An Overview of the Health Benefits, Extraction Methods and Improving the Properties of Pomegranate
Abstract
1. Introduction
2. Phytochemical Composition of Different Parts of the Plant
2.1. Fruits
2.2. Seeds
2.3. Leaves
2.4. Flowers
2.5. Peel
3. Pomegranate Extraction Techniques
3.1. Consolidated Extraction Methods
3.2. Emerging Green Extraction Methods
3.3. Perspective: Hydrodynamic Cavitation
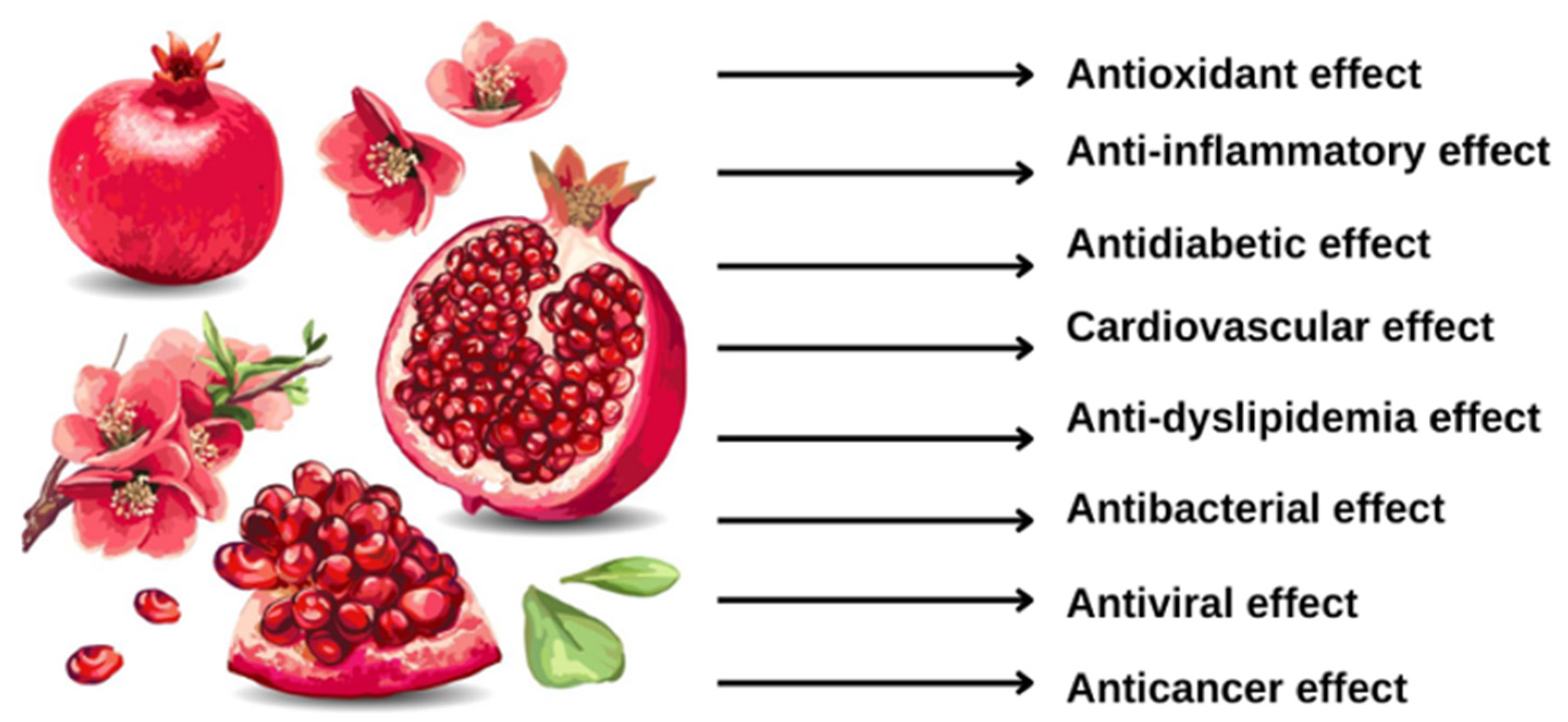
4. Beneficial Effects
4.1. Antioxidant Activity
4.2. Anti-Inflammatory Activity
4.3. Anti-Diabetic Effects
4.4. Cardiovascular Effects
4.5. Hypo-Lipidic Effects
4.6. Neuroprotective Effects
4.7. Antibacterial Activity
4.8. Antiviral Activity
4.9. Anticancer Effects
| Activity | Plant Part | Dosage | Design of study | Outcomes | Ref. |
|---|---|---|---|---|---|
| Antioxidant | PJ | 50 g/day for 4 weeks. | Placebo-controlled RCT on 40 T2D | ↓ IL-6, ↑ TAC | [89] |
| PJ | 200 mL/day for 6 weeks | Placebo-controlled RCT on diabetic patients | ↓ lipid oxidation, ↑ TAC | [87] | |
| PJ | 250 mL/day for 12 weeks | Placebo-controlled RCT on 44 patients with T2D | ↑ TAC, ↓ MDA | [88] | |
| Anti-inflammatory | PPE | 6 g PPE/day for 10 weeks | Placebo-controlled RCT on 62 adults with UC | ↓ UC symptoms (i.e., fecal incontinence) | [99] |
| PE | 2 capsules of 250 mg PE/day for 8 weeks | Placebo-controlled RCT on 55 rheumatoid arthritis patients | ↓ joints pain, ↓ inflammatory markers | [100] | |
| PJ | 250 mL/day PJ | Placebo-controlled RCT on 50 patients with T2D | ↓ hs-CRP and IL-6 | [101] | |
| Antidiabetic | PSO | 3 g/day per 8 weeks | Placebo-controlled RCT on 52 obese T2D patients | ↓ FBG level, ↑ GLUT-4 expression | [108] |
| PJ | 1.5 mL/kg | Placebo-controlled RCT on 85 T2D patients | ↓ FGB levels and insuline resistance, ↑ β-cell function | [109] | |
| PSP | 5 g twice a day for 8 weeks | Placebo-controlled RCT on 60 T2D patients | ↓ FBG and Hemoglobin A1C | [110] | |
| PJ | 200 mL/day for 6 weeks | Placebo-controlled RCT on 50 T2D patients. | ↓ FBG, cholesterol and LDL | [111] | |
| Cardiovascular | PJ | 240 mL/day for 3 months | Patients with coronary heart disease | ↑ myocardial perfusion (17%) ↓ frequency of angina episodes (50%) | [116] |
| PJ | 220 mL/day for 5 days | Patients with heart diseases | ↓ frequency, duration, severity of angina | [117] | |
| PJ | 50 mL/day for 12 months | 15 health patients | ↓ systolic blood pressure and the lipids peroxidation | [118] | |
| PJ | 240 mL/day for 18 months | 289 patients with coronary heart diseases | ↓ carotid thickness progression | [119] | |
| PJ | 50 mL/day for 2 weeks | Hypertensive adults | ↓ ACE activity (36%) | [123] | |
| PPE | 250 mg/twice in a day for 8 weeks | Placebo-controlled RCT on T2D patients | ↓ systolic and diastolic blood pressure, ↑ lipidic profile | [124] | |
| PJ | 200 mL/day for 6 weeks | Placebo-controlled RCT on 60 health adults | ↓ blood pressure, ↑ lipidic parameters | [125] | |
| PJ | 330 mL/day for 4 weeks | Placebo-controlled RCT on 51 adults | ↓ blood pressure | [126] | |
| PJ | 150 mL/day for 2 weeks | Placebo-controlled RCT on 21 adults | ↓ systolic and diastolic pressure, — inflammatory and lipidic profile | [127] | |
| PJ | 150 mL/day | Placebo-controlled RCT on 13 men | ↓ systolic and diastolic pressure, — IL-6, E-selectin, ICAM-1, CRP levels | [128] | |
| Hypo-lipidic | PPE | 500 mg for 8 weeks | Placebo-controlled RCT on obese female volunteers | ↓ cholesterol, triglycerides, LDL, body weight and blood pressure | [131] |
| PJ | 200 mL/day for 4 weeks | Placebo-controlled RCT on 24 subjects treated with PJ or lovastatin | ↓ cholesterol and LDL | [132] | |
| PE | 1 g/day PE + 20 mg/day simvastatin | Placebo-controlled RCT on volunteers with hypercholesterolemia | ↓ triglycerides, cholesterol and ROS in blood samples, ↑ protection against atherosclerosis | [133] | |
| PJ | 50 mL/day or 20–80 mL/day | Healthy adults | ↑ TAC, ↓ LDL oxidation and aggregation | [136] | |
| Antibacterial and antifunginal | HAP | 15 mL/day | Placebo-controlled RCT on 60 healthy adults | ↓ dental plaque | [147] |
| Antiviral | PJ | 200 mL/3 times a day | 182 adults presenting SARS-CoV-2 infection | ↓ COVID-19 symptoms | [152] |
| Anticancer | PJ | 200 mL/day for 3 days | 63 patients with prostate cancer or benign prostate hyperplasia | — CDKN1A, M Ki-67, c-Myc mRNA expression | [168] |
| PJ | 70 mg total polyphenol/8 once for day | Men with prostate cancer | ↑ PSA doubling time from 15 months to 54 months | [170] | |
| PE | 300 mg/2 time a day | Patients treated to prevent radiotherapy-induced mucositis and dermatitis | ↑ defense from radiotherapy damages | [171] |
| Activity | Plant Part | Dosage | Design of Study | Outcomes | Ref. |
|---|---|---|---|---|---|
| Anti-inflammatory | PGF | 10–100 μg/mL | LPS-induced RAW 264.7 macrophages | ↓ inflammatory markers (i.e., NO, PGE2, IL-1β, IL-6 and TNF-α) | [92] |
| Antidiabetic | Purified compounds from PGF | α-glucosidase, α-amylase and lipase assays | ↓ α-glucosidase activity | [107] | |
| Antibacterial and antifunginal | PPE | 0.3–1.20 µg/mL | P. aeruginosa (ATCC 9027) and S. epidermidis (ATCC 12228) | ↓ bacterial growth | [143] |
| Leaves alcohol extracts | 3–10% extract in distilled water. | C. albicans, A. niger and P. notatum | Anti-fungal and antidandruff activities | [43] | |
| Antiviral | PPE | 0.04 mg/mL | Human kidney 2 cell (HK2) | ↓ Spike-ACE2 interaction and ACE2 and TMPRSS2 gene expression | [150] |
| Polyphenols | - | Molecular docking studies | Punicalagin and EA were the most effective on Mpro interaction | [148] | |
| PPE and purified polyphenols | 62.5–1000 μg/mL | Molecular docking studies and ELISA kit | ↓ interaction between S protein and ACE2 receptor | [151] | |
| Anticancer | PJ | - | Leukemia cell lines | ↓ Cell proliferation | [160] |
| Pomegranate leaves extract | 0–200 μg/mL for 24, 48 and 72 h | Lung cancer cell lines (A549, H1299), mouse Lewis lung carcinoma (LL/2) | Arrest of cell cycle progression in G2/M phase | [44] | |
| PE | 5–60 mg/L | UVA- and UVB-damaged human skin fibroblast cells (SKU-1064) | ↓ pro-inflammatory transcription factor NF-kB, ↓ caspase-3, ↑ G0/G1 phase associated with DNA repair | [172] |
| Activity | Plant Part | Dosage | Design of Study | Outcomes | Ref. |
|---|---|---|---|---|---|
| Anti-inflammatory | PJ and purified punicalagin | 400 mg/kg (PJ) 4 mg/kg (purfied punicalagin) for 18 days | Sprague-Dawley rats affected by colitis using DNBS | ↓ DNBS damage, inflammatory genes (i.e., TNF-α, IL-1β, IL-18 and NF-κβ) | [90] |
| PE | 13.6 mg/kg and 34 mg/kg for 10 days | Mice with rheumatoid arthritis | ↓ IL-6, IL-1β and TNF-α levels, ↑ joint infiltration | [98] | |
| EA | 100 mg/day for 7 days in acute model, 25 mg/day for 56 days in chronic model | Female mice with UC induced by DSS | Prevention, in acute as well as in chronic protocol, of the progression of the UC, ↓ inflammatory intestinal markers (IL-6, COX-2, iNOS and TNF-α) | [93] | |
| EA | 10–20 mg/kg for 48 h | Wistar rats TNBS-induced colitis | ↓ expression of COX-2, iNOS and other pro-inflammatory markers and morphological alterations | [94] | |
| PE and urolithin-A | 250 mg/kg (PE) or 15 mg/kg (urolithin-A) for 25 days | Fisher rats with DSS-induced intestinal damage | ↓ Inflammatory markers expression | [95] | |
| PSO | 1 g/100 g diet for 1 month | Male prediabetic mice | ↑ expression of PPAR-γ, ↓ FBG | [97] | |
| Antidiabetic | Pomegranate seed extract | 150–600 mg/kg | STZ-induced diabetic rats | ↓ FBG | [102] |
| PSO | 1 g/kg/day for 12 weeks | C57Bl/J6 HFD mice | ↓ body weight gain, ↑ insulin sensitivity | [103] | |
| PGF | 500 mg/kg/day for 6 weeks | Zucker diabetic fatty rats | ↓ FBG, ↑ PPAR-γ and GLUT4 mRNA expression | [104] | |
| PGF | 250 mg/kg and 500 mg/kg for 21 days | Diabetic Wistar rats | ↓ FBG, cholesterol, ↑ triglycerides, GSH, LDL, VLDL and tissue LPO levels, — HDL-C and antioxidant enzymes | [105] | |
| PGF | 50–100 mg/kg for 4 weeks | STZ-induced diabetic rats | ↓ weight gain and FBG, ↑ insulin sensitivity | [106] | |
| Cardiovascular | PE (Pomanox®) | 625 mg/day (200 mg punicalagin/day) | HFD-fed pigs | ↓ vascular damage, ↑ oxidative stress | [112] |
| PGF | 500 mg/kg for 6 weeks | Zucker diabetic fatty rats | ↓ lipid absorption and fatty acids | [113] | |
| PJ | 20 mL/day for 30 days | Rats (IP)-induced cardiac damage | Protection of the hearts, ↓ oxidative stres | [114] | |
| PJ | 80 μM/day of polyphenols for 1 month | 48 rats exposed to cigarette smoke for one month. | Protection from cigarette damages, as aortic calcification, or cardiac hypertrophy | [115] | |
| PJ | 100–300 mg/kg for 4 weeks | Diabetic Wistar rats with AngII-induced hypertension | ↓ blood pressure | [122] | |
| Hypo-lipidic | PSO | Diet enriched | ApoE/LDLR−/− mice | ↓ LDL and TG plasma level, — atherosclerosis progression | [129] |
| PJ | 10 mL/kg for 8 weeks | HFD-fed Wistar rats | ↓ blood pressure, LDL and pro-inflammatory cytokines, ↑ HDL levels, E-selectine and adiponectin | [130] | |
| Pomegranate peel powder | 0.5 g/kg for 4 weeks | 28 HFD-fed albino rats. | ↓ inflammatory markers, cholesterol and LDL, ↓ aorta alteration and cardioprotective effect. | [134] | |
| PPE | 200 mg/kg for 12 weeks | ApoE−/− mice | Stabilization of the aorta necrosis area, facilitation of the plaque remodeling and the collagen content, necrosis area | [135] | |
| PJ | 0–12.5 mL for 11 weeks | Apolipoprotein E-deficient mice | ↓ LDL oxidation (90%) and atherosclerosis vessel damage (44%) | [136] | |
| Neuroprotective | PSO, PL, PP, PJ | PSO: 2 mL/kg/day; PJ, PP and PL: 250 mg/kg/day | Standard diet or HFD diet rats | ↓ cholinesterase activity, ↑ antioxidant capacity | [139] |
| EA | 50 mg/kg for 30 days | STZ-induced sporadic Alzheimer’s desease rats | ↑ cognitive behavior, protection in hippocampal CA1 pyramidal neurons, ↓ inflammation and oxidative markers | [140] | |
| EA | 50 mg/kg/day for 1 week | Intrastriatal 6-OHDA-lesioned rats | ↓ MDA, ROS, DNA fragmentation and MAO-B activity | [141] | |
| EA | 10 mg/kg /day for 12 or 18 days | Lewis rats with autoimmune encephalomyelitis | ↓ progression of the disease | [142] |
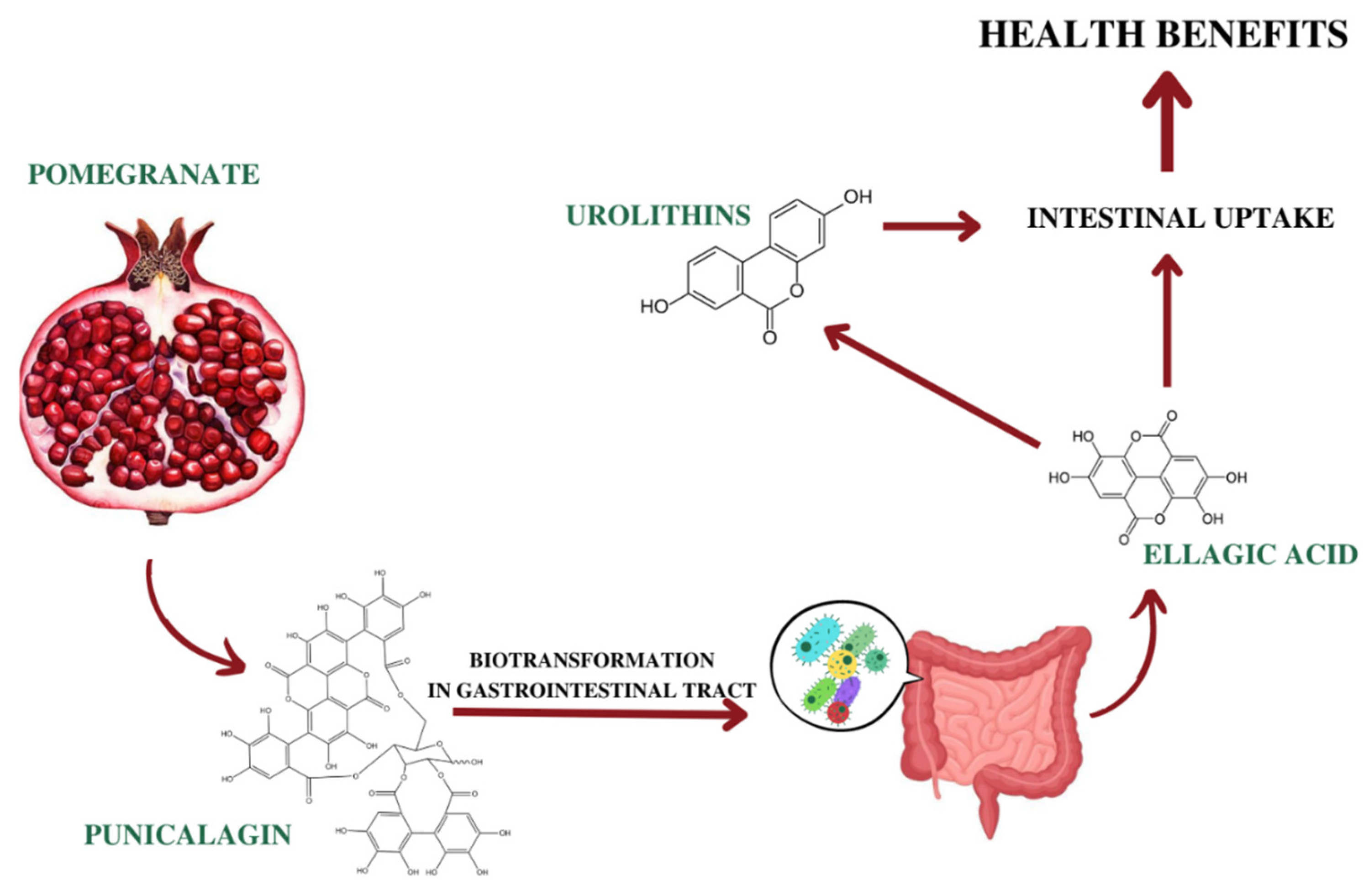
5. Pharmacokinetics of Ellagitannins
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Akhtar, S.; Ismail, T.; Fraternale, D.; Sestili, P. Pomegranate peel and peel extracts: Chemistry and food features. Food Chem. 2015, 174, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.; Hatib, K.; Bar-Ya’akov, I. Pomegranate: Botany, horticulture, breeding. Hortic. Rev. 2009, 35, 127–191. [Google Scholar]
- Rajaei, H.; Yazdanpanah, P. Buds and leaves in pomegranate (Punica granatum L.): Phenology in relation to structure and development. Flora—Morphol. Distrib. Funct. Ecol. Plants 2015, 214, 61–69. [Google Scholar] [CrossRef]
- Vučić, V.; Grabež, M.; Trchounian, A.; Arsić, A. Composition and potential health benefits of pomegranate: A review. Curr. Pharm. Des. 2019, 25, 1817–1827. [Google Scholar] [CrossRef]
- Trabelsi, A.; El Kaibi, M.A.; Abbassi, A.; Horchani, A.; Chekir-Ghedira, L.; Ghedira, K. Phytochemical Study and Antibacterial and Antibiotic Modulation Activity of Punica granatum (Pomegranate) Leaves. Scientifica 2020, 2020, 8271203. [Google Scholar] [CrossRef]
- Ko, K.; Dadmohammadi, Y.; Abbaspourrad, A. Nutritional and Bioactive Components of Pomegranate Waste Used in Food and Cosmetic Applications: A Review. Foods 2021, 10, 657. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; Fawole, O.A.; Opara, U.L. Processing factors affecting the phytochemical and nutritional properties of pomegranate (Punica granatum L.) peel waste: A review. Molecules 2020, 25, 4690. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and quantification of phenolic compounds from pomegranate (Punica granatum L.) peel, mesocarp, aril and differently produced juices by HPLC-DAD-ESI/MS(n). Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha; Neeraj; Sami, R.; Khojah, E.; Aljahani, A.H.; Al-Mushhin, A.A.M. Effects of drying methods and solvent extraction on quantification of major bioactive compounds in pomegranate peel waste using HPLC. Sci. Rep. 2022, 12, 8000. [Google Scholar] [CrossRef]
- Schwartz, E.; Tzulker, R.; Glazer, I.; Bar-Ya’akov, I.; Wiesman, Z.; Tripler, E.; Bar-Ilan, I.; Fromm, H.; Borochov-Neori, H.; Holland, D.; et al. Environmental conditions affect the color, taste, and antioxidant capacity of 11 pomegranate accessions’ fruits. J. Agric. Food Chem. 2009, 57, 9197–9209. [Google Scholar] [CrossRef]
- Young, J.E.; Pan, Z.; Teh, H.E.; Menon, V.; Modereger, B.; Pesek, J.J.; Matyska, M.T.; Dao, L.; Takeoka, G. Phenolic composition of pomegranate peel extracts using an liquid chromatography-mass spectrometry approach with silica hydride columns. J. Sep. Sci. 2017, 40, 1449–1456. [Google Scholar] [CrossRef]
- Bar-Ya’akov, I.; Tian, L.; Amir, R.; Holland, D. Primary Metabolites, Anthocyanins, and Hydrolyzable Tannins in the Pomegranate Fruit. Front. Plant Sci. 2019, 10, 620. [Google Scholar] [CrossRef]
- Yisimayili, Z.; Abdulla, R.; Tian, Q.; Wang, Y.; Chen, M.; Sun, Z.; Li, Z.; Liu, F.; Aisa, H.A.; Huang, C. A comprehensive study of pomegranate flowers polyphenols and metabolites in rat biological samples by high-performance liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2019, 1604, 460472. [Google Scholar] [CrossRef]
- Wu, S.; Tian, L. Diverse phytochemicals and bioactivities in the ancient fruit and modern functional food pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef]
- Fahmy, H.; Hegazi, N.; El-Shamy, S.; Farag, M.A. Pomegranate juice as a functional food: A comprehensive review of its polyphenols, therapeutic merits, and recent patents. Food Funct. 2020, 11, 5768–5781. [Google Scholar] [CrossRef]
- Prakash, C.V.S.; Prakash, I. Bioactive chemical constituents from pomegranate (Punica granatum) juice, seed and peel—A review. Int. J. Res. Chem. Env. 2011, 1, 1–18. [Google Scholar]
- Dafny-Yalin, M.; Glazer, I.; Bar-Ilan, I.; Kerem, Z.; Holland, D.; Amir, R. Color, sugars and organic acids composition in aril juices and peel homogenates prepared from different pomegranate accessions. J. Agric. Food Chem. 2010, 58, 4342–4352. [Google Scholar] [CrossRef]
- Hasnaoui, N.; Wathelet, B.; Jiménez-Araujo, A. Valorization of pomegranate peel from 12 cultivars: Dietary fibre composition, antioxidant capacity and functional properties. Food Chem. 2014, 160, 196–203. [Google Scholar] [CrossRef]
- Li, Y.; Gu, P.; Wang, L.; Wang, S.; Yang, H.; Zhang, B.; Zhu, B.; Ma, C. Comparison of amino acid profile in the juice of six pomegranate cultivars from two cultivation regions in China. J. Food Process. Preserv. 2017, 41, e13197. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Bălan, A.; Dima, L.; Toma, S.I.; Bîgiu, N.F.; Blidaru, A. Pharmacological and therapeutic properties of Punica granatum phytochemicals: Possible roles in breast cancer. Molecules 2021, 26, 1054. [Google Scholar] [CrossRef]
- Tzulker, R.; Glazer, I.; Bar-Ilan, I.; Holland, D.; Aviram, M.; Amir, R. Antioxidant activity, polyphenol content, and related compounds in different fruit juices and homogenates prepared from 29 different pomegranate accessions. J. Agric. Food Chem. 2007, 55, 9559–9570. [Google Scholar] [CrossRef]
- Montefusco, A.; Durante, M.; Migoni, D.; De Caroli, M.; Ilahy, R.; Pék, Z.; Helyes, L.; Fanizzi, F.P.; Mita, G.; Piro, G. Analysis of the phytochemical composition of pomegranate fruit juices, peels and kernels: A comparative study on four cultivars grown in southern Italy. Plants 2021, 10, 2521. [Google Scholar] [CrossRef]
- Noda, Y.; Kaneyuki, T.; Mori, A.; Packer, L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: Delphinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 2002, 50, 166–171. [Google Scholar] [CrossRef]
- Dumbravă, D.-G.; Moldovan, C.; Raba, D.-N.; Popa, M.-V.; Drugă, M. Evaluation of antioxidant activity, polyphenols and vitamin C content of some exotic fruits. J. Agroaliment. Process. Technol. 2016, 22, 13–16. [Google Scholar]
- Tarantino, A.; Difonzo, G.; Disciglio, G.; Frabboni, L.; Paradiso, V.M.; Gambacorta, G.; Caponio, F. Fresh pomegranate juices from cultivars and local ecotypes grown in southeastern Italy: Comparison of physicochemical properties, antioxidant activity and bioactive compounds. J. Sci. Food Agric. 2022, 102, 1185–1192. [Google Scholar] [CrossRef]
- Waheed, S.; Siddique, N.; Rahman, A.; Zaidi, J.; Ahmad, S. INAA for dietary assessment of essential and other trace elements in fourteen fruits harvested and consumed in Pakistan. J. Radioanal. Nucl. Chem. 2004, 260, 523–531. [Google Scholar] [CrossRef]
- Kim, N.D.; Mehta, R.; Yu, W.; Neeman, I.; Livney, T.; Amichay, A.; Poirier, D.; Nicholls, P.; Kirby, A.; Jiang, W. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res. Treat. 2002, 71, 203–217. [Google Scholar] [CrossRef]
- Akter, R.; Ahn, J.C.; Nahar, J.; Awais, M.; Ramadhania, Z.M.; Oh, S.-W.; Oh, J.-H.; Kong, B.M.; Rupa, E.J.; Lee, D.W. Pomegranate juice fermented by tannin acyl hydrolase and Lactobacillus vespulae DCY75 enhance estrogen receptor expression and anti-inflammatory effect. Front. Pharmacol. 2022, 13, 1010103. [Google Scholar] [CrossRef]
- Khemakhem, M.; Zarroug, Y.; Jabou, K.; Selmi, S.; Bouzouita, N. Physicochemical characterization of oil, antioxidant potential, and phenolic profile of seeds isolated from Tunisian pomegranate (Punica granatum L.) cultivars. J. Food Sci. 2021, 86, 852–859. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Fernández-López, J.; Pérez-Álvarez, J. Pomegranate and its many functional components as related to human health: A review. Compr. Rev. Food Sci. Food Saf. 2010, 9, 635–654. [Google Scholar] [CrossRef]
- Khoddami, A.; Man, Y.B.C.; Roberts, T.H. Physico-chemical properties and fatty acid profile of seed oils from pomegranate (Punica granatum L.) extracted by cold pressing. Eur. J. Lipid Sci. Technol. 2014, 116, 553–562. [Google Scholar] [CrossRef]
- Verardo, V.; Garcia-Salas, P.; Baldi, E.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Caboni, M.F. Pomegranate seeds as a source of nutraceutical oil naturally rich in bioactive lipids. Food Res. Int. 2014, 65, 445–452. [Google Scholar] [CrossRef]
- Fadavi, A.; Barzegar, M.; Azizi, M.H. Determination of fatty acids and total lipid content in oilseed of 25 pomegranates varieties grown in Iran. J. Food Compos. Anal. 2006, 19, 676–680. [Google Scholar] [CrossRef]
- Hora, J.J.; Maydew, E.R.; Lansky, E.P.; Dwivedi, C. Chemopreventive effects of pomegranate seed oil on skin tumor development in CD1 mice. J. Med. Food 2003, 6, 157–161. [Google Scholar] [CrossRef]
- Nekooeian, A.A.; Eftekhari, M.H.; Adibi, S.; Rajaeifard, A. Effects of pomegranate seed oil on insulin release in rats with type 2 diabetes. Iran. J. Med. Sci. 2014, 39, 130. [Google Scholar]
- Aruna, P.; Venkataramanamma, D.; Singh, A.K.; Singh, R. Health benefits of punicic acid: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 16–27. [Google Scholar] [CrossRef]
- Amri, Z.; Lazreg-Aref, H.; Mekni, M.; El-Gharbi, S.; Dabbaghi, O.; Mechri, B.; Hammami, M. Oil characterization and lipids class composition of pomegranate seeds. BioMed Res. Int. 2017, 2017, 2037341. [Google Scholar] [CrossRef]
- Elfalleh, W.; Tlili, N.; Ying, M.; Sheng-Hua, H.; Ferchichi, A.; Nasri, N. Organoleptic quality, minerals, proteins and amino acids from two Tunisian commercial pomegranate fruits. Int. J. Food Eng. 2011, 7. [Google Scholar] [CrossRef]
- Li, G.; Chen, M.; Chen, J.; Shang, Y.; Lian, X.; Wang, P.; Lei, H.; Ma, Q. Chemical composition analysis of pomegranate seeds based on ultra-high-performance liquid chromatography coupled with quadrupole-Orbitrap high-resolution mass spectrometry. J. Pharm. Biomed. Anal. 2020, 187, 113357. [Google Scholar] [CrossRef]
- Rowayshed, G.; Salama, A.; Abul-Fadl, M.; Akila-Hamza, S.; Emad, A.M. Nutritional and chemical evaluation for pomegranate (Punica granatum L.) fruit peel and seeds powders by products. Middle East J. Appl. Sci. 2013, 3, 169–179. [Google Scholar]
- Mestry, S.N.; Dhodi, J.B.; Kumbhar, S.B.; Juvekar, A.R. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J. Tradit. Complement. Med. 2017, 7, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Özen, C.; Abu-Reidah, I.M.; Chigurupati, S.; Patra, J.K.; Horbanczuk, J.O.; Jóźwik, A.; Tzvetkov, N.T.; Uhrin, P.; Atanasov, A.G. Vasculoprotective Effects of Pomegranate (Punica granatum L.). Front. Pharmacol. 2018, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Wadkar, G.H.; Todkar, S.S.; Savali, A.S.; Chittapurkar, H.R. Screening of hair growth promoting activity of Punica granatum L.(pomegranate) leaves extracts and its potential to exhibit antidandruff and anti-lice effect. Heliyon 2021, 7, e06903. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, F.; Zheng, W.; Hu, M.; Wang, J.; Ma, S.; Deng, Y.; Luo, Y.; Ye, T.; Yin, W. Punica granatum (pomegranate) leaves extract induces apoptosis through mitochondrial intrinsic pathway and inhibits migration and invasion in non-small cell lung cancer in vitro. Biomed. Pharmacother. 2016, 80, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Pande, G. Antioxidant Capacity and Lipid Characterization of Georgia-Grown Underutilized Fruit Crops. Ph.D. Thesis, University of Georgia, Athens, GA, USA, 2009. [Google Scholar]
- Swilam, N.; Nematallah, K.A. Polyphenols profile of pomegranate leaves and their role in green synthesis of silver nanoparticles. Sci. Rep. 2020, 10, 14851. [Google Scholar] [CrossRef]
- Ge, S.; Duo, L.; Wang, J.; Yang, J.; Li, Z.; Tu, Y. A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. J. Ethnopharmacol. 2021, 271, 113877. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, Y.; Zhang, Y.; Liu, J.; Yu, J. Changes in bioactive compounds and antioxidant activities in pomegranate leaves. Sci. Hortic. 2010, 123, 543–546. [Google Scholar] [CrossRef]
- Guerrero-Solano, J.A.; Jaramillo-Morales, O.A.; Jiménez-Cabrera, T.; Urrutia-Hernández, T.A.; Chehue-Romero, A.; Olvera-Hernández, E.G.; Bautista, M. Punica protopunica Balf., the forgotten sister of the common pomegranate (Punica granatum L.): Features and medicinal properties—A review. Plants 2020, 9, 1214. [Google Scholar] [CrossRef]
- Bekir, J.; Mars, M.; Vicendo, P.; Fterrich, A.; Bouajila, J. Chemical composition and antioxidant, anti-inflammatory, and antiproliferation activities of pomegranate (Punica granatum) flowers. J. Med. Food 2013, 16, 544–550. [Google Scholar] [CrossRef]
- Wafa, B.A.; Makni, M.; Ammar, S.; Khannous, L.; Hassana, A.B.; Bouaziz, M.; Es-Safi, N.E.; Gdoura, R. Antimicrobial effect of the Tunisian Nana variety Punica granatum L. extracts against Salmonella enterica (serovars Kentucky and Enteritidis) isolated from chicken meat and phenolic composition of its peel extract. Int. J. Food Microbiol. 2017, 241, 123–131. [Google Scholar] [CrossRef]
- Li, Y.; Guo, C.; Yang, J.; Wei, J.; Xu, J.; Cheng, S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006, 96, 254–260. [Google Scholar] [CrossRef]
- Sreekumar, S.; Sithul, H.; Muraleedharan, P.; Azeez, J.M.; Sreeharshan, S. Pomegranate fruit as a rich source of biologically active compounds. BioMed Res. Int. 2014, 2014, 686921. [Google Scholar] [CrossRef]
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of bioactive compounds from pomegranate (Punica granatum L.) peel using pressurized liquid extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef]
- Singh, J.P.; Kaur, A.; Shevkani, K.; Singh, N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J. Food Sci. Technol. 2016, 53, 4056–4066. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. From Pomegranate Byproducts Waste to Worth: A Review of Extraction Techniques and Potential Applications for Their Revalorization. Foods 2022, 11, 2596. [Google Scholar] [CrossRef]
- Lampakis, D.; Skenderidis, P.; Leontopoulos, S. Technologies and extraction methods of polyphenolic compounds derived from pomegranate (Punica granatum) peels. A mini review. Processes 2021, 9, 236. [Google Scholar] [CrossRef]
- El-Shamy, S.; Farag, M.A. Novel trends in extraction and optimization methods of bioactives recovery from pomegranate fruit biowastes: Valorization purposes for industrial applications. Food Chem. 2021, 365, 130465. [Google Scholar] [CrossRef]
- Talekar, S.; Patti, A.F.; Vijayraghavan, R.; Arora, A. Rapid, enhanced and eco-friendly recovery of punicalagin from fresh waste pomegranate peels via aqueous ball milling. J. Clean. Prod. 2019, 228, 1238–1247. [Google Scholar] [CrossRef]
- Lopez, J.; Streitengerger, S.; Penalver, M.; Martinez, P. Process and apparatus for preparing pomegranate extracts. Eur. Pat. Appl. 2008, 13. [Google Scholar]
- Man, G.; Ma, Y.; Xu, L.; Liao, X.; Zhao, L. Comparison of thermal and non-thermal extraction methods on free and bound phenolics in pomegranate peel. Innov. Food Sci. Emerg. Technol. 2023, 84, 103291. [Google Scholar] [CrossRef]
- Panda, D.; Saharan, V.K.; Manickam, S. Controlled hydrodynamic cavitation: A review of recent advances and perspectives for greener processing. Processes 2020, 8, 220. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Albanese, L.; Zabini, F. Hydrodynamic cavitation in beer and other beverage processing. Innov. Food Process. Technol. 2020, 369–394. [Google Scholar] [CrossRef]
- Ciriminna, R.; Scurria, A.; Pagliaro, M. Natural product extraction via hydrodynamic cavitation. Sustain. Chem. Pharm. 2023, 33, 101083. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Zabini, F. Agri-Food and Forestry Sectors for Sustainable Development: Innovations to Address the Ecosystems-Resources-Climate-Food-Health Nexus; Springer Nature: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Gallina, L.; Cravotto, C.; Capaldi, G.; Grillo, G.; Cravotto, G. Plant Extraction in Water: Towards Highly Efficient Industrial Applications. Processes 2022, 10, 2233. [Google Scholar] [CrossRef]
- Albanese, L.; Bonetti, A.; D’Acqui, L.P.; Meneguzzo, F.; Zabini, F. Affordable production of antioxidant aqueous solutions by hydrodynamic cavitation processing of silver fir (Abies alba Mill.) needles. Foods 2019, 8, 65. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Beer-brewing powered by controlled hydrodynamic cavitation: Theory and real-scale experiments. J. Clean. Prod. 2017, 142, 1457–1470. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Brunetti, C.; Fidalgo, A.; Ciriminna, R.; Delisi, R.; Albanese, L.; Zabini, F.; Gori, A.; dos Santos Nascimento, L.B.; De Carlo, A. Real-scale integral valorization of waste orange peel via hydrodynamic cavitation. Processes 2019, 7, 581. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Krijgsman, A.J.; Fryer, P.J.; Zuidam, N.J. Intensification of protein extraction from soybean processing materials using hydrodynamic cavitation. Innov. Food Sci. Emerg. Technol. 2017, 41, 47–55. [Google Scholar] [CrossRef]
- Faraloni, C.; Albanese, L.; Chini Zittelli, G.; Meneguzzo, F.; Tagliavento, L.; Zabini, F. New route to the production of almond beverages using hydrodynamic cavitation. Foods 2023, 12, 935. [Google Scholar] [CrossRef]
- Albanese, L.; Ciriminna, R.; Meneguzzo, F.; Pagliaro, M. Energy efficient inactivation of S accharomyces cerevisiae via controlled hydrodynamic cavitation. Energy Sci. Eng. 2015, 3, 221–238. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Zabini, F.; Albanese, L.; Crisci, A. Novel affordable, reliable and efficient technologies to help addressing the water-energy-food nexus. Eur. J. Sustain. Dev. 2019, 8, 1. [Google Scholar] [CrossRef]
- Albanese, L.; Meneguzzo, F. Hydrodynamic cavitation technologies: A pathway to more sustainable, healthier beverages, and food supply chains. In Processing and Sustainability of Beverages; Elsevier: Amsterdam, The Netherlands, 2019; pp. 319–372. [Google Scholar]
- Rivas, M.Á.; Casquete, R.; de Guía Córdoba, M.; Benito, M.J.; Hernández, A.; Ruiz-Moyano, S.; Martín, A. Functional properties of extracts and residual dietary fibre from pomegranate (Punica granatum L.) peel obtained with different supercritical fluid conditions. LWT 2021, 145, 111305. [Google Scholar] [CrossRef]
- Yang, X.; Nisar, T.; Hou, Y.; Gou, X.; Sun, L.; Guo, Y. Pomegranate peel pectin can be used as an effective emulsifier. Food Hydrocoll. 2018, 85, 30–38. [Google Scholar] [CrossRef]
- Ciriminna, R.; Fidalgo, A.; Meneguzzo, F.; Presentato, A.; Scurria, A.; Nuzzo, D.; Alduina, R.; Ilharco, L.M.; Pagliaro, M. Pectin: A Long-Neglected Broad-Spectrum Antibacterial. ChemMedChem 2020, 15, 2228–2235. [Google Scholar] [CrossRef]
- Presentato, A.; Scurria, A.; Albanese, L.; Lino, C.; Sciortino, M.; Pagliaro, M.; Zabini, F.; Meneguzzo, F.; Alduina, R.; Nuzzo, D. Superior antibacterial activity of integral lemon pectin extracted via hydrodynamic cavitation. ChemistryOpen 2020, 9, 628–630. [Google Scholar] [CrossRef]
- Nuzzo, D.; Cristaldi, L.; Sciortino, M.; Albanese, L.; Scurria, A.; Zabini, F.; Lino, C.; Pagliaro, M.; Meneguzzo, F.; Di Carlo, M. Exceptional antioxidant, non-cytotoxic activity of integral lemon pectin from hydrodynamic cavitation. ChemistrySelect 2020, 5, 5066–5071. [Google Scholar] [CrossRef]
- Meneguzzo, F.; Ciriminna, R.; Zabini, F.; Pagliaro, M. Review of evidence available on hesperidin-rich products as potential tools against COVID-19 and hydrodynamic cavitation-based extraction as a method of increasing their production. Processes 2020, 8, 549. [Google Scholar] [CrossRef]
- Nuzzo, D.; Picone, P.; Giardina, C.; Scordino, M.; Mudò, G.; Pagliaro, M.; Scurria, A.; Meneguzzo, F.; Ilharco, L.M.; Fidalgo, A. New neuroprotective effect of lemon IntegroPectin on neuronal cellular model. Antioxidants 2021, 10, 669. [Google Scholar] [CrossRef]
- Flori, L.; Albanese, L.; Calderone, V.; Meneguzzo, F.; Pagliaro, M.; Ciriminna, R.; Zabini, F.; Testai, L. Cardioprotective effects of grapefruit IntegroPectin extracted via hydrodynamic cavitation from by-products of Citrus fruits industry: Role of mitochondrial potassium channels. Foods 2022, 11, 2799. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Chander, R.; Sharma, A. Antioxidant and antimicrobial activity of pomegranate peel extract improves the shelf life of chicken products. Int. J. Food Sci. Technol. 2010, 45, 216–222. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozturk, I.; Cankurt, H.; Tornuk, F. Interaction between some phenolic compounds and probiotic bacterium in functional ice cream production. Food Bioprocess Technol. 2012, 5, 2964–2971. [Google Scholar] [CrossRef]
- Feng, X.; Yang, Q.; Wang, C.; Tong, W.; Xu, W. Punicalagin Exerts Protective Effects against Ankylosing Spondylitis by Regulating NF-kappaB-TH17/JAK2/STAT3 Signaling and Oxidative Stress. Biomed. Res. Int. 2020, 2020, 4918239. [Google Scholar] [CrossRef]
- Katz, S.R.; Newman, R.A.; Lansky, E.P. Punica granatum: Heuristic treatment for diabetes mellitus. J. Med. Food 2007, 10, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, G.; Ebrahimof, S.; Sotoudeh, G.; Neyestani, T.R.; Angoorani, P.; Hedayati, M.; Siasi, F. Effects of pomegranate juice consumption on oxidative stress in patients with type 2 diabetes: A single-blind, randomized clinical trial. Int. J. Food Sci. Nutr. 2017, 68, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Sohrab, G.; Angoorani, P.; Tohidi, M.; Tabibi, H.; Kimiagar, M.; Nasrollahzadeh, J. Pomegranate (Punicagranatum) juice decreases lipid peroxidation, but has no effect on plasma advanced glycated end-products in adults with type 2 diabetes: A randomized double-blind clinical trial. Food Nutr. Res. 2015, 59, 28551. [Google Scholar] [CrossRef]
- Shishehbor, F.; Zarei, M.; Saki, A.; Zakerkish, M.; Shirani, F.; Zare, M. Effects of concentrated pomegranate juice on subclinical inflammation and cardiometabolic risk factors for type 2 diabetes: A quasi-experimental study. Int. J. Endocrinol. Metab. 2016, 14, e33835. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.A.; Parikh, M.; Patel, K.V.; Patel, K.G.; Joshi, C.G.; Gandhi, T.R. Evaluation of the effect of Punica granatum juice and punicalagin on NFκB modulation in inflammatory bowel disease. Mol. Cell. Biochem. 2016, 419, 65–74. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Sánchez-Hidalgo, M.; Cárdeno, A.; Aparicio-Soto, M.; Sánchez-Fidalgo, S.; Villegas, I.; de la Lastra, C.A. Dietary supplementation of an ellagic acid-enriched pomegranate extract attenuates chronic colonic inflammation in rats. Pharmacol. Res. 2012, 66, 235–242. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, Y.; Aisa, H.A. Anti-inflammatory effect of pomegranate flower in lipopolysaccharide (LPS)-stimulated RAW264. 7 macrophages. Pharm. Biol. 2017, 55, 2095–2101. [Google Scholar] [CrossRef]
- Marín, M.; Giner, R.M.; Ríos, J.-L.; Recio, M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef]
- Rosillo Ramírez, M.d.l.Á.; Sánchez Hidalgo, M.; Cárdeno Galván, A.; Alarcón de la Lastra Romero, C. Protective effect of ellagic acid, a natural polyphenolic compound, in a murine model of Crohn’s disease. Biochem. Pharmacol. 2011, 82, 737–745. [Google Scholar] [CrossRef]
- Larrosa, M.; González-Sarrías, A.; Yáñez-Gascón, M.J.; Selma, M.V.; Azorín-Ortuño, M.; Toti, S.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Anti-inflammatory properties of a pomegranate extract and its metabolite urolithin-A in a colitis rat model and the effect of colon inflammation on phenolic metabolism. J. Nutr. Biochem. 2010, 21, 717–725. [Google Scholar] [CrossRef]
- Lee, C.-J.; Chen, L.-G.; Liang, W.-L.; Wang, C.-C. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010, 118, 315–322. [Google Scholar] [CrossRef]
- Hontecillas, R.; O’Shea, M.; Einerhand, A.; Diguardo, M.; Bassaganya-Riera, J. Activation of PPAR γ and α by Punicic Acid Ameliorates Glucose Tolerance and Suppresses Obesity-Related Inflammation. J. Am. Coll. Nutr. 2009, 28, 184–195. [Google Scholar] [CrossRef]
- Shukla, M.; Gupta, K.; Rasheed, Z.; Khan, K.A.; Haqqi, T.M. Consumption of hydrolyzable tannins-rich pomegranate extract suppresses inflammation and joint damage in rheumatoid arthritis. Nutrition 2008, 24, 733–743. [Google Scholar] [CrossRef]
- Kamali, M.; Tavakoli, H.; Khodadoost, M.; Daghaghzadeh, H.; Kamalinejad, M.; Gachkar, L.; Mansourian, M.; Adibi, P. Efficacy of the Punica granatum peels aqueous extract for symptom management in ulcerative colitis patients. A randomized, placebo-controlled, clinical trial. Complement. Ther. Clin. Pract. 2015, 21, 141–146. [Google Scholar] [CrossRef]
- Ghavipour, M.; Sotoudeh, G.; Tavakoli, E.; Mowla, K.; Hasanzadeh, J.; Mazloom, Z. Pomegranate extract alleviates disease activity and some blood biomarkers of inflammation and oxidative stress in Rheumatoid Arthritis patients. Eur. J. Clin. Nutr. 2017, 71, 92–96. [Google Scholar] [CrossRef]
- Sohrab, G.; Nasrollahzadeh, J.; Zand, H.; Amiri, Z.; Tohidi, M.; Kimiagar, M. Effects of pomegranate juice consumption on inflammatory markers in patients with type 2 diabetes: A randomized, placebo-controlled trial. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 215. [Google Scholar]
- Das, A.K.; Mandal, S.C.; Banerjee, S.K.; Sinha, S.; Saha, B.; Pal, M. Studies on the hypoglycaemic activity of Punica granatum seed in streptozotocin induced diabetic rats. Phytother. Res. 2001, 15, 628–629. [Google Scholar] [CrossRef]
- Vroegrijk, I.O.; van Diepen, J.A.; van den Berg, S.; Westbroek, I.; Keizer, H.; Gambelli, L.; Hontecillas, R.; Bassaganya-Riera, J.; Zondag, G.C.; Romijn, J.A.; et al. Pomegranate seed oil, a rich source of punicic acid, prevents diet-induced obesity and insulin resistance in mice. Food Chem. Toxicol. 2011, 49, 1426–1430. [Google Scholar] [CrossRef]
- Huang, T.H.; Peng, G.; Kota, B.P.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D.; Li, Y. Anti-diabetic action of Punica granatum flower extract: Activation of PPAR-gamma and identification of an active component. Toxicol. Appl. Pharm. 2005, 207, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Bagri, P.; Ali, M.; Aeri, V.; Bhowmik, M.; Sultana, S. Antidiabetic effect of Punica granatum flowers: Effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem. Toxicol. 2009, 47, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Liu, L.; Ajiakber, D.; Ye, J.; Xu, J.; Xin, X.; Aisa, H.A. Anti-diabetic effect of Punica granatum flower polyphenols extract in type 2 diabetic rats: Activation of Akt/GSK-3β and inhibition of IRE1α-XBP1 pathways. Front. Endocrinol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Tian, L. A new flavone glucoside together with known ellagitannins and flavones with anti-diabetic and anti-obesity activities from the flowers of pomegranate (Punica granatum). Nat. Prod. Res. 2019, 33, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Khajebishak, Y.; Payahoo, L.; Alivand, M.; Hamishehkar, H.; Mobasseri, M.; Ebrahimzadeh, V.; Alipour, M.; Alipour, B. Effect of pomegranate seed oil supplementation on the GLUT-4 gene expression and glycemic control in obese people with type 2 diabetes: A randomized controlled clinical trial. J. Cell. Physiol. 2019, 234, 19621–19628. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.; Makahleh, S.; El-Akawi, Z.; Al-Fashtaki, R.; Khabour, O.; Gharibeh, M.; Saadah, N.; Al-Hashimi, F.; Al-Khasieb, N. Fresh pomegranate juice ameliorates insulin resistance, enhances β-cell function, and decreases fasting serum glucose in type 2 diabetic patients. Nutr. Res. 2014, 34, 862–867. [Google Scholar] [CrossRef]
- Hashemi, M.S.; Namiranian, N.; Tavahen, H.; Dehghanpour, A.; Rad, M.H.; Jam-Ashkezari, S.; Emtiazy, M.; Hashempur, M.H. Efficacy of pomegranate seed powder on glucose and lipid metabolism in patients with type 2 diabetes: A prospective randomized double-blind placebo-controlled clinical trial. Complement. Med. Res. 2021, 28, 226–233. [Google Scholar]
- Parsaeyan, N.; Mozaffari–Khosravi, H.; Mozayan, M.R. Effect of pomegranate juice on paraoxonase enzyme activity in patients with type 2 diabetes. J. Diabetes Metab. Disord. 2012, 11, 11. [Google Scholar] [CrossRef]
- Vilahur, G.; Padró, T.; Casaní, L.; Mendieta, G.; López, J.A.; Streitenberger, S.; Badimon, L. Polyphenol-enriched diet prevents coronary endothelial dysfunction by activating the Akt/eNOS pathway. Rev. Esp. Cardiol. 2015, 68, 216–225. [Google Scholar] [CrossRef]
- Huang, T.H.W.; Peng, G.; Kota, B.P.; Li, G.Q.; Yamahara, J.; Roufogalis, B.D.; Li, Y. Pomegranate flower improves cardiac lipid metabolism in a diabetic rat model: Role of lowering circulating lipids. Br. J. Pharmacol. 2005, 145, 767–774. [Google Scholar] [CrossRef]
- Jadeja, R.N.; Thounaojam, M.C.; Patel, D.K.; Devkar, R.V.; Ramachandran, A. Pomegranate (Punica granatum L.) juice supplementation attenuates isoproterenol-induced cardiac necrosis in rats. Cardiovasc. Toxicol. 2010, 10, 174–180. [Google Scholar] [CrossRef]
- Al Hariri, M.; Zibara, K.; Farhat, W.; Hashem, Y.; Soudani, N.; Al Ibrahim, F.; Hamade, E.; Zeidan, A.; Husari, A.; Kobeissy, F. Cigarette smoking-induced cardiac hypertrophy, vascular inflammation and injury are attenuated by antioxidant supplementation in an animal model. Front. Pharmacol. 2016, 7, 397. [Google Scholar] [CrossRef]
- Sumner, M.D.; Elliott-Eller, M.; Weidner, G.; Daubenmier, J.J.; Chew, M.H.; Marlin, R.; Raisin, C.J.; Ornish, D. Effects of pomegranate juice consumption on myocardial perfusion in patients with coronary heart disease. Am. J. Cardiol. 2005, 96, 810–814. [Google Scholar] [CrossRef]
- Razani, Z.; Dastani, M.; Kazerani, H.R. Cardioprotective effects of pomegranate (Punica granatum) juice in patients with ischemic heart disease. Phytother. Res. 2017, 31, 1731–1738. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef]
- Davidson, M.H.; Maki, K.C.; Dicklin, M.R.; Feinstein, S.B.; Witchger, M.; Bell, M.; McGuire, D.K.; Provost, J.-C.; Liker, H.; Aviram, M. Effects of consumption of pomegranate juice on carotid intima–media thickness in men and women at moderate risk for coronary heart disease. Am. J. Cardiol. 2009, 104, 936–942. [Google Scholar] [CrossRef]
- Yılmaz, B.; Usta, C. Ellagic Acid-Induced Endothelium-Dependent and Endothelium-Independent Vasorelaxation in Rat Thoracic Aortic Rings and the Underlying Mechanism. Phytother. Res. 2013, 27, 285–289. [Google Scholar] [CrossRef]
- Khoo, N.K.; White, C.R.; Pozzo-Miller, L.; Zhou, F.; Constance, C.; Inoue, T.; Patel, R.P.; Parks, D.A. Dietary flavonoid quercetin stimulates vasorelaxation in aortic vessels. Free Radic. Biol. Med. 2010, 49, 339–347. [Google Scholar] [CrossRef]
- Mohan, M.; Waghulde, H.; Kasture, S. Effect of pomegranate juice on Angiotensin II-induced hypertension in diabetic Wistar rats. Phytother. Res. 2010, 24, S196–S203. [Google Scholar] [CrossRef]
- Aviram, M.; Dornfeld, L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis 2001, 158, 195–198. [Google Scholar] [CrossRef]
- Grabež, M.; Škrbić, R.; Stojiljković, M.P.; Rudić-Grujić, V.; Paunović, M.; Arsić, A.; Petrović, S.; Vučić, V.; Mirjanić-Azarić, B.; Šavikin, K. Beneficial effects of pomegranate peel extract on plasma lipid profile, fatty acids levels and blood pressure in patients with diabetes mellitus type-2: A randomized, double-blind, placebo-controlled study. J. Funct. Foods 2020, 64, 103692. [Google Scholar] [CrossRef]
- Sohrab, G.; Roshan, H.; Ebrahimof, S.; Nikpayam, O.; Sotoudeh, G.; Siasi, F. Effects of pomegranate juice consumption on blood pressure and lipid profile in patients with type 2 diabetes: A single-blind randomized clinical trial. Clin. Nutr. ESPEN 2019, 29, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.; Hamadeh, H.; Leung, W.C.; Russell, J.M.; Barker, M.E. Effects of pomegranate juice supplementation on pulse wave velocity and blood pressure in healthy young and middle-aged men and women. Plant Foods Hum. Nutr. 2012, 67, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian-Kopaei, M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 2014, 28, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Asgary, S.; Keshvari, M.; Sahebkar, A.; Hashemi, M.; Rafieian-Kopaei, M. Clinical investigation of the acute effects of pomegranate juice on blood pressure and endothelial function in hypertensive individuals. ARYA Atheroscler. 2013, 9, 326. [Google Scholar]
- Franczyk-Żarów, M.; Tarko, T.; Drahun-Misztal, A.; Czyzynska-Cichon, I.; Kus, E.; Kostogrys, R.B. Pomegranate Seed Oil as a Source of Conjugated Linolenic Acid (CLnA) Has No Effect on Atherosclerosis Development but Improves Lipid Profile and Affects the Expression of Lipid Metabolism Genes in apoE/LDLR−/− Mice. Int. J. Mol. Sci. 2023, 24, 1737. [Google Scholar] [CrossRef]
- Michicotl-Meneses, M.M.; Thompson-Bonilla, M.d.R.; Reyes-López, C.A.; García-Pérez, B.E.; López-Tenorio, I.I.; Ordaz-Pichardo, C.; Jaramillo-Flores, M.E. Inflammation markers in adipose tissue and cardiovascular risk reduction by pomegranate juice in obesity induced by a hypercaloric diet in Wistar rats. Nutrients 2021, 13, 2577. [Google Scholar] [CrossRef]
- Haghighian, M.K.; Rafraf, M.; Moghaddam, A.; Hemmati, S.; Jafarabadi, M.A.; Gargari, B.P. Pomegranate (Punica granatum L.) peel hydro alcoholic extract ameliorates cardiovascular risk factors in obese women with dyslipidemia: A double blind, randomized, placebo controlled pilot study. Eur. J. Integr. Med. 2016, 8, 676–682. [Google Scholar] [CrossRef]
- Anoosh, E.; Mojtaba, E.; Fatemeh, S. Study the effect of juice of two variety of pomegranate on decreasing plasma LDL cholesterol. Procedia-Soc. Behav. Sci. 2010, 2, 620–623. [Google Scholar] [CrossRef][Green Version]
- Hamoud, S.; Hayek, T.; Volkova, N.; Attias, J.; Moscoviz, D.; Rosenblat, M.; Aviram, M. Pomegranate extract (POMx) decreases the atherogenicity of serum and of human monocyte-derived macrophages (HMDM) in simvastatin-treated hypercholesterolemic patients: A double-blinded, placebo-controlled, randomized, prospective pilot study. Atherosclerosis 2014, 232, 204–210. [Google Scholar] [CrossRef]
- Salama, A.A.; Ismael, N.M.; Bedewy, M. The anti-inflammatory and antiatherogenic in vivo effects of pomegranate peel powder: From waste to medicinal food. J. Med. Food 2021, 24, 145–150. [Google Scholar] [CrossRef]
- Manickam, V.; Dhawan, U.K.; Singh, D.; Gupta, M.; Subramanian, M. Pomegranate Peel Extract Decreases Plaque Necrosis and Advanced Atherosclerosis Progression in Apoe-/-Mice. Front. Pharmacol. 2022, 13, 888300. [Google Scholar] [CrossRef]
- Aviram, M.; Dornfeld, L.; Rosenblat, M.; Volkova, N.; Kaplan, M.; Coleman, R.; Hayek, T.; Presser, D.; Fuhrman, B. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: Studies in humans and in atherosclerotic apolipoprotein E–deficient mice. Am. J. Clin. Nutr. 2000, 71, 1062–1076. [Google Scholar] [CrossRef]
- Xu, K.Z.-Y.; Zhu, C.; Kim, M.S.; Yamahara, J.; Li, Y. Pomegranate flower ameliorates fatty liver in an animal model of type 2 diabetes and obesity. J. Ethnopharmacol. 2009, 123, 280–287. [Google Scholar] [CrossRef]
- Konsoula, Z. A preliminary in vitro investigation of anticholinesterase activity of pomegranate peel extracts. J. Biotechnol. 2018, 280, S88. [Google Scholar] [CrossRef]
- Amri, Z.; Ghorbel, A.; Turki, M.; Akrout, F.M.; Ayadi, F.; Elfeki, A.; Hammami, M. Effect of pomegranate extracts on brain antioxidant markers and cholinesterase activity in high fat-high fructose diet induced obesity in rat model. BMC Complement. Altern. Med. 2017, 17, 339. [Google Scholar] [CrossRef]
- Jha, A.B.; Panchal, S.S.; Shah, A. Ellagic acid: Insights into its neuroprotective and cognitive enhancement effects in sporadic Alzheimer’s disease. Pharmacol. Biochem. Behav. 2018, 175, 33–46. [Google Scholar] [CrossRef]
- Baluchnejadmojarad, T.; Rabiee, N.; Zabihnejad, S.; Roghani, M. Ellagic acid exerts protective effect in intrastriatal 6-hydroxydopamine rat model of Parkinson’s disease: Possible involvement of ERβ/Nrf2/HO-1 signaling. Brain Res. 2017, 1662, 23–30. [Google Scholar] [CrossRef]
- Busto, R.; Serna, J.; Perianes-Cachero, A.; Quintana-Portillo, R.; García-Seisdedos, D.; Canfrán-Duque, A.; Paino, C.L.; Lerma, M.; Casado, M.E.; Martín-Hidalgo, A. Ellagic acid protects from myelin-associated sphingolipid loss in experimental autoimmune encephalomyelitis. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2018, 1863, 958–967. [Google Scholar] [CrossRef]
- Gosset-Erard, C.; Zhao, M.; Lordel-Madeleine, S.; Ennahar, S. Identification of punicalagin as the bioactive compound behind the antimicrobial activity of pomegranate (Punica granatum L.) peels. Food Chem. 2021, 352, 129396. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, C.; Wu, Q.; Zheng, Z.; Liu, P.; Li, G.; Peng, X.; Xia, X. Antimicrobial activity of punicalagin against Staphylococcus aureus and its effect on biofilm formation. Foodborne Pathog. Dis. 2017, 14, 282–287. [Google Scholar] [CrossRef] [PubMed]
- de Medeiros Nóbrega, D.R.; Santos, R.L.; Soares, R.d.S.C.; Alves, P.M.; Medeiros, A.C.D.; Pereira, J.V. A randomized, controlled clinical trial on the clinical and microbiological efficacy of Punica granatum Linn mouthwash. Pesqui. Bras. Odontopediatria Clínica Integr. 2015, 15, 301–308. [Google Scholar] [CrossRef]
- Goel, D.; Bansal, A.; Nigam, A.G. Effect of Achyranthes aspera, 0.2% aqueous chlorhexidine gluconate and Punica granatum oral rinse on the levels of salivary Streptococcus mutans in 8 to 12 years old children. J. Contemp. Dent. Pract. 2016, 16, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Menezes, S.M.; Cordeiro, L.N.; Viana, G.S. Punica granatum (pomegranate) extract is active against dental plaque. J. Herb. Pharmacother. 2006, 6, 79–92. [Google Scholar] [CrossRef]
- Khaerunnisa, S.; Kurniawan, H.; Awaluddin, R.; Suhartati, S.; Soetjipto, S. Potential inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints 2020, 2020, 2020030226. [Google Scholar]
- Maphetu, N.; Unuofin, J.O.; Masuku, N.P.; Olisah, C.; Lebelo, S.L. Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punica granatum L.(pomegranate) plant extracts: A review. Biomed. Pharmacother. 2022, 153, 113256. [Google Scholar] [CrossRef]
- Tito, A.; Colantuono, A.; Pirone, L.; Pedone, E.; Intartaglia, D.; Giamundo, G.; Conte, I.; Vitaglione, P.; Apone, F. Pomegranate peel extract as an inhibitor of SARS-CoV-2 spike binding to human ACE2 receptor (in vitro): A promising source of novel antiviral drugs. Front. Chem. 2021, 9, 638187. [Google Scholar] [CrossRef]
- Suručić, R.; Travar, M.; Petković, M.; Tubić, B.; Stojiljković, M.P.; Grabež, M.; Šavikin, K.; Zdunić, G.; Škrbić, R. Pomegranate peel extract polyphenols attenuate the SARS-CoV-2 S-glycoprotein binding ability to ACE2 Receptor: In silico and in vitro studies. Bioorg. Chem. 2021, 114, 105145. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Ahmadpoor, M.; Farahi, M.M.; Hadianfar, A.; Sahebkar, A.; Esmaily, H.; Nematy, M.; Rakhshandeh, H. The Effect of Pomegranate Juice and Sumac Consumption in the Treatment of Outpatients with COVID-19. Mediat. Inflamm. 2022, 2022, 6850342. [Google Scholar] [CrossRef]
- Teniente, S.L.; Flores-Gallegos, A.C.; Esparza-González, S.C.; Campos-Múzquiz, L.G.; Nery-Flores, S.D.; Rodríguez-Herrera, R. Anticancer Effect of Pomegranate Peel Polyphenols against Cervical Cancer. Antioxidants 2023, 12, 127. [Google Scholar] [CrossRef]
- Pantiora, P.D.; Balaouras, A.I.; Mina, I.K.; Freris, C.I.; Pappas, A.C.; Danezis, G.P.; Zoidis, E.; Georgiou, C.A. The Therapeutic Alliance between Pomegranate and Health Emphasizing on Anticancer Properties. Antioxidants 2023, 12, 187. [Google Scholar] [CrossRef]
- Henderson, B.E.; Feigelson, H.S. Hormonal carcinogenesis. Carcinogenesis 2000, 21, 427–433. [Google Scholar] [CrossRef]
- Adams, L.S.; Zhang, Y.; Seeram, N.P.; Heber, D.; Chen, S. Pomegranate ellagitannin–derived compounds exhibit antiproliferative and antiaromatase activity in breast cancer cells in vitro. Cancer Prev. Res. 2010, 3, 108–113. [Google Scholar] [CrossRef]
- Eroglu Ozkan, E.; Seyhan, M.F.; Kurt Sirin, O.; Yilmaz-Ozden, T.; Ersoy, E.; Hatipoglu Cakmar, S.D.; Goren, A.C.; Yilmaz Aydogan, H.; Ozturk, O. Antiproliferative effects of Turkish pomegranate (Punica granatum L.) extracts on MCF-7 human breast cancer cell lines with focus on antioxidant potential and bioactive compounds analyzed by LC-MS/MS. J. Food Biochem. 2021, 45, e13904. [Google Scholar] [CrossRef]
- Jeune, M.L.; Kumi-Diaka, J.; Brown, J. Anticancer activities of pomegranate extracts and genistein in human breast cancer cells. J. Med. Food 2005, 8, 469–475. [Google Scholar] [CrossRef]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef]
- Dahlawi, H.; Jordan-Mahy, N.; Clench, M.R.; Le Maitre, C.L. Bioactive actions of pomegranate fruit extracts on leukemia cell lines in vitro hold promise for new therapeutic agents for leukemia. Nutr. Cancer 2012, 64, 100–110. [Google Scholar] [CrossRef]
- Soslow, R.A.; Dannenberg, A.J.; Rush, D.; Woerner, B.M.; Khan, K.N.; Masferrer, J.; Koki, A.T. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 2000, 89, 2637–2645. [Google Scholar] [CrossRef]
- Shukla, M.; Gupta, K.; Rasheed, Z.; Khan, K.A.; Haqqi, T.M. Bioavailable constituents/metabolites of pomegranate (Punica granatum L.) preferentially inhibit COX2 activity ex vivo and IL-1beta-induced PGE2 production in human chondrocytes in vitro. J. Inflamm. 2008, 5, 9. [Google Scholar] [CrossRef]
- Hajleh, M.A.; Al-Dujaili, A. Anti-cancer activity of pomegranate and its biophenols; general review. EC Nutr. 2016, 6, 28–52. [Google Scholar]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Bhatia, D.; Bishayee, A. Anti-inflammatory mechanism involved in pomegranate-mediated prevention of breast cancer: The role of NF-κB and Nrf2 signaling pathways. Nutrients 2017, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.Y.; Seeram, N.P.; Heber, D. Pomegranate polyphenols down-regulate expression of androgen-synthesizing genes in human prostate cancer cells overexpressing the androgen receptor. J. Nutr. Biochem. 2008, 19, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Rettig, M.B.; Heber, D.; An, J.; Seeram, N.P.; Rao, J.Y.; Liu, H.; Klatte, T.; Belldegrun, A.; Moro, A.; Henning, S.M. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-κB-dependent mechanism. Mol. Cancer Ther. 2008, 7, 2662–2671. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Giménez-Bastida, J.A.; García-Conesa, M.T.; Gómez-Sánchez, M.B.; García-Talavera, N.V.; Gil-Izquierdo, A.; Sánchez-Álvarez, C.; Fontana-Compiano, L.O.; Morga-Egea, J.P.; Pastor-Quirante, F.A. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol. Nutr. Food Res. 2010, 54, 311–322. [Google Scholar] [CrossRef]
- Paller, C.J.; Ye, X.; Wozniak, P.J.; Gillespie, B.K.; Sieber, P.R.; Greengold, R.H.; Stockton, B.R.; Hertzman, B.L.; Efros, M.D.; Roper, R.P.; et al. A randomized phase II study of pomegranate extract for men with rising PSA following initial therapy for localized prostate cancer. Prostate Cancer Prostatic Dis. 2013, 16, 50–55. [Google Scholar] [CrossRef]
- Pantuck, A.J.; Leppert, J.T.; Zomorodian, N.; Aronson, W.; Hong, J.; Barnard, R.J.; Seeram, N.; Liker, H.; Wang, H.; Elashoff, R.; et al. Phase II study of pomegranate juice for men with rising prostate-specific antigen following surgery or radiation for prostate cancer. Clin. Cancer Res. 2006, 12, 4018–4026. [Google Scholar] [CrossRef]
- Thotambailu, A.M.; Bhandary, B.S.K.; Sharmila, K. Protective effect of punica granatum extract in head and neck cancer patients undergoing radiotherapy. Indian J. Otolaryngol. Head Neck Surg. 2019, 71, 318–320. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Noratto, G.; Hingorani, L.; Talcott, S.T.; Mertens-Talcott, S.U. Protective effects of standardized pomegranate (Punica granatum L.) polyphenolic extract in ultraviolet-irradiated human skin fibroblasts. J. Agric. Food Chem. 2008, 56, 8434–8441. [Google Scholar] [CrossRef]
- Aguilar-Zárate, P.; Wong-Paz, J.E.; Buenrostro-Figueroa, J.J.; Ascacio, J.A.; Contreras-Esquivel, J.C.; Aguilar, C.N. Ellagitannins: Bioavailability, purification and biotechnological degradation. Mini Rev. Med. Chem. 2018, 18, 1244–1252. [Google Scholar] [CrossRef]
- Cerdá, B.; Llorach, R.; Cerón, J.J.; Espín, J.C.; Tomás-Barberán, F.A. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. Eur. J. Nutr. 2003, 42, 18–28. [Google Scholar] [CrossRef]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar] [CrossRef]
- Lei, F.; Xing, D.M.; Xiang, L.; Zhao, Y.N.; Wang, W.; Zhang, L.J.; Du, L.J. Pharmacokinetic study of ellagic acid in rat after oral administration of pomegranate leaf extract. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 796, 189–194. [Google Scholar] [CrossRef]
- Doyle, B.; Griffiths, L. The metabolism of ellagic acid in the rat. Xenobiotica 1980, 10, 247–256. [Google Scholar] [CrossRef]
- Teel, R.W.; Martin, R.M. Disposition of the plant phenol ellagic acid in the mouse following oral administration by gavage. Xenobiotica 1988, 18, 397–405. [Google Scholar] [CrossRef]
- Yan, L.; Yin, P.; Ma, C.; Liu, Y. Method development and validation for pharmacokinetic and tissue distributions of ellagic acid using ultrahigh performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). Molecules 2014, 19, 18923–18935. [Google Scholar] [CrossRef]
- González-Sarrías, A.; García-Villalba, R.; Núñez-Sánchez, M.Á.; Tomé-Carneiro, J.; Zafrilla, P.; Mulero, J.; Tomás-Barberán, F.A.; Espín, J.C. Identifying the limits for ellagic acid bioavailability: A crossover pharmacokinetic study in healthy volunteers after consumption of pomegranate extracts. J. Funct. Foods 2015, 19, 225–235. [Google Scholar] [CrossRef]
- García-Villalba, R.; Beltrán, D.; Espín, J.C.; Selma, M.V.; Tomás-Barberán, F.A. Time course production of urolithins from ellagic acid by human gut microbiota. J. Agric. Food Chem. 2013, 61, 8797–8806. [Google Scholar] [CrossRef]
- Monika, P.; Chandraprabha, M.; Hari Krishna, R.; Vittal, M.; Likhitha, C.; Pooja, N.; Chaudhary, V. Recent advances in pomegranate peel extract mediated nanoparticles for clinical and biomedical applications. Biotechnol. Genet. Eng. Rev. 2022, 1–29. [Google Scholar] [CrossRef]
- Li, B.; Harich, K.; Wegiel, L.; Taylor, L.S.; Edgar, K.J. Stability and solubility enhancement of ellagic acid in cellulose ester solid dispersions. Carbohydr. Polym. 2013, 92, 1443–1450. [Google Scholar] [CrossRef]
- Chudasama, Y.N.; Lugea, A.; Lu, Q.Y.; Pandol, S.J. Beta-cyclodextrin increases bioavailability of ellagic acid in rats. Gastroenterology 2011, 5, S-860. [Google Scholar] [CrossRef]
- Mady, F.M.; Mohamed Ibrahim, S.R. Cyclodextrin-based nanosponge for improvement of solubility and oral bioavailability of Ellagic acid. Pak. J. Pharm. Sci. 2018, 31, 2069–2076. [Google Scholar] [PubMed]
- Li, Y.; Zhao, X.; Zu, Y.; Zhang, Y.; Ge, Y.; Zhong, C.; Wu, W. Preparation and characterization of micronized ellagic acid using antisolvent precipitation for oral delivery. Int. J. Pharm. 2015, 486, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Mady, F.M.; Shaker, M.A. Enhanced anticancer activity and oral bioavailability of ellagic acid through encapsulation in biodegradable polymeric nanoparticles. Int. J. Nanomed. 2017, 12, 7405. [Google Scholar] [CrossRef] [PubMed]
- Meneguzzo, F.; Zabini, F. Sustainable Exploitation of Agro-Food Waste. In Sustainable Development Goals Series; Springer: Cham, Switzerland, 2021; pp. 95–111. [Google Scholar]
- Gostiša, J.; Širok, B.; Repinc, S.K.; Levstek, M.; Stražar, M.; Bizjan, B.; Zupanc, M. Performance evaluation of a novel pilot-scale pinned disc rotating generator of hydrodynamic cavitation. Ultrason. Sonochem. 2021, 72, 105431. [Google Scholar] [CrossRef] [PubMed]
- Albanese, L.; Baronti, S.; Liguori, F.; Meneguzzo, F.; Barbaro, P.; Vaccari, F.P. Hydrodynamic cavitation as an energy efficient process to increase biochar surface area and porosity: A case study. J. Clean. Prod. 2019, 210, 159–169. [Google Scholar] [CrossRef]
- Chu, J.; Metcalfe, P.; Linford, H.V.; Zhao, S.; Goycoolea, F.M.; Chen, S.; Ye, X.; Holmes, M.; Orfila, C. Short-time acoustic and hydrodynamic cavitation improves dispersibility and functionality of pectin-rich biopolymers from citrus waste. J. Clean. Prod. 2022, 330, 129789. [Google Scholar] [CrossRef]
- Askarniya, Z.; Sun, X.; Wang, Z.; Boczkaj, G. Cavitation-based technologies for pretreatment and processing of food wastes: Major applications and mechanisms–A review. Chem. Eng. J. 2023, 454, 140388. [Google Scholar] [CrossRef]
- Scurria, A.; Pagliaro, M.; Pantaleo, G.; Meneguzzo, F.; Giordano, F.M.; Ciriminna, R. CytroCell Micronized Cellulose Enhances the Structural and Thermal Properties of IntegroPectin Cross-Linked Films. ACS Appl. Bio Mater. 2022, 5, 4942–4947. [Google Scholar] [CrossRef]
- Al Jitan, S.; Scurria, A.; Albanese, L.; Pagliaro, M.; Meneguzzo, F.; Zabini, F.; Al Sakkaf, R.; Yusuf, A.; Palmisano, G.; Ciriminna, R. Micronized cellulose from citrus processing waste using water and electricity only. Int. J. Biol. Macromol. 2022, 204, 587–592. [Google Scholar] [CrossRef]
- Avachat, A.M.; Patel, V.G. Self nanoemulsifying drug delivery system of stabilized ellagic acid-phospholipid complex with improved dissolution and permeability. Saudi Pharm. J. 2015, 23, 276–289. [Google Scholar] [CrossRef]
- Zheng, D.; Lv, C.; Sun, X.; Wang, J.; Zhao, Z. Preparation of a supersaturatable self-microemulsion as drug delivery system for ellagic acid and evaluation of its antioxidant activities. J. Drug Deliv. Sci. Technol. 2019, 53, 101209. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benedetti, G.; Zabini, F.; Tagliavento, L.; Meneguzzo, F.; Calderone, V.; Testai, L. An Overview of the Health Benefits, Extraction Methods and Improving the Properties of Pomegranate. Antioxidants 2023, 12, 1351. https://doi.org/10.3390/antiox12071351
Benedetti G, Zabini F, Tagliavento L, Meneguzzo F, Calderone V, Testai L. An Overview of the Health Benefits, Extraction Methods and Improving the Properties of Pomegranate. Antioxidants. 2023; 12(7):1351. https://doi.org/10.3390/antiox12071351
Chicago/Turabian StyleBenedetti, Giada, Federica Zabini, Luca Tagliavento, Francesco Meneguzzo, Vincenzo Calderone, and Lara Testai. 2023. "An Overview of the Health Benefits, Extraction Methods and Improving the Properties of Pomegranate" Antioxidants 12, no. 7: 1351. https://doi.org/10.3390/antiox12071351
APA StyleBenedetti, G., Zabini, F., Tagliavento, L., Meneguzzo, F., Calderone, V., & Testai, L. (2023). An Overview of the Health Benefits, Extraction Methods and Improving the Properties of Pomegranate. Antioxidants, 12(7), 1351. https://doi.org/10.3390/antiox12071351











