Effects of β-Nicotinamide Mononucleotide, Berberine, and Cordycepin on Lipid Droplet Content and Developmental Ability of Vitrified Bovine Oocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Oocyte Collection and In Vitro Maturation (IVM)
2.2. Vitrification and Warming
2.3. IVF Procedure of Oocytes
2.4. qRT-PCR Procedure
2.5. ROS Staining of Oocytes
2.6. Lipid Droplets Staining of Oocytes
2.7. PS Externalization of Oocytes
2.8. Experimental Design
2.9. Statistics Analysis
3. Result
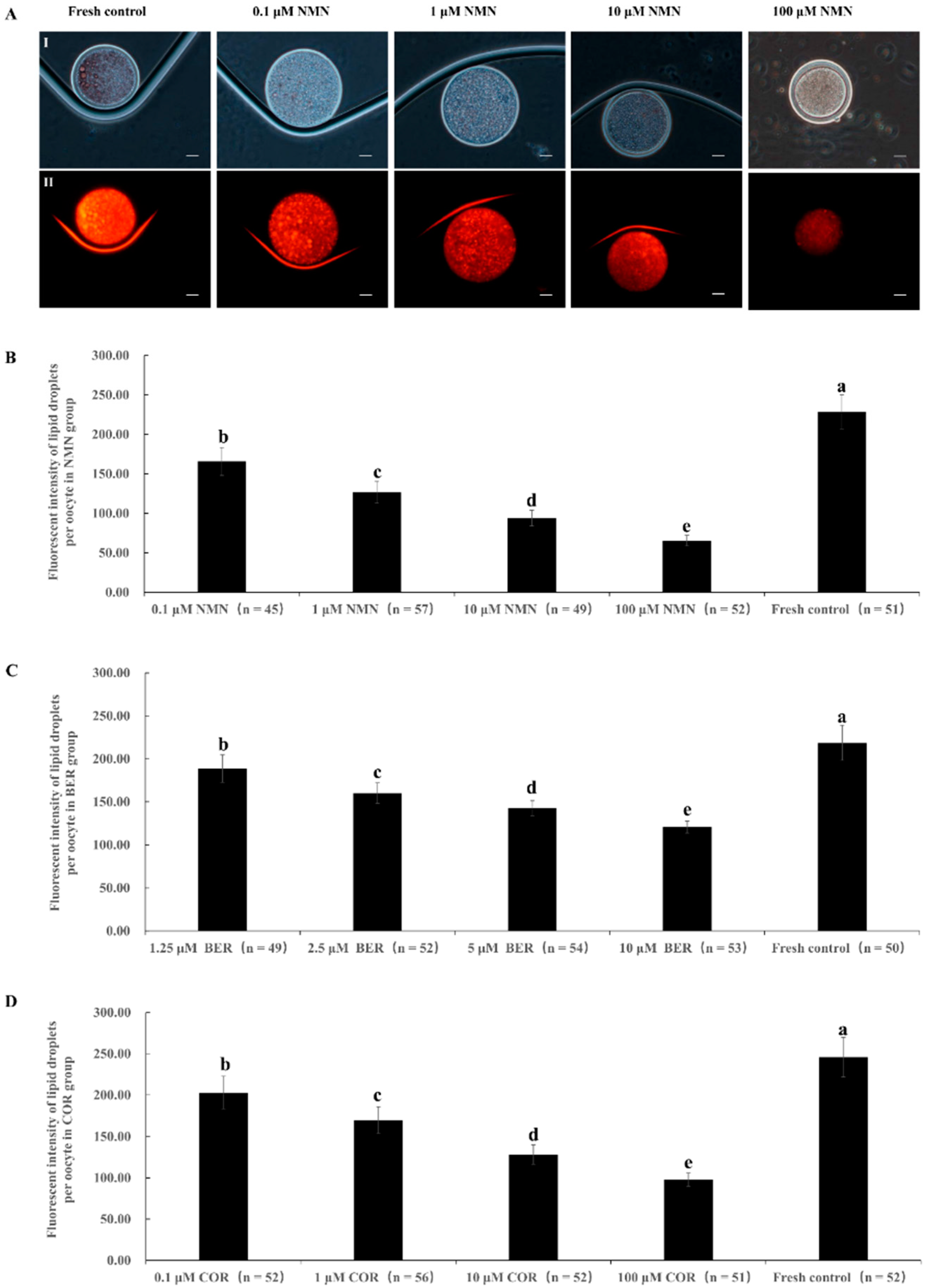
3.1. Effect of NMN, BER, or COR Supplementation during IVM on Lipid Droplet Content in Bovine Oocytes
3.2. Effect of NMN Supplementation during IVM on the Maturation Rate of Bovine Oocytes and the Survival Rate and Developmental Ability of Bovine Vitrified Oocytes
3.3. Effect of BER Supplementation during IVM on the Maturation Rate of Bovine Oocytes and the Survival Rate and Developmental Ability of Bovine Vitrified Oocytes
3.4. Effect of COR Supplementation during IVM on the Maturation Rate of Bovine Oocytes and the Survival Rate and Developmental Ability of Bovine Vitrified Oocytes
3.5. Effect of NMN, BER, or COR Supplementation during IVM on the Maturation Rate of Bovine Oocytes and the Survival Rate and Developmental Ability of Bovine Vitrified Oocytes
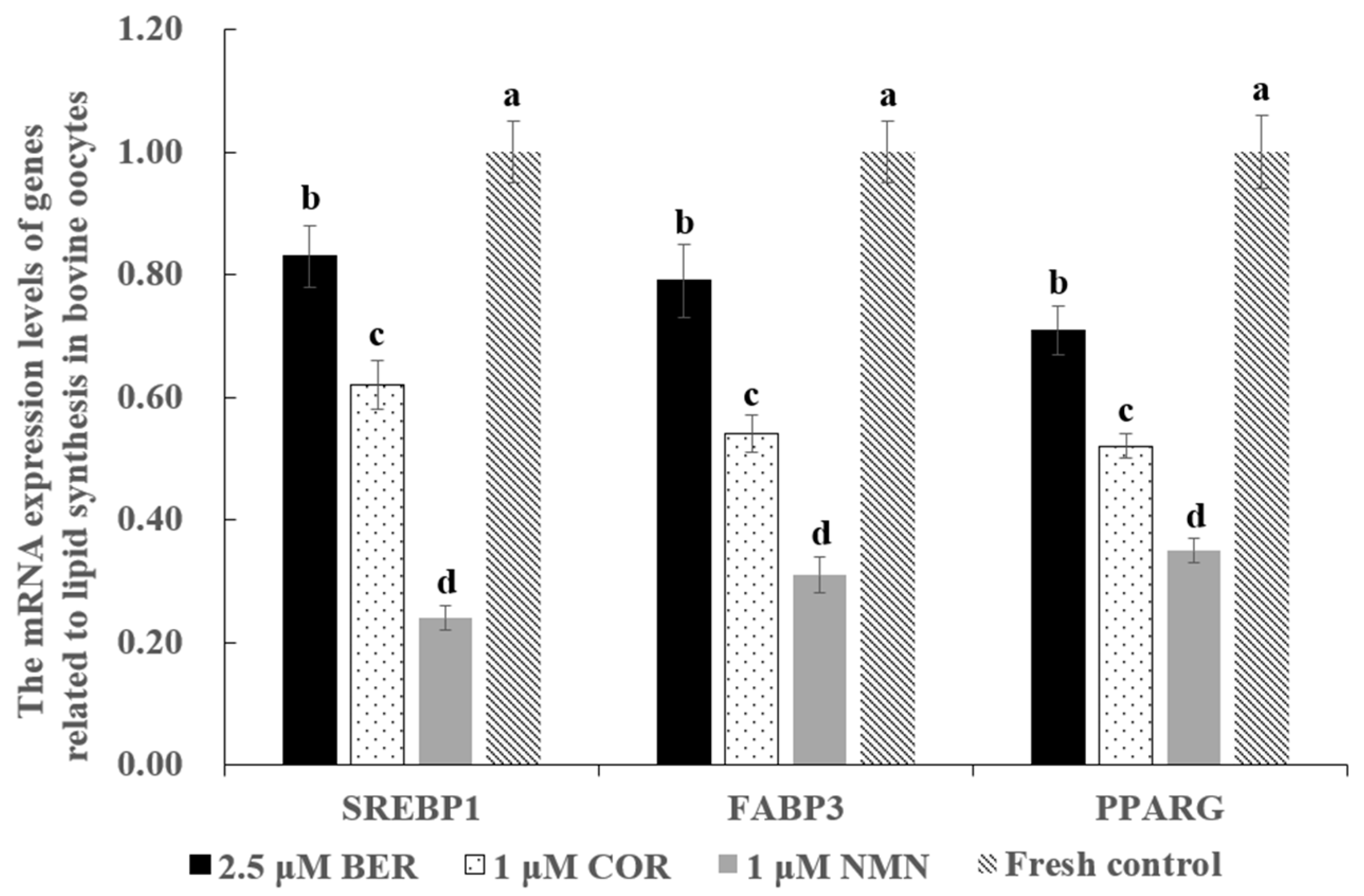
3.6. Effect of NMN, BER, or COR Supplementation during IVM on the mRNA Expression Levels of Genes Associated with Lipid Synthesis in Bovine Oocytes
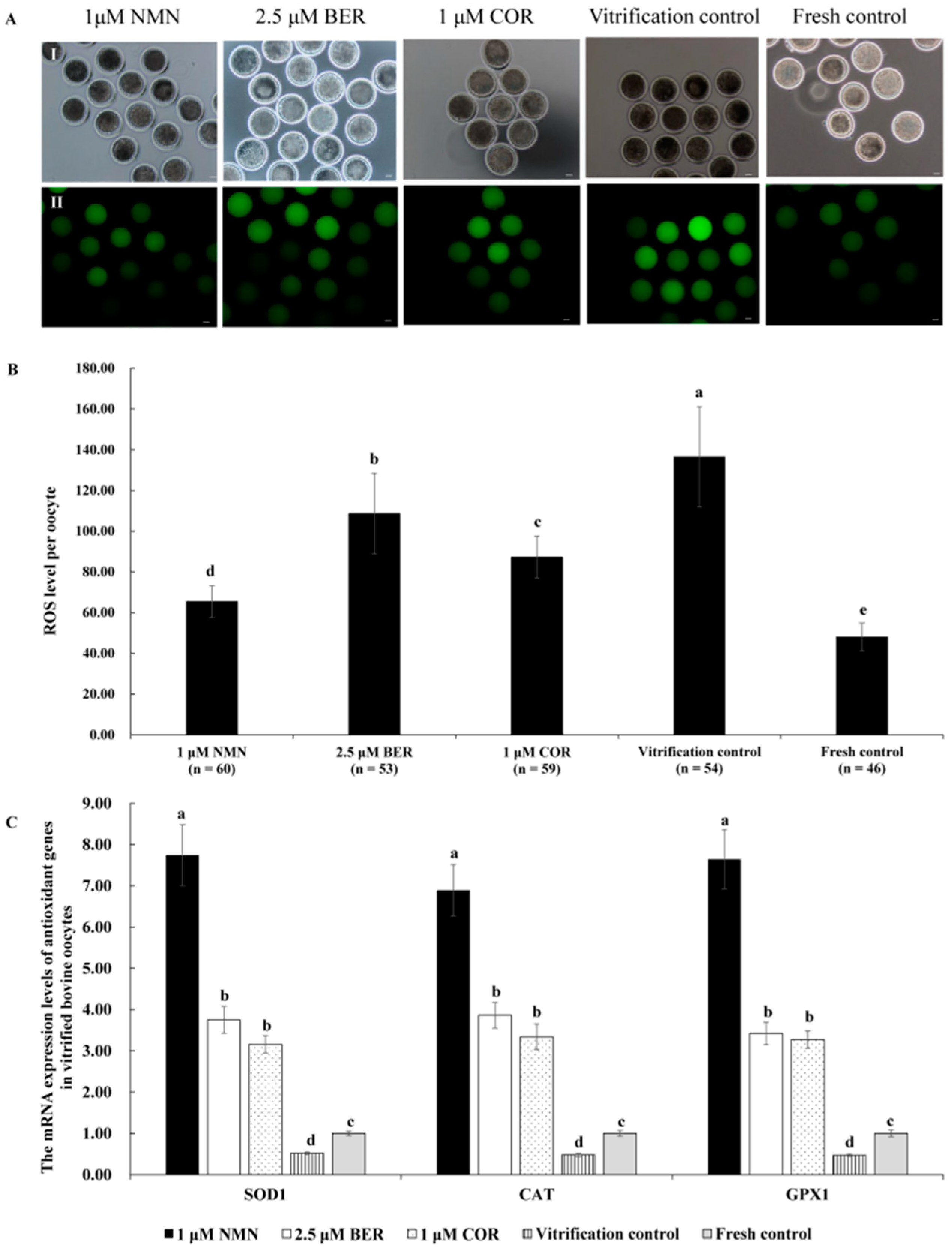
3.7. Effects of NMN, BER, or COR on ROS Level of Bovine Vitrified Oocytes
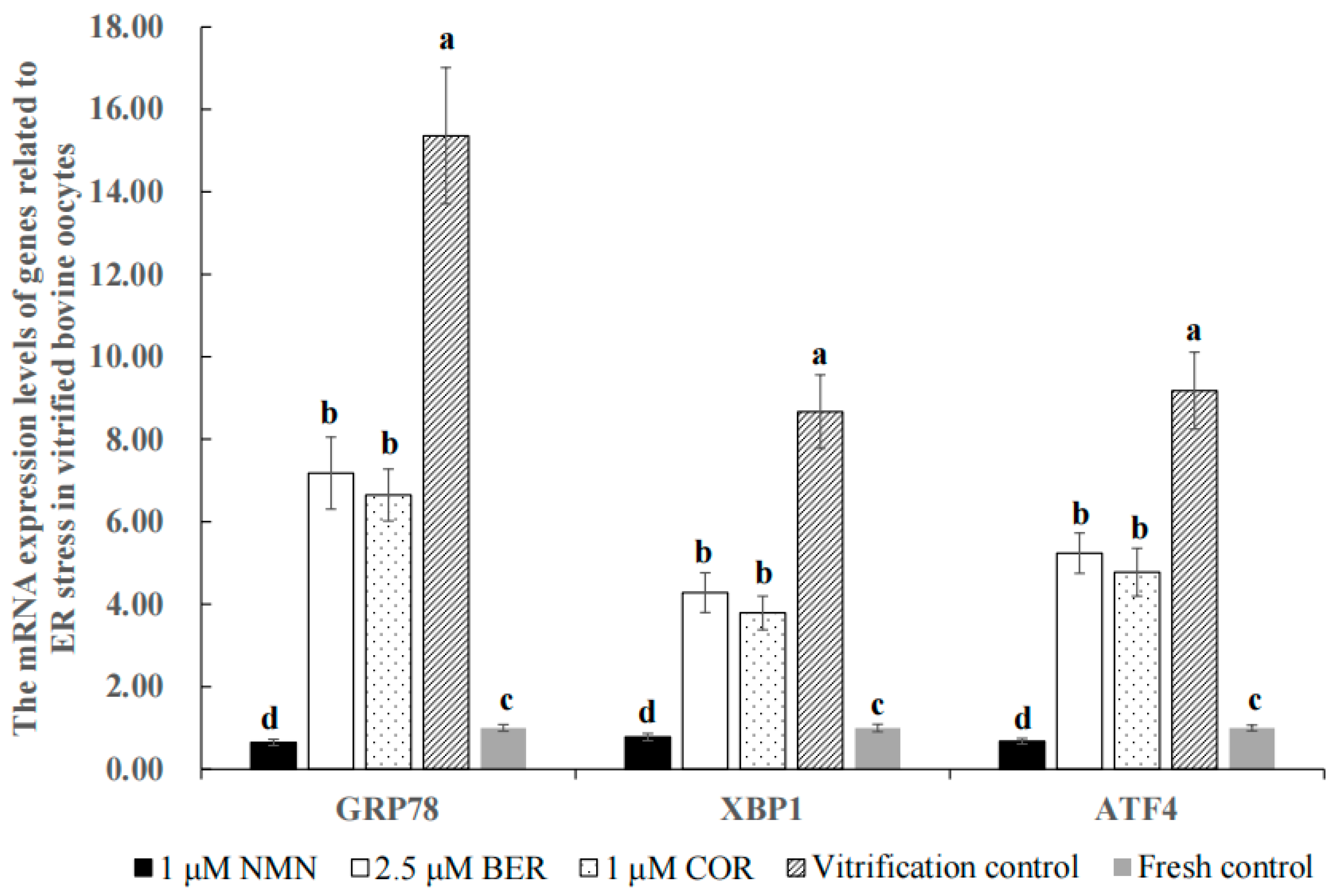
3.8. Effect of NMN, BER, or COR on ER Stress in Bovine Vitrified Oocytes
3.9. Effect of NMN, BER, or COR on Mitochondrial Function in Bovine Vitrified Oocytes
3.10. Effects of NMN, BER, or COR on Apoptosis Level of Bovine Vitrified Oocytes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sprícigo, J.F.; Morais, K.S.; Yang, B.S.; Dode, M.A. Effect of the exposure to methyl-β-cyclodextrin prior to chilling or vitrification on the viability of bovine immature oocytes. Cryobiology 2012, 65, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Morrell, J.M.; Mayer, I. Reproduction biotechnologies in germplasm banking of livestock species: A review. Zygote 2017, 25, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Mayer, I. The Role of Reproductive sciences in the preservation and breeding of commercial and threatened teleost fishes. Adv. Exp. Med. Biol. 2019, 1200, 187–224. [Google Scholar] [PubMed]
- Vining, L.M.; Zak, L.J.; Harvey, S.C.; Harvey, K.E. The role of apoptosis in cryopreserved animal oocytes and embryos. Theriogenology 2021, 173, 93–101. [Google Scholar] [CrossRef]
- Telfer, E.E.; Andersen, C.Y. In vitro growth and maturation of primordial follicles and immature oocytes. Fertil. Steril. 2021, 115, 1116–1125. [Google Scholar] [CrossRef]
- Mullen, S.F.; Fahy, G.M. A chronologic review of mature oocyte vitrification research in cattle, pigs, and sheep. Theriogenology 2012, 78, 1709–1719. [Google Scholar] [CrossRef]
- Ortiz-Escribano, N.; Bogado Pascottini, O.; Woelders, H.; Vandenberghe, L.; De Schauwer, C.; Govaere, J.; Van den Abbeel, E.; Vullers, T.; Ververs, C.; Roels, K.; et al. An improved vitrification protocol for equine immature oocytes, resulting in a first live foal. Equine Vet. J. 2018, 50, 391–397. [Google Scholar] [CrossRef]
- Yodrug, T.; Parnpai, R.; Hirao, Y.; Somfai, T. Effect of vitrification at different meiotic stages on epigenetic characteristics of bovine oocytes and subsequently developing embryos. Anim. Sci. J. 2021, 92, e13596. [Google Scholar] [CrossRef]
- McEvoy, T.G.; Coull, G.D.; Broadbent, P.J.; Hutchinson, J.S.; Speake, B.K. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J. Reprod. Fertil. 2000, 118, 163–170. [Google Scholar] [CrossRef]
- Yang, X.; Wu, L.L.; Chura, L.R.; Liang, X.; Lane, M.; Norman, R.J.; Robker, R.L. Exposure to lipid-rich follicular fluid is associated with endoplasmic reticulum stress and impaired oocyte maturation in cumulus-oocyte complexes. Fertil. Steril. 2012, 97, 1438–1443. [Google Scholar] [CrossRef]
- Del Collado, M.; da Silveira, J.C.; Sangalli, J.R.; Andrade, G.M.; Sousa, L.; Silva, L.A.; Meirelles, F.V.; Perecin, F. Fatty acid binding protein 3 and transzonal projections are involved in lipid accumulation during in vitro maturation of bovine oocytes. Sci. Rep. 2017, 7, 2645. [Google Scholar] [CrossRef]
- Quan, G.; Wu, G.; Hong, Q. Oocyte Oryopreservation based in sheep: The current status and future perspective. Biopreserv. Biobank. 2017, 15, 535–547. [Google Scholar] [CrossRef]
- Amstislavsky, S.; Mokrousova, V.; Brusentsev, E.; Okotrub, K.; Comizzoli, P. Influence of cellular lipids on cryopreservation of mammalian oocytes and preimplantation embryos: A review. Biopreserv. Biobank. 2019, 17, 76–83. [Google Scholar] [CrossRef]
- Hara, K.; Abe, Y.; Kumada, N.; Aono, N.; Kobayashi, J.; Matsumoto, H.; Sasada, H.; Sato, E. Extrusion and removal of lipid from the cytoplasm of porcine oocytes at the germinal vesicle stage: Centrifugation under hypertonic conditions influences vitrification. Cryobiology 2005, 50, 216–222. [Google Scholar] [CrossRef]
- Owen, C.M.; Johnson, M.A.; Rhodes-Long, K.A.; Gumber, D.J.; Barceló-Fimbres, M.; Altermatt, J.L.; Campos-Chillon, L.F. Novel synthetic oviductal fluid for conventional freezing 1 (SCF1) culture medium improves development and cryotolerance of in vitro produced holstein embryos. J. Anim. Sci. 2022, 100, skac043. [Google Scholar] [CrossRef]
- Ren, L.; Fu, B.; Ma, H.; Liu, D. Effects of mechanical delipation in porcine oocytes on mitochondrial distribution, ROS activity and viability after vitrification. CryoLetters 2015, 36, 30–36. [Google Scholar]
- Poddar, S.K.; Sifat, A.E.; Haque, S.; Nahid, N.A.; Chowdhury, S.; Mehedi, I. Nicotinamide mononucleotide: Exploration of diverse therapeutic applications of a potential molecule. Biomolecules 2019, 9, 34. [Google Scholar] [CrossRef]
- Zhong, O.; Wang, J.; Tan, Y.; Lei, X.; Tang, Z. Effects of NAD+ precursor supplementation on glucose and lipid metabolism in humans: A meta-analysis. Nutr. Metab. 2022, 19, 20. [Google Scholar] [CrossRef]
- Zeng, H.F.; Xu, J.; Wang, X.L.; Li, S.J.; Han, Z.Y. Nicotinamide mononucleotide alleviates heat stress-induced oxidative stress and apoptosis in BMECs through reducing mitochondrial damage and endoplasmic reticulum stress. Ecotoxicol. Environ. Saf. 2022, 235, 113441. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, C.L.; Li, P.; Li, H.R.; Liu, Q.; Deng, X.M.; Wang, J.F. Nicotinamide mononucleotide attenuates LPS-Induced acute lung injury with anti-inflammatory, anti-oxidative and anti-apoptotic effects. J. Surg. Res. 2023, 283, 9–18. [Google Scholar] [CrossRef]
- Hong, W.; Mo, F.; Zhang, Z.; Huang, M.; Wei, X. Nicotinamide mononucleotide: A promising molecule for therapy of diverse diseases by targeting NAD+ metabolism. Front. Cell. Dev. Biol. 2020, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Okabe, K.; Yaku, K.; Tobe, K.; Nakagawa, T. Implications of altered NAD metabolism in metabolic disorders. J. Biomed. Sci. 2019, 26, 34. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.F.; Baggioni, A. Berberine and its role in chronic disease. Adv. Exp. Med. Biol. 2016, 928, 27–45. [Google Scholar] [PubMed]
- Li, T.; Wen, L.; Cheng, B. Cordycepin alleviates hepatic lipid accumulation by inducing protective autophagy via PKA/mTOR pathway. Biochem. Biophys. Res. Commun. 2019, 516, 632–638. [Google Scholar] [CrossRef]
- Han, F.; Dou, M.; Wang, Y.; Xu, C.; Li, Y.; Ding, X.; Xue, W.; Zheng, J.; Tian, P.; Ding, C. Cordycepin protects renal ischemia/reperfusion injury through regulating inflammation, apoptosis, and oxidative stress. Acta Biochim. Biophys. Sin. 2020, 52, 125–132. [Google Scholar] [CrossRef]
- Uddin, G.M.; Youngson, N.A.; Chowdhury, S.S.; Hagan, C.; Sinclair, D.A.; Morris, M.J. Administration of nicotinamide mononucleotide (NMN) reduces metabolic impairment in male mouse offspring from obese mothers. Cells 2020, 9, 791. [Google Scholar] [CrossRef]
- Liu, Y.; Hua, W.; Li, Y.; Xian, X.; Zhao, Z.; Liu, C.; Zou, J.; Li, J.; Fang, X.; Zhu, Y. Berberine suppresses colon cancer cell proliferation by inhibiting the SCAP/SREBP-1 signaling pathway-mediated lipogenesis. Biochem. Pharmacol. 2020, 174, 113776. [Google Scholar] [CrossRef]
- Niu, Y.J.; Tao, R.Y.; Liu, Q.; Tian, J.Y.; Ye, F.; Zhu, P.; Zhu, H.B. Improvement on lipid metabolic disorder by 3′-deoxyadenosine in high-fat-diet-induced fatty mice. Am. J. Chin. Med. 2010, 38, 1065–1075. [Google Scholar] [CrossRef]
- Xu, H.; Wu, B.; Wang, X.; Ma, F.; Li, Y.; An, Y.; Wang, C.; Wang, X.; Luan, W.; Li, S.; et al. Cordycepin regulates body weight by inhibiting lipid droplet formation, promoting lipolysis and recruiting beige adipocytes. J. Pharm. Pharmacol. 2019, 71, 1429–1439. [Google Scholar] [CrossRef]
- Song, M.; Li, Y.; Zhou, Y.; Yan, J.; Zhou, X.; Gao, Q.; Miao, Y.; Xiong, B. Nicotinamide mononucleotide supplementation improves the quality of porcine oocytes under heat stress. J. Anim. Sci. Biotechnol. 2022, 13, 68. [Google Scholar] [CrossRef]
- Dai, J.; Huang, X.; Zhang, C.; Luo, X.; Cao, S.; Wang, J.; Liu, B.; Gao, J. Berberine regulates lipid metabolism via miR-192 in porcine oocytes matured in vitro. Vet. Med. Sci. 2021, 7, 950–959. [Google Scholar] [CrossRef]
- Liu, H.; Gao, Y.; Zhai, B.; Jiang, H.; Ding, Y.; Zhang, L.; Li, C.; Deng, Q.; Yu, X.; Zhang, J. The effects of polyadenylation status on MPFs during in vitro porcine oocyte maturation. Cell Physiol. Biochem. 2016, 39, 1735–1745. [Google Scholar] [CrossRef]
- Miao, Y.; Cui, Z.; Gao, Q.; Rui, R.; Xiong, B. Nicotinamide mononucleotide supplementation reverses the declining quality of maternally aged oocytes. Cell Rep. 2020, 32, 107987. [Google Scholar] [CrossRef]
- Huang, C.H.; Wang, F.T.; Chan, W.H. Dose-dependent beneficial and harmful effects of berberine on mouse oocyte maturation and fertilization and fetal development. Toxicol. Res. 2020, 9, 431–443. [Google Scholar] [CrossRef]
- Zhao, X.M.; Hao, H.S.; Du, W.H.; Zhao, S.J.; Wang, H.Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016, 60, 132–141. [Google Scholar] [CrossRef]
- Brackett, B.G.; Oliphant, G. Capacitation of rabbit spermatozoa in vitro. Biol. Reprod. 1975, 12, 260–274. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Buschiazzo, J.; Ríos, G.L.; Canizo, J.R.; Antollini, S.S.; Alberio, R.H. Free cholesterol and cholesterol esters in bovine oocytes: Implications in survival and membrane raft organization after cryopreservation. PLoS ONE 2017, 12, e0180451. [Google Scholar] [CrossRef]
- Anguita, B.; Vandaele, L.; Mateusen, B.; Maes, D.; Van Soom, A. Developmental competence of bovine oocytes is not related to apoptosis incidence in oocytes, cumulus cells and blastocysts. Theriogenology 2007, 67, 537–549. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Wei, J.; Guo, F.; Li, L.; Han, Z.; Wang, Z.; Zhu, H.; Zhang, X.; Li, Z.; et al. Administration of nicotinamide mononucleotide improves oocyte quality of obese mice. Cell Prolif. 2022, 55, e13303. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Ren, H.; Huang, X.; Shen, T.; Tang, W.; Dou, L.; Li, J. miR-23a/b-3p promotes hepatic lipid accumulation by regulating Srebp-1c and Fas. J. Mol. Endocrinol. 2021, 68, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.L.; Liu, Y.Y.; Li, Z.Z.; Zhuang, Q.Z.; Tang, W.Z.; Xiong, Y.; Huang, X.Z. Amentoflavone prevents ox-LDL-induced lipid accumulation by suppressing the PPARγ/CD36 signal pathway. Toxicol. Appl. Pharmacol. 2021, 431, 115733. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Wang, X.N.; Huang, C.C.; Hu, L.; Xiao, Y.F.; Guan, X.H.; Qian, Y.S.; Deng, K.Y.; Xin, H.B. Inhibition of NAMPT aggravates high fat diet-induced hepatic steatosis in mice through regulating Sirt1/AMPKα/SREBP1 signaling pathway. Lipids Health Dis. 2017, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhu, X.P.; Bai, J.Y.; Xia, P.; Li, Y.; Lu, Y.; Li, X.Y.; Gao, X. Berberine alleviates nonalcoholic fatty liver induced by a high-fat diet in mice by activating SIRT3. FASEB J. 2019, 33, 7289–7300. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Zhang, R.; Li, P.; Zuo, L.; Wang, M.; Yang, J.; Wang, J. Hydrochloride berberine ameliorates alcohol-induced liver injury by regulating inflammation and lipid metabolism. Biochem. Biophys. Res. Commun. 2022, 610, 49–55. [Google Scholar] [CrossRef]
- An, Y.; Li, Y.; Wang, X.; Chen, Z.; Xu, H.; Wu, L.; Li, S.; Wang, C.; Luan, W.; Wang, X.; et al. Cordycepin reduces weight through regulating gut microbiota in high-fat diet-induced obese rats. Lipids Health Dis. 2018, 17, 276. [Google Scholar] [CrossRef]
- Wu, C.; Guo, Y.; Su, Y.; Zhang, X.; Luan, H.; Zhang, X.; Zhu, H.; He, H.; Wang, X.; Sun, G.; et al. Cordycepin activates AMP-activated protein kinase (AMPK) via interaction with the γ1 subunit. J. Cell Mol. Med. 2014, 18, 293–304. [Google Scholar] [CrossRef]
- Gong, X.; Li, T.; Wan, R.; Sha, L. Cordycepin attenuates high-fat diet-induced non-alcoholic fatty liver disease via down-regulation of lipid metabolism and inflammatory responses. Int. Immunopharmacol. 2021, 91, 107173. [Google Scholar] [CrossRef]
- Chankitisakul, V.; Somfai, T.; Inaba, Y.; Techakumphu, M.; Nagai, T. Supplementation of maturation medium with L-carnitine improves cryo-tolerance of bovine in vitro matured oocytes. Theriogenology 2013, 79, 590–598. [Google Scholar] [CrossRef]
- Khatun, H.; Wada, Y.; Konno, T.; Tatemoto, H.; Yamanaka, K.I. Endoplasmic reticulum stress attenuation promotes bovine oocyte maturation in vitro. Reproduction 2020, 159, 361–370. [Google Scholar] [CrossRef]
- Park, H.J.; Park, J.Y.; Kim, J.W.; Yang, S.G.; Jung, J.M.; Kim, M.J.; Kang, M.J.; Cho, Y.H.; Wee, G.; Yang, H.Y.; et al. Melatonin improves the meiotic maturation of porcine oocytes by reducing endoplasmic reticulum stress during in vitro maturation. J. Pineal Res. 2018, 64, e12458. [Google Scholar] [CrossRef]
- Wu, L.L.; Russell, D.L.; Norman, R.J.; Robker, R.L. Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (DeltaPsi m), and embryo development. Mol. Endocrinol. 2012, 26, 562–573. [Google Scholar] [CrossRef]
- Li, C.J.; Lin, L.T.; Tsai, H.W.; Wen, Z.H.; Tsui, K.H. Phosphoglycerate mutase family member 5 maintains oocyte quality via mitochondrial dynamic rearrangement during aging. Aging Cell 2022, 21, e13546. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, X.; Wang, B.; Song, K.; Zhang, J.; Chen, Y.; Tian, Y.; Weng, J.; Liang, Y.; Ma, W. Adverse effects of 2-methoxyestradiol on mouse oocytes during reproductive aging. Chem. Biol. Interact. 2023, 369, 110277. [Google Scholar] [CrossRef]
- Chen, H.; Chomyn, A.; Chan, D.C. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 2005, 280, 26185–26192. [Google Scholar] [CrossRef]
- Smirnova, E.; Griparic, L.; Shurland, D.L.; van der Bliek, A.M. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell. 2001, 12, 2245–2256. [Google Scholar] [CrossRef]
- Kim, D.; Sankaramoorthy, A.; Roy, S. Downregulation of Drp1 and Fis1 inhibits mitochondrial fission and prevents high glucose-induced apoptosis in retinal endothelial Cells. Cells 2020, 9, 1662. [Google Scholar] [CrossRef]
- Chen, K.L.; Wang, H.L.; Jiang, L.Z.; Qian, Y.; Yang, C.X.; Chang, W.W.; Zhong, J.F.; Xing, G.D. Heat stress induces apoptosis through disruption of dynamic mitochondrial networks in dairy cow mammary epithelial cells. In Vitro Cell. Dev. Biol. Anim. 2020, 56, 322–331. [Google Scholar] [CrossRef]
- Klimova, N.; Fearnow, A.; Long, A.; Kristian, T. NAD(+) precursor modulates post-ischemic mitochondrial fragmentation and reactive oxygen species generation via SIRT3 dependent mechanisms. Exp. Neurol. 2020, 325, 113144. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Yu, X.L.; Cai, M.D.; Liu, Y.H.; Liu, J.Q.; Zhao, S.Y.; Li, X.X.; Li, Y.H. Relationship between bovine oocytes developmental competence and mRNA expression of apoptotic and mitochondrial genes following the change of vitrification temperatures and cryoprotectant concentrations. Cryobiology 2020, 97, 110–122. [Google Scholar] [CrossRef]
- Miao, Y.; Li, X.; Shi, X.; Gao, Q.; Chen, J.; Wang, R.; Fan, Y.; Xiong, B. Nicotinamide mononucleotide restores the meiotic competency of porcine oocytes exposed to ethylene glycol butyl ether. Front. Cell Dev. Biol. 2021, 9, 628580. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qi, Q.; Men, L.; Wang, S.; Li, M.; Xiao, M.; Chen, X.; Wang, S.; Wang, G.; Jia, H.; et al. Berberine protects Kawasaki disease-induced human coronary artery endothelial cells dysfunction by inhibiting of oxidative and endoplasmic reticulum stress. Vasc. Pharmacol. 2020, 127, 106660. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Zhao, Y.; Gong, J.; Huang, W.; Su, H.; Yuan, F.; Fang, K.; Wang, D.; Li, J.; Zou, X.; et al. Berberine protects glomerular podocytes via inhibiting Drp1-mediated mitochondrial fission and dysfunction. Theranostics 2019, 9, 1698–1713. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.L.; Park, S.Y.; Kim, Y.H.; Oh, J.I.; Lee, S.J.; Park, G. The neuroprotective effects of cordycepin inhibit glutamate-induced oxidative and ER stress-associated apoptosis in hippocampal HT22 cells. Neurotoxicology 2014, 41, 102–111. [Google Scholar] [CrossRef]
- Kitamura, M.; Kato, H.; Saito, Y.; Nakajima, S.; Takahashi, S.; Johno, H.; Gu, L.; Katoh, R. Aberrant, differential and bidirectional regulation of the unfolded protein response towards cell survival by 3′-deoxyadenosine. Cell Death Differ. 2011, 18, 1876–1888. [Google Scholar] [CrossRef]
- Zhang, X.L.; Huang, W.M.; Tang, P.C.; Sun, Y.; Zhang, X.; Qiu, L.; Yu, B.C.; Zhang, X.Y.; Hong, Y.X.; He, Y.; et al. Anti-inflammatory and neuroprotective effects of natural cordycepin in rotenone-induced PD models through inhibiting Drp1-mediated mitochondrial fission. Neurotoxicology 2021, 84, 1–13. [Google Scholar] [CrossRef]
- Krischek, C.; Meinecke, B. In vitro maturation of bovine oocytes requires polyadenylation of mRNAs coding proteins for chromatin condensation, spindle assembly, MPF and MAP kinase activation. Anim. Reprod. Sci. 2002, 73, 129–140. [Google Scholar] [CrossRef]
- Traverso, J.M.; Donnay, I.; Lequarre, A.S. Effects of polyadenylation inhibition on meiosis progression in relation to the polyadenylation status of cyclins A2 and B1 during in vitro maturation of bovine oocytes. Mol. Reprod. Dev. 2005, 71, 107–114. [Google Scholar] [CrossRef]
- Zhang, D.X.; Cui, X.S.; Kim, N.H. Involvement of polyadenylation status on maternal gene expression during in vitro maturation of porcine oocytes. Mol. Reprod. Dev. 2009, 76, 881–889. [Google Scholar] [CrossRef]
- Shirzeyli, M.H.; Eini, F.; Shirzeyli, F.H.; Majd, S.A.; Ghahremani, M.; Joupari, M.D.; Novin, M.G. Assessment of mitochondrial function and developmental potential of mouse oocytes after mitoquinone supplementation during vitrification. J. Am. Assoc. Lab. Anim. Sci. 2021, 60, 388–395. [Google Scholar] [CrossRef]
- García-Martínez, T.; Vendrell-Flotats, M.; Martínez-Rodero, I.; Ordóñez-León, E.A.; Álvarez-Rodríguez, M.; López-Béjar, M.; Yeste, M.; Mogas, T. Glutathione ethyl ester protects in vitro-maturing bovine oocytes against oxidative stress induced by subsequent vitrification/warming. Int. J. Mol. Sci. 2020, 21, 7547. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Yamaura, K.; Mifune, Y.; Inui, A.; Nishimoto, H.; Kurosawa, T.; Mukohara, S.; Hoshino, Y.; Niikura, T.; Kuroda, R. Antioxidant effect of nicotinamide mononucleotide in tendinopathy. BMC Musculoskelet. Disord. 2022, 23, 249. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, M.; Zhao, Y.; Gong, J.; Su, H.; Yuan, F.; Fang, K.; Yuan, X.; Yu, X.; Dong, H.; et al. Berberine protects against diabetic kidney disease via promoting PGC-1α-regulated mitochondrial energy homeostasis. Br. J. Pharmacol. 2020, 177, 3646–3661. [Google Scholar] [CrossRef]
- Ku, C.W.; Ho, T.J.; Huang, C.Y.; Chu, P.M.; Ou, H.C.; Hsieh, P.L. Cordycepin attenuates palmitic acid-induced inflammation and apoptosis of vascular endothelial cells through mediating PI3K/Akt/eNOS signaling pathway. Am. J. Chin. Med. 2021, 49, 1703–1722. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, A.; Gong, Y.; Sun, W.; Yan, B.; Lei, S.; Yao, L.H. Improving effect of cordycepin on insulin synthesis and secretion in normal and oxidative-damaged INS-1 cells. Eur. J. Pharmacol. 2022, 920, 174843. [Google Scholar] [CrossRef]
- Chang, C.C.; Shapiro, D.B.; Nagy, Z.P. The effects of vitrification on oocyte quality. Biol. Reprod. 2022, 106, 316–327. [Google Scholar] [CrossRef]
- Casao, A.; Mendoza, N.; Pérez-Pé, R.; Grasa, P.; Abecia, J.A.; Forcada, F.; Cebrián-Pérez, J.A.; Muino-Blanco, T. Melatonin prevents capacitation and apoptotic-like changes of ram spermatozoa and increases fertility rate. J. Pineal Res. 2010, 48, 39–46. [Google Scholar] [CrossRef]
- Pu, Q.; Guo, X.X.; Hu, J.J.; Li, A.L.; Li, G.G.; Li, X.Y. Nicotinamide mononucleotide increases cell viability and restores tight junctions in high-glucose-treated human corneal epithelial cells via the SIRT1/Nrf2/HO-1 pathway. Biomed. Pharmacother. 2022, 147, 112659. [Google Scholar] [CrossRef]
- Wu, Y.Z.; Zhang, L.; Wu, Z.X.; Shan, T.T.; Xiong, C. Berberine ameliorates doxorubicin-induced cardiotoxicity via a SIRT1/p66Shc-mediated pathway. Oxid. Med. Cell Longev. 2019, 2019, 2150394. [Google Scholar] [CrossRef]
- Song, T.; Chen, W.D. Berberine inhibited carotid atherosclerosis through PI3K/AKTmTOR signaling pathway. Bioengineered 2021, 12, 8135–8146. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Huang, L.P.; Li, Y.; Liu, C.; Wang, S.; Meng, W.; Wei, S.; Liu, X.P.; Gong, Y.; Yao, L.H. Neuroprotective effects of cordycepin inhibit Aβ-induced apoptosis in hippocampal neurons. Neurotoxicology 2018, 68, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sun, T.; Luan, Y.; Chen, Y.; Song, J.; Ling, L.; Yuan, P.; Li, R.; Cui, K.; Ruan, Y.; et al. Berberine ameliorates erectile dysfunction in rats with streptozotocin-induced diabetes mellitus through the attenuation of apoptosis by inhibiting the SPHK1/S1P/S1PR2 and MAPK pathways. Andrology 2022, 10, 404–418. [Google Scholar] [CrossRef] [PubMed]


| Gene | NCBI Accession | Primer (3′-5′) | Size (bp) |
|---|---|---|---|
| SREBP1 | NM_001113302.1 | CTACATCCGCTTCCTTCAGC | 90 |
| TTCAGCGATTTGCTTTTGTG | |||
| PPARG | NM_181024.2 | ATTTGGAAACGGACGTCTTG | 220 |
| TGAGGTCCTTGCAGACACTG | |||
| FABP3 | NM_174313.2 | TTCAAGCTGGGAGTCGAGTT | 235 |
| GCAGTCAGTGGAAGGAGAGG | |||
| XBP1 | NM_001034727.3 | GACATCCTGTTGGGCATTCT | 257 |
| ACATTGGCTTCGCTCTCTGT | |||
| ATF4 | NM_001034342.2 | AGATGACCTGGAAACCATGC | 190 |
| AGGGGGAAGAGGTTGAAAGA | |||
| GRP78 | NM_001075148.1 | TGGCATTCTTCGAGTGACAG | 84 |
| GTCAGGCGATTTTGGTCATT | |||
| MFN1 | NM_001206508.1 | GCAGACAGCACATGGAAAGA | 181 |
| CTTGCCTGAAATCCTTCTGC | |||
| MFN2 | NM_001190269.1 | GAAGTCGAGAGGCAGGTGTC | 131 |
| CGGTGCAGCTCATTCTTGTA | |||
| FIS1 | NM_001034784.2 | AACGACGACATCCGTAAAGG | 238 |
| CCATGCCCACTAGTCCATCT | |||
| DRP1 | NM_001046494.2 | TGGGGTCTTGTGTGTTACGA | 210 |
| GAGGTCTCCGGGTGACAATA | |||
| CAT | NM_001035386.2 | TGGGACCCAACTATCTCCAG | 178 |
| AAGTGGGTCCTGTGTTCCAG | |||
| GPX1 | NM_174076.3 | ACATTGAAACCCTGCTGTCC | 216 |
| TCATGAGGAGCTGTGGTCTG | |||
| SOD1 | NM_174615.2 | GGATTCCACGTCCATCAGTT | 292 |
| CAGCGTTGCCAGTCTTTGTA | |||
| BAX | NM_173894.1 | TCTGACGGCAACTTCAACTG | 205 |
| TGGGTGTCCCAAAGTAGGAG | |||
| BCL2 | NM_001166486.1 | CATCGTGGCCTTCTTTGAGT | 111 |
| CGGTTCAGGTACTCGGTCAT | |||
| β-ACTIN | NM_173979.3 | ACTTGCGCAGAAAACGAGAT | 121 |
| CACCTTCACCGTTCCAGTTT |
| Groups | No. of MII | Survival Rate after Vitrification | Cleavage Rate after IVF | Blastocyst Rate after IVF |
|---|---|---|---|---|
| 0.1 μM NMN | 83.38 ± 7.85% (271/325) b | 90.57 ± 0.33% (96/106) b | 62.50 ± 0.70% (60/96) c | 21.67 ± 0.59% (13/60) c |
| 1 μM NMN | 92.24 ± 9.59% (321/348) a | 97.25 ± 0.11% (106/109) a | 75.47 ± 1.11% (80/106) b | 33.75 ± 1.43% (27/80) b |
| 10 μM NMN | 84.53 ± 8.57% (317/375) b | 90.57 ± 1.10% (96/106) b | 64.58 ± 0.94% (62/96) c | 20.97 ± 1.84% (13/62) c |
| 100 μM NMN | 72.01 ± 7.81% (265/368) c | 82.00 ± 0.64% (82/100) c | 52.44 ± 2.15% (43/82) d | 13.95 ± 0.55% (6/43) d |
| Vitrification control | 74.73 ± 7.18% (272/364) c | 89.29 ± 1.16% (100/112) b | 52.00 ± 1.26% (52/100) d | 15.38 ± 1.73% (8/52) d |
| Fresh control | 75.42 ± 7.48% (267/354) c | - | 85.60 ± 7.59% (107/125) a | 45.79 ± 3.85% (49/107) a |
| Groups | No. of MII | Survival Rate after Vitrification | Cleavage Rate after IVF | Blastocyst Rate after IVF |
|---|---|---|---|---|
| 1.25 μM BER | 78.64 ± 9.52% (313/398) ab | 89.47 ± 1.64% (102/114) a | 59.80 ± 0.66% (61/102) c | 16.39 ± 0.92% (10/61) c |
| 2.5 μM BER | 83.16 ± 8.67% (321/386) a | 93.50 ± 0.97% (115/123) a | 69.57 ± 1.69% (80/115) b | 25.00 ± 1.80% (20/80) b |
| 5 μM BER | 79.43 ± 8.12% (305/384) ab | 89.29 ± 1.39% (100/112) a | 62.00 ± 1.73% (62/100) c | 16.13 ± 4.81% (10/62) c |
| 10 μM BER | 72.96 ± 6.87% (286/392) c | 81.73 ± 3.21% (85/104) b | 51.76 ± 5.49% (44/85) d | 11.36 ± 4.50% (5/44) d |
| Vitrification control | 75.32 ± 7.62% (293/389) bc | 89.74 ± 1.18% (105/117) a | 53.33 ± 1.71% (56/105) d | 12.50 ± 1.26% (7/56) d |
| Fresh control | 76.72 ± 7.58% (290/378) bc | - | 87.10 ± 7.59% (108/124) a | 46.30 ± 4.13% (50/108) a |
| Groups | No. of MII | Survival Rate after Vitrification | Cleavage Rate after IVF | Blastocyst Rate after IVF |
|---|---|---|---|---|
| 0.1 μM COR | 79.49 ±8.45 % (248/312) ab | 90.91 ± 1.27% (100/110) ab | 60.00 ± 3.20% (60/100) c | 15.00 ± 0.75% (9/60) c |
| 1 μM COR | 83.63 ± 7.65% (286/342) a | 94.83 ± 0.27% (110/116) a | 69.09 ± 0.46% (76/110) b | 23.68 ± 1.41% (18/76) b |
| 10 μM COR | 79.40 ± 7.49% (266/335) ab | 87.96 ± 2.04% (95/108) b | 60.00 ± 0.00% (57/95) c | 15.79 ± 1.37% (9/57) c |
| 100 μM COR | 71.64 ± 6.98% (245/342) c | 71.00 ± 9.94% (71/100) c | 47.89 ± 4.38% (34/71) e | 11.76 ± 0.80% (4/34) d |
| Vitrification control | 75.79 ± 7.49% (241/318) bc | 89.19 ± 1.66% (99/111) ab | 53.54 ± 2.94% (53/99) d | 13.21 ± 0.59% (7/53) cd |
| Fresh control | 76.07 ± 7.82% (248/326) bc | - | 84.76 ± 6.87% (89/105) a | 43.82 ± 3.59% (39/89) a |
| Groups | No. of MII | Survival Rate after vitrification | Cleavage Rate after IVF | Blastocyst Rate after IVF |
|---|---|---|---|---|
| 2.5 μM BER | 85.97 ± 9.58 % (239/278) b | 93.55 ± 2.71% (58/62) ab | 68.97 ± 7.34% (40/58) c | 22.50 ± 3.21% (9/40) c |
| 1 μM COR | 83.00 ± 8.46% (205/247) b | 86.21 ± 7.06% (75/87) b | 76.00 ± 7.23% (57/75) b | 21.05 ± 4.85% (12/57) c |
| 1 μM NMN | 93.08 ± 8.78% (269/289) a | 98.55 ± 2.25% (68/69) a | 79.41 ± 5.13% (54/68) ab | 31.48 ± 4.79% (17/54) b |
| Vitrification control | 73.23 ± 7.29% (186/254) c | 87.88 ± 7.13% (58/66) b | 50.00 ± 4.47% (29/58) d | 10.34 ± 2.75% (3/29) d |
| Fresh control | 75.46 ± 7.68% (203/269) c | - | 82.05 ± 5.83% (64/78) a | 42.19 ± 4.10% (27/64) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Yang, B.; Zhang, H.; Feng, X.; Hao, H.; Du, W.; Zhu, H.; Khan, A.; Khan, M.Z.; Zhang, P.; et al. Effects of β-Nicotinamide Mononucleotide, Berberine, and Cordycepin on Lipid Droplet Content and Developmental Ability of Vitrified Bovine Oocytes. Antioxidants 2023, 12, 991. https://doi.org/10.3390/antiox12050991
Xu X, Yang B, Zhang H, Feng X, Hao H, Du W, Zhu H, Khan A, Khan MZ, Zhang P, et al. Effects of β-Nicotinamide Mononucleotide, Berberine, and Cordycepin on Lipid Droplet Content and Developmental Ability of Vitrified Bovine Oocytes. Antioxidants. 2023; 12(5):991. https://doi.org/10.3390/antiox12050991
Chicago/Turabian StyleXu, Xi, Baigao Yang, Hang Zhang, Xiaoyi Feng, Haisheng Hao, Weihua Du, Huabin Zhu, Adnan Khan, Muhammad Zahoor Khan, Peipei Zhang, and et al. 2023. "Effects of β-Nicotinamide Mononucleotide, Berberine, and Cordycepin on Lipid Droplet Content and Developmental Ability of Vitrified Bovine Oocytes" Antioxidants 12, no. 5: 991. https://doi.org/10.3390/antiox12050991
APA StyleXu, X., Yang, B., Zhang, H., Feng, X., Hao, H., Du, W., Zhu, H., Khan, A., Khan, M. Z., Zhang, P., & Zhao, X. (2023). Effects of β-Nicotinamide Mononucleotide, Berberine, and Cordycepin on Lipid Droplet Content and Developmental Ability of Vitrified Bovine Oocytes. Antioxidants, 12(5), 991. https://doi.org/10.3390/antiox12050991








