Abstract
Justicia secunda Vahl. is a traditional medicinal plant in tropical regions, including West Africa. The present study examined the chemical profiles and biological properties of J. secunda extracts obtained with different solvents (dichloromethane, ethyl acetate, methanolic and aqueous: macerated and infused). Chemical components were characterized by liquid chromatography-mass spectrometry (LC-MS), and over 50 compounds were identified, including flavonoids, phenolic acids, and alkaloids. Antioxidant, enzyme inhibitory, cytotoxic, and antiviral properties were selected as biological properties. Total phenolic and flavonoid contents in methanol (58.07 mg gallic acid equivalent (GAE)/g and 13.07 mg rutin equivalent (RE)/g) and water (infused) (36.34 mg GAE/g and 8.52 mg RE/g) were higher than in other extracts. Consistent with the levels of total bioactive components, the methanol and water extracts exhibited stronger antioxidant abilities. However, the dichloromethane and ethyl acetate extracts were more active on α-amylase and α-glucosidase than other extracts. Aqueous extracts exerted selective anticancer properties toward human pharyngeal cancer cell lines, whereas the methanolic extract decreased the human herpesvirus type-1 (HHV-1) infectious titer by 2.16 log and the viral load by 1.21 log. Overall, J. secunda could be considered a multifunctional bioactive raw material in the preparation of potent applications to manage diseases related to oxidative stress, including cancer, diabetes, and Alzheimer’s.
1. Introduction
In the past ten years, the world population has been increasing daily, and the way of life of humans has undergone an intense change. Due to this fact, the prevalence of some diseases, including cancer [1], diabetes [2], or cardiovascular problems [3], is reaching a critical point worldwide, and we need to find global solutions. Synthetic drugs topped the list, but their long-term use raises serious concerns. In light of this, the importance of finding efficient drugs points out the opportunity to study natural products and plant species, which have attracted the interest of the scientific platform, especially, interest in un-investigated plants has been steadily increasing [4].
Justicia secunda Vahl. (eng. St. John’s bush) is a plant belonging to the Acanthaceae family. In Barbados, it is known as “Bloodroot” and in Venezuela as “Sanguinaria”, which refers to the red color of water observed when the plant is boiled. Traditional medicine of Barbadian locals describes the oral use of decoctions and infusions from the leaves of this plant to treat wound infections [5]. It is also used for bathing dogs suffering from skin rashes [6]. An ethnopharmacological survey carried out in Kikwit city (Kwilu province, southwest part of the Democratic Republic of the Congo) revealed that J. secunda leaves were used in traditional medicine to treat sickle cell disease. Importantly, further in vitro studies showed that J. secunda extract permits a change of shape of a sickle cell into a normal one with a maximal normalization rate (NRmax) of above 80% [7]. Moreover, the anthocyanin fraction isolated from J. secunda leaves extract also showed anti-sickling activity through direct binding with sickle cell deoxyhemoglobin molecules and stabilization of the sickle cell red blood cells’ membrane [8]. Traditional medicinal use of this plant to treat diabetes was also reported [9]. The anti-diabetic activity of this plant could be attributed to the presence of 2-caffeoyloxy-4-hydroxy-glutaric acid and the diastereomers secundarellone B and C, which were shown in vitro to inhibit α-glucosidase [9]. Interestingly, the literature data provide discrepant results concerning the antibacterial activity of J. secunda. Rojas et al. [10] reported that the aqueous extract exerted activity against Escherichia coli and ethanolic extract additionally against Pseudomonas aeruginosa and Candida albicans at concentrations of 25 μg/mL. The hexanoic extract inhibited only Bacillus cereus. Other Gram-positive bacteria, Staphylococcus aureus and β-hemolytic Streptococcus, were resistant to tested extracts [10]. Interestingly, Carrington et al. [5] reported that Staphylococcus aureus (ATCC 25923), Pseudomonas aeruginosa (ATCC 27853), and Enterococcus feacalis (clinical strain) were resistant to J. secunda methanolic and acetone extracts in concentrations range 1–200 mg/mL.
However, a comprehensive chemical and biological assessment of J. secunda still needs to be made available. With this in mind, we aimed to identify chemical components (by LC-MS technique) and to assess the biological activities (antioxidant, enzyme inhibitory, cytotoxic, and antiviral) of different extracts (dichloromethane, ethyl acetate, methanolic, aqueous (as macerated and infused)) of J. secunda. The obtained results could clarify the functional properties of J. secunda on the road from nature to pharmacy shelves.
2. Materials and Methods
2.1. Plant Materials and Extraction
The aerial parts of the plants (Justicia secunda) were collected from one locality (Nahio (Department of Issia, Haut-Sassandra region, Côte d’Ivoire) of Ivory Coast at the flowering period in the summer season of 2020. The plant was identified by a botanist (Dr. Kouadio Bene). Voucher specimens (KB-20-1016) were deposited at the herbarium at Selcuk University. The plant materials were thoroughly cleaned by washing with tap water and then rinsing with distilled water to remove soil and contaminants. The aerial parts were separated and dried in a well-ventilated, shaded environment at the room temperature. After 10 days of drying, the materials were ground into powder using a Retsch SM-200 laboratory mill and extracted within the same week. The powdered plant material was stored in a cool, dark, and well-ventilated area at around 20 °C.
We used four solvents (dichloromethane, ethyl acetate, methanol, and water) to prepare plant extracts. The maceration method was chosen for the extracts, and 10 g of plant material was mixed with 200 mL of the solvents for 24 h at room temperature. The mixtures were then filtered with Whatman filter paper, and the solvents were removed with a rotary evaporator under reduced pressure at 40 °C. Traditional infusion was prepared using 10 g of plant material steeped in 200 mL of boiled water for 15 min. The mixture was subsequently filtered, frozen, and lyophilized for 48 h (−80 °C, under vacuum). All extracts were kept at 4 °C until analysis.
2.2. Determination of Total Phenolic, Flavonoid and Antioxidant and Enzyme Inhibitory Effects
Total phenolic content (TPC), total flavonoid content (TFC), DPPH radical scavenging, ABTS radical scavenging, cupric reducing antioxidant capacity (CUPRAC), ferric reducing antioxidant power (FRAP), metal chelating activity (MCA), phosphomolybdenum, inhibition of acetylcholinesterase (AChE), butyrylcholinesterase (BChE), tyrosinase, amylase, and glucosidase assays were performed as previously described [11,12]. Each sample was processed in triplicate.
2.3. LC-MS Analysis
Liquid chromatography—mass spectrometry analysis was performed using Agilent 1200 Infinity HPLC coupled to Agilent 6530B QTOF system (Agilent Technologies, Santa Clara, CA, USA). Chromatographic separation was performed on the C18 Gemini® column (3 µm i.d., with TMS endcapping, 110 Å, 100 × 2 mm) supported with a guard column (Phenomenex Inc, Torrance, CA, USA). Solvent A (water with 0.1% formic acid v/v) and solvent B (acetonitrile with 0.1% formic acid) were pumped in the gradient: 0–60% B for 45 min., next 60–95% B for 1 min, and 95% B for 4 min, at the flow rate of 0.2 mL/min. A total of 10 μL of the sample was injected into the chromatographic column at 20 °C. MS detection was performed in positive and negative ion modes at a collision energies of 10 and 30 eV. Scanning was done in the range of 100–1000 m/z. The other conditions were as follows: drying gas temp: 275 °C, drying gas flow: 10 L/min, sheath gas temp: 325 °C, sheath gas flow: 12 L/min; nebulizer pressure: 35 psig, capillary V (+): 4000 V, skimmer 65 V, fragmentor 140 V. Compounds were tentatively identified based on their accurate masses and fragmentation patterns, also supported by the available literature sources.
2.4. Cytotoxicity Testing and Anticancer Selectivity
The cytotoxicity of J. secunda extracts was tested using a colorimetric microculture tetrazolium (MTT) assay according to the previously described methodology [13], and details were presented in Supplementary Materials. Briefly, the monolayers of selected cell lines in 96-well plates were incubated with serial dilutions of test extracts in cell media for 72 h. Afterwards, the MTT was performed to assess the cellular viability. Data analysis was conducted using GraphPad Prism, and the CC50 values (50% cytotoxic concentration) were evaluated. To evaluate the anticancer activity, the selectivity indexes (SI) were assessed in relation to VERO (SI = CC50VERO/CC50Cancer, SI > 1 indicates anticancer selectivity). For the FaDu cells, the influence of tested compounds on the cellular morphology was assessed during the 72 h incubation period using Olympus Provi CM20 Incubation Monitoring System (Evident Corporation, Hamburg, Germany).
2.5. Evaluation of the Anti-Herpesvirus Effect
The influence of J. secunda extracts on the Human Herpesvirus type-1 (HHV-1, aka. HSV-1) induced cytopathic effect (CPE) was assessed as previously described [13], and details are presented in the Supplementary Materials. For this purpose, the monolayers of VERO cells in 48-well plates were infected with 100-fold CCID50 (CCID50—50% cell culture infectious dose) of HHV-1 for 1 h. Afterwards, the monolayers were washed with PBS to remove unattached viral particles, tested compounds in non-toxic concentration were added, and incubation continued until the CPE was observed in the virus control (HHV-1 infected, untreated cells). Afterwards, the plates were observed using an inverted microscope (CKX41, Olympus Corporation, Tokyo, Japan) for possible inhibition of CPE by tested extracts, and the results were recorded. The plates were thrice frozen (−72 °C) and thawed; the samples were collected and stored at −72 °C until used in the end-point virus titration assay and DNA isolation. Acyclovir was used as a reference antiviral drug.
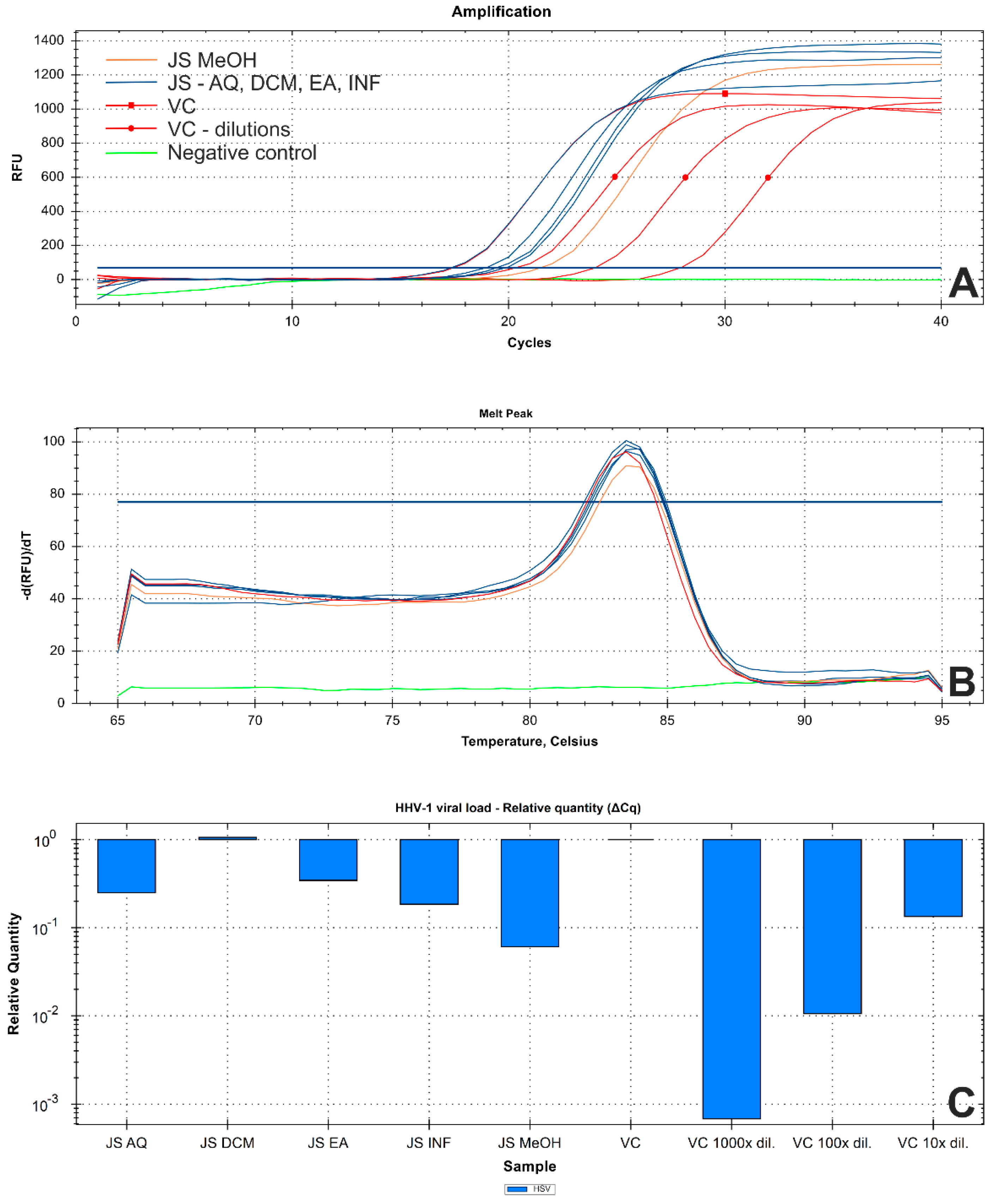
Samples collected from antiviral assays were subjected to an end-point dilution assay to evaluate the HHV-1 titers. The difference (Δlog) of HHV-1 infectious titer (logCCID50/mL) in the samples treated with J. secunda extracts and in the virus control (VC) from the same experiment (Δlog = logCCID50VC − logCCID50JS) were calculated. Data analysis was conducted using GraphPad Prism.
The DNA was isolated using a commercially available kit (QIAamp DNA Mini Kit, Cat#51304, QIAGEN GmbH, Hilden, Germany) following the manufacturer’s instructions. The real-time PCR amplification was performed using SybrAdvantage qPCR Premix (Takara Bio Inc., Kusatsu, Shiga, Japan) and primers (UL54F-5′ CGCCAAGAAAATTTCATCGAG 3′, UL54R-5′ ACATCTTGCACCACGCCAG 3′) on the CFX96 real-time thermal cycler (Bio-Rad Laboratories, Inc., Pleasanton, CA, USA). The HHV-1 viral load in the tested samples was assessed in relation to virus control based on the relative quantity (ΔCq) method using CFX Manager™ Dx Software (Bio-Rad Laboratories, Hercules, CA, USA).
2.6. Data Analysis
Data were presented as mean ± standard deviation. One-way analysis of variance with Tukey’s post-hoc test was achieved; p < 0.05 was considered statistically significant. Then Pearson correlation coefficient between the chemical compounds and the antioxidant and enzyme inhibitory activity were calculated. Pearson’s coefficient higher than 0.7 was considered statistically significant. All analysis were done under R v 4.1.2 software.
3. Results and Discussion
3.1. Total Phenolic and Flavonoid Contents
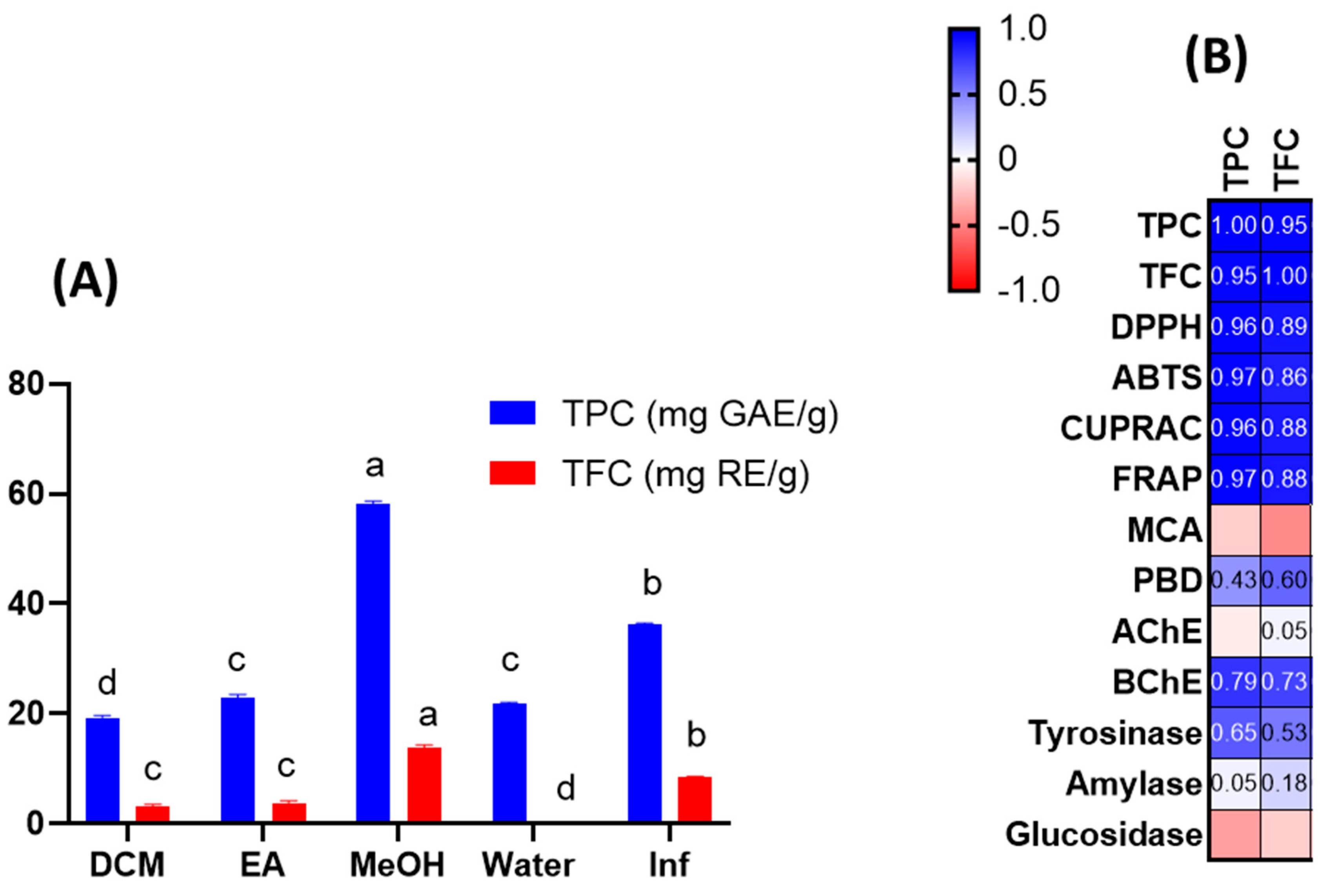
Extracts were tested for their total phenolic and flavonoid content using spectrophotometric techniques. As can be seen from Figure 1A, the highest concentration of total bioactive compounds was detected in methanolic extract (TPC: 58.07 mg gallic acid equivalent (GAE)/g: TFC: 13.70 mg rutin equivalent (RE)/g), followed by infusion (TPC: 36.34 mg GAE/g: TFC: 8.52 mg RE/g) and ethyl acetate extract (TPC: 22.98 mg GAE/g: TFC: 3.65 mg RE/g). The total amount of bioactive compounds was lowest in the dichloromethane and aqueous extracts. Total bioactive compound amounts were clearly influenced by the solvents used. Based on our results, methanol and water (as an infusion) could be effective solvents for further applications of J. secunda. Few publications in the literature have reported the total phenolic content of J. secunda extracts or fractions [14,15,16]. In the reports, the authors used different standards to explain the total content of bioactive compounds in dry or fresh samples, therefore our results could not be compared with most previous reports. In a study conducted by John et al. [16], the total phenolic and flavonoid contents were reported for six Justicia species and the highest level was reported for the leaf extract of J. adhatoda with 38.75 mg GAE/100 g extract, which was lower than those of our tested extracts. Moreover, the total phenolic and flavonoid contents were reported as 1.33–5.01 mg GAE/100 g and 0.18–1.30 mg catechin equivalent (CE)/100 g for J. spicigera extracts, respectively in another study [17]. Naqvi et al. [18] reported the total phenolic level as 30.4–80.9 mg GAE/100 g extract in J. camara and J. adhatoda extracts. Based on the results of earlier reports, we concluded that J. secunda could be considered a valuable source of phenolic compounds.
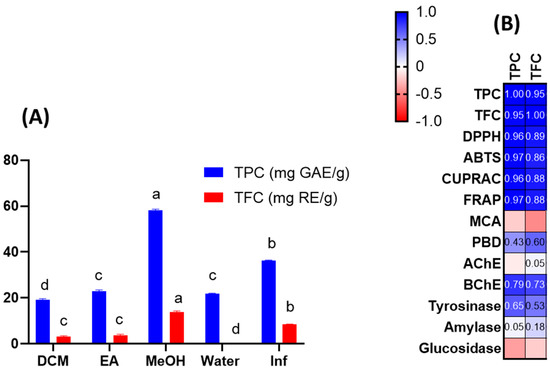
Figure 1.
Total phenolic (TPC) and total flavonoid (TFC) content of the tested extracts (A). Pearson’s correlation values between total bioactive compounds and antioxidant and enzyme assays (B). Values are reported as mean ± SD of three parallel measurements. GAE: gallic acid equivalents; RE: rutin equivalents. Different letters (a–d) in the columns indicate significant differences in the tested extracts (“a” indicated the highest content, p < 0.05). ABTS, 2,2′-azino-bis (3-ethylbenzothiazoline) 6-sulfonic acid; AChE, acetylcholinesterase; BChE, butyrylcholinesterase; CUPRAC, cupric ion reducing antioxidant capacity; DPPH, 1,1-diphenyl-2-picrylhydrazyl; FRAP, ferric ion reducing antioxidant power; MCA, metal chelating activity; PBD, phosphomolybdenum activity; AChE: acetylcholinesterase; BChE: butrylcholinesterase.
3.2. Chemical Characterization
Applying high-performance liquid chromatography combined with electrospray ionization-quadrupole time-of-flight mass spectrometry enabled the tentative identification of over numerous compounds in various extracts obtained from J. secunda leaves (Table 1). Spectrometric data collected in negative and positive ion mode for all compounds are presented in Supplementary Table S1.

Table 1.
The secondary metabolites determined in J. secunda extracts.
The first group of compounds identified in J. secunda leaves were flavonoids. They were found in infusion and methanolic extract and also in fewer number in ethyl acetate extract. Among flavonoids, mainly glycosidic derivatives of tetrahydroxyflavone, trihydroxyflavone, trihydroxymethoxyflavone, and tetrahydroxyflavonol were observed and tentatively identified as luteolin, apigenin, diosmetin, and quercetin, respectively. Di- and trisaccharidic moieties substituted on flavonoids comprised glucose, rhamnose, apiose, xylose, and acetylpentose (tentatively identified as acetylapiose). Phenolic acids substituted on flavonoids were also observed. Two compounds: luteolin 7-O-[β-glucopyranosyl-(1→2)-β-rhamnosyl-(1→6)] β-glucopyranoside and luteolin 7-O-[β-apiofuranosyl-(1→2)]-β-xylopyranoside were previously identified in J. secunda leaves by Koffi et al. [14] by nuclear magnetic resonance (1H NMR,13C NMR). Moreover, Koffi et al. [15] identified luteolin 7-O-rutinoside by HPLC-ESI-MS/IonTrap in the same plant species. The abovementioned compounds were identified in our extracts.
Polyphenols were also represented by phenolic acids. Benzoic acid derivatives were present in infusion, aqueous and methanolic extracts. Glycosides of salicylic, syringic, dihydroxy- and trihydroxybenzoic (gallic) acids were tentatively identified. Hydroxycinnamic and caffeic acid derivatives were found in methanolic extract and infusion. Moreover, non-phenolic carboxylic acids derivatives were present in infusion, aqueous, and methanolic extracts.
According to Corrêa and Alcântara [19], lignans are one of the chemical classes found in the different species of the genus Justicia. We found four isomers characterized by the same precursor ion [M-H]- at m/z 355.0612 (predicted) but with different retention times, which were tentatively identified as lignan derivatives, probably 10-(1,3-benzodioxol-5-yl)-5H-benzo[c]furo[3,2-g]chromen-5-one isomers. They were present only in the infusion. Koffi et al. [15] also found precursor ion at m/z 355 ([M-H]−), which they identified as justiflorinol with molecular formula C19H16O7. Nevertheless, the molecular formula calculated for the precursor ion [M-H]− at m/z 355.0612 (C22H12O5) found in our extract varied and was identified as a different compound. One lignan derivative ([M-H]− at m/z 505.2079, predicted) was found in the methanolic extract.
Moreover, cyclohexanone derivatives, mainly glucosides, were found in all obtained extracts except the aqueous one. According to data obtained for J. aequilabris [20], a compound characterized by the precursor ion [M+COOH]− at m/z 431.1923 (predicted) was identified as roseoside. Two different cyclohexanone derivatives were tentatively identified as dihydroroseoside and 9-hydroxy-7-megastigmen-3-one glucoside. Furthermore, decalin glucoside derivatives tentatively identified as ophiogonoside A isomers were present in all extracts except the aqueous one.
Alkaloids are one of the compound classes found in the species belonging to the genus Justicia [19,21]. NMR analyses performed by Theiler et al. [22] revealed the presence of secundarellone B/C (racemate) and secundarellone A in the leaves of J. secunda. These compounds can be classified as pyrrolidone alkaloids. Secundarellone B/C and one isomer of secundarellone A were found in dichloromethane extract, and the second one of the isomers of secundarellone A was identified in all obtained extracts.
3.3. Antioxidant Capacity
Antioxidant has been one of the most used terms in pharmaceutical, nutraceutical, and cosmetic applications over the past decade. Antioxidant compounds could be considered powerful protective agents against the attacks of free radicals, which are the main trigger for developing severe health problems such as cancer, diabetes, or cardiovascular diseases. In this sense, discovering new sources of antioxidants is crucial to treating the mentioned diseases. In the chemical industry, some compounds, including butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), and propyl gallate (PG), have been chemically produced, but their use raises public concerns such as hepatotoxicity or cancerogenic influence on the human organism [23]. Therefore, synthetic antioxidants should be swapped with natural alternatives, and plants serve as a rich source of such antioxidants.
Given the preceding information, we examined the antioxidant capacities of J. secunda extracts using various chemical assays, such as free radical quenching, reducing power, and metal chelation. The results are presented in Table 2. The DPPH and ABTS assays are the most prevalent antioxidant assays and provide information on plant extracts’ radical-scavenging capacity. In both assays, the tested methanolic and infused extracts were the most active, while the weakest ability was exerted by the dichloromethane extract. In addition, the macerated and infused aqueous extracts showed different abilities in the free radical scavenging assays. In general, the antioxidant mechanism is based on hydrogen or electron-donation abilities. Reducing power assays have been utilized to assess the electron-donation capacity of a compound or extract. CUPRAC and FRAP are the two most important reducing power assays based on the transformation of Cu2+ to Cu+ and Fe3+ to Fe2+, respectively. Table 2 shows that similar to radical scavenging assays, methanolic and infusion extracts were found to possess the most potent reducing abilities. In addition, the extracts contained higher amounts of the total phenolic and flavonoid when compared to other extracts. Both radical scavenging (R2 was 0.96 for DPPH and TPC; was 0.97 for ABTS and TPC) and reducing power assays (R2 was 0.96 for CUPRAC and TPC; was 0.97 for FRAP and TPC) correlated strongly with the total bioactive content at this point (Figure 1B). Consistent with our approach, several researchers reported a linear correlation between the levels of total bioactive compounds and these parameters [24,25,26]. In addition to the positive correlation between total bioactive compounds and free radical scavenging/reduction assays, the presence of some compounds can be attributed to the observed significant antioxidant properties of methanol and infused extracts. For example, rutin was only detected in the tested methanol extract and it has already been shown to be a powerful antioxidant [27]. Besides rutin, caffeic acid derivatives was only found in the methanol extract and some researchers have reported antioxidant properties of caffeic acid [28,29]. Regarding infused extract, particularly the presence of apigenin and hydroxycinnamic acid were only detected in infused extract and these compounds may contribute to the observed antioxidant properties [30,31]. In the literature, several researchers reported significant free radical scavenging and reducing abilities of members of the Justicia genus. For example, Koffi et al. [14] examined J. secunda leaves extracts for ABTS radical scavenging, and the ability was reported as 218 µmol TE/g for non-concentrated extract. In another study by Anyasor et al. [32], the hot-water extract of J. secunda leaves showed a stronger ability to scavenge free radicals and reduce potency than the cold-water extract, consistent with our presented results. Significant free radical scavenging and reducing potential of the methanolic extract (80%) of J. secunda leaves was reported in a previous study by Onoja et al. [33]. Phosphomolybdenum assays involve the transformation of Mo (VI) to Mo (V) under acidic conditions by antioxidant compounds. In contrast to other reducing power assays, the best ability was recorded in the ethyl acetate extract with 1.66 mmol TE/g, followed by methanol and dichloromethane. This case could be explained by the presence of non-phenolic antioxidants such as ascorbic acid and tocopherols. Thus, the correlation between total phenolic and phosphomolybdenum was weak (R2: 0.43). Several researchers who reported a weak correlation between total phenolic and phophomolybdenum assay concurred with our findings [34,35]. Transition metals play a role in the Fenton reaction, which leads to the formation of hydroxyl radicals. As a result, when transition metals are chelated, the production of this radical is inhibited. Therefore, metal chelation is considered to be an important antioxidant mechanism. In contrast to other assays, the highest metal chelating ability was provided by the aqueous extract with 35.91 mg EDTAE/g, followed by ethyl acetate and methanolic extracts. Surprisingly, the infusion showed no ability to metal chelating. The presence of non-phenolic chelating agents, such as peptides and polysaccharides in the tested extracts, could explain the conflicting results. Overall, we provided a comprehensive antioxidant profile of different extracts from J. secunda. The methanolic and infused extracts of J. secunda exhibited greater antioxidant activity than other extracts, and this finding could be used to design functional applications utilizing J. secunda in future research.

Table 2.
Antioxidant properties of the tested extracts *.
3.4. Enzyme Inhibitory Properties
Enzymes play crucial roles in developing effective therapeutic strategies to combat global health issues. In this sense, the majority of drugs have been designed to inhibit specific enzymes. This phenomenon is known as the enzyme inhibition theory [36]. For example, the main problem in diabetics is high blood glucose levels, and this could be managed by inhibiting amylase and glucosidase, the main starch-degrading enzymes [37]. Similar correlations could be made between the inhibition of acetylcholinesterase and Alzheimer’s patients, who have lower acetylcholine levels compared to healthy individuals [38]. With this in mind, some compounds have been synthesized through chemical synthesis, but they have undesirable side effects, such as gastrointestinal disturbances and toxicity [39,40,41]. Consequently, more secure, and efficient phytochemical-based inhibitors are desired.
In light of the above information, we examined the enzyme-inhibitory properties of J. secunda extracts against AChE, BChE, tyrosinase, amylase, and glucosidase. The results are presented in Table 3. The best AChE inhibitory ability was observed by the ethyl acetate extract with 1.63 mg GALAE, but it was not statistically different from dichloromethane (1.51 mg GALAE/g) (p > 0.05). Regarding the BChE inhibitory abilities, only two extracts (methanolic and ethyl acetate) were active on the enzyme, and the methanolic extract was more active than the ethyl acetate one. In both AChE and BChE assays, macerated and infused aqueous extracts were not active. The observed anti-cholinesterase ability of the methanolic extract might be explained by the presence of rutin, which was only detected in the methanolic extract. Similar to our approach, the anti-cholinesterase ability of rutin and its mechanism have been reported in several studies [42,43]. In addition to rutin, the observed ability to inhibit cholinesterase can be attributed to caffeic acid derivatives. In earlier studies [44,45], caffeic acid has been reported as a significant anti-Alzheimer’s agent. There is a dearth of data in the scientific literature about the cholinesterase-inhibiting effects of the Justicia genus. In contrast to our results, Rawa et al. [46] reported that the methanolic extract of J. gendarussa leaves was not active on both AChE and BChE. Previous research by Gupta and Gupta [47] found that both the leaf and stem of J. gendarussa showed weak AChE inhibitory properties.

Table 3.
Enzyme inhibitory effects of the tested extracts *.
Similar to the cholinesterase inhibitory properties, the aqueous extracts exhibited lower inhibitory effects on amylase and glucosidase compared to other extracts. The ethyl acetate extract was the most active on the anti-diabetic enzymes, but its activity was not statistically different from dichloromethane extract. The observed inhibitory effects on amylase and glucosidase did not correlate with the total bioactive compounds. As can be seen from Table 2, diosmetin was only detected in ethyl acetate extract and its presence can be attributed to the observed antidiabetic ability of the extract. In an earlier study by Yuan et al. [48], diosmetin as well as luteolin and quercetin exhibited a significant amylase inhibitory effect. The compounds in the methanol extracts such as rutin and caffeic acid derivatives can also contributed to the observed antidiabetic ability [49,50]. The amylase inhibitory effect of the aqueous extract of J. carnea was reported to be moderate [51], which is similar to our findings. In another study, Ani et al. [52] studied the anti-amylase and anti-glucosidase abilities of different extracts (ethanolic, methanolic, and aqueous) of J. carnea leaves and found that the ethanolic extract was the most active. In contrast to our results, the methanolic extract of J. adhatoda leaves showed low amylase inhibitory activity with an inhibitory value of less than 50% [53].
Melanogenesis is an essential process that shields humans from the sun’s ultraviolet rays. Tyrosinase is a crucial enzyme in melanogenesis reactions, and inhibiting it could alleviate hyperpigmentation problems [41]. As can be seen from the Table 3, the tested extracts can be ranked according to decrease tyrosinase inhibitory activity as follows: methanolic > aqueous > infusion > dichloromethane > ethyl acetate. As can be seen from Figure 1, the observed tyrosinase inhibitory ability correlated moderately with the total phenolic and flavonoid content of the tested extracts (R2 < 0.7). Based on the identified compounds from Table 1, the observed anti-tyrosinase ability can be attributed to some compounds. For example, several compounds, including rutin, luteolin and caffeic acid, have already been reported as effective anti-tyrosinase agents [54,55]. In a recent study by Basit et al. [56], the tyrosinase inhibitory effect of n-butanol extract of J. vahlii leaves was reported as 193.21 mg KAE/g, which was higher than our results. The anti-tyrosinase inhibitory effect of the ethanolic extract (50%) of J. adhatoda leaves was found to be less than 50% in a previous study by Ito et al. [57]. The current study is, to our knowledge, the first to describe the enzyme-inhibiting properties of J. secunda. The results could shed light on the potential applications of J. secunda in nutraceutical, cosmetic, and pharmaceutical practices.
3.5. Cytotoxicity Evaluation and Anticancer Selectivity
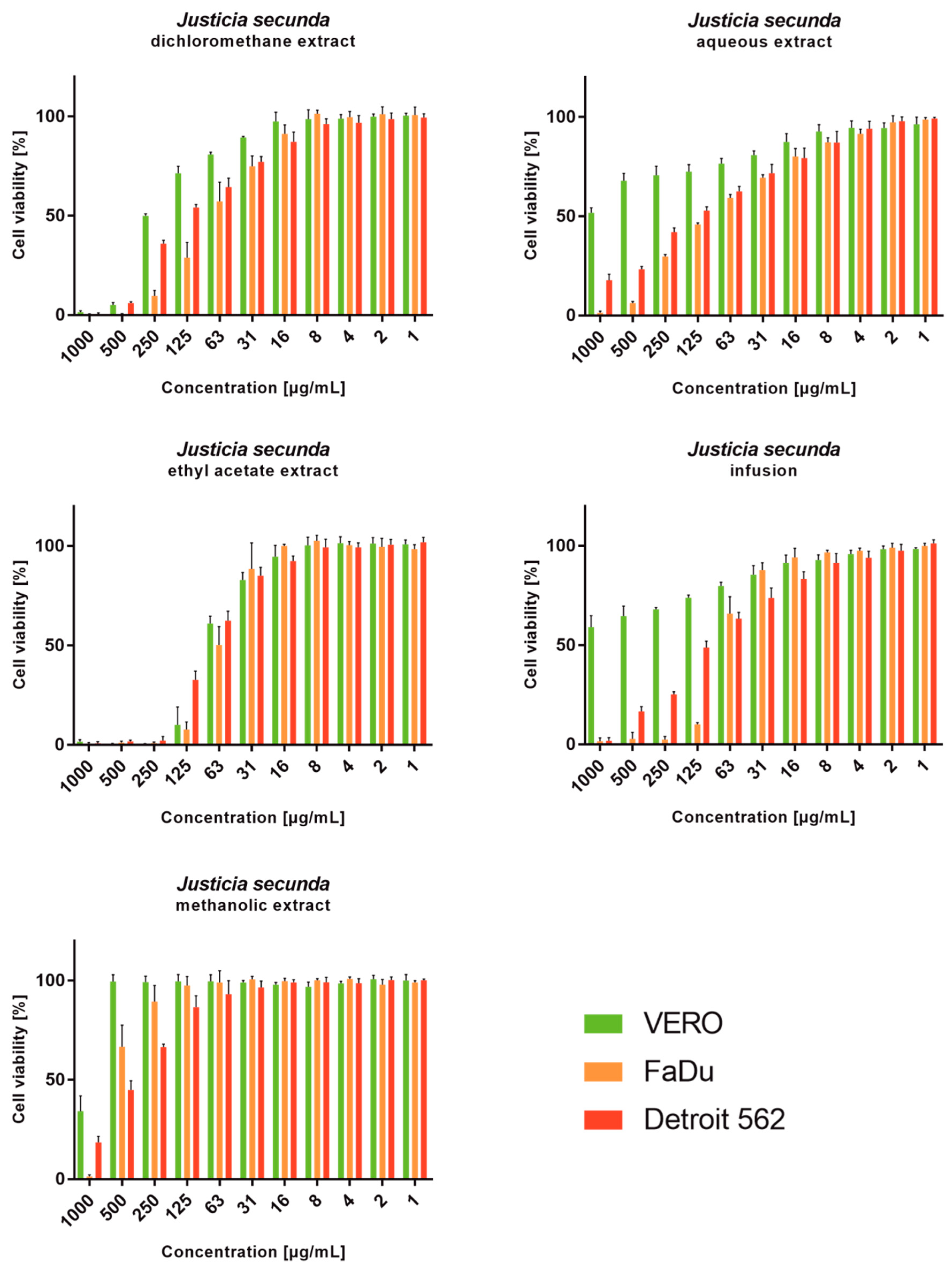
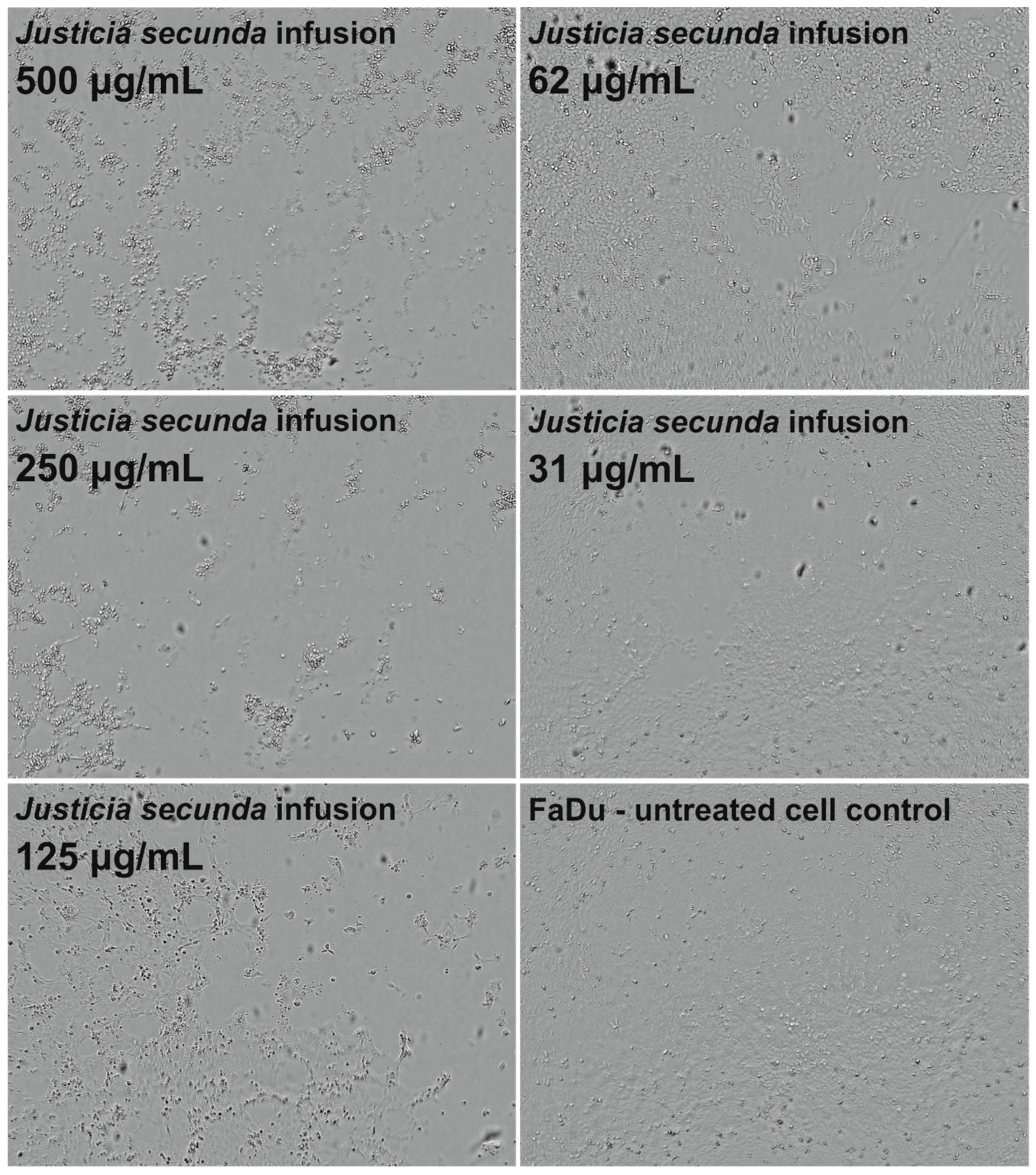
J. secunda aqueous extract and infusion showed the lowest cytotoxicity toward normal VERO cells, and the exact CC50 values could not be evaluated since they were above the tested concentration range (Figure 2). Based on the classification of plant extract’s cytotoxicity, the methanolic and aqueous extracts, as well as the infusion, can be labelled as non-toxic to normal cells [58]. The highest toxicity toward all cell types was observed for the ethyl acetate extract with CC50 between 61.18 and 79.57 μg/mL (Table 4). Despite SI of 1.11 calculated for ethyl acetate extract on FaDu, it was not found to be significant (p > 0.5). J. secunda dichloromethane extract showed substantial anticancer activity (p < 0.01) toward FaDu and Detroit with SI of 2.83 and 1.75, respectively. Notably, also methanolic extract exerted anticancer selectivity, but the observed CC50 values point to low toxicity. The highest anticancer potential and selectivity toward both pharyngeal carcinomas were found for J. secunda aqueous extract and infusion. The influence of infusion in the 500, 250, 125, 62 and 31 μg/mL concentrations on the morphology of FaDu cells monolayer is presented in Figure 3. At 500 and 250 μg/mL concentrations, the cellular monolayer was utterly destroyed after 72 h of incubation, and no FaDu cells could be noticed. The 125 μg/mL concentration also induced damage to the monolayer, but some intact cells were visible. The cellular monolayer was observed in the lower concentrations, 62 and 31 μg/mL, but it was noticeably less dense than in the cell control. Olympus Provi CM20 allowed for constant monitoring of cellular monolayer and observation of the changes induced by different concentrations of tested extracts. It was found that most of the damage to the FaDu monolayer induced by the INF 500 μg/mL occurred during 24 h of incubation. The infusion at 250 μg/mL showed a similar cytotoxic effect after 44 h of incubation.
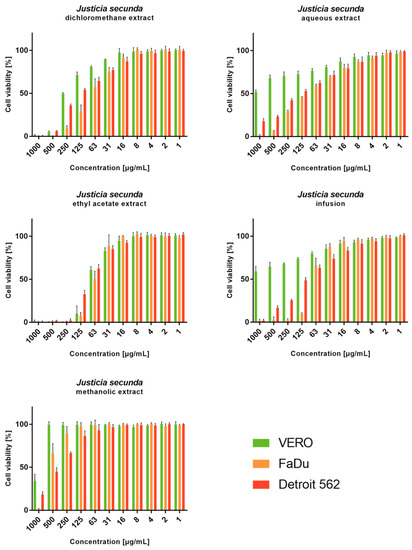
Figure 2.
Viability of cell lines treated with different concentrations of Justicia secunda extracts (VERO—cell line from the kidney of an African green monkey; FaDu—cell line from hypopharyngeal carcinoma; Detroit 562—cell line from pharyngeal carcinoma).

Table 4.
Cytotoxicity of Justicia secunda extracts after 72 h incubation.
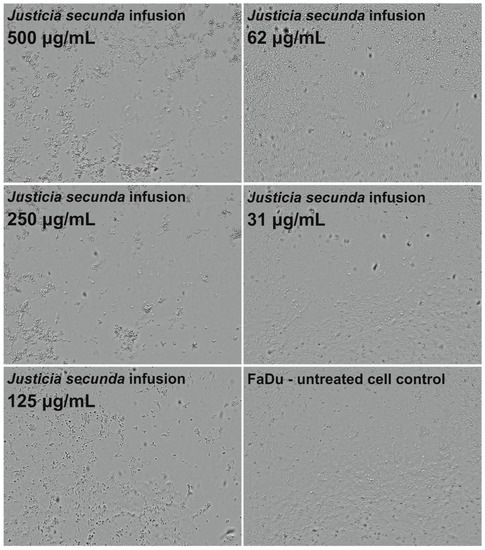
Figure 3.
Influence of Justicia secunda infusion on the morphology of FaDu cells’ monolayer after 72 h incubation.
Onochie et al. [59] reported that J. secunda leaves ethanolic extract caused increased lipid profile values, creatinine and blood urea levels in Wistar rats, which may indicate potential negative cardiac and renal influence. Moreover, the calculated LD50 (50% lethal dose) of the extract was determined to be 3800 mg/kg body weight, which also raises potential safety concerns [59]. However, these effects were only described for ethanolic extract, and it must be noted that, in most cases, traditional medicine uses J. secunda infusions or decoctions, which may exert different safety profiles. Nevertheless, our in vitro experiments also indicate lower toxicity of aqueous extract and infusion to normal cells than the extracts obtained using organic solvents, especially dichloromethane and ethyl acetate. It should be emphasized that this report is the first one depicting the cytotoxicity and anticancer potential of extracts obtained from the aerial parts of J. secunda.
3.6. Antiviral Activity
Incubation of J. secunda methanolic extract (500 μg/mL) with HHV-1 infected VERO cells decreased the intensity of virus-induced cytopathic effect (CPE). However, there were still noticeable signs of CPE present. The rest of the tested extracts did not influence the formation of CPE. The formation of CPE was observed every 2 h during a 72 h incubation period using Olympus Provi CM20. Live-cell imaging allowed for real-time monitoring of morphological changes induced by the HHV-1 infection in the VERO cells’ monolayer. A comparison between the development of CPE in virus-infected cells treated with J. secunda methanolic extract (500 μg/mL) or J. secunda ethyl acetate extract (16 μg/mL), and the CPE in the virus control (VC) is presented in Figure 4. After 36 h of incubation, the CPE was clearly visible in the VC. However, in the cells treated with J. secunda ethyl acetate extract, only early signs of CPE, including cell rounding and several areas of focal degeneration, were visible, and J. secunda methanolic extract protected cells from virus-induced damage. After the 48 h incubation period, the VC showed profound CPE, with ballooning degeneration, vacuolization, and polykaryon (syncytia) formation. Moreover, the HHV-1-infected cells treated with J. secunda ethyl acetate extract showed significant CPE, and occasional cells treated with J. secunda methanolic extract showed early signs of CPE (cell swelling, rounding, and clumping). Continued incubation (Figure 4; 60 h and 66 h) resulted in the complete destruction of the cellular monolayer in VC and infected cells treated with J. secunda ethyl acetate extract. Despite signs of CPE, J. secunda methanolic extract after 66 h exerted a noticeable decrease in virus-induced damage compared to VC. Further incubation, up to 72 h, did not induce significant changes in the observed cells. Acyclovir (60 μg/mL), a reference antiviral drug, inhibited the development of the CPE.

Figure 4.
Real-time microscopic monitoring of the antiviral assay.
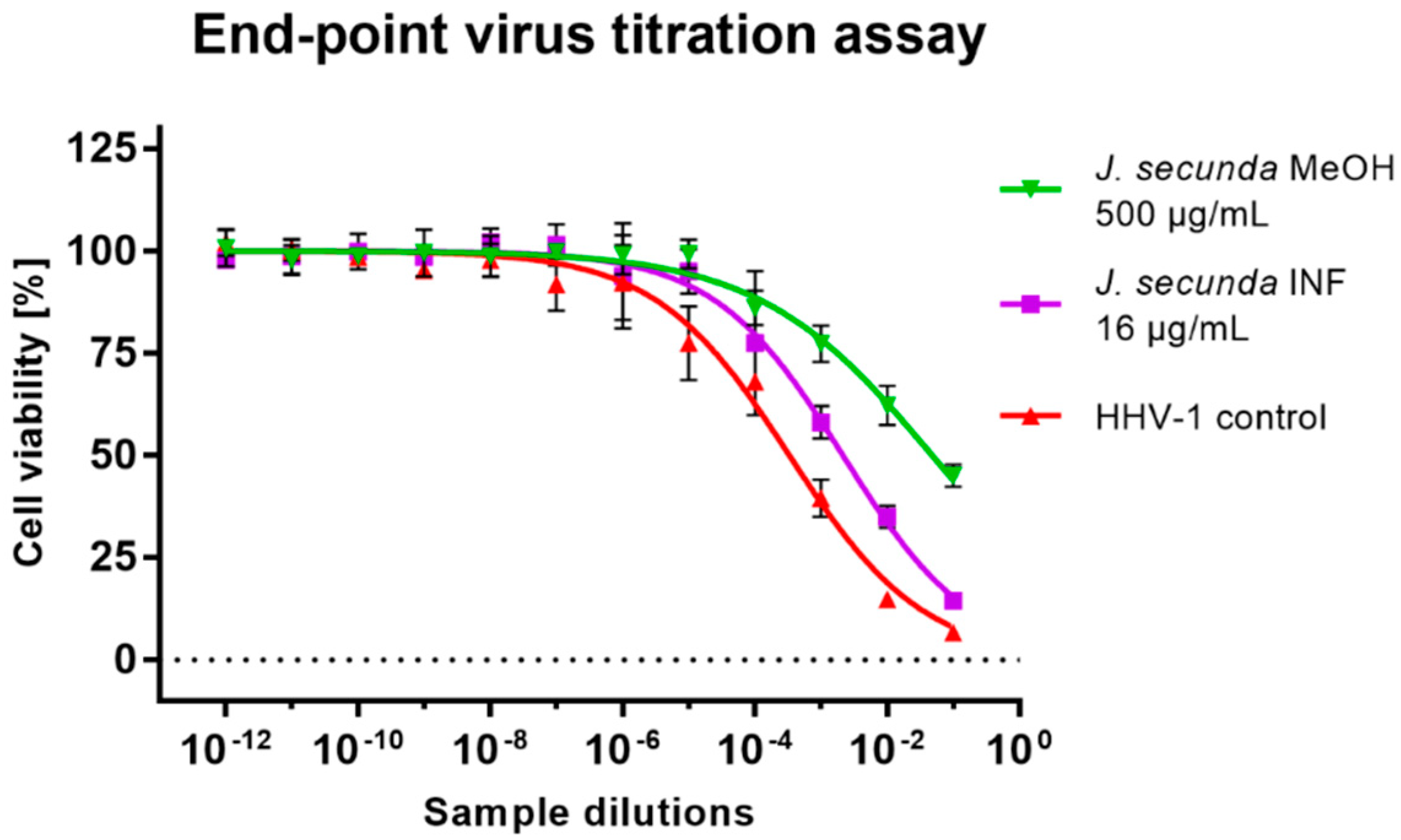
Subsequent virus titration showed that J. secunda methanolic extract (500 μg/mL) managed to decrease the HHV-1 infectious titer by 2.16 ± 0.25 log (Figure 5). Other tested extracts showed inhibition below 1 log. However, significant antiviral activity can only be reported for samples decreasing the infectious titer by at least 3 log. End-point virus titration showed that acyclovir (60 μg/mL) successfully blocked the HHV-1 replication and viral infectivity was not detected. To further evaluate the influence of J. secunda on the replication of HHV-1, the viral load was measured using the real-time PCR technique (Figure 6A). The HHV-1 viral load in the tested samples was assessed in relation to virus control based on the relative quantity (ΔCq) method (Figure 6C). It was found that J. secunda methanolic extract (500 μg/mL) inhibited the replication of HHV-1, decreasing the viral load by 1.21 log compared to the virus control. Virus-infected cells treated with J. secunda dichloromethane extract showed a titer of HHV-1 comparable to the virus control. In contrast, ethyl acetate, aqueous, and infusion extracts reduced the viral load by 0.46, 0.6, and 0.73 log, respectively. The viral DNA was not detected in samples obtained from HHV-1-infected cells treated (60 μg/mL) with acyclovir. The melt curve analysis showed a single melting peak at 83.5–84 °C (Figure 6B), confirming the presence of the same amplicon in all tested samples, except negative control (uninfected cells). Considering the presented data, it can be concluded that J. secunda extracts failed to exert a significant antiviral effect against HHV-1. However, herein the anti-herpesviral activity of extracts from the aerial parts of J. secunda has been evaluated for the first time. It was previously reported that J. procumbens var. leucantha has substantial inhibitory activity against vesicular stomatitis virus (VSV), and lignans and their glycosides (e.g., justicidins A and B, diphyllin, diphyllin apioside and diphyllin apioside-5-acetate) were found to be responsible for the observed effect [60]. Lignans from Justicia spp. also demonstrated antiviral activity against HIV-1 [61,62,63]. Arylnaphthalene lignans from J. procumbens, justatropmers A–H, showed potent anti-HIV-1 activity [63]. The anti-HIV-1 activity was also reported for 70%-fractionated ethanolic extract of J. gendarussa on HIV-infected MOLT-4 cells using syncytia formation assay and HIV p24 antigen assay [64]. Interestingly, anisotine, an alkaloid from J. adhatoda, was shown in silico to inhibit the main protease (Mpro) of the SARS-CoV-2 [65]. We have also found lignan derivatives in the J. secunda extracts, which may be related to the observed inhibition of HHV-1-induced CPE formation and reduction of infectious titer and viral load by the methanolic extract, since lignans were also reported to inhibit herpesviruses [61]. However, the concentration of lignans in J. secunda extracts might have been insufficient for higher inhibition of HHV-1 replication. That is why it could be beneficial to perform a more detailed analysis of J. secunda, including the isolation of bioactive fractions and pure compounds responsible for the observed bioactivity.
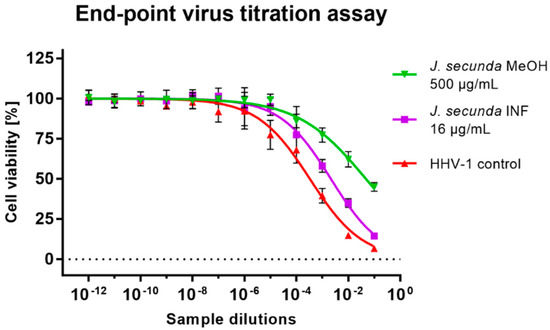
Figure 5.
The end-point titration of HHV-1 infectious titer in the samples treated with Justicia secunda methanolic extract or infusion in relation to the virus control.
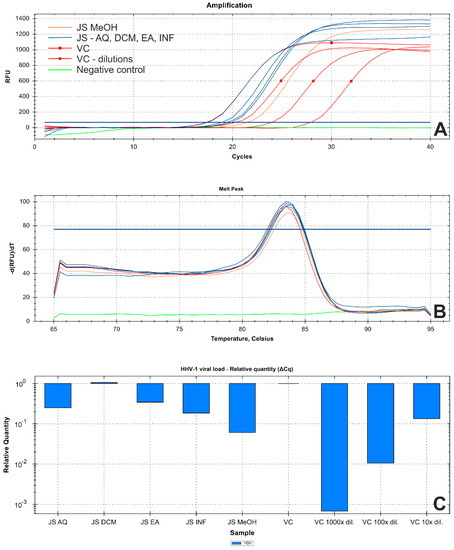
Figure 6.
Results of real−time PCR analysis of the HHV−1 viral load in tested samples ((A)—amplification curve; (B)—DNA melt peak; (C)—relative quantitation using ΔCq method; Abbreviations: JS—DNA isolates from Justicia secunda-treated HHV−1-infected cells; VC—DNA isolate from virus control, and series of tenfold dilutions; Extracts: DCM—dichloromethane; EA—ethyl acetate; MeOH—methanol; AQ—water; INF—infusion).
4. Conclusions
In the current study, we focused on the chemical profile and biological activities of extracts of J. secunda obtained with different solvents. Both chemical profiles and biological abilities were influenced by the extraction solvent. In general, the best antioxidant activity was observed for the methanolic and aqueous extracts. However, different results were obtained considering each enzyme inhibitory assay. LC-MS analysis revealed the presence of biologically active compounds, including apigenin glucosides (infusion and methanolic extract), rutin (methanolic extract), luteolin and its glucosides (infusion, ethyl acetate and methanolic extracts), and alkaloids, namely secundarellones B/C in the dichloromethane extracts and isomers of secundarellone A in all tested extracts. Lignan derivatives were found in the infusion and methanolic extract. The highest toxicity toward all cell types was observed for the ethyl acetate extract. Whereas the highest anticancer potential and selectivity toward both pharyngeal carcinomas, FaDu and Detroit 562, were found for aqueous extract and infusion. Our results show that J. secunda has significant biological properties, making it a promising candidate as a coadjutant agent for developing therapeutics, dietary supplements, and cosmetics. However, further studies are strongly suggested to isolate active compounds responsible for biological activities
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12020509/s1. Table S1. Chemical characterization of the tested extracts. The detailed description of Materials and Methods.
Author Contributions
Conceptualization, Ł.Ś., E.S. and G.Z.; methodology, Ł.Ś., E.S., K.I.S., G.Z., A.B. and M.P.-D.; software, Ł.Ś., E.S. and G.Z.; validation, G.Z., K.B., B.H. and S.D.; formal analysis, B.H. and G.Z.; investigation, Ł.Ś., E.S., B.H., G.Z., A.B. and M.P.-D.; resources, K.I.S. and K.B.; data curation, G.Z. and S.D.; writing—original draft preparation, Ł.Ś., B.H. and G.Z.; writing—review and editing, E.S., Ł.Ś. and S.D.; visualization, Ł.Ś. and E.S.; supervision, G.Z. and S.D.; project administration, G.Z.; funding acquisition, Ł.Ś. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
Ministry of Education and Science in Poland within the statutory activity of Medical University of Lublin (DS 28/2022).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Evident Corporation (Hamburg, Germany) for providing the Olympus Provi CM20 Incubation Monitoring System used during in vitro cytotoxicity and antiviral experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.-F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health; American College of Cardiology Foundation: Washington, DC, USA, 2022; Volume 80, pp. 2361–2371. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Carrington, S.; Cohall, D.; Gossell-Williams, M.; Lindo, J. The antimicrobial screening of a Barbadian medicinal plant with indications for use in the treatment of diabetic wound infections. West Indian Med. J. 2012, 61, 861. [Google Scholar] [CrossRef] [PubMed]
- Lans, C.; Harper, T.; Georges, K.; Bridgewater, E. Medicinal and ethnoveterinary remedies of hunters in Trinidad. BMC Complement. Altern. Med. 2001, 1, 10. [Google Scholar] [CrossRef]
- Kitadi, J.M.; Mazasa, P.P.; Sha-Tshibey Tshibangu, D.; Kasali, F.M.; Tshilanda, D.D.; Ngbolua, K.-T.-N.; Mpiana, P.T. Ethnopharmacological survey and antisickling activity of plants used in the management of sickle cell disease in Kikwit city, DR Congo. Evid.-Based Complement. Altern. Med. 2020, 2020, 1346493. [Google Scholar] [CrossRef]
- Mpiana, P.T.; Ngbolua, K.N.N.; Bokota, M.T.; Kasonga, T.K.; Atibu, E.K.; Tshibangu, D.S.; Mudogo, V. In vitro effects of anthocyanin extracts from Justicia secunda Vahl on the solubility of haemoglobin S and membrane stability of sickle erythrocytes. Blood Transfus. 2010, 8, 248. [Google Scholar] [PubMed]
- Theiler, B.A.; Istvanits, S.; Zehl, M.; Marcourt, L.; Urban, E.; Caisa, L.O.E.; Glasl, S. HPTLC bioautography guided isolation of α-glucosidase inhibiting compounds from Justicia secunda Vahl (Acanthaceae). Phytochem. Anal. 2017, 28, 87–92. [Google Scholar] [CrossRef]
- Rojas, J.J.; Ochoa, V.J.; Ocampo, S.A.; Muñoz, J.F. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complement. Altern. Med. 2006, 6, 1–6. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and enzyme inhibitory potential of two Potentilla species (P. speciosa L. and P. reptans Willd.) and their chemical composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Świątek, Ł.; Sieniawska, E.; Sinan, K.I.; Maciejewska-Turska, M.; Boguszewska, A.; Polz-Dacewicz, M.; Senkardes, I.; Guler, G.O.; Bibi Sadeer, N.; Mahomoodally, M.F. LC-ESI-QTOF-MS/MS Analysis, Cytotoxic, Antiviral, Antioxidant, and Enzyme Inhibitory Properties of Four Extracts of Geranium pyrenaicum Burm. f.: A Good Gift from the Natural Treasure. Int. J. Mol. Sci. 2021, 22, 7621. [Google Scholar] [CrossRef] [PubMed]
- Koffi, E.N.; Le Guernevé, C.; Lozano, P.R.; Meudec, E.; Adjé, F.A.; Bekro, Y.-A.; Lozano, Y.F. Polyphenol extraction and characterization of Justicia secunda Vahl leaves for traditional medicinal uses. Ind. Crops Prod. 2013, 49, 682–689. [Google Scholar] [CrossRef]
- Koffi, E.; Kassi, A.B.B.; Adje, F.A.; Lozano, Y.F.; Bekro, Y.-A. Effect of freeze-drying and spray-drying on total phenolics content and antioxidant activity from aqueous extract of Justicia secunda leaves. Trends Phytochem. Res. 2020, 4, 69–76. [Google Scholar]
- John, B.; Reddy, V.; Sulaiman, C. Total phenolics and flavonoids in selected Justicia species. J. Pharmacogn. Phytochem. 2013, 2, 72–73. [Google Scholar]
- Sepúlveda-Jiménez, G.; Reyna-Aquino, C.; Chaires-Martínez, L.; Bermúdez-Torres, K.; Rodríguez-Monroy, M. Antioxidant activity and content of phenolic compounds and flavonoids from Justicia spicigera. J. Biol. Sci. 2009, 9, 629–632. [Google Scholar] [CrossRef]
- Naqvi, S.A.R.; Waseem, R.; Mahmood, N.; Hussain, Z.; Khan, Z.A.; Shahzad, S.A.; Yar, M.; Amin, R.; Bukhari, S.A.; Zahoor, A.F. Phenolic acid content, antioxidant properties, and antibacterial potential of flowers and fruits from selected Pakistani indigenous medicinal plants. Sci. Asia 2013, 39, 340–345. [Google Scholar] [CrossRef]
- Corrêa, G.M.; Alcântara, A.F.d.C. Chemical constituents and biological activities of species of Justicia: A review. Rev. Bras. Farmacogn. 2012, 22, 220–238. [Google Scholar] [CrossRef]
- e Silva, J.P.; Pereira, L.C.; Abreu, L.S.; Lins, F.S.; de Souza, T.A.; do Espírito-Santo, R.F.; Barros, R.P.; Villarreal, C.F.; de Melo, J.I.; Scotti, M.T. Targeted Isolation of Anti-inflammatory Lignans from Justicia aequilabris by Molecular Networking Approach. J. Nat. Prod. 2022, 85, 2184–2191. [Google Scholar] [CrossRef]
- Calderón, A.I.; Hodel, A.; Wolfender, J.-L.; Gupta, M.P.; Correa, M.; Hostettmann, K. LC–DAD–MS-based metabolite profiling of three species of Justicia (Acanthaceae). Nat. Prod. Res. 2013, 27, 1335–1342. [Google Scholar] [CrossRef]
- Theiler, B.A.; Revoltella, S.; Zehl, M.; Dangl, C.; Caisa, L.O.E.; König, J.; Winkler, J.; Urban, E.; Glasl, S. Secundarellone A, B, and C from the leaves of Justicia secunda Vahl. Phytochem. Lett. 2014, 10, cxxix–cxxxii. [Google Scholar] [CrossRef]
- Gonçalves-Filho, D.; De Souza, D. Detection of Synthetic Antioxidants: What Factors Affect the Efficiency in the Chromatographic Analysis and in the Electrochemical Analysis? Molecules 2022, 27, 7137. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, H.; Mazhar, M.S.; Quddus, S.; Suleria, H.A.R. LC-ESI-QTOF-MS/MS profiling of phenolic compounds in Australian native plums and their potential antioxidant activities. Food Biosci. 2023, 52, 102331. [Google Scholar] [CrossRef]
- Lin, Y.; Tang, D.; Liu, X.; Cheng, J.; Wang, X.; Guo, D.; Zou, J.; Yang, H. Phenolic profile and antioxidant activity of longan pulp of different cultivars from South China. LWT 2022, 165, 113698. [Google Scholar] [CrossRef]
- Zhong, J.; Wang, Y.; Li, C.; Yu, Q.; Xie, J.; Dong, R.; Xie, Y.; Li, B.; Tian, J.; Chen, Y. Natural variation on free, esterified, glycosylated and insoluble-bound phenolics of Rubus chingii Hu: Correlation between phenolic constituents and antioxidant activities. Food Res. Int. 2022, 162, 112043. [Google Scholar] [CrossRef]
- Yang, J.; Guo, J.; Yuan, J. In vitro antioxidant properties of rutin. LWT-Food Sci. Technol. 2008, 41, 1060–1066. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Spagnol, C.M.; Assis, R.P.; Brunetti, I.L.; Isaac, V.L.B.; Salgado, H.R.N.; Corrêa, M.A. In vitro methods to determine the antioxidant activity of caffeic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 358–366. [Google Scholar] [CrossRef]
- Kashyap, P.; Shikha, D.; Thakur, M.; Aneja, A. Functionality of apigenin as a potent antioxidant with emphasis on bioavailability, metabolism, action mechanism and in vitro and in vivo studies: A review. J. Food Biochem. 2022, 46, e13950. [Google Scholar] [CrossRef]
- Abramovič, H. Antioxidant properties of hydroxycinnamic acid derivatives: A focus on biochemistry, physicochemical parameters, reactive species, and biomolecular interactions. In Coffee in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2015; pp. 843–852. [Google Scholar]
- Anyasor, G.N.; Moses, N.; Kale, O. Hepatoprotective and hematological effects of Justicia secunda Vahl leaves on carbon tetrachloride induced toxicity in rats. Biotech. Histochem. 2020, 95, 349–359. [Google Scholar] [CrossRef]
- Onoja, S.O.; Ezeja, M.I.; Omeh, Y.N.; Onwukwe, B.C. Antioxidant, anti-inflammatory and antinociceptive activities of methanolic extract of Justicia secunda Vahl leaf. Alex. J. Med. 2017, 53, 207–213. [Google Scholar] [CrossRef]
- Locatelli, M.; Yerlikaya, S.; Baloglu, M.C.; Zengin, G.; Altunoglu, Y.C.; Cacciagrano, F.; Campestre, C.; Mahomoodally, M.F.; Mollica, A. Investigations into the therapeutic potential of Asphodeline liburnica roots: In vitro and in silico biochemical and toxicological perspectives. Food Chem. Toxicol. 2018, 120, 172–182. [Google Scholar] [CrossRef] [PubMed]
- YALÇIN, H.; KAVUNCUOGLU, H.; CAPAR, T.D. Total Phenolic Contents, Antioxidant Activities and Antioxidant Capacities of Some Selected Pepper (Capsicum annuum L.) Pulps and Seeds. Avrupa Bilim Teknol. Derg. 2021, 21, 581–586. [Google Scholar]
- Holdgate, G.A.; Meek, T.D.; Grimley, R.L. Mechanistic enzymology in drug discovery: A fresh perspective. Nat. Rev. Drug Discov. 2018, 17, 115–132. [Google Scholar] [CrossRef]
- Seetaloo, A.; Aumeeruddy, M.; Kannan, R.R.; Mahomoodally, M. Potential of traditionally consumed medicinal herbs, spices, and food plants to inhibit key digestive enzymes geared towards diabetes mellitus management—A systematic review. S. Afr. J. Bot. 2019, 120, 3–24. [Google Scholar] [CrossRef]
- Mishra, P.; Kumar, A.; Panda, G. Anti-cholinesterase hybrids as multi-target-directed ligands against Alzheimer’s disease (1998–2018). Bioorganic Med. Chem. 2019, 27, 895–930. [Google Scholar]
- Bashary, R.; Vyas, M.; Nayak, S.K.; Suttee, A.; Verma, S.; Narang, R.; Khatik, G.L. An insight of alpha-amylase inhibitors as a valuable tool in the management of type 2 diabetes mellitus. Curr. Diabetes Rev. 2020, 16, 117–136. [Google Scholar]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics. Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative Properties and Effect of Quercetin and Its Glycosylated Form (Rutin) on Acetylcholinesterase and Butyrylcholinesterase Activities. J. Evid.-Based Complement. Altern. Med. 2016, 21, Np11–Np17. [Google Scholar] [CrossRef]
- Li, N.; Yang, J.; Wang, C.; Wu, L.; Liu, Y. Screening bifunctional flavonoids of anti-cholinesterase and anti-glucosidase by in vitro and in silico studies: Quercetin, kaempferol and myricetin. Food Biosci. 2023, 51, 102312. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Szwajgier, D. Anticholinesterase activity of selected phenolic acids and flavonoids-interaction testing in model solutions. Ann. Agric. Environ. Med. 2015, 22, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Rawa, M.S.A.; Hassan, Z.; Murugaiyah, V.; Nogawa, T.; Wahab, H.A. Anti-cholinesterase potential of diverse botanical families from Malaysia: Evaluation of crude extracts and fractions from liquid-liquid extraction and acid-base fractionation. J. Ethnopharmacol. 2019, 245, 112160. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gupta, R. A survey of plants for presence of cholinesterase activity. Phytochemistry 1997, 46, 827–831. [Google Scholar] [CrossRef]
- Yuan, E.; Liu, B.; Wei, Q.; Yang, J.; Chen, L.; Li, Q. Structure activity relationships of flavonoids as potent α-amylase inhibitors. Nat. Prod. Commun. 2014, 9, 1934578X1400900829. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Ayeni, P.O.; Omojokun, O.S.; Bello, F. Comparative effect of quercetin and rutin on α-amylase, α-glucosidase, and some pro-oxidant-induced lipid peroxidation in rat pancreas. Comp. Clin. Pathol. 2015, 24, 1103–1110. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Anigboro, A.A.; Avwioroko, O.J.; Ohwokevwo, O.A.; Pessu, B.; Tonukari, N.J. Phytochemical profile, antioxidant, α-amylase inhibition, binding interaction and docking studies of Justicia carnea bioactive compounds with α-amylase. Biophys. Chem. 2021, 269, 106529. [Google Scholar] [CrossRef]
- Ani, O.N.; Udedi, S.C.; Asogwa, K.K.; Enemali, M.O.; Onwelumadu, C.M.; Ikedife, K.S. Inhibitory Potential and Antidiabetic Activity of Leaf Extracts of Justicia carnea. Int. J. Biochem. Res. Rev. 2020, 29, 34–45. [Google Scholar] [CrossRef]
- Khadayat, K.; Marasini, B.P.; Gautam, H.; Ghaju, S.; Parajuli, N. Evaluation of the alpha-amylase inhibitory activity of Nepalese medicinal plants used in the treatment of diabetes mellitus. Clin. Phytosci. 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Jakimiuk, K.; Sari, S.; Milewski, R.; Supuran, C.T.; Şöhretoğlu, D.; Tomczyk, M. Flavonoids as tyrosinase inhibitors in in silico and in vitro models: Basic framework of SAR using a statistical modelling approach. J. Enzym. Inhib. Med. Chem. 2022, 37, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Angelis, A.; Antosiewicz, B.; Sakipova, Z.; Kozhanova, K.; Głowniak, K.; Kukula-Koch, W. Identification of mushroom and murine tyrosinase inhibitors from Achillea biebersteinii Afan. extract. Molecules 2021, 26, 964. [Google Scholar] [CrossRef] [PubMed]
- Basit, A.; Ahmad, S.; Sherif, A.E.; Aati, H.Y.; Ovatlarnporn, C.; Khan, M.A.; Rao, H.; Ahmad, I.; Shahzad, M.N.; Ghalloo, B.A. New mechanistic insights on Justicia vahlii Roth: UPLC-Q-TOF-MS and GC–MS based metabolomics, in-vivo, in-silico toxicological, antioxidant based anti-inflammatory and enzyme inhibition evaluation. Arab. J. Chem. 2022, 15, 104135. [Google Scholar] [CrossRef]
- Ito, J.; Hara, K.; Someya, T.; Myoda, T.; Sagane, Y.; Watanabe, T.; Wijesekara, R.; Toeda, K.; Nojima, S. Data on the inhibitory effect of traditional plants from Sri Lanka against tyrosinase and collagenase. Data Brief 2018, 20, 573–576. [Google Scholar] [CrossRef]
- Łaska, G.; Sieniawska, E.; Świątek, Ł.; Zjawiony, J.; Khan, S.; Boguszewska, A.; Stocki, M.; Angielczyk, M.; Polz-Dacewicz, M. Phytochemistry and biological activities of Polemonium caeruleum L. Phytochem. Lett. 2019, 30, 314–323. [Google Scholar] [CrossRef]
- Onochie, A.U.; Oli, A.H.; Oli, A.N.; Ezeigwe, O.C.; Nwaka, A.C.; Okani, C.O.; Okam, P.C.; Ihekwereme, C.P.; Okoyeh, J.N. The pharmacobiochemical effects of ethanol extract of Justicia secunda vahl leaves in rattus norvegicus. J. Exp. Pharmacol. 2020, 12, 423. [Google Scholar] [CrossRef]
- Asano, J.; Chiba, K.; Tada, M.; Yoshii, T. Antiviral activity of lignans and their glycosides from Justicia procumbens. Phytochemistry 1996, 42, 713–717. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Wang, D.-Y.; Li, Y.-P.; Deyrup, S.T.; Zhang, H.-J. Plant-derived lignans as potential antiviral agents: A systematic review. Phytochem. Rev. 2022, 21, 239–289. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Rumschlag-Booms, E.; Guan, Y.-F.; Liu, K.-L.; Wang, D.-Y.; Li, W.-F.; Cuong, N.M.; Soejarto, D.D.; Fong, H.H.; Rong, L. Anti-HIV diphyllin glycosides from Justicia gendarussa. Phytochemistry 2017, 136, 94–100. [Google Scholar] [CrossRef]
- Zhao, Y.; Yi Tsang, N.; Xu, X.Y.; Zhao, C.L.; Ku, C.F.; Li, W.F.; Zhu, Y.; Liu, K.L.; Rong, L.; Zhang, H.J. Axial Chirality and Antiviral Activity Evaluation of Arylnaphthalene Lignan Glycosides from Justicia procumbens. Asian J. Org. Chem. 2022, 11, e202200267. [Google Scholar] [CrossRef]
- Hikmawanti, N.P.E.; Widiyanti, P.; Prajogo EW, B. In vitro anti-HIV activity of ethanol extract from gandarusa (Justicia gendarussa Burm. f) leaves. Infect. Dis. Rep. 2020, 12, 8730. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Chakraborty, A.; Biswas, A.; Chowdhuri, S. Identification of alkaloids from Justicia adhatoda as potent SARS-CoV-2 main protease inhibitors: An in silico perspective. J. Mol. Struct. 2021, 1229, 129489. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).