Aldo-Keto Reductase 1C15 Characterization and Protection in Ischemic Brain Injury
Abstract
1. Introduction
2. Materials and Methods
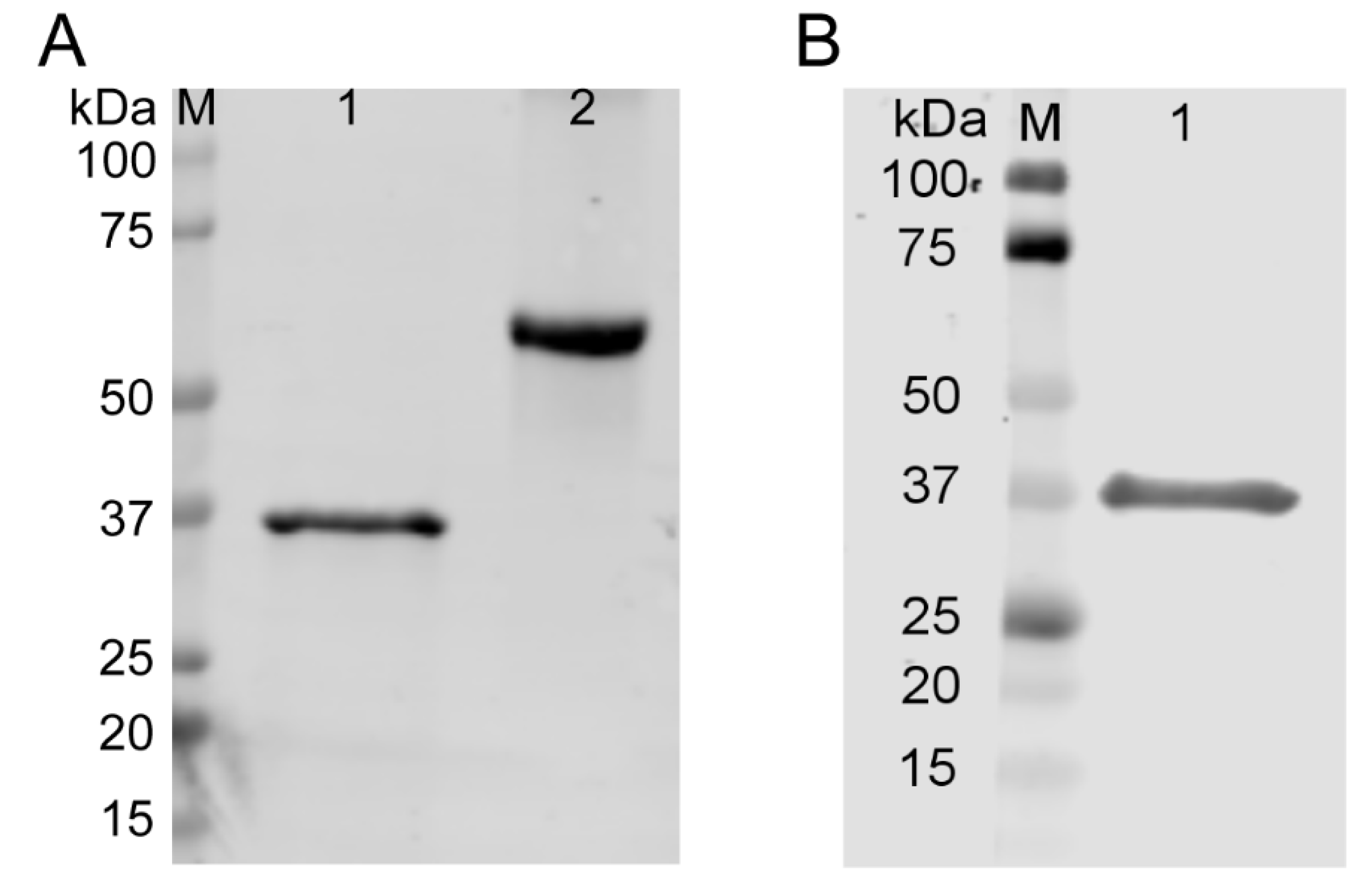
2.1. Generation and Quality Control of TAT-AKR1C15 Fusion Protein
2.2. Animals and Drug Administration
2.3. Middle Cerebral Artery Occlusion (MCAO)
2.4. Neurological Scoring
2.5. Measurement of Infarct Volume
2.6. Primary Cell Cultures
2.7. Oxygen–Glucose Deprivation (OGD)
2.8. Cell Viability and Death Assays
2.9. In Vitro Blood–Brain Barrier Model and Permeability Assay
2.10. Nitric Oxide (NO) Release Assay
2.11. Real-Time PCR (RT-PCR)
2.12. Immunocytochemistry
2.13. Electrophoresis and Western Blot
2.14. Statistical Analyses
3. Results
3.1. AKR1C15 Reduced Infarct Volumes and Neurological Deficits after Ischemic Stroke
3.2. Early Injection of AKR1C15 Abolished the Protective Role of IPC
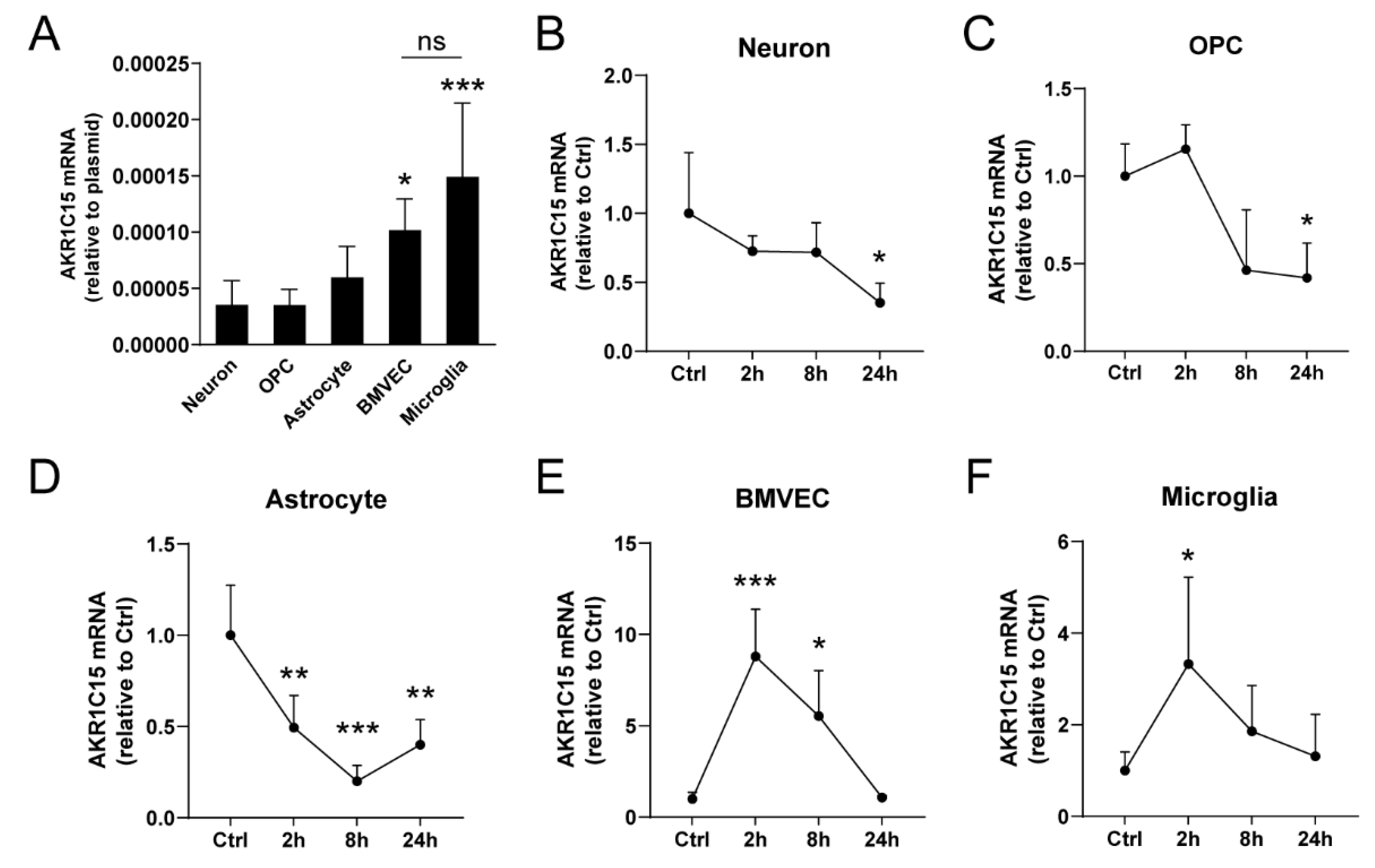
3.3. AKR1C15 Expression in Primary CNS Cell Cultures before and after OGD
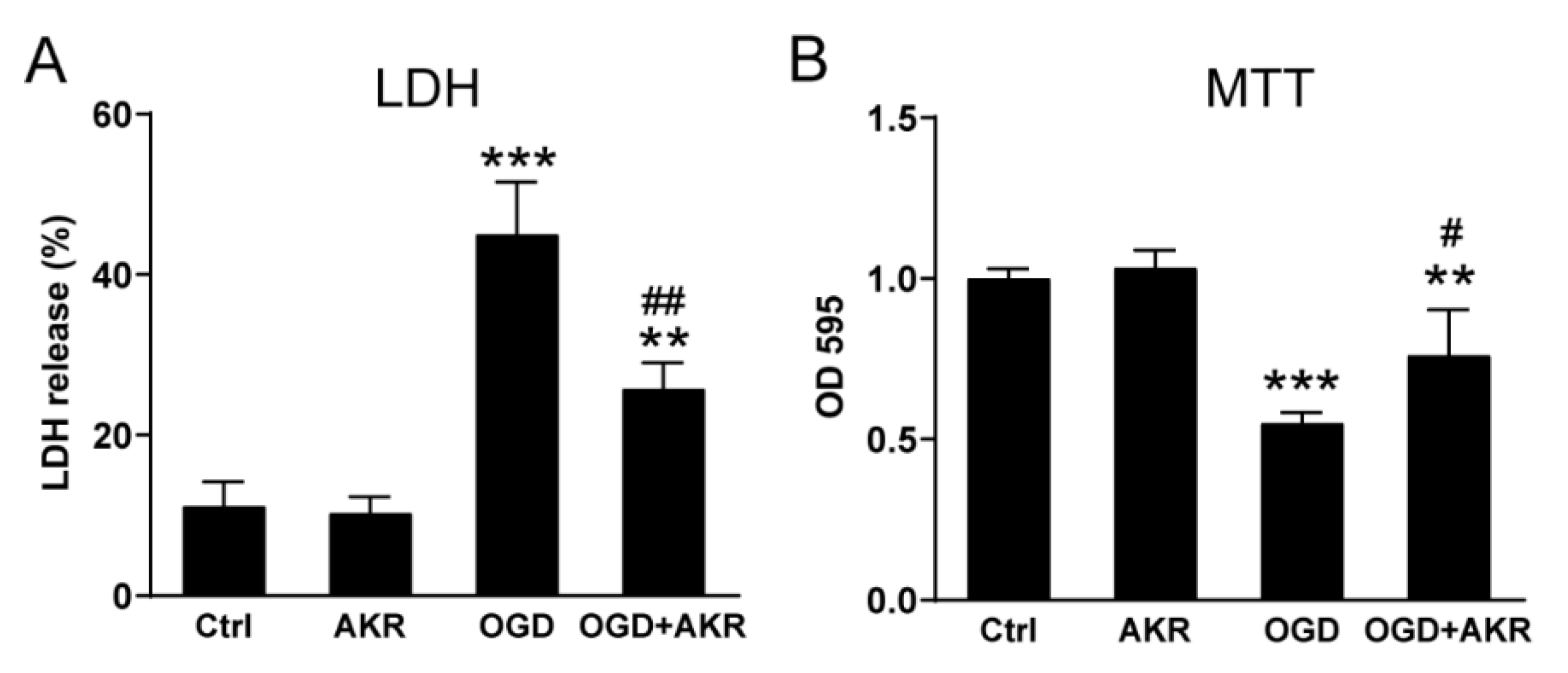
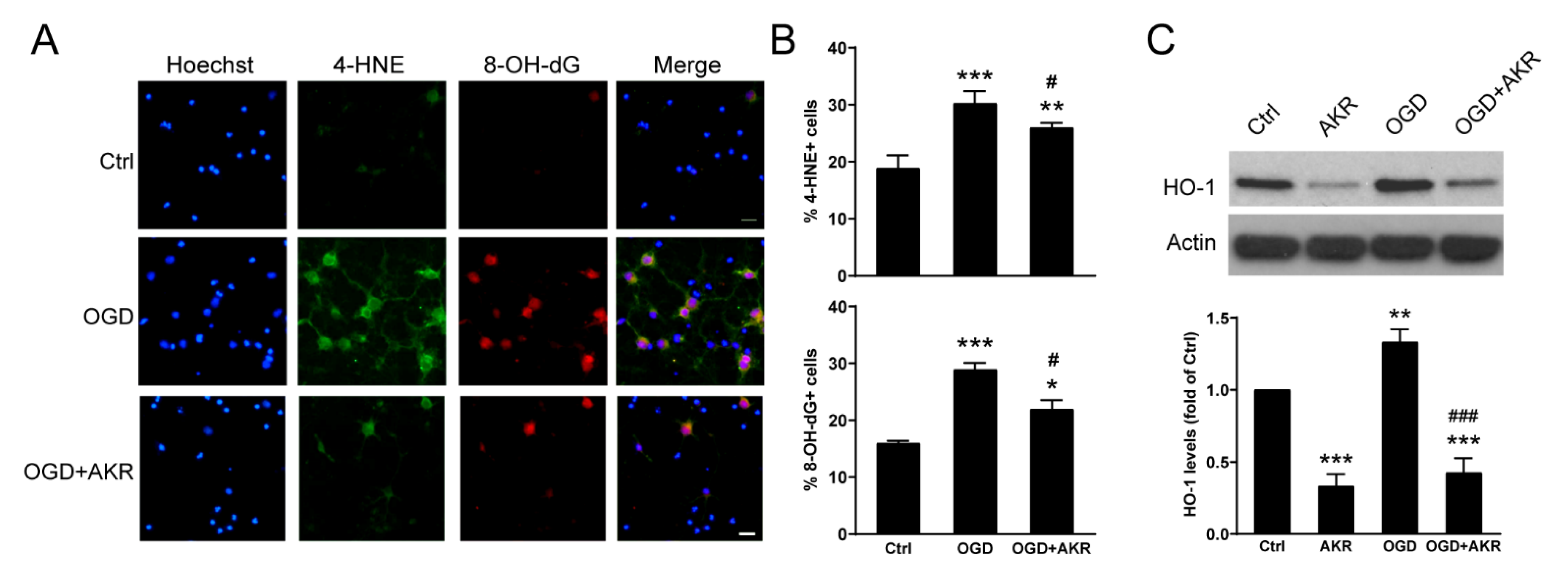
3.4. AKR1C15 Protected Neurons against OGD Associated with Reduced Lipid Electrophile and Oxidative Stress
3.5. AKR1C15 Protected BMVECs against OGD-Induced Cell Death and In Vitro BBB Leakage
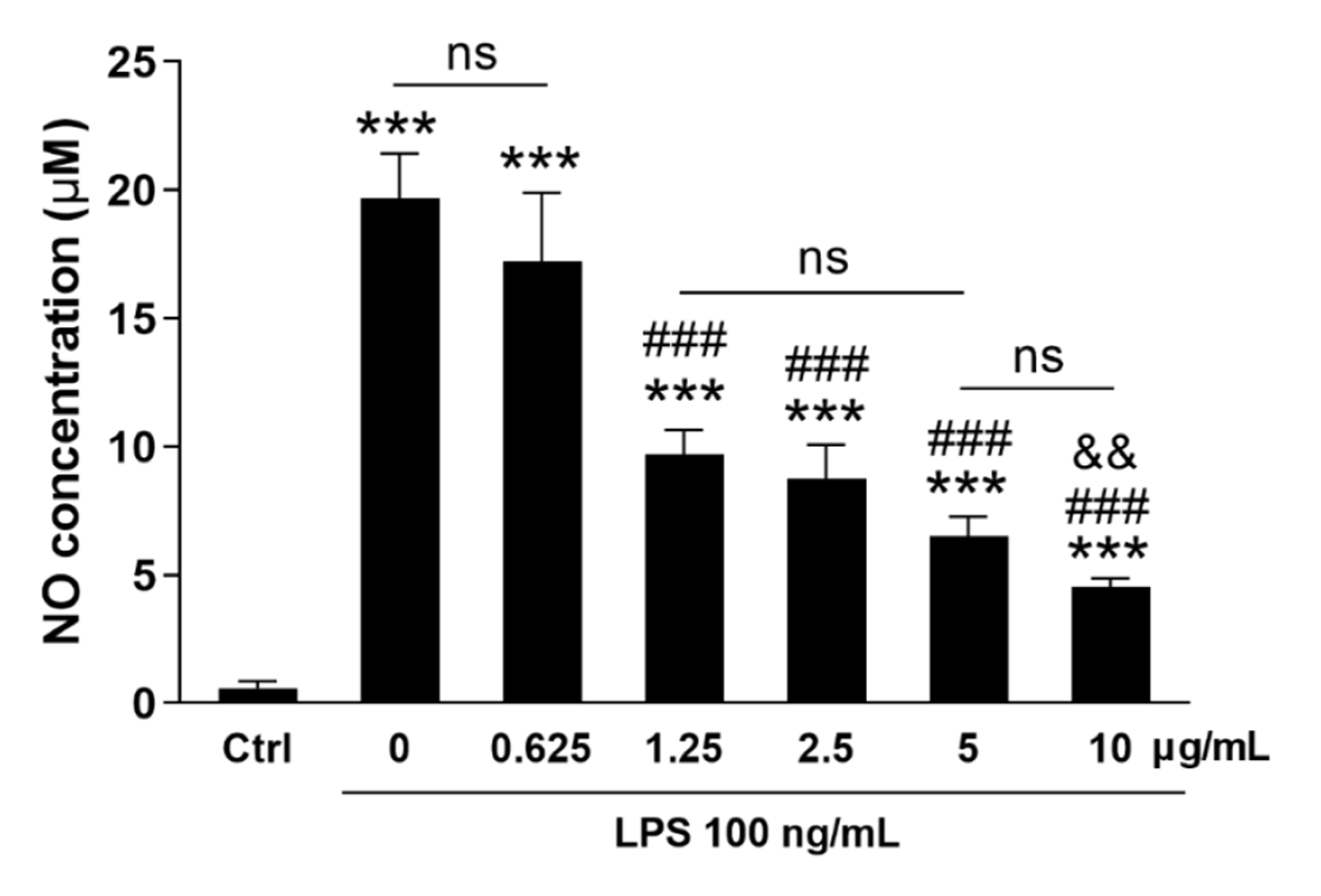
3.6. AKR1C15 Reduced Microglial NO Release upon Proinflammatory Stimulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CDC. Stroke Facts. Available online: https://www.cdc.gov/stroke/facts.htm (accessed on 1 March 2023).
- Dwivedi, S.; Sharma, A.; Patrick, B.; Sharma, R.; Awasthi, Y.C. Role of 4-hydroxynonenal and its metabolites in signaling. Redox Rep. 2007, 12, 4–10. [Google Scholar] [CrossRef]
- Parola, M.; Bellomo, G.; Robino, G.; Barrera, G.; Dianzani, M.U. 4-Hydroxynonenal as a biological signal: Molecular basis and pathophysiological implications. Antioxid. Redox Signal. 1999, 1, 255–284. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Sun, Y.; Li, Q.; Li, S.; Shi, Y.; Leak, R.K.; Chen, J.; Zhang, F. Ischemic preconditioning provides long-lasting neuroprotection against ischemic stroke: The role of Nrf2. Exp. Neurol. 2020, 325, 113142. [Google Scholar] [CrossRef]
- Yang, T.; Sun, Y.; Mao, L.; Zhang, M.; Li, Q.; Zhang, L.; Shi, Y.; Leak, R.K.; Chen, J.; Zhang, F. Brain ischemic preconditioning protects against ischemic injury and preserves the blood-brain barrier via oxidative signaling and Nrf2 activation. Redox Biol. 2018, 17, 323–337. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Mao, L.; Leak, R.K.; Shi, Y.; Zhang, W.; Hu, X.; Sun, B.; Cao, G.; Gao, Y.; et al. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J. Neurosci. 2014, 34, 1903–1915. [Google Scholar] [CrossRef]
- Smathers, R.L.; Fritz, K.S.; Galligan, J.J.; Shearn, C.T.; Reigan, P.; Marks, M.J.; Petersen, D.R. Characterization of 4-HNE modified L-FABP reveals alterations in structural and functional dynamics. PLoS ONE 2012, 7, e38459. [Google Scholar] [CrossRef] [PubMed]
- Schaur, R.J. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol. Asp. Med. 2003, 24, 149–159. [Google Scholar] [CrossRef]
- Dalleau, S.; Baradat, M.; Gueraud, F.; Huc, L. Cell death and diseases related to oxidative stress: 4-hydroxynonenal (HNE) in the balance. Cell. Death Differ. 2013, 20, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Velica, P.; Davies, N.J.; Rocha, P.P.; Schrewe, H.; Ride, J.P.; Bunce, C.M. Lack of functional and expression homology between human and mouse aldo-keto reductase 1C enzymes: Implications for modelling human cancers. Mol. Cancer 2009, 8, 121. [Google Scholar] [CrossRef]
- Davies, N.J.; Hayden, R.E.; Simpson, P.J.; Birtwistle, J.; Mayer, K.; Ride, J.P.; Bunce, C.M. AKR1C isoforms represent a novel cellular target for jasmonates alongside their mitochondrial-mediated effects. Cancer Res. 2009, 69, 4769–4775. [Google Scholar] [CrossRef]
- Desmond, J.C.; Mountford, J.C.; Drayson, M.T.; Walker, E.A.; Hewison, M.; Ride, J.P.; Luong, Q.T.; Hayden, R.E.; Vanin, E.F.; Bunce, C.M. The aldo-keto reductase AKR1C3 is a novel suppressor of cell differentiation that provides a plausible target for the non-cyclooxygenase-dependent antineoplastic actions of nonsteroidal anti-inflammatory drugs. Cancer Res. 2003, 63, 505–512. [Google Scholar] [PubMed]
- Penning, T.M.; Byrns, M.C. Steroid hormone transforming aldo-keto reductases and cancer. Ann. N. Y. Acad. Sci. 2009, 1155, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Qin, K.N.; Cheng, K.C. Structure and tissue-specific expression of the aldo-keto reductase superfamily. Biochemistry 1994, 33, 3223–3228. [Google Scholar] [CrossRef]
- Matsunaga, T.; Shinoda, Y.; Inoue, Y.; Endo, S.; El-Kabbani, O.; Hara, A. Protective effect of rat aldo-keto reductase (AKR1C15) on endothelial cell damage elicited by 4-hydroxy-2-nonenal. Chem. Biol. Interact. 2011, 191, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Matsunaga, T.; Horie, K.; Tajima, K.; Bunai, Y.; Carbone, V.; El-Kabbani, O.; Hara, A. Enzymatic characteristics of an aldo-keto reductase family protein (AKR1C15) and its localization in rat tissues. Arch. Biochem. Biophys. 2007, 465, 136–147. [Google Scholar] [CrossRef]
- Bagashev, A.; Sawaya, B.E. Roles and functions of HIV-1 Tat protein in the CNS: An overview. Virol. J. 2013, 10, 358. [Google Scholar] [CrossRef]
- Zhang, F.; Xing, J.; Liou, A.K.; Wang, S.; Gan, Y.; Luo, Y.; Ji, X.; Stetler, R.A.; Chen, J.; Cao, G. Enhanced Delivery of Erythropoietin Across the Blood-Brain Barrier for Neuroprotection against Ischemic Neuronal Injury. Transl. Stroke Res. 2010, 1, 113–121. [Google Scholar] [CrossRef]
- Fisher, M.; Feuerstein, G.; Howells, D.W.; Hurn, P.D.; Kent, T.A.; Savitz, S.I.; Lo, E.H.; Group, S. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009, 40, 2244–2250. [Google Scholar] [CrossRef]
- Stetler, R.A.; Leak, R.K.; Gan, Y.; Li, P.; Zhang, F.; Hu, X.; Jing, Z.; Chen, J.; Zigmond, M.J.; Gao, Y. Preconditioning provides neuroprotection in models of CNS disease: Paradigms and clinical significance. Prog. Neurobiol. 2014, 114, 58–83. [Google Scholar] [CrossRef]
- Longa, E.Z.; Weinstein, P.R.; Carlson, S.; Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989, 20, 84–91. [Google Scholar] [CrossRef]
- Mao, L.; Yang, T.; Li, X.; Lei, X.; Sun, Y.; Zhao, Y.; Zhang, W.; Gao, Y.; Sun, B.; Zhang, F. Protective effects of sulforaphane in experimental vascular cognitive impairment: Contribution of the Nrf2 pathway. J. Cereb. Blood Flow. Metab. 2019, 39, 352–366. [Google Scholar] [CrossRef]
- Miao, W.; Jiang, L.; Xu, F.; Lyu, J.; Jiang, X.; He, M.; Liu, Y.; Yang, T.; Leak, R.K.; Stetler, R.A.; et al. Adiponectin ameliorates hypoperfusive cognitive deficits by boosting a neuroprotective microglial response. Prog. Neurobiol. 2021, 205, 102125. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, L.; Pu, H.; Mao, L.; Hu, X.; Jiang, X.; Xu, N.; Stetler, R.A.; Zhang, F.; Liu, X.; et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nat. Commun. 2016, 7, 10523. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Ambrosio, S.; Lania, L.; Majello, B.; Amente, S. The Intertwined Role of 8-oxodG and G4 in Transcription Regulation. Int. J. Mol. Sci. 2023, 24, 2031. [Google Scholar] [CrossRef]
- Zhang, M.; An, C.; Gao, Y.; Leak, R.K.; Chen, J.; Zhang, F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog. Neurobiol. 2013, 100, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Jiang, X.; Leak, R.K.; Shi, Y.; Hu, X.; Chen, J. Microglial Responses to Brain Injury and Disease: Functional Diversity and New Opportunities. Transl. Stroke Res. 2021, 12, 474–495. [Google Scholar] [CrossRef]
- Wang, R.; Pu, H.; Ye, Q.; Jiang, M.; Chen, J.; Zhao, J.; Li, S.; Liu, Y.; Hu, X.; Rocha, M.; et al. Transforming Growth Factor Beta-Activated Kinase 1-Dependent Microglial and Macrophage Responses Aggravate Long-Term Outcomes After Ischemic Stroke. Stroke 2020, 51, 975–985. [Google Scholar] [CrossRef]
- Hu, X.; Li, P.; Guo, Y.; Wang, H.; Leak, R.K.; Chen, S.; Gao, Y.; Chen, J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 2012, 43, 3063–3070. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Stetler, A.R.; Leak, R.K.; Hu, X.; Chen, J. Phagocytic microglia and macrophages in brain injury and repair. CNS Neurosci. 2022, 28, 1279–1293. [Google Scholar] [CrossRef]
- Jez, J.M.; Bennett, M.J.; Schlegel, B.P.; Lewis, M.; Penning, T.M. Comparative anatomy of the aldo-keto reductase superfamily. Biochem. J. 1997, 326 Pt 3, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, D.; Bauman, D.R.; Heredia, V.V.; Penning, T.M. The aldo-keto reductase superfamily homepage. Chem. Biol. Interact. 2003, 143–144, 621–631. [Google Scholar] [CrossRef]
- Endo, S.; Matsunaga, T.; Ohta, C.; Soda, M.; Kanamori, A.; Kitade, Y.; Ohno, S.; Tajima, K.; El-Kabbani, O.; Hara, A. Roles of rat and human aldo-keto reductases in metabolism of farnesol and geranylgeraniol. Chem. Biol. Interact. 2011, 191, 261–268. [Google Scholar] [CrossRef]
- Schaur, R.J.; Siems, W.; Bresgen, N.; Eckl, P.M. 4-Hydroxy-nonenal-A Bioactive Lipid Peroxidation Product. Biomolecules 2015, 5, 2247–2337. [Google Scholar] [CrossRef] [PubMed]
- Poganik, J.R.; Aye, Y. Electrophile Signaling and Emerging Immuno- and Neuro-modulatory Electrophilic Pharmaceuticals. Front. Aging Neurosci. 2020, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M.; Burczynski, M.E.; Jez, J.M.; Hung, C.F.; Lin, H.K.; Ma, H.; Moore, M.; Palackal, N.; Ratnam, K. Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1-AKR1C4) of the aldo-keto reductase superfamily: Functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem. J. 2000, 351, 67–77. [Google Scholar] [CrossRef] [PubMed]



| Score | Criteria |
|---|---|
| 0 | No neurologic deficit. |
| 1 | Mild focal neurologic deficit. Failure to fully extend left forepaw. |
| 2 | Moderate focal neurologic deficit. Circling to the left. |
| 3 | Severe focal neurologic deficit. Falling to the left. |
| 4 | Could not walk spontaneously and with decreased level of consciousness. |
| mRNA | Forward | Reverse | Accession Number |
|---|---|---|---|
| Akr1c15 | GATACTTGGGAGGCACTGGA | TGGTTGAGATACGGGTGACA | NM_001109900.1 |
| Gapdh | TGTTGCCATCAACGACCCCTT | CTCCACGACATACTCAGCA | NM_017008.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Li, Q.; Fadoul, G.; Alraqmany, N.; Ikonomovic, M.; Zhang, F. Aldo-Keto Reductase 1C15 Characterization and Protection in Ischemic Brain Injury. Antioxidants 2023, 12, 909. https://doi.org/10.3390/antiox12040909
Yang T, Li Q, Fadoul G, Alraqmany N, Ikonomovic M, Zhang F. Aldo-Keto Reductase 1C15 Characterization and Protection in Ischemic Brain Injury. Antioxidants. 2023; 12(4):909. https://doi.org/10.3390/antiox12040909
Chicago/Turabian StyleYang, Tuo, Qianqian Li, George Fadoul, Nour Alraqmany, Milos Ikonomovic, and Feng Zhang. 2023. "Aldo-Keto Reductase 1C15 Characterization and Protection in Ischemic Brain Injury" Antioxidants 12, no. 4: 909. https://doi.org/10.3390/antiox12040909
APA StyleYang, T., Li, Q., Fadoul, G., Alraqmany, N., Ikonomovic, M., & Zhang, F. (2023). Aldo-Keto Reductase 1C15 Characterization and Protection in Ischemic Brain Injury. Antioxidants, 12(4), 909. https://doi.org/10.3390/antiox12040909









