Adenosine Deaminase Inhibitory Activity of Medicinal Plants: Boost the Production of Cordycepin in Cordyceps militaris
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Cultivation of C. militaris
2.3. Quantification of Cordycepin
2.4. HPLC-Q-TOF MS
2.5. Determination of ADA Inhibitory Activity
2.6. Determination of Antioxidant Activity Using DPPH Assay
2.7. LC-Q-TOF-MS
2.8. Molecular Docking Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Various Medicinal Plants on Mycelium Growth and Cordycepin Production
3.2. Effects of Medicinal Plants on ADA Inhibition
3.3. Cordycepin Production by the Regulation of ADA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, M.A.; Tania, M. Cordycepin in anticancer research: Molecular mechanism of therapeutic effects. Curr. Med. Chem. 2020, 27, 983–996. [Google Scholar] [CrossRef] [PubMed]
- Radhi, M.; Ashraf, S.; Lawrence, S.; Tranholm, A.A.; Wellham, P.A.D.; Hafeez, A.; Khamis, A.S.; Thomas, R.; McWilliams, D.; De Moor, C.H. A systematic review of the biological effects of cordycepin. Molecules 2021, 26, 5886. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Sharma, A.K.; Sandhu, S.S.; Kashyap, D. Cordycepin: A bioactive metabolite with therapeutic potential. Life Sci. 2013, 93, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Kontogiannatos, D.; Koutrotsios, G.; Xekalaki, S.; Zervakis, G.I. Biomass and cordycepin production by the medicinal mushroom Cordyceps militaris—A review of various aspects and recent trends towards the exploitation of a valuable fungus. J. Fungi. 2021, 7, 986. [Google Scholar] [CrossRef]
- Zeng, Z.; Mou, D.; Luo, L.; Zhong, W.; Duan, L.; Zou, X. Different cultivation environments affect the yield, bacterial community and metabolites of Cordyceps cicadae. Front. Microbiol. 2021, 12, 669785. [Google Scholar] [CrossRef]
- Turk, A.; Abdelhamid, M.A.; Yeon, S.W.; Ryu, S.H.; Lee, S.; Ko, S.M.; Kim, B.S.; Pack, S.P.; Hwang, B.Y.; Lee, M.K. Cordyceps mushroom with increased cordycepin content by the cultivation on edible insects. Front. Microbiol. 2022, 13, 1017576. [Google Scholar]
- Turk, A.; Kim, B.S.; Ko, S.M.; Yeon, S.W.; Ryu, S.H.; Kim, Y.-G.; Hwang, B.Y.; Lee, M.K. Optimization of cultivation and extraction conditions of pupae-Cordyceps for cordycepin production. Nat. Prod. Sci. 2021, 27, 187–192. [Google Scholar]
- Xia, Y.; Luo, F.; Shang, Y.; Chen, P.; Lu, Y.; Wang, C. Fungal cordycepin biosynthesis is coupled with the production of the safeguard molecule pentostatin. Cell Chem. Biol. 2017, 24, 1479–1489.e1474. [Google Scholar] [CrossRef]
- Yang, L.; Li, G.; Chai, Z.; Gong, Q.; Guo, J. Synthesis of cordycepin: Current scenario and future perspectives. Fungal Genet. Biol. 2020, 143, 103431. [Google Scholar] [CrossRef]
- Meyts, I.; Aksentijevich, I. Deficiency of adenosine deaminase 2 (DADA2): Updates on the phenotype, genetics, pathogenesis, and treatment. J. Clin. Immunol. 2018, 38, 569–578. [Google Scholar] [CrossRef]
- Baker, A.R.; Slack, F.J. ADAR1 and its implications in cancer development and treatment. Trends Genet. 2022, 38, 821–830. [Google Scholar] [CrossRef]
- Kutryb-Zajac, B.; Mierzejewska, P.; Slominska, E.M.; Smolenski, R.T. Therapeutic perspectives of adenosine deaminase inhibition in cardiovascular diseases. Molecules 2020, 25, 4652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-g.; Liu, J.-w.; Tang, P.; Liu, Z.-y.; Guo, G.-J.; Sun, Q.-Y.; Yin, J.-j. Identification of a new uncompetitive inhibitor of adenosine deaminase from endophyte Aspergillus niger sp. Curr. Microbiol. 2018, 75, 565–573. [Google Scholar] [CrossRef]
- Lou, H.; Lin, J.; Guo, L.; Wang, X.; Tian, S.; Liu, C.; Zhao, Y.; Zhao, R. Advances in research on Cordyceps militaris degeneration. Appl. Microbiol. Biotechnol. 2019, 103, 7835–7841. [Google Scholar] [CrossRef]
- Xiong, C.; Xia, Y.; Zheng, P.; Wang, C. Increasing oxidative stress tolerance and subculturing stability of Cordyceps militaris by overexpression of a glutathione peroxidase gene. Appl. Microbiol. Biotechnol. 2013, 97, 2009–2015. [Google Scholar] [CrossRef]
- Avcı, A.; Kaçmaz, M.; Kavutcu, M.; Göçmen, E.; Durak, I. Effects of on antioxidant extract on adenosine deaminase activities in cancerous human liver tissues. Int. J. Cancer Res. 2005, 1, 53–56. [Google Scholar]
- Moreno, E.; Canet, J.; Gracia, E.; Lluís, C.; Mallol, J.; Canela, E.I.; Cortés, A.; Casadó, V. Molecular evidence of adenosine deaminase linking adenosine A2A receptor and CD26 proteins. Front. Pharmacol. 2018, 9, 106. [Google Scholar] [CrossRef]
- Adamson, R.H.; Zaharevitz, D.W.; Johns, D.G. Enhancement of the biological activity of adenosine analogs by the adenosine deaminase inhibitor 2′-deoxycoformycin. Pharmacology 1977, 15, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Kuno, M.; Seki, N.; Tsujimoto, S.; Nakanishi, I.; Kinoshita, T.; Nakamura, K.; Terasaka, T.; Nishio, N.; Sato, A.; Fujii, T. Anti-inflammatory activity of non-nucleoside adenosine deaminase inhibitor FR234938. Eur. J. Pharmacol. 2006, 534, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, S.; Saboury, A.A.; Haertlé, T. Adenosine deaminase inhibition. Int. J. Biol. Macromol. 2019, 141, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Volpini, R.; Costanzi, S.; Vittori, S.; Cristalli, G.; Lupidi, G. Synthesis and adenosine deaminase inhibitory activity of 3′-deoxy-1-deazaadenosines. Helv. Chim. Acta 1999, 82, 2112–2118. [Google Scholar] [CrossRef]
- Montgomery, J.A.; Thomas, H.J.; Zell, A.L.; Einsphar, H.M.; Bugg, C.E. Study on the inhibition of adenosine deaminase. J. Med. Chem. 1985, 28, 1751–1753. [Google Scholar] [CrossRef]
- Arun, K.; Sharanya, C.; Abhithaj, J.; Sadasivan, C. Biochemical and computational insights of adenosine deaminase inhibition by Epigallocatechin gallate. Comput. Biol. Chem. 2019, 83, 107111. [Google Scholar]
- Li, G.; Nakagome, I.; Hirono, S.; Itoh, T.; Fujiwara, R. Inhibition of adenosine deaminase (ADA)-mediated metabolism of cordycepin by natural substances. Pharmacol. Res. Perspect. 2015, 3, e00121. [Google Scholar] [CrossRef] [PubMed]
- Arun, K.; Sharanya, C.; Sandeep, P.; Sadasivan, C. Inhibitory activity of hibifolin on adenosine deaminase-experimental and molecular modeling study. Comput. Biol. Chem. 2016, 64, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Li, Y.H.; Hao, R.L.; Li, H.; Hu, S.Q.; Li, H.H. Identification of adenosine deaminase inhibitors from Tofu wastewater and litchi peel and their synergistic anticancer and antibacterial activities with cordycepin. Int. J. Food. Sci. Technol. 2016, 51, 1168–1176. [Google Scholar] [CrossRef]
- Kim, M.J.; Ahn, J.H.; Kim, S.B.; Jo, Y.H.; Liu, Q.; Hwang, B.Y.; Lee, M.K. Effect of extraction conditions of green tea on antioxidant activity and EGCG content: Optimization using response surface methodology. Nat. Prod. Sci. 2016, 22, 270–274. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and its major compound curcumin on health: Bioactive effects and safety profiles for food, pharmaceutical, biotechnological and medicinal applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Rabail, R.; Shabbir, M.A.; Sahar, A.; Miecznikowski, A.; Kieliszek, M.; Aadil, R.M. An intricate review on nutritional and analytical profiling of coconut, flaxseed, olive, and sunflower oil blends. Molecules 2021, 26, 7187. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, Z.; Shen, M.; Zhao, X.; Xie, J.; He, X.; Li, C. A review of traditional uses, phytochemistry, and pharmacological properties of the genus Saururus. Am. J. Chin. Med. 2020, 48, 47–76. [Google Scholar] [CrossRef]
- Nazir, R.; Kumar, V.; Gupta, S.; Dwivedi, P.; Pandey, D.K.; Dey, A. Biotechnological strategies for the sustainable production of diosgenin from Dioscorea spp. Appl. Microbiol. Biotechnol. 2021, 105, 569–585. [Google Scholar] [CrossRef]
- Chen, C.; Razali, U.H.M.; Saikim, F.H.; Mahyudin, A.; Noor, N.Q.I.M. Morus alba L. plant: Bioactive compounds and potential as a functional food ingredient. Foods 2021, 10, 689. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Jo, Y.H.; Kim, S.B.; Liu, Q.; Lee, J.W.; Mo, E.J.; Lee, K.Y.; Hwang, B.Y.; Lee, M.K. Pancreatic lipase inhibitory constituents from Morus alba leaves and optimization for extraction conditions. Bioorg. Med. Chem. Lett. 2015, 25, 2269–2274. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Parveen, S.; Qureshi, M.; Subhan, F.; Lee, Y.S. Decursin and decursinol angelate: Molecular mechanism and therapeutic potential in inflammatory diseases. Inflamm. Res. 2018, 67, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kim, Y.C. Five novel neuroprotective triterpene esters of Ulmus davidiana var. japonica. J. Nat. Prod. 2001, 64, 328. [Google Scholar] [CrossRef]
- Ahn, J.H.; Ryu, S.H.; Lee, S.; Yeon, S.W.; Turk, A.; Han, Y.J.; Lee, K.Y.; Hwang, B.Y.; Lee, M.K. Aromatic constituents from the leaves of Actinidia arguta with antioxidant and α-glucosidase inhibitory activity. Antioxidants 2021, 10, 1896. [Google Scholar] [CrossRef]
- Ratech, H.; Hirschhorn, R. Serum adenosine deaminase in normals and in a patient with adenosine deaminase deficient-severe combined immunodeficiency. Clin. Chim. Acta 1981, 115, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Jäger, W.; Groh, U.A.; Plank, G. In vitro inhibition of adenosine deaminase by flavonoids and related compounds. New insight into the mechanism of action of plant phenolics. Methods. Find. Exp. Clin. Pharmacol. 1992, 14, 413–417. [Google Scholar]
- Melzig, M. Inhibition of adenosine deaminase activity of aortic endothelial cells by selected flavonoids. Planta. Med. 1996, 62, 20–21. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Lešnik, S.; Furlan, V.; Bren, U. Rosemary (Rosmarinus officinalis L.): Extraction techniques, analytical methods and health-promoting biological effects. Phytochem. Rev. 2021, 20, 1273–1328. [Google Scholar] [CrossRef]

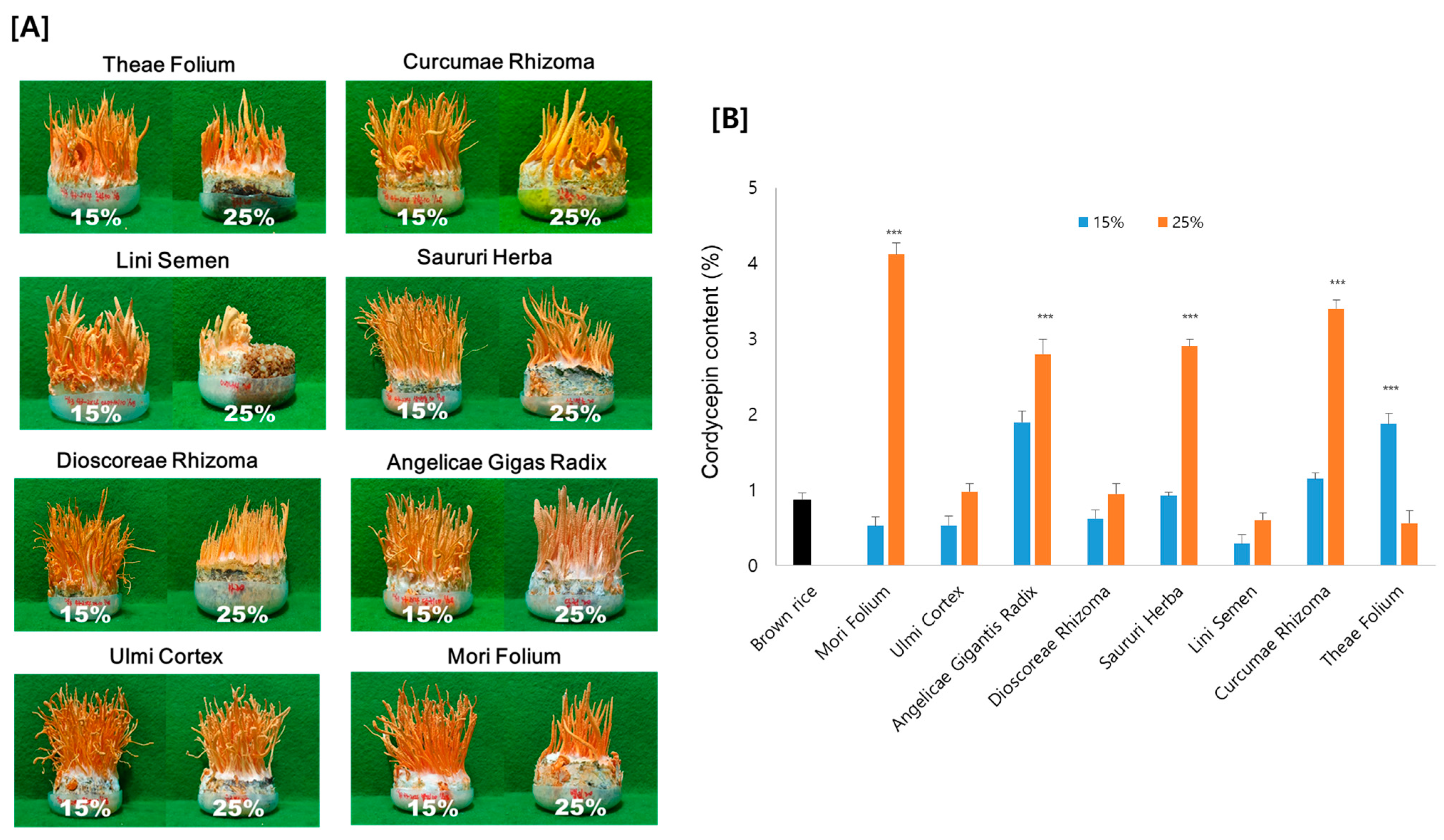

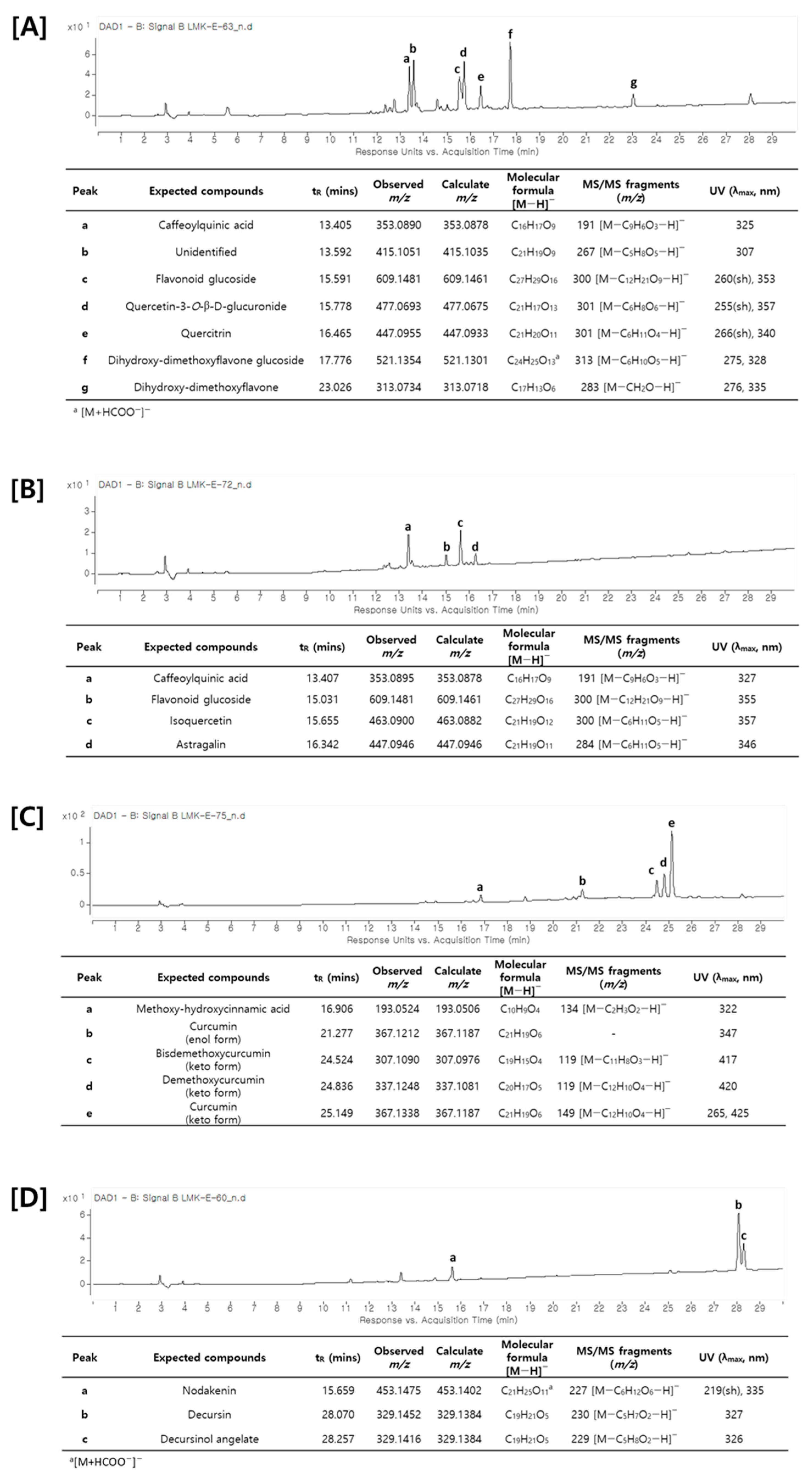
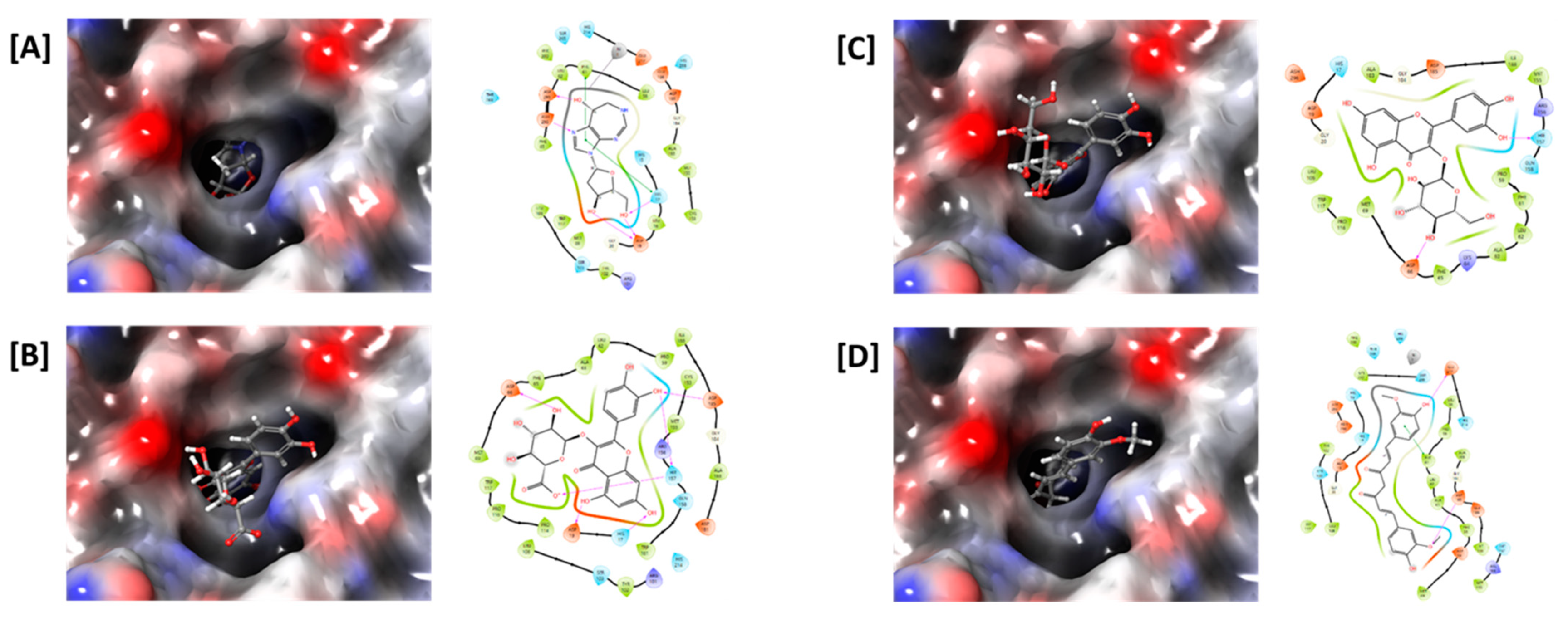
| Origin | Compounds | Docking Score |
|---|---|---|
| Positive control | Pentostatin | −7.738 |
| Saururi Herba | Caffeoylquinic acid | −5.740 |
| Quercetin-3-O-β-d-glucuronide | −7.292 | |
| Quercitrin | −4.377 | |
| Mori Folium | Caffeoylquinic acid | −5.740 |
| Isoquercetin | −6.676 | |
| Astragalin | −4.903 | |
| Curcumae Rhizoma | Bisdemethoxycurcumin | −5.598 |
| Demethoxycurcumin | −5.915 | |
| Curcumin | −6.247 | |
| Angelicae Gigantis Radix | Decursin | −5.147 |
| Decursinol angelate | −4.642 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turk, A.; Lee, S.; Yeon, S.W.; Ryu, S.H.; Han, Y.K.; Kim, Y.J.; Ko, S.M.; Kim, B.S.; Hwang, B.Y.; Lee, K.Y.; et al. Adenosine Deaminase Inhibitory Activity of Medicinal Plants: Boost the Production of Cordycepin in Cordyceps militaris. Antioxidants 2023, 12, 1260. https://doi.org/10.3390/antiox12061260
Turk A, Lee S, Yeon SW, Ryu SH, Han YK, Kim YJ, Ko SM, Kim BS, Hwang BY, Lee KY, et al. Adenosine Deaminase Inhibitory Activity of Medicinal Plants: Boost the Production of Cordycepin in Cordyceps militaris. Antioxidants. 2023; 12(6):1260. https://doi.org/10.3390/antiox12061260
Chicago/Turabian StyleTurk, Ayman, Solip Lee, Sang Won Yeon, Se Hwan Ryu, Yoo Kyong Han, Young Jun Kim, Sung Min Ko, Beom Seok Kim, Bang Yeon Hwang, Ki Yong Lee, and et al. 2023. "Adenosine Deaminase Inhibitory Activity of Medicinal Plants: Boost the Production of Cordycepin in Cordyceps militaris" Antioxidants 12, no. 6: 1260. https://doi.org/10.3390/antiox12061260
APA StyleTurk, A., Lee, S., Yeon, S. W., Ryu, S. H., Han, Y. K., Kim, Y. J., Ko, S. M., Kim, B. S., Hwang, B. Y., Lee, K. Y., & Lee, M. K. (2023). Adenosine Deaminase Inhibitory Activity of Medicinal Plants: Boost the Production of Cordycepin in Cordyceps militaris. Antioxidants, 12(6), 1260. https://doi.org/10.3390/antiox12061260







