Abstract
Brassica vegetables have demonstrated many health benefits over the years due to their composition of phenolic, flavonoid, and glucosinolate contents. However, these bioactive molecules can be easily depleted during gastronomic operations. Therefore, a sustainable method that improves their phenolic content and antioxidant activity is required for both the processors and consumers. Thermal processing has been demonstrated as a method to improve the phenolic content and antioxidant status of Brassica vegetables. In the current study, four different thermal processing methods, including freeze-drying, sautéing, steaming, and air-frying, were employed for five different Brassica vegetables, including kale, broccoli sprouts, Brussels sprouts, red cabbage, and green cabbage. The total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activities were assessed using radical scavenging activity (DPPH and ABTS•+), reducing power (FRAP), and the chelating ability of metal ions. Among the methods tested, air-frying at 160 °C for 10 min showed the highest TPC, TFC, and antioxidant activity of the Brassica vegetables, while sautéing showed the lowest. The steam treatments were preferred over the freeze-drying treatments. Within the vegetables tested, both kale and broccoli sprouts contained higher antioxidant properties in most of the employed processing treatments. The results also indicated that there is a strong correlation between the TPC, TFC, and antioxidant activity (p < 0.05). This study indicates that air-frying could be used as a sustainable thermal processing method for improving biomolecules in Brassica vegetables.
1. Introduction
Brassica vegetables, particularly Brassica oleraceae (e.g., cabbage, Brussels sprouts, broccoli sprouts, kale), have gained attention over the years due to their numerous health benefits. Their associated health benefits include protective effects on type 2 diabetes, cardiovascular disease, coronary heart disease, and hypertension [1,2,3]. These vegetables are rich sources of fiber, vitamins, carotenoids, and minerals [2,4], including rich phenolic profiles with relatively higher antioxidant properties [4,5]. Among these natural antioxidants, flavonoids provide better protective properties as reducing agents and radical scavenging agents pertaining to antioxidant activity [5].
The common understanding is that when consumed raw, these Brassica vegetables would provide better nutritional benefits, whereas heat treatments and thermal processing decrease their nutrient content [5] with prolonged cooking. This controversial statement on thermal processing should be clarified with robust scientific evidence. The research question of whether thermal processing affects the phenolic composition and antioxidant activity of Brassica vegetables has arisen. Enhanced food-processing techniques are necessary to address the improved nutritional content in these gastronomic operations and fulfill the nutritional needs of the high-risk populations for chronic diseases [6].
Among the thermal processing techniques, pressurized steam, stir-frying, and air-frying have gained attention over the period [7,8,9,10,11]. In addition, freeze-drying has been popular due to its wide applicability in different food matrixes [12,13]. A recent study reported an improvement in the phenolic composition and antioxidant profiles of some Brassica vegetables, such as canola and mustard, using air-frying techniques compared to other thermal techniques [7,8]. Whether similar findings occur in Brassica oleraceae is of interest. Therefore, the current study was designed to assess the impact of different thermal processing techniques on the total phenolic content (TPC), total flavonoid content (TFC), and antioxidant activity of Brassica oleraceae vegetables. The findings of the current study would demonstrate the importance of thermal processing of Brassica vegetables as a method to improve their phenolic and flavonoid contents, ultimately improving health and the food industry.
2. Materials and Methods
2.1. Materials
Five Brassica vegetables, including red cabbage, green cabbage, broccoli sprouts, Brussels sprouts, and kale, were selected for the current study based on their potential health benefits toward the positive outcomes of type 2 diabetes. The vegetables were selected from three different locations in Manitoba (south, central, and north) on the same date to obtain a representative sample. The grocery stores included Sobeys (south Winnipeg), Fresco (central Winnipeg), and Safeway (north Winnipeg). All of the vegetables were subjected to different processing conditions on the same day and stored at −80 °C until used for different assays.
2.2. Chemicals
A Folin–Ciocalteu’s (FC) reagent, a total phenolic content (TPC) standard, iron (II) chloride hexahydrate (98%), iron (III) chloride hexahydrate (97%), iron (II) sulphate heptahydrate (99%), hydrogen chloride (HCl, 99%), sodium acetate, 2,4,6-tris-(2-pyridyl)-s-triazine (TPTZ > 98%), sinapic acid (>97%), 2,2-diphenyl-1-picrylhydrazyl (DPPH, 97%), and formic acid were all purchased from Fisher Scientific Canada Ltd. (Ottawa, ON, Canada). Aluminum chloride (AlCl3), sodium nitrite (NaNO2), sodium carbonate (Na2CO3), sodium hydroxide (NaOH), ferrozine, and disodium ethylenediaminetetraacetic acid (Na2EDTA) were purchased from Sigma Canada Ltd. (Mississauga, ON, Canada). Quercetin hydrate (>95%) and 2-amino-ethyl-diphenyl borate (98%) were purchased from Acros (Mississauga, ON, Canada).
Extraction reagents, including methanol (optima grade) and ethanol (analytical grade), and standard compounds for high-performance liquid chromatography (HPLC) were purchased from Sigma Canada Ltd. (Mississauga, ON, Canada).
2.3. Application of Different Processing Techniques to Optimize the Phenolic Content
2.3.1. Freeze-Drying Treatment
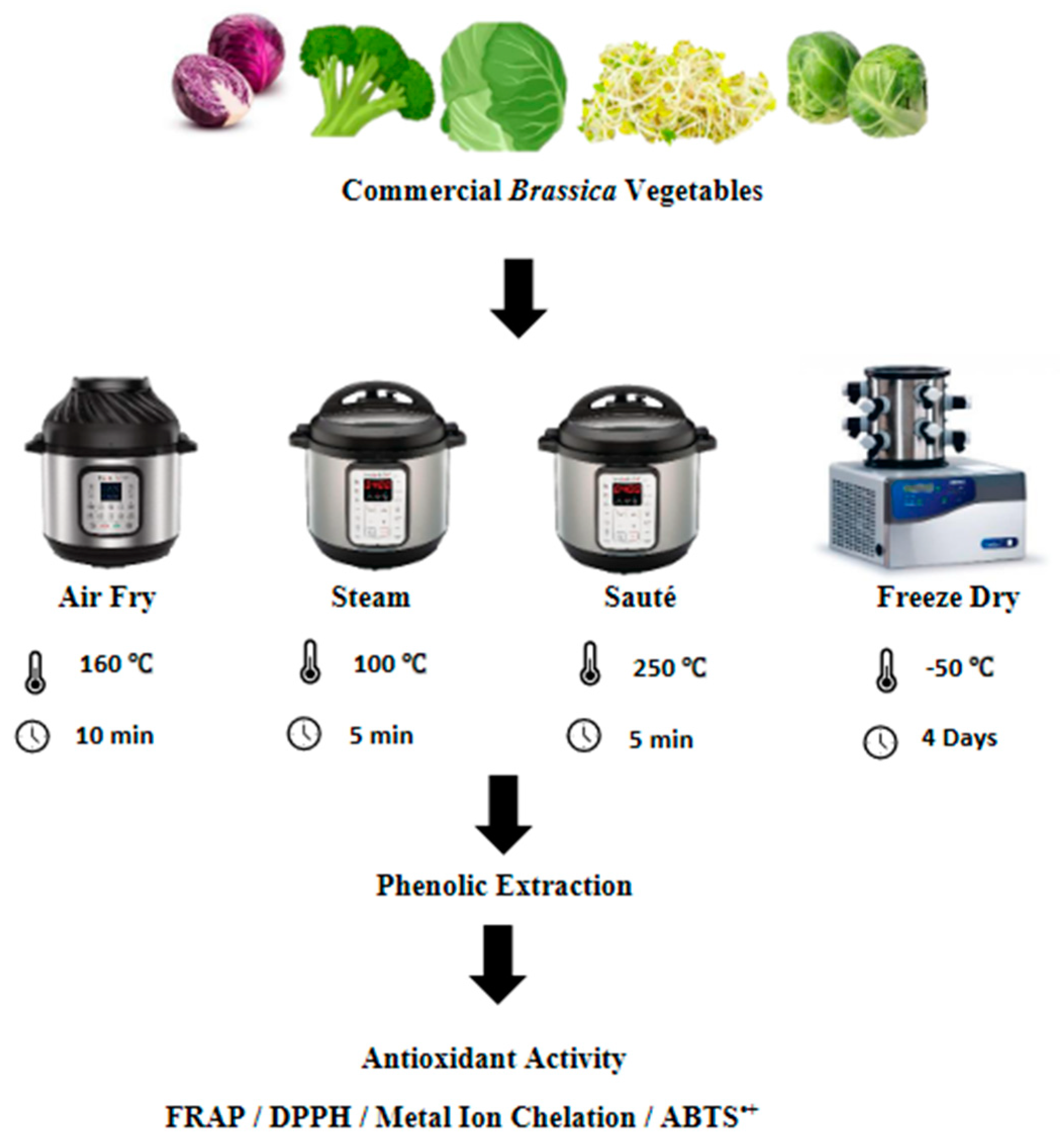
The freeze-drying of the vegetables was conducted based on the method described by Wu et al. [14], with slight modifications. Each type of vegetable was cut into pieces measuring 2 cm × 2 cm in size and stored for two hours at −80 °C prior to freeze-drying. The freeze-drying was conducted using a Labconco 4.5 Freezone freeze-dryer (Labconco Corporation, Kansas City, MO, USA) at a temperature of −50 °C for four days until the constant dry weight was recorded. After the freeze-drying, the vegetables were ground into fine particles and kept at −80 °C until further analysis (Figure 1).
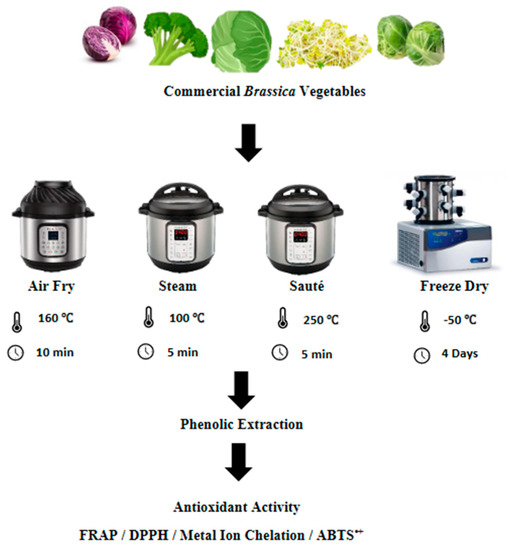
Figure 1.
Summarized experimental approach for the processing methods. FRAP, ferric reducing antioxidant power; DPPH, 2,2-diphenyl-1-picrylhydrazyl; ABTS•+, 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid.
2.3.2. Pressurized Steam Treatment
The pressurized wet extraction of the vegetables was conducted using an instant pot (Instant Pot Duo Mini 3 Qt; model number: IPDUOMINI 3 Qt) at a temperature of 100 °C at 10.2 psi for 5 min. The vegetable-to-water ratio was kept at 5:1 according to the method described by Korus et al. [15], with a few modifications. After each steam treatment, the vegetables were drained and cut into pieces measuring 2 cm × 2 cm in size. The samples were freeze-dried according to the method described in Section 2.3.1 (Figure 1).
2.3.3. Air-Frying Treatment
The air-frying/roasting of the vegetables was conducted using the method described by Fadairo et al. [7,8], with slight modifications. The same instant pot (Instant Pot Duo Mini 3 Qt; model number: IPDUOMINI 3 Qt) was used here with the air-frying extension. All of the vegetables were subjected to an air-frying temperature and time combination of 160 °C for 10 min based on the optimized results of Fadairo et al. [7] and Nandasiri et al. [16]. The samples were cut into pieces measuring 2 cm × 2 cm in size after the air-frying, and they were freeze-dried according to the method described in Section 2.3.1 (Figure 1).
2.3.4. Stir-Frying/Sautéing of the Vegetables
The same instant pot (Instant Pot Duo Mini 3 Qt; model number: IPDUOMINI 3 Qt) was used for the stir-frying operations using its in-built sauté function according to the method described by Nugrahedi et al. [17], with minor modifications. The vegetable-to-oil ratio of 10:1 was used for the stir-frying operations using canola oil (complements brand, Winnipeg, Manitoba). The stir-frying operation was conducted at 250 °C for 5 min. The vegetables were cut into pieces measuring 2 cm × 2 cm in size after the stir-frying, and they were freeze-dried as per the method described in Section 2.3.1 (Figure 1).
2.4. Sample Preparation
2.4.1. Ultrasound-Assisted Extraction (UAE) of Phenolic Compounds
The phenolic extraction was conducted using the methods described by Liu et al. [18] and Liang et al. [19], with slight modifications. In brief, 0.05 g of the freeze-dried vegetable sample was weighed and dissolved in 0.45 mL of 70% (v/v) methanol (at a solid-to-solvent ratio of 1:10). The phenolic extraction was carried out by ultrasound extraction using the SONOPLUS ultrasonic homogenizer HD 2200 system (BANDELIN electronic GmbH & Co. KG, Heinrichstraße, Berlin, Germany) with a power of 40% and a frequency of 20 kHz ± 500 Hz for 1 min at room temperature (25 °C). The extracts were centrifuged at 3000× g for 30 min at refrigeration conditions (4 °C) using the Eppendorf™ centrifuge 5804R (Fisher Scientific, Ottawa, ON, Canada). The extraction was repeated two more times, and the final volume was adjusted to 1.5 mL. The samples were concentrated using the Savant SPD 111V SpeedVac concentrator (Thermo Scientific, Mississauga, ON, Canada) for 5 h to remove the residual solvents, followed by freeze-drying at −50 °C for 2–3 h as per the method described in Section 2.3.1. The freeze-dried extracts were reconstituted in 0.5 mL of 100% (v/v) methanol and kept at −80 °C until further analysis.
2.4.2. Phenolic Extraction for Antioxidant Assays
The phenolic extractions for the antioxidant assays were conducted according to the method described by Singleton and Rossi [20], with a few modifications. In brief, 0.5 g of the freeze-dried vegetable powder was dissolved in 5.0 mL of 80% (v/v) methanol (at a sample-to-solvent ratio of 1:10) and kept at 25 °C for 15 h using an orbital shaker. After 15 h, the extracts were centrifuged at 3000× g for 30 min at refrigeration conditions (4 °C) using the Eppendorf™ centrifuge 5804R (Fisher Scientific, Ottawa, ON, Canada). The supernatant was collected and stored at −80 °C until further analysis.
2.5. Antioxidant Activity of the Vegetables
2.5.1. Assessment of Total Phenolic Content (TPC)
The total phenolic content (TPC) of the obtained extracts was estimated using the Folin–Ciocalteu method described by Thiyam et al. [21] and modified for a plate reader as described by Fadairo et al. [7]. In brief, 40 µL of the reconstituted plant extracts obtained from Section 2.4.2 were added to a Corning 9017 96-well microplate (Fisher Scientific, Ottawa, ON, Canada), followed by the addition of 120 µL of deionized water. A total of 40 µL of the FC reagent was added to the mixture and incubated for 5 min at 25 °C. After the incubation, 40 µL of Na2CO3 was added, and the sample mixture was kept in the dark for 1 h. Next, the absorbance was measured at 640 nm using a microplate reader (Bio-Tek Powerwave XS, New England, VT, USA). Methanol was substituted as the blank, and a TPC standard solution of 1000 mg/mL (Fisher Scientific, Mississauga, ON, Canada) was used to assemble the standard curve (Figure S1). The TPC was expressed as milligrams of gallic acid per gram of vegetable on a dry weight basis.
2.5.2. Assessment of Total Flavonoid Content (TFC)
The total flavonoid content (TFC) of the vegetable extracts was determined by the aluminum chloride colorimetric method described by Zhishen et al. [22], with slight modifications. In brief, 25 µL of the reconstituted plant extracts obtained from Section 2.4.2 was added to a Corning 9017 96-well microplate (Fisher Scientific, Ottawa, ON, Canada), followed by the addition of 100 µL of deionized water (at a ratio of 1:4 (v/v)). The diluted sample was then mixed with 7.5 µL of 5% (w/v) NaNO2, and the reaction mixture was held at room temperature (25 °C) for 6 min. After, 7.5 µL of 10% (w/v) AlCl3 was added and held at room temperature (25 °C) for an additional 5 min. Then, 50 µL of NaOH (1 M) was added and mixed using the VWR™ analog mini vortex mixer (Henry Troemner LLC, Thorofare, NJ, USA). The absorbance was measured at 510 nm using a microplate reader (Bio-Tek Powerwave XS, New England, VT, USA). Methanol was substituted as the blank, and a quercetin standard solution of 1.0 mg/mL (Fisher Scientific, Mississauga, ON, Canada) was used to assemble the standard curve (Figure S2). The TFC was expressed as milligrams of quercetin per gram of vegetable on a dry weight basis.
2.5.3. DPPH Free Radical Scavenging Assay
The DPPH radical scavenging activity of the extracted solution was measured using the DPPH assay described by Nandasiri et al. [23], with minor modifications. Briefly, 10 µL of the reconstituted plant extracts obtained from Section 2.4.2 was added to 290 µL of 100% (v/v) methanol in a Corning 9017 96-well microplate (Fisher Scientific, Ottawa, ON, Canada), followed by the addition of 10 µL of the prepared DPPH solution (0.05 mM). The samples were kept in the dark for 5 min to generate the radicals. The absorbance was measured at 516 nm using a microplate reader (Bio-Tek Powerwave XS, New England, VT, USA). Methanol was substituted as the blank. The free radical scavenging activity was measured using the following equation:
where Ac is the absorbance of the solvent control, and As is the absorbance of the sample.
2.5.4. Ferric Reducing Antioxidant Power Assay (FRAP Assay)
Apart from the radical scavenging activity, the reducing power of the extracts was assessed using a modified method by Benzie and Strain [24]. The working reagent of FRAP was prepared by mixing acetate buffer (300 mM, pH = 3.6) and a TPTZ (2,4,6-tri [2-pyridyl]-s-triazine) solution (10 mM in 40 mM HCl) with a 20 mM FeCl3 solution at a ratio of 10:1:1 and keeping it at 37 °C until a straw-colored solution was formed. Briefly, 10 µL of the reconstituted plant extracts obtained from Section 2.4.2 was mixed with 90 µL of deionized water, followed by 90 µL of FRAP reagent in a Corning 9017 96-well microplate (Fisher Scientific, Ottawa, ON, Canada). The reaction mixture was then left in the dark for 8 min, and the absorbance was measured at 593 nm using a microplate reader (Bio-Tek Powerwave XS, New England, VT, USA). Deionized water was used as the blank, and a 1.0 mM solution of Trolox was used to create the standard curve (Figure S3).
2.5.5. Ferrous Ion-Chelating Activity Assay Antioxidant Capacity
The chelating activity of the metals was assessed according to the method described by Dinis et al. [25], with a few modifications. In short, 10 µL of the reconstituted plant extracts obtained from Section 2.4.2 was added to a Corning 9017 96-well microplate (Fisher Scientific, Ottawa, ON, Canada), with 50 µL of a 2.0 mM FeCl2 solution and 20 µL of a 5.0 mM ferrozine solution prepared fresh daily. The total volume was adjusted to 280 µL using deionized water. The mixture was then kept at room temperature (25 °C) for 10 min, and the absorbance was measured at a wavelength of 562 nm using a microplate reader (Bio-Tek Powerwave XS, New England, VT, USA). Deionized water was used as the blank, and a 1.0 mM solution of Na2EDTA was used to create the standard curve (Figure S4).
2.5.6. Total Antioxidant Capacity (TAC) Assay
The total antioxidant activity of each extract was assessed according to a protocol using a commercial KIT (Item # Cay709001-96; Cayman Chemicals, Ann Arbor, Michigan, USA) and a microplate reader (Bio-Tek Powerwave XS, Michigan, CA, USA). Deionized water was used as the blank, and a 1.0 mM solution of Trolox was used to create the standard curve (Figure S5).
3. Statistical Analysis
The results are presented as means ± standard deviations for all of the experiments that were conducted with three replicates. The normality of the data and the constant variance were confirmed prior to the statistical analyses [26]. The differences between the mean values of the main factor were determined by a two-way analysis of variance (ANOVA). A post-hoc analysis was conducted using the Tukey’s test, with 5% statistically significant differences (p < 0.05) considered statistically significant [26]. The SPSS statistical software version 26 (IBM, New York, NY, USA) was used to analyze the data.
4. Results and Discussion
4.1. Impact of Thermal Processing on Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
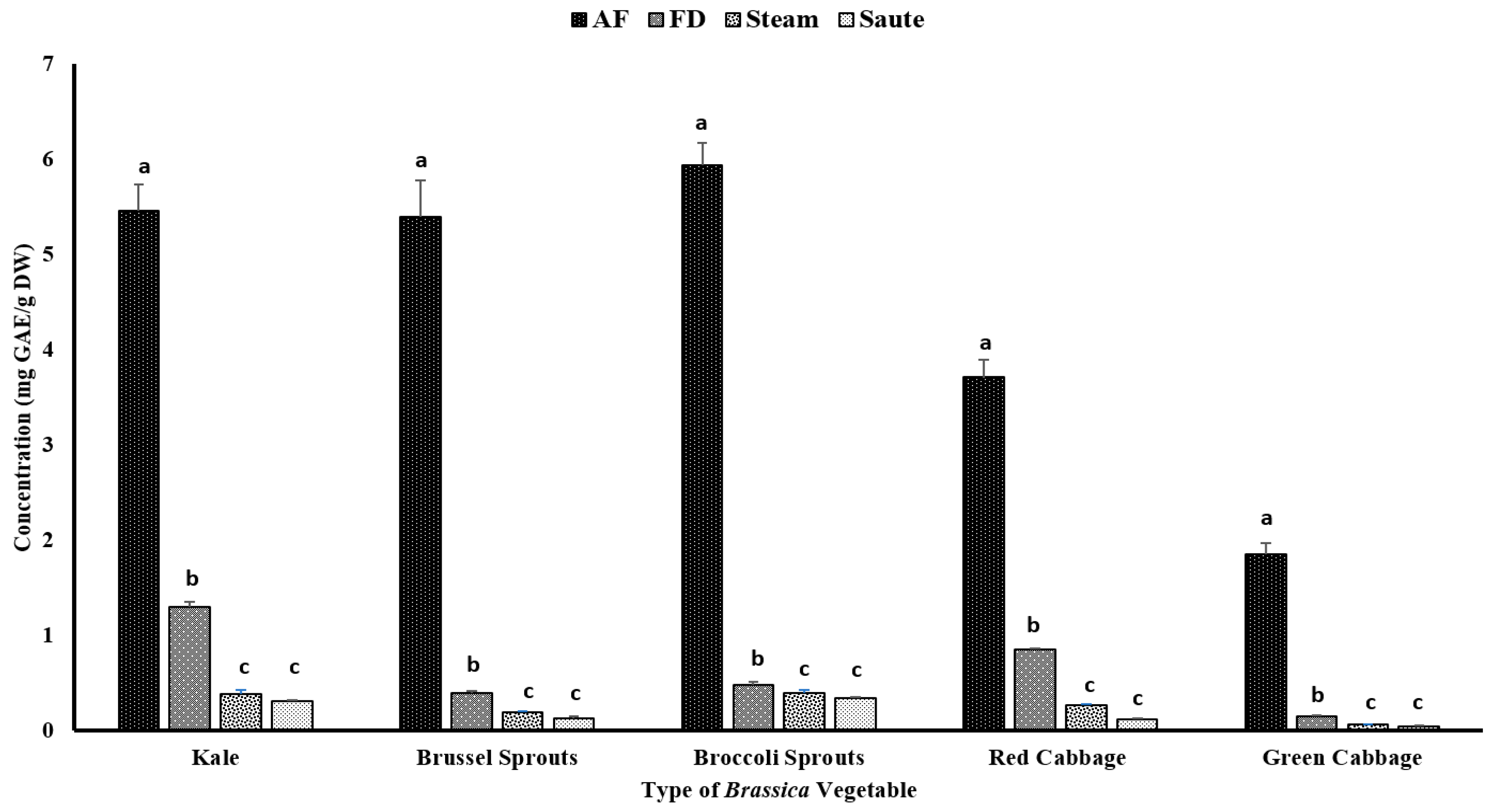
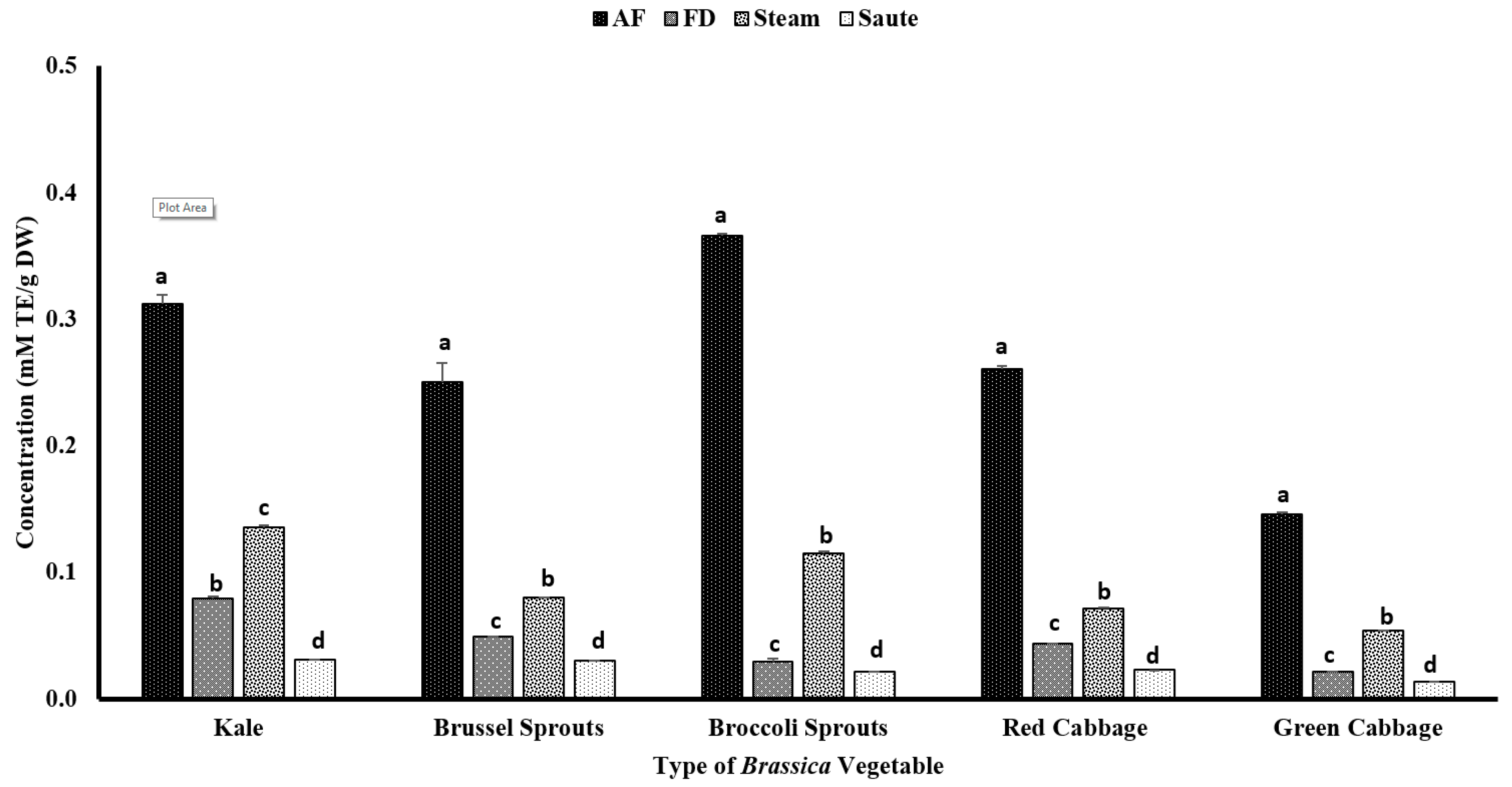
Phenolic compounds have been reported to contribute to plants’ flavor, color, and antioxidant activity. The TPC of the vegetables was determined using the Folin–Ciocalteu assay, which was based on the oxidation reaction of phenolic compounds in the presence of a mixture of phosphomolybdate and phosphotungstate [27]. The impact of the different thermal processing techniques was evaluated for the TPC. The results showed that air-frying, compared to other techniques, significantly (p < 0.05) increased the TPC of the vegetables, regardless of the variety (Figure 2). The statistical analysis indicated that the type of vegetable, the processing method, and the interactions between them were significant in the model statistics with an adjusted R2 value of 0.996 (Table 1). Previous studies by Nandasiri et al. [16] and Fadairo et al. [7,8] also showed improved TPC in canola and mustard by the application of air-frying. They observed the highest TPC values for the canola meal substrate conditions at 190 °C for 15 min (3.15 ± 0.14 mg GAE/g DW) and 20 min (3.05 ± 0.02 mg GAE/g DW), respectively. The present study optimized a condition with a lower temperature for a shorter time, 160 °C for 10 min, which exhibited much higher TPC values, ranging from 1.76 ± 0.11 mg GAE/g DW (green cabbage) to 5.87 ± 0.23 mg GAE/g DW (broccoli sprouts) (Figure 2). A study conducted by Ayaz et al. [28] reported that the TPC in kale leaves was around 1.37 mg/g on a fresh weight basis (FW), which was much lower compared to the values obtained by the current study.
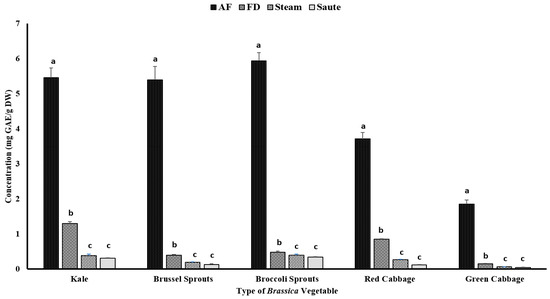
Figure 2.
The effects of thermal processing techniques on the total phenolic content (TPC) of the selected Brassica vegetables. The bars represent means ± standard deviations (n = 3). The different letters for each vegetable indicate statistical differences based on a two-way analysis of variance. GAE, gallic acid equivalents; DW, dry weight; mg, milligram; g, gram; AF, air-fry; FD, freeze-dry.

Table 1.
Impact of thermal treatments on the total phenolic (a) and total flavonoid (b) contents and different antioxidant activities (c–f) of the selected Brassica vegetables.
The application of less time in gastronomic operations is often preferred for vegetables; hence, the air-frying condition of 160 °C for 10 min is a preferred option for Brassica vegetables. The air-fryer is designed to use hot air (~200 °C) to quickly cook foods with a continuous flow (using rapid air technology) circulating through the cooking chamber [29]. This rapid air technology creates an opportunity to create faster cooking operations, creating crispy coatings on the outside of the foods. Further, this rapid air technology reduces the preparation time (by 25–50%), pre-heating time (by 50–75%), and energy consumption (by 50%) [29]. No recent reports on the impact of air-frying of Brassica vegetables have been reported up to date, and this is the first study evaluating the impact of air-frying on their compositional changes. The application of higher temperatures (>140 °C) for shorter time intervals during the process of air-frying and the consistent circulation of hot air throughout the system make it ideal to preserve the nutrients and phenolic compounds without them leaching out [7]. Furthermore, in both canola and mustard, it was observed that certain thermo-generative phenolic compounds were also formed during the thermal process of air-frying, including canolol [7,8,16,30,31]. Canolol and other thermo-generative phenolic compounds demonstrated higher antioxidant potentials in their respective studies. Although not measured, the formation of these thermo-generative compounds could be associated with the higher TPC values and antioxidant activities obtained via the air-frying method in the present study.
In the present study, both the sauté and pressurized steam operations reported the lowest TPC levels (Figure 2). Further, the statistical analyses indicated that the type of vegetable, the processing method, and the interactions between them were significant in the model statistics with an adjusted R2 value of 0.983 (Table 1). The leaching of the nutrients, glucosinolates, and other phenolic compounds during the steaming process may lead to lower levels of TPC, regardless of the types of vegetables [32,33]. Paciulli et al. [32] reported that steamed Brussels sprouts contained a TPC of 0.25 ± 0.8 mg of GAE/g, which is comparable to the values reported in the current study using the pressured steam treatment by the instant pot, 0.19 ± 0.01 mg of GAE/g DW (Figure 2). A similar study conducted by Cieślik et al. [34] evaluated the impact of boiling, blanching, cooking, and freezing on different cruciferous vegetables, including Brussel sprouts, white and green cauliflower, broccoli, and curly kale, and observed considerable losses of total glucosinolates after blanching and cooking, with 30% and 72.4%, respectively, which affected the TPC levels. However, an interesting correlation trend was observed with the TPC and other antioxidant activities (Table 2). It was observed that the FRAP (0.936), TFC (0.863), and metal ion chelating activity (0.911) had a very high correlation with the TPC value (Table 2). However, a poor correlation was observed between the TPC and the DPPH radical activity (0.234) (Table 2).

Table 2.
Pearson correlation analyses between different antioxidant activity, total phenolic content, and total flavonoid content.
Thermal processing strategies have been applied in the food industry since ancient times with the focus of delaying the inevitable deterioration of perishable foods between production and consumption [9]. Thermal processing, including steam, would destroy microbial pathogens while reducing the number of spoilage microorganisms and inactivating certain enzymes related to the relapse of foods [9]. Further, the use of oil in stir-frying operations might also have a detrimental effect on the TPC levels of the vegetables. During processing, some lipophilic phenolic compounds could leach out of the medium, resulting in losses in TPC values. A study conducted by Nugrahedi et al. [17] showed minimal differences in the quantity of glucosinolates among the treatment groups of different time–temperature combinations for Chinese cabbage. The authors reported that the inactivation of the myrosinase enzyme at higher temperatures of stir-frying would result in a negligible influence on its composition. However, in the current study, we observed low TPC values, ranging from 0.05 ± 0.00 mg GAE/g DW (green cabbage) to 0.35 ± 0.01 mg GAE/g DW (broccoli sprouts). In a different study, conducted on serrano peppers and jalapeno peppers, Mwebi and Ogendi [10] reported that the antioxidant concentration in the stir-fry, steamed, and boiled samples was nearly the same, but much higher than the raw samples. Interestingly, freeze-dried vegetables also had lower TPC levels compared to air-fried vegetables. In general, freeze-drying has been reported as a non-destructive method to preserve nutrients. Together with the above study [10], the current study demonstrated that freeze-drying operations were not an effective method compared to air-frying (Figure 2).
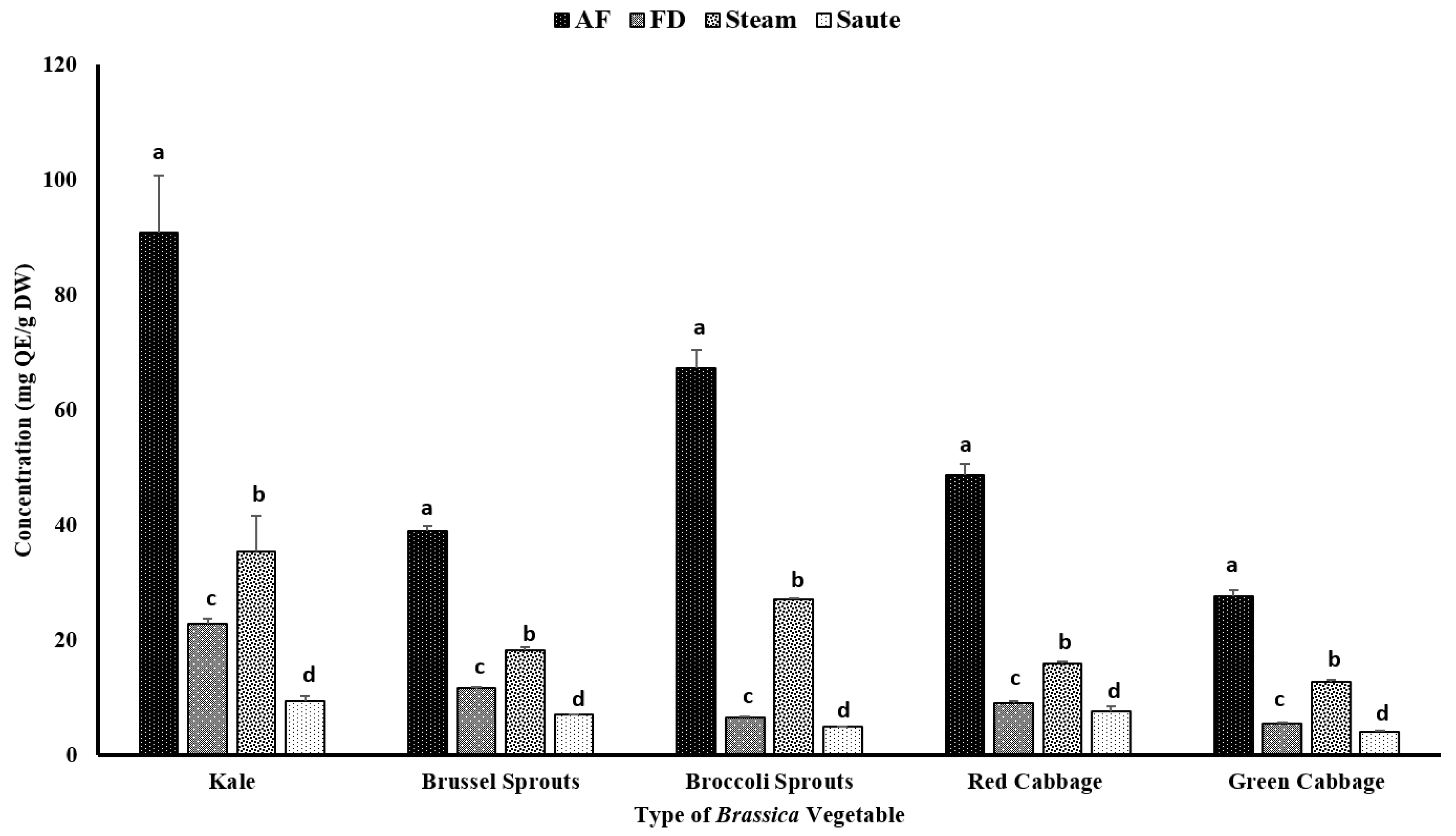
Certain phenolic compounds, including flavonoids, have also been proven to show strong antioxidant properties and health benefits [35,36]. The TFC of vegetables can be influenced by both intrinsic and extrinsic factors, including variety, maturity stage, cultivation location, and other processing conditions, such as temperature, pH, and pressure [37,38]. The current study found that the application of air-frying was able to significantly improve the TFC of vegetables, regardless of their varieties (Figure 3). For the air-fried treatment, kale contained the highest amount of TFC (90.76 ± 10.04 mg QE/g DW), followed by broccoli sprouts (67.21 ± 3.29 mg QE/g DW) and red cabbage (48.72 ± 2.01 mg QE/g DW). The TFC also had a similar correlation trend compared to the TPC (Table 2). The TFC demonstrated higher correlations among the FRAP (0.940), TPC (0.863), and metal ion chelating activity (0.939) (Table 2). However, similar to the TPC, a poor correlation was observed between the TFC and the DPPH radical activity (0.375) (Table 2).
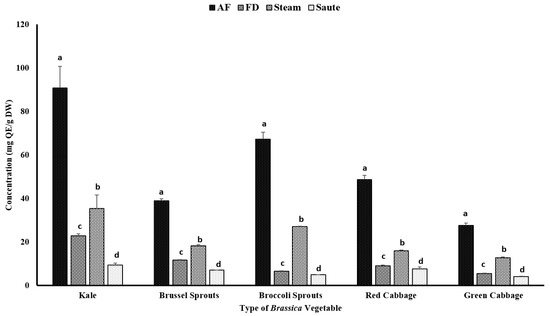
Figure 3.
The effects of thermal processing techniques on the total flavonoid content (TFC) of the selected Brassica vegetables. The bars represent means ± standard deviations (n = 3). The different letters for each vegetable indicate statistical differences based on a two-way analysis of variance. QE, quercetin equivalents; DW, dry weight; mg, milligram; g, gram; AF, air-fry; FD, freeze-dry.
These findings confirmed that air-frying is the preferred processing method for Brassica vegetables (Figure 3). It was reported that kale contains a relatively higher amount of flavonoids, including kaempferol, quercetin, isorhamntin, flavonol-3-O-glycosides, and flavonol-7-O-glycosides [39]. The higher TFC values could be associated with the distribution of these flavonoids. On the contrary, red cabbage contains a relatively higher amount of cyanidin compounds, which represents the comparable higher TFC values [40]. In addition, the processing conditions of air-frying, which involve a higher temperature for a shorter time, would further prevent them from leaching out of the vegetables during the processing. Interestingly, the sauté treatment demonstrated the lowest values for the TFC (Figure 3). The solubility of certain flavonoids in a hydrophobic medium is higher compared to an aqueous medium, and this could be associated with the lower TFC levels in the sauté treatment [41]. The formation of H-bonds with oil will further increase the solubility of the flavonoids in an oil medium, allowing the leaching out of the extractants and resulting in a lower flavonoid content in the final extracts of the sauté treatment [42]. Lemańska et al. [42] reported that both 3- and 5-hydroflavone formations with strong H-bonds with oxygen atoms from C4 = O inhibit deprotonationation and antioxidant potentials. However, with an increase in the pH, the shift would take place from the C3- and/or C5-hydroxyl groups to the C4 = carbonyl group, creating stable cations to promote electron donation for the flavonoid molecules. Interestingly, the steam treatments showed a higher TFC compared to the freeze-dry treatments (Figure 3). The shorter time exposure to heated vapor during the steam treatment would facilitate the breaking of the cell wall materials of these vegetables, thereby releasing the intracellular phenolic compounds into the medium and resulting in higher yields of TFC [43].
The current study confirms that thermal processing of Brassica vegetables for shorter durations at higher temperatures would enhance the extractability of flavonoids. These results were similar to those of Nandasiri et al. [23] from canola meal using accelerated solvent extraction. They found that there was a significant increase in the concentration of flavonoids among the extracts between the extraction temperatures of 140 °C (3.70 ± 0.11 µmol QE/g DM) and 180 °C (5.45 ± 0.27 µmol QE/g DM). The application of both pressure (1500 psi) and temperature had a favorable impact on the extractability of the flavonoids [23]. Similarly, Zago et al. [44] reported that both the pre-heating time and the pre-heating temperature had a positive effect on the extractability of the TFC on defatted hemp cake. Yet, the values were not significantly different. The TFC values ranged from 0.020 ± 0.01 µmol QE/g DM (160 °C; 15 min) to 0.23 ± 0.01 µmol QE/100 g DM (180 °C; 30 min) for the pre-treatment time/temperature combinations for the defatted hemp cake [44].
4.2. Impact of Thermal Processing on Antioxidant Activity
The antioxidant activity of the vegetable extracts was evaluated using different assays leading to different mechanisms, such as the radical scavenging activity, the chelating activity of metals, and the reducing power. Since the structures and active sites of phenolic compounds differ, each compound reacts differently, requiring different mechanisms of action to better understand their antioxidant activity.
4.2.1. DPPH Free Radical Scavenging Activity of the Brassica Vegetables
The radical scavenging activity of the vegetable extracts was evaluated primarily using the DPPH radical scavenging activity. The DPPH radical scavenging activity is one of the most widely used colorimetric methods for measuring antioxidant activity using its scavenging capacity towards DPPH• radicals via an electron donating mechanism. In general, a higher antioxidant capacity would lead to a decrease in absorbance. These radicals could react in four different pathways, including proton-coupled electron transfer (PC-ET), electron transfer–proton transfer (ET-PT), sequential proton loss electron transfer (SPLET), and adduct formation (AF) [45,46]. Among the reported mechanisms, both the PC-ET and SPLET mechanisms are considered to follow the DPPH radical formation. Hence, for the 70% (v/v) methanol extractants with a dielectric constant of = 33, the SPLET mechanism is more applicable as it encourages ionization [45,47].
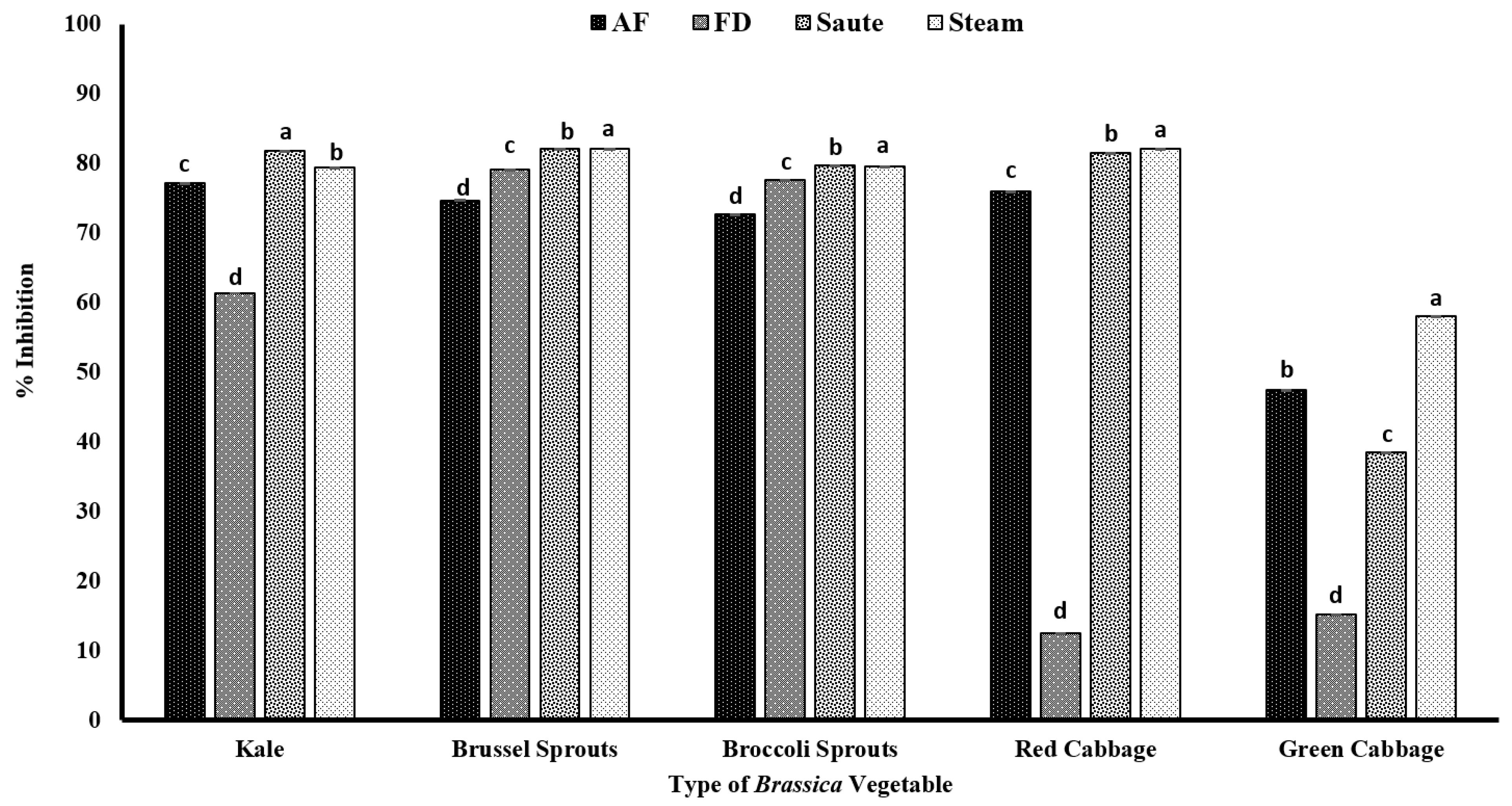
In the present study, both Brussels sprouts and broccoli sprouts had higher DPPH radical activity, despite the processing method, with an average radical scavenging activity of over 70% (Figure 4), indicating their higher antioxidant potential. The statistical analyses further indicated that the type of vegetable, the processing method, and the interactions between them were significant in the model statistics with an adjusted R2 value of 0.996 for the DPPH radical activity (Table 1). Further, it confirms that in the sprout stage, the composition of phenolics is much higher and more condensed compared to the mature stage. In addition, the minimal changes in the radical scavenging activity despite the processing operations indicate that the endogenous phenolic compounds present in both Brussels sprouts and broccoli sprouts are relatively stable and are minimally impacted by the gastronomic operations. In general, above 50% radical scavenging activity was reported for the air-fry, sauté, and steam operations, showing that thermal processing has favorable conditions toward antioxidant activity (Figure 4). A study conducted by Mwebi and Ogendi [10] also reported that the cooking operations have a different impact on the radical scavenging activity. The authors reported that the DPPH radical activity was in the order of microwaved > stir-fried > steamed > raw > boiled for both serrano peppers and jalapeno peppers. Further, in another study conducted by Turkmen et al. [48], it was reported that the radical scavenging activity of fresh vegetables was in the order of broccoli > pepper > spinach > green beans > peas > squash > leek. The authors also found a similar DPPH activity of 78.17% for fresh broccoli [48]. Similar to our findings, the authors also found a significant increase in the antioxidant activity in broccoli during the gastronomic operations of boiling (15.90%) and microwaving (16.68%) compared to its fresh form. The increments in antioxidant activity, specifically for Brassica vegetables, are reported to be due to the inactivation of peroxidases at higher processing temperatures, which reduces the pro-oxidant effects [48]. However, DPPH showed a lower correlation among TPC, TFC, and other antioxidant activities, demonstrating that the mechanism of action is different (Table 2).
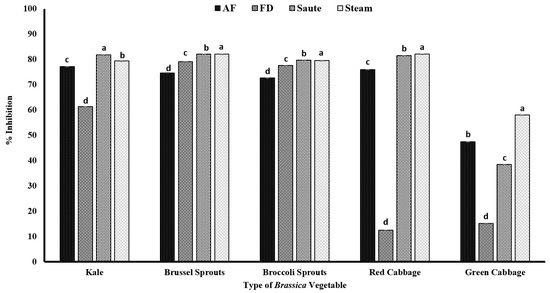
Figure 4.
The effects of thermal processing techniques on the antioxidant activity of the selected Brassica vegetables measured by DPPH radical scavenging activity. The bars represent means ± standard deviations (n = 3). The different letters for each vegetable indicate statistical differences based on a two-way analysis of variance. AF, air-fry; FD, freeze-dry; DPPH, 2,2-diphenyl-1-picrylhydrazyl.
4.2.2. Ferric Reducing Antioxidant Power (FRAP) of the Brassica Vegetables
The antioxidant activity measured by reducing power demonstrated a different pattern from the DPPH radical scavenging activity. The results showed that the air-fry treatment facilitated the ferric reducing power of the vegetable extracts (Figure 5). For the air-fry treatment, both the broccoli sprouts (0.37 ± 0.00 mM TE/g DW) and kale (0.31 ± 0.01 mM TE/g DW) showed a higher FRAP value, while the green cabbage (0.15 ± 0.00 mM TE/g DW) showed the lowest (Figure 5). The statistical analyses also indicated that the type of vegetable, the processing method, and the interaction between them were significant in the model statistics with an adjusted R2 value of 0.998 for the reducing power (Table 1). An interesting higher correlation was observed between the FRAP activity and the TPC (0.936), TFC (0.940), and metal ion chelation (0.921) (Table 2). In addition, a moderate correlation was also observed between the FRAP and the total antioxidant activity (0.530) (Table 2). The trends between the TPC, TFC, and FRAP further illustrate the relationship between the phenolic content and its antioxidant activity.
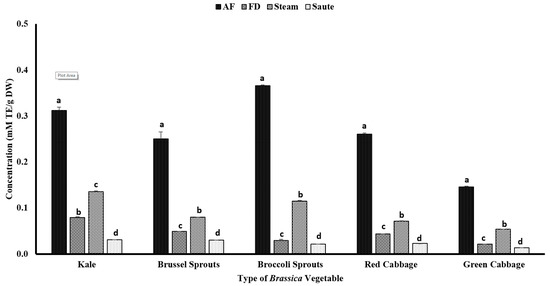
Figure 5.
The effects of thermal processing techniques on the antioxidant activity of the selected Brassica vegetables measured by the FRAP antioxidant assay. The bars represent means ± standard deviations (n = 3). The different letters for each vegetable indicate statistical differences based on a two-way analysis of variance. mM, millimoles; g, gram; TE, Trolox equivalent; AF, air-fry; FD, freeze-dry; FRAP, ferric reducing antioxidant power.
The sauté treatment yielded the lowest antioxidant activity for all of the vegetable types. The lower FRAP values with the sauté treatment could be due to the leaching out of phenolic compounds into the oil fraction due to the formation of strong H-bonds [42]. The reducing power is closely linked with the electron-donating ability of a substance, which facilitates the transformation of ferric ions (Fe3+) (light brown) into ferrous ions (Fe2+) (blue) [23]. Consequently, in sautéing, due to the formation of H-bonds with the oil fraction, the electron-donating ability is further reduced, which results in a lower FRAP activity. Similar to both the TPC and TFC, the steam treatment demonstrated a higher FRAP value compared to the freeze-dry treatment (Figure 5). The application of heat in a pressurized environment for a shorter period would deactivate the myrosinase enzyme activity of the Brassica vegetables [49,50,51]. This phenomenon will activate the glucosinolates, thereby increasing the antioxidant activity of the thermally processed vegetables [51,52].
In addition, Gaspar et al. [53] explained the electron transfer mechanism of phenolic acids, demonstrating the association between the number of hydroxyl groups and the electrochemical potential of a phenolic acid. The authors showed that if a phenolic compound contains a higher number of hydroxyl groups, it will result in a lower electrochemical potential via o-quinone formation [53]. Teh et al. [54] also reported that higher extraction temperatures were correlated with a higher phenolic content and a higher antioxidant activity. The current study showed a similar trend for the TPC, TFC, and FRAP, agreeing with the findings of Teh et al. [54].
4.2.3. Metal Ion Chelating Activity (MIC)
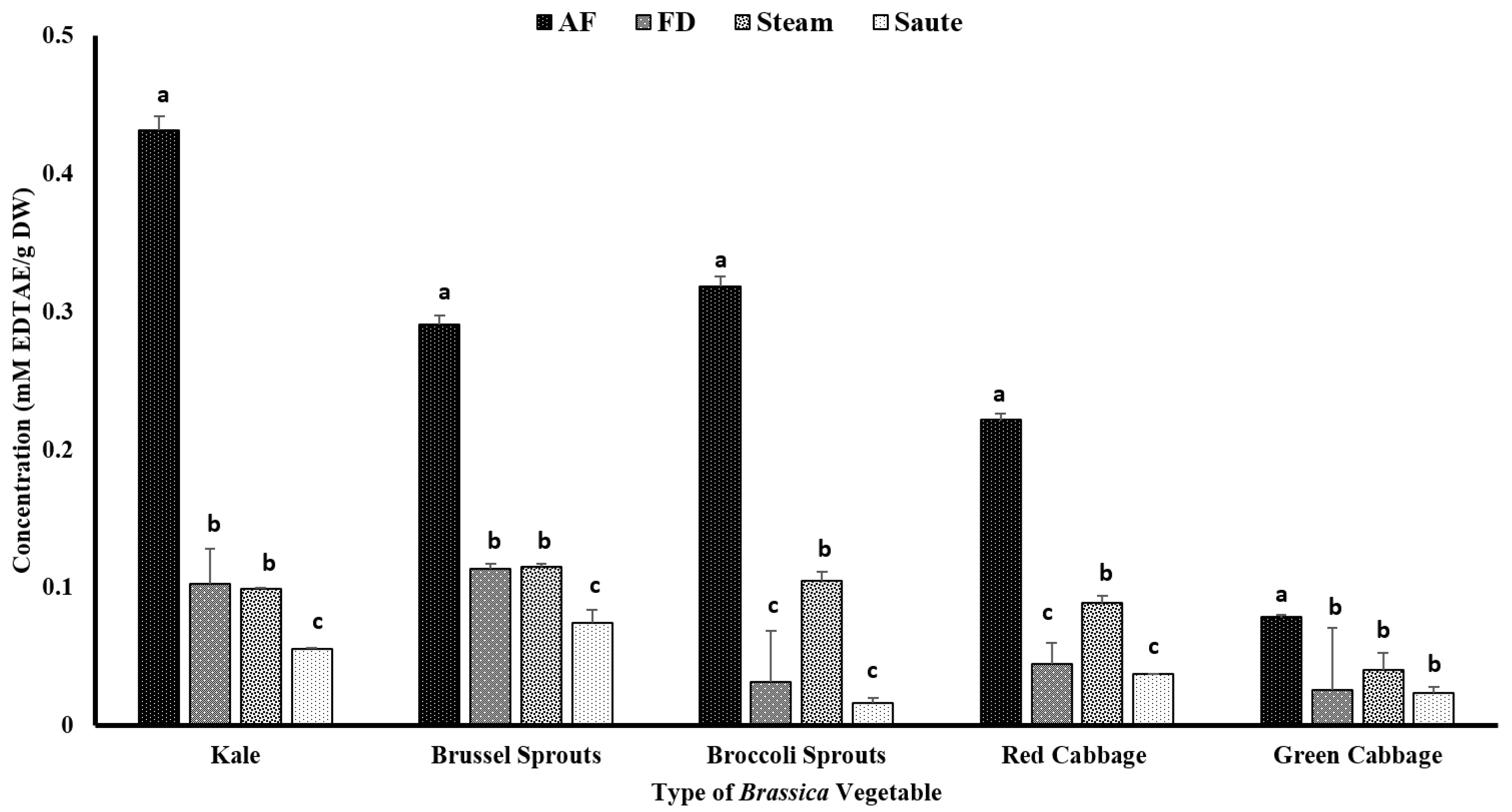
The chelating ability of the metal ions was assessed as a different mechanism of action for antioxidant activity. Similar to FRAP, the air-fried samples showed a comparatively higher antioxidant activity. Among the Brassica vegetables, kale showed the highest chelating activity for the air-fried samples (0.43 ± 0.03 mM EDTAE/g DW), while green cabbage showed the lowest (0.08 ± 0.04 mM EDTAE/g DW) (Figure 6). Sautéing showed the lowest chelating activity among the thermal processing treatments (Figure 6). The statistical analyses indicated that the type of vegetable, the processing method, and the interaction between them were significant in the model statistics with an adjusted R2 value of 0.974 for the metal ion chelating activity (Table 1). Similar to FRAP, the MIC demonstrated a higher correlation among the TPC (0.911), TFC (0.939), and FRAP (0.921) (Table 2). A moderate correlation was also observed between the total antioxidant activity (0.453) and DPPH (0.359) with MIC (Table 2). Mladěnka et al. [55] claimed that a neutral pH is favored for phenolic compounds to serve as metal ion chelators. They further stated that phenolic compounds containing 3-hydroxy-4-keto groups could create complexes, while phenolic compounds containing a catechol B ring are unable to chelate the metal ions [55]. It is speculated that in sautéing operations, more compounds containing a catechol B ring could be formed, resulting in lower metal ion chelating activity.
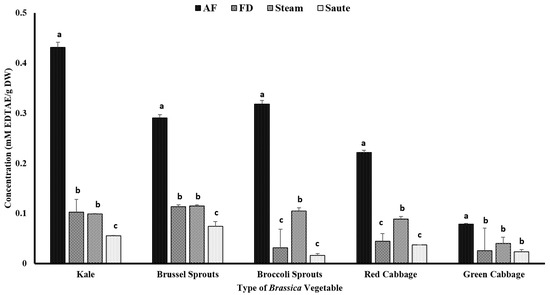
Figure 6.
The effects of thermal processing techniques on the antioxidant activity of the selected Brassica vegetables measured by the chelating ability of the metals. The bars represent means ± standard deviations (n = 3). The different letters for each vegetable indicate statistical differences based on a two-way analysis of variance. mM, millimoles; g, gram; AF, air-fry; FD, freeze-dry; EDTA, ethylenediaminetetraacetic acid.
4.2.4. Total Antioxidant Capacity
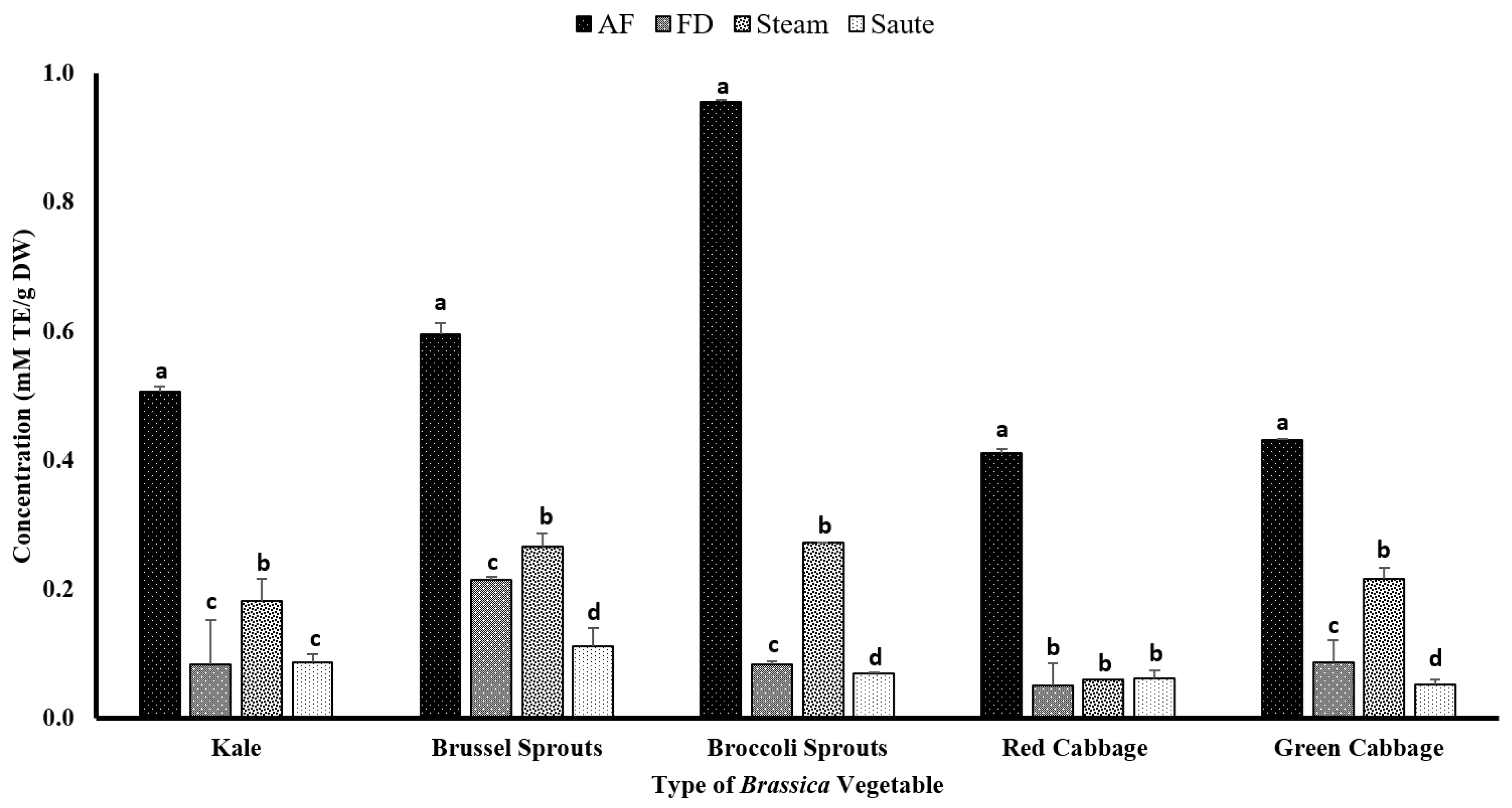
The total antioxidant capacity of the extracts was measured using ABTS+ scavenging activity commercial kits. The assay is based on a colorimetric principle evaluating the decay of ABTS+ in the presence of an antioxidant agent. For the current study, Trolox was used as the antioxidant agent. Dudonné et al. [56] illustrated a strong positive correlation among the TPC and ABTS·+ assay with an R2 value of 0.966. This was evident from the results of the current study, which showed similar trends for the TPC, TFC, FRAP, and metal ion chelation activity, with the air-frying treatment producing the highest antioxidant activity (Figure 7). The broccoli sprouts (0.96 ± 0.01 mM TE/g DW) showed the highest ABTS+ radical activity for the air-frying treatment, while both the green (0.43 ± 0.03 mM TE/g DW) and red (0.41 ± 0.03 mM TE/g DW) cabbage showed the lowest (Figure 7). The statistical analyses indicated that the type of vegetable, the processing method, and the interaction between them were significant in the model statistics. However, the adjusted R2 value for the total antioxidant activity was much lower compared to the other assays, with an adjusted R2 value of 0.528 (Table 1f). However, the correlation between the ABTS+ radical activity and DPPH was similar, with a moderate correlation among the TPC (0.541), TFC (0.464), FRAP (0.530), and MIC (0.453) (Table 2). Both the DPPH and ABTS+ radical activities showed no correlation (r = 0.013) (Table 2) for the current study. Even though both assays work on a radical scavenging mechanism, it shows that the mechanism of action between the two assays could be different.
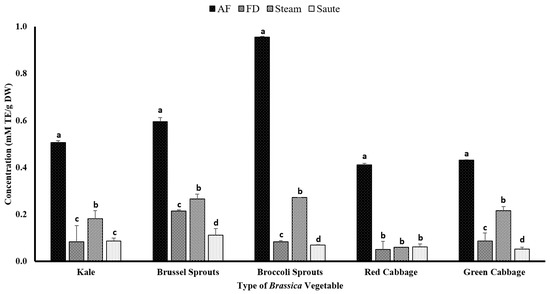
Figure 7.
The effects of thermal processing techniques on the total antioxidant capacity of the selected Brassica vegetables measured by the ABTS radical scavenging assay. The bars represent means ± standard deviations (n = 3). The different letters for each vegetable indicate statistical differences based on a two-way analysis of variance. mM, millimoles; g, gram; TE, Trolox equivalent; AF, air-fry; FD, freeze-dry; ABTS, 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid.
Interestingly, both the freeze-dried and sautéed samples showed the lowest ABTS·+ radical activity for all of the Brassica vegetables. It was also found that the ABTS activity increases with the increasing polarity of the solvent [57]. Consequently, the application of sautéing would result in a low-polar medium, creating lower ABTS·+ radical activity (Figure 7).
5. Conclusions
The current study investigated the influence of different thermal processing methods to improve the antioxidant status of selected Brassica vegetables. The findings demonstrated that the application of air-frying improved the phenolic and flavonoid statuses and the antioxidant potential of the selected Brassica vegetables. Both kale and broccoli sprouts demonstrated the highest antioxidant activities during the air-frying treatment at 160 °C for 10 min. It was further observed that the antioxidant potential of the vegetables was improved with thermal processing. The pressurized steam treatment, which is the preferred gastronomic operation method, provided significantly (p < 0.05) lower antioxidant potentials compared to air-frying. Moreover, sautéing was the least favored thermal processing method, yielding lower phenolic and flavonoid contents and antioxidant activity. To the authors’ knowledge, this is the first study to evaluate the impact of air-frying on the phenolic and flavonoid contents and antioxidant activity of the selected Brassica oleraceae vegetables (kale, broccoli sprout, Brussels sprout, green cabbage, and red cabbage). The outcome of this study will further contribute to the food industry by introducing air-frying as an innovative and sustainable method to improve the antioxidant status of Brassica vegetables. This technique could be further applied to improve people’s nutritional statuses while creating new opportunities for producing functional vegetables.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12020490/s1, Figure S1: Total phenolic content standard curve using gallic acid solution (1 mM) as standard (GAE—gallic acid equivalents, mg—miligram, mL—mililiter, nm—nanometer, R2—coefficient of variance). Figure S2: Total flavonoid content standard curve using quercetin solution (1 mM) as standard (QE—qercetin equivalents, nm—nanometer, mg—miligram, mL—mililiter, R2—coefficient of variance). Figure S3: Standard curve for antioxidant activity by FRAP using trolox stnadard solution (1 mM). (TE—trolox equivalents, nm—nanometer, mg—miligram, mL—mililiter, R2—coefficient of variance). Figure S4: Standard curve for antioxidant activity by metal ion chelating activity using Na2EDTA stnadard solution (EDTAE—EDTA equivalents, nm—nanometer, mg—miligram, mL—mililiter, R2—coefficient of variance). Figure S5: Standard curve for antioxidant activity by ABTS activity using trolox stnadard solution (TE—trolox equivalents, nm—nanometer, mg—miligram, mL—mililiter, R2—coefficient of variance).
Author Contributions
R.N. and M.S. designed the study. R.N. and B.S. performed the experiments. R.N. interpreted the results, performed the statistical analyses, and drafted the manuscript. M.S. and C.W. proofread the manuscript. M.S. was the PI, provided financial support, directed the overall studies, and had primary responsibility for the final content. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to acknowledge the Canadian Agriculture Partnership (CAP) funding, Ag Action Manitoba Program (grant number 100227192), for funding this research project. Additionally, the CIHR training fund (grant number HTP-177412) is highly acknowledged for providing training funds for the HQPs.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be available on request.
Acknowledgments
The authors would also like to acknowledge the CIHR-CGSM funding for Breanne Semenko for her master’s study. The summer research assistant Heenal Nanda and technical support provided by Khuong Lee is highly appreciated.
Conflicts of Interest
All authors read, edited, and approved the final version of the manuscript. The authors declare that there are no conflict of interest.
References
- Nagata, N.; Xu, L.; Kohno, S.; Ushida, Y.; Aoki, Y.; Umeda, R.; Fuke, N.; Zhuge, F.; Ni, Y.; Nagashimada, M.; et al. Glucoraphanin Ameliorates Obesity and Insulin Resistance Through Adipose Tissue Browning and Reduction of Metabolic Endotoxemia in Mice. Diabetes 2017, 66, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Tanongkankit, Y.; Chiewchan, N.; Devahastin, S. Effect of Processing on Antioxidants and Their Activity in Dietary Fiber Powder from Cabbage Outer Leaves. Dry. Technol. 2010, 28, 1063–1071. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Ota, T. Glucoraphanin: A Broccoli Sprout Extract That Ameliorates Obesity-Induced Inflammation and Insulin Resistance. Adipocyte 2018, 7, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Elżbieta, S. Bodziarczyk Izabela Composition and Antioxidant Activity of Kale (Brassica oleracea L. Var. Acephala) Raw and Cooked-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/22744944/ (accessed on 12 December 2022).
- Fiol, M.; Weckmüller, A.; Neugart, S.; Schreiner, M.; Rohn, S.; Krumbein, A.; Kroh, L.W. Thermal-Induced Changes of Kale’s Antioxidant Activity Analyzed by HPLC–UV/Vis-Online-TEAC Detection. Food Chem. 2013, 138, 857–865. [Google Scholar] [CrossRef]
- OCN. Opaskwayak Health Authority 2016 Adult Health Survey; Opaskwayak Health Authority: The Pas, MB, Canada, 2017. [Google Scholar]
- Fadairo, O.; Nandasiri, R.; Alashi, A.M.; Eskin, N.A.M.; Thiyam-Höllander, U. Air Frying Pretreatment and the Recovery of Lipophilic Sinapates from the Oil Fraction of Mustard Samples. J. Food Sci. 2021, 86, 3810–3823. [Google Scholar] [CrossRef] [PubMed]
- Fadairo, O.S.; Nandasiri, R.; Nguyen, T.; Eskin, N.A.M.; Aluko, R.E.; Scanlon, M.G. Improved Extraction Efficiency and Antioxidant Activity of Defatted Canola Meal Extract Phenolic Compounds Obtained from Air-Fried Seeds. Antioxidants 2022, 11, 2411. [Google Scholar] [CrossRef]
- Lafarga, T.; Bobo, G.; Viñas, I.; Collazo, C.; Aguiló-Aguayo, I. Effects of Thermal and Non-Thermal Processing of Cruciferous Vegetables on Glucosinolates and Its Derived Forms. J. Food Sci. Technol. 2018, 55, 1973–1981. [Google Scholar] [CrossRef]
- Mwebi, N.O.; Ogendi, B.M.O. Effect of Boiling, Steaming, Stir-Frying and Microwave Cooking on the Antioxidant Potential of Peppers of Varying Pungency. Cogent Food Agric. 2020, 6, 1834661. [Google Scholar] [CrossRef]
- Roy, M.K.; Juneja, L.R.; Isobe, S.; Tsushida, T. Steam Processed Broccoli (Brassica oleracea) Has Higher Antioxidant Activity in Chemical and Cellular Assay Systems. Food Chem. 2009, 114, 263–269. [Google Scholar] [CrossRef]
- Jin, X.; Oliviero, T.; van der Sman, R.G.M.; Verkerk, R.; Dekker, M.; van Boxtel, A.J.B. Impact of Different Drying Trajectories on Degradation of Nutritional Compounds in Broccoli (Brassica oleracea Var. Italica). LWT Food Sci. Technol. 2014, 59, 189–195. [Google Scholar] [CrossRef]
- Lohachoompol, V.; Srzednicki, G.; Craske, J. The Change of Total Anthocyanins in Blueberries and Their Antioxidant Effect After Drying and Freezing. J. Biomed. Biotechnol. 2004, 2004, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, J.; Yu, D.; Chen, S.; Ye, X.; Zhang, Z. Analysis of Processing Effects on Glucosinolate Profiles in Red Cabbage by LC-MS/MS in Multiple Reaction Monitoring Mode. Molecules 2021, 26, 5171. [Google Scholar] [CrossRef] [PubMed]
- Korus, A.; Słupski, J.; Gebczyński, P.; Banaś, A. Effect of Preliminary Processing and Method of Preservation on the Content of Glucosinolates in Kale (Brassica oleracea L. Var. Acephala) Leaves. LWT 2014, 59, 1003–1008. [Google Scholar] [CrossRef]
- Nandasiri, R.; Imran, A.; Thiyam-Holländer, U.; Eskin, N.A.M. Rapidoxy® 100: A Solvent-Free Pre-Treatment for Production of Canolol. Front. Nutr. 2021, 8, 687851. [Google Scholar] [CrossRef] [PubMed]
- Nugrahedi, P.Y.; Oliviero, T.; Heising, J.K.; Dekker, M.; Verkerk, R. Stir-Frying of Chinese Cabbage and Pakchoi Retains Health-Promoting Glucosinolates. Plant Foods Hum. Nutr. 2017, 72, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, J.; Wan, J.; Pham, Q.; Zhang, Z.; Sun, J.; Yu, L.; Luo, Y.; Wang, T.T.Y.; Chen, P. Profiling of Polyphenols and Glucosinolates in Kale and Broccoli Microgreens Grown under Chamber and Windowsill Conditions by Ultrahigh-Performance Liquid Chromatography High-Resolution Mass Spectrometry. ACS Food Sci. Technol. 2022, 2, 101–113. [Google Scholar] [CrossRef]
- Liang, J.; Zago, E.; Nandasiri, R.; Khattab, R.; Eskin, N.A.M.; Eck, P.; Thiyam-Holländer, U. Effect of Solvent, Preheating Temperature, and Time on the Ultrasonic Extraction of Phenolic Compounds from Cold-Pressed Hempseed Cake. J. Am. Oil Chem. Soc. 2018, 95, 1319–1327. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Thiyam, U.; Stöckmann, H.; Felde, T.Z.; Schwarz, K. Antioxidative Effect of the Main Sinapic Acid Derivatives from Rapeseed and Mustard Oil By-Products. Eur. J. Lipid Sci. Technol. 2006, 108, 239–248. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Nandasiri, R.; Eskin, N.A.M.; Thiyam-Höllander, U. Antioxidative Polyphenols of Canola Meal Extracted by High Pressure: Impact of Temperature and Solvents. J. Food Sci. 2019, 84, 3117–3128. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Dinis, T.C.; Maderia, V.M.; Almeida, L.M. Action of Phenolic Derivatives (Acetaminophen, Salicylate, and 5-Aminosalicylate) as Inhibitors of Membrane Lipid Peroxidation and as Peroxyl Radical Scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Pallant, J. SPSS Survival Manual: A Step by Step Guide to Data Analysis Using SPSS Version 18, 4th ed.; Open University Press, McGraw-Hill Education: Maidenhead, Berkshire, UK, 2011; ISBN 978-0-33-524239-9 (pb). [Google Scholar]
- Chandrasekara, A.; Shahidi, F. Content of Insoluble Bound Phenolics in Millets and Their Contribution to Antioxidant Capacity. J. Agric. Food Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, F.A.; Hayirlioglu-Ayaz, S.; Alpay-Karaoglu, S.; Grúz, J.; Valentová, K.; Ulrichová, J.; Strnad, M. Phenolic Acid Contents of Kale (Brassica oleraceae L. Var. Acephala DC.) Extracts and Their Antioxidant and Antibacterial Activities. Food Chem. 2008, 107, 19–25. [Google Scholar] [CrossRef]
- APDS What Is Rapid Air Technology? Available online: http://apds.nl/development-en/what-is-rapid-air-technology/ (accessed on 27 January 2023).
- Nandasiri, R.; Zago, E.; Thiyam-Holländer, U.; Eskin, N.A.M. Attenuation of Sinapic Acid and Sinapine-Derived Flavor-Active Compounds Using a Factorial-Based Pressurized High-Temperature Processing. J. Am. Oil Chem. Soc. 2021, 98, 779–794. [Google Scholar] [CrossRef]
- Nandasiri, R.; Eskin, N.A.M.; Komatsu, E.; Perreault, H.; Thiyam-Holländer, U. Valorization of Canola By-Products: Concomitance of Flavor-Active Bitter Phenolics Using Pressurized Heat Treatments. LWT 2021, 138, 110397. [Google Scholar] [CrossRef]
- Paciulli, M.; Dall’Asta, C.; Rinaldi, M.; Pellegrini, N.; Pugliese, A.; Chiavaro, E. Application and Optimisation of Air–Steam Cooking on Selected Vegetables: Impact on Physical and Antioxidant Properties. J. Sci. Food Agric. 2018, 98, 2267–2276. [Google Scholar] [CrossRef]
- Lafarga, T.; Bobo, G.; Viñas, I.; Zudaire, L.; Simó, J.; Aguiló-Aguayo, I. Steaming and Sous-Vide: Effects on Antioxidant Activity, Vitamin C, and Total Phenolic Content of Brassica Vegetables. Int. J. Gastron. Food Sci. 2018, 13, 134–139. [Google Scholar] [CrossRef]
- Cieślik, E.; Leszczyńska, T.; Filipiak-Florkiewicz, A.; Sikora, E.; Pisulewski, P.M. Effects of Some Technological Processes on Glucosinolate Contents in Cruciferous Vegetables. Food Chem. 2007, 105, 976–981. [Google Scholar] [CrossRef]
- Kwon, O.; Eck, P.; Chen, S.; Corpe, C.P.; Lee, J.-H.; Kruhlak, M.; Levine, M. Inhibition of the Intestinal Glucose Transporter GLUT2 by Flavonoids. FASEB J. 2007, 21, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-Antioxidant Activity Relationships of Flavonoids and Phenolic Acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Um, M.; Han, T.H.; Lee, J.W. Ultrasound-Assisted Extraction and Antioxidant Activity of Phenolic and Flavonoid Compounds and Ascorbic Acid from Rugosa Rose (Rosa Rugosa Thunb.) Fruit. Food Sci. Biotechnol. 2018, 27, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of Ultrasound-Assisted Extraction of Phenolic Compounds: Oleuropein, Phenolic Acids, Phenolic Alcohols and Flavonoids from Olive Leaves and Evaluation of Its Antioxidant Activities. Ind. Crops Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Identification of the Phenolic Components of Collard Greens, Kale, and Chinese Broccoli. J. Agric. Food Chem. 2009, 57, 7401–7408. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Ferreira, O.; Pinho, P. Solubility of Flavonoids in Pure Solvents. Ind. Eng. Chem. Res. 2012, 51, 6586–6590. [Google Scholar] [CrossRef]
- Lemańska, K.; Szymusiak, H.; Tyrakowska, B.; Zieliński, R.; Soffers, A.E.; Rietjens, I.M. The Influence of PH on Antioxidant Properties and the Mechanism of Antioxidant Action of Hydroxyflavones. Free Radic. Biol. Med. 2001, 31, 869–881. [Google Scholar] [CrossRef]
- Li, J.; Guo, Z. Concurrent Extraction and Transformation of Bioactive Phenolic Compounds from Rapeseed Meal Using Pressurized Solvent Extraction System. Ind. Crops Prod. 2016, 94, 152–159. [Google Scholar] [CrossRef]
- Zago, E.; Nandasiri, R.; Thiyam-Holländer, U.; Michael Eskin, N.A. Influence of Thermal Treatments on the Antioxidant Activity of Hemp Cake Polar Extracts. J. Food Sci. Technol. 2022, 59, 3256–3265. [Google Scholar] [CrossRef]
- Tavasi, V.; Bettens, R.P.A.; Leong, L.P. Temperature and Solvent Effects on Radical Scavenging Ability of Phenols. J. Phys. Chem. A 2009, 113, 3068–3077. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Ji, H.F. How Vitamin E Scavenges DPPH Radicals in Polar Protic Media. New J. Chem. 2006, 30, 503–504. [Google Scholar] [CrossRef]
- Friaa, O.; Brault, D. Kinetics of the Reaction between the Antioxidant Trolox® and the Free Radical DPPH in Semi-Aqueous Solution. Org. Biomol. Chem. 2006, 4, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The Effect of Cooking Methods on Total Phenolics and Antioxidant Activity of Selected Green Vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- López-Berenguer, C.; Carvajal, M.; Moreno, D.A.; García-Viguera, C. Effects of Microwave Cooking Conditions on Bioactive Compounds Present in Broccoli Inflorescences. J. Agric. Food Chem. 2007, 55, 10001–10007. [Google Scholar] [CrossRef] [PubMed]
- Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Godbout, S.; Valéro, J.R. Extraction and Analysis of Polyphenols: Recent Trends. Crit. Rev. Biotechnol. 2011, 31, 227–249. [Google Scholar] [CrossRef]
- Francisco, M.; Velasco, P.; Moreno, D.A.; García-Viguera, C.; Cartea, M.E. Cooking Methods of Brassica Rapa Affect the Preservation of Glucosinolates, Phenolics and Vitamin C. Food Res. Int. 2010, 43, 1455–1463. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, A.; Rai, P.K.; Kumar, D. Effect of Microwave Pre-Treatment on Quality Parameters in Indian Mustard. J. Food Sci. Technol. 2019, 56, 4956–4965. [Google Scholar] [CrossRef]
- Gaspar, A.; Garrido, E.M.; Rio Esteves, M.; Quezada, E.; Milhazes, N.; Garrido, J.; Borges, F. New Insights into the Antioxidant Activity of Hydroxycinnamic Acids: Synthesis and Physicochemical Characterization of Novel Halogenated Derivatives. Eur. J. Med. Chem. 2008, 44, 2092–2099. [Google Scholar] [CrossRef]
- Teh, S.S.; Birch, E.J. Effect of Ultrasonic Treatment on the Polyphenol Content and Antioxidant Capacity of Extract from Defatted Hemp, Flax and Canola Seed Cakes. Ultrason. Sonochemistry 2014, 21, 346–353. [Google Scholar] [CrossRef]
- Mladěnka, P.; MacÁková, K.; Filipský, T.; Zatloukalová, L.; Jahodář, L.; Bovicelli, P.; Silvestri, I.P.; Hrdina, R.; Saso, L. In Vitro Analysis of Iron Chelating Activity of Flavonoids. J. Inorg. Biochem. 2011, 105, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, J.; Saura-Calixto, F. Effect of Solvent and Certain Food Constituents on Different Antioxidant Capacity Assays. Food Res. Int. 2006, 39, 791–800. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).