Blood Leukocyte ROS Production Reflects Seminal Fluid Oxidative Stress and Spermatozoa Dysfunction in Idiopathic Infertile Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Seminal Fluid and Blood Collection
2.3. Intracellular ROS Levels Assessment in Blood Leukocytes and Spermatozoa by Flow Cytometry Analysis
2.4. Lipid Peroxidation Estimation in Blood and Seminal Plasma
2.5. Total Antioxidant Capacity (TAC) Estimation in Blood and Seminal Plasma
2.6. Statistical Analysis
3. Results
3.1. Subjects
3.2. Assessment of Oxidative Stress in Blood and Seminal Fluid
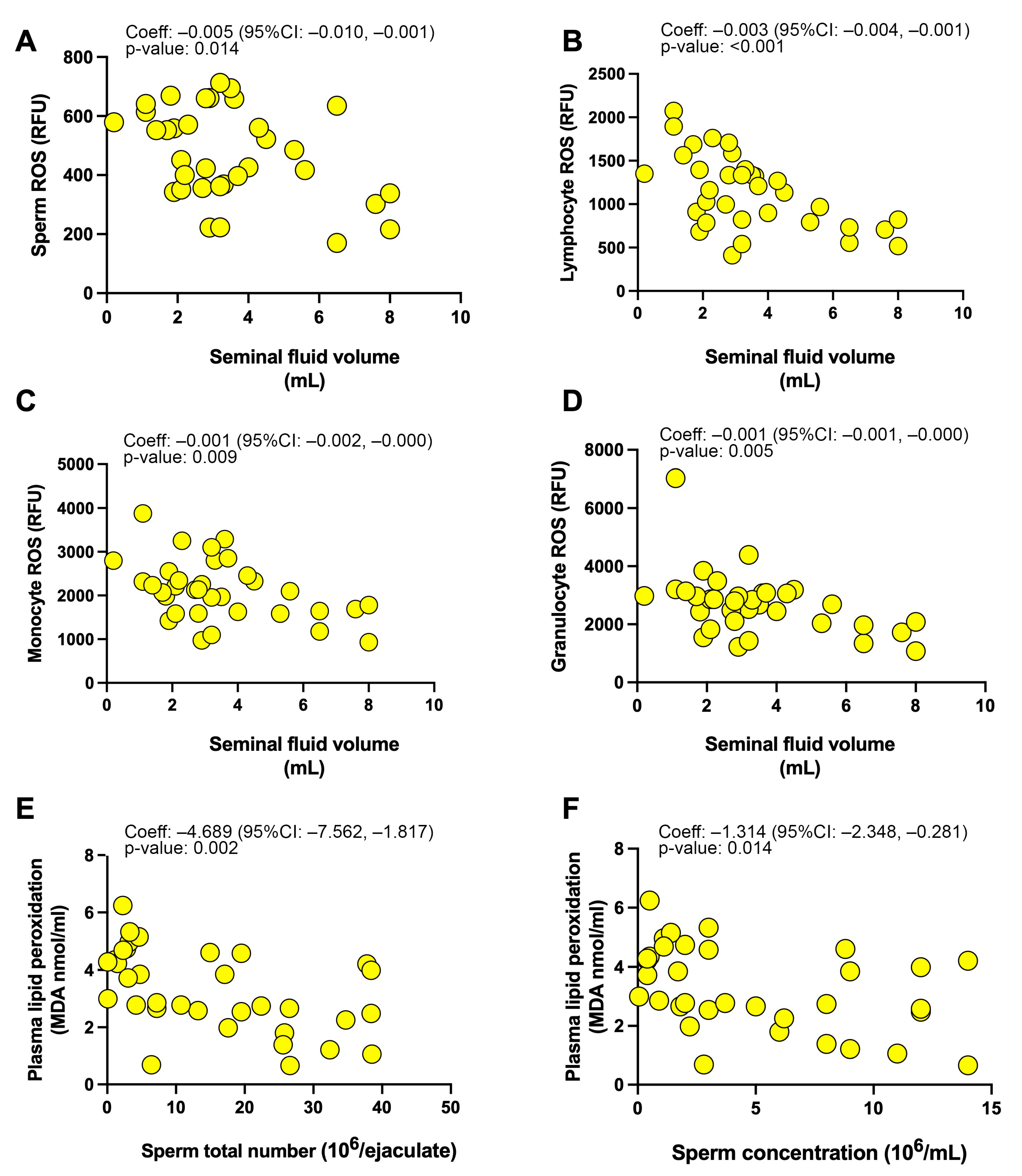
3.3. Associations between Investigated Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Mbizvo, M.; Festin, M.P.; Björndahl, L.; Toskin, I.; other Editorial Board Members of the WHO Laboratory Manual for the Examination and Processing of Human Semen. Evolution of the WHO “Semen” processing manual from the first (1980) to the sixth edition (2021). Fertil. Steril. 2022, 117, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care. Hum. Reprod. 2017, 32, 1786–1801. [Google Scholar] [CrossRef]
- Pereira, S.C.; Moreira, M.V.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Roles of Oxidative Stress in the Male Reproductive System: Potential of Antioxidant Supplementation for Infertility Treatment. Adv. Exp. Med. Biol. 2022, 1391, 259–274. [Google Scholar] [PubMed]
- Saleh, R.A.; Agarwal, A. Oxidative stress and male infertility: From research bench to clinical practice. J. Androl. 2002, 23, 737–752. [Google Scholar]
- Makker, K.; Agarwal, A.; Sharma, R. Oxidative stress & male infertility. Indian J. Med. Res. 2009, 129, 357–367. [Google Scholar]
- Agarwal, A.; Rana, M.; Qiu, E.; AlBunni, H.; Bui, A.D.; Henkel, R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia 2018, 50, e13126. [Google Scholar] [CrossRef] [PubMed]
- Cito, G.; Becatti, M.; Natali, A.; Fucci, R.; Picone, R.; Cocci, A.; Falcone, P.; Criscuoli, L.; Mannucci, A.; Argento, F.R.; et al. Redox status assessment in infertile patients with non-obstructive azoospermia undergoing testicular sperm extraction: A prospective study. Andrology 2020, 8, 364–371. [Google Scholar] [CrossRef]
- Zini, A.; Garrels, K.; Phang, D. Antioxidant activity in the semen of fertile and infertile men. Urology 2000, 55, 922–929. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.K.; Nallella, K.P.; Thomas, A.J.; Alvarez, J.G.; Sikka, S.C. Reactive oxygen species as an independent marker of male factor infertility. Fertil. Steril. 2006, 86, 878–885. [Google Scholar] [CrossRef]
- Khosrowbeygi, A.; Zarghami, N. Levels of oxidative stress biomarkers in seminal plasma and their relationship with seminal parameters. BMC Clin. Pathol. 2007, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Aitken, J.; Fisher, H. Reactive oxygen species generation and human spermatozoa: The balance of benefit and risk. Bioessays 1994, 16, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, E.; Kokot, I.; Kmieciak, A.; Gilowska, I.; Faundez, R.; Kratz, E.M. Are There Associations between Seminal Plasma Advanced Oxidation Protein Products and Selected Redox-Associated Biochemical Parameters in Infertile Male Patients? A Preliminary Report. Cells 2022, 11, 3667. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, S.S.; Agarwal, A.; Halabi, J.; Tvrda, E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015, 32, 509–520. [Google Scholar] [CrossRef]
- Kothari, S.; Thompson, A.; Agarwal, A.; du Plessis, S.S. Free radicals: Their beneficial and detrimental effects on sperm function. Indian J. Exp. Biol. 2010, 48, 425–435. [Google Scholar]
- Gosalvez, J.; Tvrda, E.; Agarwal, A. Free radical and superoxide reactivity detection in semen quality assessment: Past, present, and future. J. Assist. Reprod. Genet. 2017, 34, 697–707. [Google Scholar] [CrossRef]
- Iommiello, V.M.; Albani, E.; Di Rosa, A.; Marras, A.; Menduni, F.; Morreale, G.; Levi, S.L.; Pisano, B.; Levi-Setti, P.E. Ejaculate oxidative stress is related with sperm DNA fragmentation and round cells. Int. J. Endocrinol. 2015, 2015, 321901. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Sharma, R.; Sikka, S.; Agarwal, A. Diagnostic application of total antioxidant capacity in seminal plasma to assess oxidative stress in male factor infertility. J. Assist. Reprod. Genet. 2016, 33, 627–635. [Google Scholar] [CrossRef]
- Sharma, R.K.; Pasqualotto, F.F.; Nelson, D.R.; Thomas, A.J.; Agarwal, A. The reactive oxygen species-total antioxidant capacity score is a new measure of oxidative stress to predict male infertility. Hum. Reprod. 1999, 14, 2801–2807. [Google Scholar] [CrossRef]
- Agarwal, A.; Roychoudhury, S.; Sharma, R.; Gupta, S.; Majzoub, A.; Sabanegh, E. Diagnostic application of oxidation-reduction potential assay for measurement of oxidative stress: Clinical utility in male factor infertility. Reprod. Biomed. Online 2017, 34, 48–57. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S.; Fishel, S. Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol. Reprod. 1989, 41, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Saleh, R.A.; Bedaiwy, M.A. Role of reactive oxygen species in the pathophysiology of human reproduction. Fertil. Steril. 2003, 79, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Flesch, F.M.; Gadella, B.M. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochim. Biophys. Acta 2000, 1469, 197–235. [Google Scholar] [CrossRef]
- Shiva, M.; Gautam, A.K.; Verma, Y.; Shivgotra, V.; Doshi, H.; Kumar, S. Association between sperm quality, oxidative stress, and seminal antioxidant activity. Clin. Biochem. 2011, 44, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.K.; Bilaspuri, G.S. Impacts of oxidative stress and antioxidants on semen functions. Vet. Med. Int. 2010, 2010, 686137. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Baker, M.A. Oxidative stress, sperm survival and fertility control. Mol. Cell Endocrinol. 2006, 250, 66–69. [Google Scholar] [CrossRef]
- Venkatesh, S.; Shamsi, M.B.; Deka, D.; Saxena, V.; Kumar, R.; Dada, R. Clinical implications of oxidative stress & sperm DNA damage in normozoospermic infertile men. Indian J. Med. Res. 2011, 134, 396–398. [Google Scholar]
- Agarwal, A.; Virk, G.; Ong, C.; du Plessis, S.S. Effect of oxidative stress on male reproduction. World J. Men’s Health 2014, 32, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Bui, A.D.; Sharma, R.; Henkel, R.; Agarwal, A. Reactive oxygen species impact on sperm DNA and its role in male infertility. Andrologia 2018, 50, e13012. [Google Scholar] [CrossRef]
- Kamkar, N.; Ramezanali, F.; Sabbaghian, M. The relationship between sperm DNA fragmentation, free radicals and antioxidant capacity with idiopathic repeated pregnancy loss. Reprod. Biol. 2018, 18, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Makker, K.; Sharma, R. Clinical relevance of oxidative stress in male factor infertility: An update. Am. J. Reprod. Immunol. 2008, 59, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Vatannejad, A.; Tavilani, H.; Sadeghi, M.R.; Amanpour, S.; Shapourizadeh, S.; Doosti, M. Evaluation of ROS-TAC Score and DNA Damage in Fertile Normozoospermic and Infertile Asthenozoospermic Males. Urol. J. 2017, 14, 2973–2978. [Google Scholar] [PubMed]
- Becatti, M.; Fucci, R.; Mannucci, A.; Barygina, V.; Mugnaini, M.; Criscuoli, L.; Giachini, C.; Bertocci, F.; Picone, R.; Emmi, G.; et al. A Biochemical Approach to Detect Oxidative Stress in Infertile Women Undergoing Assisted Reproductive Technology Procedures. Int. J. Mol. Sci. 2018, 19, 592. [Google Scholar] [CrossRef]
- Sofi, F.; Dinu, M.; Pagliai, G.; Cesari, F.; Gori, A.M.; Sereni, A.; Becatti, M.; Fiorillo, C.; Marcucci, R.; Casini, A. Low-Calorie Vegetarian Versus Mediterranean Diets for Reducing Body Weight and Improving Cardiovascular Risk Profile: CARDIVEG Study (Cardiovascular Prevention With Vegetarian Diet). Circulation 2018, 137, 1103–1113. [Google Scholar] [CrossRef]
- Becatti, M.; Emmi, G.; Silvestri, E.; Bruschi, G.; Ciucciarelli, L.; Squatrito, D.; Vaglio, A.; Taddei, N.; Abbate, R.; Emmi, L.; et al. Neutrophil Activation Promotes Fibrinogen Oxidation and Thrombus Formation in Behçet Disease. Circulation 2016, 133, 302–311. [Google Scholar] [CrossRef]
- Becatti, M.; Mannucci, A.; Barygina, V.; Mascherini, G.; Emmi, G.; Silvestri, E.; Wright, D.; Taddei, N.; Galanti, G.; Fiorillo, C. Redox status alterations during the competitive season in élite soccer players: Focus on peripheral leukocyte-derived ROS. Intern. Emerg. Med. 2017, 12, 777–788. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Eggert-Kruse, W.; Hofsäss, A.; Haury, E.; Tilgen, W.; Gerhard, I.; Runnebaum, B. Relationship between local anti-sperm antibodies and sperm-mucus interaction in vitro and in vivo. Hum. Reprod. 1991, 6, 267–276. [Google Scholar] [CrossRef]
- Fiorillo, C.; Becatti, M.; Attanasio, M.; Lucarini, L.; Nassi, N.; Evangelisti, L.; Porciani, M.C.; Nassi, P.; Gensini, G.F.; Abbate, R.; et al. Evidence for oxidative stress in plasma of patients with Marfan syndrome. Int. J. Cardiol. 2010, 145, 544–546. [Google Scholar] [CrossRef]
- Whittaker, A.; Sofi, F.; Luisi, M.L.; Rafanelli, E.; Fiorillo, C.; Becatti, M.; Abbate, R.; Casini, A.; Gensini, G.F.; Benedettelli, S. An organic khorasan wheat-based replacement diet improves risk profile of patients with acute coronary syndrome: A randomized crossover trial. Nutrients 2015, 7, 3401–3415. [Google Scholar] [CrossRef]
- Whittaker, A.; Dinu, M.; Cesari, F.; Gori, A.M.; Fiorillo, C.; Becatti, M.; Casini, A.; Marcucci, R.; Benedettelli, S.; Sofi, F. A khorasan wheat-based replacement diet improves risk profile of patients with type 2 diabetes mellitus (T2DM): A randomized crossover trial. Eur. J. Nutr. 2017, 56, 1191–1200. [Google Scholar] [CrossRef]
- Shamsi, M.B.; Venkatesh, S.; Kumar, R.; Gupta, N.P.; Malhotra, N.; Singh, N.; Mittal, S.; Arora, S.; Arya, D.S.; Talwar, P.; et al. Antioxidant levels in blood and seminal plasma and their impact on sperm parameters in infertile men. Indian J. Biochem. Biophys. 2010, 47, 38–43. [Google Scholar]
- Benedetti, S.; Tagliamonte, M.C.; Catalani, S.; Primiterra, M.; Canestrari, F.; De Stefani, S.; Palini, S.; Bulletti, C. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod. Biomed. Online 2012, 25, 300–306. [Google Scholar] [CrossRef]
- Bromfield, J.J. Seminal fluid and reproduction: Much more than previously thought. J. Assist. Reprod. Genet. 2014, 31, 627–636. [Google Scholar] [CrossRef]
- Mannucci, A.; Argento, F.R.; Fini, E.; Coccia, M.E.; Taddei, N.; Becatti, M.; Fiorillo, C. The Impact of Oxidative Stress in Male Infertility. Front. Mol. Biosci. 2022, 8, 799294. [Google Scholar] [CrossRef]
- Majzoub, A.; Agarwal, A. Systematic review of antioxidant types and doses in male infertility: Benefits on semen parameters, advanced sperm function, assisted reproduction and live-birth rate. Arab. J. Urol. 2018, 16, 113–124. [Google Scholar] [CrossRef]
- Sabeti, P.; Pourmasumi, S.; Rahiminia, T.; Akyash, F.; Talebi, A.R. Etiologies of sperm oxidative stress. Int. J. Reprod. Biomed. 2016, 14, 231–240. [Google Scholar] [CrossRef]
- Barati, E.; Nikzad, H.; Karimian, M. Oxidative stress and male infertility: Current knowledge of pathophysiology and role of antioxidant therapy in disease management. Cell. Mol. Life Sci. 2020, 77, 93–113. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, R.K.; Sharma, R.; Assidi, M.; Abuzenadah, A.M.; Alshahrani, S.; Durairajanayagam, D.; Sabanegh, E. Characterizing semen parameters and their association with reactive oxygen species in infertile men. Reprod. Biol. Endocrinol. 2014, 12, 33. [Google Scholar] [CrossRef]
- Madhu, N.R.; Sarkar, B.; Slama, P.; Jha, N.K.; Ghorai, S.K.; Jana, S.K.; Govindasamy, K.; Massanyi, P.; Lukac, N.; Kumar, D.; et al. Effect of Environmental Stressors, Xenobiotics, and Oxidative Stress on Male Reproductive and Sexual Health. Adv. Exp. Med. Biol. 2022, 1391, 33–58. [Google Scholar]
- Whittington, K.; Harrison, S.C.; Williams, K.M.; Day, J.L.; McLaughlin, E.A.; Hull, M.G.; Ford, W.C. Reactive oxygen species (ROS) production and the outcome of diagnostic tests of sperm function. Int. J. Androl. 1999, 22, 236–242. [Google Scholar] [CrossRef]
- Hosseinzadeh Colagar, A.; Karimi, F.; Jorsaraei, S.G. Correlation of sperm parameters with semen lipid peroxidation and total antioxidants levels in astheno- and oligoasheno- teratospermic men. Iran. Red Crescent Med. J. 2013, 15, 780–785. [Google Scholar] [CrossRef]
- Bonanno, O.; Romeo, G.; Asero, P.; Pezzino, F.M.; Castiglione, R.; Burrello, N.; Sidoti, G.; Frajese, G.V.; Vicari, E.; D’Agata, R. Sperm of patients with severe asthenozoospermia show biochemical, molecular and genomic alterations. Reproduction 2016, 152, 695–704. [Google Scholar] [CrossRef]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Dorostghoal, M.; Kazeminejad, S.R.; Shahbazian, N.; Pourmehdi, M.; Jabbari, A. Oxidative stress status and sperm DNA fragmentation in fertile and infertile men. Andrologia 2017, 49. [Google Scholar] [CrossRef]
- Aitken, R.J.; Smith, T.B.; Lord, T.; Kuczera, L.; Koppers, A.J.; Naumovski, N.; Connaughton, H.; Baker, M.A.; De Iuliis, G.N. On methods for the detection of reactive oxygen species generation by human spermatozoa: Analysis of the cellular responses to catechol oestrogen, lipid aldehyde, menadione and arachidonic acid. Andrology 2013, 1, 192–205. [Google Scholar] [CrossRef]
- Singh, F.; Charles, A.L.; Schlagowski, A.I.; Bouitbir, J.; Bonifacio, A.; Piquard, F.; Krähenbühl, S.; Geny, B.; Zoll, J. Reductive stress impairs myoblasts mitochondrial function and triggers mitochondrial hormesis. Biochim. Biophys. Acta 2015, 1853, 1574–1585. [Google Scholar] [CrossRef] [PubMed]
- Mentor, S.; Fisher, D. Aggressive Antioxidant Reductive Stress Impairs Brain Endothelial Cell Angiogenesis and Blood Brain Barrier Function. Curr. Neurovasc. Res. 2017, 14, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Lamosová, D.; Juráni, M.; Greksák, M.; Nakano, M.; Vaneková, M. Effect of Rooibos tea (Aspalathus linearis) on chick skeletal muscle cell growth in culture. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1997, 116, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Guz, J.; Gackowski, D.; Foksinski, M.; Rozalski, R.; Zarakowska, E.; Siomek, A.; Szpila, A.; Kotzbach, M.; Kotzbach, R.; Olinski, R. Comparison of oxidative stress/DNA damage in semen and blood of fertile and infertile men. PLoS ONE 2013, 8, e68490. [Google Scholar] [CrossRef]
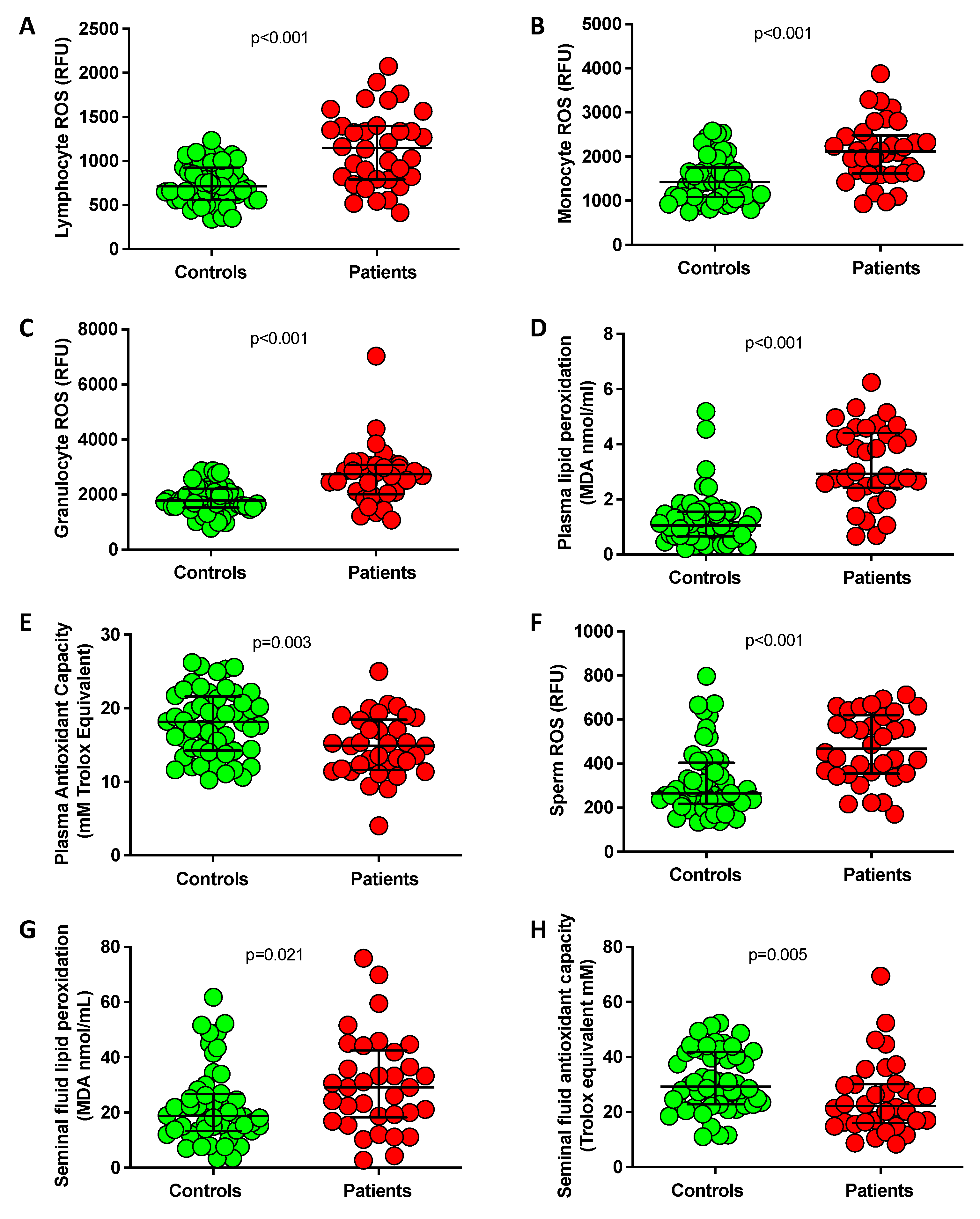
| Parameter | Controls (n = 52) | Idiopathic Infertile Men (n = 34) | p-Value |
|---|---|---|---|
| Age (years), median (IQR) | 39 (36–42) | 40 (38–45) | Matching variable |
| BMI, median (IQR) | 24 (22–26) | 23 (22–24) | 0.059 |
| Smoke, n (%) | 12 (23.1) | 9 (26.5) | 0.799 |
| Parameter | Controls (n = 52) Median (IQR) | Idiopathic Infertile Males (n = 34) Median (IQR) | p-Value |
|---|---|---|---|
| Semen volume (mL) | 3.5 (2.3–4.8) | 3.1 (2.1–4.3) | 0.344 |
| Sperm concentration (106/mL) | 47.0 (30.0–81.0) | 3.0 (1.1–8.8) | <0.001 |
| Sperm total number (106/ejaculate) | 169.5 (111.0–243.25) | 12.0 (3.2–25.8) | <0.001 |
| Progressive motility, PR, (%) | 52.5 (40.0–60.0) | 30.0 (15.0–40.0) | <0.001 |
| Non-progressive motility, NP (%) | 5.0 (5.0–10.0) | 10.0 (5.0–20.0) | 0.016 |
| Immotile sperm, IM (%) | 35.0 (30.0–50.0) | 50.0 (45.0–75.0) | <0.001 |
| Normal sperm morphology (%) | 6.0 (5.0–6.0) | 4.0 (3.0–5.0) | <0.001 |
| Semen Volume | Sperm Total Number | Sperm Concentration | Progressive Sperm Motility | |
|---|---|---|---|---|
| (a) Seminal redox status | ||||
| Sperm ROS production (RFU) | F(1, 32): 6.73 Coef: −0.005 (−0.010, −0.001) p-value: 0.014 * | F(1, 32): 0.00 Coef: +0.000 (−0.030, +0.031) p-value: 0.980 | F(1, 32): 0.28 Coef: +0.003 (−0.008, +0.013) p-value: 0.598 | F(1, 32): 0.00 Coef: −0.001 (−0.045, +0.043) p-value: 0.973 |
| Seminal plasma lipid peroxidation (MDA nmol/mL) | F(1, 32): 1.59 Coef: −0.025 (−0.066, +0.015) p-value: 0.216 | F(1, 32): 1.48 Coef: −0.159 (−0.426, +0.107) p-value: 0.232 | F(1, 32): 0.08 Coef: −0.013 (−0.106, +0.080) p-value: 0.777 | F(1, 32): 0.18 Coef: +0.080 (−0.309, +0.470) p-value: 0.677 |
| Seminal plasma antioxidant capacity (mM Trolox eq.) | F(1, 32): 1.45 Coef: −0.031 (−0.085, +0.022) p-value: 0.237 | F(1, 32): 2.50 Coef: −0.267 (−0.611, +0.077) p-value: 0.124 | F(1, 32): 1.10 Coef: −0.062 (−0.181, +0.058) p-value: 0.303 | F(1, 32): 0.01 Coef: +0.027 (−0.484, +0.539) p-value: 0.914 |
| (b) Systemic redox status | ||||
| Lymphocyte ROS (RFU) | F(1, 32): 15.52 Coef: −0.003 (−0.004, −0.001) p-value: <0.001 * | F(1, 32): 1.15 Coef: −0.006 (−0.017, +0.005) p-value: 0.292 | F(1, 32): 0.28 Coef: +0.001 (−0.003, +0.005) p-value: 0.598 | F(1, 32): 0.02 Coef: −0.001 (−0.017, +0.015) p-value: 0.901 |
| Monocyte ROS (RFU) | F(1, 32): 7.72 Coef: −0.001 (−0.002, −0.000) p-value: 0.009 * | F(1, 32): 0.09 Coef: −0.001 (−0.008, +0.006) p-value: 0.762 | F(1, 32): 0.65 Coef: +0.001 (+0.001, +0.003) p-value: 0.425 | F(1, 32): 0.90 Coef: −0.005 (−0.014, +0.005) p-value: 0.349 |
| Granulocyte ROS (RFU) | F(1, 32): 8.99 Coef: −0.001 (−0.001, −0.000) p-value: 0.005 * | F(1, 32): 0.02 Coef: −0.000 (−0.005, +0.004) p-value: 0.900 | F(1, 32): 2.44 Coef: −0.001 (−0.000, +0.003) p-value: 0.128 | F(1, 32): 0.67 Coef: −0.003 (−0.009, +0.004) p-value: 0.419 |
| Plasma lipid peroxidation (MDA nmol/mL) | F(1, 32): 0.85 Coef:−0.226 (−0.727, +0.275) p-value: 0.364 | F(1, 32): 11.06 Coef:−4.689(−7.562, −1.817) p-value: 0.002 * | F(1, 32): 6.71 Coef: −1.314 (−2.348, −0.281) p-value: 0.014 * | F(1, 32): 1.55 Coef:−2.853 (−7.514, +1.808) p-value: 0.222 |
| Plasma antioxidant capacity (mM Trolox eq.) | F(1, 32): 0.03 Coef: +0.015 (−0.157, +0.188) p-value: 0.857 | F(1, 32): 2.72 Coef: −0.883 (−1.973, +0.208) p-value: 0.109 | F(1, 32): 2.81 Coef: −0.306 (−0.678, + 0.066) p-value: 0.103 | F(1, 32): 0.36 Coef: −0.478 (−2.097, + 1.140 p-value: 0.551 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becatti, M.; Cito, G.; Argento, F.R.; Fini, E.; Bettiol, A.; Borghi, S.; Mannucci, A.; Fucci, R.; Giachini, C.; Picone, R.; et al. Blood Leukocyte ROS Production Reflects Seminal Fluid Oxidative Stress and Spermatozoa Dysfunction in Idiopathic Infertile Men. Antioxidants 2023, 12, 479. https://doi.org/10.3390/antiox12020479
Becatti M, Cito G, Argento FR, Fini E, Bettiol A, Borghi S, Mannucci A, Fucci R, Giachini C, Picone R, et al. Blood Leukocyte ROS Production Reflects Seminal Fluid Oxidative Stress and Spermatozoa Dysfunction in Idiopathic Infertile Men. Antioxidants. 2023; 12(2):479. https://doi.org/10.3390/antiox12020479
Chicago/Turabian StyleBecatti, Matteo, Gianmartin Cito, Flavia Rita Argento, Eleonora Fini, Alessandra Bettiol, Serena Borghi, Amanda Mannucci, Rossella Fucci, Claudia Giachini, Rita Picone, and et al. 2023. "Blood Leukocyte ROS Production Reflects Seminal Fluid Oxidative Stress and Spermatozoa Dysfunction in Idiopathic Infertile Men" Antioxidants 12, no. 2: 479. https://doi.org/10.3390/antiox12020479
APA StyleBecatti, M., Cito, G., Argento, F. R., Fini, E., Bettiol, A., Borghi, S., Mannucci, A., Fucci, R., Giachini, C., Picone, R., Emmi, G., Taddei, N., Coccia, M. E., & Fiorillo, C. (2023). Blood Leukocyte ROS Production Reflects Seminal Fluid Oxidative Stress and Spermatozoa Dysfunction in Idiopathic Infertile Men. Antioxidants, 12(2), 479. https://doi.org/10.3390/antiox12020479









