Manoalide Induces Intrinsic Apoptosis by Oxidative Stress and Mitochondrial Dysfunction in Human Osteosarcoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Annexin V-FITC/Propidium Iodide (PI)-PE Staining
2.5. ROS Measurement
2.5.1. Mitochondrial ROS
2.5.2. Intracellular ROS
2.5.3. CellROX® Green Staining
2.6. Seahorse Real-Time Cell Metabolic Analysis
2.7. Measurement of ΔΨm
2.7.1. DiOC6 Staining
2.7.2. JC-1 Kit
2.8. Western Blotting
2.9. Statistical Analysis
3. Results
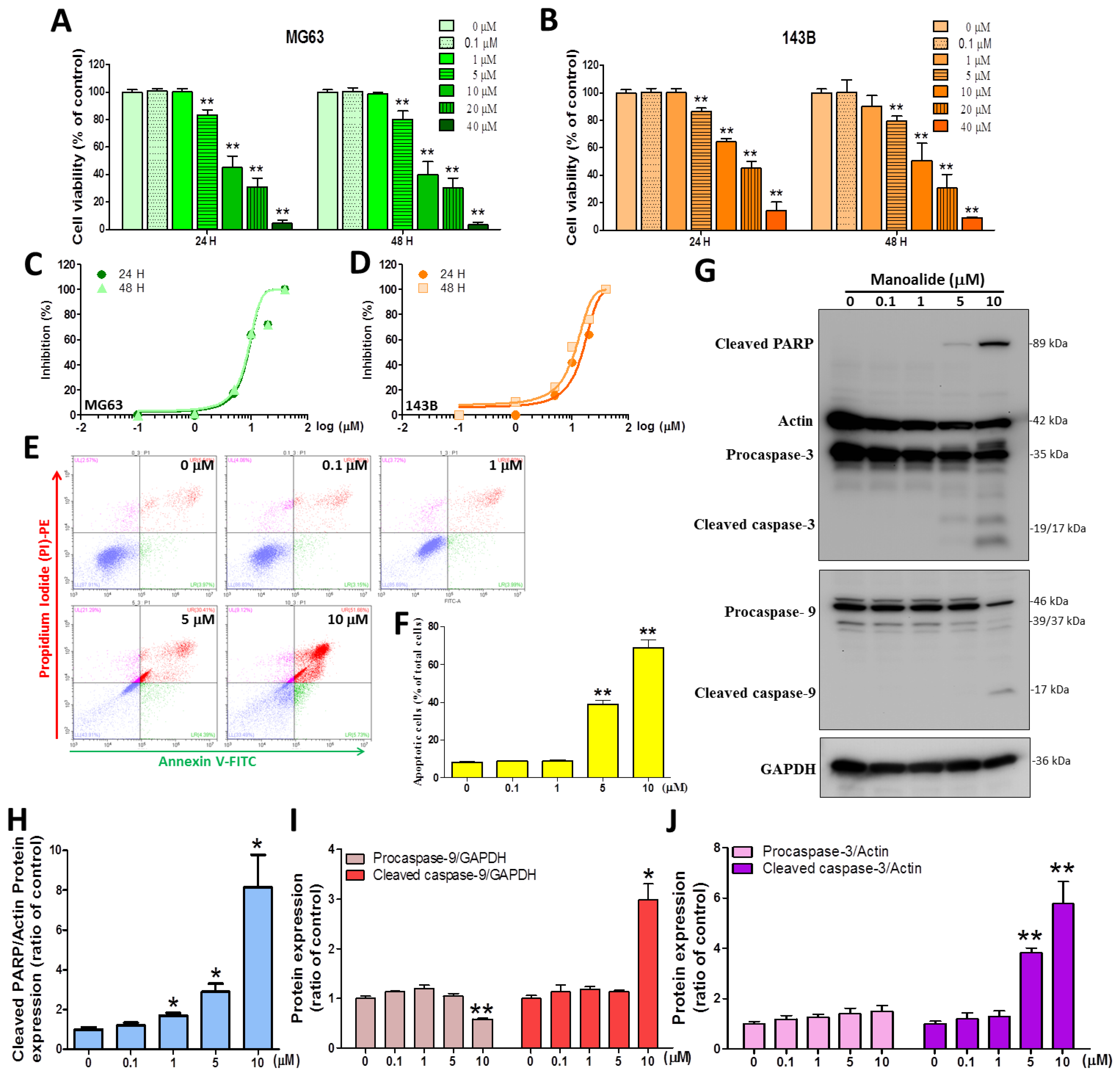
3.1. Manoalide Significantly Induces Cytotoxicity and Apoptosis through DNA Fragmentation and Intrinsic Caspase Activation in OS Cells
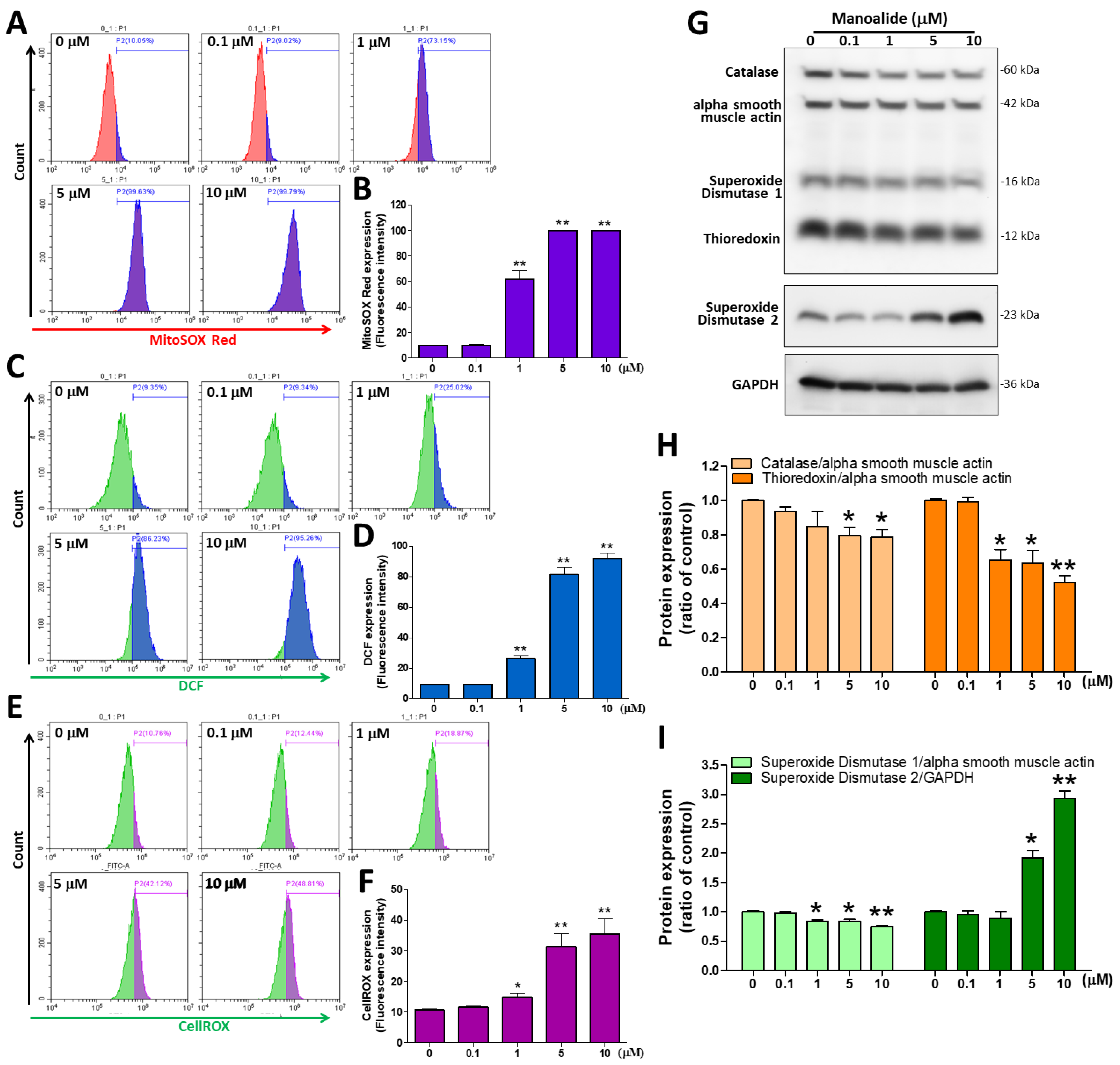
3.2. Manoalide Treatment Increased Intracellular, Mitochondrial, and Total ROS Levels but Decreased Oxidative Stress Defense Enzyme Expression in OS
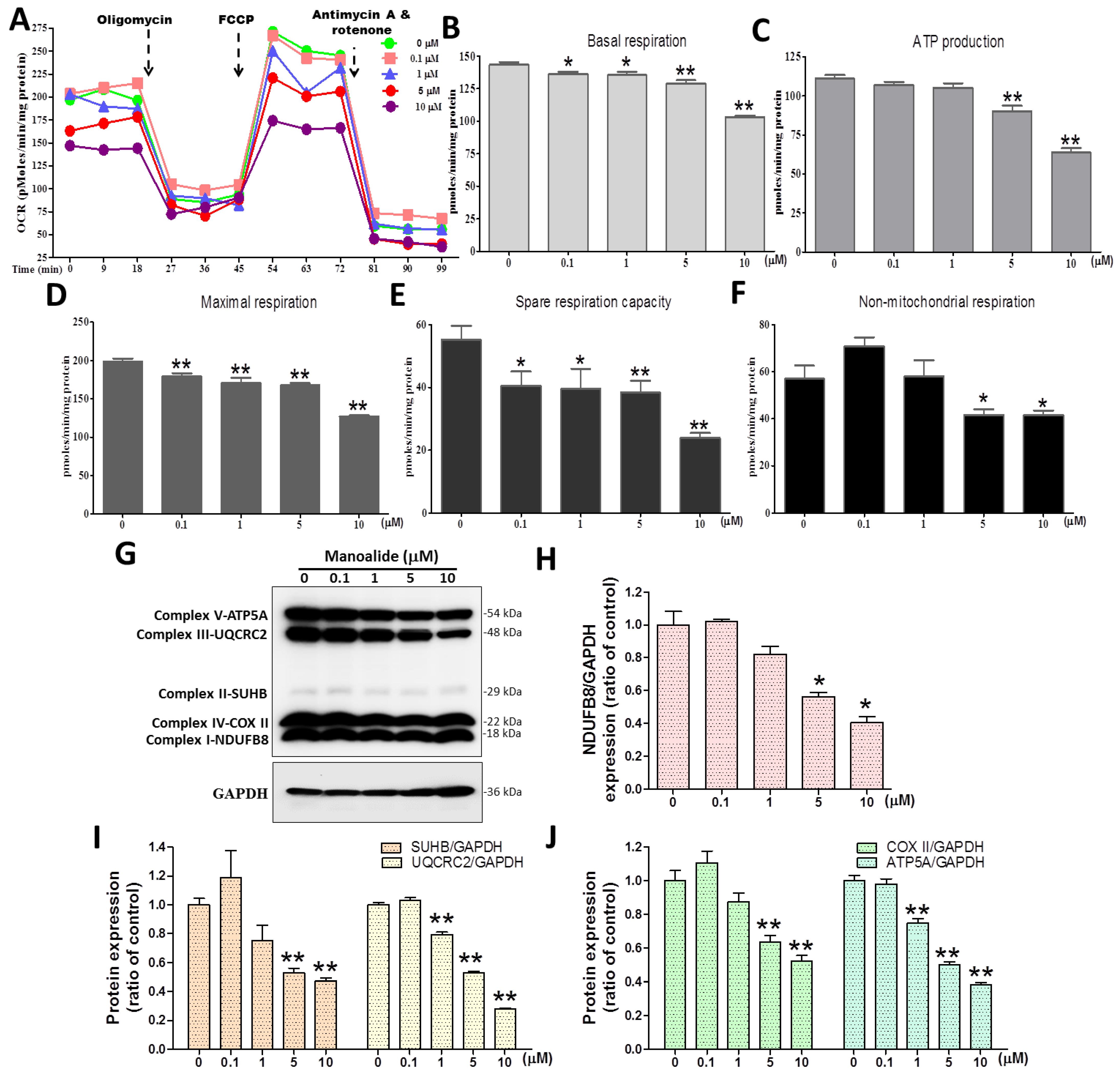
3.3. Manoalide Treatment Reduces OCR and Oxidative Phosphorylation (OXPHOS) Protein Expression in MG63 Cells
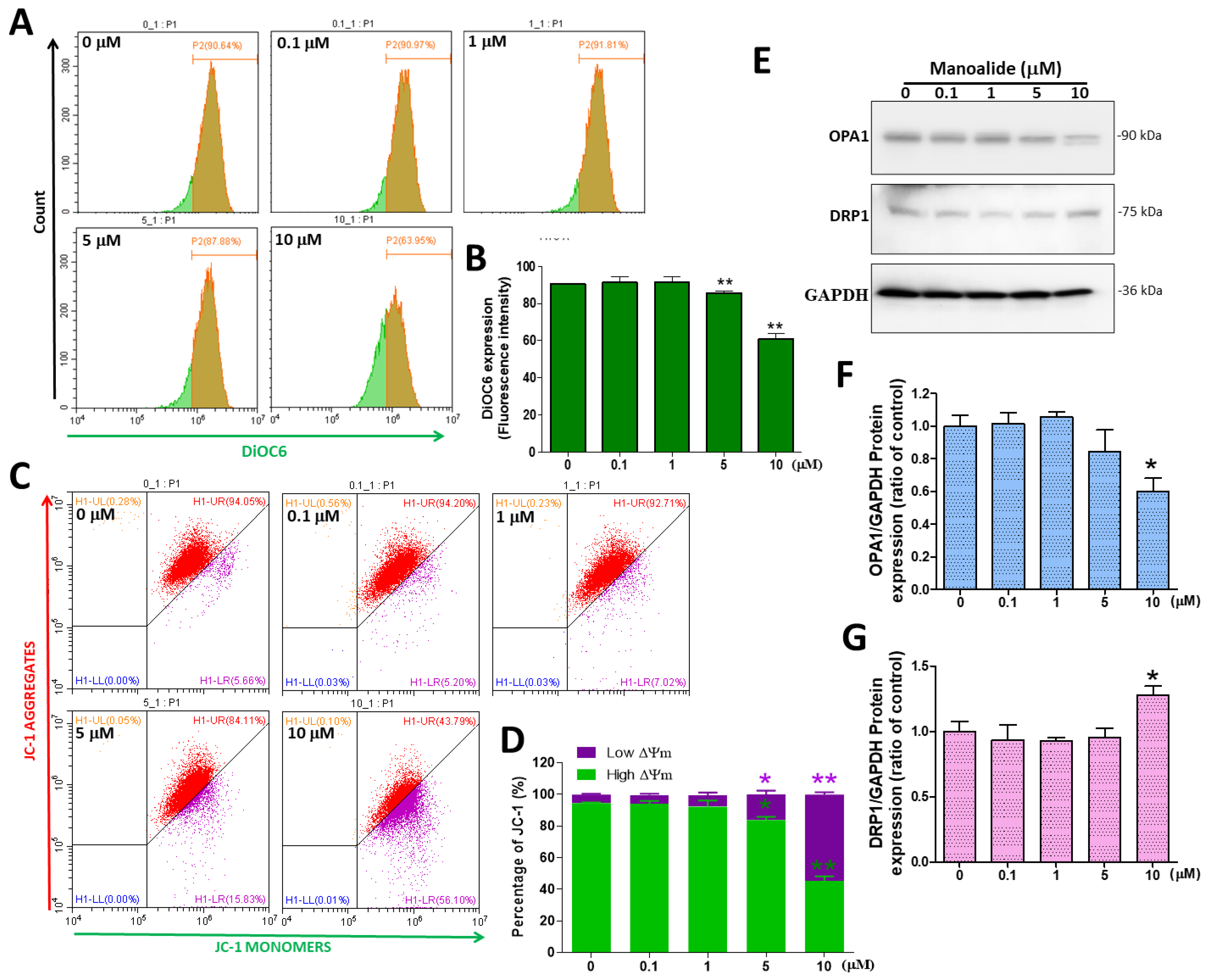
3.4. In MG63 Cells, Manoalide Regulates Mitochondrial Transmembrane Potential (ΔΨm) and Mitochondrial Dynamic Protein
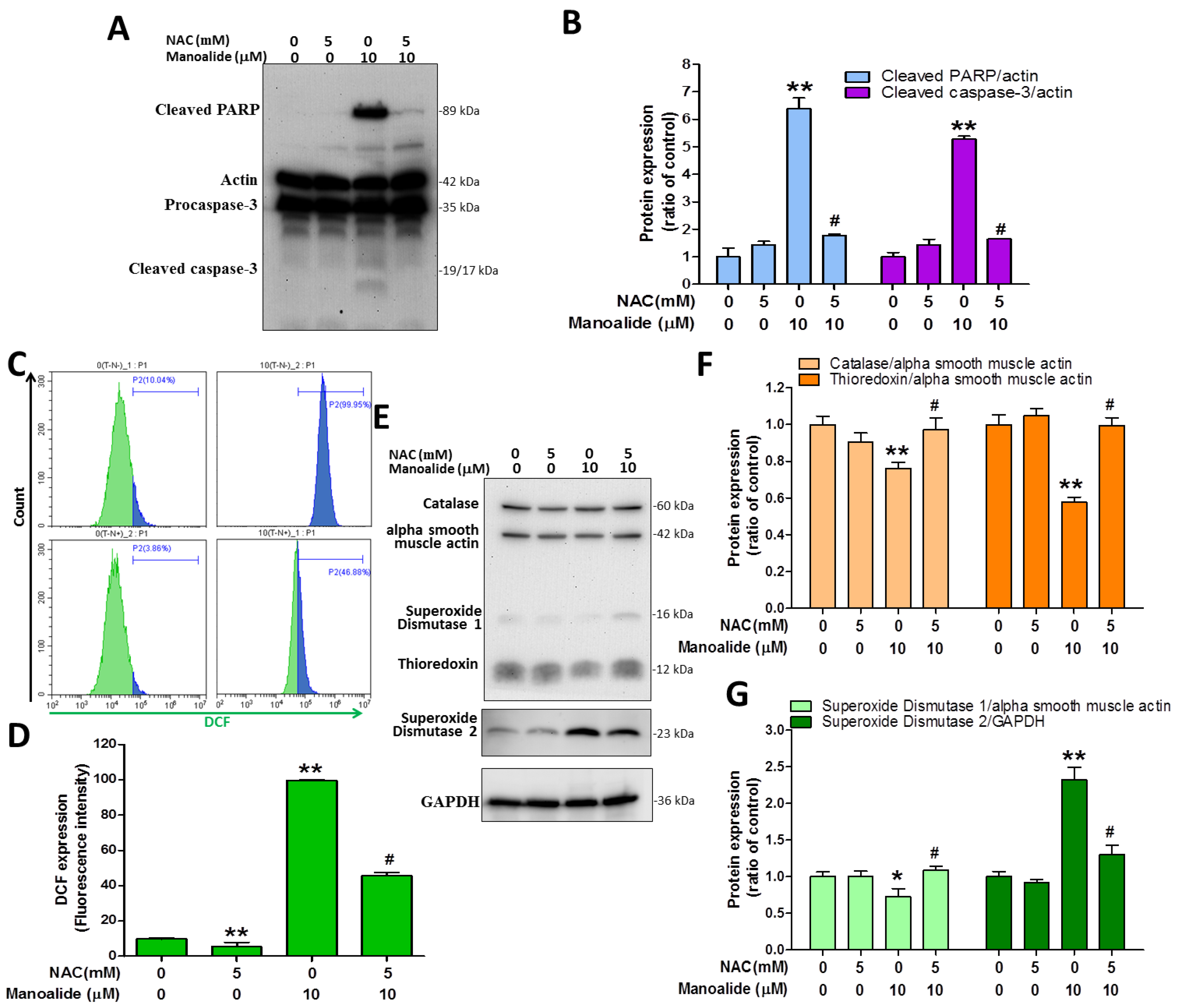
3.5. N-Acetylcysteine Pre-Treatment Reduces Manoalide-Induced Apoptosis, Cellular ROS Production, and Oxidative Stress Defense Enzyme Expression
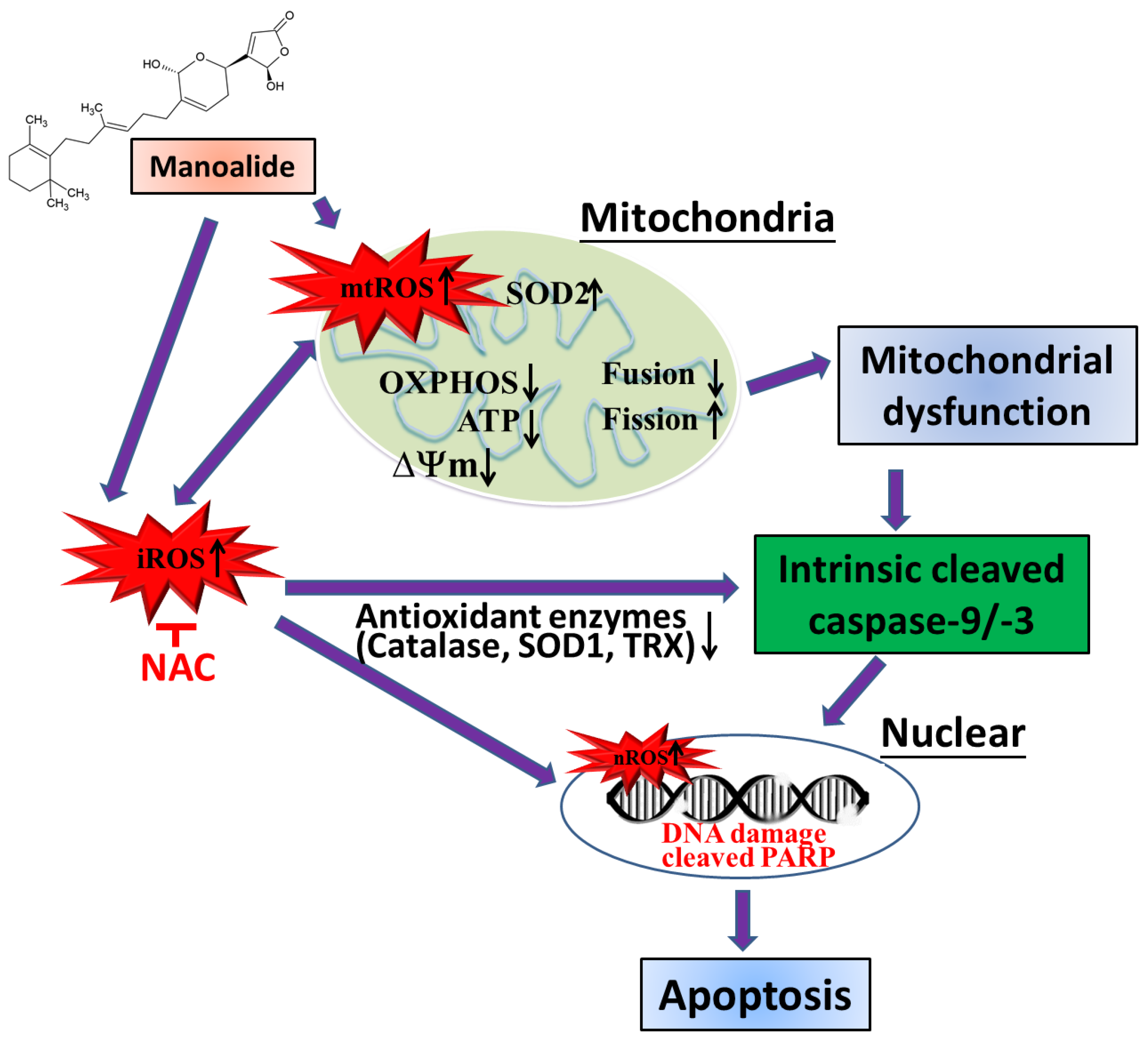
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dilipde, S.E.; Paul, J.S. Manoalide, an antibiotic sesterterpenoid from the marine sponge Luffariella variabilis (polejaeff). Tetrahedron Lett. 1980, 21, 1611–1614. [Google Scholar] [CrossRef]
- Yeom, J.H.; Kim, H.Y.; Lim, J.H.; Yoon, K.W.; Kim, H.M.; Jeong, H.J. A calcium channel blocker, manoalide exerts an anti-allergic inflammatory effect through attenuating NF-kappaB activity. Immunopharmacol. Immunotoxicol. 2021, 43, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, L.A.; Sachs, G.; De Vries, G.; Goodrum, D.; Woldemussie, E.; Muallem, S. Manoalide, a natural sesterterpenoid that inhibits calcium channels. J. Biol. Chem. 1987, 262, 6531–6538. [Google Scholar] [CrossRef] [PubMed]
- Soriente, A.; De Rosa, M.M.; Scettri, A.; Sodano, G.; Terencio, M.C.; Payá, M.; Alcaraz, M.J. Manoalide. Curr. Med. Chem. 1999, 6, 415–431. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.R.; Tang, J.Y.; Wang, Y.Y.; Farooqi, A.A.; Yen, C.Y.; Yuan, S.F.; Huang, H.W.; Chang, H.W. Manoalide Preferentially Provides Antiproliferation of Oral Cancer Cells by Oxidative Stress-Mediated Apoptosis and DNA Damage. Cancers 2019, 11, 1303. [Google Scholar] [CrossRef]
- Kobayashi, J.; Zeng, C.M.; Ishibashi, M.; Sasaki, T. Luffariolides F and G, new manoalide derivatives from the Okinawan marine sponge Luffariella sp. J. Nat. Prod. 1993, 56, 436–439. [Google Scholar] [CrossRef]
- Lombardo, D.; Dennis, E.A. Cobra venom phospholipase A2 inhibition by manoalide. A novel type of phospholipase inhibitor. J. Biol. Chem. 1985, 260, 7234–7240. [Google Scholar] [CrossRef]
- Vecchi, L.; Araujo, T.G.; Azevedo, F.; Mota, S.T.S.; Avila, V.M.R.; Ribeiro, M.A.; Goulart, L.R. Phospholipase A2 Drives Tumorigenesis and Cancer Aggressiveness through Its Interaction with Annexin A1. Cells 2021, 10, 1472. [Google Scholar] [CrossRef]
- Cummings, B.S. Phospholipase A2 as targets for anti-cancer drugs. Biochem. Pharmacol. 2007, 74, 949–959. [Google Scholar] [CrossRef]
- Wang, H.R.; Chen, P.H.; Tang, J.Y.; Yen, C.Y.; Su, Y.C.; Huang, M.Y.; Chang, H.W. Manoalide Shows Mutual Interaction between Cellular and Mitochondrial Reactive Species with Apoptosis in Oral Cancer Cells. Oxid. Med. Cell Longev. 2021, 2021, 6667355. [Google Scholar] [CrossRef]
- Pant, S.; Tripathi, S.; Dandriyal, R.; Astekar, M.; Joshi, A. Osteosarcoma: A Diagnostic Dilemma. J. Exp. Ther. Oncol. 2017, 12, 61–65. [Google Scholar]
- Puhaindran, M.E.; Pho, R.W. Biological reconstruction for children with osteosarcoma around the knee. Ann. Acad. Med. 2014, 43, 499–505. [Google Scholar] [CrossRef]
- Jafari, F.; Javdansirat, S.; Sanaie, S.; Naseri, A.; Shamekh, A.; Rostamzadeh, D.; Dolati, S. Osteosarcoma: A comprehensive review of management and treatment strategies. Ann. Diagn. Pathol. 2020, 49, 151654. [Google Scholar] [CrossRef]
- Simpson, E.; Brown, H.L. Understanding osteosarcomas. Jaapa 2018, 31, 15–19. [Google Scholar] [CrossRef]
- Belayneh, R.; Fourman, M.S.; Bhogal, S.; Weiss, K.R. Update on Osteosarcoma. Curr. Oncol. Rep. 2021, 23, 71. [Google Scholar] [CrossRef] [PubMed]
- Bishop, M.W.; Janeway, K.A.; Gorlick, R. Future directions in the treatment of osteosarcoma. Curr. Opin. Pediatr. 2016, 28, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Marina, N.M.; Smeland, S.; Bielack, S.S.; Bernstein, M.; Jovic, G.; Krailo, M.D.; Hook, J.M.; Arndt, C.; van den Berg, H.; Brennan, B.; et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): An open-label, international, randomised controlled trial. Lancet Oncol. 2016, 17, 1396–1408. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Lin, T.Y.; Lu, M.K.; Leng, P.J.; Tsao, S.M.; Wu, Y.C. Fucoidan induces Toll-like receptor 4-regulated reactive oxygen species and promotes endoplasmic reticulum stress-mediated apoptosis in lung cancer. Sci. Rep. 2017, 7, 44990. [Google Scholar] [CrossRef] [PubMed]
- Romorini, L.; Garate, X.; Neiman, G.; Luzzani, C.; Furmento, V.A.; Guberman, A.S.; Sevlever, G.E.; Scassa, M.E.; Miriuka, S.G. AKT/GSK3beta signaling pathway is critically involved in human pluripotent stem cell survival. Sci. Rep. 2016, 6, 35660. [Google Scholar] [CrossRef]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef]
- Zhou, L.; Jiang, L.; Xu, M.; Liu, Q.; Gao, N.; Li, P.; Liu, E.H. Miltirone exhibits antileukemic activity by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction pathways. Sci. Rep. 2016, 6, 20585. [Google Scholar] [CrossRef] [PubMed]
- Romanov, V.; Whyard, T.C.; Waltzer, W.C.; Grollman, A.P.; Rosenquist, T. Aristolochic acid-induced apoptosis and G2 cell cycle arrest depends on ROS generation and MAP kinases activation. Arch. Toxicol. 2015, 89, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Feniouk, B.A.; Skulachev, V.P. Cellular and Molecular Mechanisms of Action of Mitochondria-Targeted Antioxidants. Curr. Aging Sci. 2017, 10, 41–48. [Google Scholar] [CrossRef]
- Ahn, H.J.; Kim, K.I.; Kim, G.; Moon, E.; Yang, S.S.; Lee, J.S. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS ONE 2011, 6, e28154. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, M.; Robert, E.; Lerondel, S.; Sarron, V.; Ries, D.; Dozias, S.; Sobilo, J.; Gosset, D.; Kieda, C.; Legrain, B.; et al. ROS implication in a new antitumor strategy based on non-thermal plasma. Int. J. Cancer 2012, 130, 2185–2194. [Google Scholar] [CrossRef]
- Dranka, B.P.; Hill, B.G.; Darley-Usmar, V.M. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free Radic. Biol. Med. 2010, 48, 905–914. [Google Scholar] [CrossRef]
- Dranka, B.P.; Benavides, G.A.; Diers, A.R.; Giordano, S.; Zelickson, B.R.; Reily, C.; Zou, L.; Chatham, J.C.; Hill, B.G.; Zhang, J.; et al. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free Radic. Biol. Med. 2011, 51, 1621–1635. [Google Scholar] [CrossRef]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, X.; Shen, X.; Xie, T.; Xu, C.; Zou, Z.; Dong, J.; Liao, L. Different sulfonylureas induce the apoptosis of proximal tubular epithelial cell differently via closing KATP channel. Mol. Med. 2018, 24, 47. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef]
- Candas, D.; Li, J.J. MnSOD in oxidative stress response-potential regulation via mitochondrial protein influx. Antioxid. Redox Signal 2014, 20, 1599–1617. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Biol. Protoc. 2019, 9, 3128. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Sun, S.Y. N-acetylcysteine, reactive oxygen species and beyond. Cancer Biol. Ther. 2010, 9, 109–110. [Google Scholar] [CrossRef]
- Durfee, R.A.; Mohammed, M.; Luu, H.H. Review of Osteosarcoma and Current Management. Rheumatol. Ther. 2016, 3, 221–243. [Google Scholar] [CrossRef] [PubMed]
- Folmer, F.; Jaspars, M.; Schumacher, M.; Dicato, M.; Diederich, M. Marine natural products targeting phospholipases A2. Biochem. Pharmacol. 2010, 80, 1793–1800. [Google Scholar] [CrossRef]
- Fellenberg, J.; Dechant, M.J.; Ewerbeck, V.; Mau, H. Identification of drug-regulated genes in osteosarcoma cells. Int. J. Cancer 2003, 105, 636–643. [Google Scholar] [CrossRef]
- Eichhorst, S.T. Modulation of apoptosis as a target for liver disease. Expert Opin. Ther. Targets 2005, 9, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Radogna, F.; Dicato, M.; Diederich, M. Cancer-type-specific crosstalk between autophagy, necroptosis and apoptosis as a pharmacological target. Biochem. Pharmacol. 2015, 94, 1–11. [Google Scholar] [CrossRef]
- Riedl, S.J.; Shi, Y. Molecular mechanisms of caspase regulation during apoptosis. Nat. Rev. Mol. Cell Biol. 2004, 5, 897–907. [Google Scholar] [CrossRef]
- Boulares, A.H.; Yakovlev, A.G.; Ivanova, V.; Stoica, B.A.; Wang, G.; Iyer, S.; Smulson, M. Role of poly(ADP-ribose) polymerase (PARP) cleavage in apoptosis. Caspase 3-resistant PARP mutant increases rates of apoptosis in transfected cells. J. Biol. Chem. 1999, 274, 22932–22940. [Google Scholar] [CrossRef] [PubMed]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Ozben, T. Oxidative stress and apoptosis: Impact on cancer therapy. J. Pharm. Sci. 2007, 96, 2181–2196. [Google Scholar] [CrossRef] [PubMed]
- Chaban, Y.; Boekema, E.J.; Dudkina, N.V. Structures of mitochondrial oxidative phosphorylation supercomplexes and mechanisms for their stabilisation. Biochim. Biophys. Acta 2014, 1837, 418–426. [Google Scholar] [CrossRef]
- Fleury, C.; Mignotte, B.; Vayssiere, J.L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef]
- Detmer, S.A.; Chan, D.C. Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 2007, 8, 870–879. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
| Antibody | Host Animal | Supplier/Catalogue | Dilution Ratio |
|---|---|---|---|
| Apoptosis Western Blot Cocktail (pro/p17-caspase-3, cleaved PARP1, muscle actin) | Rabbit, polyclonal+ monoclonal | Abcam/ab136812 | 1:250 |
| Anti-caspase 9 | Rabbit, polyclonal | Cell Signaling/9502 | 1:1000 |
| Oxidative Stress Defense (Catalase, SOD1, TRX, smooth muscle Actin) Western Blot Cocktail | Rabbit, polyclonal | Abcam/ab179843 | 1:1000 |
| Anti-SOD2 | Rabbit, monoclonal | Abcam/ab68155 | 1:1000 |
| Total OXPHOS Human WB Antibody Cocktail | Mouse, monoclonal | Abcam/ab110411 | 1:1000 |
| Anti-DRP1 | Rabbit, polyclonal | Santa Cruz/SC-32898 | 1:500 |
| Anti-OPA1 | Rabbit, polyclonal | Millipore/ABN95 | 1:1000 |
| Anti-GADPH | Rabbit, polyclonal | GeneTex/GTX10018 | 1:5000 |
| Cell Line | MG63 Cells | 143B Cells | ||
|---|---|---|---|---|
| Time (h) | 24 | 48 | 24 | 48 |
| IC50 (µM) | 8.88 | 8.66 | 15.07 | 10.93 |
| Standard error (µM) | 1.10 | 1.12 | 1.54 | 1.28 |
| R-square | 0.9272 | 0.9273 | 0.96 | 0.96 |
| 95% confidence intervals | 6.04–11.72 | 5.79–11.53 | 11.12–19.01 | 7.64–14.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Z.-K.; Jean, Y.-H.; Lin, S.-C.; Lai, Y.-C.; Chen, N.-F.; Tseng, C.-C.; Chen, W.-F.; Wen, Z.-H.; Kuo, H.-M. Manoalide Induces Intrinsic Apoptosis by Oxidative Stress and Mitochondrial Dysfunction in Human Osteosarcoma Cells. Antioxidants 2023, 12, 1422. https://doi.org/10.3390/antiox12071422
Yao Z-K, Jean Y-H, Lin S-C, Lai Y-C, Chen N-F, Tseng C-C, Chen W-F, Wen Z-H, Kuo H-M. Manoalide Induces Intrinsic Apoptosis by Oxidative Stress and Mitochondrial Dysfunction in Human Osteosarcoma Cells. Antioxidants. 2023; 12(7):1422. https://doi.org/10.3390/antiox12071422
Chicago/Turabian StyleYao, Zhi-Kang, Yen-Hsuan Jean, Sung-Chun Lin, Yu-Cheng Lai, Nan-Fu Chen, Chung-Chih Tseng, Wu-Fu Chen, Zhi-Hong Wen, and Hsiao-Mei Kuo. 2023. "Manoalide Induces Intrinsic Apoptosis by Oxidative Stress and Mitochondrial Dysfunction in Human Osteosarcoma Cells" Antioxidants 12, no. 7: 1422. https://doi.org/10.3390/antiox12071422
APA StyleYao, Z.-K., Jean, Y.-H., Lin, S.-C., Lai, Y.-C., Chen, N.-F., Tseng, C.-C., Chen, W.-F., Wen, Z.-H., & Kuo, H.-M. (2023). Manoalide Induces Intrinsic Apoptosis by Oxidative Stress and Mitochondrial Dysfunction in Human Osteosarcoma Cells. Antioxidants, 12(7), 1422. https://doi.org/10.3390/antiox12071422







