Large-Scale Qualitative and Quantitative Assessment of Dityrosine Crosslinking Omics in Response to Endogenous and Exogenous Hydrogen Peroxide in Escherichia coli
Abstract
1. Introduction
2. Materials and Methods
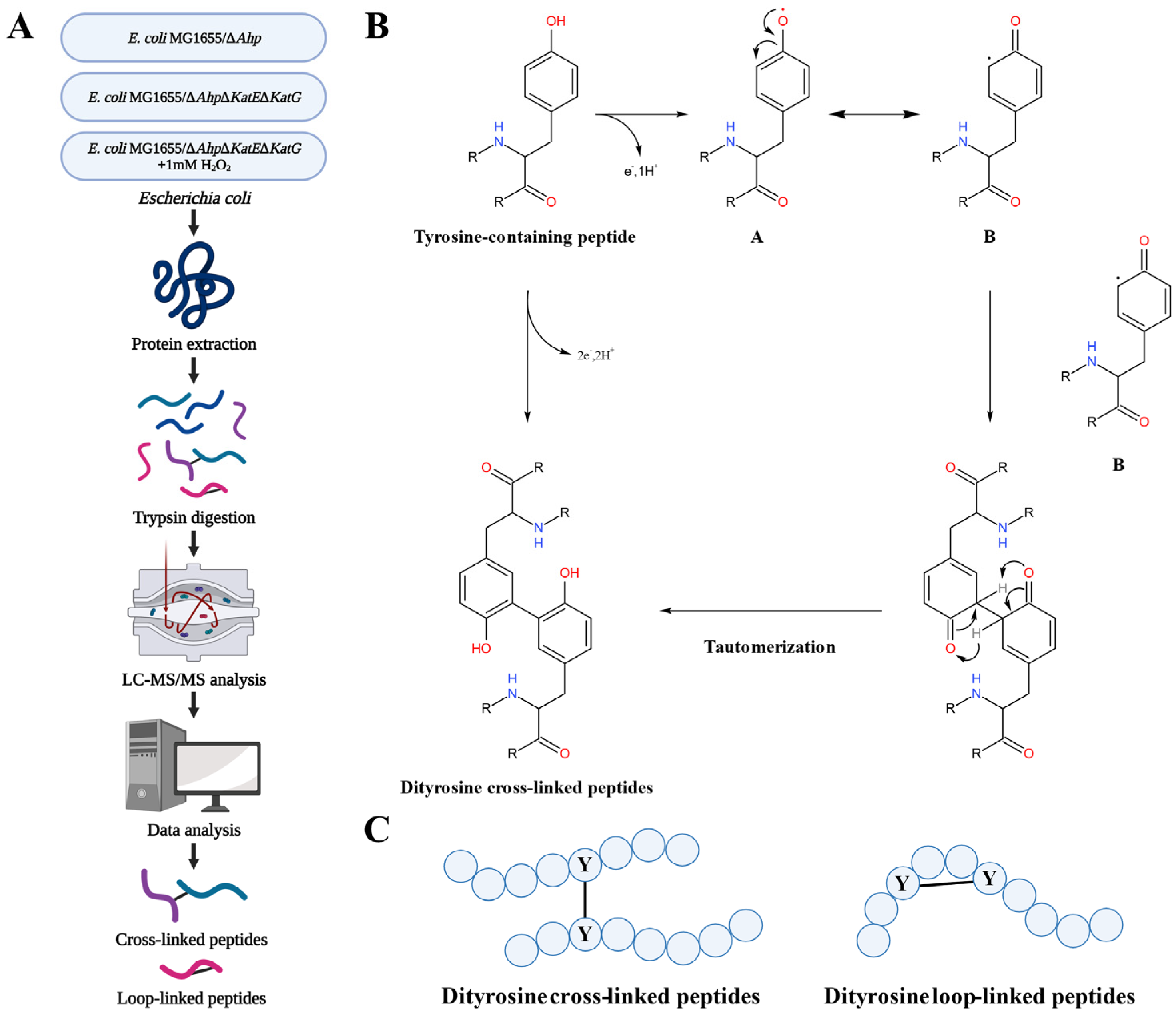
2.1. Strains and Growth Conditions
2.2. Protein Extraction, Digestion, and Desalting
2.3. LC–MS/MS Analysis
2.4. Data Analysis
2.5. Bioinformatic Analysis
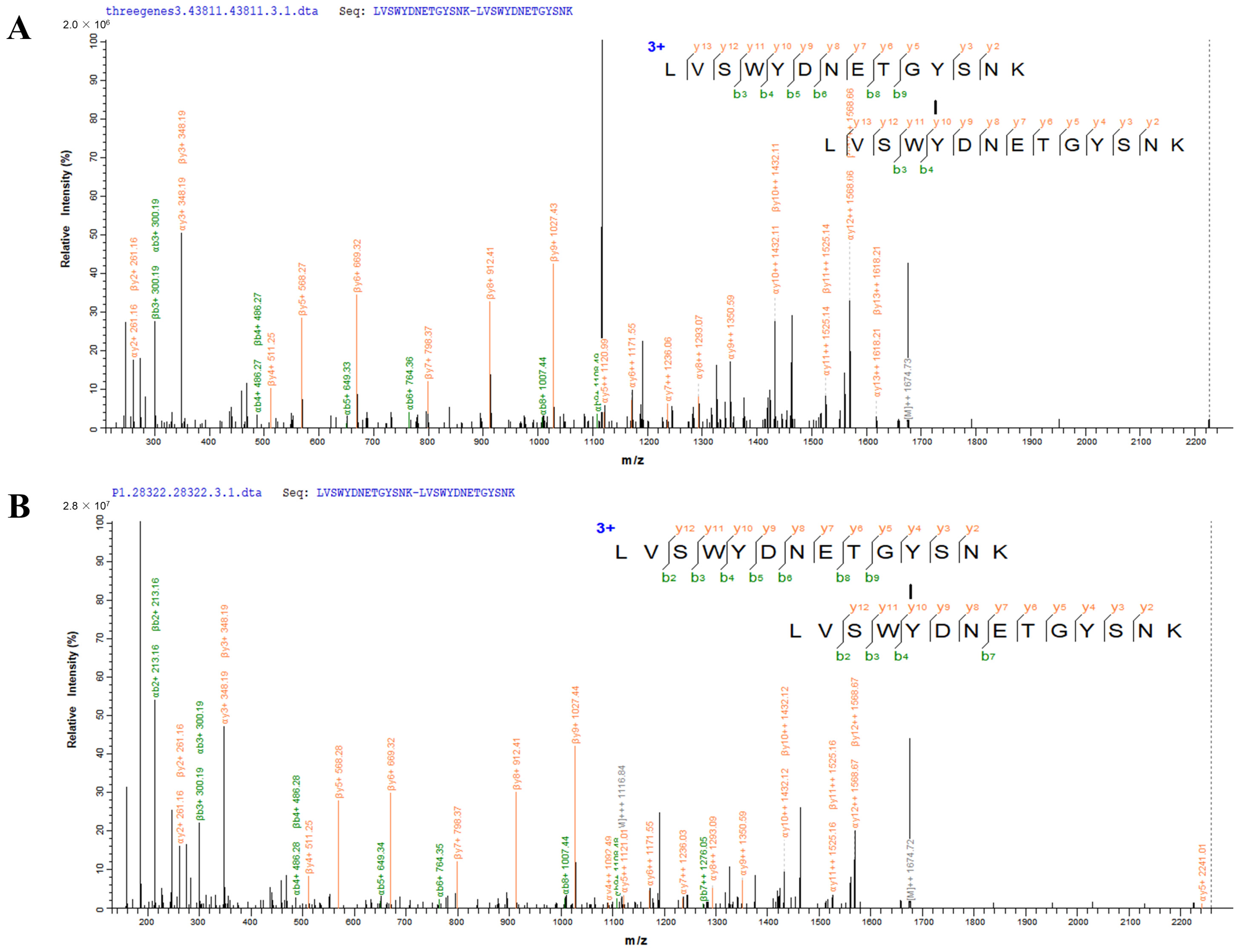
2.6. Validation of Dityrosine-Crosslinked Peptides by Mass Spectrometry
2.7. Data Availability
3. Results
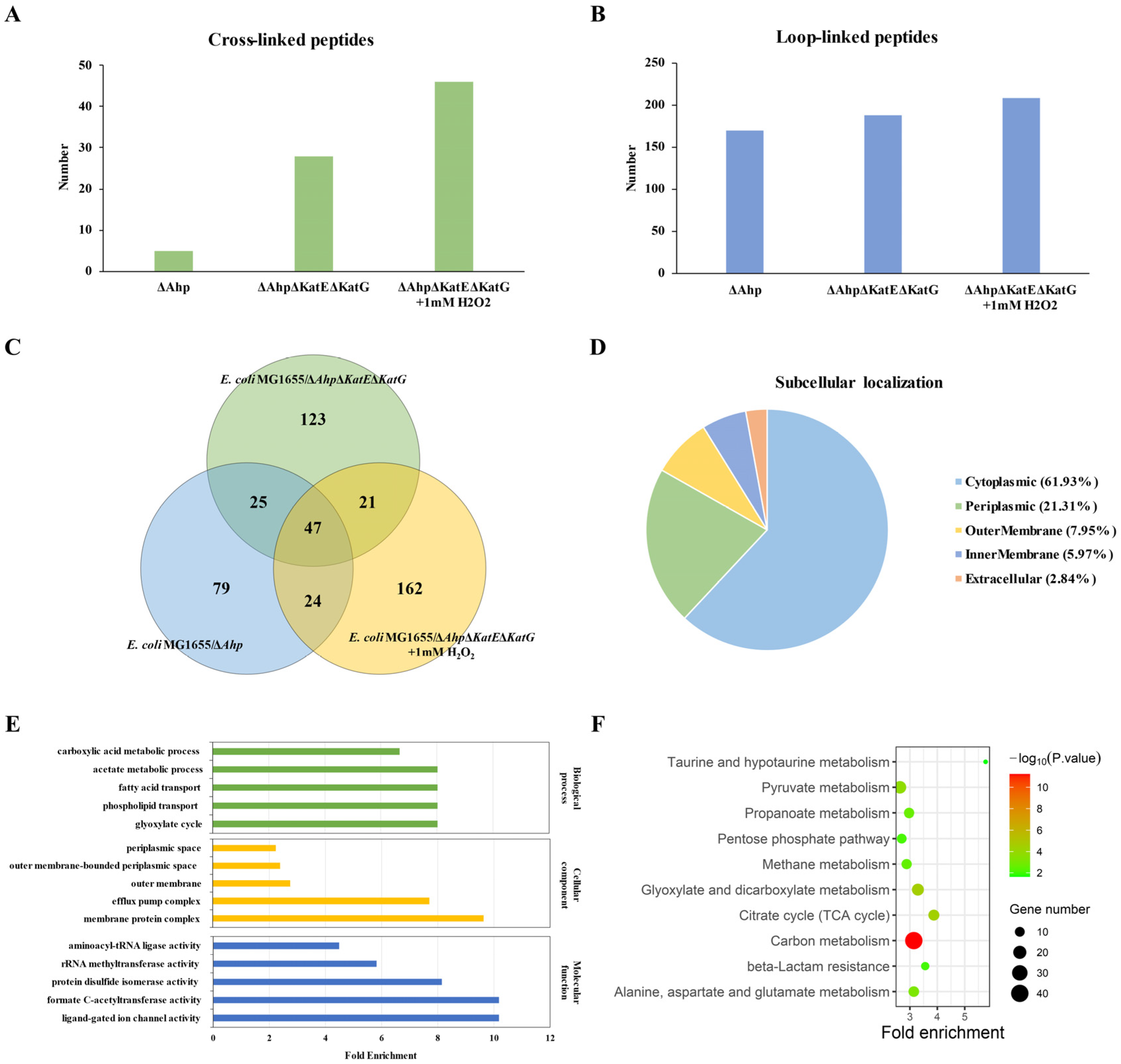
3.1. Qualitative Analysis of Dityrosine Crosslinking
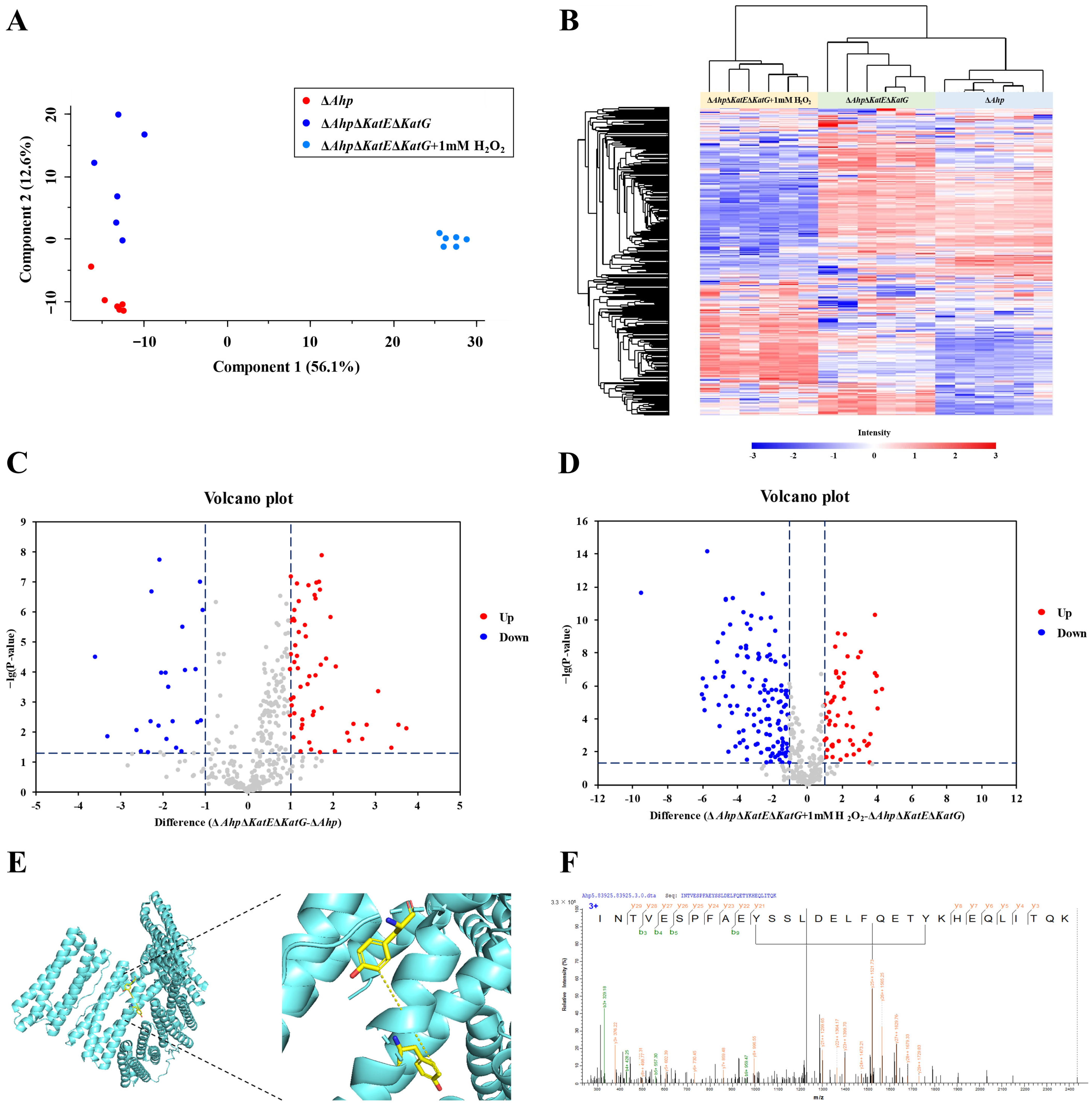
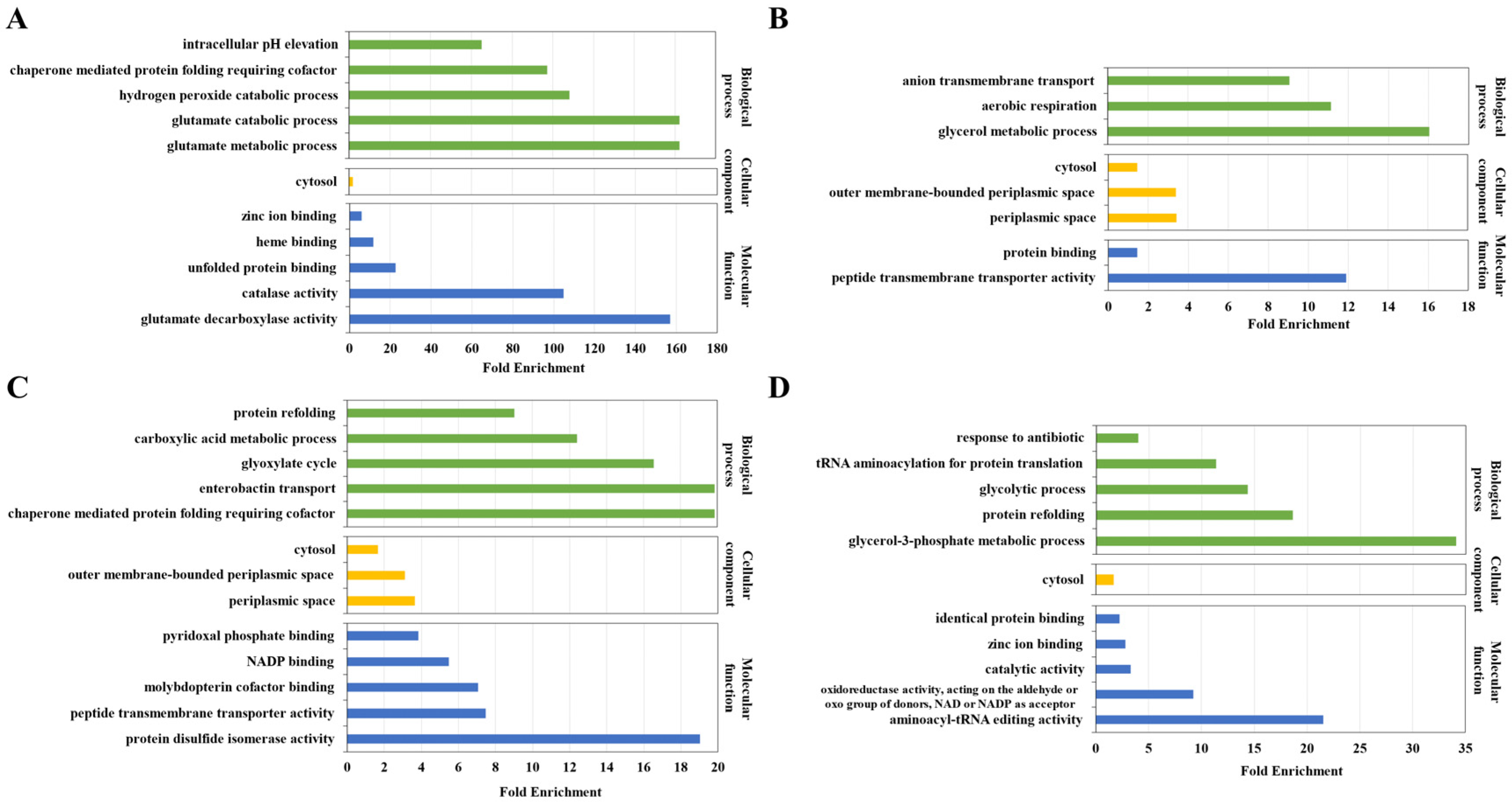
3.2. Quantitative Analysis of Dityrosine Crosslinking
3.3. Validation of Dityrosine Crosslinked Peptides In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wensien, M.; von Pappenheim, F.R.; Funk, L.-M.; Kloskowski, P.; Curth, U.; Diederichsen, U.; Uranga, J.; Ye, J.; Fang, P.; Pan, K.-T.; et al. A lysine–cysteine redox switch with an NOS bridge regulates enzyme function. Nature 2021, 593, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Ursem, R.; Swarge, B.; Abhyankar, W.R.; Buncherd, H.; de Koning, L.J.; Setlow, P.; Brul, S.; Kramer, G. Identification of Native Cross-Links in Bacillus subtilis Spore Coat Proteins. J. Proteome Res. 2021, 20, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Fan, S.-B.; Yang, B.; Li, Y.-X.; Meng, J.-M.; Wu, L.; Li, P.; Zhang, K.; Zhang, M.-J.; Fu, Y.; et al. Mapping native disulfide bonds at a proteome scale. Nat. Methods 2015, 12, 329–331. [Google Scholar] [CrossRef] [PubMed]
- Giulivi, C.; Traaseth, N.J.; Davies, K.J.A. Tyrosine oxidation products: Analysis and biological relevance. Amino Acids 2003, 25, 227–232. [Google Scholar] [CrossRef]
- Giulivi, C.; Davies, K.J. Mechanism of the Formation and Proteolytic Release of H2O2-induced Dityrosine and Tyrosine Oxidation Products in Hemoglobin and Red Blood Cells. J. Biol. Chem. 2001, 276, 24129–24136. [Google Scholar] [CrossRef]
- Li, X.; Li, S.; Liang, X.; McClements, D.J.; Liu, X.; Liu, F. Applications of oxidases in modification of food molecules and colloidal systems: Laccase, peroxidase and tyrosinase. Trends Food Sci. Technol. 2020, 103, 78–93. [Google Scholar] [CrossRef]
- Partlow, B.P.; Applegate, M.B.; Omenetto, F.G.; Kaplan, D.L. Dityrosine Cross-Linking in Designing Biomaterials. ACS Biomater. Sci. Eng. 2016, 2, 2108–2121. [Google Scholar] [CrossRef]
- Andersen, S.O. Characterization of a new type of cross-linkage in resilin, a rubber-like protein. Biochim. Biophys. Acta 1963, 69, 249–262. [Google Scholar] [CrossRef]
- Eyre, D.R.; Paz, M.A.; Gallop, P.M. Cross-linking in collagen and elastin. Annu. Rev. Biochem. 1984, 53, 717–748. [Google Scholar] [CrossRef]
- Locy, M.L.; Rangarajan, S.; Yang, S.; Johnson, M.R.; Bernard, K.; Kurundkar, A.; Bone, N.B.; Zmijewski, J.W.; Byun, J.; Pennathur, S.; et al. Oxidative cross-linking of fibronectin confers protease resistance and inhibits cellular migration. Sci. Signal. 2020, 13, eaau2803. [Google Scholar] [CrossRef]
- Al-Hilaly, Y.K.; Williams, T.L.; Stewart-Parker, M.; Ford, L.; Skaria, E.; Cole, M.; Bucher, W.G.; Morris, K.L.; Sada, A.A.; Thorpe, J.R.; et al. A central role for dityrosine crosslinking of Amyloid-β in Alzheimer’s disease. Acta Neuropathol. Commun. 2013, 1, 83. [Google Scholar] [CrossRef]
- Seaver, L.C.; Imlay, J.A. Alkyl Hydroperoxide Reductase Is the Primary Scavenger of Endogenous Hydrogen Peroxide in Escherichia coli. J. Bacteriol. 2001, 183, 7173–7181. [Google Scholar] [CrossRef]
- Liu, F.; Min, R.; Hong, J.; Cheng, G.; Zhang, Y.; Deng, Y. Quantitative proteomic analysis of ahpC/F and katE and katG knockout Escherichia coli—A useful model to study endogenous oxidative stress. Appl. Microbiol. Biotechnol. 2021, 105, 2399–2410. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Genet. 2013, 11, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2013, 10, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; DELLA-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2021, 50, D543–D552. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Kapp, E.A.; Lothian, A.; Roberts, A.M.; Vasil’Ev, Y.V.; Boughton, B.A.; Barnham, K.J.; Kok, W.M.; Hutton, C.A.; Masters, C.L.; et al. Characterization and Identification of Dityrosine Cross-Linked Peptides Using Tandem Mass Spectrometry. Anal. Chem. 2017, 89, 6136–6145. [Google Scholar] [CrossRef]
- Malencik, D.A.; Anderson, S.R. Dityrosine as a product of oxidative stress and fluorescent probe. Amino Acids 2003, 25, 233–247. [Google Scholar] [CrossRef]
- Smail, E.H.; Briza, P.; Panagos, A.; Berenfeld, L. Candida albicans cell walls contain the fluorescent cross-linking amino acid dityrosine. Infect. Immun. 1995, 63, 4078–4083. [Google Scholar] [CrossRef]
- Gu, M.; Bode, D.C.; Viles, J.H. Copper Redox Cycling Inhibits Aβ Fibre Formation and Promotes Fibre Fragmentation, while Generating a Dityrosine Aβ Dimer. Sci. Rep. 2018, 8, 16190. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Maruyama, W.; Naoi, M.; Hashizume, Y.; Osawa, T. Immunohistochemical detection of dityrosine in lipofuscin pigments in the aged human brain. FEBS Lett. 1998, 439, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Wu, X.; Naito, M.; Nomura, H.; Kitamoto, N.; Osawa, T. Immunochemical detection of protein dityrosine in atherosclerotic lesion of apo-E-deficient mice using a novel monoclonal antibody. Biochem. Biophys. Res. Commun. 2000, 275, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Koike-Takeshita, A.; Arakawa, T.; Taguchi, H.; Shimamura, T. Crystal Structure of a Symmetric Football-Shaped GroEL:GroES2-ATP14 Complex Determined at 3.8 Å Reveals Rearrangement between Two GroEL Rings. J. Mol. Biol. 2014, 426, 3634–3641. [Google Scholar] [CrossRef] [PubMed]
- Stillman, T.; Hempstead, P.; Artymiuk, P.; Andrews, S.; Hudson, A.; Treffry, A.; Guest, J.; Harrison, P. The high-resolution X-ray crystallographic structure of the ferritin (EcFtnA) of Escherichia coli; comparison with human H ferritin (HuHF) and the structures of the Fe3+ and Zn2+ derivatives. J. Mol. Biol. 2001, 307, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Leinisch, F.; Mariotti, M.; Hägglund, P.; Davies, M.J. Structural and functional changes in RNAse A originating from tyrosine and histidine cross-linking and oxidation induced by singlet oxygen and peroxyl radicals. Free Radic. Biol. Med. 2018, 126, 73–86. [Google Scholar] [CrossRef]
- de la Torre, A.V.; Gay, M.; Vilaprinyó-Pascual, S.; Mazzucato, R.; Serra-Batiste, M.; Vilaseca, M.; Carulla, N. Direct Evidence of the Presence of Cross-Linked Aβ Dimers in the Brains of Alzheimer’s Disease Patients. Anal. Chem. 2018, 90, 4552–4560. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Altomare, A.; Rusconi, F.; Giustarini, D.; Portinaro, N.; Garavaglia, M.L.; Rossi, R.; Dalle-Donne, I.; Milzani, A. Thiol oxidation and di-tyrosine formation in human plasma proteins induced by inflammatory concentrations of hypochlorous acid. J. Proteom. 2017, 152, 22–32. [Google Scholar] [CrossRef]
- Wu, G.-R.; Cheserek, M.; Shi, Y.-H.; Shen, L.-Y.; Yu, J.; Le, G.-W. Elevated Plasma Dityrosine in Patients with Hyperlipidemia Compared to Healthy Individuals. Ann. Nutr. Metab. 2015, 66, 44–50. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Hansen, P.A.; Holloszy, J.O.; Heinecke, J.W. Oxidized amino acids in the urine of aging rats: Potential markers for assessing oxidative stress in vivo. Am. J. Physiol. Integr. Comp. Physiol. 1999, 276, R128–R135. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Pennathur, S.; Byun, J.; Crowley, J.; Mueller, D.; Gischler, J.; Hotchkiss, R.S.; Heinecke, J.W. NADPH Oxidase of Neutrophils Elevates o,o′-Dityrosine Cross-Links in Proteins and Urine during Inflammation. Arch. Biochem. Biophys. 2001, 395, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Wells-Knecht, M.; Huggins, T.; Dyer, D.; Thorpe, S.; Baynes, J. Oxidized amino acids in lens protein with age. Measurement of o-tyrosine and dityrosine in the aging human lens. J. Biol. Chem. 1993, 268, 12348–12352. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.A.; Linton, S.M.; Davies, M. Detection of HOCl-mediated protein oxidation products in the extracellular matrix of human atherosclerotic plaques. Biochem. J. 2003, 370, 729–735. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Liu, F.; Li, N.; Zhang, Y. Large-Scale Qualitative and Quantitative Assessment of Dityrosine Crosslinking Omics in Response to Endogenous and Exogenous Hydrogen Peroxide in Escherichia coli. Antioxidants 2023, 12, 786. https://doi.org/10.3390/antiox12040786
Zhou X, Liu F, Li N, Zhang Y. Large-Scale Qualitative and Quantitative Assessment of Dityrosine Crosslinking Omics in Response to Endogenous and Exogenous Hydrogen Peroxide in Escherichia coli. Antioxidants. 2023; 12(4):786. https://doi.org/10.3390/antiox12040786
Chicago/Turabian StyleZhou, Xiangzhe, Feng Liu, Nuomin Li, and Yongqian Zhang. 2023. "Large-Scale Qualitative and Quantitative Assessment of Dityrosine Crosslinking Omics in Response to Endogenous and Exogenous Hydrogen Peroxide in Escherichia coli" Antioxidants 12, no. 4: 786. https://doi.org/10.3390/antiox12040786
APA StyleZhou, X., Liu, F., Li, N., & Zhang, Y. (2023). Large-Scale Qualitative and Quantitative Assessment of Dityrosine Crosslinking Omics in Response to Endogenous and Exogenous Hydrogen Peroxide in Escherichia coli. Antioxidants, 12(4), 786. https://doi.org/10.3390/antiox12040786





