The Prolyl Oligopeptidase Inhibitor KYP-2047 Is Cytoprotective and Anti-Inflammatory in Human Retinal Pigment Epithelial Cells with Defective Proteasomal Clearance
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Treatments
2.3. Cell Viability Assays
2.4. ROS Detection
2.5. PREP Activity Measurement
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Western Blot
2.8. Statistical Analysis
3. Results
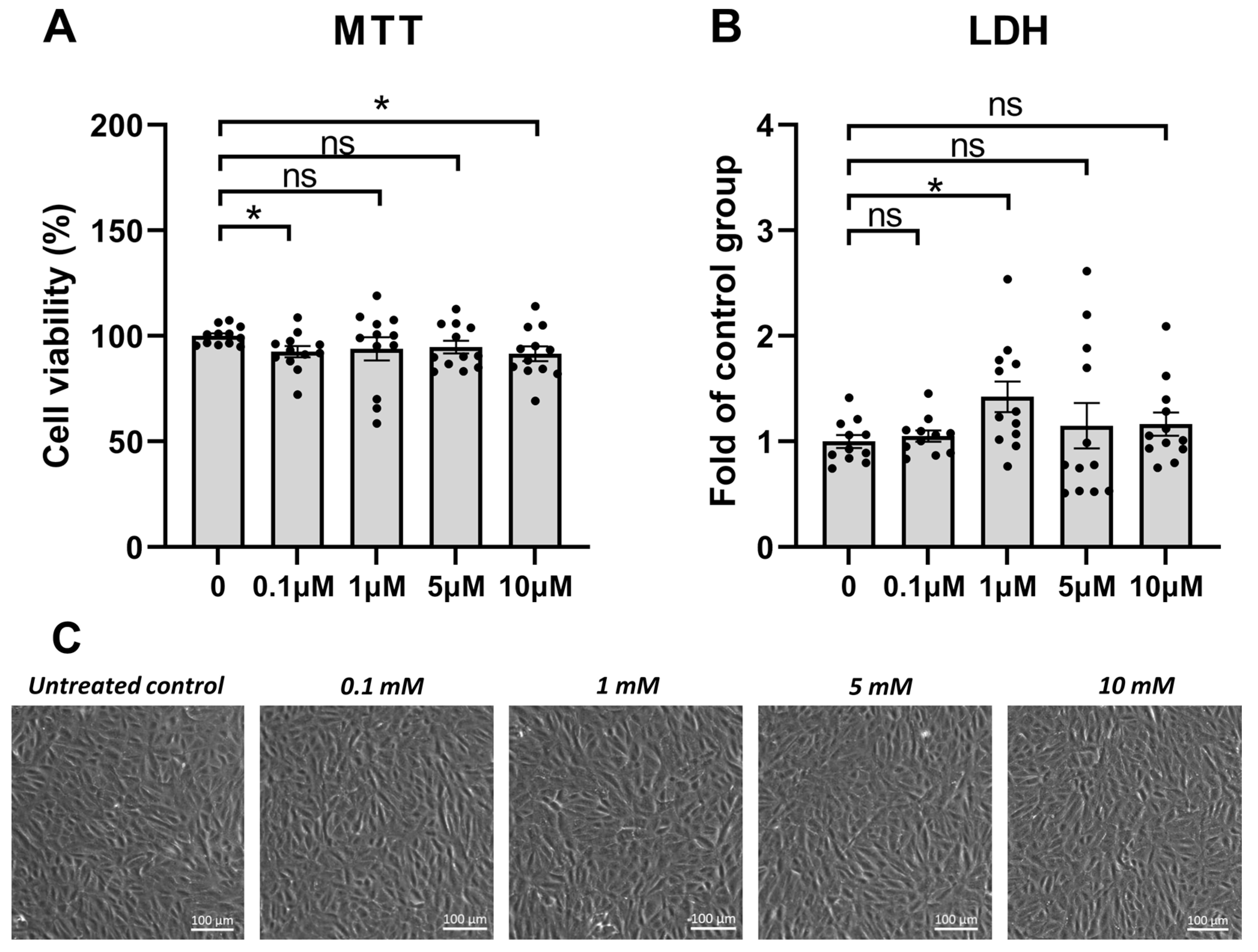
3.1. PREP Inhibitor KYP-2047 Was Tolerated Well by the ARPE-19 Cells
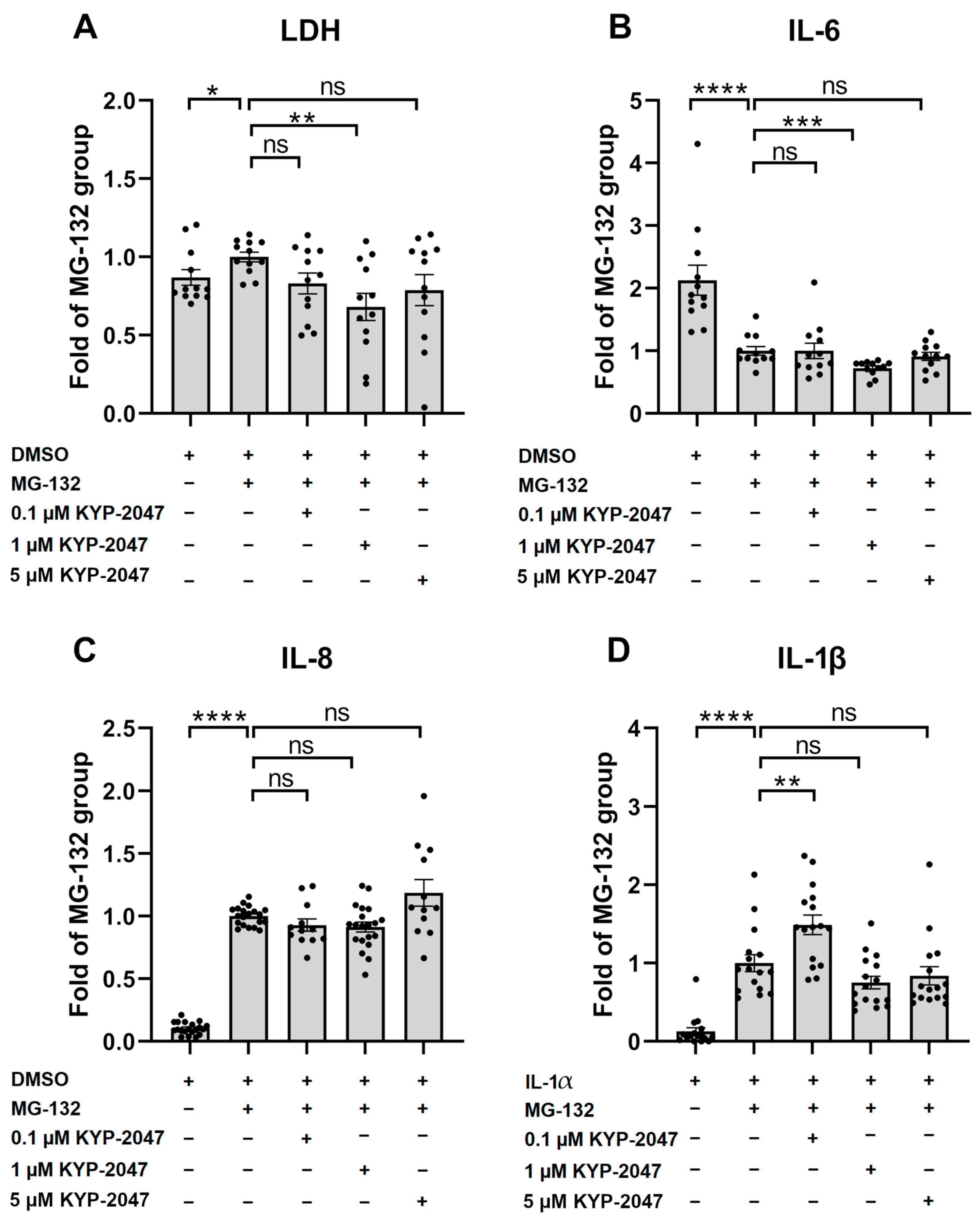
3.2. PREP Inhibitor KYP-2047 Reduced the Production of Pro-Inflammatory Cytokines and Protected the ARPE-19 Cells from MG-132-Induced Cytotoxicity
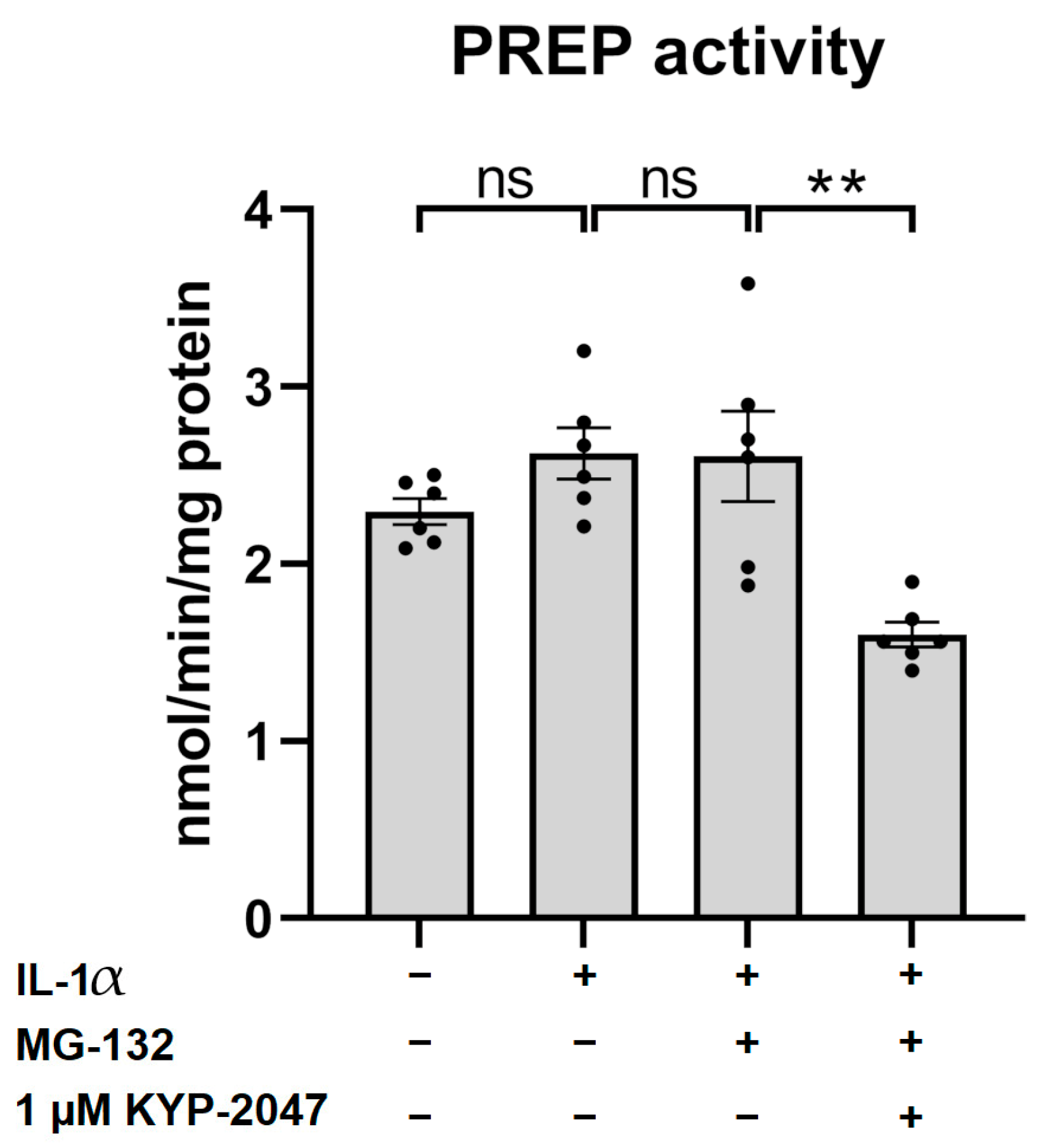
3.3. KYP-2047 Inhibited Prolyl Oligopeptidase Activity in the ARPE-19 Cells
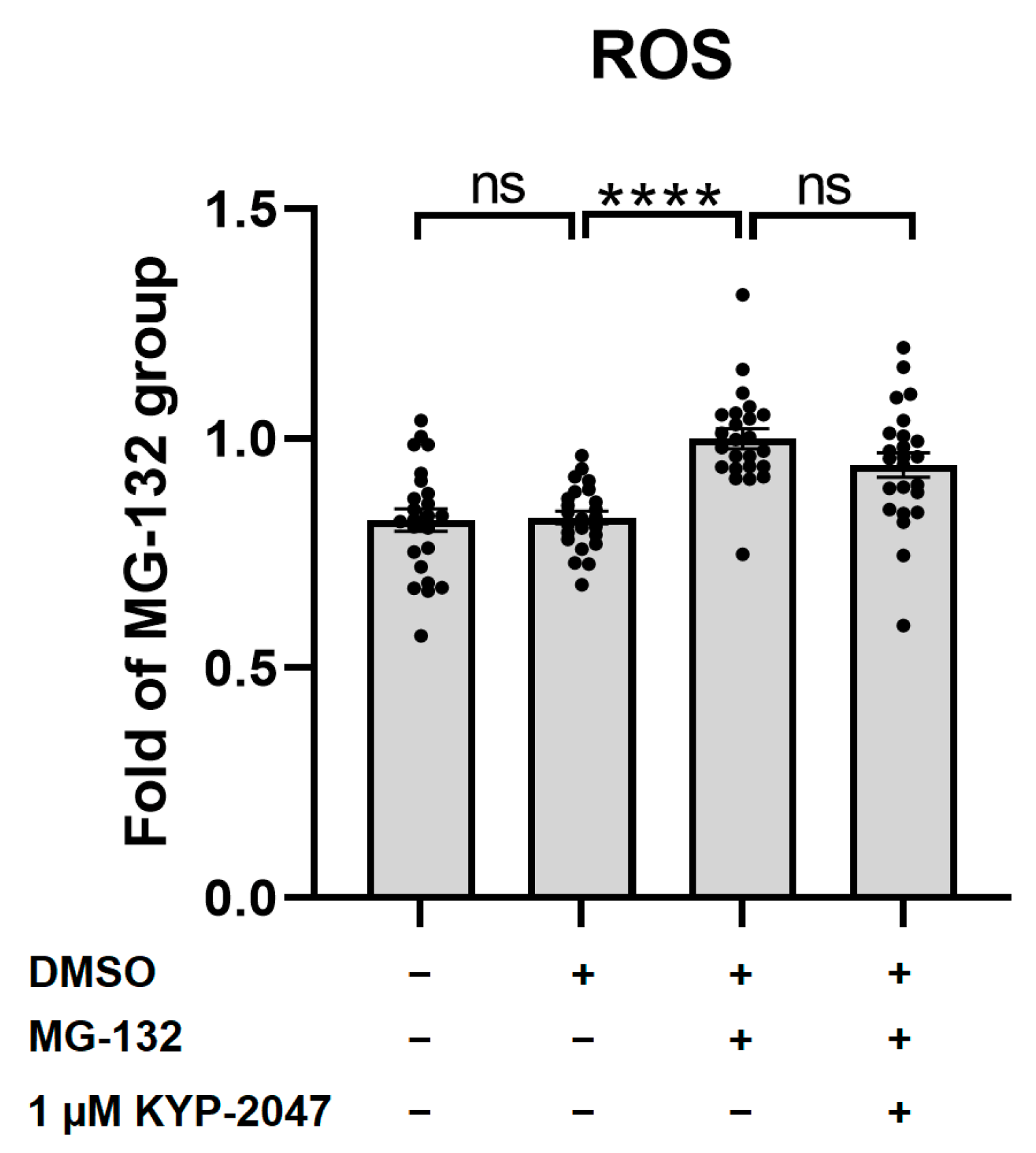
3.4. MG-132 Increased ROS Production in ARPE-19, and KYP-2047 Tended to Prevent It
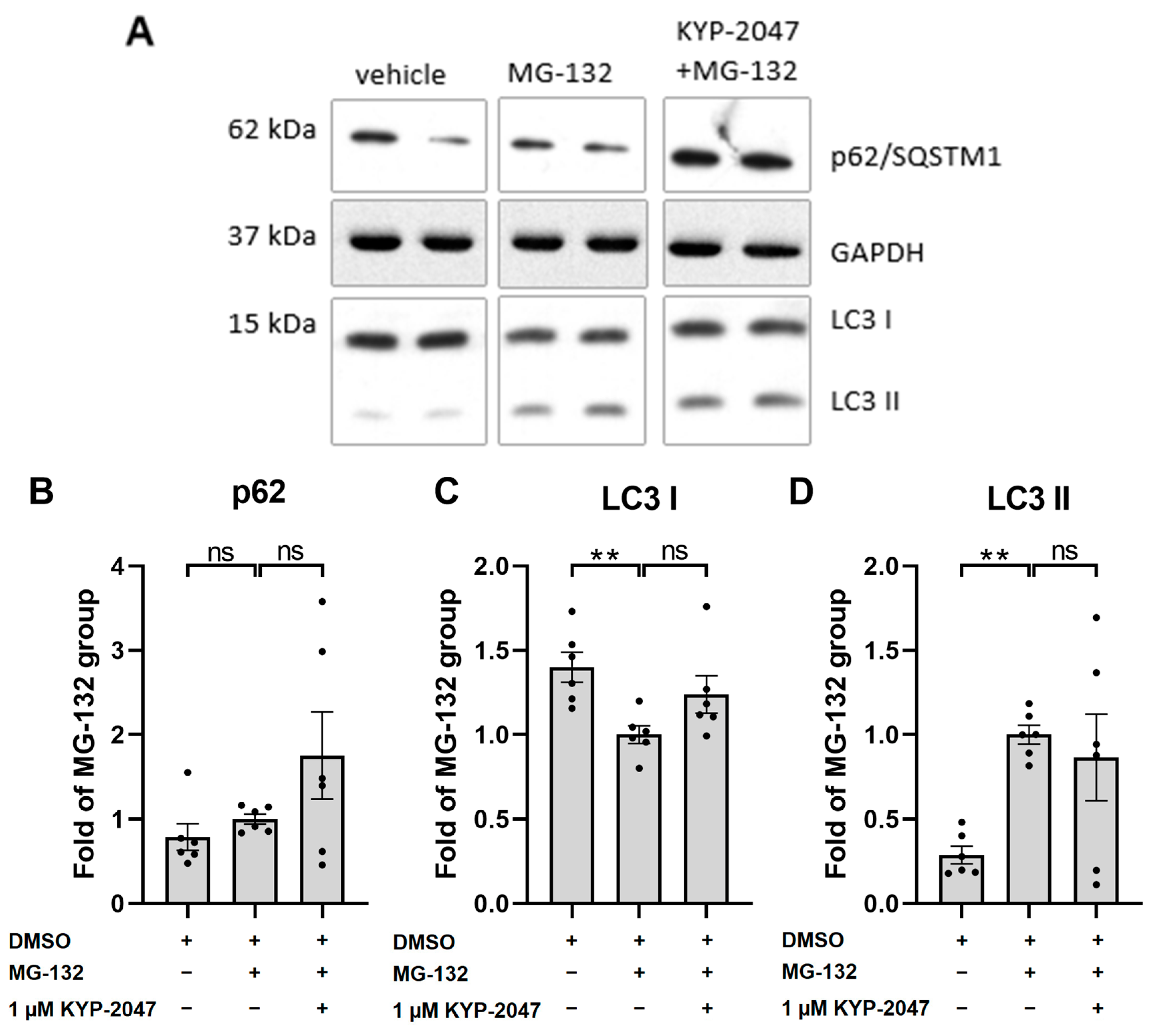
3.5. KYP-2047 Did Not Induce Autophagy in ARPE-19 Cells upon Exposure to MG-132
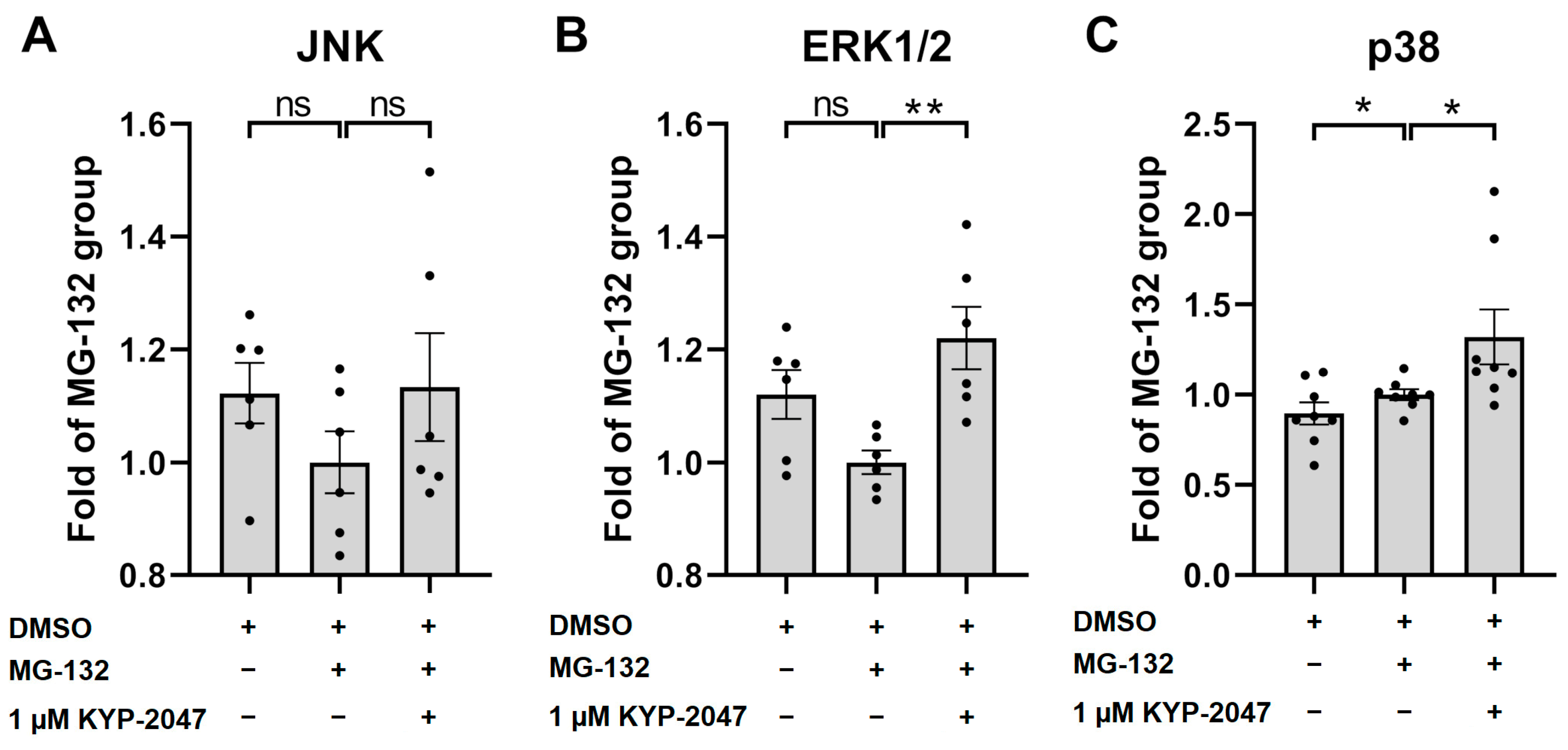
3.6. Exposure of ARPE-19 Cells to KYP-2047 Led to Phosphorylation of MAPK p38 and ERK1/2
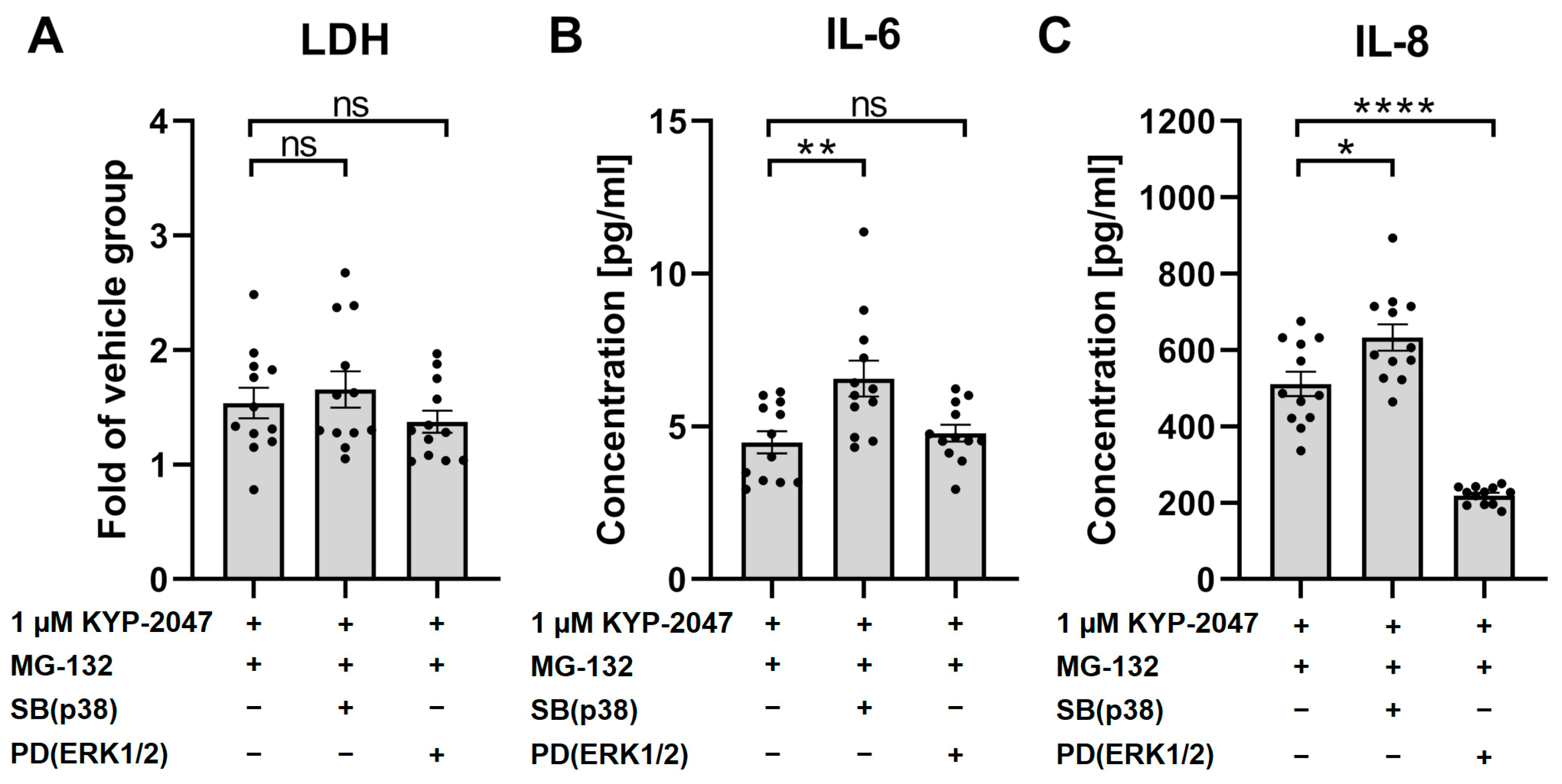
3.7. p38 Regulated the Anti-Inflammatory Effects of KYP-2047
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cunningham, D.F.; O’Connor, B. Proline specific peptidases. Biochim. Biophys. Acta 1997, 1343, 160–186. [Google Scholar] [CrossRef]
- Brandt, I.; Gérard, M.; Sergeant, K.; Devreese, B.; Baklelandt, V.; Augustyns, K.; Scharpé, S.; Engelborghs, Y.; Lambeir, A.-M. Prolyl oligopeptidase stimulates the aggregation of α-synuclein. Peptides 2008, 29, 1472–1478. [Google Scholar] [CrossRef]
- Jalkanen, A.J.; Piepponen, T.P.; Hakkarainen, J.J.; De Meester, I.; Lambeir, A.; Forsberg, M.M. The effect of prolyl oligopeptidase inhibition on extracellular acetylcholine and dopamine levels in the rat striatum. Neurochem. Int. 2012, 60, 301–309. [Google Scholar] [CrossRef]
- Myöhänen, T.; Tenorio-Laranga, J.; Jokinen, B.; Vázquez-Sánchez, R.; Moreno-Baylach, M.J.; García-Horsman, J.A.; Männistö, P.T. Prolyl oligopeptidase induces angiogenesis bothin vitroandin vivoin a novel regulatory manner. Br. J. Pharmacol. 2011, 163, 1666–1678. [Google Scholar] [CrossRef]
- Savolainen, M.H.; Richie, C.T.; Harvey, B.K.; Männistö, P.T.; Maguire-Zeiss, K.A.; Myöhänen, T.T. The beneficial effect of a prolyl oligopeptidase inhibitor, KYP-2047, on alpha-synuclein clearance and autophagy in A30P transgenic mouse. Neurobiol. Dis. 2014, 68, 1–15. [Google Scholar] [CrossRef]
- Gaggar, A.; Jackson, P.L.; Noerager, B.D.; O’Reilly, P.J.; McQuaid, D.B.; Rowe, S.M.; Clancy, J.P.; Blalock, J.E. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J. Immunol. 2008, 180, 5662–5669. [Google Scholar] [CrossRef]
- Eteläinen, T.; Kulmala, V.; Svarcbahs, R.; Jäntti, M.; Myöhänen, T.T. Prolyl oligopeptidase inhibition reduces oxidative stress via reducing NADPH oxidase activity by activating protein phosphatase 2A. Free Radic. Biol. Med. 2021, 169, 14–23. [Google Scholar] [CrossRef]
- Penttinen, A.; Tenorio-Laranga, J.; Siikanen, A.; Morawski, M.; Rossner, S.; García-Horsman, J.A. Prolyl oligopeptidase: A rising star on the stage of neuroinflammation research. CNS Neurol. Disord. Drug. Targets 2011, 10, 340–348. [Google Scholar] [CrossRef]
- Tenorio-Laranga, J.; Coret-Ferrer, F.; Casanova-Estruch, B.; Burgal, M.; García-Horsman, J.A. Prolyl oligopeptidase is inhibited in relapsing-remitting multiple sclerosis. J. Neuroinflamm. 2010, 7, 23. [Google Scholar] [CrossRef]
- Tenorio-Laranga, J.; Montoliu, C.; Urios, A.; Hernandez-Rabaza, V.; Ahabrach, H.; García-Horsman, J.A.; Felipo, V. The expression levels of prolyl oligopeptidase responds not only to neuroinflammation but also to systemic inflammation upon liver failure in rat models and cirrhotic patients. J. Neuroinflamm. 2015, 12, 183. [Google Scholar] [CrossRef]
- Dunaevsky, Y.E.; Tereshchenkova, V.F.; Oppert, B.; Belozersky, M.A.; Filippova, I.Y.; Elpidina, E.N. Human proline specific peptidases: A comprehensive analysis. Biochim. Biophys. Acta Gen. Subj. 2020, 1864, 129636. [Google Scholar] [CrossRef]
- Kilpeläinen, T.P.; Hellinen, L.; Vrijdag, J.; Yan, X.; Svarcbahs, R.; Vellonen, K.-S.; Lambeir, A.-M.; Huttunen, H.; Urtti, A.; Wallen, E.A.A.; et al. The effect of prolyl oligopeptidase inhibitors on alpha-synuclein aggregation and autophagy cannot be predicted by their inhibitory efficacy. Biomed. Pharmacother. 2020, 128, 110253. [Google Scholar] [CrossRef]
- Agirregoitia, N.; Gil, J.; Ruiz, F.; Irazusta, J.; Casis, L. Effect of aging on rat tissue peptidase activities. The journals of gerontology. J. Gerontol. A Biol. Sci. Med. Sci. 2003, 58, B792–B797. [Google Scholar] [CrossRef]
- Roßner, S.; Schulz, I.; Zeitschel, U.; Schliebs, R.; Bigl, V.; Demuth, H. Brain prolyl endopeptidase expression in aging, APP transgenic mice and alzheimer’s disease. Neurochem. Res. 2005, 30, 695–702. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Sinha, D.; Blasiak, J.; Kauppinen, A.; Veréb, Z.; Salminen, A.; Boulton, M.E.; Petrovski, G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 2013, 9, 973–984. [Google Scholar] [CrossRef]
- Viiri, J.; Amadio, M.; Marchesi, N.; Hyttinen, J.M.T.; Kivinen, N.; Sironen, R.; Rilla, K.; Akhtar, S.; Provenzani, A.; D’Agostino, V.G.; et al. Autophagy activation clears ELAVL1/HuR-mediated accumulation of SQSTM1/p62 during proteasomal inhibition in human retinal pigment epithelial cells. PLoS ONE 2013, 8, e69563. [Google Scholar] [CrossRef]
- Kauppinen, A.; Paterno, J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef]
- Natunen, T.A.; Gynther, M.; Rostalski, H.; Jaako, K.; Jalkanen, A.J. Extracellular prolyl oligopeptidase derived from activated microglia is a potential neuroprotection target. Basic Clin. Pharmacol. Toxicol. 2019, 124, 40–49. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT keratinocytes response on antimicrobial atelocollagen substrates: Extent of cytotoxicity, cell viability and proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef]
- Piippo, N.; Korkmaz, A.; Hytti, M.; Kinnunen, K.; Salminen, A.; Atalay, M.; Kaarniranta, K.; Kauppinen, A. Decline in cellular clearance systems induces inflammasome signaling in human ARPE-19 cells. Biochim. Biophys. Acta 2014, 1843, 3038–3046. [Google Scholar] [CrossRef]
- Svarcbahs, R.; Jäntti, M.; Kilpeläinen, T.; Julku, U.H.; Urvas, L.; Kivioja, S.; Norrbacka, S.; Myöhänen, T. Prolyl oligopeptidase inhibition activates autophagy via protein phosphatase 2A. Pharmacol. Res. 2020, 151, 104558. [Google Scholar] [CrossRef]
- Blasiak, J.; Pawlowska, E.; Sobczuk, A.; Szczepanska, J.; Kaarniranta, K. The aging stress response and its implication for AMD pathogenesis. Int. J. Mol. Sci. 2020, 21, 8840. [Google Scholar] [CrossRef]
- Uniprot 2021, September 29-Last Update, H0Y5Y0_HUMAN. Available online: https://www.uniprot.org/uniprot/H0Y5Y0 (accessed on 14 March 2023).
- Myöhänen, T.T.; Pyykkö, E.; Männistö, P.T.; Carpen, O. Distribution of prolyl oligopeptidase in human peripheral tissues and in ovarian and colorectal tumors. J. Histochem. Cytochem. 2012, 60, 706–715. [Google Scholar] [CrossRef]
- Hellinen, L.; Koskela, A.; Vattulainen, E.; Liukkonen, M.; Wegler, C.; Treyer, A.; Handin, N.; Svensson, R.; Myöhänen, T.; Poso, A.; et al. Inhibition of prolyl oligopeptidase: A promising pathway to prevent the progression of age-related macular degeneration. Biomed. Pharmacother. 2022, 146, 112501. [Google Scholar] [CrossRef]
- Norrbacka, S.; Lindholm, D.; Myöhänen, T.T. Prolyl oligopeptidase inhibition reduces PolyQ aggregation and improves cell viability in cellular model of huntington’s disease. J. Cell. Mol. Med. 2019, 23, 8511–8515. [Google Scholar] [CrossRef]
- Piippo, N.; Korhonen, E.; Hytti, M.; Kinnunen, K.; Kaarniranta, K.; Kauppinen, A. Oxidative Stress is the Principal Contributor to Inflammasome Activation in Retinal Pigment Epithelium Cells with Defunct Proteasomes and Autophagy. Cell. Physiol. Biochem. 2018, 49, 359–367. [Google Scholar] [CrossRef]
- Puttonen, K.A.; Lehtonen, S.; Raasmaja, A.; Männistö, P.T. A prolyl oligopeptidase inhibitor, Z-Pro-Prolinal, inhibits glyceraldehyde-3-phosphate dehydrogenase translocation and production of reactive oxygen species in CV1-P cells exposed to 6-hydroxydopamine. Toxicol. In Vitro 2006, 20, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Cavasin, M.A.; Rhaleb, N.; Yang, X.; Carretero, O.A. Prolyl oligopeptidase is involved in release of the antifibrotic peptide ac-SDKP. Hypertension 2004, 43, 1140–1145. [Google Scholar] [CrossRef]
- Kumar, N.; Yin, C. The anti-inflammatory peptide ac-SDKP: Synthesis, role in ACE inhibition, and its therapeutic potential in hypertension and cardiovascular diseases. Pharmacol. Res. 2018, 134, 268–279. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, P.J.; Hardison, M.T.; Jackson, P.L.; Xu, X.; Snelgrove, R.J.; Gaggar, A.; Galin, F.S.; Blalock, J.E. Neutrophils contain prolyl endopeptidase and generate the chemotactic peptide, PGP, from collagen. J. Neuroimmunol. 2009, 217, 51–54. [Google Scholar] [CrossRef]
- Moutzouris, J.P.; Che, W.; Ramsay, E.E.; Manetsch, M.; Alkhouri, H.; Bjorkman, A.M.; Schuster, F.; Ge, Q.; Ammit, A.A. Proteasomal inhibition upregulates the endogenous MAPK deactivator MKP-1 in human airway smooth muscle: Mechanism of action and effect on cytokine secretion. Biochim. Biophys. Acta 2010, 1803, 416–423. [Google Scholar] [CrossRef]
- Tsunoda, M.; Fukasawa, M.; Nishihara, A.; Takada, L.; Asano, M. JunB can enhance the transcription of IL-8 in oral squamous cell carcinoma. J. Cell. Physiol. 2021, 236, 309–317. [Google Scholar] [CrossRef]
- Vijayaraj, S.L.; Feltham, R.; Rashidi, M.; Frank, D.; Liu, Z.; Simpson, D.S.; Ebert, G.; Vince, A.; Herold, M.J.; Kueh, A.; et al. The ubiquitylation of IL-1β limits its cleavage by caspase-1 and targets it for proteasomal degradation. Nat. Commun. 2021, 12, 2713. [Google Scholar] [CrossRef]
- Qin, T.; Gao, S. Inhibition of proteasome activity upregulates IL-6 expression in RPE cells through the activation of P38 MAPKs. J. Ophthalmol. 2018, 2018, 5392432. [Google Scholar] [CrossRef]
- Hommes, D.W.; Peppelenbosch, M.P.; van Deventer, S.J.H. Mitogen activated protein (MAP) kinase signal transduction pathways and novel anti-inflammatory targets. Gut 2003, 52, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R.; Dean, J.L. The p38 MAPK pathway in rheumatoid arthritis: A sideways look. Open. Rheumatol. J. 2012, 6, 209–219. [Google Scholar] [CrossRef]
- Saklatvala, J. The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr. Opin. Pharmacol. 2004, 4, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Terajima, M.; Inoue, T.; Magari, K.; Yamazaki, H.; Higashi, Y.; Mizuhara, H. Anti-inflammatory effect and selectivity profile of AS1940477, a novel and potent p38 mitogen-activated protein kinase inhibitor. Eur. J. Pharmacol. 2013, 698, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Munoz, L.; Ramsay, E.E.; Manetsch, M.; Ge, Q.; Peifer, C.; Laufer, S.; Ammit, A.J. Novel p38 MAPK inhibitor ML3403 has potent anti-inflammatory activity in airway smooth muscle. Eur. J. Pharmacol. 2010, 635, 212–218. [Google Scholar] [CrossRef]
- Clark, A.R.; Dean, J.L.E.; Saklatvala, J. The p38 MAPK pathway mediates both antiinflammatory and proinflammatory processes: Comment on the article by damjanov and the editorial by genovese. Arthritis Rheum. 2009, 60, 3513–3514. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.L.W.; Baumgarth, N.; Yu, D.; Barry, P.A. Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J. Virol. 2004, 78, 8720–8731. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Ross, E.A.; Naylor, A.J.; O’Neil, J.D.; Crowley, T.; Ridley, M.L.; Crowe, J.; Smallie, T.; Tang, T.J.; Turner, J.D.; Norling, L.V.; et al. Treatment of inflammatory arthritis via targeting of tristetraprolin, a master regulator of pro-inflammatory gene expression. Ann. Rheum. Dis. 2017, 76, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Uzquiza, Á.; Arechederra, M.; Bragado, P.; Aguirre-Ghiso, J.A.; Porras, A. p38α mediates cell survival in response to oxidative stress via induction of antioxidant genes: Effect on the p70S6K pathway. J. Biol. Chem. 2012, 287, 2632–2642. [Google Scholar] [CrossRef]
- Hytti, M.; Piippo, N.; Korhonen, E.; Honkakoski, P.; Kaarniranta, K.; Kauppinen, A. Fisetin and luteolin protect human retinal pigment epithelial cells from oxidative stress-induced cell death and regulate inflammation. Sci. Rep. 2015, 5, 17645. [Google Scholar] [CrossRef]
- Andrade, F.E.C.; Correia-Silva, R.D.; Covre, J.L.; Lice, I.; Gomes, J.Á.P.; Gil, C.D. Effects of galectin-3 protein on UVA-induced damage in retinal pigment epithelial cells. Photochem. Photobiol. Sci. 2023, 22, 21–32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toppila, M.; Hytti, M.; Korhonen, E.; Ranta-aho, S.; Harju, N.; Forsberg, M.M.; Kaarniranta, K.; Jalkanen, A.; Kauppinen, A. The Prolyl Oligopeptidase Inhibitor KYP-2047 Is Cytoprotective and Anti-Inflammatory in Human Retinal Pigment Epithelial Cells with Defective Proteasomal Clearance. Antioxidants 2023, 12, 1279. https://doi.org/10.3390/antiox12061279
Toppila M, Hytti M, Korhonen E, Ranta-aho S, Harju N, Forsberg MM, Kaarniranta K, Jalkanen A, Kauppinen A. The Prolyl Oligopeptidase Inhibitor KYP-2047 Is Cytoprotective and Anti-Inflammatory in Human Retinal Pigment Epithelial Cells with Defective Proteasomal Clearance. Antioxidants. 2023; 12(6):1279. https://doi.org/10.3390/antiox12061279
Chicago/Turabian StyleToppila, Maija, Maria Hytti, Eveliina Korhonen, Sofia Ranta-aho, Niina Harju, Markus M. Forsberg, Kai Kaarniranta, Aaro Jalkanen, and Anu Kauppinen. 2023. "The Prolyl Oligopeptidase Inhibitor KYP-2047 Is Cytoprotective and Anti-Inflammatory in Human Retinal Pigment Epithelial Cells with Defective Proteasomal Clearance" Antioxidants 12, no. 6: 1279. https://doi.org/10.3390/antiox12061279
APA StyleToppila, M., Hytti, M., Korhonen, E., Ranta-aho, S., Harju, N., Forsberg, M. M., Kaarniranta, K., Jalkanen, A., & Kauppinen, A. (2023). The Prolyl Oligopeptidase Inhibitor KYP-2047 Is Cytoprotective and Anti-Inflammatory in Human Retinal Pigment Epithelial Cells with Defective Proteasomal Clearance. Antioxidants, 12(6), 1279. https://doi.org/10.3390/antiox12061279






.png)


