Pharmacologic Comparison of High-Dose Hesperetin and Quercetin on MDCK II Cell Viability, Tight Junction Integrity, and Cell Shape
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture and Morphology Analysis
2.3. Immunofluorescence Microscopy
2.4. Western Blotting
2.5. Real-Time PCR
2.6. Protein Expression and Purification
2.7. NMR Titration Experiments
2.8. Molecular Docking Based on NMR Chemical Shift Perturbations
2.9. Statistical Analysis
3. Results
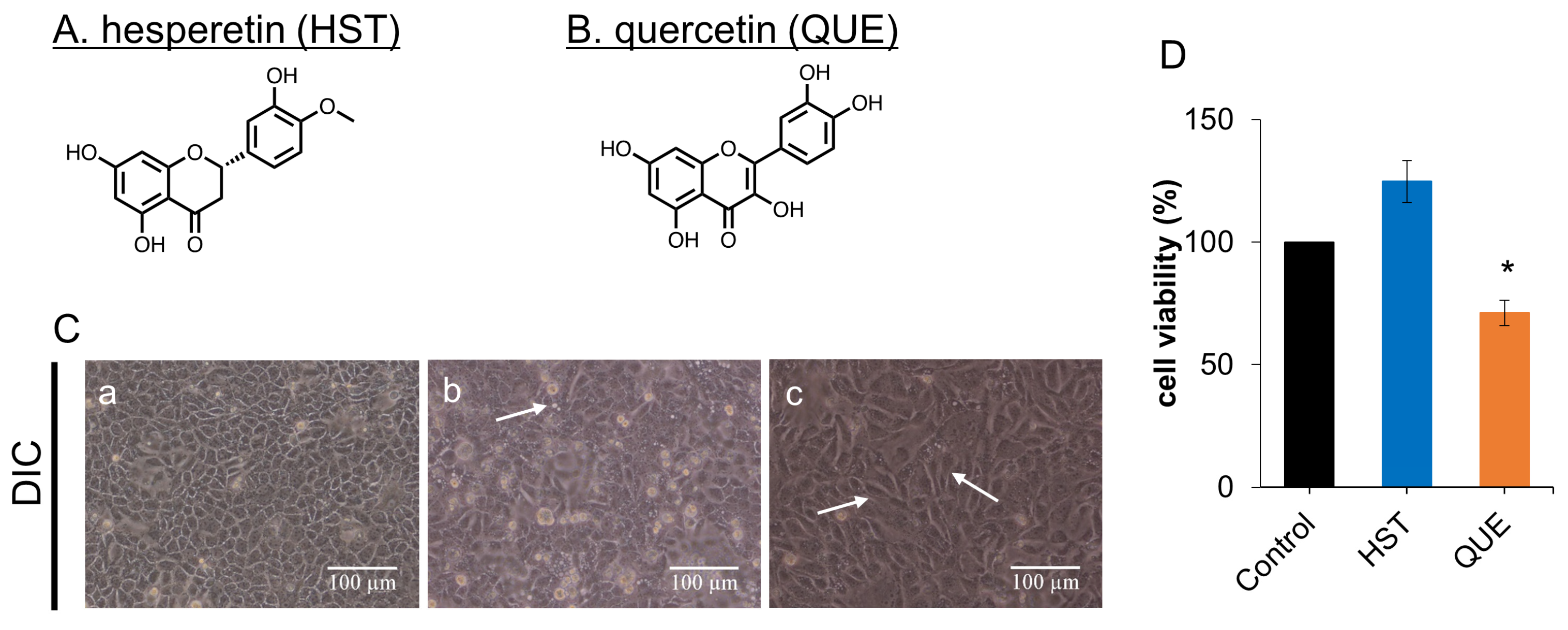
3.1. Cell Viability of HST or QUE
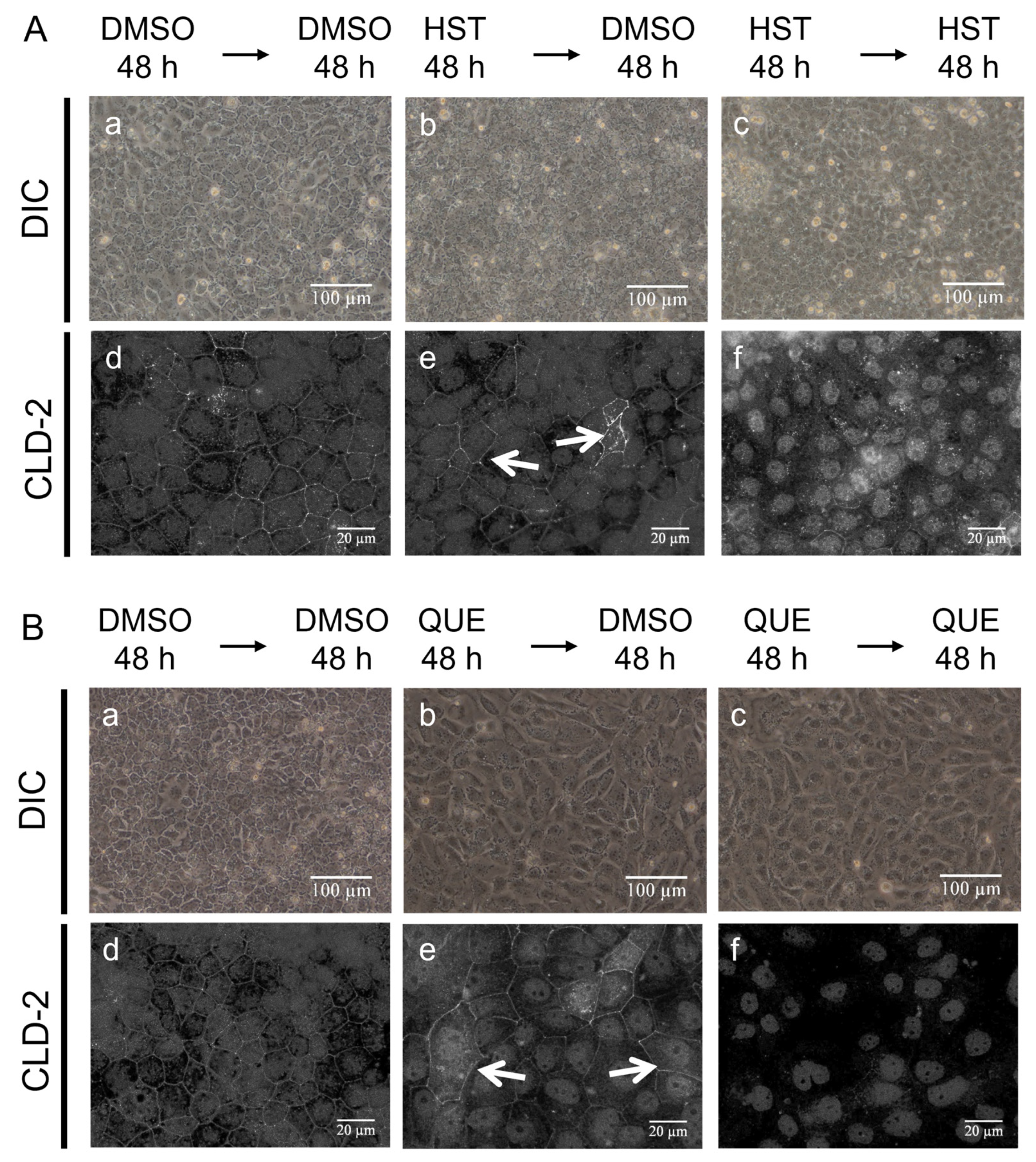
3.2. Changes in Cell Morphology Induced by QUE
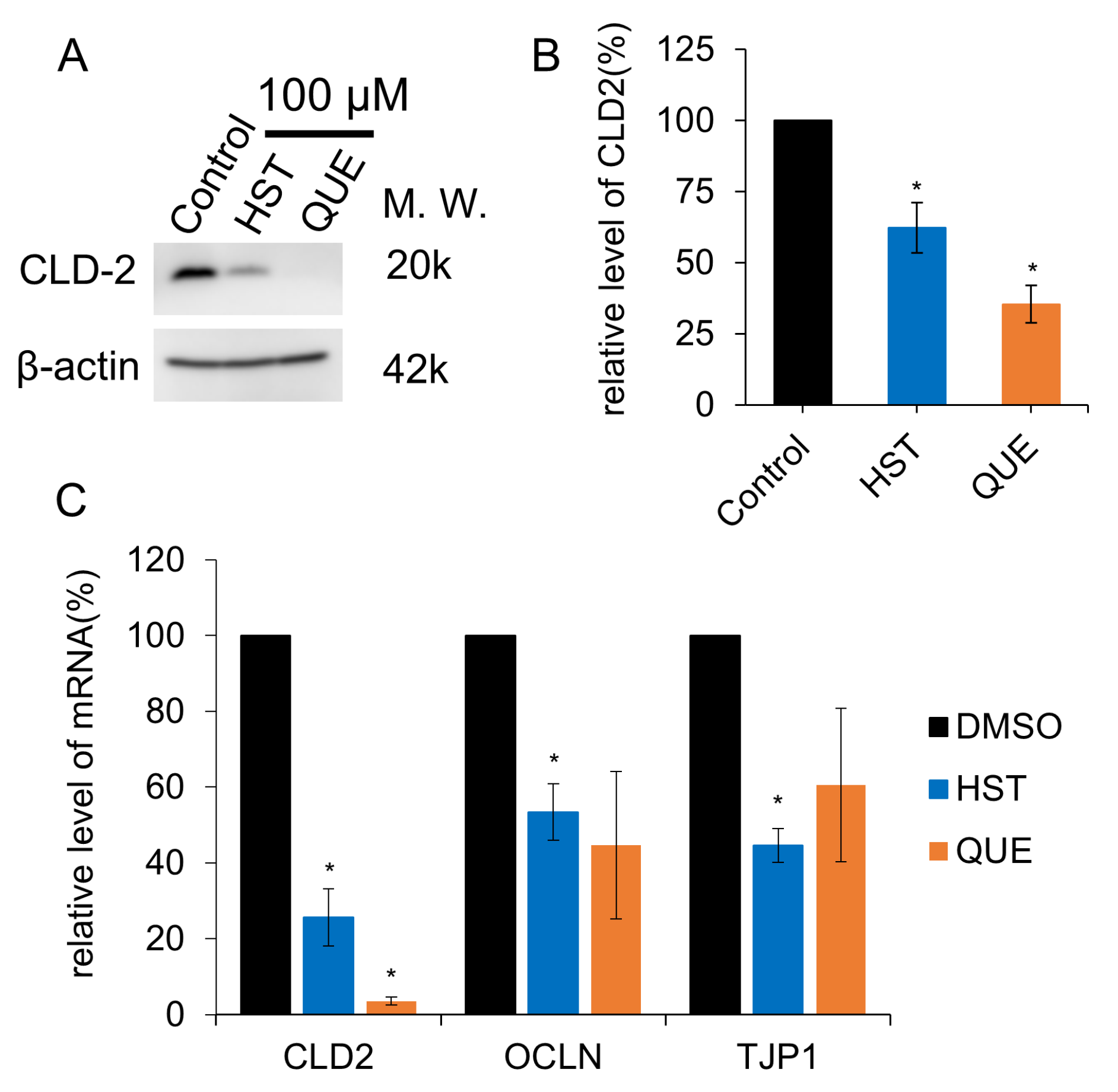
3.3. TJ Reduction Activity of HST or QUE
3.4. NMR Evidence of Direct Binding of HST but Not QUE to ZO-1(PDZ1)
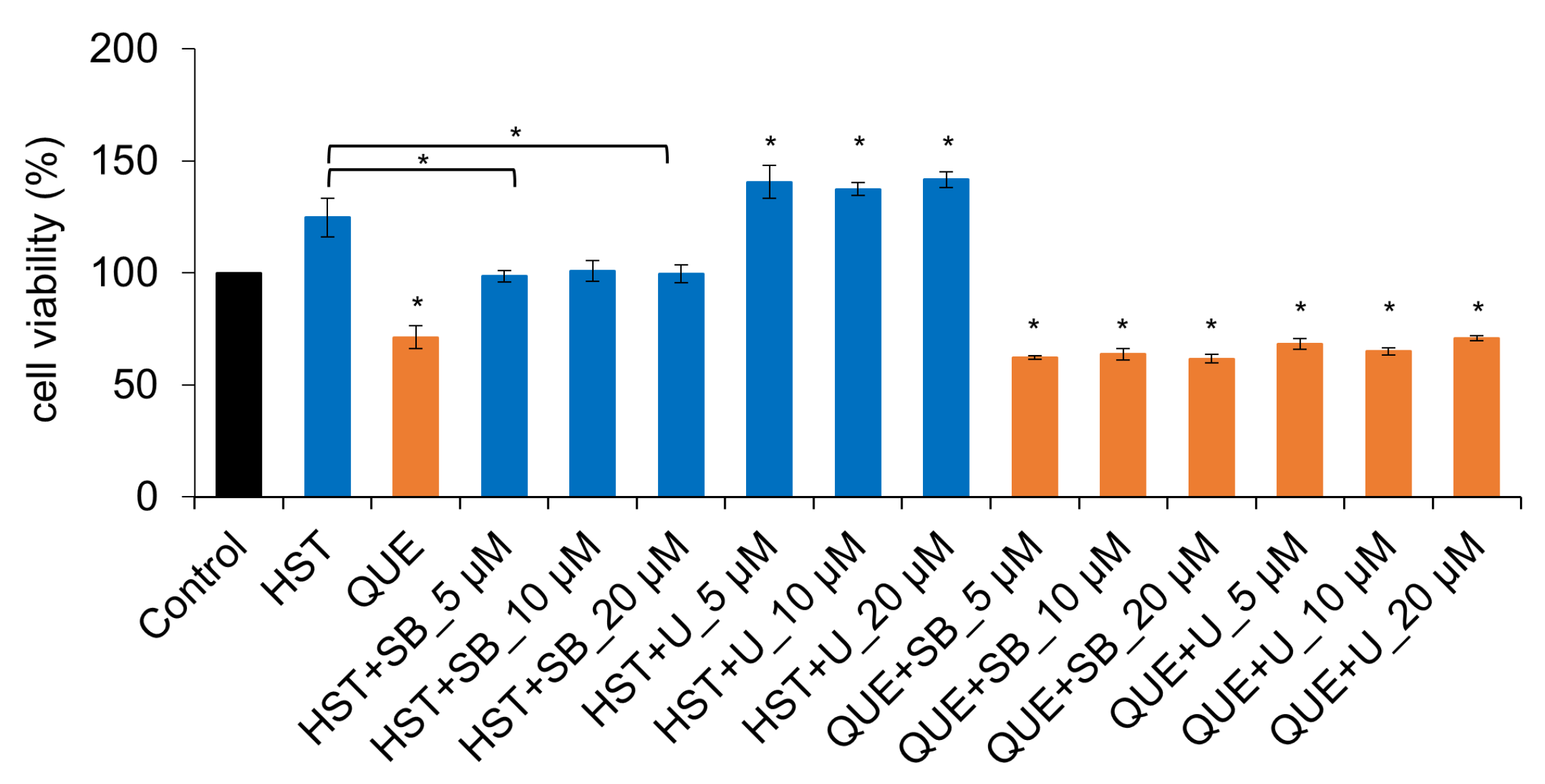
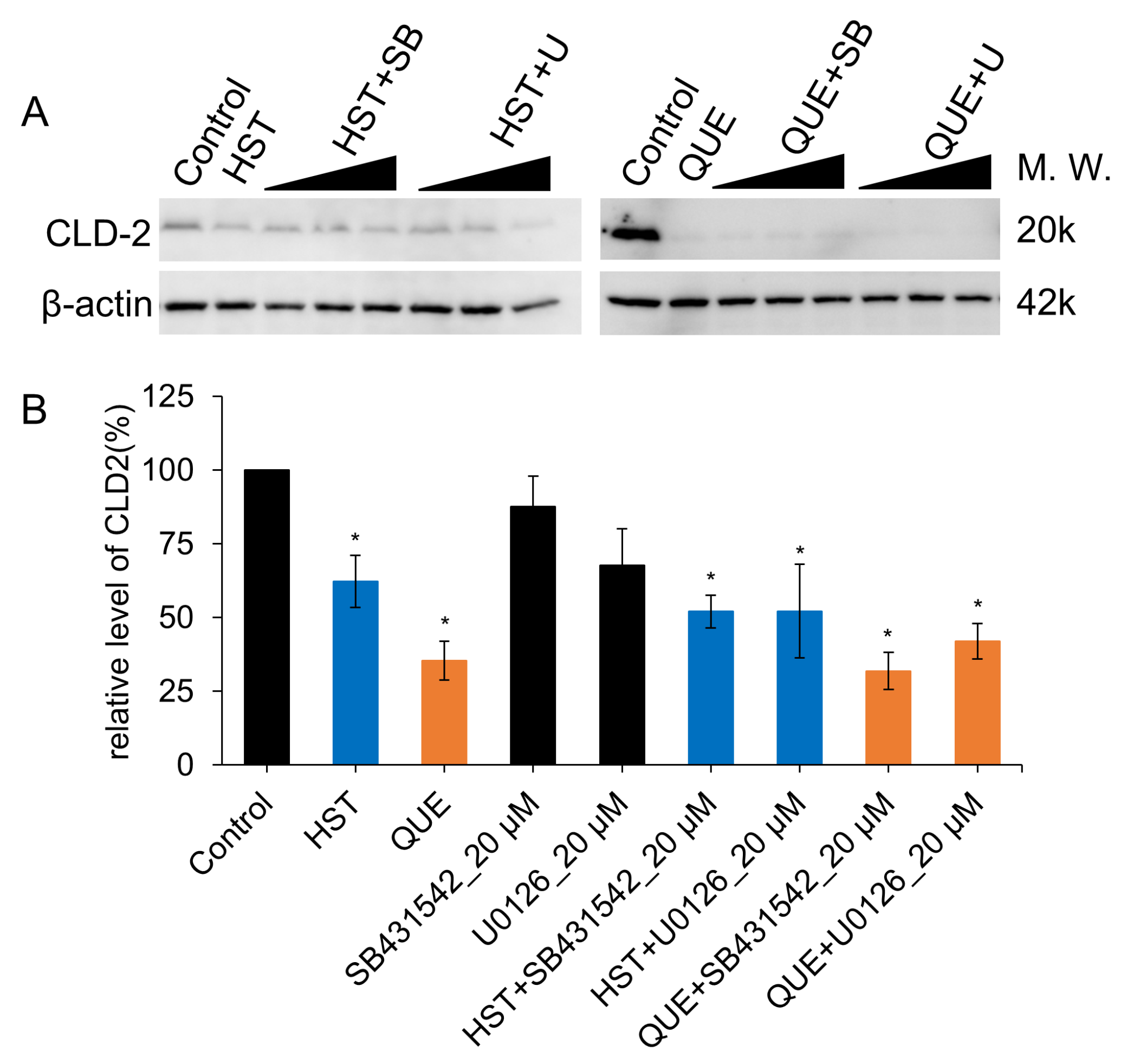
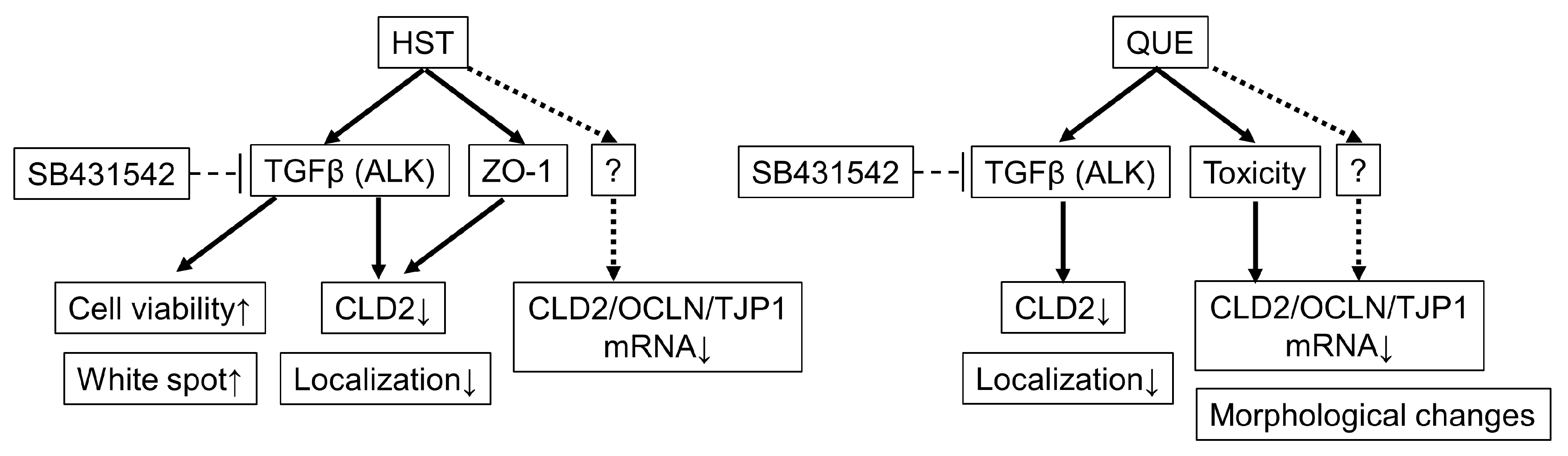
3.5. Pharmacological Investigation of the Mechanism of TJ-Opening Using HST or QUE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Förster, C. Tight Junctions and the Modulation of Barrier Function in Disease. Histochem. Cell Biol. 2008, 130, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Hata, M.; Furuse, K.; Yoshida, Y.; Haratake, A.; Sugitani, Y.; Noda, T.; Kubo, A.; Tsukita, S. Claudin-Based Tight Junctions Are Crucial for the Mammalian Epidermal Barrier. J. Cell Biol. 2002, 156, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Tsukita, S.; Furuse, M.; Itoh, M. Multifunctional Strands in Tight Junctions. Nat. Rev. Mol. Cell Biol. 2001, 2, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Fanning, A.S.; Mitic, L.L.; Anderson, J.M. Transmembrane Proteins in the Tight Junction Barrier. J. Am. Soc. Nephrol. 1999, 10, 1337–1345. [Google Scholar] [CrossRef]
- Furuse, M.; Fujita, K.; Hiiragi, T.; Fujimoto, K.; Tsukita, S. Claudin-1 and -2: Novel Integral Membrane Proteins Localizing at Tight Junctions with No Sequence Similarity to Occludin. J. Cell Biol. 1998, 141, 1539–1550. [Google Scholar] [CrossRef]
- Paris, L.; Tonutti, L.; Vannini, C.; Bazzoni, G. Structural Organization of the Tight Junctions. Biochim. Biophys. Acta 2008, 1778, 646–659. [Google Scholar] [CrossRef]
- Itoh, M.; Furuse, M.; Morita, K.; Kubota, K.; Saitou, M.; Tsukita, S. Direct Binding of Three Tight Junction-Associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH Termini of Claudins. J. Cell Biol. 1999, 147, 1351–1363. [Google Scholar] [CrossRef]
- Tokuda, S.; Higashi, T.; Furuse, M. ZO-1 Knockout by TALEN-Mediated Gene Targeting in MDCK Cells: Involvement of ZO-1 in the Regulation of Cytoskeleton and Cell Shape. PLoS ONE 2014, 9, e104994. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Betanzos, A.; Nava, P.; Jaramillo, B.E. Tight Junction Proteins. Prog. Biophys. Mol. Biol. 2003, 81, 1–44. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Betanzos, A.; Avila-Flores, A. MAGUK Proteins: Structure and Role in the Tight Junction. Semin. Cell Dev. Biol. 2000, 11, 315–324. [Google Scholar] [CrossRef]
- Nomme, J.; Antanasijevic, A.; Caffrey, M.; van Itallie, C.M.; Anderson, J.M.; Fanning, A.S.; Lavie, A. Structural Basis of a Key Factor Regulating the Affinity between the Zonula Occludens First PDZ Domain and Claudins. J. Biol. Chem. 2015, 290, 16595–16606. [Google Scholar] [CrossRef] [PubMed]
- Fanning, A.S.; van Itallie, C.M.; Anderson, J.M. Zonula Occludens-1 and -2 Regulate Apical Cell Structure and the Zonula Adherens Cytoskeleton in Polarized Epithelia. Mol. Biol. Cell 2012, 23, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Ikenouchi, J.; Umeda, K.; Tsukita, S.; Furuse, M.; Tsukita, S. Requirement of ZO-1 for the Formation of Belt-like Adherens Junctions during Epithelial Cell Polarization. J. Cell Biol. 2007, 176, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M. Knockout Animals and Natural Mutations as Experimental and Diagnostic Tool for Studying Tight Junction Functions in Vivo. Biochim. Biophys. Acta 2009, 1788, 813–819. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, H.; Xia, M.; Deng, B.; Shen, H.; Ji, G.; Li, G.; Xie, Y. The Effect of Phytic Acid on Tight Junctions in the Human Intestinal Caco-2 Cell Line and Its Mechanism. Eur. J. Pharm. Sci. 2015, 80, 1–8. [Google Scholar] [CrossRef]
- Hiroaki, H.; Satomura, K.; Goda, N.; Nakakura, Y.; Hiranuma, M.; Tenno, T.; Hamada, D.; Ikegami, T. Spatial Overlap of Claudin- and Phosphatidylinositol Phosphate-Binding Sites on the First PDZ Domain of Zonula Occludens 1 Studied by NMR. Molecules 2018, 23, 2465. [Google Scholar] [CrossRef]
- Tenno, T.; Kataoka, K.; Goda, N.; Hiroaki, H. NMR-Guided Repositioning of Non-Steroidal Anti-Inflammatory Drugs into Tight Junction Modulators. Int. J. Mol. Sci. 2021, 22, 2583. [Google Scholar] [CrossRef]
- Hisada, M.; Hiranuma, M.; Nakashima, M.; Goda, N.; Tenno, T.; Hiroaki, H. High Dose of Baicalin or Baicalein Can Reduce Tight Junction Integrity by Partly Targeting the First PDZ Domain of Zonula Occludens-1 (ZO-1). Eur. J. Pharmacol. 2020, 887, 173436. [Google Scholar] [CrossRef]
- Tenno, T.; Goda, N.; Umetsu, Y.; Ota, M.; Kinoshita, K.; Hiroaki, H. Accidental Interaction between PDZ Domains and Diclofenac Revealed by NMR-Assisted Virtual Screening. Molecules 2013, 18, 9567–9581. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and Anti-Inflammatory Properties of the Citrus Flavonoids Hesperidin and Hesperetin: An Updated Review of Their Molecular Mechanisms and Experimental Models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Nakashima, M.; Hisada, M.; Goda, N.; Tenno, T.; Kotake, A.; Inotsume, Y.; Kameoka, I.; Hiroaki, H. Opposing Effect of Naringenin and Quercetin on the Junctional Compartment of MDCK II Cells to Modulate the Tight Junction. Nutrients 2020, 12, 3285. [Google Scholar] [CrossRef] [PubMed]
- Gamero-Estevez, E.; Andonian, S.; Jean-Claude, B.; Gupta, I.; Ryan, A.K. Temporal Effects of Quercetin on Tight Junction Barrier Properties and Claudin Expression and Localization in MDCK II Cells. Int. J. Mol. Sci. 2019, 20, 4889. [Google Scholar] [CrossRef] [PubMed]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; deTakats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I Clinical Trial of the Flavonoid Quercetin: Pharmacokinetics and Evidence for in Vivo Tyrosine Kinase Inhibition. Clin. Cancer Res. 1996, 2, 659–668. [Google Scholar] [PubMed]
- Umetsu, Y.; Goda, N.; Taniguchi, R.; Satomura, K.; Ikegami, T.; Furuse, M.; Hiroaki, H. 1H, 13C, and 15N Resonance Assignment of the First PDZ Domain of Mouse ZO-1. Biomol. NMR Assign. 2011, 5, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Grzesiek, S.; Bax, A.; Clore, G.M.; Gronenborn, A.M.; Hu, J.S.; Kaufman, J.; Palmer, I.; Stahl, S.J.; Wingfield, P.T. The Solution Structure of HIV-1 Nef Reveals an Unexpected Fold and Permits Delineation of the Binding Surface for the SH3 Domain of Hck Tyrosine Protein Kinase. Nat. Struct. Biol. 1996, 3, 340–345. [Google Scholar] [CrossRef]
- Schumann, F.H.; Riepl, H.; Maurer, T.; Gronwald, W.; Neidig, K.-P.; Kalbitzer, H.R. Combined Chemical Shift Changes and Amino Acid Specific Chemical Shift Mapping of Protein-Protein Interactions. J. Biomol. NMR 2007, 39, 275–289. [Google Scholar] [CrossRef]
- Dominguez, C.; Boelens, R.; Bonvin, A.M.J.J. HADDOCK: A Protein−Protein Docking Approach Based on Biochemical or Biophysical Information. J. Am. Chem. Soc. 2003, 125, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Van Zundert, G.C.P.; Rodrigues, J.P.G.L.M.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; Van Dijk, M.; De Vries, S.J.; Bonvin, A.M.J.J. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef]
- Morimoto, R.; Hanada, A.; Matsubara, C.; Horio, Y.; Sumitani, H.; Ogata, T.; Isegawa, Y. Anti-Influenza A Virus Activity of Flavonoids in Vitro: A Structure–Activity Relationship. J. Nat. Med. 2023, 77, 219–227. [Google Scholar] [CrossRef]
- Bhat, F.A.; Sharmila, G.; Balakrishnan, S.; Arunkumar, R.; Elumalai, P.; Suganya, S.; Raja Singh, P.; Srinivasan, N.; Arunakaran, J. Quercetin Reverses EGF-Induced Epithelial to Mesenchymal Transition and Invasiveness in Prostate Cancer (PC-3) Cell Line via EGFR/PI3K/Akt Pathway. J. Nutr. Biochem. 2014, 25, 1132–1139. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Tsai, P.-H.; Kandaswami, C.C.; Cheng, C.-H.; Ke, F.-C.; Lee, P.-P.; Hwang, J.-J.; Lee, M.-T. Effects of Dietary Flavonoids, Luteolin, and Quercetin on the Reversal of Epithelial-Mesenchymal Transition in A431 Epidermal Cancer Cells. Cancer Sci. 2011, 102, 1829–1839. [Google Scholar] [CrossRef]
- Furuse, M.; Furuse, K.; Sasaki, H.; Tsukita, S. Conversion of Zonulae Occludentes from Tight to Leaky Strand Type by Introducing Claudin-2 into Madin-Darby Canine Kidney I Cells. J. Cell Biol. 2001, 153, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Hibino, E.; Goda, N.; Hisada, M.; Tenno, T.; Hiroaki, H. Direct Inhibition of the First PDZ Domain of ZO-1 by Glycyrrhizin Is a Possible Mechanism of Tight Junction Opening of Caco-2 Cells. Food Funct. 2022, 13, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Quantitation of Flavonoid Constituents in Citrus Fruits. J. Agric. Food Chem. 1999, 47, 3565–3571. [Google Scholar] [CrossRef] [PubMed]
- Morin, B.; Nichols, L.A.; Zalasky, K.M.; Davis, J.W.; Manthey, J.A.; Holland, L.J. The Citrus Flavonoids Hesperetin and Nobiletin Differentially Regulate Low Density Lipoprotein Receptor Gene Transcription in HepG2 Liver Cells. J. Nutr. 2008, 138, 1274–1281. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, Y.; Liu, H.; Ren, H.; Cui, Z. Hesperetin Protects against H2O2-Triggered Oxidative Damage via Upregulation of the Keap1-Nrf2/HO-1 Signal Pathway in ARPE-19 Cells. Biomed. Pharmacother. 2017, 88, 124–133. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Kong, L.; Tang, Z.-Z.; Zhang, Y.-M.; Liu, Y.; Wang, T.-Y.; Liu, Y.-W. Hesperetin Ameliorates Diabetic Nephropathy in Rats by Activating Nrf2/ARE/Glyoxalase 1 Pathway. Biomed. Pharmacother. 2019, 111, 1166–1175. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin Ameliorates Hepatic Oxidative Stress and Inflammation via the PI3K/AKT-Nrf2-ARE Pathway in Oleic Acid-Induced HepG2 Cells and a Rat Model of High-Fat Diet-Induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef]
- Vander Dos Santos, R.; Villalta-Romero, F.; Stanisic, D.; Borro, L.; Neshich, G.; Tasic, L. Citrus Bioflavonoid, Hesperetin, as Inhibitor of Two Thrombin-like Snake Venom Serine Proteases Isolated from Crotalus Simus. Toxicon 2018, 143, 36–43. [Google Scholar] [CrossRef]
- Eberle, R.J.; Olivier, D.S.; Amaral, M.S.; Willbold, D.; Arni, R.K.; Coronado, M.A. Promising Natural Compounds against Flavivirus Proteases: Citrus Flavonoids Hesperetin and Hesperidin. Plants 2021, 10, 2183. [Google Scholar] [CrossRef]
- Lim, W.Z.; Cheng, P.G.; Abdulrahman, A.Y.; Teoh, T.C. The Identification of Active Compounds in Ganoderma Lucidum Var. Antler Extract Inhibiting Dengue Virus Serine Protease and Its Computational Studies. J. Biomol. Struct. Dyn. 2020, 38, 4273–4288. [Google Scholar] [CrossRef] [PubMed]
- Eberle, R.J.; Olivier, D.S.; Pacca, C.C.; Avilla, C.M.S.; Nogueira, M.L.; Amaral, M.S.; Willbold, D.; Arni, R.K.; Coronado, M.A. In Vitro Study of Hesperetin and Hesperidin as Inhibitors of Zika and Chikungunya Virus Proteases. PLoS ONE 2021, 16, e0246319. [Google Scholar] [CrossRef]
- Alvarez-Collazo, J.; López-Requena, A.; Galán, L.; Talavera, A.; Alvarez, J.L.; Talavera, K. The Citrus Flavanone Hesperetin Preferentially Inhibits Slow-Inactivating Currents of a Long QT Syndrome Type 3 Syndrome Na+ Channel Mutation. Br. J. Pharmacol. 2019, 176, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.A.; Mendonca, P.; Soliman, K.F.A. Neuroprotective Effects and Therapeutic Potential of the Citrus Flavonoid Hesperetin in Neurodegenerative Diseases. Nutrients 2022, 14, 2228. [Google Scholar] [CrossRef]
- Nagira, M.; Tomita, M.; Mizuno, S.; Kumata, M.; Ayabe, T.; Hayashi, M. Ischemia/Reperfusion Injury in the Monolayers of Human Intestinal Epithelial Cell Line Caco-2 and Its Recovery by Antioxidants. Drug Metab. Pharm. 2006, 21, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, E.K.; Park, M.H.; Ha, Y.M.; Jung, K.J.; Kim, M.-S.; Kim, M.K.; Yu, B.P.; Chung, H.Y. Ferulate Protects the Epithelial Barrier by Maintaining Tight Junction Protein Expression and Preventing Apoptosis in Tert-Butyl Hydroperoxide-Induced Caco-2 Cells. Phytother. Res. 2013, 27, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Chu, L.; Liu, Y.; Wang, A.; Ji, B.; Wu, W.; Zhou, F.; Wei, Y.; Cheng, Q.; Cai, S.; et al. Analysis of the Antioxidant Capacities of Flavonoids under Different Spectrophotometric Assays Using Cyclic Voltammetry and Density Functional Theory. J. Agric. Food Chem. 2011, 59, 10277–10285. [Google Scholar] [CrossRef]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the Antioxidant Activity of Flavonoids by “Ferric Reducing Antioxidant Power” Assay and Cyclic Voltammetry. Biochim. Biophys. Acta 2005, 1721, 174–184. [Google Scholar] [CrossRef]
- Rao, R. Oxidative Stress-Induced Disruption of Epithelial and Endothelial Tight Junctions. Front. Biosci. 2008, 13, 7210–7226. [Google Scholar] [CrossRef]
- Jia, X.; Yuan, Z.; Yang, Y.; Huang, X.; Han, N.; Liu, X.; Lin, X.; Ma, T.; Xu, B.; Wang, P.; et al. Multi-Functional Self-Assembly Nanoparticles Originating from Small Molecule Natural Product for Oral Insulin Delivery through Modulating Tight Junctions. J. Nanobiotechnol. 2022, 20, 116. [Google Scholar] [CrossRef]


| HST | QUE | BLI a | BLE a | |
|---|---|---|---|---|
| CLD-2 | ||||
| TJ localization | − − | − − − | − − − | − − − |
| mRNA expression | − | − − − | − | − |
| OCLN | ||||
| TJ localization | − | − | +/− | +/− |
| mRNA expression | − | − − − | n.ex. | n.ex. |
| ZO-1 expression | ||||
| TJ localization | − | − | +/− | +/− |
| mRNA expression | − | − − − | n.ex. | n.ex. |
| MDCK II cell | ||||
| Proliferation | + + | − − | +/− | − |
| Slenderer cell shape | no | yes | no | yes |
| Direct ZO1(PDZ1) interaction | yes | no | yes | yes |
| Other signaling pathways | ||||
| TGFβ | partially | partially | yes | yes |
| MEK/ERK | partially | no | partially | yes |
| Antioxidant capacity from literature | ||||
| TEAC (trolox equiv./mmol) b | 2.01 ± 0.04 | 5.72 ± 0.16 | ||
| FCR (chlorogenic acid equiv./mmol b | 0.53 ± 0.03 | 1.24 ± 0.09 | ||
| DPPH (trolox equiv./mmol) b | 0.48 ± 0.04 | 2.25 ± 0.09 | ||
| FRAP (ferrous equiv./μmol) c | 21.1 ± 0.4 | 95.9 ± 5.4 | 38.9 ± 2.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakashima, M.; Goda, N.; Tenno, T.; Kotake, A.; Inotsume, Y.; Amaya, M.; Hiroaki, H. Pharmacologic Comparison of High-Dose Hesperetin and Quercetin on MDCK II Cell Viability, Tight Junction Integrity, and Cell Shape. Antioxidants 2023, 12, 952. https://doi.org/10.3390/antiox12040952
Nakashima M, Goda N, Tenno T, Kotake A, Inotsume Y, Amaya M, Hiroaki H. Pharmacologic Comparison of High-Dose Hesperetin and Quercetin on MDCK II Cell Viability, Tight Junction Integrity, and Cell Shape. Antioxidants. 2023; 12(4):952. https://doi.org/10.3390/antiox12040952
Chicago/Turabian StyleNakashima, Mio, Natsuko Goda, Takeshi Tenno, Ayaka Kotake, Yuko Inotsume, Minako Amaya, and Hidekazu Hiroaki. 2023. "Pharmacologic Comparison of High-Dose Hesperetin and Quercetin on MDCK II Cell Viability, Tight Junction Integrity, and Cell Shape" Antioxidants 12, no. 4: 952. https://doi.org/10.3390/antiox12040952
APA StyleNakashima, M., Goda, N., Tenno, T., Kotake, A., Inotsume, Y., Amaya, M., & Hiroaki, H. (2023). Pharmacologic Comparison of High-Dose Hesperetin and Quercetin on MDCK II Cell Viability, Tight Junction Integrity, and Cell Shape. Antioxidants, 12(4), 952. https://doi.org/10.3390/antiox12040952







