Metallothioneins, a Part of the Retinal Endogenous Protective System in Various Ocular Diseases
Abstract
1. Introduction
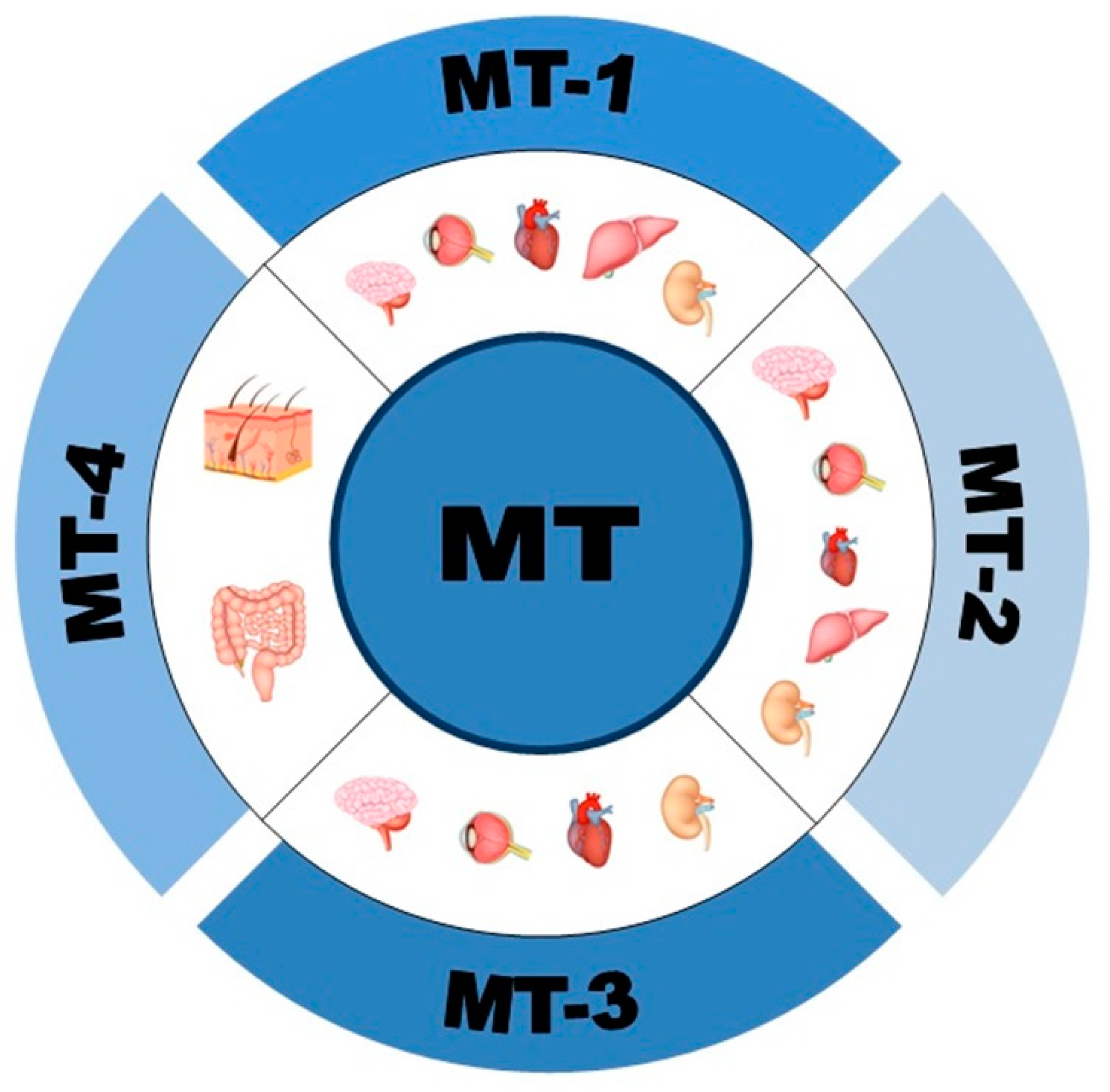
1.1. Structure, Expression and Isoforms of Metallothioneins (MTs)
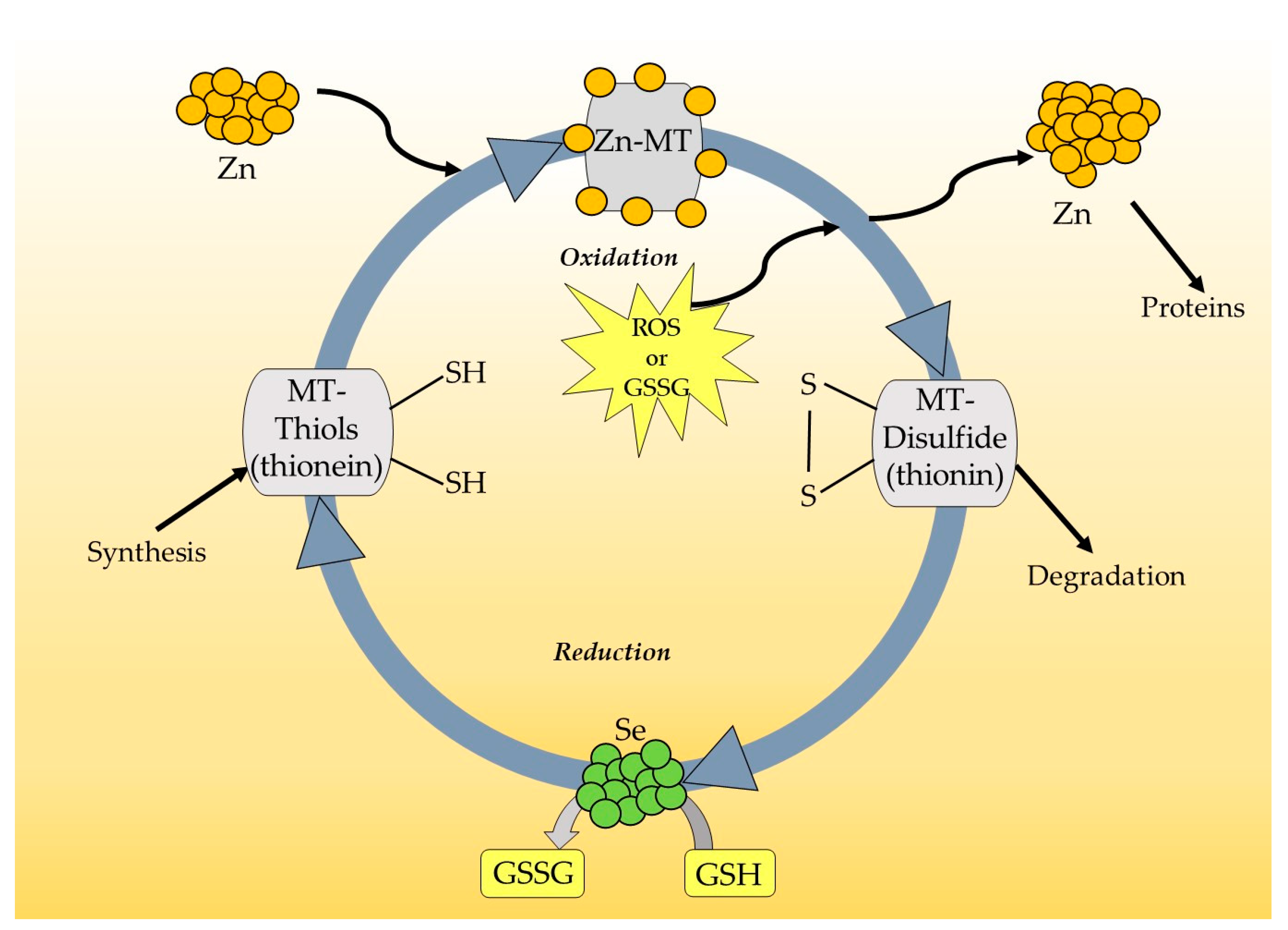
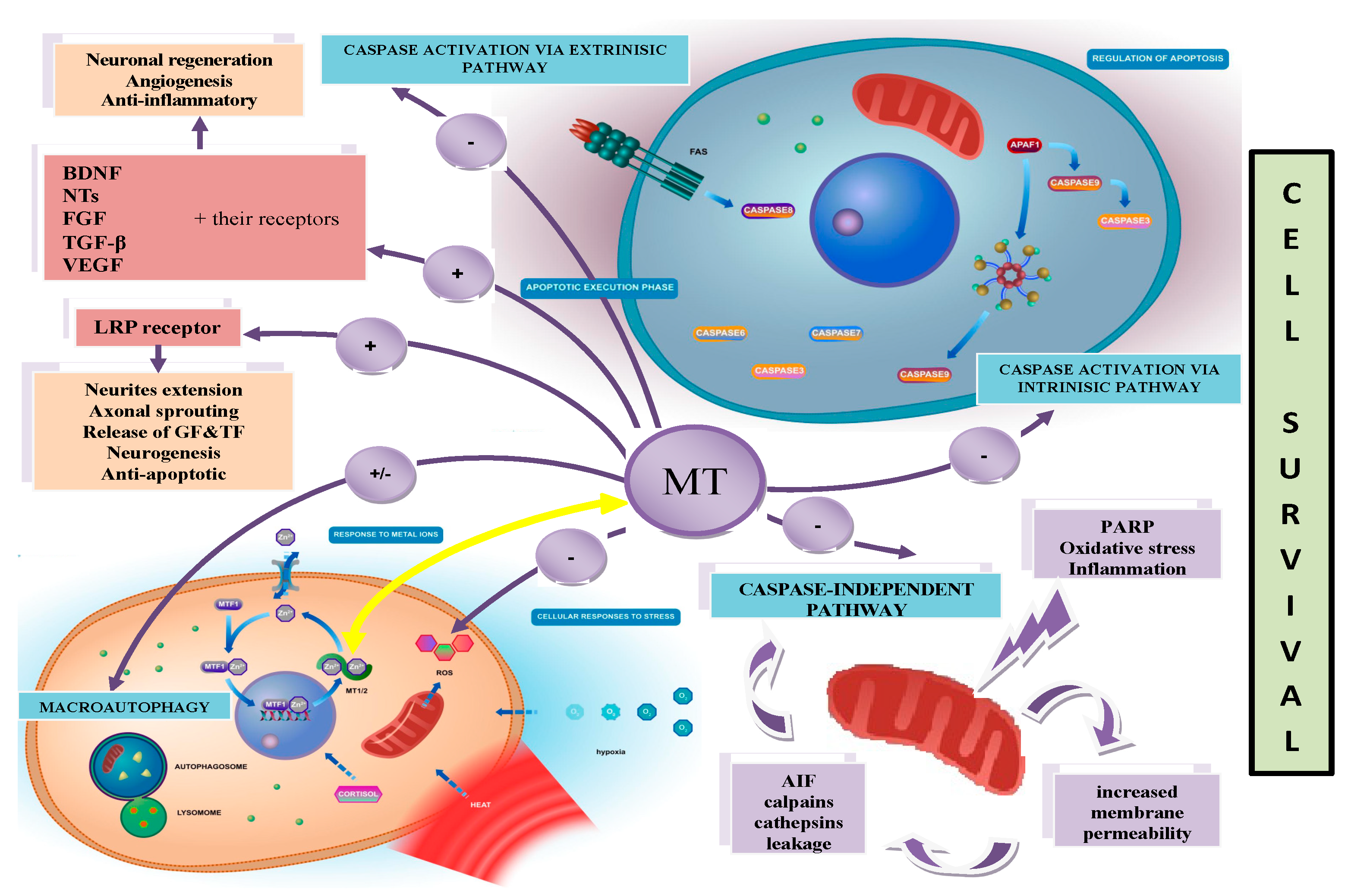
1.2. Functions of MTs
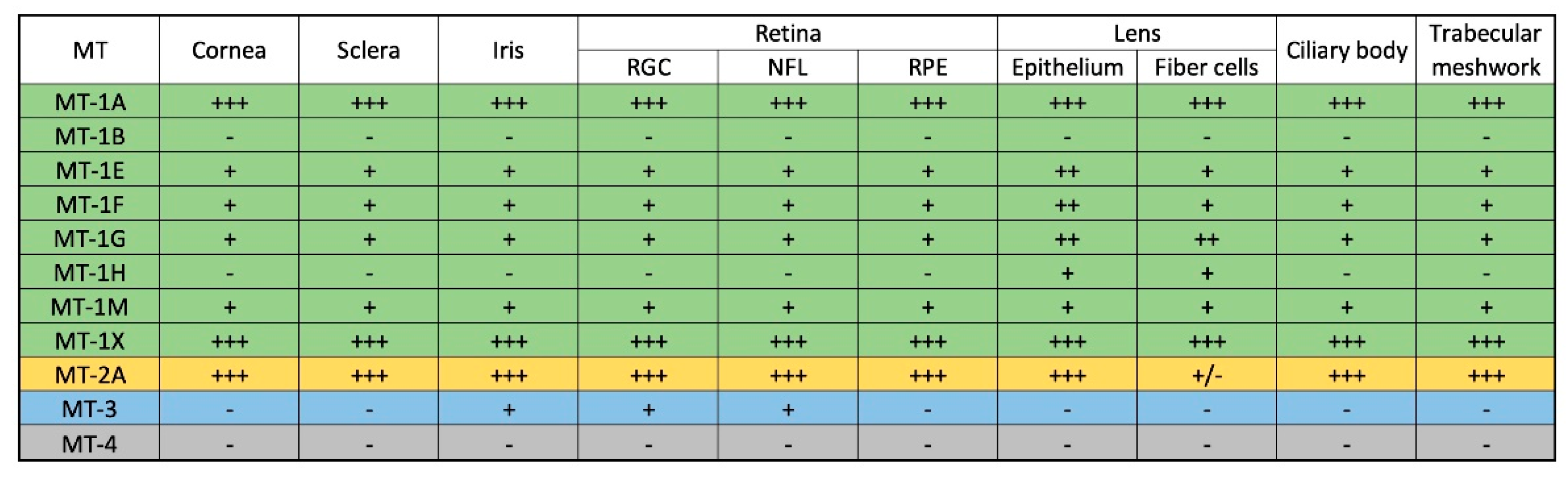
1.3. Expression of MTs in the Ocular Environment (Human Eye)
2. MTs in Ocular Diseases
2.1. Age-Related Macular Degeneration (AMD)
2.2. Retinitis Pigmentosa (RP)
2.3. Diabetic Retinopathy (DR)
2.4. Glaucoma
2.5. Cataracts
3. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Zalewska, M.; Trefon, J.; Milnerowicz, H. The Role of Metallothionein Interactions with Other Proteins. Proteomics 2014, 14, 1343–1356. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.Y.K.; Bennett, W.R.; Herbert, R.P.; West, A.K.; Lee, P.R.; Wake, H.; Fields, R.D.; Inn Chuah, M.; Chung, R.S. Metallothionein Promotes Regenerative Axonal Sprouting of Dorsal Root Ganglion Neurons after Physical Axotomy. Cell. Mol. Life Sci. 2012, 69, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Shimazawa, M.; Hara, H. Physiological Roles of Metallothioneins in Central Nervous System Diseases. Biol. Pharm. Bull. 2018, 41, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, K.S.; Deepe, G.S. Metallothioneins: Emerging Modulators in Immunity and Infection. Int. J. Mol. Sci. 2017, 18, 2197. [Google Scholar] [CrossRef]
- Margoshes, M.; Valiee, B.L. A Cadmium Protein from Equine Kidney Cortex. J. Am. Chem. Soc. 1957, 79, 4813–4814. [Google Scholar] [CrossRef]
- Coyle, P.; Philcox, J.C.; Carey, L.C.; Rofe, A.M. Metallothionein: The Multipurpose Protein. Cell. Mol. Life Sci. 2002, 59, 627–647. [Google Scholar] [CrossRef]
- Takahashi, S. Molecular Functions of Metallothionein and Its Role in Hematological Malignancies. J. Hematol. Oncol. 2012, 5, 41. [Google Scholar] [CrossRef]
- Portbury, S.D.; Adlard, P.A. Zinc Signal in Brain Diseases. Int. J. Mol. Sci. 2017, 18, 2506. [Google Scholar] [CrossRef]
- Chung, R.S.; Penkowa, M.; Dittmann, J.; King, C.E.; Bartlett, C.; Asmussen, J.W.; Hidalgo, J.; Carrasco, J.; Leung, Y.K.J.; Walker, A.K.; et al. Redefining the Role of Metallothionein within the Injured Brain: Extracellular Metallothioneins Play an Important Role in the Astrocyte-Neuron Response to Injury*. J. Biol. Chem. 2008, 283, 15349–15358. [Google Scholar] [CrossRef]
- Si, M.; Lang, J. The Roles of Metallothioneins in Carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef]
- Haq, F.; Mahoney, M.; Koropatnick, J. Signaling Events for Metallothionein Induction. Mutat. Res. Mol. Mech. Mutagen. 2003, 533, 211–226. [Google Scholar] [CrossRef] [PubMed]
- Méplan, C.; Richard, M.J.; Hainaut, P. Metalloregulation of the Tumor Suppressor Protein P53: Zinc Mediates the Renaturation of P53 after Exposure to Metal Chelators in Vitro and in Intact Cells. Oncogene 2000, 19, 5227–5236. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Liu, L.; Yi, X.; Wang, J. Studies of Interaction of Tumor Suppressor P53 with Apo-MT Using Surface Plasmon Resonance. Anal. Bioanal. Chem. 2009, 395, 2569–2575. [Google Scholar] [CrossRef] [PubMed]
- Ostrakhovitch, E.A.; Olsson, P.E.; Jiang, S.; Cherian, M.G. Interaction of Metallothionein with Tumor Suppressor P53 Protein. FEBS Lett. 2006, 580, 1235–1238. [Google Scholar] [CrossRef] [PubMed]
- Hijova, E. Metallothioneins and Zinc: Their Functions and Interactions. Bratisl. Lek. List. 2004, 105, 230–234. [Google Scholar]
- Abdel-Mageed, A.B.; Agrawal, K.C. Activation of Nuclear Factor KappaB: Potential Role in Metallothionein-Mediated Mitogenic Response. Cancer Res. 1998, 58, 2335–2338. [Google Scholar]
- Butcher, H.L.; Kennette, W.A.; Collins, O.; Zalups, R.K.; Koropatnick, J. Metallothionein Mediates the Level and Activity of Nuclear Factor Kappa B in Murine Fibroblasts. J. Pharmacol. Exp. Ther. 2004, 310, 589–598. [Google Scholar] [CrossRef]
- Rana, U.; Kothinti, R.; Meeusen, J.; Tabatabai, N.M.; Krezoski, S.; Petering, D.H. Zinc Binding Ligands and Cellular Zinc Trafficking: Apo-Metallothionein, Glutathione, TPEN, Proteomic Zinc, and Zn-Sp1. J. Inorg. Biochem. 2008, 102, 489–499. [Google Scholar] [CrossRef]
- Huang, M.; Shaw, C.F.; Petering, D.H. Interprotein Metal Exchange between Transcription Factor IIIa and Apo-Metallothionein. J. Inorg. Biochem. 2004, 98, 639–648. [Google Scholar] [CrossRef]
- Choi, S.; Liu, X.; Pan, Z. Zinc Deficiency and Cellular Oxidative Stress: Prognostic Implications in Cardiovascular Diseases. Acta Pharmacol. Sin. 2018, 39, 1120–1132. [Google Scholar] [CrossRef]
- Milnerowicz, H.; Jabłonowska, M.; Bizoń, A. Change of Zinc, Copper, and Metallothionein Concentrations and the Copper-Zinc Superoxide Dismutase Activity in Patients with Pancreatitis. Pancreas 2009, 38, 681–688. [Google Scholar] [CrossRef]
- Piacenza, F.; Malavolta, M.; Cipriano, C.; Costarelli, L.; Giacconi, R.; Muti, E.; Tesei, S.; Pierpaoli, S.; Basso, A.; Bracci, M.; et al. L-Arginine Normalizes NOS Activity and Zinc-MT Homeostasis in the Kidney of Mice Chronically Exposed to Inorganic Mercury. Toxicol. Lett. 2009, 189, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Flora, G.; Bhatnagar, P.; Flora, S.J.S. Comparative Oxidative Stress, Metallothionein Induction and Organ Toxicity Following Chronic Exposure to Arsenic, Lead and Mercury in Rats. Cell. Mol. Biol. 2014, 60, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Aquime, J.R.H.S.; Zampieri, L.C.D.A.P.; Kataoka, M.S.d.S.; Ribeiro, N.A.B.; Jaeger, R.G.; da Silva, A.L.; Ramos, R.T.J.; Alves Júnior, S.d.M.; Pinheiro, J.d.J.V. Metallothionein Expression and Its Influence on the In Vitro Biological Behavior of Mucoepidermoid Carcinoma. Cells 2020, 9, 157. [Google Scholar] [CrossRef]
- Chen, Y.; Maret, W. Catalytic Selenols Couple the Redox Cycles of Metallothionein and Glutathione. Eur. J. Biochem. 2001, 268, 3346–3353. [Google Scholar] [CrossRef] [PubMed]
- Maret, W.; Krȩzel, A. Cellular Zinc and Redox Buffering Capacity of Metallothionein/Thionein in Health and Disease. Mol. Med. 2007, 13, 371–375. [Google Scholar] [CrossRef]
- Álvarez-Barrios, A.; Álvarez, L.; García, M.; Artime, E.; Pereiro, R.; González-Iglesias, H. Antioxidant Defenses in the Human Eye: A Focus on Metallothioneins. Antioxidants 2021, 10, 89. [Google Scholar] [CrossRef]
- Krezel, A.; Hao, Q.; Maret, W. The Zinc/Thiolate Redox Biochemistry of Metallothionein and the Control of Zinc Ion Fluctuations in Cell Signaling. Arch. Biochem. Biophys. 2007, 463, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Muraoka, S.; Ogiso, T. Antioxidant Activity of Metallothionein Compared with Reduced Glutathione. Life Sci. 1997, 60, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.K.J.; Pankhurst, M.; Dunlop, S.A.; Ray, S.; Dittmann, J.; Eaton, E.D.; Palumaa, P.; Sillard, R.; Chuah, M.I.; West, A.K.; et al. Metallothionein Induces a Regenerative Reactive Astrocyte Phenotype via JAK/STAT and RhoA Signalling Pathways. Exp. Neurol. 2010, 221, 98–106. [Google Scholar] [CrossRef]
- West, A.K.; Leung, J.Y.K.; Chung, R.S. Neuroprotection and Regeneration by Extracellular Metallothionein via Lipoprotein-Receptor-Related Proteins. J. Biol. Inorg. Chem. 2011, 16, 1115–1122. [Google Scholar] [CrossRef]
- Lewis, K.E.A.; Bennett, W.; Blizzard, C.L.; West, A.K.; Chung, R.S.; Chuah, M.I. The Influence of Metallothionein Treatment and Treadmill Running Exercise on Disease Onset and Survival in SOD1G93A Amyotrophic Lateral Sclerosis Mice. Eur. J. Neurosci. 2020, 52, 3223–3241. [Google Scholar] [CrossRef]
- Landowski, L.M.; Pavez, M.; Brown, L.S.; Gasperini, R.; Taylor, B.V.; West, A.K.; Foa, L. Low-Density Lipoprotein Receptor-Related Proteins in a Novel Mechanism of Axon Guidance and Peripheral Nerve Regeneration. J. Biol. Chem. 2016, 291, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Morellini, N.M.; Fear, M.W.; Rea, S.; West, A.K.; Wood, F.M.; Dunlop, S.A. Burn Injury Has a Systemic Effect on Reinnervation of Skin and Restoration of Nociceptive Function. Wound Repair Regen. 2012, 20, 367–377. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Nairn, P.; Bartlett, C.A.; Chung, R.S.; West, A.K.; Beazley, L.D. Metallothionein-IIA Promotes Neurite Growth via the Megalin Receptor. Exp. Brain Res. 2007, 183, 171–180. [Google Scholar] [CrossRef]
- Suemori, S.; Shimazawa, M.; Kawase, K.; Satoh, M.; Nagase, H.; Yamamoto, T.; Hara, H. Metallothionein, an Endogenous Antioxidant, Protects against Retinal Neuron Damage in Mice. Investig. Ophthalmol. Vis. Sci. 2006, 47, 3975–3982. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, M.; Larsen, A.; Stoltenberg, M.; Penkowa, M. Cell Death in the Injured Brain: Roles of Metallothioneins. Prog. Histochem. Cytochem. 2009, 44, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Penkowa, M.; Tio, L.; Giralt, M.; Quintana, A.; Molinero, A.; Atrian, S.; Vašák, M.; Hidalgo, J. Specificity and Divergence in the Neurobiologic Effects of Different Metallothioneins after Brain Injury. J. Neurosci. Res. 2006, 83, 974–984. [Google Scholar] [CrossRef]
- Pietrucha-Dutczak, M.; Smedowski, A.; Liu, X.; Matuszek, I.; Varjosalo, M.; Lewin-Kowalik, J. Candidate Proteins from Predegenerated Nerve Exert Time-Specific Protection of Retinal Ganglion Cells in Glaucoma. Sci. Rep. 2017, 7, 14540. [Google Scholar] [CrossRef]
- Arriaga, J.M.; Levy, E.M.; Bravo, A.I.; Bayo, S.M.; Amat, M.; Aris, M.; Hannois, A.; Bruno, L.; Roberti, M.P.; Loria, F.S.; et al. Metallothionein Expression in Colorectal Cancer: Relevance of Different Isoforms for Tumor Progression and Patient Survival. Hum. Pathol. 2012, 43, 197–208. [Google Scholar] [CrossRef]
- Zheng, Y.; Jiang, L.; Hu, Y.; Xiao, C.; Xu, N.; Zhou, J.; Zhou, X. Metallothionein 1H (MT1H) Functions as a Tumor Suppressor in Hepatocellular Carcinoma through Regulating Wnt/β-Catenin Signaling Pathway. BMC Cancer 2017, 17, 161. [Google Scholar] [CrossRef]
- Pedersen, M.; Larsen, A.; Stoltenberg, M.; Penkowa, M. The Role of Metallothionein in Oncogenesis and Cancer Prognosis. Prog. Histochem. Cytochem. 2009, 44, 29–64. [Google Scholar] [CrossRef] [PubMed]
- Demidenko, R.; Daniunaite, K.; Bakavicius, A.; Sabaliauskaite, R.; Skeberdyte, A.; Petroska, D.; Laurinavicius, A.; Jankevicius, F.; Lazutka, J.R.; Jarmalaite, S. Decreased Expression of MT1E Is a Potential Biomarker of Prostate Cancer Progression. Oncotarget 2017, 8, 61709–61718. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Iglesias, H.; Alvarez, L.; García, M.; Petrash, C.; Sanz-Medel, A.; Coca-Prados, M. Metallothioneins (MTs) in the Human Eye: A Perspective Article on the Zinc-MT Redox Cycle. Metallomics 2014, 6, 201–208. [Google Scholar] [CrossRef]
- Alvarez, L.; Gonzalez-Iglesias, H.; Garcia, M.; Ghosh, S.; Sanz-Medel, A.; Coca-Prados, M. The Stoichiometric Transition from Zn6Cu1-Metallothionein to Zn7-Metallothionein Underlies the Up-Regulation of Metallothionein (MT) Expression: Quantitative Analysis of Mt-Metal Load in Eye Cells*. J. Biol. Chem. 2012, 287, 28456–28469. [Google Scholar] [CrossRef]
- Oppermann, B.; Zhang, W.; Magabo, K.; Kantorow, M. Identification and Spatial Analysis of Metallothioneins Expressed by the Adult Human Lens. Investig. Ophthalmol. Vis. Sci. 2001, 42, 188–193. [Google Scholar]
- Tate, D.J.; Miceli, M.V.; Newsome, D.A. Expression of Metallothionein Isoforms in Human Chorioretinal Complex. Curr. Eye Res. 2002, 24, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Koh, H.H.; Phil, M.; Henson, D.; Boulton, M. The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Tanito, M.; Kaidzu, S.; Takai, Y.; Ohira, A. Association between Systemic Oxidative Stress and Visual Field Damage in Open-Angle Glaucoma. Sci. Rep. 2016, 6, 25792. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kowluru, A.; Mishra, M.; Kumar, B. Oxidative Stress and Epigenetic Modifications in the Pathogenesis of Diabetic Retinopathy. Prog. Retin. Eye Res. 2015, 48, 40–61. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Strauss, R.W.; Lu, L.; Hafiz, G.; Wolfson, Y.; Shah, S.M.; Sophie, R.; Mir, T.A.; Scholl, H.P. Is There Excess Oxidative Stress and Damage in Eyes of Patients with Retinitis Pigmentosa? Antioxid. Redox Signal. 2015, 23, 643–648. [Google Scholar] [CrossRef]
- Domènech, E.B.; Marfany, G. The Relevance of Oxidative Stress in the Pathogenesis and Therapy of Retinal Dystrophies. Antioxidants 2020, 9, 347. [Google Scholar] [CrossRef]
- Ezquerra-Inchausti, M.; Anasagasti, A.; Barandika, O.; Garai-Aramburu, G.; Galdós, M.; López de Munain, A.; Irigoyen, C.; Ruiz-Ederra, J. A New Approach Based on Targeted Pooled DNA Sequencing Identifies Novel Mutations in Patients with Inherited Retinal Dystrophies. Sci. Rep. 2018, 8, 15457. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; Bramanti, P.; Scimone, C.; Rinaldi, C.; D’Angelo, R.; Sidoti, A. miRNAexpression Profile of Retinal Pigment Epithelial Cells under Oxidative Stress Conditions. FEBS Open Bio 2018, 8, 219–233. [Google Scholar] [CrossRef]
- Tuson, M.; Garanto, A.; Gonzàlez-Duarte, R.; Marfany, G. Overexpression of CERKL, a Gene Responsible for Retinitis Pigmentosa in Humans, Protects Cells from Apoptosis Induced by Oxidative Stress. Mol. Vis. 2009, 15, 168–180. [Google Scholar] [PubMed]
- Golestaneh, N.; Chu, Y.; Xiao, Y.Y.; Stoleru, G.L.; Theos, A.C. Dysfunctional Autophagy in RPE, a Contributing Factor in Age-Related Macular Degeneration. Cell Death Dis. 2017, 8, e2537. [Google Scholar] [CrossRef] [PubMed]
- Decanini, A.; Nordgaard, C.L.; Feng, X.; Ferrington, D.A.; Olsen, T.W. Changes in Select Redox Proteins of the Retinal Pigment Epithelium in Age-Related Macular Degeneration. Am. J. Ophthalmol. 2007, 143, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.W.T.; Wong, T.Y.; Kreis, A.J.; Simpson, J.A.; Guymer, R.H. Dietary Antioxidants and Primary Prevention of Age Related Macular Degeneration: Systematic Review and Meta-Analysis. BMJ 2007, 335, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Moriarty-Craige, S.E.; Adkison, J.; Lynn, M.; Gensler, G.; Bressler, S.; Jones, D.P.; Sternberg, P. Antioxidant Supplements Prevent Oxidation of Cysteine/Cystine Redox in Patients with Age-Related Macular Degeneration. Am. J. Ophthalmol. 2005, 140, 1020–1026. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant Vitamin and Mineral Supplements for Preventing Age-Related Macular Degeneration. Cochrane Database Syst. Rev. 2017, 7, CD000253. [Google Scholar] [CrossRef]
- Gil-Martínez, M.; Santos-Ramos, P.; Fernández-Rodríguez, M.; Abraldes, M.J.; Rodríguez-Cid, M.J.; Santiago-Varela, M.; Fernández-Ferreiro, A.; Gómez-Ulla, F. Pharmacological Advances in the Treatment of Age-Related Macular Degeneration. Curr. Med. Chem. 2020, 27, 583–598. [Google Scholar] [CrossRef]
- Carneiro, Â.; Andrade, J.P. Nutritional and Lifestyle Interventions for Age-Related Macular Degeneration: A Review. Oxid. Med. Cell. Longev. 2017, 2017, 6469138. [Google Scholar] [CrossRef]
- Miceli, M.V.; Tatejr, D.J.; Alcock, N.W.; Newsome, D.A. Zinc Deficiency and Oxidative Stress in the Retina of Pigmented Rats. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1238–1244. [Google Scholar]
- Kassoff, A.; Kassoff, J.; Buehler, J.; Eglow, M.; Kaufman, F.; Mehu, M.; Kieval, S.; Mairs, M.; Graig, B.; Quattrocchi, A.; et al. A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation with Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss: AREDS Report No. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.; Gu, S.; Yu, Y.; Huang, H.; Mo, K.; Xu, H.; Zeng, F.; Xiao, Y.; Peng, L.; et al. D609 Protects Retinal Pigmented Epithelium as a Potential Therapy for Age-Related Macular Degeneration. Signal Transduct. Target. Ther. 2020, 5, 20. [Google Scholar] [CrossRef]
- Ahmed, C.M.; Biswal, M.R.; Li, H.; Han, P.; Ildefonso, C.J.; Lewin, A.S. Repurposing an Orally Available Drug for the Treatment of Geographic Atrophy. Mol. Vis. 2016, 22, 294–310. [Google Scholar]
- Biswal, M.R.; Ahmed, C.M.; Ildefonso, C.J.; Han, P.; Li, H.; Jivanji, H.; Mao, H.; Lewin, A.S. Systemic Treatment with a 5HT1a Agonist Induces Anti-Oxidant Protection and Preserves the Retina from Mitochondrial Oxidative Stress. Exp. Eye Res. 2015, 140, 94–105. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis Pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef] [PubMed]
- Boughman, J.A.; Conneally, P.M.; Nance, W.E. Population Genetic Studies of Retinitis Pigmentosa. Am. J. Hum. Genet. 1980, 32, 223–235. [Google Scholar]
- Wunderlich, K.A.; Leveillard, T.; Penkowa, M.; Zrenner, E.; Perez, M.T. Altered Expression of Metallothionein-I and -II and Their Receptor Megalin in Inherited Photoreceptor Degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4809–4820. [Google Scholar] [CrossRef]
- Lee, R.; Wong, T.Y.; Sabanayagam, C. Epidemiology of Diabetic Retinopathy, Diabetic Macular Edema and Related Vision Loss. Eye Vis. 2015, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.P.; Di Marco, E.; Okabe, J.; Szyndralewiez, C.; Heitz, F.; Montezano, A.C.; De Haan, J.B.; Koulis, C.; El-Osta, A.; Andrews, K.L.; et al. NADPH Oxidase 1 Plays a Key Role in Diabetes Mellitus-Accelerated Atherosclerosis. Circulation 2013, 127, 1888–1902. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, A.S.; Kelly, M.; Berg, R.M.G.; Møller, K.; Pedersen, B.K. Type 2 Diabetes Is Associated with Altered NF-ΚB DNA Binding Activity, JNK Phosphorylation, and AMPK Phosphorylation in Skeletal Muscle after LPS. PLoS ONE 2011, 6, e23999. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Zhuo, L. Longitudinal in Vivo Imaging of Retinal Gliosis in a Diabetic Mouse Model. Exp. Eye Res. 2010, 91, 530–536. [Google Scholar] [CrossRef]
- Frank, R.N. Diabetic Retinopathy. N. Engl. J. Med. 2004, 350, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Kan, E.; Alici, Ö.; Kan, E.K.; Ayar, A. Effects of Alpha-Lipoic Acid on Retinal Ganglion Cells, Retinal Thicknesses, and VEGF Production in an Experimental Model of Diabetes. Int. Ophthalmol. 2017, 37, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, Q.; Xia, X.; Zhang, S.; Gu, Q.; Luo, D. Blood-Retinal Barrier Breakdown Induced by Activation of Protein Kinase C via Vascular Endothelial Growth Factor in Streptozotocin-Induced Diabetic Rats. Curr. Eye Res. 2004, 28, 251–256. [Google Scholar] [CrossRef]
- Pe’er, J.; Shweiki, D.; Itin, A.; Hemo, I.; Gnessin, H.; Keshet, E. Hypoxia-Induced Expression of Vascular Endothelial Growth Factor by Retinal Cells Is a Common Factor in Neovascularizing Ocular Diseases. Lab. Investig. 1995, 72, 638–645. [Google Scholar]
- Barber, A.J.; Gardner, T.W.; Abcouwer, S.F. The Significance of Vascular and Neural Apoptosis to the Pathology of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Midena, E. Retinal Layers Changes in Human Preclinical and Early Clinical Diabetic Retinopathy Support Early Retinal Neuronal and Müller Cells Alterations. J. Diabetes Res. 2013, 2013, 905058. [Google Scholar] [CrossRef]
- Fresta, C.G.; Fidilio, A.; Caruso, G.; Caraci, F.; Giblin, F.J.; Leggio, G.M.; Salomone, S.; Drago, F.; Bucolo, C. A New Human Blood-Retinal Barrier Model Based on Endothelial Cells, Pericytes, and Astrocytes. Int. J. Mol. Sci. 2020, 21, 1636. [Google Scholar] [CrossRef]
- Catalani, E.; Cervia, D. Diabetic Retinopathy: A Matter of Retinal Ganglion Cell Homeostasis. Neural Regen. Res. 2020, 15, 1253–1254. [Google Scholar] [CrossRef]
- Nakamura, S.; Shimazawa, M.; Inoue, Y.; Takata, S.; Ito, Y.; Tsuruma, K.; Ikeda, T.; Honda, A.; Satoh, M.; Hara, H. Role of Metallothioneins 1 and 2 in Ocular Neovascularization. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6851–6860. [Google Scholar] [CrossRef]
- Van Wouwe, J.P.; Uijlenbroek, J.J.M. The Role of the Pancreas in the Regulation of Zinc Status. Biol. Trace Elem. Res. 1994, 42, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.V. Zinc and Insulin in Pancreatic Beta-Cells. Endocrine 2014, 45, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Chausmer, A.B. Zinc, Insulin and Diabetes. J. Am. Coll. Nutr. 1998, 17, 109–115. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc: Role in Immunity, Oxidative Stress and Chronic Inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 646–652. [Google Scholar] [CrossRef]
- Miao, X.; Sun, W.; Miao, L.; Fu, Y.; Wang, Y.; Su, G.; Liu, Q. Zinc and Diabetic Retinopathy. J. Diabetes Res. 2013, 2013, 425854. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, S.A. Zinc Might Protect Oxidative Changes in the Retina and Pancreas at the Early Stage of Diabetic Rats. Toxicol. Appl. Pharmacol. 2004, 201, 149–155. [Google Scholar] [CrossRef]
- Zangger, K.; Öz, G.; Haslinger, E.; Kunert, O.; Armitage, I.M. Nitric Oxide Selectively Releases Metals from the Amino-Terminal Domain of Metallothioneins: Potential Role at Inflammatory Sites. FASEB J. 2001, 15, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Spahl, D.U.; Berendji-Grün, D.; Suschek, C.V.; Kolb-Bachofen, V.; Kröncke, K.D. Regulation of Zinc Homeostasis by Inducible NO Synthase-Derived NO: Nuclear Metallothionein Translocation and Intranuclear Zn2+ Release. Proc. Natl. Acad. Sci. USA 2003, 100, 13952–13957. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Luo, L.J.; Yang, C.J.; Lai, J.Y. Highly Retina-Permeating and Long-Acting Resveratrol/Metformin Nanotherapeutics for Enhanced Treatment of Macular Degeneration. ACS Nano 2023, 17, 168–183. [Google Scholar] [CrossRef]
- Dziedziak, J.; Kasarełło, K.; Cudnoch-Jędrzejewska, A. Dietary Antioxidants in Age-Related Macular Degeneration and Glaucoma. Antioxidants 2021, 10, 1743. [Google Scholar] [CrossRef] [PubMed]
- Kumaramanickavel, G.; Jadhav, S.; Ramsait, S.P.; Davey, P.G. Molecular Genomics of Glaucoma: An Update. Intechopen 2022, 1, 1–47. [Google Scholar]
- Kamińska, A.; Romano, G.L.; Rejdak, R.; Zweifel, S.; Fiedorowicz, M.; Rejdak, M.; Bajka, A.; Amato, R.; Bucolo, C.; Avitabile, T.; et al. Influence of Trace Elements on Neurodegenerative Diseases of the Eye—The Glaucoma Model. Int. J. Mol. Sci. 2021, 22, 4323. [Google Scholar] [CrossRef]
- Aranaz, M.; Costas-Rodríguez, M.; Lobo, L.; García, M.; González-Iglesias, H.; Pereiro, R.; Vanhaecke, F. Homeostatic Alterations Related to Total Antioxidant Capacity, Elemental Concentrations and Isotopic Compositions in Aqueous Humor of Glaucoma Patients. Anal. Bioanal. Chem. 2022, 414, 515–524. [Google Scholar] [CrossRef]
- Iwase, A.; Suzuki, Y.; Araie, M.; Yamamoto, T.; Abe, H.; Shirato, S.; Kuwayama, Y.; Mishima, H.K.; Shimizu, H.; Tomita, G.; et al. The Prevalence of Primary Open-Angle Glaucoma in Japanese: The Tajimi Study. Ophthalmology 2004, 111, 1641–1648. [Google Scholar] [CrossRef]
- Hohberger, B.; Chaudhri, M.A.; Michalke, B.; Lucio, M.; Nowomiejska, K.; Schlötzer-Schrehardt, U.; Grieb, P.; Rejdak, R.; Jünemann, A.G.M. Levels of Aqueous Humor Trace Elements in Patients with Open-Angle Glaucoma. J. Trace Elem. Med. Biol. 2017, 45, 150–155. [Google Scholar] [CrossRef]
- Gonzalez, P.; Epstein, D.L.; Borrás, T. Genes Upregulated in the Human Trabecular Meshwork in Response to Elevated Intraocular Pressure. Investig. Ophthalmol. Vis. Sci. 2000, 41, 352–361. [Google Scholar]
- DeToma, A.S.; Dengler-Crish, C.M.; Deb, A.; Braymer, J.J.; Penner-Hahn, J.E.; van der Schyf, C.J.; Lim, M.H.; Crish, S.D. Abnormal Metal Levels in the Primary Visual Pathway of the DBA/2J Mouse Model of Glaucoma. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Vecino, E.; Ugarte, M.; Nash, M.S.; Osborne, N.N. NMDA Induces BDNF Expression in the Albino Rat Retina in vivo. Neuroreport 1999, 10, 1103–1106. [Google Scholar] [CrossRef] [PubMed]
- Vecino, E.; García-Grespo, D.; García, M.; Martinez-Millán, L.; Sharma, S.C.; Carrascal, E. Rat Retinal Ganglion Cells Co-Express Brain Derived Neurotrophic Factor (BDNF) and Its Receptor TrkB. Vision Res. 2002, 42, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, J.M.; Taylor, A.; Jacques, P.; Willett, W.C.; Rosner, B.; Colditz, G.A.; Chylack, L.T.; Hankinson, S.E. Postmenopausal Hormone Use and Lens Opacities. Ophthalmic Epidemiol. 2002, 9, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Asbell, P.A.; Dualan, I.; Mindel, J.; Brocks, D.; Ahmad, M.; Epstein, S. Age-Related Cataract. Lancet 2005, 365, 599–609. [Google Scholar] [CrossRef]
- Lee, C.M.; Afshari, N.A. The Global State of Cataract Blindness. Curr. Opin. Ophthalmol. 2017, 28, 98–103. [Google Scholar] [CrossRef]
- Kantorow, M.; Kays, T.; Horwitz, J.; Huang, Q.; Sun, J.; Piatigorsky, J.; Carper, D. Differential Display Detects Altered Gene Expression between Cataractous and Normal Human Lenses. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2344–2354. [Google Scholar]
- Hawse, J.R.; Padgaonkar, V.A.; Leverenz, V.R.; Pelliccia, S.E.; Kantorow, M.; Giblin, F.J. The Role of Metallothionein IIa in Defending Lens Epithelial Cells against Cadmium and TBHP Induced Oxidative Stress. Mol. Vis. 2006, 12, 342–349. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamrozik, D.; Dutczak, R.; Machowicz, J.; Wojtyniak, A.; Smędowski, A.; Pietrucha-Dutczak, M. Metallothioneins, a Part of the Retinal Endogenous Protective System in Various Ocular Diseases. Antioxidants 2023, 12, 1251. https://doi.org/10.3390/antiox12061251
Jamrozik D, Dutczak R, Machowicz J, Wojtyniak A, Smędowski A, Pietrucha-Dutczak M. Metallothioneins, a Part of the Retinal Endogenous Protective System in Various Ocular Diseases. Antioxidants. 2023; 12(6):1251. https://doi.org/10.3390/antiox12061251
Chicago/Turabian StyleJamrozik, Daniel, Radosław Dutczak, Joanna Machowicz, Alicja Wojtyniak, Adrian Smędowski, and Marita Pietrucha-Dutczak. 2023. "Metallothioneins, a Part of the Retinal Endogenous Protective System in Various Ocular Diseases" Antioxidants 12, no. 6: 1251. https://doi.org/10.3390/antiox12061251
APA StyleJamrozik, D., Dutczak, R., Machowicz, J., Wojtyniak, A., Smędowski, A., & Pietrucha-Dutczak, M. (2023). Metallothioneins, a Part of the Retinal Endogenous Protective System in Various Ocular Diseases. Antioxidants, 12(6), 1251. https://doi.org/10.3390/antiox12061251