Autophagy Activation Promoted by Pulses of Light and Phytochemicals Counteracting Oxidative Stress during Age-Related Macular Degeneration
Abstract
1. Introduction
1.1. Outline of the Main Objectives
1.2. The Main Functions of Retinal Pigment Epithelium and a Target of Reactive Oxygen Species (ROS) in the Course of Retinal Degeneration
1.3. The RPE from Melanin Accumulation to Formation of Oxygen-Dependent Inclusions (Figure 3)
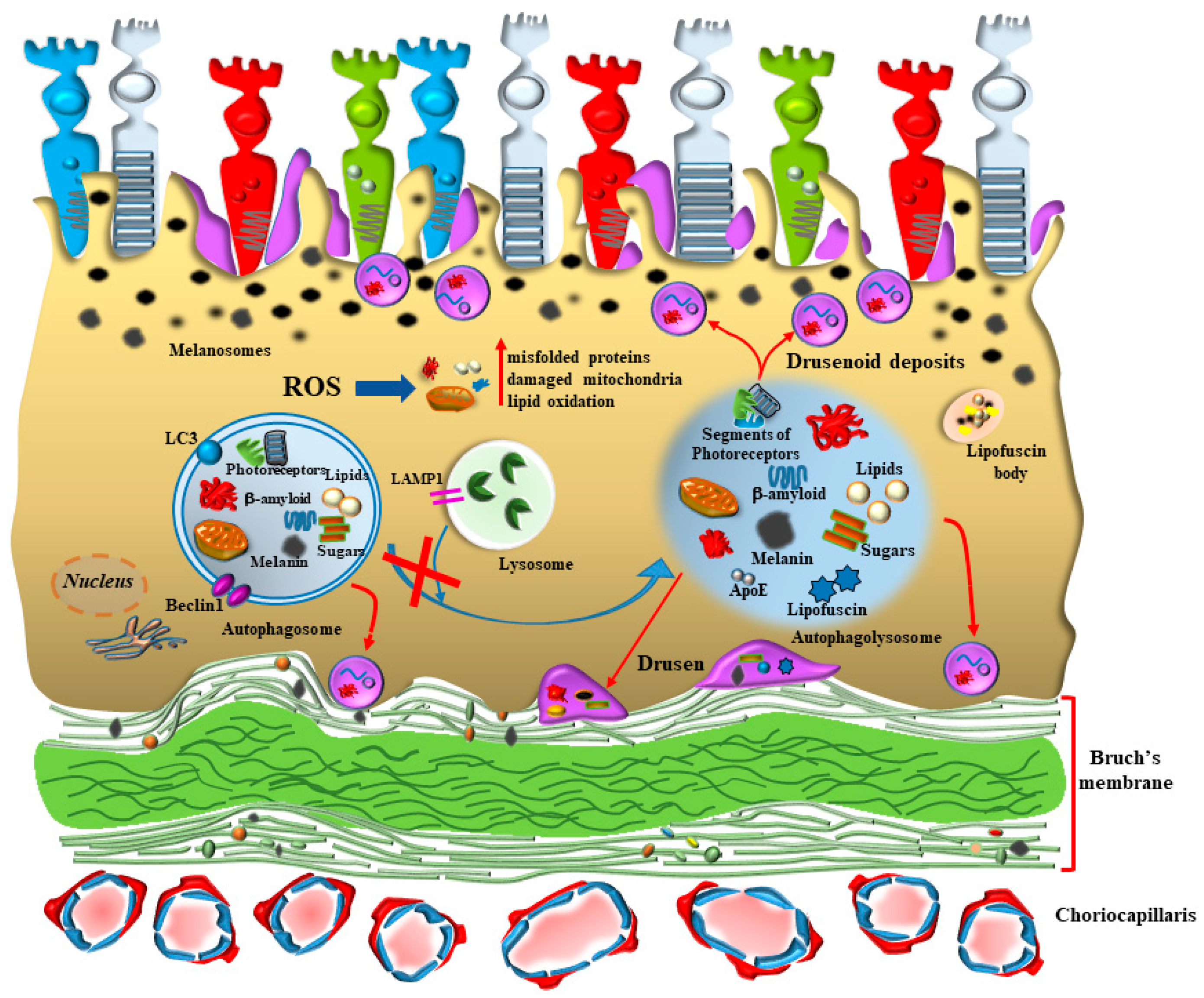
2. The Specific Role of RPE beyond Baseline Conditions at Onset and during AMD
2.1. The Seminal Role of Autophagy within AMD-RPE
2.2. RPE Autophagy Defect and Inclusions
2.3. RPE Autophagy Defect and Visual Function
3. Natural Light Stimulation and Photobiomodulation (Figure 4)
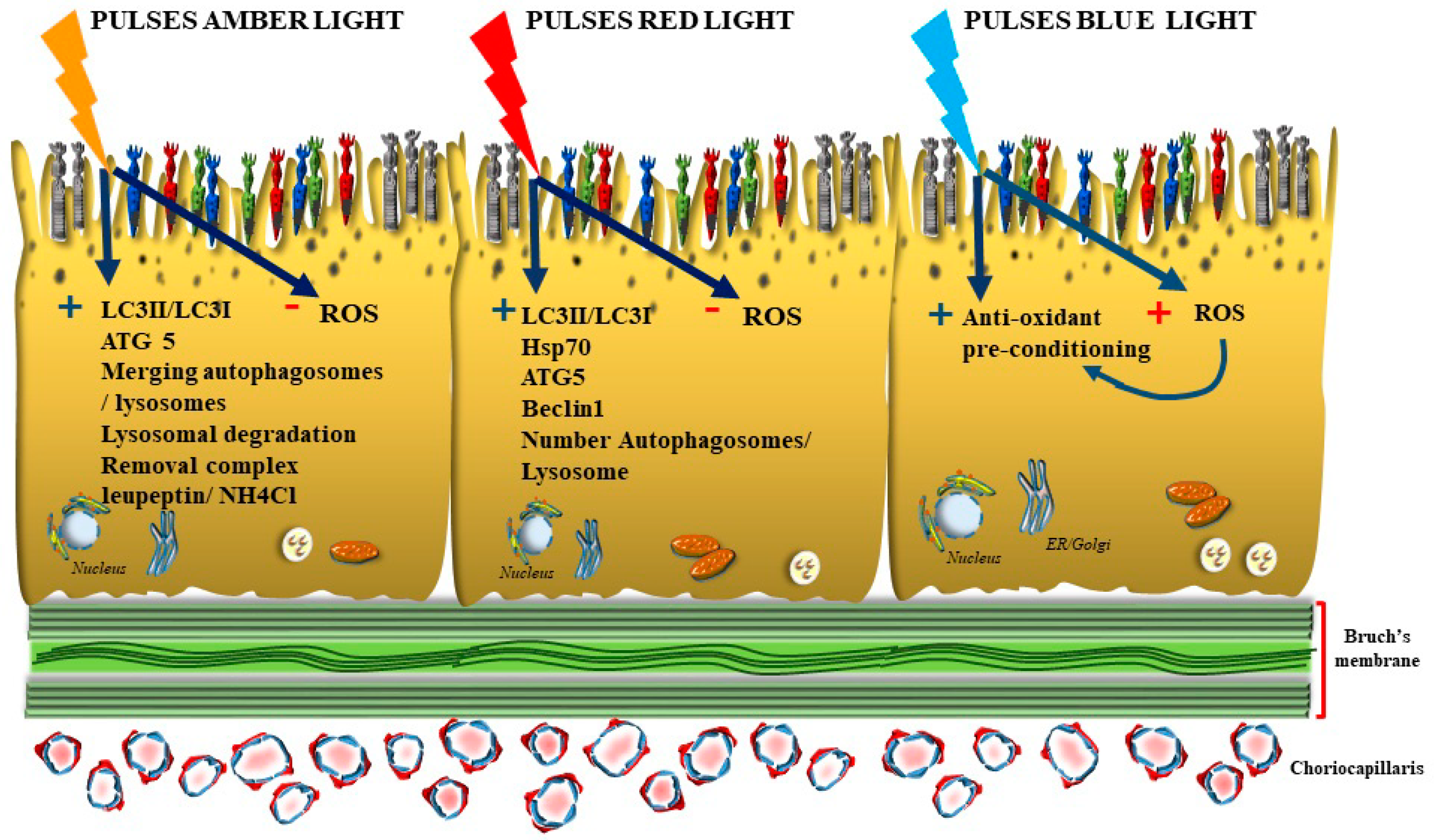
Oxidative Preconditioning, the Paradoxical Benefit of Quick Pulses of Blue Short Wavelengths Light
4. The Autophagy-Inducing Effects of Phytochemicals
4.1. Lutein
4.2. Resveratrol
4.3. Bilberry
4.4. Curcumin
5. The Archaic Nature of Synergism between Natural Light and Phytochemicals May Work as a Disease Modifier in AMD (Figure 5)
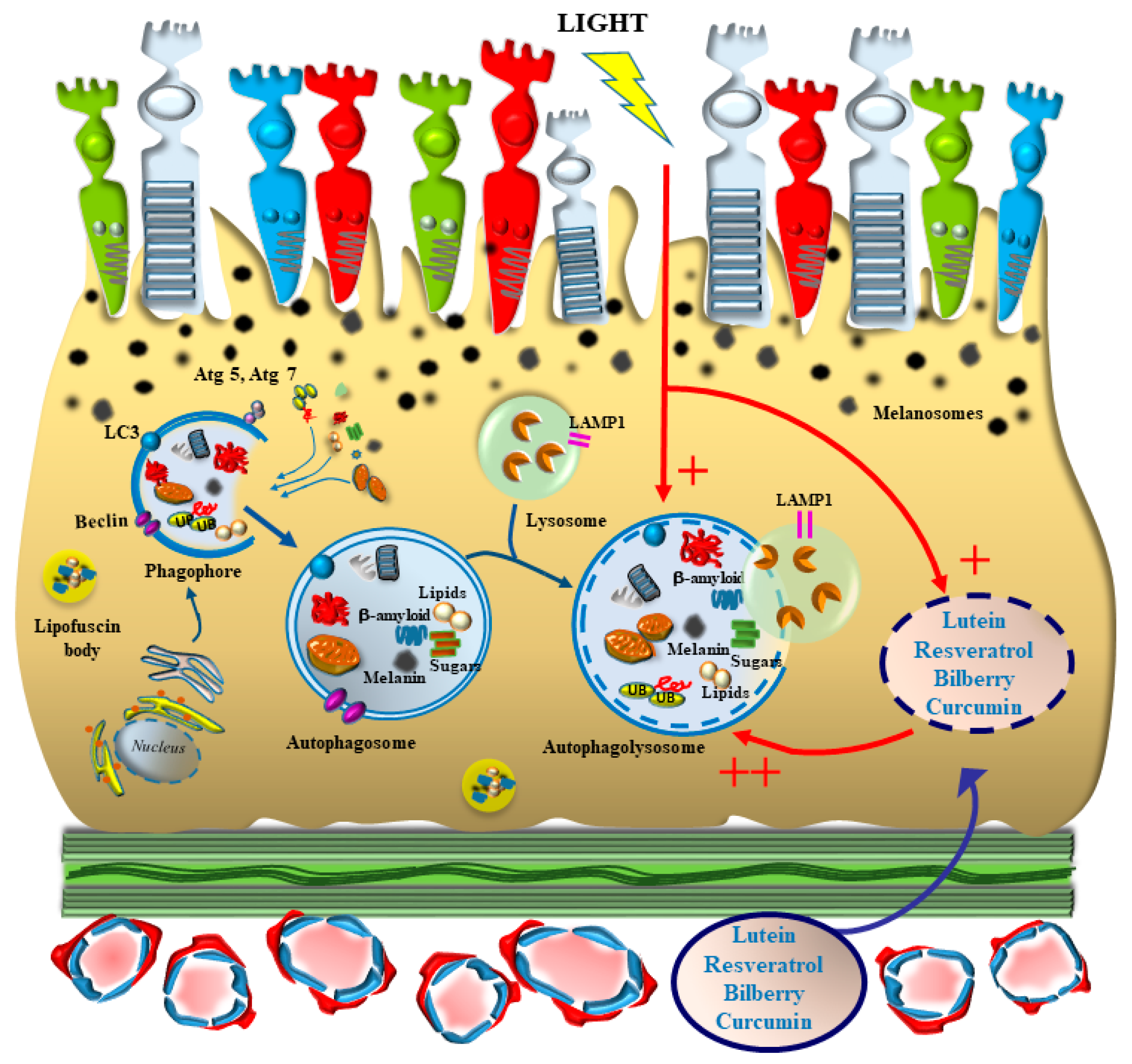
Light-Induced Chemical Changes in Phytochemicals
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jager, R.D.; Mieler, W.F.; Miller, J.W. Age-related macular degeneration. N. Engl. J. Med. 2008, 358, 2606–2617. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, R.; Bertelli, M.; Scaffidi, E.; Fulceri, F.; Busceti, C.L.; Biagioni, F.; Fornai, F. Measurement of drusen and their correlation with visual symptoms in patients affected by age-related macular degeneration. Arch. Ital. Biol. 2020, 158, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, R.; Biagioni, F.; Limanaqi, F.; Bertelli, M.; Scaffidi, E.; Polzella, M.; Busceti, C.L.; Fornai, F. A Re-Appraisal of Pathogenic Mechanisms Bridging Wet and Dry Age-Related Macular Degeneration Leads to Reconsider a Role for Phytochemicals. Int. J. Mol. Sci. 2020, 21, 5563. [Google Scholar] [CrossRef] [PubMed]
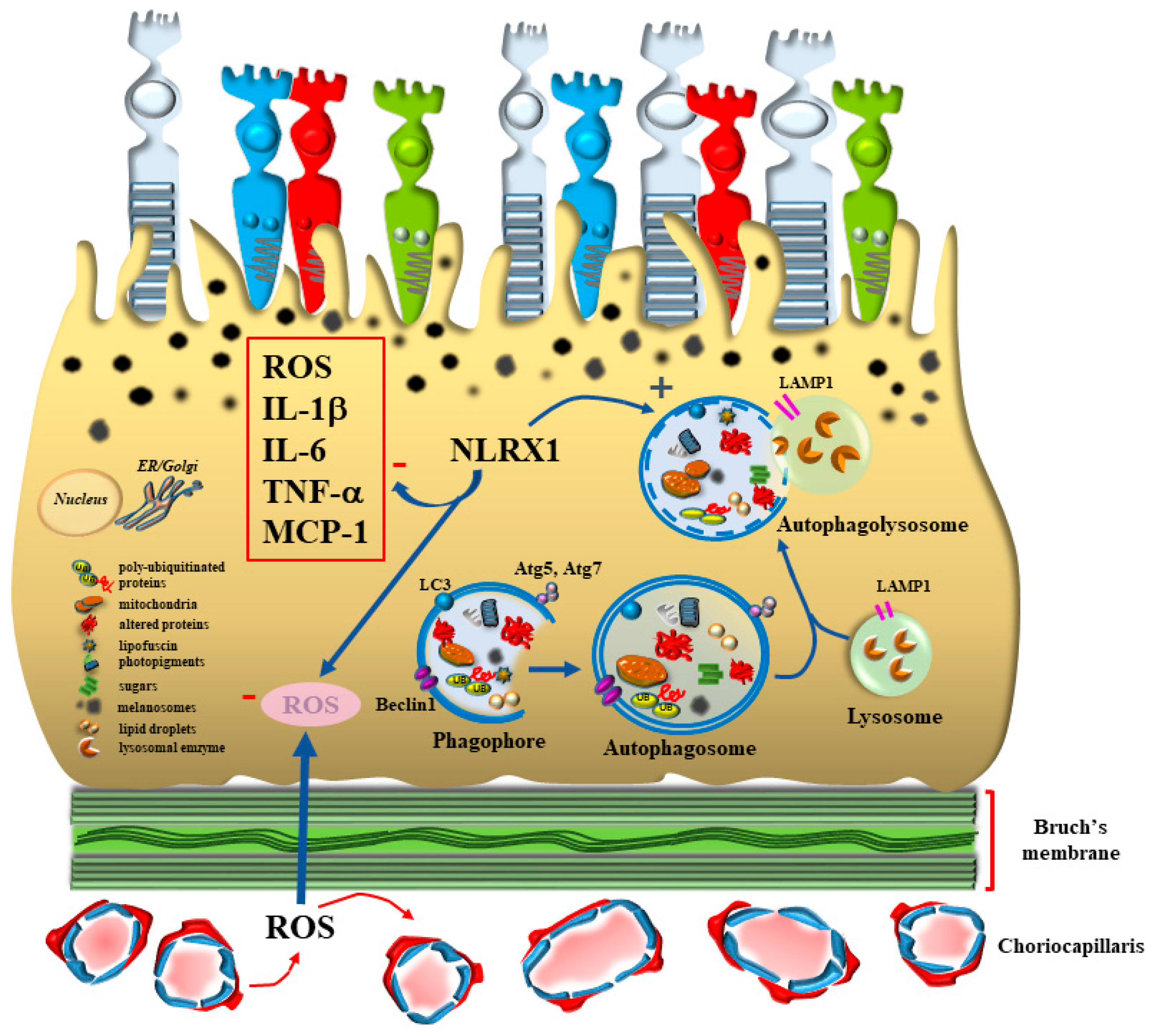
- Wang, Q.; He, F.; Wu, L. NLRX1 increases human retinal pigment epithelial autophagy and reduces H2O2-induced oxidative stress and inflammation by suppressing FUNDC1 phosphorylation and NLRP3 activation. Allergol. Immunopathol. (Madr) 2023, 51, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.C.; Shpaner, M.; Molholm, S.; Foxe, J.J. Visual object processing as a function of stimulus energy, retinal eccentricity and Gestalt configuration: A high-density electrical mapping study. Neuroscience 2012, 221, 1–11. [Google Scholar] [CrossRef]
- Monaco, S.; Gallivan, J.P.; Figley, T.D.; Singhal, A.; Culham, J.C. Recruitment of Foveal Retinotopic Cortex During Haptic Exploration of Shapes and Actions in the Dark. J. Neurosci. 2017, 37, 11572–11591. [Google Scholar] [CrossRef]
- Tao, J.X.; Zhou, W.C.; Zhu, X.G. Mitochondria as Potential Targets and Initiators of the Blue Light Hazard to the Retina. Oxid Med. Cell Longev. 2019, 2019, 6435364. [Google Scholar] [CrossRef]
- Lin, C.W.; Yang, C.M.; Yang, C.H. Protective Effect of Astaxanthin on Blue Light Light-Emitting Diode-Induced Retinal Cell Damage via Free Radical Scavenging and Activation of PI3K/Akt/Nrf2 Pathway in 661W Cell Model. Mar. Drugs 2020, 18, 387. [Google Scholar] [CrossRef]
- Lin, Y.H.; Sheu, S.J.; Liu, W.; Hsu, Y.T.; He, C.X.; Wu, C.Y.; Chen, K.J.; Lee, P.Y.; Chiu, C.C.; Cheng, K.C. Retinal protective effect of curcumin metabolite hexahydrocurcumin against blue light-induced RPE damage. Phytomedicine 2023, 110, 154606. [Google Scholar] [CrossRef]
- Cheng, K.C.; Hsu, Y.T.; Liu, W.; Huang, H.L.; Chen, L.Y.; He, C.X.; Sheu, S.J.; Chen, K.J.; Lee, P.Y.; Lin, Y.H.; et al. The Role of Oxidative Stress and Autophagy in Blue-Light-Induced Damage to the Retinal Pigment Epithelium in Zebrafish In Vitro and In Vivo. Int. J. Mol. Sci. 2021, 22, 1338. [Google Scholar] [CrossRef]
- Domalpally, A.; Xing, B.; Pak, J.W.; Agrón, E.; Ferris, F.L., 3rd; Clemons, T.E.; Chew, E.Y. Extramacular Drusen and Progression of Age-Related Macular Degeneration: Age Related Eye Disease Study 2 Report 30. Ophthalmol. Retina. 2023, 7, 111–117. [Google Scholar] [CrossRef]
- Intartaglia, D.; Giamundo, G.; Conte, I. Autophagy in the retinal pigment epithelium: A new vision and future challenges. FEBS J. 2022, 289, 7199–7212. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Boulton, M.E. Consequences of oxidative stress in age-related macular degeneration. Mol. Aspects Med. 2012, 33, 399–417. [Google Scholar] [CrossRef]
- Matsumoto, B.; Defoe, D.M.; Besharse, J.C. Membrane turnover in rod photoreceptors: Ensheathment and phagocytosis of outer segment distal tips by pseudopodia of the retinal pigment epithelium. Proc. R Soc. Lond. B Biol. Sci. 1987, 230, 339–354. [Google Scholar] [CrossRef]
- Kwon, W.; Freeman, S.A. Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling. Front Immunol. 2020, 11, 604205. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Sinha, D.; Blasiak, J.; Kauppinen, A.; Veréb, Z.; Salminen, A.; Boulton, M.E.; Petrovski, G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular de-generation. Autophagy 2013, 9, 973–984. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Tokarz, P.; Koskela, A.; Paterno, J.; Blasiak, J. Autophagy regulates death of retinal pigment epithelium cells in age-related macular degeneration. Cell Biol. Toxicol. 2017, 33, 113–128. [Google Scholar] [CrossRef]
- Hyttinen, J.M.T.; Niittykoski, M.; Salminen, A.; Kaarniranta, K. Maturation of autophagosomes and endosomes: A key role for Rab7. Biochim. Biophys Acta. 2013, 1833, 503–510. [Google Scholar] [CrossRef]
- Hyttinen, J.M.T.; Amadio, M.; Viiri, J.; Pascale, A.; Salminen, A.; Kaarniranta, K. Clearance of misfolded and aggregated proteins by aggrephagy and implication for aggregation diseases. Ageing Res. Rev. 2014, 18, 16–28. [Google Scholar] [CrossRef]
- Pinelli, R.; Bertelli, M.; Scaffidi, E.; Busceti, C.L.; Biagioni, F.; Fornai, F. Exosomes and alpha-synuclein within retina from autophagy to protein spreading in neurodegeneration. Arch. Ital. Biol. 2021, 159, 38–50. [Google Scholar] [CrossRef]
- Intartaglia, D.; Giamundo, G.; Naso, F.; Nusco, E.; Di Giulio, S.; Salierno, F.G.; Polishchuk, E.; Conte, I. Induction of Autophagy Promotes Clearance of RHOP23H Aggregates and Protects From Retinal Degeneration. Front. Aging Neurosci. 2022, 14, 878958. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.S.; Kim, H.J.; Ham, M.; Choi, D.H.; Lee, T.R.; Shin, D.W. Amber Light (590 nm) Induces the Breakdown of Lipid Droplets through Autophagy-Related Lysosomal Degradation in Differentiated Adipocytes. Sci. Rep. 2016, 6, 28476. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, T.A.; Green, D.R. Autophagy and phagocytosis converge for better vision. Autophagy 2014, 10, 165–167. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wei, L.; Liu, D.; Zhang, Q.; Xia, X.; Ding, L.; Xiong, S. Identification and Validation of Autophagy-Related Genes in Diabetic Retinopathy. Front. Endocrinol. 2022, 13, 867600. [Google Scholar] [CrossRef]
- Roffler-Tarlov, S.; Liu, J.H.; Naumova, E.N.; Bernal-Ayala, M.M.; Mason, C.A. L-Dopa and the albino riddle: Content of L-Dopa in the developing retina of pigmented and albino mice. PLoS ONE 2013, 8, e57184. [Google Scholar] [CrossRef]
- Roberts, J.E. Ocular phototoxicity. J. Photochem. Photobiol. B. 2001, 64, 136–143. [Google Scholar] [CrossRef]
- Peters, S.; Lamah, T.; Kokkinou, D.; Bartz-Schmidt, K.U.; Schraermeyer, U. Melanin protects choroidal blood vessels against light toxicity. Z Nat. C J. Biosci. 2006, 61, 427–433. [Google Scholar] [CrossRef]
- Zareba, M.; Szewczyk, G.; Sarna, T.; Hong, L.; Simon, J.D.; Henry, M.M.; Burke, J.M. Effects of photodegradation on the physical and antioxidant properties of melanosomes isolated from retinal pigment epithelium. Photochem. Photobiol. 2006, 82, 1024–1029. [Google Scholar] [CrossRef]
- Yamagishi, S.; Nakamura, K.; Matsui, T.; Inagaki, Y.; Takenaka, K.; Jinnouchi, Y.; Yoshida, Y.; Matsuura, T.; Narama, I.; Motomiya, Y.; et al. Pigment epithelium-derived factor inhibits advanced glycation end product-induced retinal vascular hyperpermeability by blocking reactive oxygen species-mediated vascular endothelial growth factor expression. J. Biol. Chem. 2006, 281, 20213–20220. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.J.; Kim, M.Y.; Bang, E.; Moon, S.K.; Yun, S.J.; Kim, W.J.; Noh, J.S.; Kim, G.Y.; Cho, S.; et al. Phloroglucinol Attenuates DNA Damage and Apoptosis Induced by Oxidative Stress in Human Retinal Pigment Epithelium ARPE-19 Cells by Blocking the Production of Mitochondrial ROS. Antioxidants 2022, 11, 2353. [Google Scholar] [CrossRef]
- Sarangarajan, R.; Apte, S.P. Melanization and phagocytosis: Implications for age related macular degeneration. Mol. Vis. 2005, 11, 482–490. [Google Scholar]
- Choi, E.H.; Daruwalla, A.; Suh, S.; Leinonen, H.; Palczewski, K. Retinoids in the visual cycle: Role of the retinal G protein-coupled receptor. J. Lipid Res. 2021, 62, 100040. [Google Scholar] [CrossRef]
- Michelis, G.; German, O.L.; Villasmil, R.; Soto, T.; Rotstein, N.P.; Politi, L.; Becerra, S.P. Pigment epithelium-derived factor (PEDF) and derived peptides promote survival and differentiation of photoreceptors and induce neurite-outgrowth in amacrine neurons. J. Neurochem. 2021, 159, 840–856. [Google Scholar] [CrossRef]
- Angelova, P.R. Sources and triggers of oxidative damage in neurodegeneration. Free Radic. Biol. Med. 2021, 173, 52–63. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.; Shin, C.; Lee, S. Intersection between Redox Homeostasis and Autophagy: Valuable Insights into Neurodegeneration. Antioxidants 2021, 10, 694. [Google Scholar] [CrossRef]
- Georgieva, E.; Ivanova, D.; Zhelev, Z.; Bakalova, R.; Gulubova, M.; Aoki, I. Mitochondrial Dysfunction and Redox Imbalance as a Diagnostic Marker of “Free Radical Diseases”. Anticancer. Res. 2017, 37, 5373–5381. [Google Scholar] [CrossRef]
- Ravikumar, B.; Rubinsztein, D.C. Can autophagy protect against neurodegeneration caused by aggregate-prone proteins? Neuroreport 2004, 15, 2443–2445. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Kroemer, G. Autophagy for the avoidance of neurodegeneration. Genes Dev. 2009, 23, 2253–2259. [Google Scholar] [CrossRef]
- Haidar, M.; Loix, M.; Bogie, J.F.J.; Hendriks, J.J.A. Lipophagy: A new player in CNS disorders. Trends Endocrinol. Metab. 2021, 32, 941–951. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Coupling mitogenesis and mitophagy for longevity. Autophagy 2015, 11, 1428–1430. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature 2015, 521, 525–528. [Google Scholar] [CrossRef] [PubMed]
- LeDouarin, N.M. The Neural Crest, 1st ed.; Cambridge University Press: Cambridge, CA, USA, 1982; p. 259. [Google Scholar]
- Jeffery, G. The albino retina: An abnormality that provides insight into normal retinal development. Trends Neurosci. 1997, 20, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Hearing, V.J. The melanosome: The perfect model for cellular responses to the environment. Pigment. Cell Res. 2000, 13, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Seagle, B.L.; Rezai, K.A.; Gasyna, E.M.; Kobori, Y.; Rezaei, K.A.; Norris, J.R., Jr. Time-resolved detection of melanin free radicals quenching reactive oxygen species. J. Am. Chem. Soc. 2005, 127, 11220–11221. [Google Scholar] [CrossRef]
- Rios, M.; Habecker, B.; Sasaoka, T.; Eisenhofer, G.; Tian, H.; Landis, S.; Chikaraishi, D.; Roffler-Tarlov, S. Catecholamine synthesis is mediated by tyrosinase in the absence of tyrosine hydroxylase. J. Neurosci. 1999, 19, 3519–3526. [Google Scholar] [CrossRef] [PubMed]
- Iwai-Takekoshi, L.; Ramos, A.; Schaler, A.; Weinreb, S.; Blazeski, R.; Mason, C. Retinal pigment epithelial integrity is compromised in the developing albino mouse retina. J. Comp. Neurol. 2016, 524, 3696–3716. [Google Scholar] [CrossRef]
- Dieguez, H.H.; Romeo, H.E.; González Fleitas, M.F.; Aranda, M.L.; Milne, G.A.; Rosenstein, R.E.; Dorfman, D. Superior cervical gangliectomy induces non-exudative age-related macular degeneration in mice. Dis. Model. Mech. 2018, 11, dmm031641. [Google Scholar] [CrossRef]
- Ishibashi, T.; Murata, T.; Hangai, M.; Nagai, R.; Horiuchi, S.; Lopez, P.F.; Hinton, D.R.; Ryan, S.J. Advanced glycation end products in age-related macular degeneration. Arch. Ophthalmol. 1998, 116, 1629–1632. [Google Scholar] [CrossRef]
- Lee, E.J.; Kim, J.Y.; Oh, S.H. Advanced glycation end products (AGEs) promote melanogenesis through receptor for AGEs. Sci. Rep. 2016, 6, 27848. [Google Scholar] [CrossRef]
- Fang, J.; Ouyang, M.; Qu, Y.; Wang, M.; Huang, X.; Lan, J.; Lai, W.; Xu, Q. Advanced Glycation End Products Promote Melanogenesis by Activating NLRP3 Inflammasome in Human Dermal Fibroblasts. J. Invest. Dermatol. 2022, 142, 2591–2602.e8. [Google Scholar] [CrossRef]
- Fornai, F.; Lenzi, P.; Gesi, M.; Ferrucci, M.; Lazzeri, G.; Busceti, C.L.; Ruffoli, R.; Soldani, P.; Ruggieri, S.; Alessandrì, M.G.; et al. Fine structure and biochemical mechanisms underlying nigrostriatal inclusions and cell death after proteasome inhibition. J. Neurosci. 2003, 23, 8955–8966. [Google Scholar] [CrossRef]
- Fornai, F.; Soldani, P.; Lazzeri, G.; Di Poggio, A.B.; Biagioni, F.; Fulceri, F.; Batini, S.; Ruggieri, S.; Paparelli, A. Neuronal inclusions in degenerative disorders Do they represent static features or a key to understand the dynamics of the disease? Brain Res. Bull. 2005, 65, 275–290. [Google Scholar] [CrossRef]
- Herrera, A.S.; Beeraka, N.M.; Solis, L.F.T.; Mikhaleva, L.M.; Somasundaram, S.G.; Kirkland, C.E.; Aliev, G. The Efficacy of Melanin Precursor QIAPI 1© Against Age-related Macular Degenration (AMD): A Case Report. Cent. Nerv. Syst. Agents Med. Chem. 2020, 20, 218–225. [Google Scholar] [CrossRef]
- Lazzeri, G.; Lenzi, P.; Busceti, C.L.; Ferrucci, M.; Falleni, A.; Bruno, V.; Paparelli, A.; Fornai, F. Mechanisms involved in the formation of dopamine-induced intracellular bodies within striatal neurons. J. Neurochem. 2007, 101, 1414–1427. [Google Scholar] [CrossRef]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef]
- Henning, Y.; Blind, U.S.; Larafa, S.; Matschke, J.; Fandrey, J. Hypoxia aggravates ferroptosis in RPE cells by promoting the Fenton reaction. Cell Death Dis. 2022, 13, 662. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, C.; Dong, X.; Wang, H. A novel miR-338-3p/SLC1A5 axis reprograms retinal pigment epithelium to increases its resistance to high glucose-induced cell ferroptosis. J. Mol. Histol. 2022, 53, 561–571. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, D.; Fu, Q.; Hao, S.; Gu, Y.; Zhao, W.; Chen, S.; Sheng, F.; Xu, Y.; Chen, Z.; et al. CHAC1 as a Novel Contributor of Ferroptosis in Retinal Pigment Epithelial Cells with Oxidative Damage. Int. J. Mol. Sci. 2023, 24, 1582. [Google Scholar] [CrossRef]
- Notomi, S.; Ishihara, K.; Efstathiou, N.E.; Lee, J.J.; Hisatomi, T.; Tachibana, T.; Konstantinou, E.K.; Ueta, T.; Murakami, Y.; Maidana, D.E.; et al. Genetic LAMP2 deficiency accelerates the age-associated formation of basal laminar deposits in the retina. Proc. Natl. Acad. Sci. USA 2019, 116, 23724–23734. [Google Scholar] [CrossRef]
- Kurzawa-Akanbi, M.; Whitfield, P.; Burté, F.; Bertelli, P.M.; Pathak, V.; Doherty, M.; Hilgen, B.; Gliaudelytė, L.; Platt, M.; Queen, R.; et al. Retinal pigment epithelium extracellular vesicles are potent inducers of age-related macular degeneration disease phenotype in the outer retina. J. Extracell Vesicles. 2022, 11, e12295. [Google Scholar] [CrossRef]
- Zou, G.P.; Wang, T.; Xiao, J.X.; Wang, X.Y.; Jiang, L.P.; Tou, F.F.; Chen, Z.P.; Qu, X.H.; Han, X.J. Lactate protects against oxidative stress-induced retinal degeneration by activating autophagy. Free Radic. Biol. Med. 2023, 194, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Hyttinen, J.M.T.; Błasiak, J.; Niittykoski, M.; Kinnunen, K.; Kauppinen, A.; Salminen, A.; Kaarniranta, K. DNA damage response and autophagy in the degeneration of retinal pigment epithelial cells-Implications for age-related macular degeneration (AMD). Ageing Res. Rev. 2017, 36, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Querques, G.; Rosenfeld, P.J.; Cavallero, E.; Borrelli, E.; Corvi, F.; Querques, L.; Bandello, F.M.; Zarbin, M.A. Treatment of dry age-related macular degeneration. Ophthalmic. Res. 2014, 52, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Bales, K.L.; Gross, A.K. Aberrant protein trafficking in retinal degenerations: The initial phase of retinal remodeling. Exp. Eye Res. 2016, 150, 71–80. [Google Scholar] [CrossRef]
- Terman, A.; Brunk, U.T. Lipofuscin. Int. J. Biochem. Cell Biol. 2004, 36, 1400–1404. [Google Scholar] [CrossRef]
- Kaemmerer, E.; Schutt, F.; Krohne, T.U.; Holz, F.G.; Kopitz, J. Effects of lipid peroxidation-related protein modifications on RPE lysosomal functions and POS phagocytosis. Invest. Ophthalmol. Vis. Sci. 2007, 48, 1342–1347. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Salminen, A.; Eskelinen, E.-L.; Kopitz, J. Heat-shock proteins as gatekeepers, of proteolytic pathways: Implications for age-related macular degeneration. Ageing Res. Rev. 2009, 8, 128–139. [Google Scholar] [CrossRef]
- Luzio, J.P.; Pryor, P.R.; Bright, N.A. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2007, 8, 622–632. [Google Scholar] [CrossRef]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W., Jr.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef]
- Shahmoradian, S.H.; Lewis, A.J.; Genoud, C.; Hench, J.; Moors, T.E.; Navarro, P.P.; Castaño-Díez, D.; Schweighauser, G.; Graff-Meyer, A.; Goldie, K.N.; et al. Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat. Neurosci. 2019, 22, 1099–1109. [Google Scholar] [CrossRef]
- Spraul, C.W.; Lang, G.E.; Grossniklaus, H.E.; Lang, G.K. Histologic and morphometric analysis of the choroid, Bruch’s membrane, and retinal pigment epithelium in postmortem eyes with age-related macular degeneration and histologic ex-amination of surgically excised choroidal neovascular membranes. Surv. Ophthalmol. 1999, 44, S10–S32. [Google Scholar] [CrossRef]
- Kaarniranta, K.; Hyttinen, J.; Ryhänen, T.; Viiri, J.; Paimela, T.; Toropainen, E.; Sorri, I.; Salminen, A. Mechanism of protein aggregation on the retinal pigment epitelial cells. Front. Biosci. 2010, 2, 1374–1384. [Google Scholar] [CrossRef]
- Boyer, N.P.; Tang, P.H.; Higbee, D.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. Lipofuscin and A2E accumulate with age in the retinal pigment epithelium of Nrl-/- mice. Photochem. Photobiol. 2012, 88, 1373–1377. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.; Park, H.J.; Kim, S.H.; Lew, H.; Kim, G.J. PEDF-Mediated Mitophagy Triggers the Visual Cycle by Enhancing Mitochondrial Functions in a H2O2-Injured Rat Model. Cells 2021, 10, 1117. [Google Scholar] [CrossRef]
- Datta, S.; Cano, M.; Satyanarayana, G.; Liu, T.; Wang, L.; Wang, J.; Cheng, J.; Itoh, K.; Sharma, A.; Bhutto, I.; et al. Mitophagy initiates retrograde mitochondrial-nuclear signaling to guide retinal pigment cell heterogeneity. Autophagy 2023, 19, 966–983. [Google Scholar] [CrossRef]
- Wen, R.H.; Stanar, P.; Tam, B.; Moritz, O.L. Autophagy in Xenopus laevis rod photoreceptors is independently regulated by phototransduction and misfolded RHOP23H. Autophagy 2019, 15, 1970–1989. [Google Scholar] [CrossRef]
- Santo, M.; Conte, I. Emerging Lysosomal Functions for Photoreceptor Cell Homeostasis and Survival. Cells 2021, 11, 60. [Google Scholar] [CrossRef]
- Ramachandra Rao, S.; Fliesler, S.J. Monitoring basal autophagy in the retina utilizing CAG-mRFP-EGFP-MAP1LC3B reporter mouse: Technical and biological considerations. Autophagy 2022, 18, 1187–1201. [Google Scholar] [CrossRef]
- Vessey, K.A.; Jobling, A.I.; Tran, M.X.; Wang, A.Y.; Greferath, U.; Fletcher, E.L. Treatments targeting autophagy ameliorate the age-related macular degeneration phenotype in mice lacking APOE (apolipoprotein E). Autophagy 2022, 18, 2368–2384. [Google Scholar] [CrossRef]
- Lee, J.J.; Ishihara, K.; Notomi, S.; Efstathiou, N.E.; Ueta, T.; Maidana, D.; Chen, X.; Iesato, Y.; Caligiana, A.; Vavvas, D.G. Lysosome-associated membrane protein-2 deficiency increases the risk of reactive oxygen species-induced ferroptosis in retinal pigment epithelial cells. Biochem. Biophys Res. Commun. 2020, 521, 414–419. [Google Scholar] [CrossRef]
- Kim, J.Y.; Zhao, H.; Martinez, J.; Doggett, T.A.; Kolesnikov, A.V.; Tang, P.H.; Ablonczy, Z.; Chan, C.C.; Zhou, Z.; Green, D.R.; et al. Noncanonical autophagy promotes the visual cycle. Cell 2013, 154, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Frost, L.S.; Lopes, V.S.; Bragin, A.; Reyes-Reveles, J.; Brancato, J.; Cohen, A.; Mitchell, C.H.; Williams, D.S.; Boesze-Battaglia, K. The Contribution of Melanoregulin to Microtubule-Associated Protein 1 Light Chain 3 (LC3) Associated Phagocytosis in Retinal Pigment Epithelium. Mol. Neurobiol. 2015, 52, 1135–1151. [Google Scholar] [CrossRef] [PubMed]
- Muniz-Feliciano, L.; Doggett, T.A.; Zhou, Z.; Ferguson, T.A. RUBCN/rubicon and EGFR regulate lysosomal degradative processes in the retinal pigment epithelium (RPE) of the eye. Autophagy 2017, 13, 2072–2085. [Google Scholar] [CrossRef]
- Rojas, J.C.; Lee, J.; John, J.M.; Gonzalez-Lima, F. Neuroprotective effects of nearinfrared light in an in vivo model of mitochondrial optic neuropathy. J. Neurosci. 2008, 28, 13511–13521. [Google Scholar] [CrossRef] [PubMed]
- Tata, D.B.; Waynant, R.W. Laser therapy: A review of its mechanism of action and potential medical applications. Laser Photonics Rev. 2011, 5, 1–12. [Google Scholar] [CrossRef]
- Rojas, J.C.; Gonzalaz-Lima, F. Low level light therapy of the eye and brain. Eye Brain. 2011, 3, 49–67. [Google Scholar] [CrossRef]
- Stefenon, L.; Boasquevisque, M.; Garcez, A.S.; de Araújo, V.C.; Soares, A.B.; Santos-Silva, A.R.; Sperandio, F.; Brod, J.M.M.; Sperandio, M. Autophagy upregulation may explain inhibition of oral carcinoma in situ by photobiomodulation in vitro. J. Photochem. Photobiol. B. 2021, 221, 112245. [Google Scholar] [CrossRef]
- Comerota, M.M.; Tumurbaatar, B.; Krishnan, B.; Kayed, R.; Taglialatela, G. Near Infrared Light Treatment Reduces Synaptic Levels of Toxic Tau Oligomers in Two Transgenic Mouse Models of Human Tauopathies. Mol. Neurobiol. 2019, 56, 3341–3355. [Google Scholar] [CrossRef]
- Yang, K.L.; Khoo, B.Y.; Ong, M.T.; Yoong, I.C.K.; Sreeramanan, S. In vitro anti-breast cancer studies of LED red light therapy through autophagy. Breast Cancer 2021, 28, 60–66. [Google Scholar] [CrossRef]
- Su, A.C.; Zhang, L.Y.; Zhang, J.G.; Hu, Y.Y.; Liu, X.Y.; Li, S.C.; Xian, X.H.; Li, W.B.; Zhang, M. The Regulation of Autophagy by p38 MAPK-PPARγ Signaling During the Brain Ischemic Tolerance Induced by Cerebral Ischemic Preconditioning. DNA Cell Biol. 2022, 41, 838–849. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, L.; Yan, Y.; Zhao, L.; Han, S.; Wu, D.; Borlongan, C.V.; Li, J.; Ji, X. cPKCγ-Modulated Autophagy Contributes to Ischemic Preconditioning-Induced Neuroprotection in Mice with Ischemic Stroke via mTOR-ULK1 Pathway. Transl. Stroke Res. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Wang, L.; Yu, X.; Zhang, D.; Wen, Y.; Zhang, L.; Xia, Y.; Chen, J.; Xie, C.; Zhu, H.; Tong, J.; et al. Long-term blue light exposure impairs mitochondrial dynamics in the retina in light-induced retinal degeneration in vivo and in vitro. J. Photochem. Photobiol. B 2023, 240, 112654. [Google Scholar] [CrossRef]
- Abdouh, M.; Lu, M.; Chen, Y.; Goyeneche, A.; Burnier, J.V.; Burnier, M.N., Jr. Filtering blue light mitigates the deleterious effects induced by the oxidative stress in human retinal pigment epithelial cells. Exp. Eye Res. 2022, 217, 108978. [Google Scholar] [CrossRef]
- Ren, C.; Hu, W.; Wei, Q.; Cai, W.; Jin, H.; Yu, D.; Liu, C.; Shen, T.; Zhu, M.; Liang, X.; et al. MicroRNA-27a Promotes Oxidative-Induced RPE Cell Death through Targeting FOXO1. Biomed. Res. Int. 2021, 2021, 6666506. [Google Scholar] [CrossRef]
- Hall, H.; Ma, J.; Shekhar, S.; Leon-Salas, W.D.; Weake, V.M. Blue light induces a neuroprotective gene expression program in Drosophila photoreceptors. BMC Neurosci. 2018, 19, 43. [Google Scholar] [CrossRef]
- Otsu, W.; Ishida, K.; Nakamura, S.; Shimazawa, M.; Tsusaki, H.; Hara, H. Blue light-emitting diode irradiation promotes transcription factor EB-mediated lysosome biogenesis and lysosomal cell death in murine photoreceptor-derived cells. Biochem. Biophys Res. Commun. 2020, 526, 479–484. [Google Scholar] [CrossRef]
- Mathew, B.; Chennakesavalu, M.; Sharma, M.; Torres, L.A.; Stelman, C.R.; Tran, S.; Patel, R.; Burg, N.; Salkovski, M.; Kadzielawa, K.; et al. Autophagy and post-ischemic conditioning in retinal ischemia. Autophagy 2021, 17, 1479–1499. [Google Scholar] [CrossRef]
- Pinelli, R.; Bertelli, M.; Scaffidi, E.; Polzella, M.; Fulceri, F.; Biagioni, F.; Fornai, F. Nutraceuticals for dry age-related macular degeneration: A case report based on novel pathogenic and morphological insights. Arch Ital. Biol. 2020, 158, 24–34. [Google Scholar] [CrossRef]
- Dong, L.; He, J.; Luo, L.; Wang, K. Targeting the Interplay of Autophagy and ROS for Cancer Therapy: An Updated Overview on Phytochemicals. Pharmaceuticals 2023, 16, 92. [Google Scholar] [CrossRef]
- Patra, S.; Mishra, S.R.; Behera, B.P.; Mahapatra, K.K.; Panigrahi, D.P.; Bhol, C.S.; Praharaj, P.P.; Sethi, G.; Patra, S.K.; Bhutia, S.K. Autophagy-modulating phytochemicals in cancer therapeutics: Current evidences and future perspectives. Semin Cancer Biol. 2022, 80, 205–217. [Google Scholar] [CrossRef]
- Shannar, A.; Sarwar, M.S.; Kong, A.T. A New Frontier in Studying Dietary Phytochemicals in Cancer and in Health: Metabolic and Epigenetic Reprogramming. Prev. Nutr. Food Sci. 2022, 27, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Jafari-Nozad, A.M.; Jafari, A.; Zangooie, A.; Behdadfard, M.; Zangouei, A.S.; Aschner, M.; Farkhondeh, T.; Samarghandian, S. Curcumin Combats Against Gastrointestinal Cancer: A Review of Current Knowledge Regarding Epigenetics Mechanisms with a Focus on DNA Methylation. Curr. Med. Chem. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Dong, X.; Nao, J. Relationship between the therapeutic potential of various plant-derived bioactive compounds and their related microRNAs in neurological disorders. Phytomedicine 2023, 108, 154501. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Yu, P.; Luo, L.; Sun, J.; Tao, H.; Wang, X.; Meng, X. Berberis dictyophylla F. inhibits angiogenesis and apoptosis of diabetic retinopathy via suppressing HIF-1α/VEGF/DLL-4/Notch-1 pathway. J. Ethnopharmacol. 2022, 296, 115453. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Dai, H.; Jiang, S.; Yu, L. Advanced glycation end products in diabetic retinopathy and phytochemical therapy. Front. Nutr. 2022, 9, 1037186. [Google Scholar] [CrossRef]
- Takkar, B.; Sheemar, A.; Jayasudha, R.; Soni, D.; Narayanan, R.; Venkatesh, P.; Shivaji, S.; Das, T. Unconventional avenues to decelerate diabetic retinopathy. Surv. Ophthalmol. 2022, 67, 1574–1592. [Google Scholar] [CrossRef]
- Bosch-Morell, F.; Villagrasa, V.; Ortega, T.; Acero, N.; Muñoz-Mingarro, D.; González-Rosende, M.E.; Castillo, E.; Sanahuja, M.A.; Soriano, P.; Martínez-Solís, I. Medicinal plants and natural products as neuroprotective agents in age-related macular degeneration. Neural. Regen. Res. 2020, 15, 2207–2216. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, J.F.; Hsiao, C.Y.; Chang, H.H.; Li, H.J.; Chang, H.H.; Lee, G.A.; Hung, C.F. Lutein Induces Autophagy via Beclin-1 Upregulation in IEC-6 Rat Intestinal Epithelial Cells. Am. J. Chin. Med. 2017, 45, 1273–1291. [Google Scholar] [CrossRef]
- Muangnoi, C.; Phumsuay, R.; Jongjitphisut, N.; Waikasikorn, P.; Sangsawat, M.; Rashatasakhon, P.; Paraoan, L.; Rojsitthisak, P. Protective Effects of a Lutein Ester Prodrug, Lutein Diglutaric Acid, against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 4722. [Google Scholar] [CrossRef]
- Minasyan, L.; Sreekumar, P.G.; Hinton, D.R.; Kannan, R. Protective Mechanisms of the Mitochondrial-Derived Peptide Humanin in Oxidative and Endoplasmic Reticulum Stress in RPE Cells. Oxid Med. Cell Longev. 2017, 2017, 1675230. [Google Scholar] [CrossRef]
- Jin, C.; Ou, Q.; Chen, J.; Wang, T.; Zhang, J.; Wang, Z.; Wang, Y.; Tian, H.; Xu, J.Y.; Gao, F.; et al. Chaperone-mediated autophagy plays an important role in regulating retinal progenitor cell homeostasis. Stem. Cell Res. Ther. 2022, 13, 136. [Google Scholar] [CrossRef]
- Munia, I.; Gafray, L.; Bringer, M.A.; Goldschmidt, P.; Proukhnitzky, L.; Jacquemot, N.; Cercy, C.; Ramchani Ben Otman, K.; Errera, M.H.; Ranchon-Cole, I. Cytoprotective Effects of Natural Highly Bio-Available Vegetable Derivatives on Human-Derived Retinal Cells. Nutrients 2020, 12, 879. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Z.Z.; Cheng, Y.L.; Lin, W.; Qu, C. Resveratrol protects against oxidative damage of retinal pigment epithelium cells by modulating SOD/MDA activity and activating Bcl-2 expression. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 378–388. [Google Scholar] [CrossRef]
- Wang, N.; Luo, Z.; Jin, M.; Sheng, W.; Wang, H.T.; Long, X.; Wu, Y.; Hu, P.; Xu, H.; Zhang, X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging 2019, 11, 3117–3137. [Google Scholar] [CrossRef]
- Sun, J.; Pu, C.; Yang, E.; Zhang, H.; Feng, Y.; Luo, P.; Yang, Y.; Zhang, L.; Li, X.; Jiang, X.; et al. Macrophage/Microglia Sirt3 Contributes to the Anti-inflammatory Effects of Resveratrol Against Experimental Intracerebral Hemorrhage in Mice. Cell Mol. Neurobiol. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Li, H.; Zheng, T.; Lian, F.; Xu, T.; Yin, W.; Jiang, Y. Anthocyanin-rich blueberry extracts and anthocyanin metabolite protocatechuic acid promote autophagy-lysosomal pathway and alleviate neurons damage in in vivo and in vitro models of Alzheimer’s disease. Nutrition 2022, 93, 111473. [Google Scholar] [CrossRef]
- Osada, H.; Okamoto, T.; Kawashima, H.; Toda, E.; Miyake, S.; Nagai, N.; Kobayashi, S.; Tsubota, K.; Ozawa, Y. Neuroprotective effect of bilberry extract in a murine model of photo-stressed retina. PLoS ONE 2017, 12, e0178627. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Lu, F.; Yang, X.; Deng, Q.; Ji, B.; Huang, F. Retinoprotective Effects of Bilberry Anthocyanins via Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Mechanisms in a Visible Light-Induced Retinal Degeneration Model in Pigmented Rabbits. Molecules 2015, 20, 22395–22410. [Google Scholar] [CrossRef]
- Jin, Q.H.; Hu, X.J.; Zhao, H.Y. Curcumin activates autophagy and attenuates high glucose-induced apoptosis in HUVECs through the ROS/NF-κB signaling pathway. Exp. Ther. Med. 2022, 24, 596. [Google Scholar] [CrossRef]
- Algan, A.H.; Gungor-Ak, A.; Karatas, A. Nanoscale Delivery Systems of Lutein: An Updated Review from a Pharmaceutical Perspective. Pharmaceutics 2022, 14, 1852. [Google Scholar] [CrossRef]
- Li, X.; Holt, R.R.; Keen, C.L.; Morse, L.S.; Zivkovic, A.M.; Yiu, G.; Hackman, R.M. Potential roles of dietary zeaxanthin and lutein in macular health and function. Nutr. Rev. 2022, 81, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Kotagiri, S.R.; Morde, A.; Rai, D.; Babji, K.; Lal, M.; Padigaru, M.; Khatri, C. Superior Bioavailability of a Novel Lutein and Zeaxanthin Formulation in Healthy Human Subjects. Ophthalmol. Ther. 2022, 11, 1463–1477. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration-Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.C.; Fung, F.K.C.; Lai, A.K.W.; Wong, I.Y.H.; Shih, K.C.; Lo, A.C.Y. Autophagic Upregulation Is Cytoprotective in Ischemia/Reperfusion-Injured Retina and Retinal Progenitor Cells. Int. J. Mol. Sci. 2021, 22, 8446. [Google Scholar] [CrossRef]
- Sharma, R.; George, A.; Nimmagadda, M.; Ortolan, D.; Karla, B.S.; Qureshy, Z.; Bose, D.; Dejene, R.; Liang, G.; Wan, Q.; et al. Epithelial phenotype restoring drugs suppress macular degeneration phenotypes in an iPSC model. Nat. Commun. 2021, 12, 7293. [Google Scholar] [CrossRef]
- Georgiou, M.; Yang, C.; Atkinson, R.; Pan, K.T.; Buskin, A.; Molina, M.M.; Collin, J.; Al-Aama, J.; Goertler, F.; Ludwig, S.E.J.; et al. Activation of autophagy reverses progressive and deleterious protein aggregation in PRPF31 patient-induced pluripotent stem cell-derived retinal pigment epithelium cells. Clin. Transl. Med. 2022, 12, e759. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E.; Tavernarakis, N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018, 20, 1013–1022. [Google Scholar] [CrossRef]
- Hyttinen, J.; Blasiak, J.; Tavi, P.; Kaarniranta, K. Therapeutic potential of PGC-1α in age-related macular degeneration (AMD)—the involvement of mitochondrial quality control, autophagy, and antioxidant response. Expert Opin. Ther. Target. 2021, 25, 773–785. [Google Scholar] [CrossRef]
- Yao, Y.; Zhu, J.; Qin, S.; Zhou, Z.; Zeng, Q.; Long, R.; Mao, Z.; Dong, X.; Zhao, R.; Zhang, R.; et al. Resveratrol induces autophagy impeding BAFF-stimulated B-cell proliferation and survival by inhibiting the Akt/mTOR pathway. Biochem. Pharmacol. 2022, 202, 115139. [Google Scholar] [CrossRef]
- Jassey, A.; Wagner, M.A.; Galitska, G.; Paudel, B.; Miller, K.; Jackson, W.T. Starvation after infection restricts enterovirus D68 replication. Autophagy 2023, 19, 112–125. [Google Scholar] [CrossRef]
- Josifovska, N.; Albert, R.; Nagymihály, R.; Lytvynchuk, L.; Moe, M.C.; Kaarniranta, K.; Veréb, Z.J.; Petrovski, G. Resveratrol as Inducer of Autophagy, Pro-Survival, and Anti-Inflammatory Stimuli in Cultured Human RPE Cells. Int. J. Mol. Sci. 2020, 21, 813. [Google Scholar] [CrossRef]
- Hu, W.H.; Dai, D.K.; Zheng, B.Z.; Duan, R.; Dong, T.T.; Qin, Q.W.; Tsim, K.W. Piceatannol, a Natural Analog of Resveratrol, Exerts Anti-angiogenic Efficiencies by Blockage of Vascular Endothelial Growth Factor Binding to Its Receptor. Molecules 2020, 25, 3769. [Google Scholar] [CrossRef]
- Surya, K.; Manickam, N.; Jayachandran, K.S.; Kandasamy, M.; Anusuyadevi, M. Resveratrol Mediated Regulation of Hippocampal Neuroregenerative Plasticity via SIRT1 Pathway in Synergy with Wnt Signaling: Neurotherapeutic Implications to Mitigate Memory Loss in Alzheimer’s Disease. J. Alzheimers Dis 2022, 1–17, Epub ahead of print. [Google Scholar] [CrossRef]
- Choo, P.P.; Woi, P.J.; Bastion, M.C.; Omar, R.; Mustapha, M.; Md Din, N. Review of Evidence for the Usage of Antioxidants for Eye Aging. Biomed. Res. Int. 2022, 2022, 5810373. [Google Scholar] [CrossRef]
- Vepsäläinen, S.; Koivisto, H.; Pekkarinen, E.; Mäkinen, P.; Dobson, G.; McDougall, G.J.; Stewart, D.; Haapasalo, A.; Karjalainen, R.O.; Tanila, H.; et al. Anthocyanin-enriched bilberry and blackcurrant extracts modulate amyloid precursor protein processing and alleviate behavioral abnormalities in the APP/PS1 mouse model of Alzheimer’s disease. J. Nutr. Biochem. 2013, 24, 360–370. [Google Scholar] [CrossRef]
- Zhuge, Q.; Zhang, Y.; Liu, B.; Wu, M. Blueberry polyphenols play a preventive effect on alcoholic fatty liver disease C57BL/6 J mice by promoting autophagy to accelerate lipolysis to eliminate excessive TG accumulation in hepatocytes. Ann. Palliat. Med. 2020, 9, 1045–1054. [Google Scholar] [CrossRef]
- Ooe, E.; Kuse, Y.; Yako, T.; Sogon, T.; Nakamura, S.; Hara, H.; Shimazawa, M. Bilberry extract and anthocyanins suppress unfolded protein response induced by exposure to blue LED light of cells in photoreceptor cell line. Mol. Vis. 2018, 24, 621–632. [Google Scholar]
- Kim, J.; Kim, C.S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.S. Vaccinium myrtillus extract prevents or delays the onset of diabetes--induced blood-retinal barrier breakdown. Int. J. Food Sci. Nutr. 2015, 66, 236–242. [Google Scholar] [CrossRef]
- Farajipour, H.; Rahimian, S.; Taghizadeh, M. Curcumin: A new candidate for retinal disease therapy? J. Cell Biochem. 2019, 120, 6886–6893. [Google Scholar] [CrossRef]
- Aoki, H.; Takada, Y.; Kondo, S.; Sawaya, R.; Aggarwal, B.B.; Kondo, Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: Role of Akt and extracellular signal-regulated kinase signaling pathways. Mol. Pharmacol. 2007, 72, 29–39. [Google Scholar] [CrossRef]
- Shinojima, N.; Yokoyama, T.; Kondo, Y.; Kondo, S. Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in curcumin-induced autophagy. Autophagy 2007, 3, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Limanaqi, F.; Biagioni, F.; Busceti, C.L.; Ryskalin, L.; Polzella, M.; Frati, A.; Fornai, F. Phytochemicals Bridging Autophagy Induction and Alpha-Synuclein Degradation in Parkinsonism. Int. J. Mol. Sci. 2019, 20, 3274. [Google Scholar] [CrossRef] [PubMed]
- Ryskalin, L.; Biagioni, F.; Busceti, C.L.; Lazzeri, G.; Frati, A.; Fornai, F. The Multi-Faceted Effect of Curcumin in Glioblastoma from Rescuing Cell Clearance to Autophagy-Independent Effects. Molecules 2020, 25, 4839. [Google Scholar] [CrossRef] [PubMed]
- Ryskalin, L.; Puglisi-Allegra, S.; Lazzeri, G.; Biagioni, F.; Busceti, C.L.; Balestrini, L.; Fornasiero, A.; Leone, S.; Pompili, E.; Ferrucci, M.; et al. Neuroprotective Effects of Curcumin in Methamphetamine-Induced Toxicity. Molecules 2021, 26, 2493. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, M.B.; Bao, S.; Gao, Z.; Mei, J.; Ge, H.; Jiang, L.; Geng, C.; Li, Q.; Shi, X.; Liu, Y.; et al. Curcumin attenuates Cr (VI)-induced cell growth and migration by targeting autophagy-dependent reprogrammed metabolism. J. Biochem. Mol. Toxicol. 2022, 36, e23193. [Google Scholar] [CrossRef]
- Teng, S.; Joseph, M.J.; Yu, H.; Hu, C.; Li, X.; Hu, C. A narrative review of the protective effects of curcumin in treating ischemia-reperfusion injury. Ann. Transl. Med. 2022, 10, 807. [Google Scholar] [CrossRef]
- Chandrasekaran, P.R.; Madanagopalan, V.G. Role of Curcumin in Retinal Diseases-A review. Graefes. Arch. Clin. Exp. Ophthalmol. 2022, 260, 1457–1473. [Google Scholar] [CrossRef]
- Hassanzadeh, K.; Vahabzadeh, Z.; Bucarello, L.; Dragotto, J.; Corbo, M.; Maccarone, R.; Feligioni, M. Protective Effect of Curcuma Extract in an Ex Vivo Model of Retinal Degeneration via Antioxidant Activity and Targeting the SUMOylation. Oxid. Med. Cell Longev. 2022, 2022, 8923615. [Google Scholar] [CrossRef]
- Allegrini, D.; Raimondi, R.; Angi, M.; Ricciardelli, G.; Montericcio, A.; Borgia, A.; Romano, M.R. Curcuma-Based Nutritional Supplement in Patients with Neovascular Age-Related Macular Degeneration. J. Med. Food 2021, 24, 1191–1196. [Google Scholar] [CrossRef]
- Allegrini, D.; Raimondi, R.; Borgia, A.; Sorrentino, T.; Montesano, G.; Tsoutsanis, P.; Cancian, G.; Verma, Y.; De Rosa, F.P.; Romano, M.R. Curcumin in Retinal Diseases: A Comprehensive Review from Bench to Bedside. Int. J. Mol. Sci. 2022, 23, 3557. [Google Scholar] [CrossRef]
- Vallée, A. Curcumin and Wnt/β-catenin signaling in exudative age-related macular degeneration (Review). Int J Mol Med. 2022, 49, 79. [Google Scholar] [CrossRef]
- Tang, M.Y.; Blazes, M.S.; Lee, C.S. Imaging Amyloid and Tau in the Retina: Current Research and Future Directions. J. Neuroophthalmol. 2023. Epub ahead of print. [Google Scholar] [CrossRef]
- Nadeem, U.; Xie, B.; Xie, E.F.; D’Souza, M.; Dao, D.; Sulakhe, D.; Skondra, D. Using Advanced Bioinformatics Tools to Identify Novel Therapeutic Candidates for Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2022, 11, 10. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef]
- Zhou, H.; Beevers, C.S.; Huang, S. The targets of curcumin. Curr. Drug Target. 2011, 12, 332–347. [Google Scholar] [CrossRef]
- Wang, W.; Xu, J. Curcumin Attenuates Cerebral Ischemia-reperfusion Injury Through Regulating Mitophagy and Preserving Mitochondrial Function. Curr. Neurovasc. Res. 2020, 17, 113–122. [Google Scholar] [CrossRef]
- Khater, S.I.; Dowidar, M.F.; Abdel-Aziz, A.E.; Khamis, T.; Dahran, N.; Alqahtani, L.S.; Metwally, M.M.M.; Al-Hady Abd-Elrahamn, A.S.; Alsieni, M.; Alosaimi, M.E.; et al. β-Cell Autophagy Pathway and Endoplasmic Reticulum Stress Regulating-Role of Liposomal Curcumin in Experimental Diabetes Mellitus: A Molecular and Morphometric Study. Antioxidants 2022, 11, 2400. [Google Scholar] [CrossRef]
- Liu, X.; Ye, M.; Ma, L. The emerging role of autophagy and mitophagy in tauopathies: From pathogenesis to translational implications in Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1022821. [Google Scholar] [CrossRef]
- Fabrizi, C.; Somma, F.; Pompili, E.; Biagioni, F.; Lenzi, P.; Fornai, F.; Fumagalli, L. Role of autophagy inhibitors and inducers in modulating the toxicity of trimethyltin in neuronal cell cultures. J. Neural. Transm. 2012, 119, 1295–1305. [Google Scholar] [CrossRef]
- Arcella, A.; Biagioni, F.; Antonietta Oliva, M.; Bucci, D.; Frati, A.; Esposito, V.; Cantore, G.; Giangaspero, F.; Fornai, F. Rapamycin inhibits the growth of glioblastoma. Brain Res. 2013, 1495, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Lazzeri, G.; Biagioni, F.; Fulceri, F.; Busceti, C.L.; Scavuzzo, M.C.; Ippolito, C.; Salvetti, A.; Lenzi, P.; Fornai, F. mTOR Modulates Methamphetamine-Induced Toxicity through Cell Clearing Systems. Oxid Med. Cell Longev. 2018, 2018, 6124745. [Google Scholar] [CrossRef] [PubMed]
- Walters, H.E.; Cox, L.S. mTORC Inhibitors as Broad-Spectrum Therapeutics for Age-Related Diseases. Int. J. Mol. Sci. 2018, 19, 2325. [Google Scholar] [CrossRef] [PubMed]
- Thellung, S.; Corsaro, A.; Nizzari, M.; Barbieri, F.; Florio, T. Autophagy Activator Drugs: A New Opportunity in Neuroprotection from Misfolded Protein Toxicity. Int. J. Mol. Sci. 2019, 20, 901. [Google Scholar] [CrossRef]
- Muangnoi, C.; Sharif, U.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Paraoan, L. Protective Effects of Curcumin Ester Prodrug, Curcumin Diethyl Disuccinate against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells: Potential Therapeutic Avenues for Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 3367. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chang, W.C.; Hung, K.H.; Yang, D.M.; Cheng, Y.H.; Liao, Y.W.; Woung, L.C.; Tsai, C.Y.; Hsu, C.C.; Lin, T.C.; et al. The generation of induced pluripotent stem cells for macular degeneration as a drug screening platform: Identification of curcumin as a protective agent for retinal pigment epithelial cells against oxidative stress. Front. Aging Neurosci. 2014, 6, 191. [Google Scholar] [CrossRef]
- Pinelli, R.; Berti, C.; Scaffidi, E.; Lazzeri, G.; Bumah, V.V.; Ruffoli, R.; Biagioni, F.; Busceti, C.L.; Puglisi-Allegra, S.; Fornai, F. Combined pulses of light and sound in the retina with nutraceuticals may enhance the recovery of foveal holes. Arch Ital. Biol. 2022, 160, 1–19. [Google Scholar] [CrossRef]
- Pinelli, R.; Biagioni, F.; Scaffidi, E.; Vakunseth Bumah, V.; Busceti, C.L.; Puglisi-Allegra, S.; Lazzeri, G.; Fornai, F. The potential effects of nutrients and light on autophagy-mediated visual function and clearance of retinal aggregates. Arch. Ital. Biol. 2022, 160, 115–135. [Google Scholar] [CrossRef]
- Pinelli, R.; Bertelli, M.; Scaffidi, E.; Bumah, V.V.; Biagioni, F.; Busceti, C.L.; Puglisi-Allegra, S.; Fornai, F. The neurobiology of nutraceuticals combined with light exposure, a case report in the course of retinal degeneration. Arch. Ital. Biol. 2021, 159, 134–150. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, J.; Zhao, X.; Song, Y.; Qin, Y.; Liu, Y. Improvement of the Biosynthesis of Resveratrol in Endophytic Fungus (Alternaria sp. MG1) by the Synergistic Effect of UV Light and Oligomeric Proanthocyanidins. Front. Microbiol. 2021, 12, 770734. [Google Scholar] [CrossRef]
- Bajwa, M.N.; Khanum, M.; Zaman, G.; Ullah, M.A.; Farooq, U.; Waqas, M.; Ahmad, N.; Hano, C.; Abbasi, B.H. Effect of Wide-Spectrum Monochromatic Lights on Growth, Phytochemistry, Nutraceuticals, and Antioxidant Potential of In Vitro Callus Cultures of Moringa oleifera. Molecules 2023, 28, 1497. [Google Scholar] [CrossRef]
- Morello, V.; Brousseau, V.D.; Wu, N.; Wu, B.S.; MacPherson, S.; Lefsrud, M. Light Quality Impacts Vertical Growth Rate, Phytochemical Yield and Cannabinoid Production Efficiency in Cannabis sativa. Plants 2022, 11, 2982. [Google Scholar] [CrossRef]
- Aziz, E.; Batool, R.; Akhtar, W.; Rehman, S.; Shahzad, T.; Malik, A.; Shariati, M.A.; Laishevtcev, A.; Plygun, S.; Heydari, M.; et al. Ahmed Arif S. Xanthophyll: Health benefits and therapeutic insights. Life Sci. 2020, 240, 117104. [Google Scholar] [CrossRef]
- Karppinen, K.; Zoratti, L.; Nguyenquynh, N.; Häggman, H.; Jaakola, L. On the Developmental and Environmental Regulation of Secondary Metabolism in Vaccinium spp. Berries. Front. Plant Sci. 2016, 7, 655. [Google Scholar] [CrossRef]
- Yu, X.Q.; Su, W.; Zhang, H.; Niu, M.; Liu, X.; Li, Z.; Liu, C.; Wang, H.L.; Yin, W.; Xia, X. Genome-wide analysis of autophagy-related gene family and PagATG18a enhances salt tolerance by regulating ROS homeostasis in poplar. Int. J. Biol. Macromol. 2023, 224, 1524–1540. [Google Scholar] [CrossRef]
- Goto-Yamada, S.; Oikawa, K.; Hayashi, Y.; Mano, S.; Yamada, K.; Nishimura, M. Pexophagy in plants: A mechanism to remit cells from oxidative damage caused under high-intensity light. Autophagy 2023, 9, 1–3. [Google Scholar] [CrossRef]

| Patient | BCVA * | Metamorphopsia | Contrast Sensitivity | Central Thickness (μm) | Drusenoid Area (mm2) |
|---|---|---|---|---|---|
| 1: F, 83 years | 20/63 | 0.4° | 2.0 | 185 | 7.00 |
| 2: M, 74 years | 20/80 | 0.3° | 1.7/1.8 | 182 | 7.54 |
| 3: F, 61 years | 20/25 | 0.3° | 1.9 | 206 | 2.00 |
| 4: F, 85 years | 20/25 | 0.4° | 1.9 | 205 | 4.52 |
| 5: F, 57 years | 20/32 | 0.3° | 1.9 | 183 | 18.80 |
| 6: F, 81 years | 20/32 | 0.4° | 1.8/1.9 | 225 | 3.14 |
| 7: F, 75 years | 20/25 | 0.4° | 2.0 | 200 | 1.32 |
| 8: F, 68 years | 20/32 | 0.5° | 1.8 | 222 | 11.30 |
| 9: F, 81 years | 20/32 | 0.5° | 1.9 | 235 | 1.32 |
| 10: F, 59 years | 20/25 | 0.5° | 1.9 | 238 | 1.53 |
| 11: M, 78 years | 20/80 | 0.3° | 1.8 | 208 | 13.10 |
| 12: F, 72 years | 20/25 | 0.5° | 1.9 | 195 | 1.76 |
| 13: M, 70 years | 20/50 | 0.4° | 1.8 | 212 | 7.00 |
| 14: F, 62 years | 20/25 | 0.5° | 1.8 | 215 | 4.15 |
| 15: F, 78 years | 20/25 | 0.4° | 1.8 | 218 | 12.50 |
| 16: F, 72 years | 20/25 | 0.5° | 1.9/1.0 | 261 | 3.46 |
| 17: F, 72 years | 20/32 | 0.5° | 1.8 | 222 | 1.13 |
| 18: F, 70 years | 20/40 | 0.4° | 1.9 | 211 | 0.78 |
| Phytochemicals | Effects | Authors and References |
|---|---|---|
| Lutein | activates autophagy | Chang et al., Am J Chin Med, 2017; [109] |
| counteracts oxidative stress (ROS) | Muangnoi et al., Int J Mol Sci, 2021; [110] | |
| increases mitochondrial turnover | Minasyan et al., Oxid Med Cell Longev, 2017; [111] | |
| exerts anti-inflammatory effects | Pinelli et al., Int J Mol Sci, 2020; [3] | |
| induces retinal stem cells | Jin et al., Stem Cell Res Ther, 2022; [112] | |
| Resveratrol | activates autophagy | Munia et al., Nutrients, 2020; [113] |
| counteracts oxidative stress (ROS) | Yang et al., Eur Rev Med Pharmacol Sci, 2019; [114] | |
| increases mitochondrial turnover | Wang et al., Aging (Albany NY), 2019; [115] | |
| exerts anti-inflammatory effects | Sun et al., Cell Mol Neurobiol, 2023; [116] | |
| Bilberry | activates autophagy | Li et al., Nutrition, 2022; [117] |
| counteracts oxidative stress (ROS) | Osada et al., PLoS One, 2017; [118] | |
| exerts anti-inflammatory effects | Wang et al., Molecules, 2015; [119] | |
| Curcumin | activates autophagy | Jin et al., Exp Ther Med, 2022; [120] |
| counteracts oxidative stress (ROS) | Lin et al., Phytomedicine, 2023; [9] |
| Patient | BCVA | Metamorphopsia | Contrast Sensitivity | Central Thickness (μm) | Drusenoid Area (mm2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| 1 | 20/63 | 20/50 | 0.4° | 0.4° | 2.0 | 2.0 | 185 | 213 | 7.0 | 0.7 |
| 2 | 20/80 | 20/50 | 0.3° | 0.4° | 1.8 | 1.8 | 208 | 228 | 13.1 | 5.72 |
| 3 | 20/50 | 20/40 | 0.4° | 0.4° | 1.8 | 1.8 | 212 | 210 | 7.0 | 3.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinelli, R.; Ferrucci, M.; Biagioni, F.; Berti, C.; Bumah, V.V.; Busceti, C.L.; Puglisi-Allegra, S.; Lazzeri, G.; Frati, A.; Fornai, F. Autophagy Activation Promoted by Pulses of Light and Phytochemicals Counteracting Oxidative Stress during Age-Related Macular Degeneration. Antioxidants 2023, 12, 1183. https://doi.org/10.3390/antiox12061183
Pinelli R, Ferrucci M, Biagioni F, Berti C, Bumah VV, Busceti CL, Puglisi-Allegra S, Lazzeri G, Frati A, Fornai F. Autophagy Activation Promoted by Pulses of Light and Phytochemicals Counteracting Oxidative Stress during Age-Related Macular Degeneration. Antioxidants. 2023; 12(6):1183. https://doi.org/10.3390/antiox12061183
Chicago/Turabian StylePinelli, Roberto, Michela Ferrucci, Francesca Biagioni, Caterina Berti, Violet Vakunseth Bumah, Carla Letizia Busceti, Stefano Puglisi-Allegra, Gloria Lazzeri, Alessandro Frati, and Francesco Fornai. 2023. "Autophagy Activation Promoted by Pulses of Light and Phytochemicals Counteracting Oxidative Stress during Age-Related Macular Degeneration" Antioxidants 12, no. 6: 1183. https://doi.org/10.3390/antiox12061183
APA StylePinelli, R., Ferrucci, M., Biagioni, F., Berti, C., Bumah, V. V., Busceti, C. L., Puglisi-Allegra, S., Lazzeri, G., Frati, A., & Fornai, F. (2023). Autophagy Activation Promoted by Pulses of Light and Phytochemicals Counteracting Oxidative Stress during Age-Related Macular Degeneration. Antioxidants, 12(6), 1183. https://doi.org/10.3390/antiox12061183








