, 3801. [Google Scholar] [CrossRef] [PubMed]
- Herbas, M.S.; Shichiri, M.; Ishida, N.; Kume, A.; Hagihara, Y.; Yoshida, Y.; Suzuki, H. Probucol-Induced α-Tocopherol Deficiency Protects Mice against Malaria Infection. PLoS ONE 2015, 10, e0136014. [Google Scholar] [CrossRef]
- Jaganjac, M.; Cindrić, M.; Jakovčević, A.; Žarković, K.; Žarković, N. Lipid peroxidation in brain tumors. Neurochem. Int. 2021, 149, 105118. [Google Scholar] [CrossRef]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef]
- Jedeszko, C.; Sloane, B.F. Cysteine cathepsins in human cancer. Biol. Chem. 2004, 385, 1017–1027. [Google Scholar] [CrossRef]
- Senjor, E.; Kos, J.; Nanut, M.P. Cysteine Cathepsins as Therapeutic Targets in Immune Regulation and Immune Disorders. Biomedicines 2023, 11, 476. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Liu, Y.; Qiao, Z.; Bai, T.; Yang, L.; Liu, B. Dihydroartemisinin triggers ferroptosis in primary liver cancer cells by promoting and unfolded protein response-induced upregulation of CHAC1 expression. Oncol. Rep. 2021, 46, 240. [Google Scholar] [CrossRef]
- Golizeh, M.; Geib, T.; Sleno, L. Identification of 4-hydroxynonenal protein targets in rat, mouse and human liver microsomes by two-dimensional liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 1488–1494. [Google Scholar] [CrossRef]
- Skorokhod, O.; Triglione, V.; Barrera, V.; Di Nardo, G.; Valente, E.; Ulliers, D.; Schwarzer, E.; Gilardi, G. Posttranslational Modification of Human Cytochrome CYP4F11 by 4-Hydroxynonenal Impairs ω-Hydroxylation in Malaria Pigment Hemozoin-Fed Monocytes: The Role in Malaria Immunosuppression. Int. J. Mol. Sci. 2023, 24, 10232. [Google Scholar] [CrossRef]
- Sijwali, P.S.; Kato, K.; Seydel, K.B.; Gut, J.; Lehman, J.; Klemba, M.; Goldberg, D.E.; Miller, L.H.; Rosenthal, P.J. Plasmodium falciparum cysteine protease falcipain-1 is not essential in erythrocytic stage malaria parasites. Proc. Natl. Acad. Sci. USA 2004, 101, 8721–8726. [Google Scholar] [CrossRef]
- Rosenthal, P.J. Falcipain cysteine proteases of malaria parasites: An update. Biochim. Biophys. Acta Proteins Proteom 2020, 1868, 140362. [Google Scholar] [CrossRef]
- Greenbaum, D.C.; Baruch, A.; Grainger, M.; Bozdech, Z.; Medzihradszky, K.F.; Engel, J.; DeRisi, J.; Holder, A.A.; Bogyo, M. A Role for the Protease Falcipain 1 in Host Cell Invasion by the Human Malaria Parasite. Science 2002, 298, 2002–2006. [Google Scholar] [CrossRef]


| 4-HNE Binding Site (UniProt) | Oxidation (UniProt) | Stage-Dependent Expression/Assessment | Known Molecular Function (UniProt) | Amino Acid Residues in Cysteine Proteases Corresponding to 4-HNE Binding Sites in Falcipain-1 |
|---|---|---|---|---|
| K88, K126, K147, K209, K211, K224/K227, K288/K300, H292, H296, K375/K390, C388, K436, K446 | M204, M208, M243, M297, M545 | Whole parasite cycle including blood stage/transcriptome | Cysteine-type peptidase activity | P.f. falcipain-2: C316 (C388), K365 (K436); P.f. falcipain 3: K105 (K147), H238 (H292), K311 (K375), C324 (C388); Human cathepsin K: H99 (H296), C170 (C388); Human cathepsin B: C141 (C388); Human cathepsin S: C170 (C388); |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skorokhod, O.; Valente, E.; Mandili, G.; Ulliers, D.; Schwarzer, E. Micromolar Dihydroartemisinin Concentrations Elicit Lipoperoxidation in Plasmodium falciparum-Infected Erythrocytes. Antioxidants 2023, 12, 1468. https://doi.org/10.3390/antiox12071468
Skorokhod O, Valente E, Mandili G, Ulliers D, Schwarzer E. Micromolar Dihydroartemisinin Concentrations Elicit Lipoperoxidation in Plasmodium falciparum-Infected Erythrocytes. Antioxidants. 2023; 12(7):1468. https://doi.org/10.3390/antiox12071468
Chicago/Turabian StyleSkorokhod, Oleksii, Elena Valente, Giorgia Mandili, Daniela Ulliers, and Evelin Schwarzer. 2023. "Micromolar Dihydroartemisinin Concentrations Elicit Lipoperoxidation in Plasmodium falciparum-Infected Erythrocytes" Antioxidants 12, no. 7: 1468. https://doi.org/10.3390/antiox12071468
APA StyleSkorokhod, O., Valente, E., Mandili, G., Ulliers, D., & Schwarzer, E. (2023). Micromolar Dihydroartemisinin Concentrations Elicit Lipoperoxidation in Plasmodium falciparum-Infected Erythrocytes. Antioxidants, 12(7), 1468. https://doi.org/10.3390/antiox12071468