Human Sperm as an In Vitro Model to Assess the Efficacy of Antioxidant Supplements during Sperm Handling: A Narrative Review
Abstract
1. Introduction
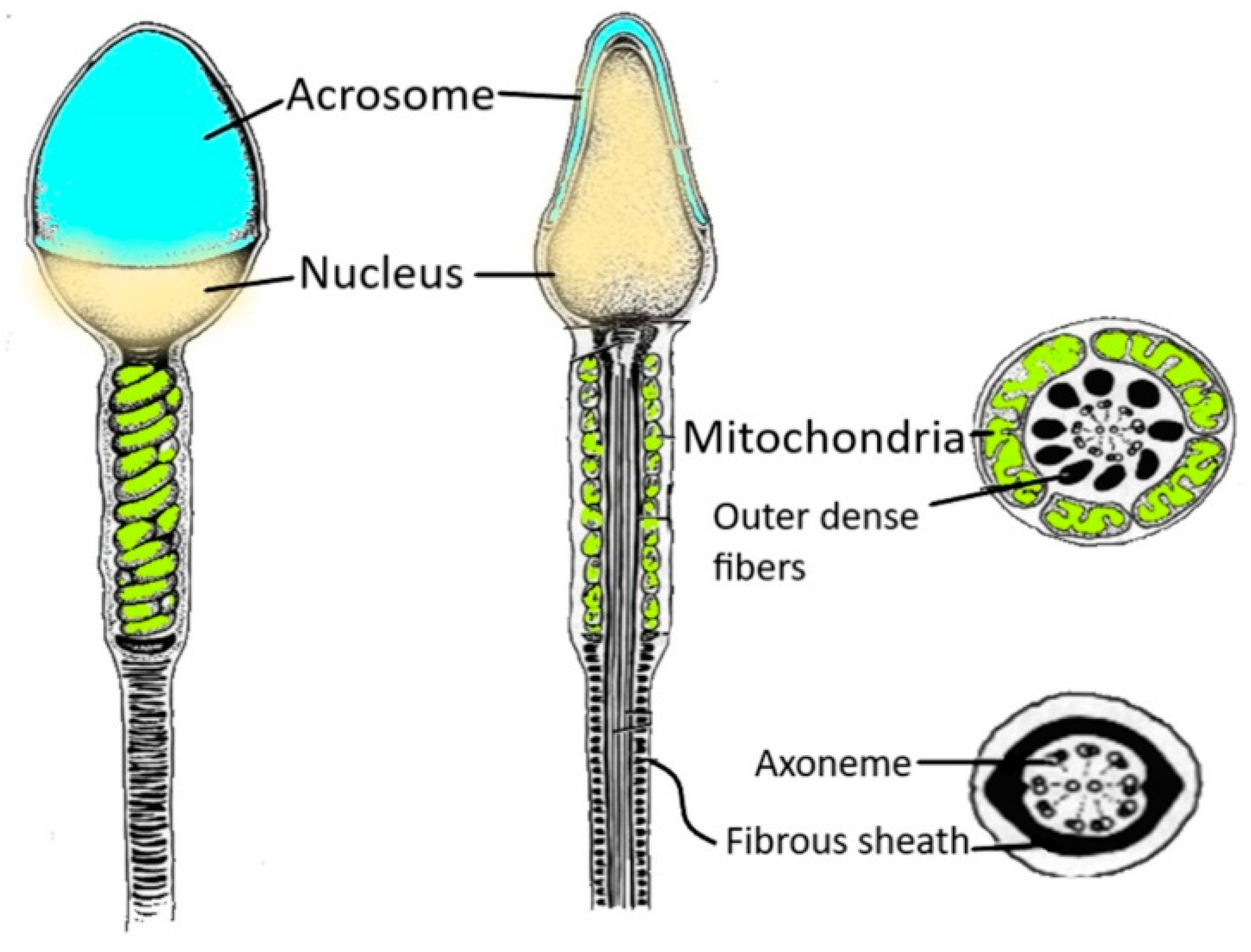
2. Structure of Human Spermatozoa
3. Main Items of OS
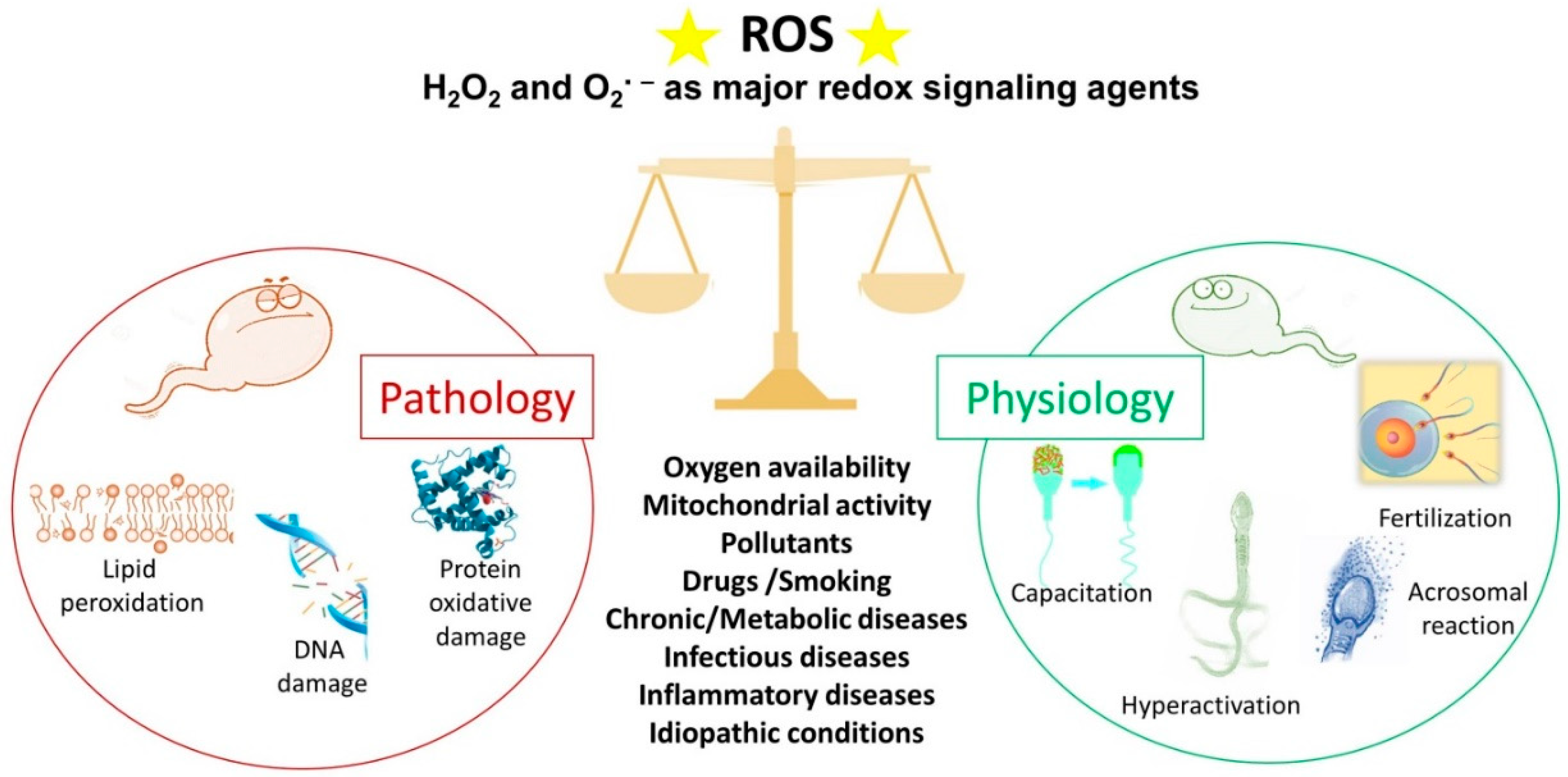
4. Sperm and ROS: An Ambivalent Relationship
4.1. ROS and Sperm Physiology
4.2. ROS and Sperm Pathology
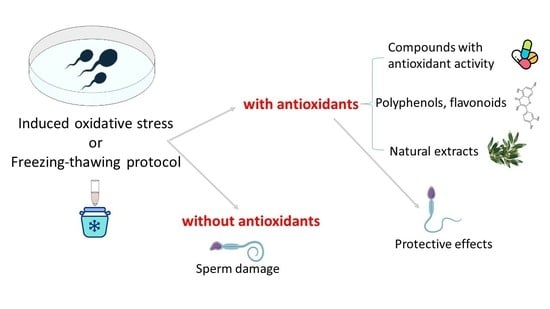
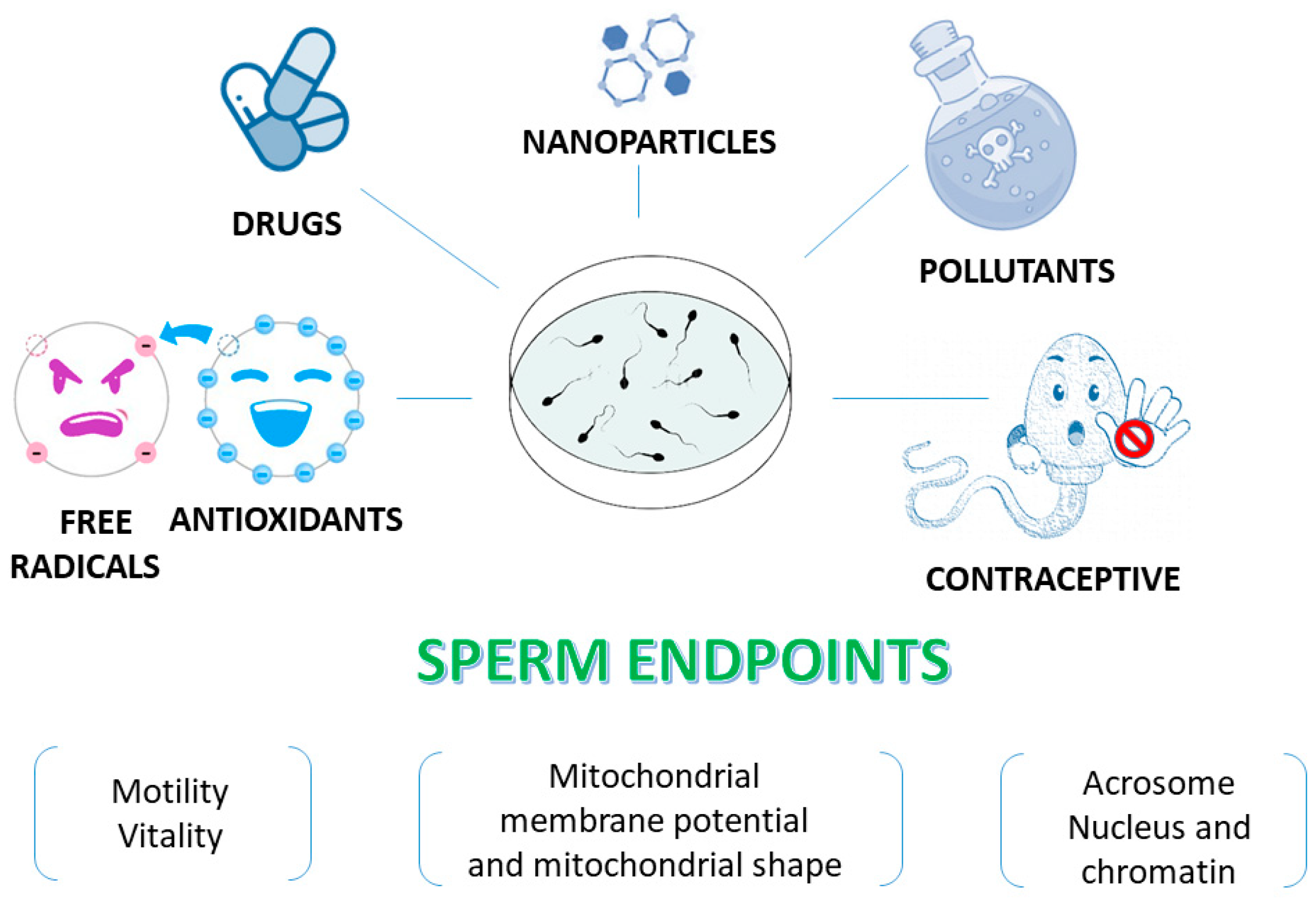
5. Human Spermatozoa as a Model for In Vitro Studies
6. Human Sperm as a Model to Assess the Antioxidant Activity of Different Compounds
| Investigated Compounds | Effects | Freezing Protocol |
|---|---|---|
| Pentoxifylline | Motility positive effect [114,116,118,119,120,121,122] * Antioxidant activity [115,117] | [123] b, [177] b, [178] ab, [179] b |
| Papaverine | Motility positive effect [131] | [131] a |
| Zinc | Motility positive effect [132] Antioxidant activity [132,139,140,141] | [180] b, [181] b |
| Selenium | Motility positive effect [133] Antioxidant activity [133] | |
| Vitamin E (α tocopherol, liposoluble) Trolox (hydrosoluble) | Motility positive effect [135,136] Motility negative effect [137] Antioxidant activity [135,144,145,146,147] | [182] b, [183] b, [184] b, [185] b, [186] b |
| D-aspartate | Motility positive effect [132] Antioxidant activity [132] | |
| Ascorbic acid | Motility positive effect [138] Motility negative effect [137]; Antioxidant activity [138,144,148] | [187] b, [188] b, [189] b |
| N-acetylcysteine | [190] b, [191] b | |
| S-glutathione | Antioxidant activity [152] | [196] ab, [197] b |
| Hypotaurine | Antioxidant activity [152] | [198] b |
| Oleoylethanolamide | Motility positive effect [153] Antioxidant activity [153] | |
| Ethylenediaminetetraacetic acid (EDTA) | Motility positive effect [154] Antioxidant activity [154] | |
| Catalase | Motility positive effect [154] Antioxidant activity [154] | [188] b, [199] b, [200] b |
| Coenzyme Q | Motility positive effect [132,143] Antioxidant activity [132,144] | [194] b |
| L-carnitine | Motility positive effect [134] | [190] b, [192] b, [193] b, [194] b, [195] b |
| Phosphatidylcholine | [195] b | |
| Myoinositol/Inositol | Motility positive effect [157,158,159,163] | [160] b, [161] b, [162] b, [164] ab, [165] b |
| Melatonin | Motility positive effect [166,170,171,172,174,176] Antioxidant activity [166,171,172] Anti-apoptotic effect [171,176] | [167] b, [168] b, [169] ab, [175] b |
| Curcumin | Antioxidant activity [87] | [201] b, [202] b, [203] b |
| Lycopene | Antioxidant activity [155,156] |
| Investigated Flavonoids and Polyphenols | Effects | Freezing Protocol |
|---|---|---|
| Rosmarinic acid | Negative effect on motility and acrosome reaction [226] | |
| Quercetin | Negative effect on motility [225] Positive effect on sperm motility [210,213,214] * Positive effects on membrane integrity, sperm vitality, acrosome [213,214] * Positive effect on DNA integrity [145] Negative effect on DNA integrity [214] * Antioxidant activity [85] | [219] b, [220] b, [223] b |
| Genistein | Positive effects on membrane integrity [224] | [217] a |
| Equol | Positive effect on DNA integrity [215] | |
| Rutin | Positive effect on sperm motility, membrane integrity, sperm vitality [213] Positive effect on DNA integrity [145] | |
| Naringenin | Positive effect on sperm motility, membrane integrity, sperm vitality [213] Positive effect on DNA integrity [145] | |
| Epigallocatechin-3-gallate | Antioxidant activity [207] | |
| Hydroxytyrosol | Positive effect on sperm motility, vitality, DNA integrity [211] Antioxidant activity [211] | |
| Caffeic acid phenethyl ester | Positive effect on DNA integrity [208] Antioxidant activity [208] | |
| Procyanidine | Positive effect on sperm motility [209] | [209] b |
| Ellagic acid | Positive effect on sperm motility, vitality, DNA integrity [212] | |
| Resveratrol | Positive effect on sperm motility [85,218] Positive effect on membrane integrity [224] Antioxidant activity [85] | [187] b, [216] b, [218] b, [221] b, [222] b |
| Cholorogenic acid | Positive effect on sperm motility, vitality, DNA integrity [88] | [88] b |
| Natural Extract | Extract Characterization and Effects on Human Sperm | Freezing Protocol |
|---|---|---|
| Propolfenol® | Extract characterization, antioxidant activity [239] | |
| Chilean propolis ethanolic extract | Positive effect on DNA integrity, antioxidant activity [240] | |
| Withania somnifera aqueous ethanol extract | Extract characterization, positive effect on sperm parameters, antioxidant activity [91] | |
| Prunus japonica seed ethanolic extract | Extract characterization, positive effect on sperm parameters [242] | |
| Moringa oleifera aqueous extract | Positive effect on sperm parameters, DNA integrity, antioxidant activity [238] | |
| Capparis spinosa L. hydroalcoholic extract | Extract characterization, positive effect on sperm parameters, DNA integrity [245] | |
| Origanum vulgare | Positive effect on sperm parameter [237] essential oil obtained by hydrodistillation | [251] b aqueous extract |
| Castanea sativa Mill. ethanolic extract | Extract characterization, positive effect on sperm parameters, antioxidant activity [241] | |
| Eruca sativa aqueous extract | Extract characterization, positive effect on sperm parameters, antioxidant activity [236] | |
| Tribulus terrestris aqueous extract | Positive effect on sperm parameters [234] | [249] ab |
| Terminalia arjuna bark aqueous extract | Extract characterization, positive effect on sperm parameters, antioxidant activity [235] | [250] b |
| Morinda officinalis | Extract characterization, positive effect on DNA integrity, antioxidant activity [244] | |
| Date seed oil | Positive effect on sperm parameters, DNA integrity, antioxidant activity [248] | |
| Mondia whitei aqueous extract | Positive effect on sperm parameters [233] | |
| Aqueous extract of herbal medicines | Positive effect on sperm parameters [232] * | |
| The red alga Gelidiella acerosa | Extract characterization, positive effect on sperm parameters [243] | |
| Opuntia ficus-indica | Extract characterization [218] | [218] b, [252] a |
7. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; WHO Press: Geneva, Switzerland, 2021. [Google Scholar]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Nieschlag, E.; Lenzi, A. The conventional management of male infertility. Int. J. Gynaecol. Obstet. 2013, 123 (Suppl. S2), S31–S35. [Google Scholar] [CrossRef] [PubMed]
- Leslie, S.W.; Soon-Sutton, T.L.; Khan, M.A.B. Male infertility. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Wang, K.; Gao, Y.; Wang, C.; Liang, M.; Liao, Y.; Hu, K. Role of oxidative stress in varicocele. Front. Genet. 2022, 13, 850114. [Google Scholar] [CrossRef] [PubMed]
- Pellati, D.; Mylonakis, I.; Bertoloni, G.; Fiore, C.; Andrisani, A.; Ambrosini, G.; Armanini, D. Genital tract infections and infertility. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 140, 3–11. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Yao, Y.; Li, J.; Deng, S. Bacterial infections affect male fertility: A focus on the oxidative stress-autophagy axis. Front. Cell Dev. Biol. 2021, 9, 727812. [Google Scholar] [CrossRef]
- Walczak-Jedrzejowska, R.; Wolski, J.K.; Slowikowska-Hilczer, J. The role of oxidative stress and antioxidants in male fertility. Cent. Eur. J. Urol. 2013, 66, 60–67. [Google Scholar] [CrossRef]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Longobardi, S.; Di Rella, F.; Adiga, S.K.; Talevi, R. Sperm oxidative stress during in vitro manipulation and its effects on sperm function and embryo development. Antioxidants 2021, 10, 1025. [Google Scholar] [CrossRef]
- Aitken, R.J.; Gibb, Z.; Baker, M.A.; Drevet, J.; Gharagozloo, P. Causes and consequences of oxidative stress in spermatozoa. Reprod. Fertil. Dev. 2016, 28, 1–10. [Google Scholar] [CrossRef]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative stress and sperm function: A systematic review on evaluation and management. Arab J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R.; Moazamian, A.; Gharagozloo, P. Male infertility and oxidative stress: A focus on the underlying mechanisms. Antioxidants 2022, 11, 306. [Google Scholar] [CrossRef]
- Ford, W.C. Regulation of sperm function by reactive oxygen species. Hum. Reprod. Update 2004, 10, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Milne, S.; Leeson, H. Sperm DNA damage caused by oxidative stress: Modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod. Biomed. Online 2014, 28, 684–703. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.A.; Mohanty, T.K.; Baithalu, R.K.; Yadav, H.P. Sperm protein carbonylation. Andrologia 2019, 51, e13233. [Google Scholar] [CrossRef]
- Agarwal, A.; Maldonado Rosas, I.; Anagnostopoulou, C.; Cannarella, R.; Boitrelle, F.; Munoz, L.V.; Finelli, R.; Durairajanayagam, D.; Henkel, R.; Saleh, R. Oxidative stress and assisted reproduction: A comprehensive review of its pathophysiological role and strategies for optimizing embryo culture environment. Antioxidants 2022, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Martin-Hidalgo, D.; Bragado, M.J.; Batista, A.R.; Oliveira, P.F.; Alves, M.G. Antioxidants and male fertility: From molecular studies to clinical evidence. Antioxidants 2019, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Symeonidis, E.N.; Evgeni, E.; Palapelas, V.; Koumasi, D.; Pyrgidis, N.; Sokolakis, I.; Hatzichristodoulou, G.; Tsiampali, C.; Mykoniatis, I.; Zachariou, A.; et al. Redox balance in male infertility: Excellence through moderation—“Μέτρον ἄριστον”. Antioxidants 2021, 10, 1534. [Google Scholar] [CrossRef]
- Amidi, F.; Pazhohan, A.; Shabani Nashtaei, M.; Khodarahmian, M.; Nekoonam, S. The role of antioxidants in sperm freezing: A review. Cell Tissue Bank 2016, 17, 745–756. [Google Scholar] [CrossRef]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The role of resveratrol in mammalian reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef]
- Abou-Haila, A.; Tulsiani, D.R. Mammalian sperm acrosome: Formation, contents, and function. Arch. Biochem. Biophys. 2000, 379, 173–182. [Google Scholar] [CrossRef]
- Bowker, Z.; Goldstein, S.; Breitbart, H. Protein acetylation protects sperm from spontaneous acrosome reaction. Theriogenology 2022, 191, 231–238. [Google Scholar] [CrossRef]
- Fujihara, Y.; Murakami, M.; Inoue, N.; Satouh, Y.; Kaseda, K.; Ikawa, M.; Okabe, M. Sperm equatorial segment protein 1, SPESP1, is required for fully fertile sperm in mouse. J. Cell Sci. 2010, 123 Pt 9, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Kashir, J.; Heindryckx, B.; Jones, C.; De Sutter, P.; Parrington, J.; Coward, K. Oocyte activation, phospholipase C zeta and human infertility. Hum. Reprod. Update 2010, 16, 690–703. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Kashir, J.; Thanassoulas, A.; Safieh-Garabedian, B.; Lai, F.A.; Nomikos, M. Essential role of sperm-specific PLC-zeta in egg activation and male factor infertility: An update. Front. Cell Dev. Biol. 2020, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.S. Organization of sperm DNA by the nuclear matrix. Am. J. Clin. Exp. Urol. 2018, 6, 87–92. [Google Scholar]
- Ribas-Maynou, J.; Nguyen, H.; Wu, H.; Ward, W.S. Functional aspects of sperm chromatin organization. Results Probl. Cell Differ. 2022, 70, 295–311. [Google Scholar] [CrossRef]
- Chemes, H.E.; Rawe, V.Y. The making of abnormal spermatozoa: Cellular and molecular mechanisms underlying pathological spermiogenesis. Cell Tissue Res. 2010, 341, 349–357. [Google Scholar] [CrossRef]
- Avidor-Reiss, T.; Carr, A.; Fishman, E.L. The sperm centrioles. Mol. Cell Endocrinol. 2020, 518, 110987. [Google Scholar] [CrossRef]
- Zabeo, D.; Heumann, J.M.; Schwartz, C.L.; Suzuki-Shinjo, A.; Morgan, G.; Widlund, P.O.; Höög, J.L. A lumenal interrupted helix in human sperm tail microtubules. Sci. Rep. 2018, 8, 2727. [Google Scholar] [CrossRef]
- Boguenet, M.; Bouet, P.E.; Spiers, A.; Reynier, P.; May-Panloup, P. Mitochondria: Their role in spermatozoa and in male infertility. Hum. Reprod. Update 2021, 27, 697–719. [Google Scholar] [CrossRef]
- Toure, A.; Rode, B.; Hunnicutt, G.R.; Escalier, D.; Gacon, G. Septins at the annulus of mammalian sperm. Biol. Chem. 2011, 392, 799–803. [Google Scholar] [CrossRef]
- Kim, Y.H.; Haidl, G.; Schaefer, M.; Egner, U.; Mandal, A.; Herr, J.C. Compartmentalization of a unique ADP/ATP carrier protein SFEC (Sperm Flagellar Energy Carrier, AAC4) with glycolytic enzymes in the fibrous sheath of the human sperm flagellar principal piece. Dev. Biol. 2007, 302, 463–476. [Google Scholar] [CrossRef]
- James, E.R.; Carrell, D.T.; Aston, K.I.; Jenkins, T.G.; Yeste, M.; Salas-Huetos, A. The Role of the Epididymis and the Contribution of Epididymosomes to Mammalian Reproduction. Int. J. Mol. Sci. 2020, 21, 5377. [Google Scholar] [CrossRef]
- Barrachina, F.; Battistone, M.A.; Castillo, J.; Mallofré, C.; Jodar, M.; Breton, S.; Oliva, R. Sperm acquire epididymis-derived proteins through epididymosomes. Hum. Reprod. 2022, 37, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Prabhakar, N.R.; Semenza, G.L. Systems biology of oxygen homeostasis. Wiley Interdiscip. Rev. Syst. Biol. Med. 2017, 9, e1382. [Google Scholar] [CrossRef]
- Hadanny, A.; Efrati, S. The hyperoxic-hypoxic paradox. Biomolecules 2020, 10, 958. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Belousov, V.V.; Chandel, N.S.; Davies, M.J.; Jones, D.P.; Mann, G.E.; Murphy, M.P.; Yamamoto, M.; Winterbourn, C. Defining roles of specific reactive oxygen species (ROS) in cell biology and physiology. Nat. Rev. Mol. Cell Biol. 2022, 23, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Torres, M.; Fukuto, J. Redox signaling. Mol. Cell Biochem. 2002, 234–235, 49–62. [Google Scholar] [CrossRef]
- Forman, H.J.; Ursini, F.; Maiorino, M. An overview of mechanisms of redox signaling. J. Mol. Cell Cardiol. 2014, 73, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Ursini, F. Homeostatic control of redox status and health. IUBMB Life 2022, 74, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Biochemistry of oxidative stress. Angew. Chem. Int. Ed. Engl. 1986, 25, 1058–1071. [Google Scholar] [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms, and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar] [CrossRef]
- Davies, K.J. Oxidative stress: The paradox of aerobic life. Biochem. Soc. Symp. 1995, 61, 1–31. [Google Scholar] [CrossRef]
- Mannucci, A.; Argento, F.R.; Fini, E.; Coccia, M.E.; Taddei, N.; Becatti, M.; Fiorillo, C. The impact of oxidative stress in male infertility. Front. Mol. Biosci. 2022, 8, 799294. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Takei, G.L.; Tourzani, D.A.; Paudel, B.; Visconti, P.E. Activation of cAMP-dependent phosphorylation pathways is independent of ROS production during mouse sperm capacitation. Mol. Reprod. Dev. 2021, 88, 544–557. [Google Scholar] [CrossRef]
- O’Flaherty, C. Redox regulation of mammalian sperm capacitation. Asian J. Androl. 2015, 17, 583–590. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, C. Biological basis for human capacitation-revisited. Hum. Reprod. Update 2017, 23, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Carrasquel Martínez, G.; Aldana, A.; Carneiro, J.; Treviño, C.L.; Darszon, A. Acrosomal alkalinization occurs during human sperm capacitation. Mol. Hum. Reprod. 2022, 28, gaac005. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Bermúdez, A.; Yeste, M.; Bonet, S.; Pinart, E. A review on the role of bicarbonate and proton transporters during sperm capacitation in mammals. Int. J. Mol. Sci. 2022, 23, 6333. [Google Scholar] [CrossRef]
- Herrero, M.B.; Chatterjee, S.; Lefièvre, L.; de Lamirande, E.; Gagnon, C. Nitric oxide interacts with the cAMP pathway to modulate capacitation of human spermatozoa. Free Radic. Biol. Med. 2000, 29, 522–536. [Google Scholar] [CrossRef]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Positive role of reactive oxygen species in mammalian sperm capacitation: Triggering and modulation of phosphorylation events. Free Radic. Biol. Med. 2006, 41, 528–540. [Google Scholar] [CrossRef]
- Cordero-Martínez, J.; Jimenez-Gutierrez, G.E.; Aguirre-Alvarado, C.; Alacántara-Farfán, V.; Chamorro-Cevallos, G.; Roa-Espitia, A.L.; Hernández-González, E.O.; Rodríguez-Páez, L. Participation of signaling proteins in sperm hyperactivation. Syst. Biol. Reprod. Med. 2022, 68, 315–330. [Google Scholar] [CrossRef]
- de Lamirande, E.; Gagnon, C. Human sperm hyperactivation and capacitation as parts of an oxidative process. Free Radic. Biol. Med. 1993, 14, 157–166. [Google Scholar] [CrossRef]
- Suarez, S.S. Control of hyperactivation in sperm. Hum. Reprod. Update 2008, 14, 647–657. [Google Scholar] [CrossRef]
- de Lamirande, E.; Tsai, C.; Harakat, A.; Gagnon, C. Involvement of reactive oxygen species in human sperm arcosome reaction induced by A23187, lysophosphatidylcholine, and biological fluid ultrafiltrates. J. Androl. 1998, 19, 585–594. [Google Scholar]
- Rivlin, J.; Mendel, J.; Rubinstein, S.; Etkovitz, N.; Breitbart, H. Role of hydrogen peroxide in sperm capacitation and acrosome reaction. Biol. Reprod. 2004, 70, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Cheng, J.W.; Ko, E.Y. Role of reactive oxygen species in male infertility: An updated review of literature. Arab J. Urol. 2017, 16, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Drevet, J.R. The importance of oxidative stress in determining the functionality of mammalian spermatozoa: A two-edged sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Peroxiredoxin 6: The protector of male fertility. Antioxidants 2018, 7, 173. [Google Scholar] [CrossRef]
- Micheli, L.; Cerretani, D.; Collodel, G.; Menchiari, A.; Moltoni, L.; Fiaschi, A.I.; Moretti, E. Evaluation of enzymatic and non-enzymatic antioxidants in seminal plasma of men with genitourinary infections, varicocele and idiopathic infertility. Andrology 2016, 4, 456–464. [Google Scholar] [CrossRef]
- Lazzarino, G.; Listorti, I.; Bilotta, G.; Capozzolo, T.; Amorini, A.M.; Longo, S.; Caruso, G.; Lazzarino, G.; Tavazzi, B.; Bilotta, P. Water- and fat-soluble antioxidants in human seminal plasma and serum of fertile males. Antioxidants 2019, 8, 96. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Oxidative stress, spermatozoa and leukocytic infiltration: Relationships forged by the opposing forces of microbial invasion and the search for perfection. J. Reprod. Immunol. 2013, 100, 11–19. [Google Scholar] [CrossRef]
- Henkel, R.R. Leukocytes and oxidative stress: Dilemma for sperm function and male fertility. Asian J. Androl. 2011, 13, 43–52. [Google Scholar] [CrossRef]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, e13126. [Google Scholar] [CrossRef]
- Gil-Guzman, E.; Ollero, M.; Lopez, M.C.; Sharma, R.K.; Alvarez, J.G.; Thomas, A.J., Jr.; Agarwal, A. Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum. Reprod. 2001, 16, 1922–1930. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, A.I.S.B.; Netherton, J.; Baker, M.A. From past to present: The link between reactive oxygen species in sperm and male infertility. Antioxidants 2019, 8, 616. [Google Scholar] [CrossRef] [PubMed]
- Wood, G.J.A.; Cardoso, J.P.G.; Paluello, D.V.; Nunes, T.F.; Cocuzza, M. Varicocele-associated infertility and the role of oxidative stress on sperm DNA fragmentation. Front. Reprod. Health 2021, 3, 695992. [Google Scholar] [CrossRef]
- Signorini, C.; Moretti, E.; Noto, D.; Micheli, L.; Ponchia, R.; Collodel, G. Fatty acid oxidation and pro-resolving lipid mediators are related to male infertility. Antioxidants 2022, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Whiting, S.; De Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Cerretani, D.; Noto, D.; Signorini, C.; Iacoponi, F.; Collodel, G. Relationship between semen IL-6, IL-33 and malondialdehyde generation in human seminal plasma and spermatozoa. Reprod. Sci. 2021, 28, 2136–2143. [Google Scholar] [CrossRef]
- Moretti, E.; Signorini, C.; Ferretti, F.; Noto, D.; Collodel, G. A study to validate the relevance of semen F2-isoprostanes on human male infertility. Int. J. Environ. Res. Public Health 2022, 19, 1642. [Google Scholar] [CrossRef]
- Aitken, R.J.; De Iuliis, G.N. On the possible origins of DNA damage in human spermatozoa. Mol. Hum. Reprod. 2010, 16, 3–13. [Google Scholar] [CrossRef]
- Jimbo, M.; Kunisaki, J.; Ghaed, M.; Yu, V.; Flores, H.A.; Hotaling, J.M. Fertility in the aging male: A systematic review. Fertil. Steril. 2022, 118, 1022–1034. [Google Scholar] [CrossRef]
- Santiago, J.; Silva, J.V.; Howl, J.; Santos, M.A.S.; Fardilha, M. All you need to know about sperm RNAs. Hum. Reprod. Update 2021, 28, 67–91. [Google Scholar] [CrossRef]
- Vollmer, T.; Ljungberg, B.; Jankowski, V.; Jankowski, J.; Glorieux, G.; Stegmayr, B.G. An in-vitro assay using human spermatozoa to detect toxicity of biologically active substances. Sci. Rep. 2019, 9, 14525. [Google Scholar] [CrossRef] [PubMed]
- Setti, A.S.; Braga, D.P.A.F.; Provenza, R.R.; Iaconelli, A., Jr.; Borges, E., Jr. Oocyte ability to repair sperm DNA fragmentation: The impact of maternal age on intracytoplasmic sperm injection outcomes. Fertil. Steril. 2021, 116, 123–129. [Google Scholar] [CrossRef]
- Collodel, G.; Federico, M.G.; Geminiani, M.; Martini, S.; Bonechi, C.; Rossi, C.; Figura, N.; Moretti, E. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod. Toxicol. 2011, 31, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, F.; Momeni, H.R. Silymarin protects plasma membrane and acrosome integrity in sperm treated with sodium arsenite. Int. J. Reprod. Biomed. 2016, 14, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Diao, R.Y.; Duan, Y.G.; Yi, T.H.; Cai, Z.M. In vitro antioxidant effect of curcumin on human sperm quality in leucocytospermia. Andrologia 2017, 49, e12760. [Google Scholar] [CrossRef]
- Noto, D.; Collodel, G.; Cerretani, D.; Signorini, C.; Gambera, L.; Menchiari, A.; Moretti, E. Protective effect of chlorogenic acid on human sperm: In vitro studies and frozen-thawed protocol. Antioxidants 2021, 10, 744. [Google Scholar] [CrossRef]
- Amirjannaty, S.; Gashti, N.G.; Mojtahedi, A.; Ashouri, A.; Bahadori, M.H. An in vitro study on the protective effect of melatonin on human sperm parameters treated by cadmium. J. Hum. Reprod. Sci. 2022, 15, 21–26. [Google Scholar] [CrossRef]
- Mottola, F.; Iovine, C.; Carannante, M.; Santonastaso, M.; Rocco, L. In vitro combination of ascorbic and ellagic acids in sperm oxidative damage inhibition. Int. J. Mol. Sci. 2022, 23, 14751. [Google Scholar] [CrossRef]
- Munir, N.; Mahmood, Z.; Shahid, M.; Afzal, M.N.; Jahangir, M.; Ali Shah, S.M.; Tahir, I.M.; Riaz, M.; Hussain, S.; Akram, M.; et al. Withania somnifera chemical constituents’ in vitro antioxidant potential and their response on spermatozoa parameters. Dose Response 2022, 20, 15593258221074936. [Google Scholar] [CrossRef]
- Paul, S.; Kang, S.C. In vitro determination of the contraceptive spermicidal activity of essential oil of Trachyspermum ammi (L.) Sprague ex Turrill fruits. New Biotechnol. 2011, 28, 684–690. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, N.; Wang, M.; Rao, M.; Su, P. In vitro study evaluating the instantaneous treatment of ozonised olive oil on human sperm. Eur. J. Contracept. Reprod. Health Care 2018, 23, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.; Chang, X.; Han, Y.; Miao, H.; Yang, Y. In vitro effect of ulipristal acetate on human sperm parameters and function. Pak. J. Pharm. Sci. 2019, 32, 1419–1422. [Google Scholar] [PubMed]
- Mondal, P.; Maity, R.; Mallick, C. In vitro spermicidal effect of Thevetia Peruviana leaves on human spermatozoa. Andrologia 2022, 54, e14323. [Google Scholar] [CrossRef]
- Marchiani, S.; Tamburrino, L.; Farnetani, G.; Muratori, M.; Vignozzi, L.; Baldi, E. Acute effects on human sperm exposed in vitro to cadmium chloride and diisobutyl phthalate. Reproduction 2019, 158, 281–290. [Google Scholar] [CrossRef]
- Xie, F.; Chen, X.; Weng, S.; Xia, T.; Sun, X.; Luo, T.; Li, P. Effects of two environmental endocrine disruptors di-n-butyl phthalate (DBP) and mono-n-butyl phthalate (MBP) on human sperm functions in vitro. Reprod. Toxicol. 2019, 83, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, B.; Huang, R.; Dong, S.; Zhou, Y.; Song, J.; Zeng, X.; Zhang, X. Involvement of Ca2+ and ROS signals in nickel-impaired human sperm function. Ecotoxicol. Environ. Saf. 2022, 231, 113181. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Terzuoli, G.; Renieri, T.; Iacoponi, F.; Castellini, C.; Giordano, C.; Collodel, G. In vitro effect of gold and silver nanoparticles on human spermatozoa. Andrologia 2013, 45, 392–396. [Google Scholar] [CrossRef]
- Préaubert, L.; Tassistro, V.; Auffan, M.; Sari-Minodier, I.; Rose, J.; Courbiere, B.; Perrin, J. Very low concentration of cerium dioxide nanoparticles induce DNA damage, but no loss of vitality, in human spermatozoa. Toxicol. Vitr. 2018, 50, 236–241. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Iovine, C.; Cesaroni, F.; Colacurci, N.; Rocco, L. In vitro effects of titanium dioxide nanoparticles (TiO2NPs) on cadmium chloride (CdCl2) genotoxicity in human sperm cells. Nanomaterials 2020, 10, 1118. [Google Scholar] [CrossRef]
- Tan, Z.; Zhou, J.; Chen, H.; Zou, Q.; Weng, S.; Luo, T.; Tang, Y. Toxic effects of 2,4-dichlorophenoxyacetic acid on human sperm function in vitro. J. Toxicol. Sci. 2016, 41, 543–549. [Google Scholar] [CrossRef]
- Xu, B.; Wang, Z.P.; Wang, Y.J.; Lu, P.H.; Wang, L.J.; Wang, X.H. The toxic effect of opioid analgesics on human sperm motility in vitro. Drug Chem. Toxicol. 2013, 36, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Pascarelli, N.A.; Fioravanti, A.; Moretti, E.; Guidelli, G.M.; Mazzi, L.; Collodel, G. The effects in vitro of TNF-α and its antagonist ‘etanercept’ on ejaculated human sperm. Reprod. Fertil. Dev. 2017, 29, 1169–1177. [Google Scholar] [CrossRef] [PubMed]
- Ali Banihani, S.; Al-Khawalde, A.A. Omeprazole does not alter human sperm motility, viability or DNA integrity in vitro. Andrologia 2019, 51, e13260. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Toshimori, K. Acrosome markers of human sperm. Anat. Sci. Int. 2016, 91, 128–142. [Google Scholar] [CrossRef]
- Zoppino, F.C.; Halón, N.D.; Bustos, M.A.; Pavarotti, M.A.; Mayorga, L.S. Recording and sorting live human sperm undergoing acrosome reaction. Fertil. Steril. 2012, 97, 1309–1315. [Google Scholar] [CrossRef]
- Farkouh, A.; Salvio, G.; Kuroda, S.; Saleh, R.; Vogiatzi, P.; Agarwal, A. Sperm DNA integrity and male infertility: A narrative review and guide for the reproductive physicians. Transl. Androl. Urol. 2022, 11, 1023–1044. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Freire-Brito, L.; Oliveira, P.F.; Alves, M.G. Evaluation of human spermatozoa mitochondrial membrane potential using the JC-1 dye. Curr. Protoc. 2022, 2, e531. [Google Scholar] [CrossRef]
- van der Horst, G.; Maree, L.; du Plessis, S.S. Current perspectives of CASA applications in diverse mammalian spermatozoa. Reprod. Fertil. Dev. 2018, 30, 875–888. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.K.; Gupta, S.; Boitrelle, F.; Finelli, R.; Parekh, N.; Durairajanayagam, D.; Saleh, R.; Arafa, M.; Cho, C.L.; et al. Sperm vitality and necrozoospermia: Diagnosis, management, and results of a global survey of clinical practice. World J. Mens Health 2022, 40, 228–242. [Google Scholar] [CrossRef]
- Hassan, M.A.E.; Khalil, W.A.; Abdelnour, S.A.; Aman, R.M. Supplementation of alpha-lipoic acid-loaded nanoliposomes in semen extender improves freezability of buffalo spermatozoa. Sci. Rep. 2022, 12, 22464. [Google Scholar] [CrossRef]
- Hezavehei, M.; Sharafi, M.; Kouchesfahani, H.M.; Henkel, R.; Agarwal, A.; Esmaeili, V.; Shahverdi, A. Sperm cryopreservation: A review on current molecular cryobiology and advanced approaches. Reprod. Biomed. Online 2018, 37, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.C.; Williams, D.B.; Huang, T.; Wang, C. Effects of pentoxifylline on sperm motility and hyperactivated motility in vitro: A preliminary report. Fertil. Steril. 1993, 59, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Gavella, M.; Lipovac, V. Effect of pentoxifylline on experimentally induced lipid peroxidation in human spermatozoa. Int. J. Androl. 1994, 17, 308–313. [Google Scholar] [CrossRef] [PubMed]
- McKinney, K.A.; Lewis, S.E.; Thompson, W. Persistent effects of pentoxifylline on human sperm motility, after drug removal, in normozoospermic and asthenozoospermic individuals. Andrologia 1994, 26, 235–240. [Google Scholar] [CrossRef]
- McKinney, K.A.; Lewis, S.E.; Thompson, W. The effects of pentoxifylline on the generation of reactive oxygen species and lipid peroxidation in human spermatozoa. Andrologia 1996, 28, 15–20. [Google Scholar] [CrossRef]
- Lewis, S.E.; McKinney, K.A.; Thompson, W. Influence of pentoxifylline on human sperm motility in asthenozoospermic individuals using computer-assisted analysis. Arch. Androl. 1994, 32, 175–183. [Google Scholar]
- Mladenovic, I.; Micic, S.; Pearson, R.M.; Genbacev, O.; Papic, N. Effects of pentoxifylline on human sperm parameters in vitro. J. Assist. Reprod. Genet. 1994, 11, 495–499. [Google Scholar] [CrossRef]
- Paul, M.; Sumpter, J.P.; Lindsay, K.S. Factors affecting pentoxifylline stimulation of sperm kinematics in suspensions. Hum. Reprod. 1996, 11, 1929–1935. [Google Scholar] [CrossRef]
- Patrizio, P.; Liu, Y.; Sonek, G.J.; Berns, M.W.; Tadir, Y. Effect of pentoxifylline on the intrinsic swimming forces of human sperm assessed by optical tweezers. J. Androl. 2000, 21, 753–756. [Google Scholar]
- Satish, M.; Kumari, S.; Deeksha, W.; Abhishek, S.; Nitin, K.; Adiga, S.K.; Hegde, P.; Dasappa, J.P.; Kalthur, G.; Rajakumara, E. Structure-based redesigning of pentoxifylline analogs against selective phosphodiesterases to modulate sperm functional competence for assisted reproductive technologies. Sci. Rep. 2021, 11, 12293. [Google Scholar] [CrossRef]
- Xian, Y.; Jiang, M.; Liu, B.; Zhao, W.; Zhou, B.; Liu, X.; Li, S.; Li, F. A cryoprotectant supplemented with pentoxifylline can improve the effect of freezing on the motility of human testicular sperm. Zygote 2022, 30, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Banihani, S.A.; Abu-Alhayjaa, R.F.; Amarin, Z.O.; Alzoubi, K.H. Pentoxifylline increases the level of nitric oxide produced by human spermatozoa. Andrologia 2018, 50, e12859. [Google Scholar] [CrossRef] [PubMed]
- Tournaye, H.; Devroey, P.; Camus, M.; Van der Linden, M.; Janssens, R.; Van Steirteghem, A. Use of pentoxifylline in assisted reproductive technology. Hum. Reprod. 1995, 10 (Suppl. S1), 72–79. [Google Scholar] [CrossRef] [PubMed]
- Matson, P.L.; Yovich, J.M.; Edirisinghe, W.R.; Junk, S.M.; Yovich, J.L. An argument for the past and continued use of pentoxifylline in assisted reproductive technology. Hum. Reprod. 1995, 10 (Suppl. S1), 67–71. [Google Scholar] [CrossRef] [PubMed]
- Terriou, P.; Hans, E.; Giorgetti, C.; Spach, J.L.; Salzmann, J.; Urrutia, V.; Roulier, R. Pentoxifylline initiates motility in spontaneously immotile epididymal and testicular spermatozoa and allows normal fertilization, pregnancy, and birth after intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2000, 17, 194–199. [Google Scholar] [CrossRef]
- Mahaldashtian, M.; Khalili, M.A.; Nottola, S.A.; Woodward, B.; Macchiarelli, G.; Miglietta, S. Does in vitro application of pentoxifylline have beneficial effects in assisted male reproduction? Andrologia 2021, 53, e13722. [Google Scholar] [CrossRef]
- Hattori, H.; Nakajo, Y.; Ito, C.; Toyama, Y.; Toshimori, K.; Kyono, K. Birth of a healthy infant after intracytoplasmic sperm injection using pentoxifylline-activated sperm from a patient with Kartagener’s syndrome. Fertil. Steril. 2011, 95, 2431.e9–2431.e11. [Google Scholar] [CrossRef]
- Montjean, D.; Courageot, J.; Altié, A.; Amar-Hoffet, A.; Rossin, B.; Geoffroy-Siraudin, C.; Tourame, P.; Boyer, P. Normal live birth after vitrified/warmed oocytes intracytoplasmic sperm injection with immotile spermatozoa in a patient with Kartagener’s syndrome. Andrologia 2015, 47, 839–845. [Google Scholar] [CrossRef]
- Terriou, P.; Hans, E.; Cortvrindt, R.; Avon, C.; Charles, O.; Salzmann, J.; Lazdunski, P.; Giorgetti, C. Papaverine as a replacement for pentoxifylline to select thawed testicular or epididymal spermatozoa before ICSI. Gynecol. Obstet. Fertil. 2015, 43, 786–790. [Google Scholar] [CrossRef]
- Talevi, T.; Barbato, V.; Fiorentino, I.; Braun, S.; Longobardi, S.; Gualtieri, R. Protective effects of in vitro treatment with zinc, d-aspartate and coenzyme q10 on human sperm motility, lipid peroxidation and DNA fragmentation. Reprod. Biol. Endocrinol. 2013, 11, 81. [Google Scholar] [CrossRef]
- Ghafarizadeh, A.A.; Vaezi, G.; Shariatzadeh, M.A.; Malekirad, A.A. Effect of in vitro selenium supplementation on sperm quality in asthenoteratozoospermic men. Andrologia 2018, 50, e12869. [Google Scholar] [CrossRef]
- Banihani, S.; Sharma, R.; Bayachou, M.; Sabanegh, E.; Agarwal, A. Human sperm DNA oxidation, motility and viability in the presence of L-carnitine during in vitro incubation and centrifugation. Andrologia 2012, 44 (Suppl. S1), 505–512. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Kanwar, K.C. Effect of vitamin E on human sperm motility and lipid peroxidation in vitro. Asian J. Androl. 1999, 1, 151–154. [Google Scholar] [PubMed]
- Keshtgar, S.; Fanaei, H.; Bahmanpour, S.; Azad, F.; Ghannadi, A.; Kazeroni, M. In vitro effects of α-tocopherol on teratozoospermic semen samples. Andrologia 2012, 44 (Suppl. S1), 721–727. [Google Scholar] [CrossRef]
- Donnelly, E.T.; McClure, N.; Lewis, S.E. Antioxidant supplementation in vitro does not improve human sperm motility. Fertil. Steril. 1999, 72, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Fanaei, H.; Khayat, S.; Halvaei, I.; Ramezani, V.; Azizi, Y.; Kasaeian, A.; Mardaneh, J.; Parvizi, M.R.; Akrami, M. Effects of ascorbic acid on sperm motility, viability, acrosome reaction and DNA integrity in teratozoospermic samples. Iran. J. Reprod. Med. 2014, 12, 103–110. [Google Scholar] [PubMed]
- Gavella, M.; Lipovac, V. In vitro effect of zinc on oxidative changes in human semen. Andrologia 1998, 30, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Gavella, M.; Lipovac, V.; Vucić, M.; Sverko, V. In vitro inhibition of superoxide anion production and superoxide dismutase activity by zinc in human spermatozoa. Int. J. Androl. 1999, 22, 266–274. [Google Scholar] [CrossRef]
- Ajina, T.; Sallem, A.; Haouas, Z.; Mehdi, M. Total antioxidant status and lipid peroxidation with and without in vitro zinc supplementation in infertile men. Andrologia 2017, 49, e12703. [Google Scholar] [CrossRef]
- Appiah, M.O.; Asante-Badu, B.; Zhao, J.; Liu, H.; Wang, J.; Lu, W. Possible Protective Mechanisms of Coenzyme Q10 Action on Spermatozoa During Cryopreservation or Cooled-Stored Condition. Cryoletters 2020, 41, 246–256. [Google Scholar]
- Boonsimma, K.; Ngeamvijawat, J.; Sukcharoen, N.; Boonla, C. Supplementing post-wash asthenozoospermic human spermatozoa with coenzyme Q10 for 1 hr in vitro improves sperm motility, but not oxidative stress. Andrologia 2020, 52, e13818. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.M.; Lewis, S.E.; McKelvey-Martin, V.J.; Thompson, W. The effects of antioxidant supplementation during Percoll preparation on human sperm DNA integrity. Hum. Reprod. 1998, 13, 1240–1247. [Google Scholar] [CrossRef] [PubMed]
- Celik, H.; Arinç, E. Evaluation of the protective effects of quercetin, rutin, naringenin, resveratrol and trolox against idarubicin-induced DNA damage. J. Pharm. Pharm. Sci. 2010, 13, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Adami, L.N.G.; Belardin, L.B.; Lima, B.T.; Jeremias, J.T.; Antoniassi, M.P.; Okada, F.K.; Bertolla, R.P. Effect of in vitro vitamin E (alpha-tocopherol) supplementation in human spermatozoon submitted to oxidative stress. Andrologia 2018, 50, e12959. [Google Scholar] [CrossRef] [PubMed]
- Ghafarizadeh, A.A.; Malmir, M.; Noreini, S.N.; Faraji, T.; Ebrahimi, Z. The effect of vitamin E on sperm motility and viability in asthenoteratozoospermic men: In vitro study. Andrologia 2021, 53, e13891. [Google Scholar] [CrossRef]
- Ahmad, G.; Agarwal, A.; Esteves, S.C.; Sharma, R.; Almasry, M.; Al-Gonaim, A.; AlHayaza, G.; Singh, N.; Al Kattan, L.; Sannaa, W.N.; et al. Ascorbic acid reduces redox potential in human spermatozoa subjected to heat-induced oxidative stress. Andrologia 2017, 49, e12773. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M. Lipid peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic. Biol. Med. 2020, 152, 175–185. [Google Scholar] [CrossRef]
- Majzoub, A.; Agarwal, A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef]
- Traber, M.G.; Head, B. Vitamin E: How much is enough, too much and why! Free Radic. Biol. Med. 2021, 177, 212–225. [Google Scholar] [CrossRef]
- Donnelly, E.T.; McClure, N.; Lewis, S.E. Glutathione and hypotaurine in vitro: Effects on human sperm motility, DNA integrity and production of reactive oxygen species. Mutagenesis 2000, 15, 61–68. [Google Scholar] [CrossRef]
- Ambrosini, A.; Zolese, G.; Ambrosi, S.; Ragni, L.; Tiano, L.; Littarru, G.; Bertoli, E.; Mantero, F.; Boscaro, M.; Balercia, G. Oleoylethanolamide protects human sperm cells from oxidation stress: Studies on cases of idiopathic infertility. Biol. Reprod. 2006, 74, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.J.; Kim, J.H.; Ryu, C.S.; Lee, J.Y.; Park, J.S.; Chung, D.Y.; Choi, S.Y.; Kim, M.H.; Chun, E.K.; Roh, S.I. Protective effect of antioxidant supplementation in sperm-preparation medium against oxidative stress in human spermatozoa. Hum. Reprod. 2008, 23, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Delves, G.H.; Chopra, M.; Lwaleed, B.A.; Cooper, A.J. Can lycopene be delivered into semen via prostasomes? In vitro incorporation and retention studies. Int. J. Androl. 2006, 29, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Zini, A.; Gabriel, M.S.; Libman, J. Lycopene supplementation in vitro can protect human sperm deoxyribonucleic acid from oxidative damage. Fertil. Steril. 2010, 94, 1033–1036. [Google Scholar] [CrossRef]
- Colone, M.; Marelli, G.; Unfer, V.; Bozzuto, G.; Molinari, A.; Stringaro, A. Inositol activity in oligoasthenoteratospermia—An in vitro study. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 891–896. [Google Scholar]
- Condorelli, R.A.; La Vignera, S.; Di Bari, F.; Unfer, V.; Calogero, A.E. Effects of myoinositol on sperm mitochondrial function in-vitro. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 129–134. [Google Scholar]
- Artini, P.G.; Casarosa, E.; Carletti, E.; Monteleone, P.; Di Noia, A.; Di Berardino, O.M. In vitro effect of myo-inositol on sperm motility in normal and oligoasthenospermia patients undergoing in vitro fertilization. Gynecol. Endocrinol. 2017, 33, 109–112. [Google Scholar] [CrossRef]
- Saleh, R.; Assaf, H.; El Maged, W.M.A.; Elsuity, M.; Fawzy, M. Increased cryo-survival rate in ejaculated human sperm from infertile men following pre-freeze in vitro myo-inositol supplementation. Clin. Exp. Reprod. Med. 2018, 45, 177–182. [Google Scholar] [CrossRef]
- Mohammadi, F.; Varanloo, N.; Nasrabadi, M.H.; Vatannejad, A.; Amjadi, F.S.; Masroor, M.J.; Bajelan, L.; Mehdizadeh, M.; Aflatoonian, R.; Zandieh, Z. Supplementation of sperm freezing medium with myoinositol improve human sperm parameters and protects it against DNA fragmentation and apoptosis. Cell Tissue Bank 2019, 20, 77–86. [Google Scholar] [CrossRef]
- Abdolsamadi, M.; Mohammadi, F.; Nashtaei, M.S.; Teimouri, M.; Sardar, R.; Dayani, M.; Haghighi, M.; Ghasemi, S.; Vatannejad, A.; Zandieh, Z. Does myoinositol supplement improve sperm parameters and DNA integrity in patients with oligoasthenoteratozoospermia after the freezing-thawing process? Cell Tissue Bank 2020, 21, 99–106. [Google Scholar] [CrossRef]
- Governini, L.; Ponchia, R.; Artini, P.G.; Casarosa, E.; Marzi, I.; Capaldo, A.; Luddi, A.; Piomboni, P. Respiratory mitochondrial efficiency and DNA oxidation in human sperm after in vitro myo-inositol treatment. J. Clin. Med. 2020, 9, 1638. [Google Scholar] [CrossRef] [PubMed]
- Ponchia, R.; Bruno, A.; Renzi, A.; Landi, C.; Shaba, E.; Luongo, F.P.; Haxhiu, A.; Artini, P.G.; Luddi, A.; Governini, L.; et al. Oxidative stress measurement in frozen/thawed human sperm: The protective role of an in vitro treatment with myo-inositol. Antioxidants 2021, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Azizi, M.; Cheraghi, E.; Mehranjani, M.S. Effect of Myo-inositol on sperm quality and biochemical factors in cryopreserved semen of patients with asthenospermia. Andrologia 2022, 54, e14528. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, S.S.; Hagenaar, K.; Lampiao, F. The in vitro effects of melatonin on human sperm function and its scavenging activities on NO and ROS. Andrologia 2010, 42, 112–116. [Google Scholar] [CrossRef]
- Karimfar, M.H.; Niazvand, F.; Haghani, K.; Ghafourian, S.; Shirazi, R.; Bakhtiyari, S. The protective effects of melatonin against cryopreservation-induced oxidative stress in human sperm. Int. J. Immunopathol. Pharmacol. 2015, 28, 69–76. [Google Scholar] [CrossRef]
- Najafi, A.; Adutwum, E.; Yari, A.; Salehi, E.; Mikaeili, S.; Dashtestani, F.; Abolhassani, F.; Rashki, L.; Shiasi, S.; Asadi, E. Melatonin affects membrane integrity, intracellular reactive oxygen species, caspase3 activity and AKT phosphorylation in frozen thawed human sperm. Cell Tissue Res. 2018, 372, 149–159. [Google Scholar] [CrossRef]
- Pariz, J.R.; Ranéa, C.; Monteiro, R.A.C.; Evenson, D.P.; Drevet, J.R.; Hallak, J. Melatonin and caffeine supplementation used, respectively, as protective and stimulating agents in the cryopreservation of human sperm improves survival, viability, and motility after thawing compared to traditional TEST-yolk buffer. Oxid. Med. Cell Longev. 2019, 2019, 6472945. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Xiong, Y.M.; Tan, Y.J.; Wang, L.; Li, R.; Zhang, Y.; Liu, X.M.; Lin, X.H.; Jin, L.; Hu, Y.T.; et al. Melatonin rescues impaired penetration ability of human spermatozoa induced by mitochondrial dysfunction. Reproduction 2019, 158, 465–475. [Google Scholar] [CrossRef]
- Malmir, M.; Noreini, S.N.; Ghafarizadeh, A.; Faraji, T.; Asali, Z. Ameliorative effect of melatonin on apoptosis, DNA fragmentation, membrane integrity and lipid peroxidation of spermatozoa in the idiopathic asthenoteratospermic men: In vitro. Andrologia 2021, 53, e13944. [Google Scholar] [CrossRef]
- Minucci, S.; Venditti, V. New Insight on the In Vitro Effects of Melatonin in Preserving Human Sperm Quality. Int. J. Mol. Sci. 2022, 23, 5128. [Google Scholar] [CrossRef]
- Luboshitzky, R.; Shen-Orr, Z.; Herer, P. Seminal plasma melatonin and gonadal steroids concentrations in normal men. Arch. Androl. 2002, 48, 225–232. [Google Scholar] [CrossRef]
- Ortiz, A.; Espino, J.; Bejarano, I.; Lozano, G.M.; Monllor, F.; García, J.F.; Pariente, J.A.; Rodríguez, A.B. High endogenous melatonin concentrations enhance sperm quality and short-term in vitro exposure to melatonin improves aspects of sperm motility. J. Pineal Res. 2011, 50, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.L.; Sun, T.C.; Yu, K.; Wang, Z.P.; Zhang, B.L.; Zhang, Y.; Wang, X.X.; Lian, Z.X.; Liu, Y.X. Melatonin reduces oxidative damage and upregulates heat shock protein 90 expression in cryopreserved human semen. Free Radic. Biol. Med. 2017, 113, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Monllor, F.; Espino, J.; Marchena, A.M.; Ortiz, A.; Lozano, G.; García, J.F.; Pariente, J.A.; Rodríguez, A.B.; Bejarano, I. Melatonin diminishes oxidative damage in sperm cells, improving assisted reproductive techniques. Turk. J. Biol. 2017, 41, 881–889. [Google Scholar] [CrossRef]
- Esteves, S.C.; Sharma, R.K.; Thomas, A.J., Jr.; Agarwal, A. Cryopreservation of human spermatozoa with pentoxifylline improves the post-thaw agonist-induced acrosome reaction rate. Hum. Reprod. 1998, 13, 3384–3389. [Google Scholar] [CrossRef] [PubMed]
- Stanic, P.; Sonicki, Z.; Suchanek, E. Effect of pentoxifylline on motility and membrane integrity of cryopreserved human spermatozoa. Int. J. Androl. 2002, 25, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Nabi, A.; Khalili, M.A.; Talebi, A.R.; Mangoli, E.; Yari, N.; Nottola, S.A.; Miglietta, S.; Taheri, F. In-vitro application of pentoxifylline preserved ultrastructure of spermatozoa after vitrification in asthenozoospermic patients. Urol. J. 2017, 14, 4038–4043. [Google Scholar]
- Kotdawala, A.P.; Kumar, S.; Salian, S.R.; Thankachan, P.; Govindraj, K.; Kumar, P.; Kalthur, G.; Adiga, S.K. Addition of zinc to human ejaculate prior to cryopreservation prevents freeze-thaw-induced DNA damage and preserves sperm function. J. Assist. Reprod. Genet. 2012, 29, 1447–1453. [Google Scholar] [CrossRef]
- Isaac, A.V.; Kumari, S.; Nair, R.; Urs, D.R.; Salian, S.R.; Kalthur, G.; Adiga, S.K.; Manikkath, J.; Mutalik, S.; Sachdev, D.; et al. Supplementing zinc oxide nanoparticles to cryopreservation medium minimizes the freeze-thaw-induced damage to spermatozoa. Biochem. Biophys. Res. Commun. 2017, 494, 656–662. [Google Scholar] [CrossRef]
- Taylor, K.; Roberts, P.; Sanders, K.; Burton, P. Effect of antioxidant supplementation of cryopreservation medium on post-thaw integrity of human spermatozoa. Reprod. Biomed. Online 2009, 18, 184–189. [Google Scholar] [CrossRef]
- Minaei, M.B.; Barbarestani, M.; Nekoonam, S.; Abdolvahabi, M.A.; Takzare, N.; Asadi, M.H.; Hedayatpour, A.; Amidi, F. Effect of Trolox addition to cryopreservation media on human sperm motility. Iran. J. Reprod. Med. 2012, 10, 99–104. [Google Scholar] [PubMed]
- Nekoonam, S.; Nashtaei, M.S.; Naji, M.; Zangi, B.M.; Amidi, F. Effect of Trolox on sperm quality in normozospermia and oligozospermia during cryopreservation. Cryobiology 2016, 72, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Seifi, S.; Shahverd, A.; Topraggaleh, T.R.; Sharafi, M.; Esmaeili, V.; Choobineh, H.; Zamiri, M.J.; Habibi, M.; Alizadeh, A.R. Inclusion of ovine enriched serum with vitamin E and polyunsaturated fatty acids in the freezing medium: A new strategy to improve human frozen-thawed sperm parameters. Andrologia 2020, 52, e13541. [Google Scholar] [CrossRef] [PubMed]
- Zerbinati, C.; Caponecchia, L.; Fiori, C.; Sebastianelli, A.; Salacone, P.; Ciacciarelli, M.; Iuliano, L. Alpha- and gamma-tocopherol levels in human semen and their potential functional implications. Andrologia 2020, 52, e13543. [Google Scholar] [CrossRef] [PubMed]
- Branco, C.S.; Garcez, M.E.; Pasqualotto, F.F.; Erdtman, B.; Salvador, M. Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology 2010, 60, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lin, Q.; Liu, R.; Xiao, W.; Liu, W. Protective effects of ascorbate and catalase on human spermatozoa during cryopreservation. J. Androl. 2010, 31, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Mangoli, E.; Talebi, A.R.; Anvari, M.; Taheri, F.; Vatanparast, M.; Rahiminia, T.; Hosseini, A. Vitamin C attenuates negative effects of vitrification on sperm parameters, chromatin quality, apoptosis and acrosome reaction in neat and prepared normozoospermic samples. Taiwan J. Obstet. Gynecol. 2018, 57, 200–204. [Google Scholar] [CrossRef]
- Ghorbani, F.; Nasiri, Z.; Koohestanidehaghi, Y.; Lorian, K. The antioxidant roles of L-carnitine and N-acetyl cysteine against oxidative stress on human sperm functional parameters during vitrification. Clin. Exp. Reprod. Med. 2021, 48, 316–321. [Google Scholar] [CrossRef]
- Jannatifar, R.; Asa, E.; Sahraei, S.S.; Verdi, A.; Piroozmanesh, H. N-acetyl-l-cysteine and alpha lipoic acid are protective supplement on human sperm parameters in cryopreservation of asthenoteratozoospermia patients. Andrologia 2022, 54, e14612. [Google Scholar] [CrossRef]
- Banihani, S.; Agarwal, A.; Sharma, R.; Bayachou, M. Cryoprotective effect of L-carnitine on motility, vitality and DNA oxidation of human spermatozoa. Andrologia 2014, 46, 637–641. [Google Scholar] [CrossRef]
- Aliabadi, E.; Jahanshahi, S.; Talaei-Khozani, T.; Banaei, M. Comparison and evaluation of capacitation and acrosomal reaction in freeze-thawed human ejaculated spermatozoa treated with L-carnitine and pentoxifylline. Andrologia 2018, 50, e12845. [Google Scholar] [CrossRef]
- Nezhad, N.C.; Vahabzadeh, Z.; Allahveisie, A.; Rahmani, K.; Raoofi, A.; Rezaie, M.J.; Rezae, M.; Partovyan, M. The effect of L-carnitine and coenzyme Q10 on the sperm motility, DNA fragmentation, chromatin structure and oxygen free radicals during, before and after freezing in oligospermia men. Urol. J. 2021, 18, 330–336. [Google Scholar] [CrossRef]
- Sicchieri, F.; Silva, A.B.; Santana, V.P.; Vasconcelos, M.A.C.; Ferriani, R.A.; Vireque, A.A.; Dos Reis, R.M. Phosphatidylcholine and L-acetyl-carnitine-based freezing medium can replace egg yolk and preserves human sperm function. Transl. Androl. Urol. 2021, 10, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Gadea, J.; Molla, M.; Selles, E.; Marco, M.A.; Garcia-Vazquez, F.A.; Gardon, J.C. Reduced glutathione content in human sperm is decreased after cryopreservation: Effect of the addition of reduced glutathione to the freezing and thawing extenders. Cryobiology 2011, 62, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, M.; Vatannejad, A.; Khodadadi, I.; Amiri, I.; Tavilani, H. Protective effects of glutathione supplementation against oxidative stress during cryopreservation of human spermatozoa. Cryoletters 2016, 37, 34–40. [Google Scholar]
- Seify, M.; Zarabadipour, M.; Ghaleno, L.R.; Alizadeh, A.R.; Valojerdi, M.R. The anti-oxidant roles of Taurine and Hypotaurine on acrosome integrity, HBA and HSPA2 of the human sperm during vitrification and post warming in two different temperature. Cryobiology 2019, 90, 89–95. [Google Scholar] [CrossRef]
- Rossi, T.; Mazzilli, F.; Delfino, M.; Dondero, F. Improved human sperm recovery using superoxide dismutase and catalase supplementation in semen cryopreservation procedure. Cell Tissue Bank 2001, 2, 9–13. [Google Scholar] [CrossRef]
- Moubasher, A.E.; El Din, A.M.E.; Ali, M.E.; El-Sherif, W.T.; Gaber, H.D. Catalase improves motility, vitality and DNA integrity of cryopreserved human spermatozoa. Andrologia 2013, 45, 135–139. [Google Scholar] [CrossRef]
- Karakus, F.N.; Kuran, S.B.; Solakoglu, S. Effect of curcumin on sperm parameters after the cryopreservation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 161–166. [Google Scholar] [CrossRef]
- Riahi, M.M.; Behnam, B.; Henney, N.C.; Jamialahmadi, T.; Sahebkar, A. Protective effects of curcumin in the reproductive system: Anti-toxic, semen cryopreservative, and contraceptive actions. Adv. Exp. Med. Biol. 2021, 1328, 223–242. [Google Scholar] [CrossRef]
- Santonastaso, M.; Mottola, F.; Iovine, C.; Colacurci, N.; Rocco, L. Protective effects of curcumin on the outcome of cryopreservation in human sperm. Reprod. Sci. 2021, 28, 2895–2905. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From theory to practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Agarwal, A.; Virk, G.; Cho, C.L. Potential role of green tea catechins in the management of oxidative stress-associated infertility. Reprod. Biomed. Online 2017, 34, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Ayla, S.; Tunalı, G.; Bilgiç, B.E.; Sofuoğlu, K.; Özdemir, A.A.; Tanrıverdi, G.; Özdemir, S.; Soner, B.C.; Öztürk, B.; Karahüseyinoğlu, S.; et al. Antioxidant activity of CAPE (caffeic acid phenethyl ester) in vitro can protect human sperm deoxyribonucleic acid from oxidative damage. Acta Histochem. 2018, 120, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Awaga, H.A.; Lymperi, S.; Bosdou, J.K.; Makedos, A.; Mitsoli, A.; Bazioti, M.G.; Savvaidou, D.; Goulis, D.G.; Chatzimeletiou, K.; Salem, M.N.; et al. Addition of procyanidine to semen preserves progressive sperm motility up to three hours of incubation. Reprod. Biol. 2019, 19, 255–260. [Google Scholar] [CrossRef]
- Diao, R.; Gan, H.; Tian, F.; Cai, X.; Zhen, W.; Song, X.; Duan, Y.G. In vitro antioxidation effect of Quercetin on sperm function from the infertile patients with leukocytospermia. Am. J. Reprod. Immunol. 2019, 82, e13155. [Google Scholar] [CrossRef]
- Kedechi, S.; Zribi, N.; Louati, N.; Menif, H.; Sellami, A.; Lassoued, S.; Mansour, R.B.; Keskes, L.; Rebai, T.; Chakroun, N. Antioxidant effect of hydroxytyrosol on human sperm quality during in vitro incubation. Andrologia 2017, 49, e12595. [Google Scholar] [CrossRef]
- Iovine, C.; Mottola, F.; Santonastaso, M.; Finelli, R.; Agarwal, A.; Rocco, L. In vitro ameliorative effects of ellagic acid on vitality, motility and DNA quality in human spermatozoa. Mol. Reprod. Dev. 2021, 88, 167–174. [Google Scholar] [CrossRef]
- Moretti, E.; Mazzi, L.; Terzuoli, G.; Bonechi, C.; Iacoponi, F.; Martini, S.; Rossi, C.; Collodel, G. Effect of quercetin, rutin, naringenin and epicatechin on lipid peroxidation induced in human sperm. Reprod. Toxicol. 2012, 34, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Moretti, E.; Mazzi, L.; Bonechi, C.; Salvatici, M.C.; Iacoponi, F.; Rossi, C.; Collodel, G. Effect of Quercetin-loaded liposomes on induced oxidative stress in human spermatozoa. Reprod. Toxicol. 2016, 60, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Sierens, J.; Hartley, J.A.; Campbell, M.J.; Leathem, A.J.C.; Woodside, J.V. In vitro isoflavone supplementation reduces hydrogen peroxide-induced DNA damage in sperm. Teratog. Carcinog. Mutagen. 2002, 22, 227–234. [Google Scholar] [CrossRef]
- Garcez, M.E.; Branco, C.S.; Venturin, L.; Pasqualotto, F.F.; Salvador, M. Effects of resveratrol supplementation on cryopreservation medium of human semen. Fertil. Steril. 2010, 94, 2118–2121. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Soto, J.C.; de DiosHourcade, J.; Gutiérrez-Adán, A.; Landeras, J.L.; Gadea, J. Effect of genistein supplementation of thawing medium on characteristics of frozen human spermatozoa. Asian J. Androl. 2010, 12, 431–441. [Google Scholar] [CrossRef]
- Meamar, M.; Zribi, N.; Cambi, M.; Tamburrino, L.; Marchiani, S.; Filimberti, E.; Fino, M.G.; Biggeri, A.; Menezo, Y.; Forti, G.; et al. Sperm DNA fragmentation induced by cryopreservation: New insights and effect of a natural extract from Opuntia ficus-indica. Fertil. Steril. 2012, 98, 326–333. [Google Scholar] [CrossRef]
- Zribi, N.; Chakroun, N.F.; Ben Abdallah, F.; Elleuch, H.; Sellami, A.; Gargouri, J.; Rebai, T.; Fakhfakh, F.; Keskes, L.A. Effect of freezing-thawing process and quercetin on human sperm survival and DNA integrity. Cryobiology 2012, 65, 326–331. [Google Scholar] [CrossRef]
- Azadi, L.; Tavalaee, M.; Deemeh, M.R.; Arbabian, M.; Nasr-Esfahani, M.H. Effects of tempol and quercetin on human sperm function after cryopreservation. Cryoletters 2017, 38, 29–36. [Google Scholar]
- Nashtaei, M.S.; Amidi, F.; Sedighi Gilani, M.A.; Aleyasin, A.; Bakhshalizadeh, S.H.; Naji, M.; Nekoonam, S. Protective features of resveratrol on human spermatozoa cryopreservation may be mediated through 5′ AMP-activated protein kinase activation. Andrology 2017, 5, 313–326. [Google Scholar] [CrossRef]
- Nashtaei, M.S.; Nekoonam, S.; Naji, M.; Bakhshalizadeh, S.; Amidi, F. Cryoprotective effect of resveratrol on DNA damage and crucial human sperm messenger RNAs, possibly through 5′ AMP-activated protein kinase activation. Cell Tissue Bank 2018, 19, 87–95. [Google Scholar] [CrossRef]
- Cheraghi, E.; Sajadi, S.M.S.; Mehranjani, M.S. The effect of quercetin on the quality of sperm parameters in frozen-thawed semen of patients with asthenospermia. Andrologia 2021, 53, e14167. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Muscio, L.; Whiting, S.; Connaughton, H.S.; Fraser, B.A.; Nixon, B.; Smith, N.D.; De Iuliis, G.N. Analysis of the effects of polyphenols on human spermatozoa reveals unexpected impacts on mitochondrial membrane potential, oxidative stress and DNA integrity; implications for assisted reproductive technology. Biochem. Pharmacol. 2016, 121, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Khanduja, K.L.; Verma, A.; Bhardwaj, A. Impairment of human sperm motility and viability by quercetin is independent of lipid peroxidation. Andrologia 2001, 33, 277–281. [Google Scholar] [PubMed]
- Lv, M.G.; Chen, W.Q.; Weng, S.Q.; Chen, H.Y.; Cheng, Y.M.; Luo, T. Rosmarinic acid compromises human sperm functions by an intracellular Ca 2+ concentration-related mechanism. Reprod. Toxicol. 2018, 81, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Guido, C.; Perrotta, I.; Panza, S.; Middea, E.; Avena, P.; Santoro, M.; Marsico, S.; Imbrogno, P.; Andò, S.; Aquila, S. Human sperm physiology: Estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ) influence sperm metabolism and may be involved in the pathophysiology of varicocele-associated male infertility. J. Cell. Physiol. 2011, 226, 3403–3412. [Google Scholar] [CrossRef]
- Galluzzo, P.; Ascenzi, P.; Bulzomi, P.; Marino, M. The nutritional flavanone naringenin triggers antiestrogenic effects by regulating estrogen receptor alpha-palmitoylation. Endocrinology 2008, 149, 2567–2575. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Sun, B.; Ross, S.M.; Trask, O.J.; Carmichael, P.L.; Dent, M.; White, A.; Andersen, M.E.; Clewell, R.A. Assessing dose-dependent differences in DNA-damage, p53 response and genotoxicity for quercetin and curcumin. Toxicol. In Vitro 2013, 27, 1877–1887. [Google Scholar] [CrossRef]
- Hasa, D.; Perissutti, B.; Dall’Acqua, S.; Chierotti, M.R.; Gobetto, R.; Grabnar, I.; Cepek, C.; Voinovich, D. Rationale of using Vinca minor Linne dry extract phytocomplex as a vincamine’s oral bioavailability enhancer. Eur. J. Pharm. Biopharm. 2013, 84, 138–144. [Google Scholar] [CrossRef]
- Liu, J.; Liang, P.; Yin, C.; Wang, T.; Li, H.; Li, Y.; Ye, Z. Effects of several Chinese herbal aqueous extracts on human sperm motility in vitro. Andrologia 2004, 36, 78–83. [Google Scholar] [CrossRef]
- Lampiao, F.; Krom, D.; du Plessis, S.S. The in vitro effects of Mondia whitei on human sperm motility parameters. Phytother. Res. 2008, 22, 1272–1273. [Google Scholar] [CrossRef]
- Khaleghi, S.; Bakhtiari, M.; Asadmobini, A.; Esmaeili, F. Tribulus terrestris extract improves human sperm parameters in vitro. J. Evid. Based Complement. Altern. Med. 2017, 22, 407–412. [Google Scholar] [CrossRef]
- Parameswari, R.; Rao, K.A.; Mano, K.; Aruna, M.; Vickram, A.S.; Rameshpathy, M.; Sridharan, T.B. Human sperm DNA damage inhibition and antioxidant activity of T. arjuna bark: An in vitro study. 3 Biotech 2017, 7, 188. [Google Scholar]
- Grami, D.; Rtibi, K.; Selmi, S.; Jridi, M.; Sebai, H.; Marzouki, L.; Sabovic, I.; Foresta, C.; De Toni, L. Aqueous extract of Eruca sativa protects human spermatozoa from mitochondrial failure due to bisphenol A exposure. Reprod. Toxicol. 2018, 82, 103–110. [Google Scholar] [CrossRef]
- Mbaye, M.M.; El Khalfi, B.; Ouzamode, S.; Saadani, B.; Louanjli, N.; Soukri, A. Effect of Origanum vulgare essential oil supplementation on the advanced parameters of mobility and on the integrity of human sperm DNA. Int. J. Reprod. Med. 2020, 2020, 1230274. [Google Scholar] [CrossRef]
- Moichela, F.T.; Adefolaju, G.A.; Henkel, R.R.; Opuwari, C.S. Aqueous leaf extract of Moringa oleifera reduced intracellular ROS production, DNA fragmentation and acrosome reaction in Human spermatozoa in vitro. Andrologia 2021, 53, e13903. [Google Scholar] [CrossRef]
- Biagi, M.; Collodel, G.; Corsini, M.; Pascarelli, N.A.; Moretti, E. Protective effect of Propolfenol® on induced oxidative stress in human spermatozoa. Andrologia 2018, 50, e12807. [Google Scholar] [CrossRef]
- Russo, A.; Troncoso, N.; Sanchez, F.; Garbarino, J.A.; Vanella, A. Propolis protects human spermatozoa from DNA damage caused by benzo[a]pyrene and exogenous reactive oxygen species. Life Sci. 2006, 78, 1401–1406. [Google Scholar] [CrossRef]
- Biagi, M.; Noto, D.; Corsini, M.; Baini, G.; Cerretani, C.; Cappellucci, G.; Moretti, E. Antioxidant effect of the Castanea sativa Mill. leaf extract on oxidative stress induced upon human spermatozoa. Oxid. Med. Cell Longev. 2019, 2019, 8926075. [Google Scholar] [CrossRef]
- Kim, J.H.; Chae, M.R.; Wijerathne, T.D.; Cooray, A.D.; Kim, C.Y.; Lee, S.W.; Lee, K.P. In vitro assessment of Prunus japonica seed extract on human spermatozoa hypermotility and intracellular alkalization. Andrologia 2022, 54, e14471. [Google Scholar] [CrossRef]
- Premakumara, G.A.; Ratnasooriya, W.D.; Tillekeratne, L.M.; Amarasekare, A.S.; Rahman, A.U. Human sperm motility stimulating activity of a sulfono glycolipid isolated from Sri Lankan marine red alga Gelidiella acerosa. Asian J. Androl. 2001, 3, 27–31. [Google Scholar]
- Chen, D.L.; Li, N.; Lin, L.; Long, H.M.; Lin, H.; Chen, J.; Zhang, H.M.; Zeng, C.C.; Liu, S.H. Confocal mirco-Raman spectroscopic analysis of the antioxidant protection mechanism of the oligosaccharides extracted from Morinda officinalis on human sperm DNA. J. Ethnopharmacol. 2014, 153, 119–124. [Google Scholar] [CrossRef]
- Rad, M.K.; Ghani, A.; Ghani, E. In vitro effects of Capparis spinosa L. extract on human sperm function, DNA fragmentation, and oxidative stress. J. Ethnopharmacol. 2021, 269, 113702. [Google Scholar]
- Shalaweh, S.M.; Erasmus, N.; Weitz, F.; Henkel, R.R. Effect of Cissampelos capensis rhizome extract on human spermatozoa in vitro. Andrologia 2015, 47, 318–327. [Google Scholar] [CrossRef]
- Takalani, N.B.; Adefolaju, G.A.; Henkel, R.; Opuwari, C.S. In vitro effects of aqueous extract of unfermented rooibos on human sperm function. Andrologia 2022, 54, e14452. [Google Scholar] [CrossRef]
- Fatma, B.A.; Nozha, C.F.; Ines, D.; Hamadi, A.; Basma, H.; Leila, A.K. Sperm quality improvement after date seed oil in vitro supplementation in spontaneous and induced oxidative stress. Asian J. Androl. 2009, 11, 393–398. [Google Scholar] [CrossRef]
- Asadmobini, A.; Bakhtiari, M.; Khaleghi, S.; Esmaeili, F.; Mostafaei, A. The effect of Tribulus terrestris extract on motility and viability of human sperms after cryopreservation. Cryobiology 2017, 75, 154–159. [Google Scholar] [CrossRef]
- Parameswari, R.; Rao, K.A.; Manigandan, P.; Vickram, A.S.; Archana, A.; Sridharan, T.B. Tea polyphenol-T. arjuna bark as sperm antioxidant extender in infertile smokers. Cryoletters 2017, 38, 95–99. [Google Scholar]
- Shiri, E.; Abolhassani, F.; Khosravizadeh, Z.; Najafi, A.; Khanezad, M.; Vazirian, M.; Fallahi, P.; Rezaeian, Z.; Hedayatpour, A. Aqueous Origanum vulgare extract improves the quality of cryopreserved human spermatozoa through its antioxidant effects. Biopreserv. Biobank. 2020, 18, 329–336. [Google Scholar] [CrossRef]
- Contino, M.; Leonardi, C.; Genovese, C.; Scalisi, E.M.; Pecoraro, R.; Ignoto, S.; Failla, C.; Ferruggia, G.; Salvaggio, A.; Asero, P.; et al. Antioxidant activity of two Opuntia Mill. species fruit extracts on human sperm quality after a freeze-thaw cycle. Nat. Prod. Res. 2022, 1–7. [Google Scholar] [CrossRef]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of fruit industrial by-products—A case study on circular economy approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, E.; Signorini, C.; Corsaro, R.; Giamalidi, M.; Collodel, G. Human Sperm as an In Vitro Model to Assess the Efficacy of Antioxidant Supplements during Sperm Handling: A Narrative Review. Antioxidants 2023, 12, 1098. https://doi.org/10.3390/antiox12051098
Moretti E, Signorini C, Corsaro R, Giamalidi M, Collodel G. Human Sperm as an In Vitro Model to Assess the Efficacy of Antioxidant Supplements during Sperm Handling: A Narrative Review. Antioxidants. 2023; 12(5):1098. https://doi.org/10.3390/antiox12051098
Chicago/Turabian StyleMoretti, Elena, Cinzia Signorini, Roberta Corsaro, Maria Giamalidi, and Giulia Collodel. 2023. "Human Sperm as an In Vitro Model to Assess the Efficacy of Antioxidant Supplements during Sperm Handling: A Narrative Review" Antioxidants 12, no. 5: 1098. https://doi.org/10.3390/antiox12051098
APA StyleMoretti, E., Signorini, C., Corsaro, R., Giamalidi, M., & Collodel, G. (2023). Human Sperm as an In Vitro Model to Assess the Efficacy of Antioxidant Supplements during Sperm Handling: A Narrative Review. Antioxidants, 12(5), 1098. https://doi.org/10.3390/antiox12051098