Sanguinarine Improves Intestinal Health in Grass Carp Fed High-Fat Diets: Involvement of Antioxidant, Physical and Immune Barrier, and Intestinal Microbiota
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Ethics Statement
2.2. Feeding Trial and Experimental Diets
2.3. Sampling
2.4. Proximate Composition
2.5. Serum Immnue Parameters
2.6. Histopathological Analysis
2.7. qRT-PCR Analysis
2.8. Western Blot
2.9. Intestinal Microbiota
2.10. Calculations and Statistical Analysis
3. Results
3.1. Immune Indices of Serum
3.2. Intestinal Antioxidant Enzyme Activities
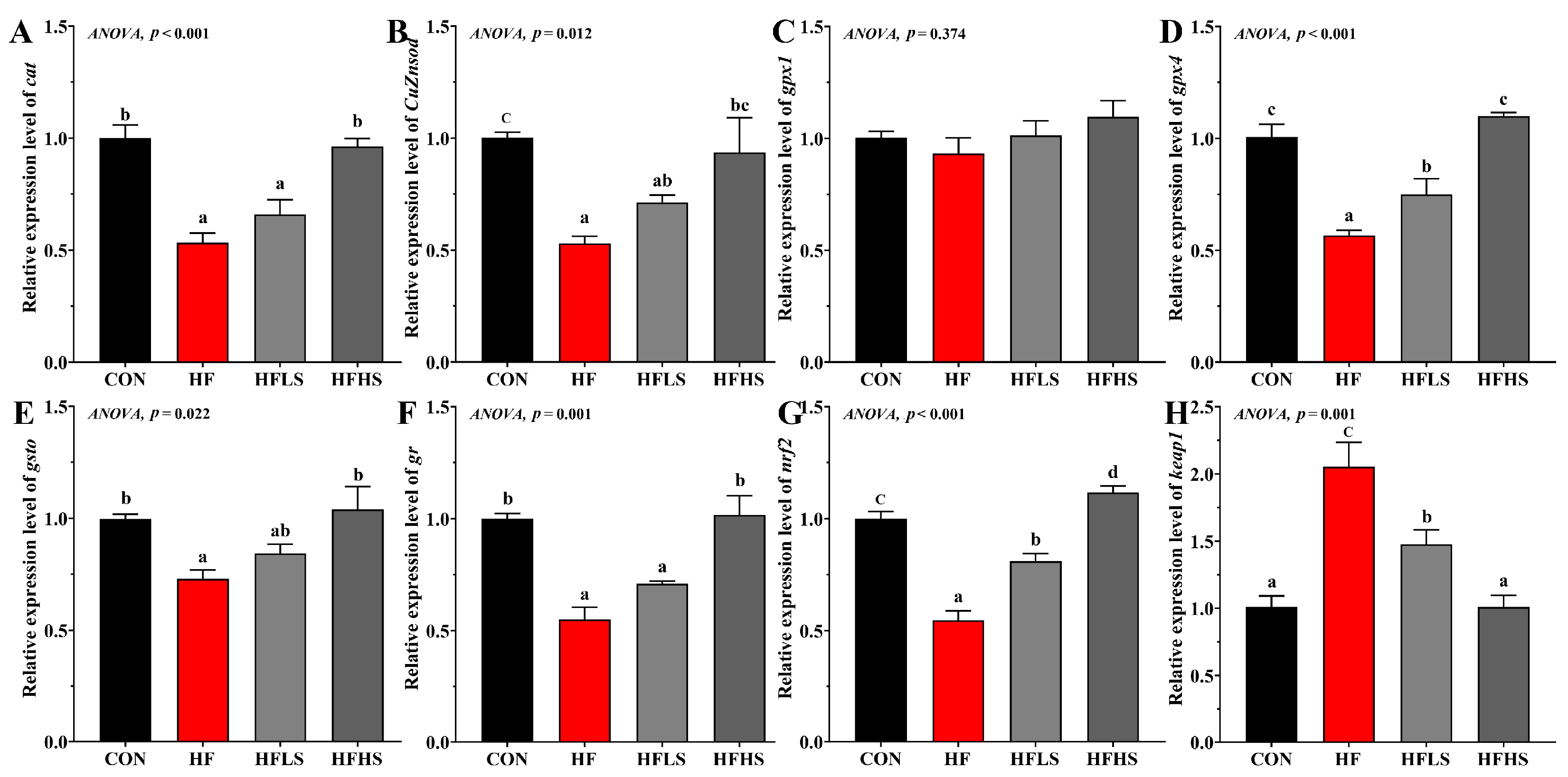
3.3. Intestinal Antioxidant-Related Gene Expression
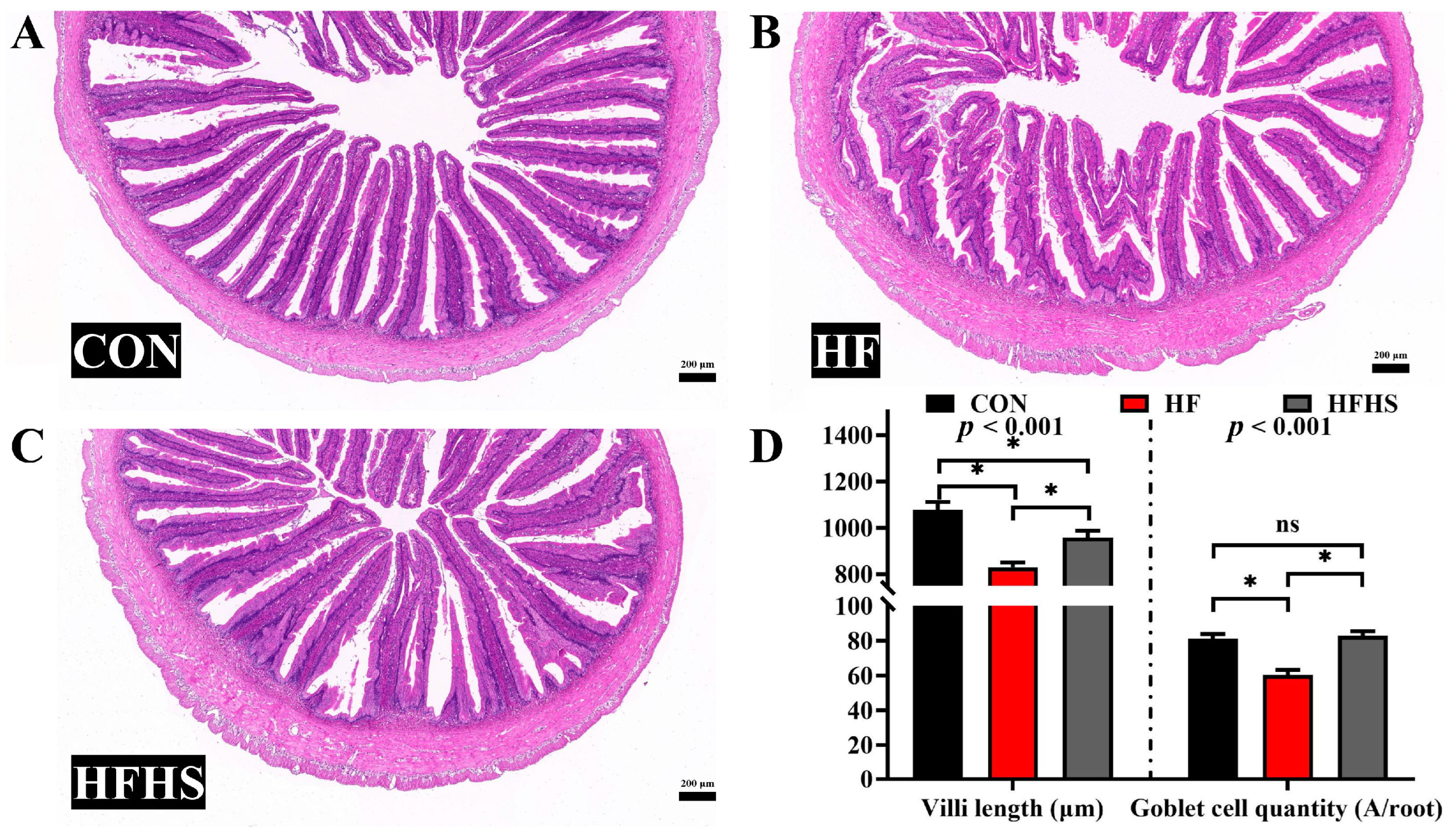
3.4. Intestinal Morphology
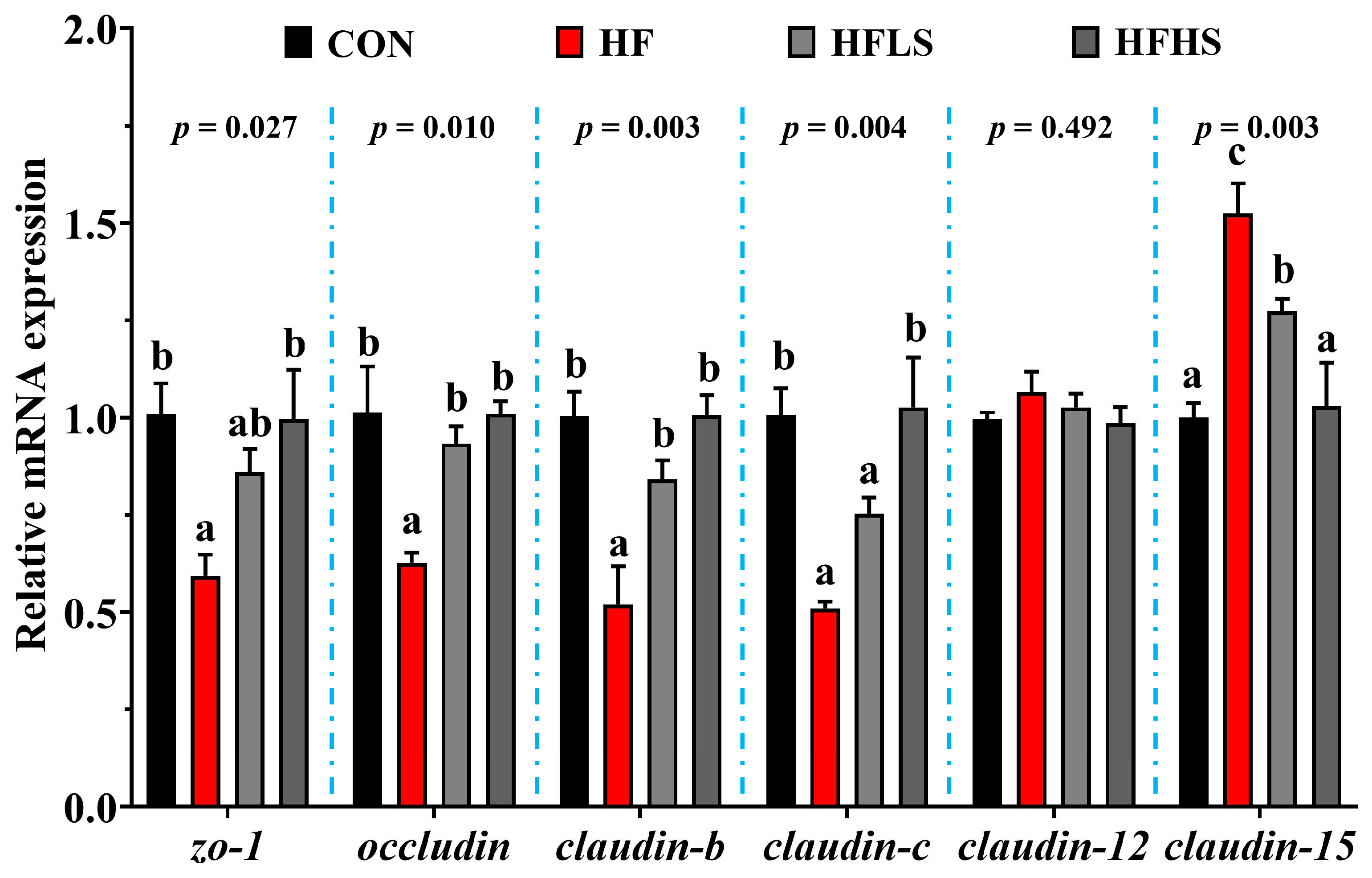
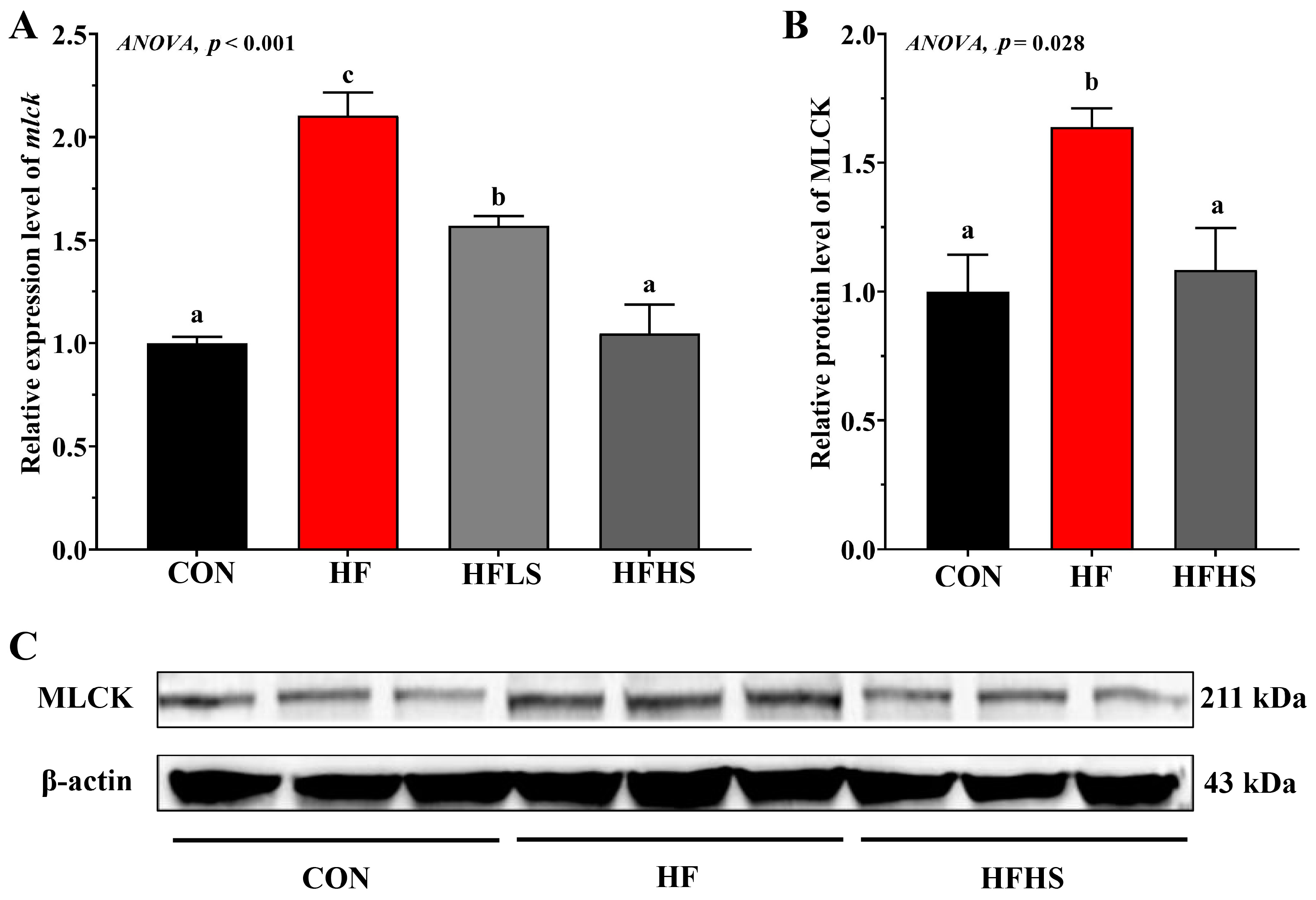
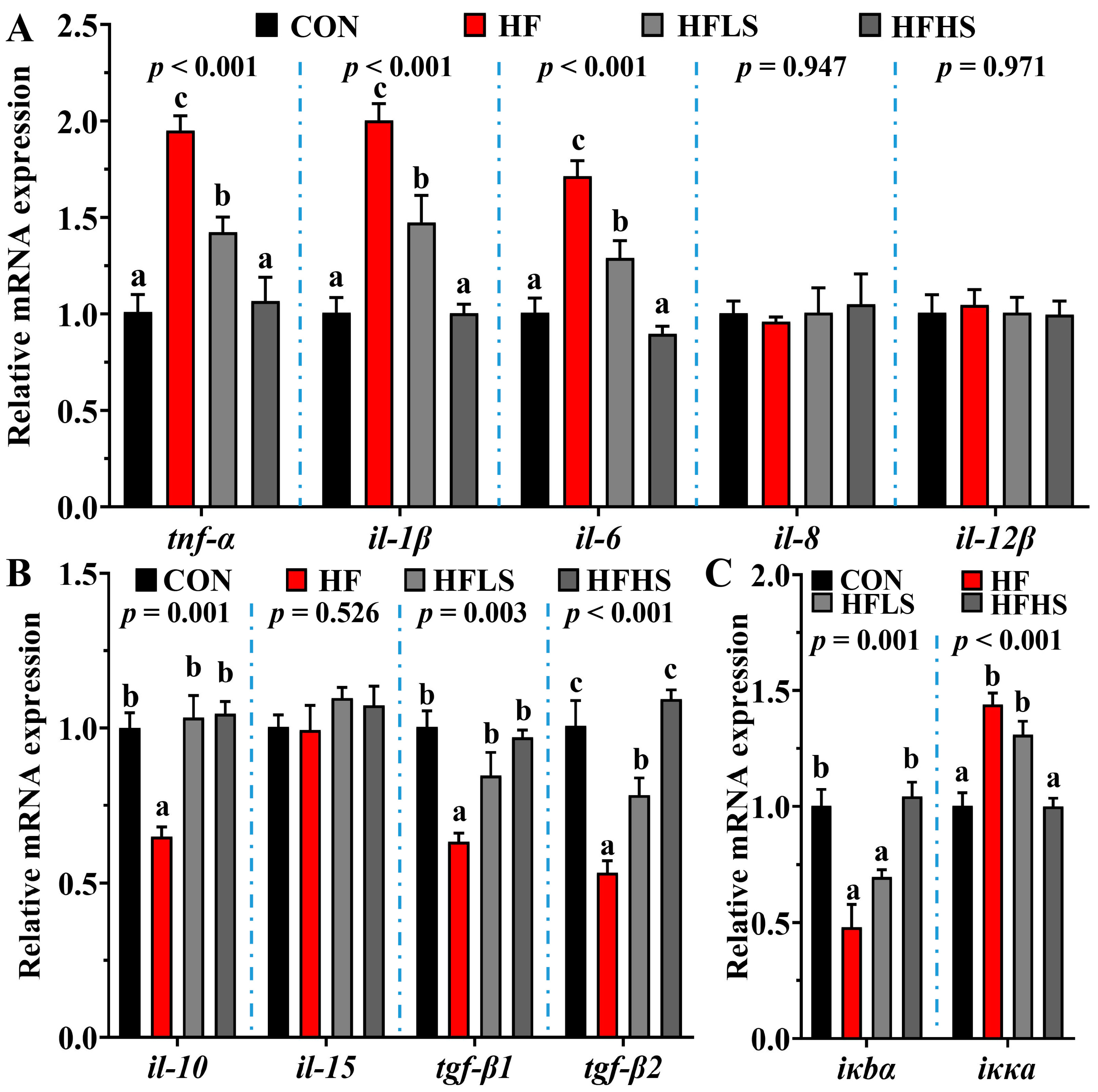
3.5. Intestinal Physical Barrier-Related Gene Expression and MLCK Protein Expression
3.6. Intestinal Immune Barrier-Related Gene Expression and NF-κB Protein Expression
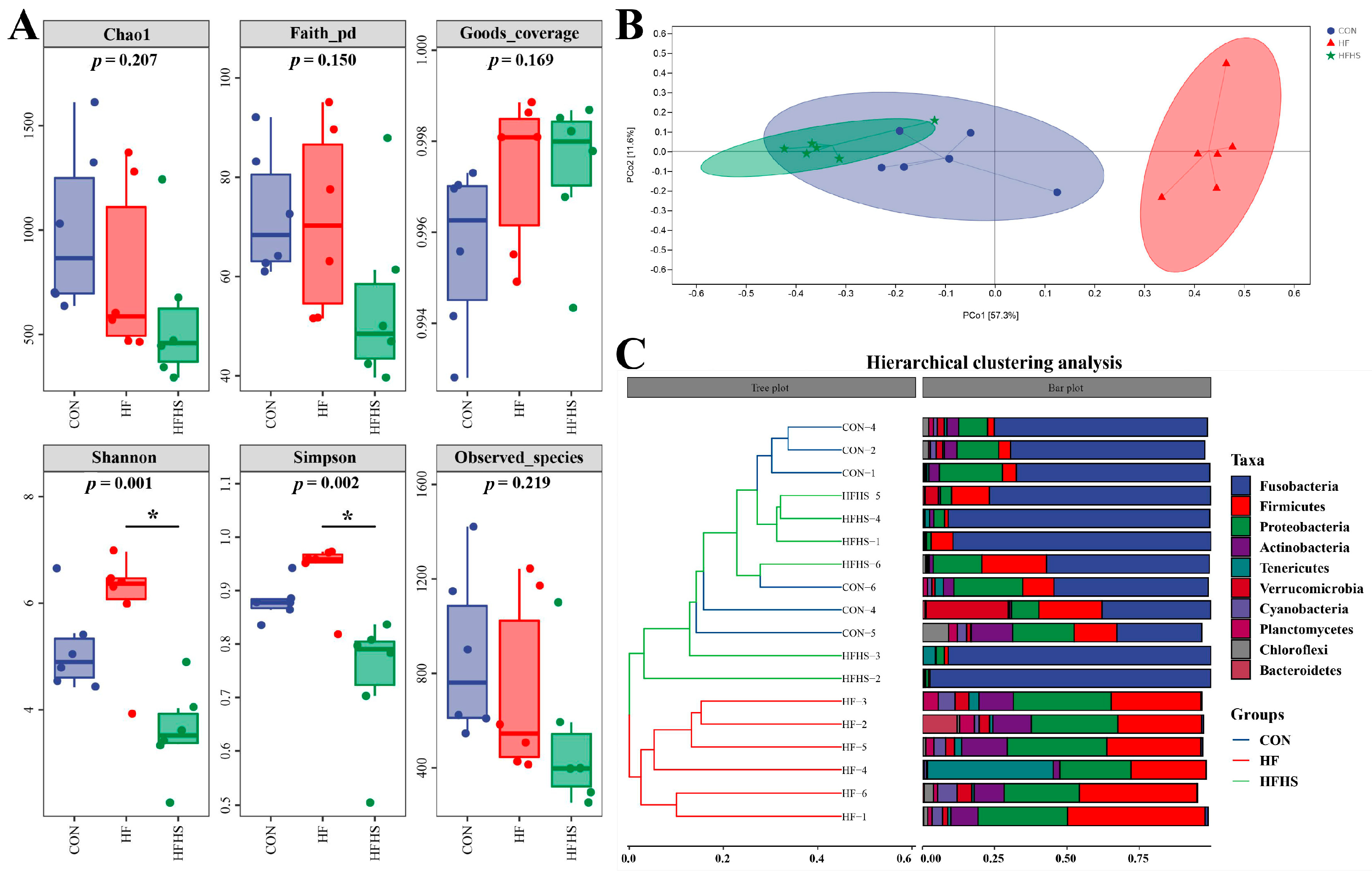
3.7. Diversity Analysis of Intestinal Microbiota
3.8. Composition of Intestinal Microbiota
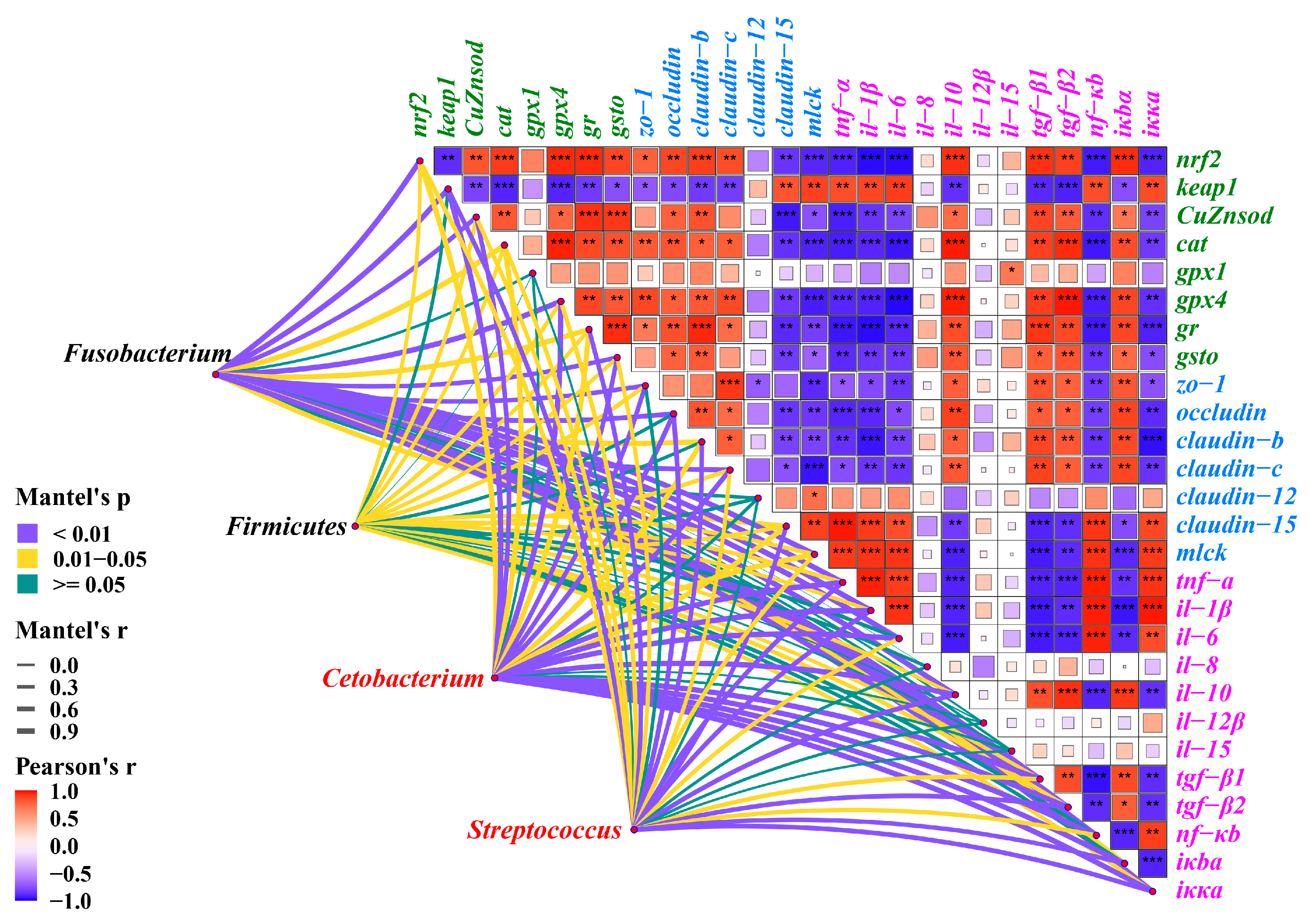
3.9. Multidimensional Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Obling, J.M. National Research Council (NRC): Nutrient requirements of fish and shrimp. Aquac. Int. 2012, 20, 601–602. [Google Scholar] [CrossRef]
- Du, Z.Y.; Clouet, P.; Zheng, W.H.; Degrace, P.; Tian, L.X.; Liu, Y.J. Biochemical hepatic alterations and body lipid composition in the herbivorous grass carp (Ctenopharyngodon idella) fed high-fat diets. Brit. J. Nutr. 2006, 951, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Liu, W.B.; Jiang, Y.Y.; Zhu, H.; Ge, X.P. Effects of dietary protein and lipid levels in practical diets on growth performance and body composition of blunt snout bream (Megalobrama amblycephala) fingerlings. Aquaculture 2010, 303, 65–70. [Google Scholar] [CrossRef]
- Li, X.; Zheng, S.; Ma, X.; Cheng, K.; Wu, G. Effects of dietary protein and lipid levels on the growth performance, feed utilization, and liver histology of largemouth bass (Micropterus salmoides). Amino Acids 2020, 52, 1043–1061. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, Y.J.; Tian, L.X.; Mai, K.S.; Liang, G.Y.; Yang, H.J.; Huai, M.Y.; Luo, W.J. Protein-sparing capability of dietary lipid in herbivorous and omnivorous freshwater in fish: A comparative case study on grass carp (Ctenopharyngodon idella) and tilapia (Oreochromis niloticus × O. aureus). Aquacult. Nutr. 2011, 17, 2–12. [Google Scholar] [CrossRef]
- Du, Z.Y.; Clouet, P.; Huang, L.M.; Degrace, P.; Zheng, W.H.; He, J.G.; Tian, L.X.; Liu, Y.J. Utilization of different dietary lipid sources at high level in herbivorous grass carp (Ctenopharyngodon idella): Mechanism related to hepatic fatty acid oxidation. Aquacult. Nutr. 2008, 14, 77–92. [Google Scholar] [CrossRef]
- Li, X.; Jiang, Y.; Liu, W.; Ge, X. Protein-sparing effect of dietary lipid in practical diets for blunt snout bream (Megalobrama amblycephala) fingerlings: Effects on digestive and metabolic responses. Fish Physiol. Biochem. 2012, 38, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, Y.; Zhao, H.; Chen, W.; Chen, Y.; Lin, S. Effect of dietary lipid level on growth, lipid metabolism and oxidative status of largemouth bass, Micropterus salmoides. Aquaculture 2019, 506, 394–400. [Google Scholar] [CrossRef]
- Xie, S.; Lin, Y.; Wu, T.; Tian, L.; Liang, J.; Tan, B. Dietary lipid levels affected growth performance, lipid accumulation, inflammatory response and apoptosis of Japanese seabass (Lateolabrax japonicus). Aquacult. Nutr. 2021, 27, 807–816. [Google Scholar] [CrossRef]
- Zhang, W.; Dan, Z.; Zhuang, Y.; Zheng, J.; Gong, Y.; Lium, Y.; Mai, K.; Ai, Q. Effects of Dietary lipid levels on growth, digestive enzyme activities, antioxidant capacity, and lipid metabolism in turbot (Scophthalmus maximus L.) at three different stages. Aquacult. Nutr. 2022, 2022, 1042263. [Google Scholar] [CrossRef]
- Lv, H.B.; Ma, Y.Y.; Hu, C.T.; Liu, Q.Y.; Yue, J.J.Y.; Chen, L.Q.; Zhang, M.L.; Du, Z.Y.; Qiao, F. The individual and combined effects of hypoxia and high-fat diet feeding on nutrient composition and flesh quality in Nile tilapia (Oreochromis niloticus). Food Chem. 2021, 343, 128479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Limbu, S.M.; Zhao, S.H.; Chen, L.Q.; Luo, Y.; Zhang, M.L.; Qiao, F.; Du, Z.Y. Dietary L-carnitine supplementation recovers the increased pH and hardness in fillets caused by high-fat diet in Nile tilapia (Oreochromis niloticus). Food Chem. 2022, 382, 132367. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.Y.; Liu, Y.J.; Tian, L.X.; Wang, J.T.; Wang, Y.; Liang, G.Y. Effect of dietary lipid level on growth, feed utilization and body composition by juvenile grass carp (Ctenopharyngodon idella). Aquacult. Nutr. 2005, 11, 139–146. [Google Scholar] [CrossRef]
- Ma, Q.; Li, L.Y.; Le, J.Y.; Lu, D.L.; Qian, F.; Zhang, M.L.; Du, Z.Y.; Li, D.L. Dietary microencapsulated oil improves immune function and intestinal health in Nile tilapia fed with high-fat diet. Aquaculture 2018, 496, 19–29. [Google Scholar] [CrossRef]
- Jia, Y.; Jing, Q.; Niu, H.; Huang, B. Ameliorative effect of vitamin E on hepatic oxidative stress and hypoimmunity induced by high-fat diet in turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2017, 67, 634–642. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhong, L.; Zhong, H.; Zhang, J.; Che, C.; Fu, G.; Hu, Y.; Mai, K. Taurine supplements in high-fat diets improve survival of juvenile Monopterus albus by reducing lipid deposition and intestinal damage. Aquaculture 2022, 547, 737431. [Google Scholar] [CrossRef]
- Falcinelli, S.; Rodiles, A.; Hatef, A.; Picchietti, S.; Cossignani, L.; Merrifield, D.L.; Unniappan, S.; Carnevali, O. Dietary lipid content reorganizes gut microbiota and probiotic L. Rhamnosus attenuates obesity and enhances catabolic hormonal milieu in zebrafish. Sci. Rep. 2017, 7, 5512. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Meng, D.; Hao, Q.; Xia, R.; Zhang, Q.; Ran, C.; Yang, Y.; Li, D.; Liu, W.; Zhang, Z.; et al. Effect of supplementation of solid-state fermentation product of Bacillus subtilis HGcc-1 to high-fat diet on growth, hepatic lipid metabolism, epidermal mucus, gut and liver health and gut microbiota of zebrafish. Aquaculture 2022, 560, 738542. [Google Scholar] [CrossRef]
- Peng, M.; Xue, J.; Hu, Y.; Wen, C.; Hu, B.; Jian, S.; Liang, L.; Yang, G. Disturbance in the homeostasis of intestinal microbiota by a high-fat diet in the rice field eel (Monopterus albus). Aquaculture 2019, 502, 347–355. [Google Scholar] [CrossRef]
- Hou, B.; Zeng, J.G. Biolological activities of Sanguinarine and application of Macleaya cordata extract in animal production. Chin. J. Anim. Nutr. 2018, 30, 413–420. [Google Scholar]
- Wu, Y.; Zhao, N.; Cao, Y.; Sun, Z.; Wang, Q.; Liu, Z.; Sun, Z. Sanguinarine metabolism and pharmacokinetics study in vitro and in vivo. J. Vet. Pharm. Ther. 2020, 43, 208–214. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Liu, Y.Y.; Che, C.B.; Zhong, L. Effects of sanguinarine on immune and intestinal inflammation related to gene expression in rice field eels (Monopterus albus) induced by LPS. J. Fish Sci. China 2020, 27, 125–137. [Google Scholar]
- Shi, Y.; Zhong, L.; Chen, K.; Fan, Y.; Xie, K.; Zhang, J.; Dai, J.; Hu, Y. Sanguinarine attenuates hydrogen peroxide-induced toxicity in liver of Monopterus albus: Role of oxidative stress, inflammation and apoptosis. Fish Shellfish Immunol. 2022, 125, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Zhong, L.; Chen, T.; Shi, Y.; Hu, Y.; Zeng, J.; Liu, H.; Xu, S. Dietary sanguinarine supplementation on the growth performance, immunity and intestinal health of grass carp (Ctenopharyngodon idellus) fed cottonseed and rapeseed meal diets. Aquaculture 2020, 528, 735521. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.W.; Zhu, J.Y.; Liu, L.L.; Liu, Y.C.; Zhu, H. Dietary sanguinarine affected immune response, digestive enzyme activity and intestinal microbiota of Koi carp (Cryprinus carpiod). Aquaculture 2019, 502, 72–79. [Google Scholar] [CrossRef]
- Aguilar-Hernández, J.A.; Urías-Estrada, J.D.; López-Soto, M.A.; Barreras, A.; Plascencia, A.; Montaño, M.; González-Vizcarra, V.M.; Estrada-Angulo, A.; Castro-Pérez, B.I.; Barajas, R.; et al. Evaluation of isoquinoline alkaloid supplementation levels on ruminal fermentation, characteristics of digestion, and microbial protein synthesis in steers fed a high-energy diet. J. Anim. Sci. 2016, 94, 267–274. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Wang, X.L.; Ou, S.Q.; Hou, D.X.; He, J.H. Sanguinarine modulate gut microbiome and intestinal morphology to enhance growth performance in broilers. PLoS ONE 2020, 15, e0234920. [Google Scholar] [CrossRef]
- Liu, G.; Guan, G.; Fang, J.; Martínez, Y.; Chen, S.; Bin, P.; Duraipandiyan, V.; Gong, T.; Tossou, M.C.B.; Al-Dhabi, N.A.; et al. Macleaya cordata extract decreased diarrhea score and enhanced intestinal barrier function in growing piglets. Bio. Med. Res. Int. 2016, 2016, 1069585. [Google Scholar]
- Shi, Y.; Zhong, L.; Ma, X.; Liu, Y.; Tang, T.; Hu, Y. Effect of replacing fishmeal with stickwater hydrolysate on the growth, serum biochemical indexes, immune indexes, intestinal histology and microbiota of rice field eel (Monopterus albus). Aquac. Rep. 2019, 15, 100223. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Zhang, J.Z.; Ma, X.K.; Zhong, H.; Peng, M.; He, H.; Hu, Y. Substitution of fish meal with krill meal in rice field eel (Monopterus albus) diets: Effects on growth, immunity, muscle textural quality, and expression of myogenic regulation factors. Anim. Feed Sci. Technol. 2021, 280, 115047. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Liu, Y.L.; Zhang, J.Z.; Lv, Z.; Li, Y.; Hu, Y. Effects of dietary andrographolide levels on growth performance, antioxidant capacity, intestinal immune function and microbioma of rice field eel (Monopterus albus). Animals 2020, 10, 1744. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Yang, Q.; Tan, B.; Dong, X.; Chi, S.; Liu, H.; Zhang, S. Effects of replacing soybean meal with cottonseed meal on growth, feed utilization and non-specific immune enzyme activities for juvenile white shrimp, Litopenaeus vannamei. Aquac. Rep. 2020, 16, 100255. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, L.; Wang, H.; Xie, F.; Wang, T. Effect of dietary vitamin C on the growth performance and innate immunity of juvenile cobia (Rachycentron canadum). Fish Shellfish Immunol. 2013, 34, 1688. [Google Scholar] [CrossRef]
- Pilström, L.; Bengtén, E. Immunoglobulin in fifish-genes, expression and structure. Fish Shellfish Immunol. 1996, 6, 243–262. [Google Scholar] [CrossRef]
- Mori, K.; Nakanishi, T.; Suzuki, T.; Oohara, I. Defense mechanisms in invertebrates and fish. Tanpakushitsu Kakusan Koso Protein Nucleic Acid Enzym. 1989, 34, 214. [Google Scholar]
- Chen, Q.Q.; Liu, W.B.; Zhou, M.; Dai, Y.J.; Xu, C.; Tian, H.Y.; Xu, W.N. Effects of berberine on the growth and immune performance in response to ammonia stress and high-fat dietary in blunt snout bream Megalobrama amblycephala. Fish Shellfish Immunol. 2016, 55, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Pan, T.; Cheng, X.; Zhu, T.T.; Sun, P.; Zhou, F.; Ding, X.; Zhou, Q. Effects of supplemental dietary L-carnitine and bile acids on growth performance, antioxidant and immune ability, histopathological changes and inflammatory response in juvenile black seabream (Acanthopagrus schlegelii) fed high-fat diet. Aquaculture 2019, 504, 199–209. [Google Scholar] [CrossRef]
- Nhu, T.Q.; Dam, N.P.; Hang, B.T.B.; Hue, B.T.B.; Scippo, M.L.; Phuong, N.T.; Quetin-Leclercq, J.; Kestemont, P. Immunomodulatory potential of extracts, fractions and pure compounds from Phyllanthus amarus and Psidium guajava on striped catfish (Pangasianodon hypophthalmus) head kidney leukocytes. Fish Shellfish Immunol. 2020, 104, 289–303. [Google Scholar] [CrossRef]
- Chen, J.S.; Kang, B.J.; Zeng, J.G.; Hu, H.B.; Zhao, Y.R.; Chen, L.; Yao, K.; Fu, C.X. Effects of dietary sanguinarine on growth performance, intestinal mucosal morphology and immune function of small intestinal mucosa of weaned piglets. Chin. J. Anim. Nutr. 2018, 30, 1845–1853. [Google Scholar]
- Lushchak, V.I. Contaminant-induced oxidative stress in fish: A mechanistic approach. Fish Physiol. Biochem. 2016, 42, 711–747. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Intestinal redox biology and oxidative stress. Semin. Cell Dev. Biol. 2012, 23, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Dinu, D.; Marinescu, D.; Munteanu, M.C.; Staicu, A.C.; Costache, M.; Dinischiotu, A. Modulatory effects of deltamethrin on antioxidant defense mechanisms and lipid peroxidation in Carassius auratus gibelio liver and intestine. Arch. Environ. Contam. Toxicol. 2010, 58, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.C.; Wang, X.; Ren, J.; Wang, J.; Limbu, S.M.; Li, R.X.; Zhou, W.H.; Qiao, F.; Zhang, M.L.; Du, Z.Y. Different effects of two dietary levels of tea polyphenols on the lipid deposition, immunity and antioxidant capacity of juvenile GIFT tilapia (Oreochromis niloticus) fed a high-fat diet. Aquaculture 2021, 542, 736896. [Google Scholar] [CrossRef]
- Lu, K.L.; Xu, W.N.; Liu, W.B.; Wang, L.N.; Zhang, C.N.; Li, X.F. Association of mitochondrial dysfunction with oxidative stress and immune suppression in blunt snout bream Megalobrama amblycephala fed a high-fat diet. J. Aquat. Anim. Health 2014, 26, 100–112. [Google Scholar] [CrossRef]
- Chen, J.; Kang, B.; Yao, K.; Fu, C.; Zhao, Y. Effects of dietary Macleaya cordata extract on growth performance, immune responses, antioxidant capacity, and intestinal development in weaned piglets. J. Appl. Anim. Res. 2019, 47, 349–356. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, J.S.; Oh, S.T.; Kang, C.W.; An, B.K. Effects of dietary sanguinarine on growth performance, relative organ weight, cecal microflora, serum cholesterol level and meat quality in broiler chickens. J. Poult. Sci. 2015, 52, 15–22. [Google Scholar] [CrossRef]
- Vrba, J.; Orolinova, E.; Ulrichova, J. Induction of heme oxygenase-1 by Macleaya cordata extract and its constituent sanguinarine in RAW264.7 cells. Fitoterapia 2012, 83, 329–335. [Google Scholar] [CrossRef]
- Hoyle, I.; Shaw, B.J.; Handy, R.D. Dietary copper exposure in the African walking catfish, Clarias gariepinus: Transient osmoregulatory disturbances and oxidative stress. Aquat. Toxicol. 2007, 83, 62–72. [Google Scholar] [CrossRef]
- Niklasson, L.; Sundh, H.; Fridell, F.; Taranger, G.L.; Sundell, K. Disturbance of the intestinal mucosal immune system of farmed Atlantic salmon (Salmo salar), in response to long-term hypoxic conditions. Fish Shellfish Immunol. 2011, 31, 1072–1080. [Google Scholar] [CrossRef]
- Luo, J.B.; Lin, F.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Zhang, Y.A.; Zhou, X.Q. The impaired intestinal mucosal immune system by valine deficiency for young grass carp (Ctenopharyngodon idella) is associated with decreasing immune status and regulating tight junction proteins transcript abundance in the intestine. Fish Shellfish Immunol. 2014, 40, 197–207. [Google Scholar] [CrossRef]
- Xu, H.J.; Jiang, W.D.; Feng, L.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary vitamin C deficiency depresses the growth, head kidney and spleen immunity and structural integrity by regulating NF-κB, TOR, Nrf2, apoptosis and MLCK signaling in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2016, 52, 111–138. [Google Scholar] [CrossRef]
- Camilleri, M. Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut 2019, 68, 1516–1526. [Google Scholar] [CrossRef]
- Tian, B.; Zhao, J.; Zhang, M.; Chen, Z.; Ma, Q.; Liu, H.; Nie, C.; Li, J. Lycium ruthenicum anthocyanins attenuate high-fat diet-induced colonic barrier dysfunction and inflammation in mice by modulating the gut microbiota. Mol. Nutr. Food. Res. 2021, 65, 2000745. [Google Scholar] [CrossRef]
- de La Serre, C.B.; Ellis, C.L.; Lee, J.; Hartman, A.L.; Rutledge, J.C.; Raybould, H.E. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol-Gastr. Liver Physiol. 2010, 299, G440–G448. [Google Scholar] [CrossRef] [PubMed]
- Abbasi-Oshaghi, E.; Mirzaei, F.; Mirzaei, A. Effects of ZnO nanoparticles on intestinal function and structure in normal/high fat diet-fed rats and Caco-2 cells. Nanomedicine 2018, 13, 2791–2816. [Google Scholar] [CrossRef]
- Wang, F.; Zou, P.; Xu, S.; Wang, Q.; Zhou, Y.; Li, X.; Tang, L.; Wang, B.; Jin, Q.; Yu, D.; et al. Dietary supplementation of Macleaya cordata extract and Bacillus in combination improve laying performance by regulating reproductive hormones, intestinal microbiota and barrier function of laying hens. J. Animal Sci. Biotechnol. 2022, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, S.S.; Xiong, X.Y.; Su, D.D.; He, J.H. Effects of macleaya cordata alkaloids on intestinal porcine epithelial cells proliferation. Chin. J. Anim Nutr. 2014, 26, 1632–1637. [Google Scholar]
- Shen, L.; Black, E.D.; Witkowski, E.D.; Lencer, W.I.; Guerriero, V.; Schneeberger, E.E.; Turner, J.R. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J. Cell Sci. 2006, 119, 2095–2106. [Google Scholar] [CrossRef]
- Song, Z.X.; Jiang, W.D.; Liu, Y.; Wu, P.; Jiang, J.; Zhou, X.Q.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Dietary zinc deficiency reduced growth performance, intestinal immune and physical barrier functions related to NF-κB, TOR, Nrf2, JNK and MLCK signaling pathway of young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017, 66, 497–523. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.H.; Lu, Z.X.; Polya, G.M. Inhibition of eukaryote protein kinases by isoquinoline and oxazine alkaloids. Planta. Med. 1997, 63, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Cao, L.P.; Du, J.L.; He, Q.; Gu, Z.Y.; Jeney, G.; Xu, P.; Yin, G.J. Effects of high-fat diet on antioxidative status, apoptosis and inflammation in liver of tilapia (Oreochromis niloticus) via Nrf2, TLRs and JNK pathways. Fish Shellfish Immunol. 2020, 104, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, J.; Qin, Q.; Liu, J.; Xu, J.; Xu, W. Berberine improved intestinal barrier function by modulating the intestinal microbiota in blunt snout bream (Megalobrama amblycephala) under dietary high-fat and high-carbohydrate stress. Fish Shellfish Immunol. 2020, 102, 336–349. [Google Scholar] [CrossRef] [PubMed]
- Elewaut, D.; DiDonato, J.A.; Kim, J.M.; Truong, F.; Eckmann, L.; Kagnoff, M.F. NF-κB is a central regulator of the intestinal epithelial cell innate immune response induced by infection with enteroinvasive bacteria. J. Immunol. 1999, 163, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Y.; Jiang, J.; Wu, P.; Jiang, W.; Li, S.; Tang, L.; Kuang, S.; Feng, L.; Zhou, X. Effects of dietary isoleucine on the immune response, antioxidant status and gene expression in the head kidney of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 2013, 35, 572–580. [Google Scholar] [CrossRef]
- Xie, J.; Liao, S.; Wang, R.; He, X.; Fang, H.; Zhuang, Z.; Wei, D.; Tian, L.; Liu, Y.; Niu, J. Molecular cloning, functional characterization and expression analysis of p65 subunit of golden pompano (Trachinotus ovatus) and response to high fat diet and LPS administration. Aquaculture 2020, 514, 734508. [Google Scholar] [CrossRef]
- Xu, R.; Ding, F.F.; Zhou, N.N.; Wang, T.; Wu, H.X.; Qiao, F.; Chen, L.Q.; Du, Z.Y.; Zhang, M.L. Bacillus amyloliquefaciens protects Nile tilapia against Aeromonas hydrophila infection and alleviates liver inflammation induced by high-carbohydrate diet. Fish Shellfish Immunol. 2022, 127, 836–842. [Google Scholar] [CrossRef]
- Zhang, F.; Luan, Y.; Hao, Q.; Zhang, Q.; Yang, Y.; Ran, C.; Zhang, Z.; Zhou, Z. Nuclease treatment enhances the probiotic effect of Bacillus velezensis T23 on hepatic steatosis and inflammation induced by high-fat diet in zebrafish. Aquaculture 2023, 562, 738801. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, Y.; Hu, Z.; Zhang, Y.; Ni, J.; Ma, Z.; Liao, H.; Wu, Q.; Tang, Q. Sanguinarine attenuates lipopolysaccharide-induced inflammation and apoptosis by inhibiting the TLR4/NF-κB pathway in H9c2 cardiomyocytes. Curr. Med. Sci. 2018, 38, 204–211. [Google Scholar] [CrossRef]
- Gerritsen, J.; Smidt, H.; Rijkers, G.T.; de Vos, W.M. Intestinal microbiota in human health and disease: The impact of probiotics. Genes. Nutr. 2011, 6, 209–240. [Google Scholar] [CrossRef]
- Wei, L.; Yue, F.; Xing, L.; Wu, S.; Shi, Y.; Li, J.; Xiang, X.; Lam, S.M.; Shui, G.; Russell, R.; et al. Constant light exposure alters gut microbiota and promotes the progression of steatohepatitis in high fat diet rats. Front. Microbiol. 2020, 11, 1975. [Google Scholar] [CrossRef]
- Shi, F.; Lu, Z.; Yang, M.; Li, F.; Zhan, F.; Zhao, L.; Li, Y.; Li, Q.; Li, J.; Li, J.; et al. Astragalus polysaccharides mediate the immune response and intestinal microbiota in grass carp (Ctenopharyngodon idellus). Aquaculture 2021, 534, 736205. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, J.; Yi, K.; Peng, L.; Xie, J.; Gou, X.; Peng, T.; Tang, L. Phlorizin ameliorates obesity-associated endotoxemia and insulin resistance in high-fat diet-fed mice by targeting the gut microbiota and intestinal barrier integrity. Gut Microbes 2020, 12, 1842990. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhang, Z.; Ding, Q.; Yang, Y.; Bindelle, J.; Ran, C.; Zhou, Z. Intestinal Cetobacterium and acetate modify glucose homeostasis via parasympathetic activation in zebrafish. Gut Microbes 2021, 13, 1–15. [Google Scholar] [CrossRef]
- Zhang, Z.; Ran, C.; Ding, Q.W.; Liu, H.L.; Xie, M.X.; Yang, Y.L.; Xie, Y.D.; Gao, C.C.; Zhang, H.L.; Zhou, Z.G. Ability of prebiotic polysaccharides to activate a HIF1α-antimicrobial peptide axis determines liver injury risk in zebrafish. Commun. Biol. 2019, 2, 274. [Google Scholar] [CrossRef] [PubMed]
- Fachi, J.L.; de Souza Felipe, J.; Pral, L.P.; da Silva, B.K.; Corrêa, R.O.; de Andrade, M.C.P.; da Fonseca, D.M.; Basso, P.J.; Câmara, N.O.S.; de Sales e Souza, É.L.; et al. Butyrate protects mice from Clostridium difficile-induced colitis through an HIF-1-dependent mechanism. Cell Rep. 2019, 27, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.T.; Wen, B.; Shen, X.C.; Bian, Z.X. Potential of plant-sourced phenols for inflammatory bowel disease. Curr. Med. Chem. 2018, 25, 5191–5217. [Google Scholar] [CrossRef]



| CON | HF | HFLS | HFHS | |
|---|---|---|---|---|
| Fish meal (%) | 6 | 6 | 6 | 6 |
| Soybean meal (%) | 26 | 26 | 26 | 26 |
| Cottonseed meal (%) | 10 | 10 | 10 | 10 |
| Rapeseed meal (%) | 15 | 15 | 15 | 15 |
| Flour (%) | 27 | 27 | 27 | 27 |
| DDGS (%) | 4 | 4 | 4 | 4 |
| Microcrystalline cellulose (%) | 6.06 | 1.06 | 1.06 | 1.06 |
| Choline chloride (%) | 0.2 | 0.2 | 0.2 | 0.2 |
| Soybean oil (%) | 3.2 | 8.2 | 8.2 | 8.2 |
| Ca(H2PO4)2 (%) | 1.5 | 1.5 | 1.5 | 1.5 |
| Premix a (%) | 1 | 1 | 1 | 1 |
| Mould inhibitor (%) | 0.03 | 0.03 | 0.03 | 0.03 |
| Antioxidants (%) | 0.01 | 0.01 | 0.01 | 0.01 |
| Sanguinarine (μg/kg) | 0 | 0 | 600 | 1200 |
| Proximate composition (%) | ||||
| Saturated fatty acids | 22.48 | 17.35 | 17.19 | 17.24 |
| Unsaturated fatty acids | 73.56 | 78.17 | 78.26 | 78.34 |
| Crude protein | 31.72 | 31.32 | 31.98 | 31.75 |
| Crude lipid | 4.95 | 10.27 | 10.36 | 10.09 |
| Ash | 6.42 | 6.35 | 6.42 | 6.64 |
| Carbohydrate | 34.27 | 34.32 | 34.18 | 34.26 |
| Crude fiber | 12.48 | 7.17 | 7.26 | 7.21 |
| Gene | Forward Sequences (5′→3′) | Reverse Sequences (5′→3′) | Accession Number |
|---|---|---|---|
| nrf2 | CTGGACGAGGAGACTGGA | ATCTGTGGTAGGTGGAAC | KF733814 |
| keap1 | TTCCACGCCCTCCTCAA | TGTACCCTCCCGCTATG | KF811013 |
| gpx1 | GGGCTGGTTATTCTGGGC | AGGCGATGTCATTCCTGTTC | EU828796 |
| gpx4 | TACGCTGAGAGAGGTTTACACAT | CTTTTCCATTGGGTTGTTCC | KU255598 |
| gr | GTGTCCAACTTCTCCTGTG | ACTCTGGGGTCCAAAACG | JX854448 |
| cat | GAAGTTCTACACCGATGAGG | CCAGAAATCCCAAACCAT | FJ560431 |
| CuZnsod | CGCACTTCAACCCTTACA | ACTTTCCTCATTGCCTCC | GU901214 |
| gsto | GGTGCTCAATGCCAAGGGAA | CTCAAACGGGTCGGATGGAA | KT757314 |
| zo-1 | CGGTGTCTTCGTAGTCGG | CAGTTGGTTTGGGTTTCAG | KJ000055 |
| occludin | TATCTGTATCACTACTGCGTCG | CATTCACCCAATCCTCCA | KF193855 |
| claudin-b | GAGGGAATCTGGATGAGC | ATGGCAATGATGGTGAGA | KF193860 |
| claudin-c | GAGGGAATCTGGATGAGC | CTGTTATGAAAGCGGCAC | KF193859 |
| claudin-12 | CCCTGAAGTGCCCACAA | GCGTATGTCACGGGAGAA | KF998571 |
| claudin-15 | TGCTTTATTTCTTGGCTTTC | CTCGTACAGGGTTGAGGTG | KF193857 |
| mlck | GAAGGTCAGGGCATCTCA | GGGTCGGGCTTATCTACT | KM279719 |
| tnf-α | CGCTGCTGTCTGCTTCAC | CCTGGTCCTGGTTCACTC | HQ696609 |
| il-1β | AGAGTTTGGTGAAGAAGAGG | TTATTGTGGTTACGCTGGA | JQ692172 |
| il-6 | CAGCAGAATGGGGGAGTTATC | CTCGCAGAGTCTTGACATCCTT | KC535507.1 |
| il-8 | ATGAGTCTTAGAGGTCTGGGT | ACAGTGAGGGCTAGGAGGG | JN663841 |
| il-10 | AATCCCTTTGATTTTGCC | GTGCCTTATCCTACAGTATGTG | HQ388294 |
| il-12β | ACAAAGATGAAAAACTGGAGGC | GTGTGTGGTTTAGGTAGGAGCC | KF944668.1 |
| il-15 | CCTTCCAACAATCTCGCTTC | AACACATCTTCCAGTTCTCCTT | KT445872 |
| tgf-β1 | TTGGGACTTGTGCTCTAT | AGTTCTGCTGGGATGTTT | EU099588 |
| nf-κb | GAAGAAGGATGTGGGAGATG | TGTTGTCGTAGATGGGCTGAG | KJ526214 |
| iκbα | TCTTGCCATTATTCACGAGG | TGTTACCACAGTCATCCACCA | KJ125069 |
| iκκα | GGCTACGCCAAAGACCTG | CGGACCTCGCCATTCATA | KM279718 |
| iκκβ | GTGGCGGTGGATTATTGG | GCACGGGTTGCCAGTTTG | KP125491 |
| β-actin | GGCTGTGCTGTCCCTGTA | GGGCATAACCCTCGTAGAT | M25013 |
| Index | CON | HF | HFLS | HFHS | p-Value |
|---|---|---|---|---|---|
| ACP 1 (King’s unit/1000 mL) | 18.29 ± 0.12 | 18.08 ± 0.19 | 18.16 ± 0.16 | 18.24 ± 0.24 | 0.853 |
| AKP 2 (King’s unit/1000 mL) | 14.88 ± 0.54 b | 11.58 ± 0.47 a | 12.79 ± 0.37 a | 14.40 ± 0.56 b | 0.005 |
| C3 3 (g/L) | 0.39 ± 0.04 bc | 0.24 ± 0.02 a | 0.34 ± 0.02 b | 0.45 ± 0.03 c | 0.005 |
| C4 4 (g/L) | 0.14 ± 0.01 c | 0.06 ± 0.00 a | 0.09 ± 0.01 b | 0.14 ± 0.00 c | <0.001 |
| IgA 5 (g/L) | 0.35 ± 0.02 c | 0.14 ± 0.01 a | 0.21 ± 0.02 b | 0.31 ± 0.03 c | <0.001 |
| IgM 6 (g/L) | 1.32 ± 0.08 b | 0.70 ± 0.04 a | 0.81 ± 0.03 a | 1.24 ± 0.10 b | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Liu, Y.; Xie, K.; Zhang, J.; Wang, Y.; Hu, Y.; Zhong, L. Sanguinarine Improves Intestinal Health in Grass Carp Fed High-Fat Diets: Involvement of Antioxidant, Physical and Immune Barrier, and Intestinal Microbiota. Antioxidants 2023, 12, 1366. https://doi.org/10.3390/antiox12071366
Shi Y, Liu Y, Xie K, Zhang J, Wang Y, Hu Y, Zhong L. Sanguinarine Improves Intestinal Health in Grass Carp Fed High-Fat Diets: Involvement of Antioxidant, Physical and Immune Barrier, and Intestinal Microbiota. Antioxidants. 2023; 12(7):1366. https://doi.org/10.3390/antiox12071366
Chicago/Turabian StyleShi, Yong, Yuanxiang Liu, Kai Xie, Junzhi Zhang, Ya Wang, Yi Hu, and Lei Zhong. 2023. "Sanguinarine Improves Intestinal Health in Grass Carp Fed High-Fat Diets: Involvement of Antioxidant, Physical and Immune Barrier, and Intestinal Microbiota" Antioxidants 12, no. 7: 1366. https://doi.org/10.3390/antiox12071366
APA StyleShi, Y., Liu, Y., Xie, K., Zhang, J., Wang, Y., Hu, Y., & Zhong, L. (2023). Sanguinarine Improves Intestinal Health in Grass Carp Fed High-Fat Diets: Involvement of Antioxidant, Physical and Immune Barrier, and Intestinal Microbiota. Antioxidants, 12(7), 1366. https://doi.org/10.3390/antiox12071366





