Melatonin Improves Turbot Oocyte Meiotic Maturation and Antioxidant Capacity, Inhibits Apoptosis-Related Genes mRNAs In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Conditions
2.2. Chemical and Reagents
2.3. Isolation and Incubation of Oocyte
2.4. Antioxidant Capacity Assay
2.5. RNA Isolation, Reverse Transcription, and Quantitative Real-Time RT-PCR (qRT-PCR)
2.6. Statistical Analysis
3. Results
3.1. Stage-Dependent Maturational Competence of Turbot Oocytes
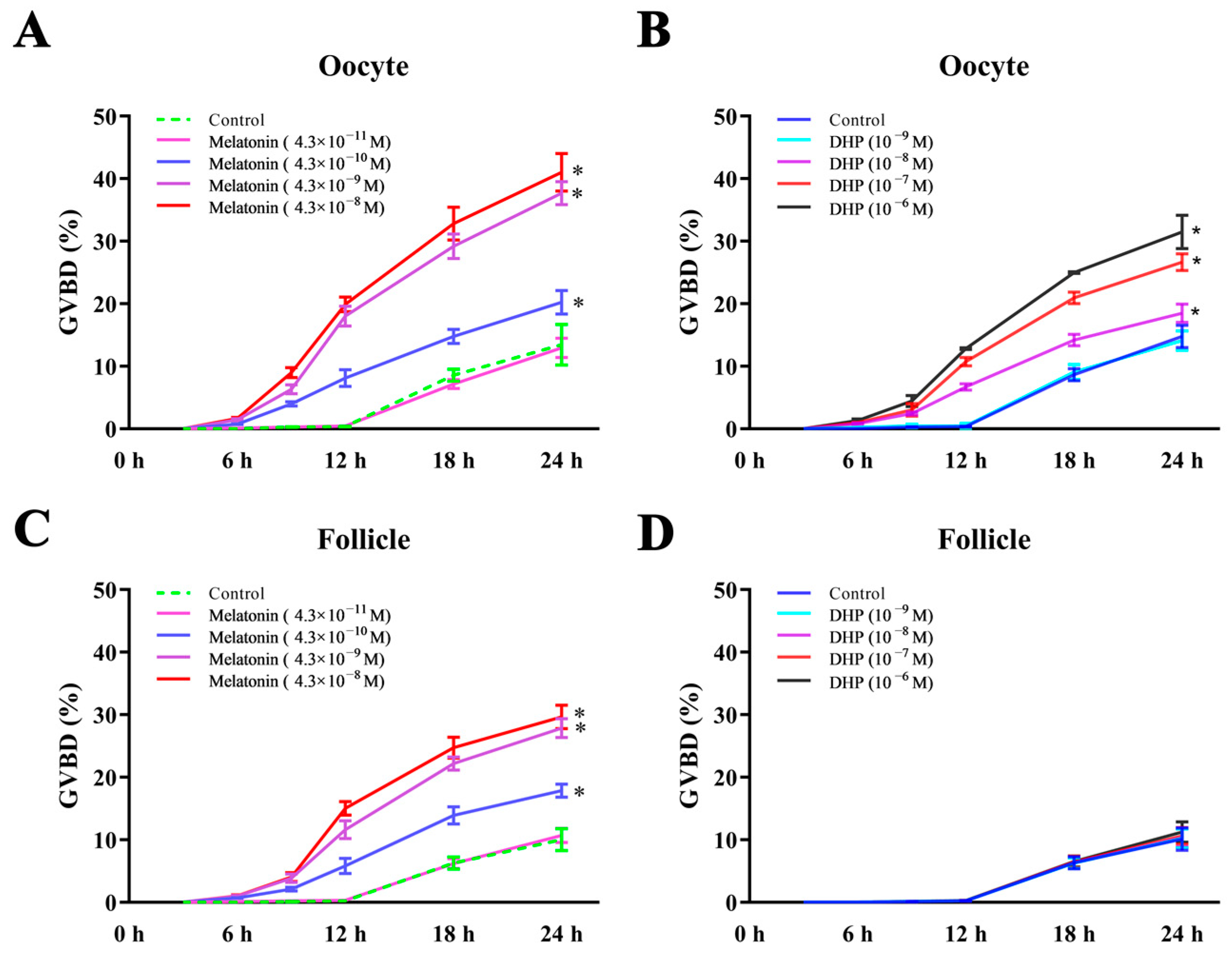
3.2. Effects of Melatonin on Meiotic Maturation of Turbot Oocytes
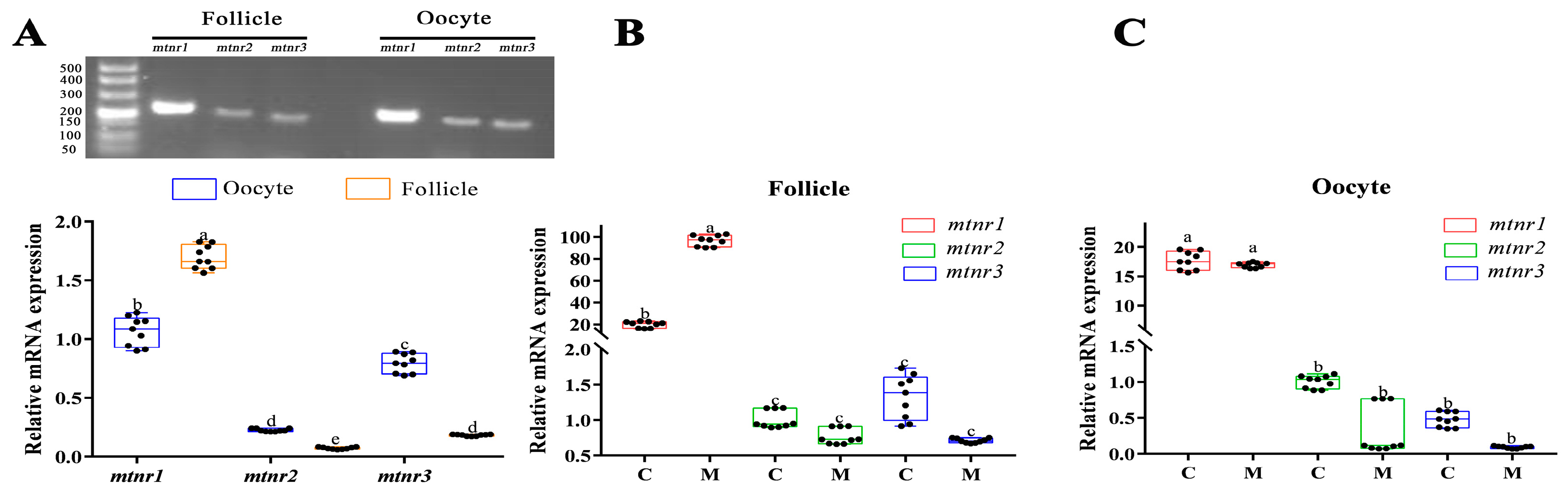
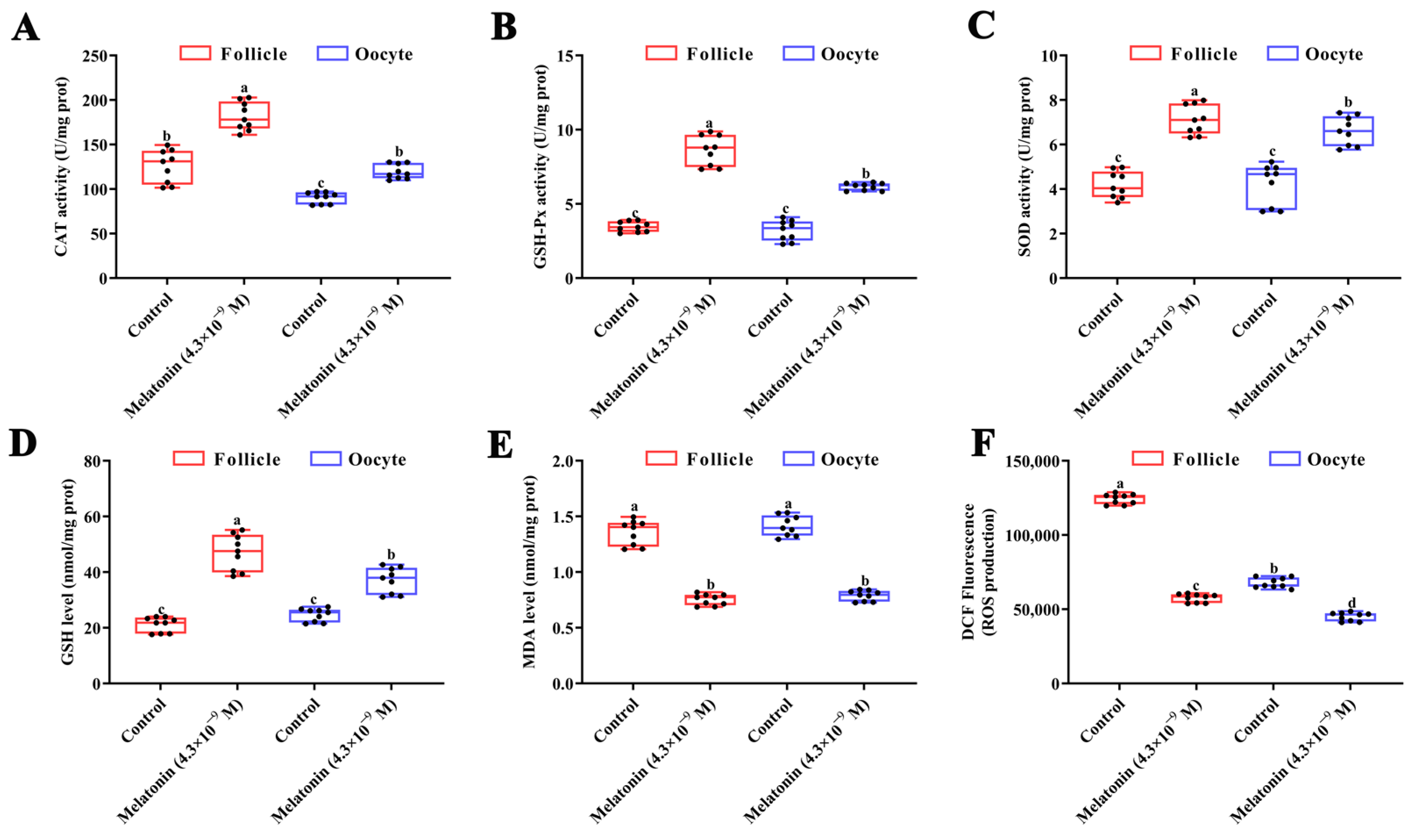
3.3. Effects of Melatonin on Mtnrs Expression and Antioxidant Capacity in Turbot Oocytes
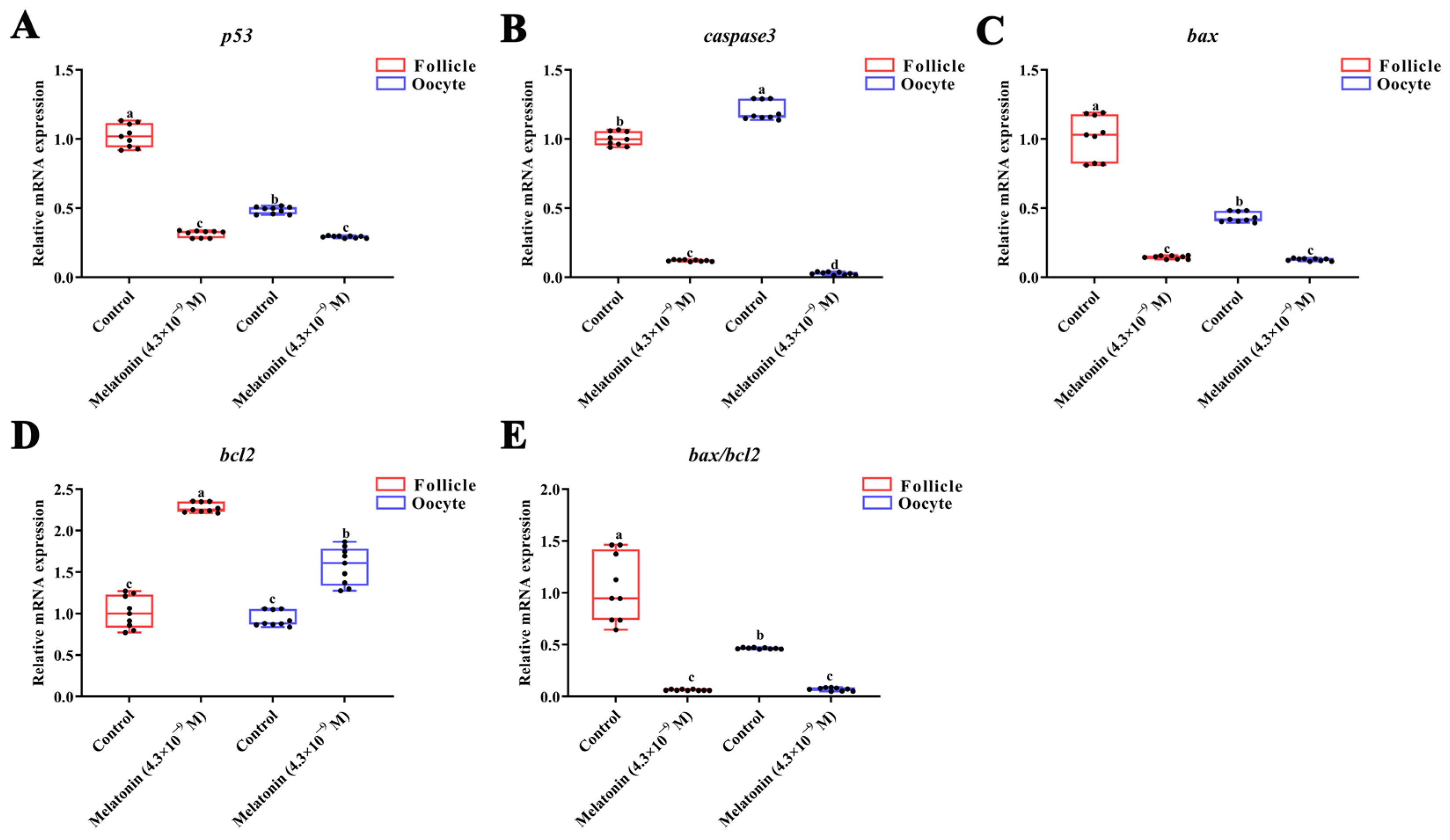
3.4. Effects of Melatonin on Apoptosis-Related Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Migaud, H.; Bell, G.; Cabrita, E.; McAndrew, B.; Davie, A.; Bobe, J.; Herráez, M.P.; Carrillo, M. Gamete quality and broodstock management in temperate fish. Rev. Aquac. 2013, 5, S194–S223. [Google Scholar] [CrossRef]
- Bobe, J. Egg quality in fish: Present and future challenges. Anim. Front. 2015, 5, 66–72. [Google Scholar] [CrossRef]
- Bobe, J.; Labbe, C. Egg and sperm quality in fish. Gen. Comp. Endocrinol. 2010, 165, 535–548. [Google Scholar] [CrossRef]
- Lubzens, E.; Young, G.; Bobe, J.; Cerda, J. Oogenesis in teleosts: How eggs are formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Wang, Y.; Niu, C. The dual role of cGMP in oocyte maturation of zebrafish. Biochem. Biophys. Res. Commun. 2018, 499, 998–1003. [Google Scholar] [CrossRef]
- Patino, R.; Sullivan, C.V. Ovarian follicle growth, maturation, and ovulation in teleost fish. Fish Physiol. Biochem. 2002, 26, 57–70. [Google Scholar] [CrossRef]
- Nagahama, Y.; Yamashita, M. Regulation of oocyte maturation in fish. Dev. Growth Differ. 2008, 50 (Suppl. S1), S195–S219. [Google Scholar] [CrossRef] [PubMed]
- Skoblina, M.N. In vitro stimulation of oocyte ovulation in teleosts by gonadotropic and steroid hormones. Russ. J. Dev. Biol. 2009, 40, 191–197. [Google Scholar] [CrossRef]
- Chian, R.-C.; Buckett, W.M.; Tan, S.-L. In-vitro maturation of human oocytes. Reprod. Biomed. Online 2004, 8, 148–166. [Google Scholar] [CrossRef]
- Hirao, Y.; Nagai, T.; Kubo, M.; Miyano, T.; Miyake, M.; Kato, S. In vitro growth and maturation of pig oocytes. Reproduction 1994, 100, 333–339. [Google Scholar] [CrossRef]
- Niwa, K.; Chang, M. Fertilization of rat eggs in vitro at various times before and after ovulation with special reference to fertilization of ovarian oocytes matured in culture. Reproduction 1975, 43, 435–451. [Google Scholar] [CrossRef] [PubMed]
- Brackett, B.G.; Mills, J.A.; Jeitles, G.G. In Vitro Fertilization of Rabbit Ova Recovered from Ovarian Follicles. Fertil. Steril. 1972, 23, 898–909. [Google Scholar] [CrossRef] [PubMed]
- Yaacobi-Artzi, S.; Shimoni, C.; Kalo, D.; Hansen, P.J.; Roth, Z. Melatonin slightly alleviates the effect of heat shock on bovine oocytes and resulting blastocysts. Theriogenology 2020, 158, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, K.; Haider, S. Effects of actinomycin d and cycloheximide on gonadotropin-or 17α, 20 β-dihydroxy-4-pregnen-3-one-induced in vitro maturation in oocytes of the catfish, Clarias batrachus. J. Biosci. 1995, 20, 151–156. [Google Scholar] [CrossRef]
- Chaube, S.K.; Haider, S. Effects of cAMP forskolin and cyanoketone on in vitro oocyte maturation in the catfish, Clarias batrachus. J. Biosci. 1997, 22, 255–265. [Google Scholar] [CrossRef]
- Pang, Y.F.; Ge, W. Activin stimulation of zebrafish oocyte maturation in vitro and its potential role in mediating gonadotropin-induced oocyte maturation. Biol. Reprod. 1999, 61, 987–992. [Google Scholar] [CrossRef]
- Chattoraj, A.; Bhattacharyya, S.; Basu, D.; Bhattacharya, S.; Bhattacharya, S.; Maitra, S.K. Melatonin accelerates maturation inducing hormone (MIH): Induced oocyte maturation in carps. Gen. Comp. Endocrinol. 2005, 140, 145–155. [Google Scholar] [CrossRef]
- Seki, S.; Kouya, T.; Tsuchiya, R.; Valdez, D.M., Jr.; Jin, B.; Hara, T.; Saida, N.; Kasai, M.; Edashige, K. Development of a reliable in vitro maturation system for zebrafish oocytes. Reproduction 2008, 135, 285–292. [Google Scholar] [CrossRef]
- Abe, T.; Ijiri, S.; Adachi, S.; Yamauchi, K. Development of an in vitro culture system for producing eel larvae from immature ovarian follicles in Japanese eel Anguilla japonica. Fish. Sci. 2010, 76, 257–265. [Google Scholar] [CrossRef]
- Skoblina, M.N.; Minin, A.A. Hormonal induction of in vitro maturation and ovulation of loach oocytes and obtaining egg cells capable of fertilization and development. Russ. J. Dev. Biol. 2015, 46, 159–167. [Google Scholar] [CrossRef]
- Nath, P.; Das, D.; Pal, S.; Maitra, S. Nitric oxide (NO) inhibition of meiotic G2-M1 transition in Anabas testudineus oocytes: Participation of cAMP-dependent protein kinase (PKA) in regulation of intra-oocyte signaling events. Mol. Cell Endocrinol. 2018, 460, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Falcon, J.; Migaud, H.; Munoz-Cueto, J.A.; Carrillo, M. Current knowledge on the melatonin system in teleost fish. Gen. Comp. Endocrinol. 2010, 165, 469–482. [Google Scholar] [CrossRef]
- Mondal, P.; Hasan, K.N.; Pal, P.K.; Maitra, S.K. Influences of exogenous melatonin on the oocyte growth and oxidative status of ovary during different reproductive phases of an annual cycle in carp Catla catla. Theriogenology 2017, 87, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, K.; Takahashi, T. A dual role for melatonin in medaka ovulation: Ensuring prostaglandin synthesis and actin cytoskeleton rearrangement in follicular cells. Biol. Reprod. 2016, 94, 64. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S.K.; Hasan, K.N. The Role of Melatonin as a Hormone and an Antioxidant in the Control of Fish Reproduction. Front. Endocrinol. 2016, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Manchester, L.C.; Paredes, S.D.; Mayo, J.C.; Sainz, R.M. Melatonin and reproduction revisited. Biol. Reprod. 2009, 81, 445–456. [Google Scholar] [CrossRef]
- Falcon, J.; Besseau, L.; Sauzet, S.; Boeuf, G. Melatonin effects on the hypothalamo-pituitary axis in fish. Trends Endocrinol. Metab. 2007, 18, 81–88. [Google Scholar] [CrossRef]
- Shi, L.; Li, N.; Bo, L.; Xu, Z. Melatonin and hypothalamic-pituitary-gonadal axis. Curr. Med. Chem. 2013, 20, 2017–2031. [Google Scholar] [CrossRef]
- Takahashi, T.; Ogiwara, K. Roles of melatonin in the teleost ovary: A review of the current status. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 254, 110907. [Google Scholar] [CrossRef]
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef]
- Maitra, S.K.; Chattoraj, A.; Mukherjee, S.; Moniruzzaman, M. Melatonin: A potent candidate in the regulation of fish oocyte growth and maturation. Gen. Comp. Endocrinol. 2013, 181, 215–222. [Google Scholar] [CrossRef]
- Sakai, K.; Yamamoto, Y.; Ikeuchi, T. Vertebrates originally possess four functional subtypes of G protein-coupled melatonin receptor. Sci. Rep. 2019, 9, 9465. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Nakamura, Y.; Korkmaz, A.; Manchester, L.C.; Tan, D.X.; Sugino, N.; Reiter, R.J. Melatonin and the ovary: Physiological and pathophysiological implications. Fertil. Steril. 2009, 92, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Lei, J. Molecular function of gonadotrophins and their receptors in the ovarian development of turbot (Scophthalmus maximus). Gen. Comp. Endocrinol. 2019, 277, 17–19. [Google Scholar] [CrossRef] [PubMed]
- FAO. Global Aquaculture Production Quantity (1950–2020). Available online: https://www.fao.org/fishery/statistics-query/en/aquaculture/aquaculturequantity (accessed on 18 July 2022).
- Girin, M.; Devauchelle, N. Décalage de la période de reproduction par raccourcissement des cycles photopériodique et thermique chez des poissons marins. Ann. Biol. Anim. Biochim. Biophys. 1978, 18, 1059–1065. [Google Scholar] [CrossRef]
- Jia, Y.; Meng, Z.; Niu, H.; Hu, P.; Lei, J. Molecular cloning, characterization, and expression analysis of luteinizing hormone receptor gene in turbot (Scophthalmus maximus). Fish Physiol. Biochem. 2014, 40, 1639–1650. [Google Scholar] [CrossRef]
- Taboada, X.; Robledo, D.; Bouza, C.; Piferrer, F.; Viñas, A.M.; Martínez, P. Reproduction and sex control in turbot. In Sex Control in Aquaculture; Wiley: Hoboken, NJ, USA, 2018; pp. 565–582. [Google Scholar]
- Imsland, A.K.; Dragsnes, M.; Stefansson, S.O. Exposure to continuous light inhibits maturation in turbot (Scophthalmus maximus). Aquaculture 2003, 219, 911–919. [Google Scholar] [CrossRef]
- Nissling, A.; Johansson, U.; Jacobsson, M. Effects of salinity and temperature conditions on the reproductive success of turbot (Scophthalmus maximus) in the Baltic Sea. Fish. Res. 2006, 80, 230–238. [Google Scholar] [CrossRef]
- Polat, H.; Ozturk, R.C.; Terzi, Y.; Aydin, I.; Kucuk, E. Effect of Photoperiod Manipulation on Spawning Time and Performance of Turbot (Scophthalmus maximus). Aquac. Stud. 2021, 21, 109–115. [Google Scholar] [CrossRef]
- Alvariño, J.; Rebollar, P.; Olmedo, M.; Alvarez-Blázquez, B.; Ubilla, E.; Peleteiro, J. Effects of melatonin implants on reproduction and growth of turbot broodstock. Aquac. Int. 2001, 9, 477–487. [Google Scholar] [CrossRef]
- Huang, B.; Wang, N.; Wang, L.; Jia, Y.; Liu, B.; Gao, X.; Liu, B.; Wang, W. Vitamin E stimulates the expression of gonadotropin hormones in primary pituitary cells of turbot (Scophthalmus maximus). Aquaculture 2019, 509, 47–51. [Google Scholar] [CrossRef]
- Gao, Y.; Jing, Q.; Huang, B.; Jia, Y. Molecular cloning, characterization, and mRNA expression of gonadotropins during larval development in turbot (Scophthalmus maximus). Fish Physiol. Biochem. 2019, 45, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Meng, Z.; Jia, Y. Molecular characterization and quantification of estrogen receptors in turbot (Scophthalmus maximus). Gen. Comp. Endocrinol. 2018, 257, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Jing, Q.; Gao, Y.; Huang, B. Involvement and expression of growth hormone/insulin-like growth factor member mRNAs in the ovarian development of turbot (Scophthalmus maximus). Fish Physiol. Biochem. 2019, 45, 955–964. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, A.; Meng, Z.; Liu, B.; Lei, J. Molecular characterization and quantification of the follicle-stimulating hormone receptor in turbot (Scophthalmus maximus). Fish Physiol. Biochem. 2016, 42, 179–191. [Google Scholar] [CrossRef]
- Ai, N.; Liu, L.; Lau, E.S.-W.; Tse, A.C.-K.; Ge, W. Separation of Oocyte and Follicle Layer for Gene Expression Analysis in Zebrafish. In Germline Development in the Zebrafish: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–9. [Google Scholar]
- Pang, Y.; Ge, W. Gonadotropin and activin enhance maturational competence of oocytes in the zebrafish (Danio rerio). Biol. Reprod. 2002, 66, 259–265. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Y.; Huang, B.; Meng, Z.; Jia, Y. Reference gene validation for quantification of gene expression during ovarian development of turbot (Scophthalmus maximus). Sci. Rep. 2020, 10, 823. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Menn, F.L.; Cerdà, J.; Babin, P.J. Ultrastructural aspects of the ontogeny and differentiation of ray-finned fish ovarian follicles. In The Fish Oocyte: From Basic Studies to Biotechnological Applications; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–37. [Google Scholar]
- Khan, I.; Thomas, P. Ovarian cycle, teleost fish. Encycl. Reprod. 1999, 3, 552–564. [Google Scholar]
- Reading, B.J.; Andersen, L.K.; Ryu, Y.-W.; Mushirobira, Y.; Todo, T.; Hiramatsu, N. Oogenesis and egg quality in finfish: Yolk formation and other factors influencing female fertility. Fishes 2018, 3, 45. [Google Scholar] [CrossRef]
- Żarski, D.; Palińska, K.; Targońska, K.; Bokor, Z.; Kotrik, L.; Krejszeff, S.; Kupren, K.; Horváth, Á.; Urbányi, B.; Kucharczyk, D. Oocyte quality indicators in Eurasian perch, Perca fluviatilis L., during reproduction under controlled conditions. Aquaculture 2011, 313, 84–91. [Google Scholar] [CrossRef]
- Żarski, D.; Kucharczyk, D.; Targońska, K.; Palińska, K.; Kupren, K.; Fontaine, P.; Kestemont, P. A new classification of pre-ovulatory oocyte maturation stages in pikeperch, Sander lucioperca (L.), and its application during artificial reproduction. Aquac. Res. 2012, 43, 713–721. [Google Scholar] [CrossRef]
- Kucharczyk, D.; Malinovskyi, O.; Nowosad, J.; Kowalska, A.; Cejko, B.I. Comparison of responses to artificial spawning of ruffe (Gymnocephalus cernua) specimens captured from their natural habitat to those produced in cultured conditions. Anim. Reprod. Sci. 2021, 225, 106684. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, D.; Piech, P.; Nowosad, J.; Abdel-Latif, H.M.; Ablaisanova, G.M.; Sikora, M. Final oocyte maturation (FOM) model and artificial reproduction of burbot spawners (Lota lota) originating from the F1 generation of a cultured stock in comparison to wild stock. Aquaculture 2022, 548, 737679. [Google Scholar] [CrossRef]
- Selman, K.; Petrino, T.R.; Wallace, R.A. Experimental conditions for oocyte maturation in the zebrafish, Brachydanio rerio. J. Exp. Zool. 1994, 269, 538–550. [Google Scholar] [CrossRef]
- Elkouby, Y.M.; Mullins, M.C. Coordination of cellular differentiation, polarity, mitosis and meiosis–new findings from early vertebrate oogenesis. Dev. Biol. 2017, 430, 275–287. [Google Scholar] [CrossRef]
- Palstra, A.; Cohen, E.; Niemantsverdriet, P.; Van Ginneken, V.; Van den Thillart, G. Artificial maturation and reproduction of European silver eel: Development of oocytes during final maturation. Aquaculture 2005, 249, 533–547. [Google Scholar] [CrossRef]
- Lee, W.K.; Yang, S.W. Relationship between ovarian development and serum levels of gonadal steroid hormones, and induction of oocyte maturation and ovulation in the cultured female Korean spotted sea bass Lateolabrax maculatus (Jeom-nong-eo). Aquaculture 2002, 207, 169–183. [Google Scholar] [CrossRef]
- Shiraishi, T.; Okamoto, K.; Yoneda, M.; Sakai, T.; Ohshimo, S.; Onoe, S.; Yamaguchi, A.; Matsuyama, M. Age validation, growth and annual reproductive cycle of chub mackerel Scomber japonicus off the waters of northern Kyushu and in the East China Sea. Fish. Sci. 2008, 74, 947–954. [Google Scholar] [CrossRef]
- Sorbera, L.A.; Asturiano, J.F.; Carrillo, M.; Cerda, J.; Kime, D.E.; Zanuy, S. In vitro oocyte maturation in the sea bass: Effects of hCG, pituitary extract and steroids. J. Fish Biol. 1999, 55, 9–25. [Google Scholar] [CrossRef]
- Bromage, N.; Porter, M.; Randall, C. The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 2001, 197, 63–98. [Google Scholar] [CrossRef]
- Tokumoto, T.; Tokumoto, M.; Horiguchi, R.; Ishikawa, K.; Nagahama, Y. Diethylstilbestrol induces fish oocyte maturation. Proc. Natl. Acad. Sci. USA 2004, 101, 3686–3690. [Google Scholar] [CrossRef] [PubMed]
- Bayarri, M.J.; Zanuy, S.; Yilmaz, O.; Carrillo, M. Effects of continuous light on the reproductive system of European sea bass gauged by alterations of circadian variations during their first reproductive cycle. Chronobiol. Int. 2009, 26, 184–199. [Google Scholar] [CrossRef]
- Ikegami, T.; Takeuchi, Y.; Hur, S.-P.; Takemura, A. Impacts of moonlight on fish reproduction. Mar. Genom. 2014, 14, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Malpaux, B.; Migaud, M.; Tricoire, H.; Chemineau, P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J. Biol. Rhythm. 2001, 16, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Porter, M.; Stefansson, S.; Nyhammer, G.; Karlsen, Ø.; Norberg, B.; Bromage, N. Environmental influences on melatonin secretion in Atlantic cod (Gadus morhua L.) and their relevance to commercial culture. Fish Physiol. Biochem. 2000, 23, 191–200. [Google Scholar] [CrossRef]
- Maitra, S.K.; Chattoraj, A.; Bhattacharyya, S. Implication of melatonin in oocyte maturation in Indian major carp Catla catla. Fish Physiol. Biochem. 2005, 31, 201–207. [Google Scholar] [CrossRef]
- Carnevali, O.; Gioacchini, G.; Maradonna, F.; Olivotto, I.; Migliarini, B. Melatonin induces follicle maturation in Danio rerio. PLoS ONE 2011, 6, e19978. [Google Scholar] [CrossRef]
- Lombardo, F.; Giorgini, E.; Gioacchini, G.; Maradonna, F.; Ferraris, P.; Carnevali, O. Melatonin effects on Fundulus heteroclitus reproduction. Reprod. Fertil. Dev. 2012, 24, 794–803. [Google Scholar] [CrossRef]
- Dubocovich, M.L.; Delagrange, P.; Krause, D.N.; Sugden, D.; Cardinali, D.P.; Olcese, J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010, 62, 343–380. [Google Scholar] [CrossRef]
- Li, D.Y.; Smith, D.G.; Hardeland, R.; Yang, M.Y.; Xu, H.L.; Zhang, L.; Yin, H.D.; Zhu, Q. Melatonin receptor genes in vertebrates. Int. J. Mol. Sci. 2013, 14, 11208–11223. [Google Scholar] [CrossRef] [PubMed]
- Chattoraj, A.; Seth, M.; Maitra, S.K. Localization and dynamics of Mel1a melatonin receptor in the ovary of carp Catla catla in relation to serum melatonin levels. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 152, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xu, S.; Liu, Y.; Feng, C.; Xiao, Y.; Wang, Y.; Liu, Q.; Li, J. Changes of melatonin and its receptors in synchronizing turbot (Scophthalmus maximus) seasonal reproduction and maturation rhythm. Acta Oceanol. Sin. 2021, 41, 84–98. [Google Scholar] [CrossRef]
- Sampaio, R.V.; Conceição, D.S.B.; Miranda, M.S.; Sampaio, L.d.F.S.; Ohashi, O.M. MT3 melatonin binding site, MT1 and MT2 melatonin receptors are present in oocyte, but only MT1 is present in bovine blastocyst produced in vitro. Reprod. Biol. Endocrinol. 2012, 10, 1–7. [Google Scholar] [CrossRef]
- Soto-Heras, S.; Catala, M.G.; Roura, M.; Menendez-Blanco, I.; Piras, A.R.; Izquierdo, D.; Paramio, M.T. Effects of melatonin on oocyte developmental competence and the role of melatonin receptor 1 in juvenile goats. Reprod. Domest. Anim. 2019, 54, 381–390. [Google Scholar] [CrossRef]
- Wang, J.; Zhuo, Z.; Ma, X.; Liu, Y.; Xu, J.; He, C.; Fu, Y.; Wang, F.; Ji, P.; Zhang, L.; et al. Melatonin Alleviates the Suppressive Effect of Hypoxanthine on Oocyte Nuclear Maturation and Restores Meiosis via the Melatonin Receptor 1 (MT1)-Mediated Pathway. Front. Cell Dev. Biol. 2021, 9, 648148. [Google Scholar] [CrossRef]
- Takahashi, T.; Ogiwara, K. cAMP signaling in ovarian physiology in teleosts: A review. Cell. Signal. 2022, 101, 110499. [Google Scholar] [CrossRef]
- Rizzo, A.; Roscino, M.T.; Binetti, F.; Sciorsci, R.L. Roles of reactive oxygen species in female reproduction. Reprod. Domest. Anim. 2012, 47, 344–352. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuoka, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J. Pineal. Res. 2008, 44, 280–287. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Manchester, L.C.; Tan, D.X. Peripheral reproductive organ health and melatonin: Ready for prime time. Int. J. Mol. Sci. 2013, 14, 7231–7272. [Google Scholar] [CrossRef]
- Tamura, H.; Takasaki, A.; Taketani, T.; Tanabe, M.; Kizuka, F.; Lee, L.; Tamura, I.; Maekawa, R.; Aasada, H.; Yamagata, Y. The role of melatonin as an antioxidant in the follicle. J. Ovarian Res. 2012, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.-X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Jozaki, M.; Tanabe, M.; Shirafuta, Y.; Mihara, Y.; Shinagawa, M.; Tamura, I.; Maekawa, R.; Sato, S.; Taketani, T. Importance of melatonin in assisted reproductive technology and ovarian aging. Int. J. Mol. Sci. 2020, 21, 1135. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.C.; Sun, T.C.; Li, H.Y.; Si, L.N.; Wei, M.; Chen, Z.H.; Cheng, L.Y.; Yang, S.H. Antioxidative effect of melatonin on cryopreserved ovarian tissue in mice. Cryobiology 2020, 96, 99–105. [Google Scholar] [CrossRef]
- Sun, T.C.; Liu, X.C.; Yang, S.H.; Song, L.L.; Zhou, S.J.; Deng, S.L.; Tian, L.; Cheng, L.Y. Melatonin inhibits oxidative stress and apoptosis in cryopreserved ovarian tissues via Nrf2/HO-1 signaling pathway. Front. Mol. Biosci. 2020, 7, 163. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Zhang, Z.Z.; Yi, J.Y.; He, C.J.; Wang, F.; Tian, X.Z.; Yang, M.H.; Song, Y.K.; He, P.L.; et al. Resveratrol compares with melatonin in improving in vitro porcine oocyte maturation under heat stress. J. Anim. Sci. Biotechnol. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Park, H.J.; Park, J.Y.; Kim, J.W.; Yang, S.G.; Jung, J.M.; Kim, M.J.; Kang, M.J.; Cho, Y.H.; Wee, G.; Yang, H.Y. Melatonin improves the meiotic maturation of porcine oocytes by reducing endoplasmic reticulum stress during in vitro maturation. J. Pineal Res. 2018, 64, e12458. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Hasan, K.N.; Maitra, S.K. Melatonin actions on ovaprim (synthetic GnRH and domperidone)-induced oocyte maturation in carp. Reproduction 2016, 151, 285–296. [Google Scholar] [CrossRef]
- Zhao, X.M.; Wang, N.; Hao, H.S.; Li, C.Y.; Zhao, Y.H.; Yan, C.L.; Wang, H.Y.; Du, W.H.; Wang, D.; Liu, Y. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J. Pineal Res. 2018, 64, e12445. [Google Scholar] [CrossRef]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell. Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Miyashita, T.; Krajewski, S.; Krajewska, M.; Wang, H.G.; Lin, H.; Liebermann, D.A.; Hoffman, B.; Reed, J.C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 1994, 9, 1799–1805. [Google Scholar]
- Xu, X.; Lai, Y.; Hua, Z.-C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef] [PubMed]
- Obeng, E. Apoptosis (programmed cell death) and its signals—A review. Braz. J. Biol. 2020, 81, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Yang, S.; Zeng, J.; Lv, J.; Zhang, L.; Du, X.; Hu, J.; Zhang, Y.; Zhao, X. The effect of melatonin on sheep endometrial epithelial cell apoptosis through the receptor and non-receptor pathways. Gen. Comp. Endocrinol. 2023, 333, 114182. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.-H.; Wang, Y.; Liu, J.-C.; Pan, Z.-N.; Zhang, H.-L.; Sun, S.-C.; Zhang, Y. Melatonin reverses mitochondria dysfunction and oxidative stress-induced apoptosis of Sudan I-exposed mouse oocytes. Ecotoxicol. Environ. Saf. 2021, 225, 112783. [Google Scholar] [CrossRef] [PubMed]
- Boumela, I.; Guillemin, Y.; Guérin, J.; Aouacheria, A. The Bcl-2 family pathway in gametes and preimplantation embryos. Gynécologie Obs. Fertil. 2009, 37, 720–732. [Google Scholar] [CrossRef]
- Jin, Y.; Zheng, S.; Pu, Y.; Shu, L.; Sun, L.; Liu, W.; Fu, Z. Cypermethrin has the potential to induce hepatic oxidative stress, DNA damage and apoptosis in adult zebrafish (Danio rerio). Chemosphere 2011, 82, 398–404. [Google Scholar] [CrossRef]
- Kim, H.G.; Song, H.; Yoon, D.H.; Song, B.-W.; Park, S.M.; Sung, G.H.; Cho, J.-Y.; Park, H.I.; Choi, S.; Song, W.O. Cordyceps pruinosa extracts induce apoptosis of HeLa cells by a caspase dependent pathway. J. Ethnopharmacol. 2010, 128, 342–351. [Google Scholar] [CrossRef]
- Lan, M.; Zhang, Y.; Wan, X.; Pan, M.-H.; Xu, Y.; Sun, S.-C. Melatonin ameliorates ochratoxin A-induced oxidative stress and apoptosis in porcine oocytes. Environ. Pollut. 2020, 256, 113374. [Google Scholar] [CrossRef]


| Primers | Accession No. | Primer Sequence (5′ to 3′) | Product Length (bp) |
|---|---|---|---|
| Mtnr1F | MK738109 | AACCTGGGTTACGTCCACTG | 224 bp |
| Mtnr1R | AGCGAACCCACAAAGAGGTT | ||
| Mtnr2F | MK738110 | CGTGGTCTTTGTGCTGTTCG | 20 bp |
| Mtnr2R | ATGCGCTTGTACTCGTTCCT | ||
| Mtnr3F | MK738111 | CAAGACGATCCTCCTCGCTC | 185 bp |
| Mtnr3R | GGCGAGGCTCCAGATAACAA | ||
| P53F | EU711045.1 | GCGGGCTCAGTATTTTGAAGAC | 94 bp |
| P53R | GCTCAGCAGGATGGTCGTCA | ||
| Caspase3F | JQ394697.1 | TCGTTCGTCTGTGTCCTGTTGAG | 91 bp |
| Caspase3R | GCTGTGGAGAAGGCGTAGAGG | ||
| BaxF | XM_020094597.1 | GCTCCAGAGGATGATAAATAAC | 124 bp |
| BaxR | AAAGTAGAAGAGTGCGACCA | ||
| Bcl2F | XM_020104180.1 | TTATCAGCGGCATCTTCATCTC | 113 bp |
| Bcl2R | TTGGCGAGGCGGTGTAATC | ||
| β-actinF | AY008305 | CATGTACGTTGCCATCCAAG | 138 bp |
| β-actinR | ACCAGAGGCATACAGGGACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Li, F.; Zhang, X.; Xie, T.; Qin, H.; Lv, J.; Gao, Y.; Li, M.; Gao, Y.; Jia, Y. Melatonin Improves Turbot Oocyte Meiotic Maturation and Antioxidant Capacity, Inhibits Apoptosis-Related Genes mRNAs In Vitro. Antioxidants 2023, 12, 1389. https://doi.org/10.3390/antiox12071389
Zhang J, Li F, Zhang X, Xie T, Qin H, Lv J, Gao Y, Li M, Gao Y, Jia Y. Melatonin Improves Turbot Oocyte Meiotic Maturation and Antioxidant Capacity, Inhibits Apoptosis-Related Genes mRNAs In Vitro. Antioxidants. 2023; 12(7):1389. https://doi.org/10.3390/antiox12071389
Chicago/Turabian StyleZhang, Jiarong, Feixia Li, Xiaoyu Zhang, Ting Xie, Hongyu Qin, Junxian Lv, Yunhong Gao, Mingyue Li, Yuntao Gao, and Yudong Jia. 2023. "Melatonin Improves Turbot Oocyte Meiotic Maturation and Antioxidant Capacity, Inhibits Apoptosis-Related Genes mRNAs In Vitro" Antioxidants 12, no. 7: 1389. https://doi.org/10.3390/antiox12071389
APA StyleZhang, J., Li, F., Zhang, X., Xie, T., Qin, H., Lv, J., Gao, Y., Li, M., Gao, Y., & Jia, Y. (2023). Melatonin Improves Turbot Oocyte Meiotic Maturation and Antioxidant Capacity, Inhibits Apoptosis-Related Genes mRNAs In Vitro. Antioxidants, 12(7), 1389. https://doi.org/10.3390/antiox12071389







