Exploiting Kinetic Features of ORAC Assay for Evaluation of Radical Scavenging Capacity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. ORAC-FL Procedure under Microplate Format
2.3. Calculation of ORAC-FL Parameters to Assess Antioxidant Capacity
3. Results
3.1. Lag Time Parameter
3.2. Probe Fluorescence Decay Rate
3.3. Effect of Ethanol in Parameter Assessment
3.4. Antioxidant Capacity Reported as Trolox Equivalents (TE)
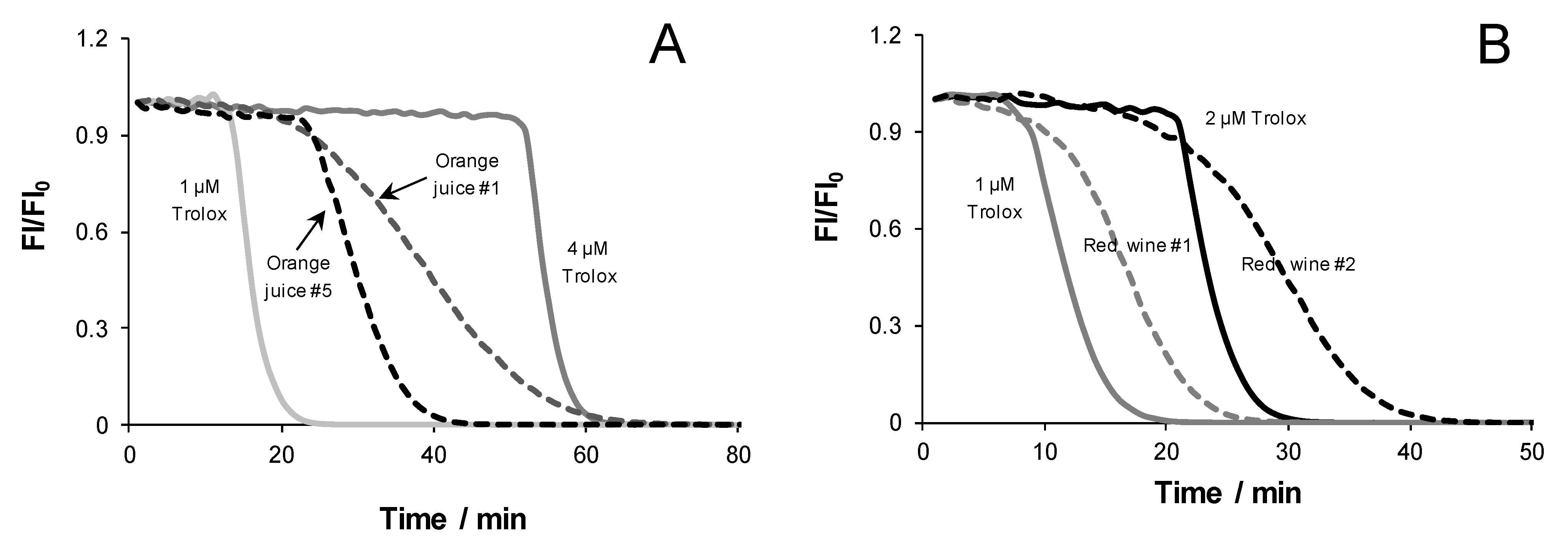
3.5. Analysis of Antioxidant-Containing Beverages
3.6. Analysis of AGREE Metric
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apak, R. Current Issues in Antioxidant Measurement. J. Agric. Food Chem. 2019, 67, 9187–9202. [Google Scholar] [CrossRef]
- Andrewes, P. Modifications can make antioxidant assays more informative but not necessarily more correct: An illustration in heat-treated milk. Int. Dairy J. 2022, 133, 105421. [Google Scholar] [CrossRef]
- Mendonca, J.D.; Guimaraes, R.D.A.; Zorgetto-Pinheiro, V.A.; Fernandes, C.D.; Marcelino, G.; Bogo, D.; Freitas, K.D.; Hiane, P.A.; Melo, E.S.D.; Vilela, M.L.B.; et al. Natural Antioxidant Evaluation: A Review of Detection Methods. Molecules 2022, 27, 3563. [Google Scholar] [CrossRef]
- Brainina, K.; Stozhko, N.; Vidrevich, M. Antioxidants: Terminology, Methods, and Future Considerations. Antioxidants 2019, 8, 297. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays (Reprinted). J. Funct. Foods 2015, 18, 782–796. [Google Scholar] [CrossRef]
- Cao, G.H.; Alessio, H.M.; Cutler, R.G. Oxigen-Radical Absorbency Capacity Assay for Antioxidants. Free. Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Apak, R.; Ozyurek, M.; Guclu, K.; Capanoglu, E. Antioxidant activity/capacity measurement. 3. Reactive oxygen and nitrogen species (ROS/RNS) scavenging assays, oxidative stress biomarkers, and chromatographic/chemometric assays. J. Agric. Food Chem. 2016, 64, 1046–1070. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Lopez-Alarcon, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Apak, R.; Calokerinos, A.; Gorinstein, S.; Segundo, M.A.; Hibbert, D.B.; Gulcin, I.; Cekic, S.D.; Guclu, K.; Ozyurek, M.; Celik, S.E.; et al. Methods to evaluate the scavenging activity of antioxidants toward reactive oxygen and nitrogen species (IUPAC Technical Report). Pure Appl. Chem. 2022, 94, 87–144. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [PubMed]
- Takashima, M.; Horie, M.; Shichiri, M.; Hagihara, Y.; Yoshida, Y.; Niki, E. Assessment of antioxidant capacity for scavenging free radicals in vitro: A rational basis and practical application. Free Radical Biol. Med. 2012, 52, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Takashima, M.; Shichiri, M.; Hagihara, Y.; Yoshida, Y.; Niki, E. Capacity of peroxyl radical scavenging and inhibition of lipid peroxidation by β-carotene, lycopene, and commercial tomato juice. Food Funct. 2012, 3, 1153–1160. [Google Scholar] [CrossRef]
- Guclu, K.; Kibrislioglu, G.; Ozyurek, M.; Apak, R. Development of a Fluorescent Probe for Measurement of Peroxyl Radical Scavenging Activity in Biological Samples. J. Agric. Food Chem. 2014, 62, 1839–1845. [Google Scholar] [CrossRef]
- Morita, M.; Naito, Y.; Yoshikawa, T.; Niki, E. Antioxidant capacity of blueberry extracts: Peroxyl radical scavenging and inhibition of plasma lipid oxidation induced by multiple oxidants. J. Berry Res. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Morita, M.; Naito, Y.; Yoshikawa, T.; Niki, E. Assessment of radical scavenging capacity of antioxidants contained in foods and beverages in plasma solution. Food Funct. 2015, 6, 1591–1599. [Google Scholar] [CrossRef]
- Jiménez-Morales, W.A.; del Pilar Cañizares-Macias, M.; Pedraza-Chaverri, J. Fast ORAC-SIA method for antioxidant capacity determination in food samples. Food Chem. 2022, 384, 132524. [Google Scholar] [CrossRef]
- Ou, B.; Chang, T.; Huang, D.; Prior, R.L. Determination of total antioxidant capacity by oxygen radical absorbance capacity (ORAC) using fluorescein as the fluorescence probe: First action 2012.23. J. AOAC Int. 2013, 96, 1372–1376. [Google Scholar] [CrossRef]
- Marques, S.S.; Magalhães, L.M.; Mota, A.I.; Soares, T.R.; Korsak, B.; Reis, S.; Segundo, M.A. Myoglobin microplate assay to evaluate prevention of protein peroxidation. J. Pharm. Biomed. Anal. 2015, 114, 305–311. [Google Scholar] [CrossRef]
- Babazadeh, A.; Taghvimi, A.; Hamishehkar, H.; Tabibiazar, M. Development of new ultrasonic-solvent assisted method for determination of trans-resveratrol from red grapes: Optimization, characterization, and antioxidant activity (ORAC assay). Food Biosci. 2017, 20, 36–42. [Google Scholar] [CrossRef]
- Magalhães, L.; Ramos, I.; Reis, S.; Segundo, M. Antioxidant profile of commercial oenological tannins determined by multiple chemical assays. Aust. J. Grape Wine Res. 2014, 20, 72–79. [Google Scholar] [CrossRef]
- Omata, Y.; Ogawa, Y.; Saito, Y.; Yoshida, Y.; Niki, E. Assessment of the antioxidant capacity of a fermented grain food product, Antioxidant Biofactor (AOB), by using pyranine and pyrogallol red as a combined probe. Food Chem. 2009, 114, 429–433. [Google Scholar] [CrossRef]
- Ogawa, Y.; Omata, Y.; Nishio, K.; Saito, Y.; Yoshida, Y.; Niki, E. Assessment of antioxidative activity of extract from fermented grain food mixture using chemical and cellular systems. Biofactors 2007, 31, 237–248. [Google Scholar] [CrossRef]
- Gregorio, B.J.R.; Ramos, I.I.; Magalhaes, L.M.; Silva, E.M.P.; Reis, S.; Segundo, M.A. Microplate ORAC-pyranine spectrophotometric assay for high-throughput assessment of antioxidant capacity. Microchem. J. 2020, 158, 105156. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Conesa, I.; Matencio, A.; Rodríguez-Bonilla, P.; García-Carmona, F.; López-Nicolás, J.M. The use of cyclodextrins as solubility enhancers in the ORAC method may cause interference in the measurement of antioxidant activity. Talanta 2022, 243, 123336. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC− fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.J.; Ou, B.X.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate flourescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Y.; Chu, L.; Wei, Y.; Wang, D.; Cai, S.; Zhou, F.; Ji, B. Relationship between the structures of flavonoids and oxygen radical absorbance capacity values: A quantum chemical analysis. J. Phys. Chem. A 2013, 117, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- López-Alarcón, C.; Aspée, A.; Lissi, E. Influence of the target molecule on the ORAC index. In Emerging Trends in Dietary Components for Preventing and Combating Disease; Bhimanagoud, S., Patil, G.K., Kotamballi, J.N., Murthy, C., Seeram, N.P., Eds.; ACS Publications: Washington, DC, USA, 2012; pp. 417–429. [Google Scholar]
- Martin, I.; Aspee, A.; Torres, P.; Lissi, E.; Lopez-Alarcon, C. Influence of the target molecule on the oxygen radical absorbance capacity index: A comparison between alizarin red-and fluorescein-based methodologies. J. Med. Food 2009, 12, 1386–1392. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Ramos, I.I.; Barreiros, L.; Reis, S.; Segundo, M.A. Kinetic matching approach for rapid assessment of endpoint antioxidant capacity. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications; Resat Apak, E.C., Shahidi, F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 321–331. [Google Scholar]
- Magalhães, L.M.; Barreiros, L.; Maia, M.A.; Reis, S.; Segundo, M.A. Rapid assessment of endpoint antioxidant capacity of red wines through microchemical methods using a kinetic matching approach. Talanta 2012, 97, 473–483. [Google Scholar] [CrossRef]
- Osakwe, O.N.; Siegel, A. A novel standardized oxygen radical absorbance assay for evaluating antioxidant natural products. J. AOAC Int. 2013, 96, 1365–1371. [Google Scholar] [CrossRef]
- Nenadis, N.; Lazaridou, O.; Tsimidou, M.Z. Use of reference compounds in antioxidant activity assessment. J. Agric. Food Chem. 2007, 55, 5452–5460. [Google Scholar] [CrossRef]
- Liao, W.; Gu, L.; Zheng, Y.; Zhu, Z.; Zhao, M.; Liang, M.; Ren, J. Correction: Analysis of the quantitative structure–activity relationship of glutathione-derived peptides based on different free radical scavenging systems. MedChemComm 2016, 7, 2193. [Google Scholar] [CrossRef]
- Perez, D.D.; Leighton, F.; Aspee, A.; Aliaga, C.; Lissi, E. A comparison of methods employed to evaluate antioxidant capabilities. Biol. Res. 2000, 33, 71–77. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Effect of solvent and certain food constituents on different antioxidant capacity assays. Food Res. Int. 2006, 39, 791–800. [Google Scholar] [CrossRef]
- Pedrielli, P.; Pedulli, G.F.; Skibsted, L.H. Antioxidant mechanism of flavonoids. Solvent effect on rate constant for chain-breaking reaction of quercetin and epicatechin in autoxidation of methyl linoleate. J. Agric. Food Chem. 2001, 49, 3034–3040. [Google Scholar] [CrossRef] [PubMed]
- Çelik, S.E.; Özyürek, M.; Güçlü, K.; Apak, R. Solvent effects on the antioxidant capacity of lipophilic and hydrophilic antioxidants measured by CUPRAC, ABTS/persulphate and FRAP methods. Talanta 2010, 81, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Villaño, D.; Fernández-Pachón, M.S.; Troncoso, A.M.; García-Parrilla, M.C. Comparison of antioxidant activity of wine phenolic compounds and metabolites in vitro. Anal. Chim. Acta 2005, 538, 391–398. [Google Scholar] [CrossRef]
- Klonis, N.; Sawyer, W.H. Spectral properties of the prototropic forms of fluorescein in aqueous solution. J. Fluoresc. 1996, 6, 147–157. [Google Scholar] [CrossRef]
- Kevers, C.; Sipel, A.; Pincemail, J.; Dommes, J. Antioxidant capacity of hydrophilic food matrices: Optimization and validation of ORAC assay. Food Anal. Methods 2014, 7, 409–416. [Google Scholar] [CrossRef]
- Bartosz, G. Non-enzymatic antioxidant capacity assays: Limitations of use in biomedicine. Free Radic. Res 2010, 44, 711–720. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Haytowitz, D.B. USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods, Release 2. Available online: http://www.drmarcofranzreb.com/wp-content/uploads/2013/04/ORAC-de-alimentos-2 (accessed on 27 December 2022).

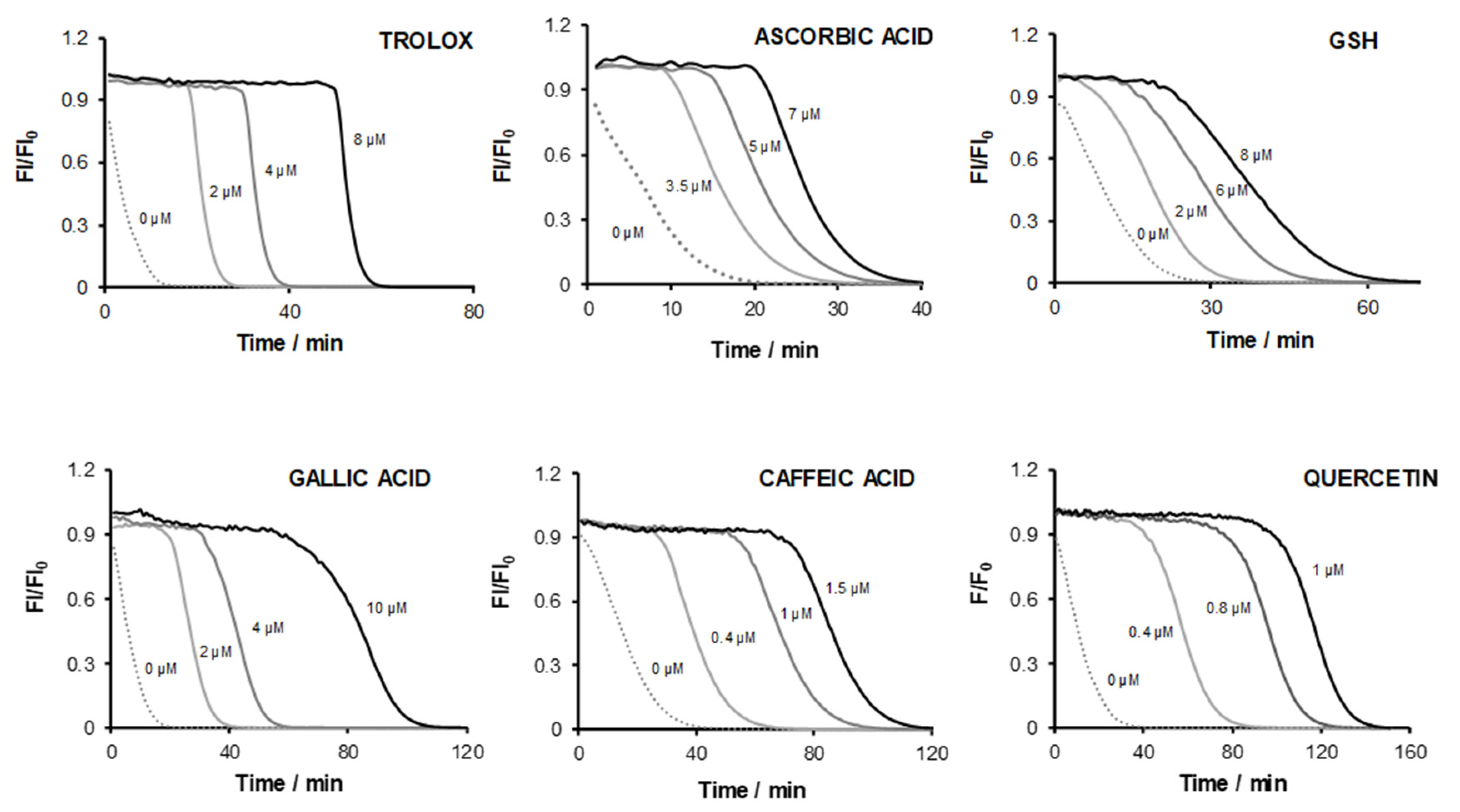
| AO Compounds | Linear Range (μM) | SlopeLT (min μM−1) | SlopeAUC (min μM−1) |
|---|---|---|---|
| Trolox | 2.0–10 | 5.51 ± 0.18 | 5.25 ± 0.18 |
| Ascorbic acid | 3.5–9.0 | 2.89 ± 0.06 | 2.78 ± 0.13 |
| GSH | 2.0–8.0 | 1.90 ± 0.11 | 2.56 ± 0.12 |
| Gallic acid | 2.0–10 | 6.16 ± 0.16 | 6.54 ± 0.21 |
| Quercetin | 0.40–2.0 | 88.30 ± 2.77 | 99.22 ± 2.48 |
| Caffeic acid | 0.40–1.0 | 33.63 ± 1.29 | 49.34 ± 1.81 |
| Phosphate Buffer | Phosphate Buffer:Ethanol (95:5, v/v) | |||||
|---|---|---|---|---|---|---|
| [Trolox] (μM) | AUC (min) | Lag Time (min) | FL Decay Rate (min−1) | AUC (min) | Lag Time (min) | FL Decay Rate (min−1) |
| 0 | 5.9 ± 3.1 | N/A | 0.066 ± 0.016 | 12.2 ± 2.9 | N/A | 0.040 ± 0.004 |
| 2.0 | 24.7 ± 3.0 | 20.5 ± 2.8 | 0.115 ± 0.036 | 40.3 ± 2.1 | 30.5 ± 3.1 | 0.053 ± 0.011 |
| 4.0 | 37.2 ± 3.3 | 33.5 ± 3.7 | 0.123 ± 0.040 | 55.2 ± 2.9 | 46.7 ± 3.2 | 0.052 ± 0.012 |
| 6.0 | 47.8 ± 4.0 | 44.5 ± 5.0 | 0.121 ± 0.043 | 68.2 ± 3.8 | 58.9 ± 3.7 | 0.051 ± 0.014 |
| 8.0 | 57.7 ± 4.6 | 55.3 ± 6.5 | 0.114 ± 0.035 | 80.9 ± 4.7 | 69.7 ± 4.7 | 0.050 ± 0.016 |
| 10 | 66.9 ± 6.1 | 64.7 ± 7.8 | 0.121 ± 0.045 | 87.2 ± 5.5 | 77.2 ± 5.1 | 0.048 ± 0.017 |
| AO Compounds | TEAUC | TELT | TE Literature |
|---|---|---|---|
| Ascorbic acid | 0.53 ± 0.03 | 0.52 ± 0.02 | 0.30–0.95 a–c |
| GSH | 0.49 ± 0.03 | 0.34 ± 0.02 | 0.62–0.65 a,d |
| Gallic acid | 1.25 ± 0.06 | 1.12 ± 0.05 | 0.90–1.7 c,e,f |
| Quercetin | 16.62 ± 1.32 | 15.15 ± 1.32 | 4.38–10.7 a,e–h |
| Caffeic acid | 8.26 ± 0.69 | 5.77 ± 0.69 | 2.70–6.63 a,c,h |
| Trolox | 1.0 | 1.0 | 1.0 |
| Sample | TEAUC a | TELT b |
|---|---|---|
| Orange juice 1 | 1.16 ± 0.02 | 0.59 ± 0.04 |
| Orange juice 2 | 0.52 ± 0.01 | 0.34 ± 0.01 |
| Orange juice 3 | 1.02 ± 0.13 | 0.57 ± 0.02 |
| Orange juice 4 | 0.54 ± 0.02 | 0.32 ± 0.01 |
| Orange juice 5 | 0.36 ± 0.01 | 0.29 ± 0.01 |
| Red wine 1 | 4.02 ± 0.32 | 2.98 ± 0.11 |
| Red wine 2 | 3.81 ± 0.27 | 2.34 ± 0.25 |
| Red wine 3 | 3.98 ± 0.29 | 2.54 ± 0.11 |
| Red wine 4 | 4.03 ± 0.33 | 2.52 ± 0.13 |
| Red wine 5 | 3.15 ± 0.35 | 2.64 ± 0.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, J.R.B.; Meireles, A.N.; Marques, S.S.; Gregório, B.J.R.; Ramos, I.I.; Silva, E.M.P.; Barreiros, L.; Segundo, M.A. Exploiting Kinetic Features of ORAC Assay for Evaluation of Radical Scavenging Capacity. Antioxidants 2023, 12, 505. https://doi.org/10.3390/antiox12020505
Carvalho JRB, Meireles AN, Marques SS, Gregório BJR, Ramos II, Silva EMP, Barreiros L, Segundo MA. Exploiting Kinetic Features of ORAC Assay for Evaluation of Radical Scavenging Capacity. Antioxidants. 2023; 12(2):505. https://doi.org/10.3390/antiox12020505
Chicago/Turabian StyleCarvalho, Joana R. B., Andreia N. Meireles, Sara S. Marques, Bruno J. R. Gregório, Inês I. Ramos, Eduarda M. P. Silva, Luisa Barreiros, and Marcela A. Segundo. 2023. "Exploiting Kinetic Features of ORAC Assay for Evaluation of Radical Scavenging Capacity" Antioxidants 12, no. 2: 505. https://doi.org/10.3390/antiox12020505
APA StyleCarvalho, J. R. B., Meireles, A. N., Marques, S. S., Gregório, B. J. R., Ramos, I. I., Silva, E. M. P., Barreiros, L., & Segundo, M. A. (2023). Exploiting Kinetic Features of ORAC Assay for Evaluation of Radical Scavenging Capacity. Antioxidants, 12(2), 505. https://doi.org/10.3390/antiox12020505