Changes in Dietary Inflammatory Index Score over Time and Cancer Development in Rural Post-Menopausal Women
Abstract
1. Introduction
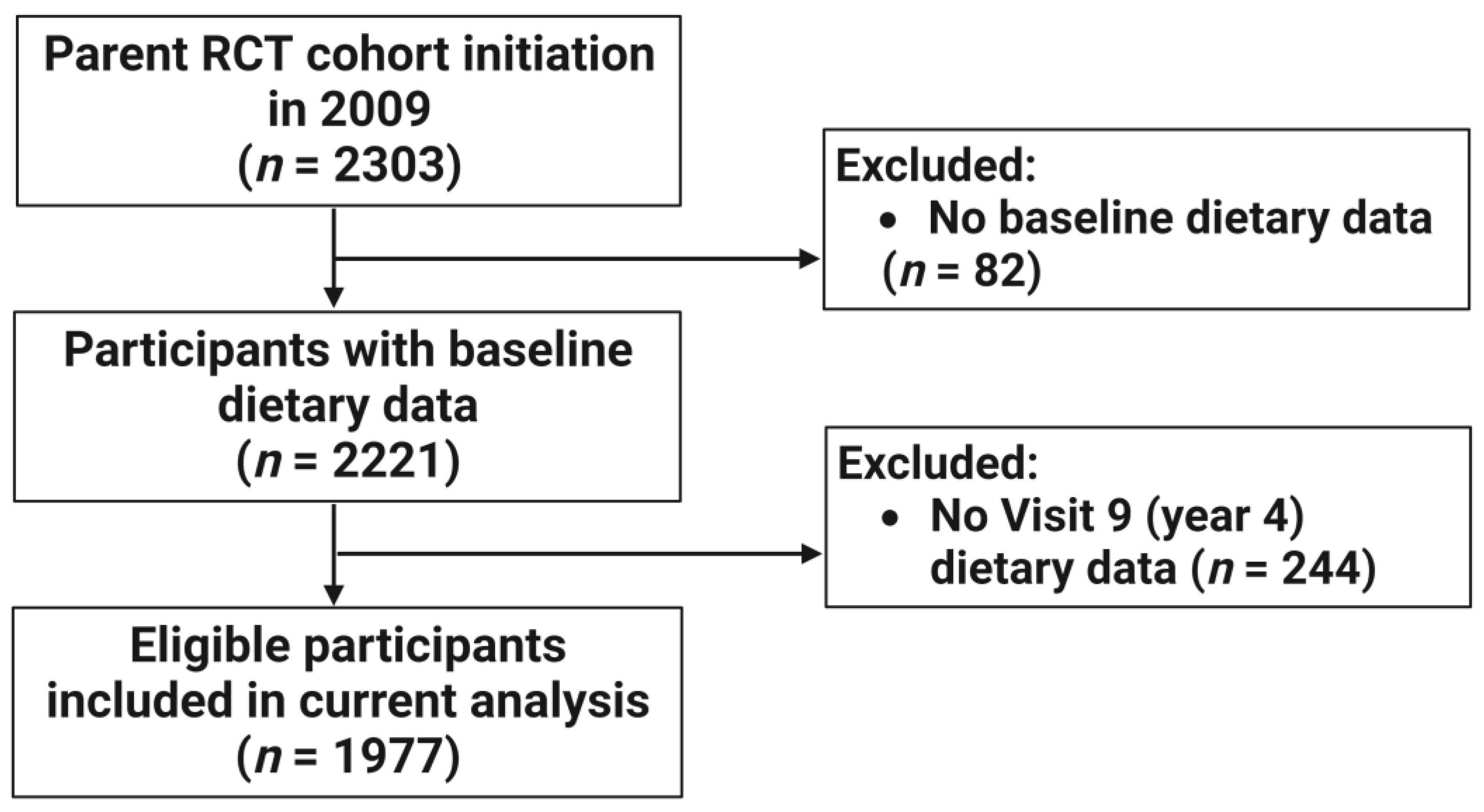
2. Materials and Methods
3. Results
3.1. Characteristics of the Study Population
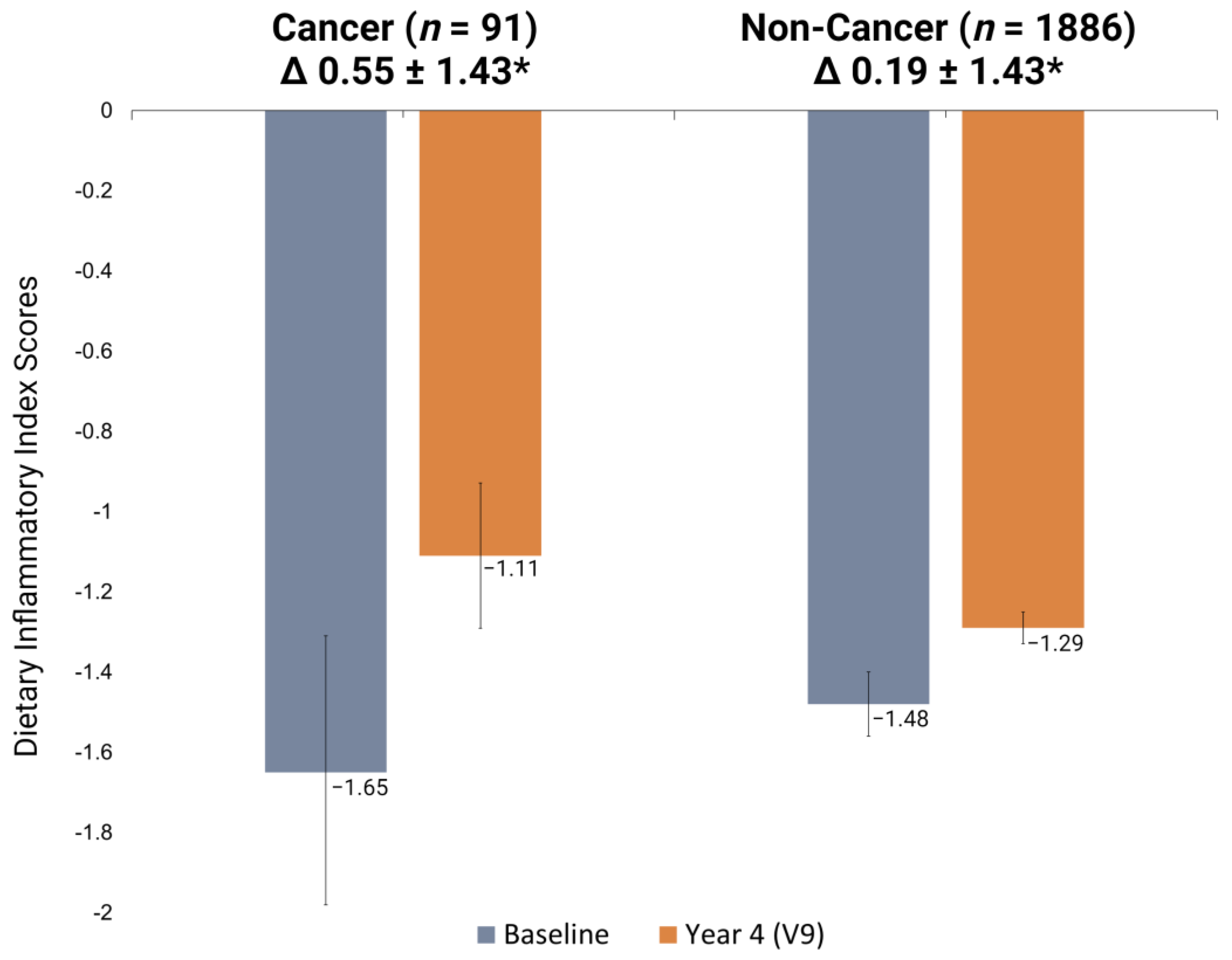
3.2. E-DII Scores and Cancer Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef]
- Murata, M. Inflammation and cancer. Environ. Health Prev. Med. 2018, 23, 50. [Google Scholar] [CrossRef] [PubMed]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018: The Cancer Process. Available online: www.dietandcancerreport.org (accessed on 5 January 2023).
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary patterns and biomarkers of oxidative stress and inflammation: A systematic review of observational and intervention studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Martinez-Gonzalez, M.A.; Corella, D.; Salas-Salvado, J.; Ruiz-Gutierrez, V.; Covas, M.I.; Fiol, M.; Gomez-Gracia, E.; Lopez-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Turpin, W.; Dong, M.; Sasson, G.; Raygoza Garay, J.A.; Espin-Garcia, O.; Lee, S.H.; Neustaeter, A.; Smith, M.I.; Leibovitzh, H.; Guttman, D.S.; et al. Mediterranean-Like Dietary Pattern Associations With Gut Microbiome Composition and Subclinical Gastrointestinal Inflammation. Gastroenterology 2022, 163, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: A systematic literature review. Nutr. Rev. 2013, 71, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Johansson-Persson, A.; Ulmius, M.; Cloetens, L.; Karhu, T.; Herzig, K.H.; Onning, G. A high intake of dietary fiber influences C-reactive protein and fibrinogen, but not glucose and lipid metabolism, in mildly hypercholesterolemic subjects. Eur. J. Nutr. 2014, 53, 39–48. [Google Scholar] [CrossRef]
- Wood, A.D.; Strachan, A.A.; Thies, F.; Aucott, L.S.; Reid, D.M.; Hardcastle, A.C.; Mavroeidi, A.; Simpson, W.G.; Duthie, G.G.; Macdonald, H.M. Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. Br. J. Nutr. 2014, 112, 1341–1352. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hebert, J.R. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Hayati, Z.; Montazeri, V.; Shivappa, N.; Hebert, J.R.; Pirouzpanah, S. The association between the inflammatory potential of diet and the risk of histopathological and molecular subtypes of breast cancer in northwestern Iran: Results from the Breast Cancer Risk and Lifestyle study. Cancer 2022, 128, 2298–2312. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Blair, C.K.; Prizment, A.E.; Jacobs, D.R.; Hebert, J.R. Prospective study of the dietary inflammatory index and risk of breast cancer in postmenopausal women. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hébert, J.R.; Rosato, V.; Montella, M.; Serraino, D.; La Vecchia, C. Association between the dietary inflammatory index and breast cancer in a large Italian case-control study. Mol. Nutr Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hebert, J.R.; Rosato, V.; Rossi, M.; Montella, M.; Serraino, D.; La Vecchia, C. Dietary inflammatory index and ovarian cancer risk in a large Italian case-control study. Cancer Causes Control 2016, 27, 897–906. [Google Scholar] [CrossRef]
- Shivappa, N.; Sandin, S.; Löf, M.; Hébert, J.R.; Adami, H.O.; Weiderpass, E. Prospective study of dietary inflammatory index and risk of breast cancer in Swedish women. Br. J. Cancer 2015, 113, 1099–1103. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Zhang, J.; Ma, Y.; Liese, A.D.; Agalliu, I.; Hingle, M.; Hou, L.; Hurley, T.G.; Jiao, L.; et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann. Epidemiol. 2015, 25, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E.; Morey, M.C.; Hartman, T.J.; Snyder, D.C.; Sloane, R.; Cohen, H.J.; Demark-Wahnefried, W. Dietary patterns differ between urban and rural older, long-term survivors of breast, prostate, and colorectal cancer and are associated with body mass index. J. Acad. Nutr. Diet. 2012, 112, 824–831.e1. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, T.; Liu, J.; Probst, J.; Merchant, A.; Jhones, S.; Martin, A.B. Obesity and obesity-related behaviors among rural and urban adults in the USA. Rural Remote Health 2015, 15, 3267. [Google Scholar] [CrossRef]
- Bhatia, S.; Landier, W.; Paskett, E.D.; Peters, K.B.; Merrill, J.K.; Phillips, J.; Osarogiagbon, R.U. Rural-Urban Disparities in Cancer Outcomes: Opportunities for Future Research. J. Natl. Cancer Inst. 2022, 114, 940–952. [Google Scholar] [CrossRef] [PubMed]
- Lappe, J.; Watson, P.; Travers-Gustafson, D.; Recker, R.; Garland, C.; Gorham, E.; Baggerly, K.; McDonnell, S.L. Effect of Vitamin D and Calcium Supplementation on Cancer Incidence in Older Women: A Randomized Clinical Trial. JAMA 2017, 317, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S.A.; Friday, J.E.; Moshfegh, A.J. MyPyramid Eqivalents Database, 2.0 for USDA Survey Foods 2003–2004; Food Surveys Research Group, Beltsville Human Nutrition Research Center, Agricultural Research Service, U.S. Department of Agriculture: Beltsville, MD, USA, 2008. [Google Scholar]
- Hebert, J.R.; Shivappa, N.; Wirth, M.D.; Hussey, J.R.; Hurley, T.G. Perspective: The Dietary Inflammatory Index (DII)-Lessons Learned, Improvements Made, and Future Directions. Adv. Nutr. 2019, 10, 185–195. [Google Scholar] [CrossRef] [PubMed]
- USDA Department of Health and Human Services. Physical Activity Guidelines for Americans, 2nd ed.; DHHS, Ed.; U.S. Department of Health and Human Services: Washington, DC, USA, 2018. [Google Scholar]
- Tabung, F.K.; Steck, S.E.; Liese, A.D.; Zhang, J.; Ma, Y.; Johnson, K.C.; Lane, D.S.; Qi, L.; Snetselaar, L.; Vitolins, M.Z.; et al. Patterns of change over time and history of the inflammatory potential of diet and risk of breast cancer among postmenopausal women. Breast Cancer Res. Treat. 2016, 159, 139–149. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Ma, Y.; Liese, A.D.; Zhang, J.; Lane, D.S.; Ho, G.Y.F.; Hou, L.; Snetselaar, L.; Ockene, J.K.; et al. Changes in the Inflammatory Potential of Diet Over Time and Risk of Colorectal Cancer in Postmenopausal Women. Am. J. Epidemiol. 2017, 186, 514–523. [Google Scholar] [CrossRef]
- Boden, S.; Myte, R.; Wennberg, M.; Harlid, S.; Johansson, I.; Shivappa, N.; Hebert, J.R.; Van Guelpen, B.; Nilsson, L.M. The inflammatory potential of diet in determining cancer risk; A prospective investigation of two dietary pattern scores. PLoS ONE 2019, 14, e0214551. [Google Scholar] [CrossRef] [PubMed]
- Bours, M.J.; Beijer, S.; Winkels, R.M.; van Duijnhoven, F.J.; Mols, F.; Breedveld-Peters, J.J.; Kampman, E.; Weijenberg, M.P.; van de Poll-Franse, L.V. Dietary changes and dietary supplement use, and underlying motives for these habits reported by colorectal cancer survivors of the Patient Reported Outcomes Following Initial Treatment and Long-Term Evaluation of Survivorship (PROFILES) registry. Br. J. Nutr. 2015, 114, 286–296. [Google Scholar] [CrossRef]
- Ghelfi, F.; Tieri, M.; Gori, S.; Nicolis, F.; Petrella, M.C.; Filiberti, A.; Apolone, G.; Titta, L. Do cancer patients change their diet in the e-health information era? A review of the literature and a survey as a proposal for the Italian population. Food Res. Int. 2018, 104, 59–68. [Google Scholar] [CrossRef]
- Hagen, K.B.; Aas, T.; Kvaloy, J.T.; Soiland, H.; Lind, R. Diet in women with breast cancer compared to healthy controls—What is the difference? Eur. J. Oncol. Nurs. 2018, 32, 20–24. [Google Scholar] [CrossRef]
- Maunsell, E.; Drolet, M.; Brisson, J.; Robert, J.; Deschenes, L. Dietary change after breast cancer: Extent, predictors, and relation with psychological distress. J. Clin. Oncol. 2002, 20, 1017–1025. [Google Scholar] [CrossRef]
- Patterson, R.E.; Neuhouser, M.L.; Hedderson, M.M.; Schwartz, S.M.; Standish, L.J.; Bowen, D.J. Changes in diet, physical activity, and supplement use among adults diagnosed with cancer. J. Am. Diet. Assoc. 2003, 103, 323–328. [Google Scholar] [CrossRef]
- Sun, Y.; Bao, W.; Liu, B.; Caan, B.J.; Lane, D.S.; Millen, A.E.; Simon, M.S.; Thomson, C.A.; Tinker, L.F.; Van Horn, L.V.; et al. Changes in Overall Diet Quality in Relation to Survival in Postmenopausal Women with Breast Cancer: Results from the Women’s Health Initiative. J. Acad. Nutr. Diet. 2018, 118, 1855–1863.e6. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.D.; Hebert, J.R.; Shivappa, N.; Hand, G.A.; Hurley, T.G.; Drenowatz, C.; McMahon, D.; Shook, R.P.; Blair, S.N. Anti-inflammatory Dietary Inflammatory Index scores are associated with healthier scores on other dietary indices. Nutr. Res. 2016, 36, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Jin, X.; Man, C.; Gao, Z.; Wang, X. Meta-analysis of the association between the inflammatory potential of diet and colorectal cancer risk. Oncotarget 2017, 8, 59592–59600. [Google Scholar] [CrossRef]
- Tabung, F.K.; Steck, S.E.; Ma, Y.; Liese, A.D.; Zhang, J.; Caan, B.; Hou, L.; Johnson, K.C.; Mossavar-Rahmani, Y.; Shivappa, N.; et al. The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: Results from the Women’s Health Initiative. Cancer Causes Control 2015, 26, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Moradi, S.; Issah, A.; Mohammadi, H.; Mirzaei, K. Associations between dietary inflammatory index and incidence of breast and prostate cancer: A systematic review and meta-analysis. Nutrition 2018, 55–56, 168–178. [Google Scholar] [CrossRef]
- Nagle, C.M.; Ibiebele, T.; Shivappa, N.; Hebert, J.R.; DeFazio, A.; Webb, P.M.; Australian Ovarian Cancer Study. The association between the inflammatory potential of diet and risk of developing, and survival following, a diagnosis of ovarian cancer. Eur. J. Nutr. 2019, 58, 1747–1756. [Google Scholar] [CrossRef]
- Wang, L.; Liu, C.; Zhou, C.; Zhuang, J.; Tang, S.; Yu, J.; Tian, J.; Feng, F.; Liu, L.; Zhang, T.; et al. Meta-analysis of the association between the dietary inflammatory index (DII) and breast cancer risk. Eur. J. Clin. Nutr. 2019, 73, 509–517. [Google Scholar] [CrossRef]
- Hayati, Z.; Jafarabadi, M.A.; Pirouzpanah, S. Dietary inflammatory index and breast cancer risk: An updated meta-analysis of observational studies. Eur. J. Clin. Nutr. 2022, 76, 1073–1087. [Google Scholar] [CrossRef]
- Mazul, A.L.; Shivappa, N.; Hébert, J.R.; Steck, S.E.; Rodriguez-Ormaza, N.; Weissler, M.; Olshan, A.F.; Zevallos, J.P. Proinflammatory diet is associated with increased risk of squamous cell head and neck cancer. Int. J. Cancer 2018, 143, 1604–1610. [Google Scholar] [CrossRef]
- Mazidi, M.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Kengne, A.P. Greater Dietary Inflammatory Index score is associated with higher likelihood of chronic kidney disease. Br. J. Nutr. 2018, 120, 204–209. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Chan, D.S.; Lau, R.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2011, 343, d6617. [Google Scholar] [CrossRef]
- Gaesser, G.A. Whole Grains, Refined Grains, and Cancer Risk: A Systematic Review of Meta-Analyses of Observational Studies. Nutrients 2020, 12, 3756. [Google Scholar] [CrossRef] [PubMed]
- Molina-Montes, E.; Ubago-Guisado, E.; Petrova, D.; Amiano, P.; Chirlaque, M.D.; Agudo, A.; Sánchez, M.J. The Role of Diet, Alcohol, BMI, and Physical Activity in Cancer Mortality: Summary Findings of the EPIC Study. Nutrients 2021, 13, 4293. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; Jonkers, D.M.; Bast, A.; Vanhoutvin, S.A.; Fischer, M.A.; Kodde, A.; Troost, F.J.; Venema, K.; Brummer, R.J. Butyrate modulates oxidative stress in the colonic mucosa of healthy humans. Clin. Nutr. 2009, 28, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Ebert, M.N.; Klinder, A.; Peters, W.H.; Schäferhenrich, A.; Sendt, W.; Scheele, J.; Pool-Zobel, B.L. Expression of glutathione S-transferases (GSTs) in human colon cells and inducibility of GSTM2 by butyrate. Carcinogenesis 2003, 24, 1637–1644. [Google Scholar] [CrossRef]
- Knapp, B.K.; Bauer, L.L.; Swanson, K.S.; Tappenden, K.A.; Fahey, G.C., Jr.; De Godoy, M.R. Soluble fiber dextrin and soluble corn fiber supplementation modify indices of health in cecum and colon of Sprague-Dawley rats. Nutrients 2013, 5, 396–410. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, Y.; Jiang, H.; Robbins, G.T.; Nie, D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int. J. Cancer 2011, 128, 847–856. [Google Scholar] [CrossRef]
- Frugé, A.D.; Smith, K.S.; Riviere, A.J.; Tenpenny-Chigas, R.; Demark-Wahnefried, W.; Arthur, A.E.; Murrah, W.M.; van der Pol, W.J.; Jasper, S.L.; Morrow, C.D.; et al. A Dietary Intervention High in Green Leafy Vegetables Reduces Oxidative DNA Damage in Adults at Increased Risk of Colorectal Cancer: Biological Outcomes of the Randomized Controlled Meat and Three Greens (M3G) Feasibility Trial. Nutrients 2021, 13, 1220. [Google Scholar] [CrossRef]
- Gualtieri, P.; Marchetti, M.; Frank, G.; Smeriglio, A.; Trombetta, D.; Colica, C.; Cianci, R.; De Lorenzo, A.; Di Renzo, L. Antioxidant-Enriched Diet on Oxidative Stress and Inflammation Gene Expression: A Randomized Controlled Trial. Genes 2023, 14, 206. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Heidari, Z.; Teimori, A.; Ghazvini, M.; Imani, Z.F.; Naeini, A.A. The Association Between the Dietary Inflammatory Index (DII) and Some Serum Oxidative Stress Markers in Non-Alcoholic Fatty Liver Disease: Case-Control. Int. J. Prev. Med. 2022, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Blair, C.K.; Prizment, A.E.; Jacobs, D.R., Jr.; Hebert, J.R. Dietary inflammatory index and risk of renal cancer in the Iowa Women’s Health Study. Eur. J. Nutr. 2018, 57, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.R. Social Desirability Trait: Biaser or Driver of Self-Reported Dietary Intake? J. Acad. Nutr. Diet. 2016, 116, 1895–1898. [Google Scholar] [CrossRef]
- Hebert, J.R.; Ebbeling, C.B.; Matthews, C.E.; Hurley, T.G.; Ma, Y.; Druker, S.; Clemow, L. Systematic errors in middle-aged women’s estimates of energy intake: Comparing three self-report measures to total energy expenditure from doubly labeled water. Ann. Epidemiol. 2002, 12, 577–586. [Google Scholar] [CrossRef]
- Hebert, J.R.; Ma, Y.; Clemow, L.; Ockene, I.S.; Saperia, G.; Stanek, E.J., 3rd; Merriam, P.A.; Ockene, J.K. Gender differences in social desirability and social approval bias in dietary self-report. Am. J. Epidemiol. 1997, 146, 1046–1055. [Google Scholar] [CrossRef]
| Alcohol | Energy (kcal) | MUFA | Riboflavin | Vitamin C |
|---|---|---|---|---|
| Vitamin B12 | Total Fat | Niacin | Saturated Fat | Vitamin D |
| Vitamin B6 | Fiber | n-3 fatty acids | Selenium | Vitamin E |
| β- Carotene | Folic Acid | n-6 fatty acids | Thiamin | Zinc |
| Carbohydrate | Iron | Protein | Trans fat | Isoflavones |
| Cholesterol | Magnesium | PUFA | Vitamin A | |
| * 29 of 45 components available for calculation. Components not available for calculation: caffeine, eugenol, garlic, ginger, onion, saffron, turmeric, green/black tea, flavon-3-ol, flavones, flavonols, flavonones, anthocyanidins, pepper, thyme/oregano, rosemary. | ||||
| Characteristics | Total Population (n = 1977) | Non-Cancer (n = 1886) | Cancer (n = 91) |
|---|---|---|---|
| Age (years; Mean ± Std.) | 65.2 ± 6.8 | 65.1 ± 6.8 | 68.1 ± 7.8 |
| Age (years) 55–59 60–64 65–74 ≥75 | 529 (26.8) 579 (29.3) 656 (33.2) 213 (10.8) | 511 (27.1) 565 (30.0) 615 (32.6) 195 (10.3) | 18 (19.8) 14 (15.4) 41 (45.0) 18 (19.8) |
| Race White Non-white/not available | 9 (0.5) 1968 (99.5) | 9 (0.5) 1877 (99.5) | 0 91 (100) |
| BMI (kg/m2; Mean ± Std.) | 29.9 ± 6.5 | 29.9 ± 6.5 | 29.0 ± 6.5 |
| BMI (kg/m2) <25.0 25–29.9 ≥30.0 | 465 (23.6) 667 (33.8) 842 (42.6) | 439 (23.3) 634 (33.7) 810 (43.0) | 26 (28.6) 33 (36.3) 32 (35.2) |
| Smoking Never Ever | 1338 (67.7) 639 (32.3) | 1278 (67.8) 608 (32.2) | 60 (65.9) 31 (34.1) |
| Physical activity (min) <150 ≥150 | 1146 (58.4) 815 (41.6) | 650 (34.7) 1223 (65.3) | 23 (26.1) 65 (73.9) |
| HRT No Yes | 1614 (81.6) 363 (18.4) | 1537 (81.5) 349 (18.5) | 77 (84.6) 14 (15.4) |
| E-DII (Mean ± Std.) | Non-Cancer (n = 1886) | Cancer (n = 91) | p-Value * |
|---|---|---|---|
| Baseline | –1.48 ± 1.74 | –1.65 ± 1.62 | 0.78 |
| Visit 9 | –1.29 ± 1.72 | –1.11 ± 1.70 | 0.75 |
| E-DII change (V9–baseline) | 0.19 ± 1.43 | 0.55 ± 1.43 | 0.02 |
| E-DII | Unadjusted OR [95% CI] | p-Value | Adjusted OR * [95% CI] | p-Value |
|---|---|---|---|---|
| E-DII Baseline E-DII V9 visit E-DII change (V9–baseline) | 0.94 [0.83, 1.06] 1.07 [0.94 1.20] 1.20 [1.04 1.40] | 0.31 0.31 0.01 | 1.04 [0.90, 1.19] - 1.21 [1.02, 1.42] | 0.64 - 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jackson, M.K.; Lappe, J.; Ma, J.; Timmerman, M.; Lyden, E.R.; Shivappa, N.; Hébert, J.R.; Travers Gustafson, D.; Graeff-Armas, L.; Hanson, C. Changes in Dietary Inflammatory Index Score over Time and Cancer Development in Rural Post-Menopausal Women. Antioxidants 2023, 12, 946. https://doi.org/10.3390/antiox12040946
Jackson MK, Lappe J, Ma J, Timmerman M, Lyden ER, Shivappa N, Hébert JR, Travers Gustafson D, Graeff-Armas L, Hanson C. Changes in Dietary Inflammatory Index Score over Time and Cancer Development in Rural Post-Menopausal Women. Antioxidants. 2023; 12(4):946. https://doi.org/10.3390/antiox12040946
Chicago/Turabian StyleJackson, Mariah Kay, Joan Lappe, Jihyun Ma, Megan Timmerman, Elizabeth R. Lyden, Nitin Shivappa, James R. Hébert, Dianne Travers Gustafson, Laura Graeff-Armas, and Corrine Hanson. 2023. "Changes in Dietary Inflammatory Index Score over Time and Cancer Development in Rural Post-Menopausal Women" Antioxidants 12, no. 4: 946. https://doi.org/10.3390/antiox12040946
APA StyleJackson, M. K., Lappe, J., Ma, J., Timmerman, M., Lyden, E. R., Shivappa, N., Hébert, J. R., Travers Gustafson, D., Graeff-Armas, L., & Hanson, C. (2023). Changes in Dietary Inflammatory Index Score over Time and Cancer Development in Rural Post-Menopausal Women. Antioxidants, 12(4), 946. https://doi.org/10.3390/antiox12040946








