NAT10, an RNA Cytidine Acetyltransferase, Regulates Ferroptosis in Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Transfection, and Treatment
2.2. Cell Viability Assay
2.3. Flow Cytometry
2.4. Biochemical Analysis
2.4.1. Glutathione Activity Assay
2.4.2. Lipid Peroxidation
2.4.3. Superoxide Dismutase Activity Assay
2.4.4. Catalase Activity Assay
2.5. Extraction for Metabolomics
2.6. Untargeted Metabolomics for Lipids Profiling
2.7. Total RNA Extraction, cDNA Synthesis and Quantitative RT-PCR
2.8. ac4C Dot Blot
2.9. RNA Immunoprecipitation (RIP)-PCR
2.10. mRNA Stability Assay
2.11. Bioinformatics Analysis
2.12. Statistical Analysis
3. Results
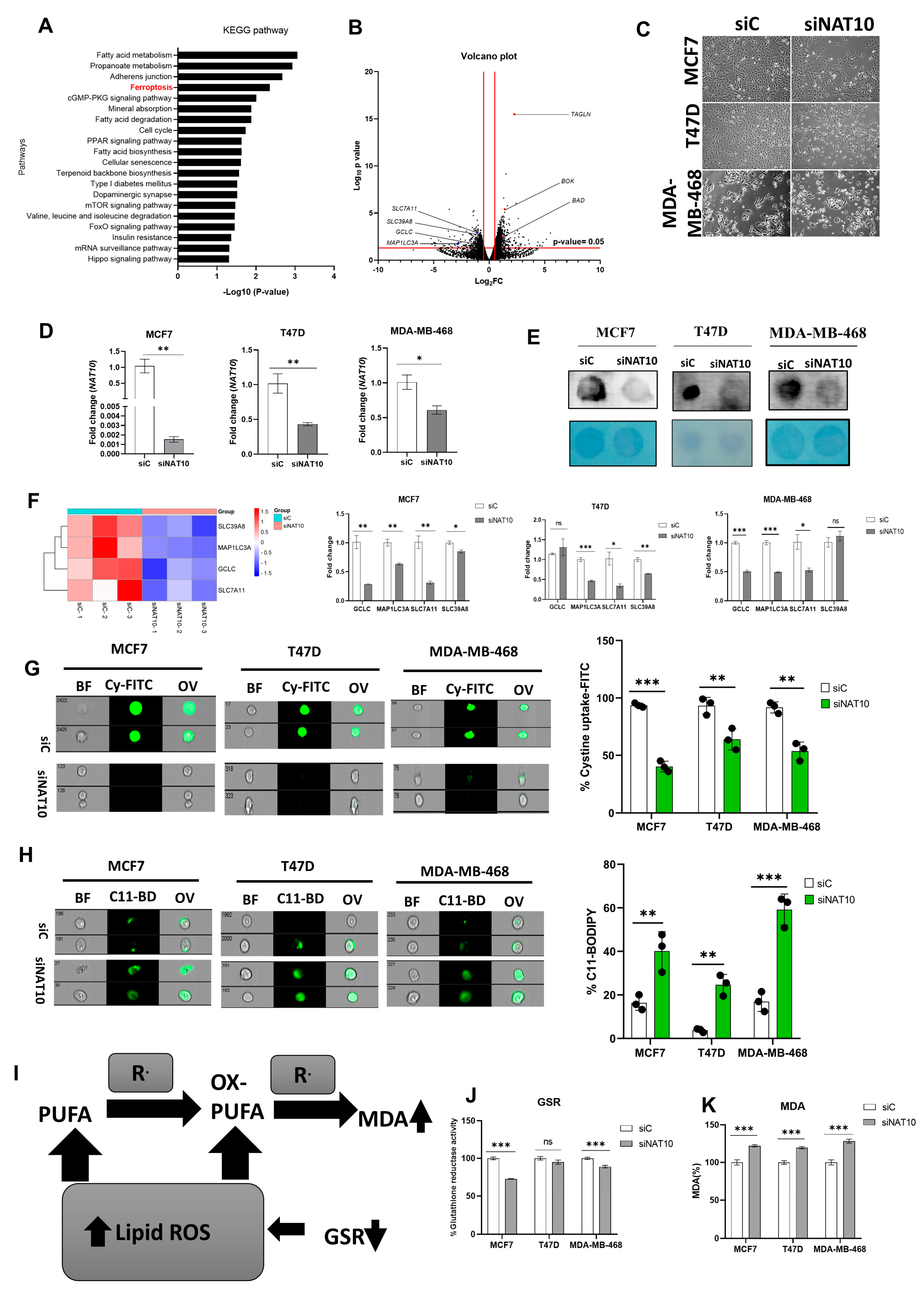
3.1. Depletion of NAT10 Induces Ferroptosis in Cancer Cells
3.2. Depletion of NAT10 Promotes Excessive Intracellular Production of PUFAs and oxPL
3.3. Depletion of NAT10 Induces Oxidative Stress in Cancer Cells
3.4. Depletion of NAT10 Induces Ferroptosis via the SLC7A11/GSH/PLOOH Axis in ac4C Dependent Manner
3.5. Remodelin, a Small Molecule Inhibitor of NAT10 Induces Ferroptosis
3.6. Remodelin Promotes Oxidative Stress in Cancer Cells
3.7. Remodelin Induces Ferroptosis via Reducing SLC7A11 RNA Acetylation and Stability
3.8. Clinicopathological Analysis Revealed Positive Correlation between NAT10 with SLC7A11 and GCLC in Breast Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arango, D.; Sturgill, D.; Alhusaini, N.; Dillman, A.A.; Sweet, T.J.; Hanson, G.; Hosogane, M.; Sinclair, W.R.; Nanan, K.K.; Mandler, M.D.; et al. Acetylation of Cytidine in mRNA Promotes Translation Efficiency. Cell 2018, 175, 1872–1886.e24. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Liu, X.; Zhang, C.; Xing, B.; Du, X. Autoacetylation of NAT10 is critical for its function in rRNA transcription activation. Biochem. Biophys. Res. Commun. 2017, 483, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Dalhat, M.H.; Altayb, H.N.; Khan, M.I.; Choudhry, H. Structural insights of human N-acetyltransferase 10 and identification of its potential novel inhibitors. Sci. Rep. 2021, 11, 6051. [Google Scholar] [CrossRef] [PubMed]
- Larrieu, D.; Britton, S.; Demir, M.; Rodriguez, R.; Jackson, S.P. Chemical inhibition of NAT10 corrects defects of laminopathic cells. Science 2014, 344, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ling, Y.; Gong, Y.; Sun, Y.; Hou, L.; Zhang, B. DNA damage induces N-acetyltransferase NAT10 gene expression through transcriptional activation. Mol. Cell Biochem. 2007, 300, 249–258. [Google Scholar] [CrossRef]
- Liu, H.Y.; Liu, Y.Y.; Yang, F.; Zhang, L.; Zhang, F.L.; Hu, X.; Shao, Z.M.; Li, D.Q. Acetylation of MORC2 by NAT10 regulates cell-cycle checkpoint control and resistance to DNA-damaging chemotherapy and radiotherapy in breast cancer. Nucleic Acids Res. 2020, 48, 3638–3656. [Google Scholar] [CrossRef]
- Liu, H.Y.; Liu, Y.Y.; Zhang, Y.L.; Ning, Y.; Zhang, F.L.; Li, D.Q. Poly(ADP-ribosyl)ation of acetyltransferase NAT10 by PARP1 is required for its nucleoplasmic translocation and function in response to DNA damage. Cell Commun. Signal. 2022, 20, 127. [Google Scholar] [CrossRef]
- Cao, Y.; Yao, M.; Wu, Y.; Ma, N.; Liu, H.; Zhang, B. N-Acetyltransferase 10 Promotes Micronuclei Formation to Activate the Senescence-Associated Secretory Phenotype Machinery in Colorectal Cancer Cells. Transl. Oncol. 2020, 13, 100783. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Z.; Sun, S.; Xie, S.; Jiang, M.; Chen, B.; Gu, C.; Yang, Y. NAT10 acetylates BCL-XL mRNA to promote the proliferation of multiple myeloma cells through PI3K-AKT pathway. Front. Oncol. 2022, 12, 967811. [Google Scholar] [CrossRef]
- Wei, R.; Cui, X.; Min, J.; Lin, Z.; Zhou, Y.; Guo, M.; An, X.; Liu, H.; Janz, S.; Gu, C.; et al. NAT10 promotes cell proliferation by acetylating CEP170 mRNA to enhance translation efficiency in multiple myeloma. Acta Pharm. Sin. B 2022, 12, 3313–3325. [Google Scholar] [CrossRef]
- Zi, J.; Han, Q.; Gu, S.; McGrath, M.; Kane, S.; Song, C.; Ge, Z. Targeting NAT10 Induces Apoptosis Associated With Enhancing Endoplasmic Reticulum Stress in Acute Myeloid Leukemia Cells. Front. Oncol. 2020, 10, 598107. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, C.; Liu, Z.; He, X.; Tang, W.; He, L.; Feng, Y.; Liu, D.; Yin, Y.; Li, T. Ferroptosis and Its Multifaceted Role in Cancer: Mechanisms and Therapeutic Approach. Antioxidants 2022, 11, 1504. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Nam, M.; Son, H.Y.; Hyun, K.; Jang, S.Y.; Kim, J.W.; Kim, M.W.; Jung, Y.; Jang, E.; Yoon, S.J.; et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 32433–32442. [Google Scholar] [CrossRef]
- Hu, W.; Liang, K.; Zhu, H.; Zhao, C.; Hu, H.; Yin, S. Ferroptosis and Its Role in Chronic Diseases. Cells 2022, 11, 40. [Google Scholar] [CrossRef]
- Li, F.J.; Long, H.Z.; Zhou, Z.W.; Luo, H.Y.; Xu, S.G.; Gao, L.C. System Xc (-)/GSH/GPX4 axis: An important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy. Front Pharm. 2022, 13, 910292. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Chen, A.; Li, L.; Liang, Q.; Wang, S.; Dong, Q.; Fu, M.; Lan, Z.; Li, Y.; Liu, X.; et al. Repression of the antiporter SLC7A11/glutathione/glutathione peroxidase 4 axis drives ferroptosis of vascular smooth muscle cells to facilitate vascular calcification. Kidney Int. 2022, 102, 1259–1275. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, P.; Li, Z.; Xu, C.; Wang, H.; Jia, B.; Gong, A.; Xu, M. Vitamin C Sensitizes Pancreatic Cancer Cells to Erastin-Induced Ferroptosis by Activating the AMPK/Nrf2/HMOX1 Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 5361241. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Shen, Z.; Lu, Y.; Sun, F.; Shi, H. p53 Promotes Ferroptosis in Macrophages Treated with Fe3O4 Nanoparticles. ACS Appl. Mater. Interfaces 2022, 14, 42791–42803. [Google Scholar] [CrossRef]
- Xuan, W.; Lu, X.; Yang, Z.; Li, J.; Jin, W.; Li, Y. Propofol Protects Against Erastin-Induced Ferroptosis in HT-22 Cells. J. Mol. Neurosci. 2022, 72, 1797–1808. [Google Scholar] [CrossRef]
- Dalhat, M.H.; Mohammed, M.R.S.; Ahmad, A.; Khan, M.I.; Choudhry, H. Remodelin, a N-acetyltransferase 10 (NAT10) inhibitor, alters mitochondrial lipid metabolism in cancer cells. J. Cell. Biochem. 2021, 122, 1936–1945. [Google Scholar] [CrossRef]
- Dalhat, M.H.; Mohammed, M.R.S.; Alkhatabi, H.A.; Rehan, M.; Ahmad, A.; Choudhry, H.; Khan, M.I. NAT10: An RNA cytidine transferase regulates fatty acid metabolism in cancer cells. Clin. Transl. Med. 2022, 12, e1045. [Google Scholar] [CrossRef] [PubMed]
- Abdulrahman, A.O.; Kuerban, A.; Alshehri, Z.A.; Abdulaal, W.H.; Khan, J.A.; Khan, M.I. Urolithins Attenuate Multiple Symptoms of Obesity in Rats Fed on a High-Fat Diet. Diabetes Metab. Syndr. Obes. 2020, 13, 3337–3348. [Google Scholar] [CrossRef] [PubMed]
- Ratnadiwakara, M.; Anko, M.L. mRNA Stability Assay Using transcription inhibition by Actinomycin D in Mouse Pluripotent Stem Cells. Bio Protoc. 2018, 8, e3072. [Google Scholar] [CrossRef] [PubMed]
- Ahmadpour, S.T.; Maheo, K.; Servais, S.; Brisson, L.; Dumas, J.F. Cardiolipin, the Mitochondrial Signature Lipid: Implication in Cancer. Int. J. Mol. Sci. 2020, 21, 8031. [Google Scholar] [CrossRef]
- Rawling, T.; Choucair, H.; Koolaji, N.; Bourget, K.; Allison, S.E.; Chen, Y.J.; Dunstan, C.R.; Murray, M. A Novel Arylurea Fatty Acid That Targets the Mitochondrion and Depletes Cardiolipin To Promote Killing of Breast Cancer Cells. J. Med. Chem. 2017, 60, 8661–8666. [Google Scholar] [CrossRef]
- Ren, M.; Phoon, C.K.; Schlame, M. Metabolism and function of mitochondrial cardiolipin. Prog. Lipid Res. 2014, 55, 1–16. [Google Scholar] [CrossRef]
- Aslan, M.; Afsar, E.; Kirimlioglu, E.; Ceker, T.; Yilmaz, C. Antiproliferative Effects of Thymoquinone in MCF-7 Breast and HepG2 Liver Cancer Cells: Possible Role of Ceramide and ER Stress. Nutr. Cancer 2021, 73, 460–472. [Google Scholar] [CrossRef]
- James, B.N.; Oyeniran, C.; Sturgill, J.L.; Newton, J.; Martin, R.K.; Bieberich, E.; Weigel, C.; Maczis, M.A.; Palladino, E.N.D.; Lownik, J.C.; et al. Ceramide in apoptosis and oxidative stress in allergic inflammation and asthma. J. Allergy Clin. Immunol. 2021, 147, 1936–1948.e9. [Google Scholar] [CrossRef]
- Jeffries, K.A.; Krupenko, N.I. Ceramide Signaling and p53 Pathways. Adv. Cancer Res. 2018, 140, 191–215. [Google Scholar] [CrossRef]
- Wiernicki, B.; Dubois, H.; Tyurina, Y.Y.; Hassannia, B.; Bayir, H.; Kagan, V.E.; Vandenabeele, P.; Wullaert, A.; Vanden Berghe, T. Excessive phospholipid peroxidation distinguishes ferroptosis from other cell death modes including pyroptosis. Cell Death Dis. 2020, 11, 922. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.; Zhou, J.; Gu, J.; Wu, H.; Jiang, Y.; Gao, S.; Liao, Y.; Shen, R.; Miao, C.; et al. NAT10 regulates neutrophil pyroptosis in sepsis via acetylating ULK1 RNA and activating STING pathway. Commun. Biol. 2022, 5, 916. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cao, L.; Han, K.; Zhang, H.; Cui, J.; Ma, X.; Zhao, S.; Zhao, C.; Yin, S.; Fan, L.; et al. Patulin disrupts SLC7A11-cystine-cysteine-GSH antioxidant system and promotes renal cell ferroptosis both in vitro and in vivo. Food Chem. Toxicol. 2022, 166, 113255. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, C.; Han, D.; Zhou, W.; Cui, Y.; Tang, X.; Xiao, C.; Wang, Y.; Gao, Y. SLC7A11/GPX4 Inactivation-Mediated Ferroptosis Contributes to the Pathogenesis of Triptolide-Induced Cardiotoxicity. Oxid. Med. Cell. Longev. 2022, 2022, 3192607. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wei, D.G.; Li, R.S. Capsaicin induces ferroptosis of NSCLC by regulating SLC7A11/GPX4 signaling in vitro. Sci. Rep. 2022, 12, 11996. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Fan, Y.Q.; Liu, B.H.; Zhou, H.; Wang, J.M.; Chen, Q.X. ACSL4 suppresses glioma cells proliferation via activating ferroptosis. Oncol. Rep. 2020, 43, 147–158. [Google Scholar] [CrossRef]
- Doll, S.; Proneth, B.; Tyurina, Y.Y.; Panzilius, E.; Kobayashi, S.; Ingold, I.; Irmler, M.; Beckers, J.; Aichler, M.; Walch, A.; et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 2017, 13, 91–98. [Google Scholar] [CrossRef]
- Gan, B. ACSL4, PUFA, and ferroptosis: New arsenal in anti-tumor immunity. Signal Transduct. Target 2022, 7, 128. [Google Scholar] [CrossRef]
- Guo, N. Identification of ACSL4 as a biomarker and contributor of ferroptosis in clear cell renal cell carcinoma. Transl. Cancer Res. 2022, 11, 2688–2699. [Google Scholar] [CrossRef]
- Liu, L.; Kang, X.X. ACSL4 is overexpressed in psoriasis and enhances inflammatory responses by activating ferroptosis. Biochem. Biophys. Res. Commun. 2022, 623, 1–8. [Google Scholar] [CrossRef]
- Grube, J.; Woitok, M.M.; Mohs, A.; Erschfeld, S.; Lynen, C.; Trautwein, C.; Otto, T. ACSL4-dependent ferroptosis does not represent a tumor-suppressive mechanism but ACSL4 rather promotes liver cancer progression. Cell Death Dis. 2022, 13, 704. [Google Scholar] [CrossRef]
- Lee, H.; Gan, B. Ferroptosis execution: Is it all about ACSL4? Cell Chem. Biol. 2022, 29, 1363–1365. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhang, Y.; Shi, J.; Li, W.; Gan, B. The glutamate/cystine antiporter SLC7A11/xCT enhances cancer cell dependency on glucose by exporting glutamate. J. Biol. Chem. 2017, 292, 14240–14249. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dalhat, M.H.; Choudhry, H.; Khan, M.I. NAT10, an RNA Cytidine Acetyltransferase, Regulates Ferroptosis in Cancer Cells. Antioxidants 2023, 12, 1116. https://doi.org/10.3390/antiox12051116
Dalhat MH, Choudhry H, Khan MI. NAT10, an RNA Cytidine Acetyltransferase, Regulates Ferroptosis in Cancer Cells. Antioxidants. 2023; 12(5):1116. https://doi.org/10.3390/antiox12051116
Chicago/Turabian StyleDalhat, Mahmood Hassan, Hani Choudhry, and Mohammad Imran Khan. 2023. "NAT10, an RNA Cytidine Acetyltransferase, Regulates Ferroptosis in Cancer Cells" Antioxidants 12, no. 5: 1116. https://doi.org/10.3390/antiox12051116
APA StyleDalhat, M. H., Choudhry, H., & Khan, M. I. (2023). NAT10, an RNA Cytidine Acetyltransferase, Regulates Ferroptosis in Cancer Cells. Antioxidants, 12(5), 1116. https://doi.org/10.3390/antiox12051116






