Effects of Microalgae on Metabolic Syndrome
Abstract
1. Introduction
2. Effect of Microalgae on MetS
2.1. In Vitro Study
Effects of Oxohexadecenoic Acids Derived from Chaetoceros karianus on MetS
2.2. In Vivo Studies
2.2.1. Tetraselmis chuii
2.2.2. Arthrospira platensis (spirulina, Sp)
2.2.3. Diacronema lutheri
2.2.4. Nannochloropsis oceanica
2.2.5. Tisochrysis lutea
2.2.6. DHA-Rich Microalgae Mixture
2.2.7. Phaeodactylum tricornutum
2.2.8. Coccomyxa gloeobotrydiformis
3. Limitation
4. Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.H.; Lee, D.K.; Liu, M.; Portincasa, P.; Wang, D.Q.H. Novel insights into the pathogenesis and management of the metabolic syndrome. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 189–230. [Google Scholar] [CrossRef]
- Ghee, L.K.; Kooi, C.W. A Review of Metabolic Syndrome Research in Malaysia. Med. J. Malays. 2016, 71, 19–27. [Google Scholar]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Manaf, M.R.A.; Nawi, A.M.; Tauhid, N.M.; Othman, H.; Rahman, M.R.A.; Yusoff, H.M.; Safian, N.; Ng, P.Y.; Manaf, Z.A.; Kadir, N.B.A.; et al. Prevalence of metabolic syndrome and its associated risk factors among staffs in a Malaysian public university. Sci. Rep. 2021, 11, 8132. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K. A Review of Current Evidence on the Relationship between Phosphate Metabolism and Metabolic Syndrome. Nutrients 2022, 14, 4525. [Google Scholar] [CrossRef]
- Mayer, C.; Richard, L.; Côme, M.; Ulmann, L.; Nazih, H.; Chénais, B.; Ouguerram, K.; Mimouni, V. The marine microalga, tisochrysis lutea, protects against metabolic disorders associated with metabolic syndrome and obesity. Nutrients 2021, 13, 430. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Ramli, F.F.; Ali, A.; Ibrahim, N.I. Genetics of Cholesterol-Related Genes in Metabolic Syndrome: A Review of Current Evidence. Biomedicines 2022, 10, 3239. [Google Scholar] [CrossRef]
- di Marzo, V.; Silvestri, C. Lifestyle and metabolic syndrome: Contribution of the endocannabinoidome. Nutrients 2019, 11, 1956. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Tuomilehto, J.; Rydén, L. The metabolic syndrome–What is it and how should it be managed? Eur. J. Prev. Cardiol. 2019, 26, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Côme, M.; Ulmann, L.; Zittelli, G.C.; Faraloni, C.; Nazih, H.; Ouguerram, K.; Chénais, B.; Mimouni, V. Preventive effects of the marine microalga phaeodactylum tricornutum, used as a food supplement, on risk factors associated with metabolic syndrome in wistar rats. Nutrients 2019, 11, 1069. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Li, X.; Adams, H.; Kubena, K.; Guo, S. Etiology of metabolic syndrome and dietary intervention. Int. J. Mol. Sci. 2018, 20, 128. [Google Scholar] [CrossRef] [PubMed]
- Fatourou, E.M.; Tsochatzis, E.A. Management of metabolic syndrome and cardiovascular risk post-liver transplantation. Lancet Gastroenterol. Hepatol. 2019, 4, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Díaz, L.A.; Ruiz-Pacheco, J.A.; Elsawy, M.A.; Reyes-Martínez, J.E.; Enríquez-Rodríguez, A.I. Self-assembling peptides as an emerging platform for the treatment of metabolic syndrome. Int. J. Nanomed. 2020, 15, 10349–10370. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, Y.D.; Hajialyani, M.; Naseri, R.; Hesari, M.; Mohammadi, P.; Stefanucci, A.; Mollica, A.; Farzaei, M.H.; Abdollahi, M. Nanoformulations of natural products for management of metabolic syndrome. Int. J. Nanomed. 2019, 14, 5303–5321. [Google Scholar] [CrossRef] [PubMed]
- Laamanen, C.A.; Desjardins, S.M.; Senhorinho, G.N.A.; Scott, J.A. Harvesting microalgae for health beneficial dietary supplements. Algal Res. 2021, 54, 102189. [Google Scholar] [CrossRef]
- Vale, M.A.; Ferreira, A.; Pires, J.C.M.; Gonçalves, G.A.L. CO2 capture using microalgae. In Advances in Carbon Capture: Methods, Technologies and Applications; Woodhead Publishing: Sawston, UK, 2020; pp. 381–405. [Google Scholar]
- Eltanahy, E.; Torky, A. Microalgae as Cell Factories: Food and Feed-grade High-value Metabolites. In Microalgal Biotechnology: Recent Advances, Market Potential, and Sustainability; Shekh, A., Schenk, P., Sarada, R., Eds.; The Royal Society of Chemistry: London, UK, 2021. [Google Scholar]
- de Oliveira, A.P.F.; Bragotto, A.P.A. Microalgae-Based Products: Food and Public Health. Future Foods 2022, 6, 100157. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Nethravathy, M.U.; Mehar, J.G.; Mudliar, S.N.; Shekh, A.Y. Recent Advances in Microalgal Bioactives for Food, Feed, and Healthcare Products: Commercial Potential, Market Space, and Sustainability. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1882–1897. [Google Scholar] [CrossRef]
- Mourelle, M.L.; Gómez, C.P.; Legido, J.L. Cosmetics the Potential Use of Marine Microalgae and Cyanobacteria in Cosmetics and Thalassotherapy. Cosmetics 2017, 4, 46. [Google Scholar] [CrossRef]
- Yarkent, Ç.; Gürlek, C.; Oncel, S.S. Potential of microalgal compounds in trending natural cosmetics: A review. Sustain. Chem. Pharm. 2020, 17, 100304. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible Microalgae and Their Bioactive Compounds in the Prevention and Treatment of Metabolic Alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Castro-Barquero, S.; Ruiz-León, A.M.; Sierra-Pérez, M.; Estruch, R.; Casas, R. Dietary Strategies for Metabolic Syndrome: A Comprehensive Review. Nutrients 2020, 12, 2983. [Google Scholar] [CrossRef]
- Lim, Y.C.; Hoe, V.C.W.; Darus, A.; Bhoo-Pathy, N. Association between Night-Shift Work, Sleep Quality and Metabolic Syndrome. Occup. Environ. Med. 2018, 75, 716–723. [Google Scholar] [CrossRef]
- Rajauria, G.; Yuan, Y.V. Recent Advances in Micro and Macroalgal Processing: Food and Health Perspectives; Wiley Blackwell: Hoboken, NJ, USA, 2021; ISBN 9781119542582. [Google Scholar]
- Krohn, I.; Menanteau-Ledouble, S.; Hageskal, G.; Astafyeva, Y.; Jouannais, P.; Nielsen, J.L.; Pizzol, M.; Wentzel, A.; Streit, W.R. Health benefits of microalgae and their microbiomes. Microb. Biotechnol. 2022, 15, 1966–1983. [Google Scholar] [CrossRef]
- Azlan, N.Z.; Mohd Yusof, Y.A.; Makpol, S. Chlorella Vulgaris Ameliorates Oxidative Stress and Improves the Muscle Regenerative Capacity of Young and Old Sprague-Dawley Rats. Nutrients 2020, 12, 3752. [Google Scholar] [CrossRef] [PubMed]
- Azlan, N.Z.; Mohd Yusof, Y.A.; Alias, E.; Makpol, S. Chlorella Vulgaris Improves the Regenerative Capacity of Young and Senescent Myoblasts and Promotes Muscle Regeneration. Oxid. Med. Cell Longev. 2019, 2019, 520789. [Google Scholar] [CrossRef]
- Yang, C.; Qiao, Z.; Xu, Z.; Wang, X.; Deng, Q.; Chen, W.; Huang, F. Algal Oil Rich in Docosahexaenoic Acid Alleviates Intestinal Inflammation Induced by Antibiotics Associated with the Modulation of the Gut Microbiome and Metabolome. J. Agric. Food Chem. 2021, 69, 9124–9136. [Google Scholar] [CrossRef] [PubMed]
- Doughman, S.D.; Krupanidhi, S.; Sanjeevi, C.B. Omega-3 Fatty Acids for Nutrition and Medicine: Considering Microalgae Oil as a Vegetarian Source of EPA and DHA. Curr. Diabetes Rev. 2007, 3, 198–203. [Google Scholar] [CrossRef]
- Xu, Z.; Tang, H.; Huang, F.; Qiao, Z.; Wang, X.; Yang, C.; Deng, Q. Algal Oil Rich in N-3 PUFA Alleviates DSS-Induced Colitis via Regulation of Gut Microbiota and Restoration of Intestinal Barrier. Front. Microbiol 2020, 11, 615404. [Google Scholar] [CrossRef]
- Na, B.R.; Lee, J.H. In Vitro and In Vivo Digestibility of Soybean, Fish, and Microalgal Oils, and Their Influences on Fatty Acid Distribution in Tissue Lipid of Mice. Molecules 2020, 25, 5357. [Google Scholar] [CrossRef] [PubMed]
- Khavari, F.; Saidijam, M.; Taheri, M.; Nouri, F. Microalgae: Therapeutic potentials and applications. Mol. Biol. Rep. 2021, 48, 4757–4765. [Google Scholar] [CrossRef] [PubMed]
- Skjånes, K.; Aesoy, R.; Herfindal, L.; Skomedal, H.; Jensen, P.-E. Bioactive peptides from microalgae: Focus on anti-cancer and immunomodulating activity. Physiol. Plant. 2021, 173, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Sæther, T.; Paulsen, S.M.; Tungen, J.E.; Vik, A.; Aursnes, M.; Holen, T.; Hansen, T.V.; Nebb, H.I. Synthesis and biological evaluations of marine oxohexadecenoic acids: PPARα/γ dual agonism and anti-diabetic target gene effects. Eur. J. Med. Chem. 2018, 155, 736–753. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, X.B.; Liu, H.Y.; Xu, C.; Wang, L.L.; Li, S. P633H, a novel dual agonist at peroxisome proliferator-activated receptors α and γ, with different anti-diabetic effects in db/db and KK-A y mice. Br. J. Pharmacol. 2009, 157, 724–735. [Google Scholar] [CrossRef]
- Brown, J.D.; Plutzky, J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation 2007, 115, 518–533. [Google Scholar] [CrossRef]
- Moldes-Anaya, A.; Sæther, T.; Uhlig, S.; Nebb, H.I.; Larsen, T.; Eilertsen, H.C.; Paulsen, S.M. Two Isomeric C16 Oxo-Fatty Acids from the Diatom Chaetoceros Karianus Show Dual Agonist Activity towards Human Pe-roxisome Proliferator-Activated Receptors (PPARs) α/γ. Mar. Drugs 2017, 15, 148. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Pellegrino, M.; Carluccio, M.A.; Calabriso, N.; Wabitsch, M.; Storelli, C.; Wright, M.; de Caterina, R. Therapeutic potential of the dual peroxisome proliferator activated receptor (PPAR)α/γ agonist aleglitazar in attenuating TNF-α-mediated inflammation and insulin resistance in human adipocytes. Pharmacol. Res. 2016, 107, 125–136. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef]
- Kim, M.; Furuzono, T.; Yamakuni, K.; Li, Y.; Kim, Y.L.; Takahashi, H.; Ohue-Kitano, R.; Jheng, H.F.; Takahashi, N.; Kano, Y.; et al. 10-oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, enhances energy metabolism by activation of TRPV1. FASEB J. 2017, 31, 5036–5048. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Kim, Y.L.; Furuzono, T.; Takahashi, N.; Yamakuni, K.; Yang, H.E.; Li, Y.; Ohue, R.; Nomura, W.; Sugawara, T.; et al. 10-oxo-12(Z)-octadecenoic acid, a linoleic acid metabolite produced by gut lactic acid bacteria, potently activates PPARγ and stimulates adipogenesis. Biochem. Biophys. Res. Commun. 2015, 459, 597–603. [Google Scholar] [CrossRef]
- Hira, T.; Ogasawara, S.; Yahagi, A.; Kamachi, M.; Li, J.; Nishimura, S.; Sakaino, M.; Yamashita, T.; Kishino, S.; Ogawa, J.; et al. Novel Mechanism of Fatty Acid Sensing in Enteroendocrine Cells: Specific Structures in Oxo-Fatty Acids Produced by Gut Bacteria Are Responsible for CCK Secretion in STC-1 Cells via GPR40. Mol. Nutr. Food Res. 2018, 62, 1800146. [Google Scholar] [CrossRef]
- Gao, H.; Geng, T.; Huang, T.; Zhao, Q. Fish Oil Supplementation and Insulin Sensitivity: A Systematic Review and Meta-Analysis. Lipids Health Dis. 2017, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Padanad, M.S.; Konstantinidou, G.; Venkateswaran, N.; Melegari, M.; Rindhe, S.; Mitsche, M.; Yang, C.; Bat-ten, K.; Huffman, K.E.; Liu, J.; et al. Fatty Acid Oxidation Mediated by Acyl-CoA Synthetase Long Chain 3 Is Required for Mutant KRAS Lung Tumorigenesis. Cell Rep. 2016, 16, 1614–1628. [Google Scholar] [CrossRef]
- Ortega-Senovilla, H.; van Poppel, M.N.M.; Desoye, G.; Herrera, E. Angiopoietin-like protein 4 (ANGPTL4) is related to gestational weight gain in pregnant women with obesity. Sci. Rep. 2018, 8, 12428. [Google Scholar] [CrossRef] [PubMed]
- Kersten, S. Role and mechanism of the action of angiopoietin-like protein ANGPTL4 in plasma lipid metabolism. J. Lipid Res. 2021, 62, 100150. [Google Scholar] [CrossRef]
- Xu, A.; Lam, M.C.; Chan, K.W.; Wang, Y.; Zhang, J.; Hoo, R.L.C.; Xu, J.Y.; Chen, B.; Chow, W.-S.; Tso, A.W.K.; et al. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 6086–6091. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, G.; Xu, C.; Liu, L.; Zhang, Q.; Xu, Q.; Jia, H.; Li, X.; Li, X. Perilipin 1 mediates lipid metabolism homeostasis and inhibits inflammatory cytokine synthesis in bovine adipocytes. Front. Immunol. 2018, 9, 467. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lee, P.; Chisholm, D.J.; James, D.E. Control of adipocyte differentiation in different fat depots; Implications for pathophysiology or therapy. Front. Endocrinol. 2015, 6, 1. [Google Scholar] [CrossRef]
- Prentice, K.J.; Saksi, J.; Hotamisligil, G.S. Adipokine FABP4 integrates energy stores and counterregulatory metabolic responses. J. Lipid Res. 2019, 60, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Hanschkow, M.; Boulet, N.; Kempf, E.; Bouloumié, A.; Kiess, W.; Stein, R.; Körner, A.; Landgraf, K. Expression of the Adipocyte Progenitor Markers MSCA1 and CD36 is Associated with Adipose Tissue Function in Children. J. Clin. Endocrinol. Metab. 2022, 107, E836–E851. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Strable, M.S.; Ntambi, J.M. Stearoyl CoA desaturase 1: Role in cellular inflammation and stress. Adv. Nutr. 2011, 2, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.; Berry, A.; Cirulli, F.; Caprio, M. Molecular mechanisms underlying metabolic syndrome: The expanding role of the adipocyte. FASEB J. 2017, 31, 4240–4255. [Google Scholar] [CrossRef] [PubMed]
- Kahn, C.R.; Wang, G.; Lee, K.Y. Altered adipose tissue and adipocyte function in the pathogenesis of metabolic syndrome. J. Clin. Investig. 2019, 129, 3990–4000. [Google Scholar] [CrossRef]
- Gil-Cardoso, K.; del Bas, J.M.; Caimari, A.; Lama, C.; Torres, S.; Mantecón, L.; Infante, C. TetraSOD®, a Unique Marine Microalgae Ingredient, Promotes an Antioxidant and Anti-Inflammatory Status in a Metabolic Syndrome-Induced Model in Rats. Nutrients 2022, 14, 4028. [Google Scholar] [CrossRef]
- Lugarà, R.; Renner, S.; Wolf, E.; Liesegang, A.; Bruckmaier, R.; Giller, K. Crossbred Sows Fed a Western Diet during Pre-Gestation, Gestation, Lactation, and Post-Lactation Periods Develop Signs of Lean Metabolic Syndrome That Are Partially Attenuated by Spirulina Supplementation. Nutrients 2022, 14, 3574. [Google Scholar] [CrossRef]
- Mayer, C.; Côme, M.; Ulmann, L.; Martin, I.; Zittelli, G.C.; Faraloni, C.; Ouguerram, K.; Chénais, B.; Mimouni, V. The Potential of the Marine Microalga Diacronema lutheri in the Prevention of Obesity and Metabolic Syndrome in High-Fat-Fed Wistar Rats. Molecules 2022, 27, 4246. [Google Scholar] [CrossRef]
- du Preez, R.; Majzoub, M.E.; Thomas, T.; Panchal, S.K.; Brown, L. Nannochloropsis oceanica as a microalgal food intervention in diet-induced metabolic syndrome in rats. Nutrients 2021, 13, 3991. [Google Scholar] [CrossRef]
- Elzinga, S.E.; Betancourt, A.; Stewart, J.C.; Altman, M.H.; Barker, V.D.; Muholland, M.; Bailey, S.; Brennan, K.M.; Adams, A.A. Effects of Docosahexaenoic Acid–Rich Microalgae Supplementation on Metabolic and Inflammatory Parameters in Horses With Equine Metabolic Syndrome. J. Equine Vet. Sci. 2019, 83, 102811. [Google Scholar] [CrossRef]
- Zheng, N.; Ding, X.; Wei, D.; Dai, B.; Zheng, L.; Sumi, R.; Hu, D.; Jahane, R.; Sun, L. Therapeutic effects of coccomyxagloeobotrydiformis on the metabolic syndrome in rats. Cell. Physiol. Biochem. 2018, 48, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Lalanza, J.F.; Snoeren, E.M.S. The cafeteria diet: A standardized protocol and its effects on behavior. Neurosci. Biobehav. Rev. 2021, 122, 92–119. [Google Scholar] [CrossRef]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: Comparison to high-fat diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Florentino, D.F.; Zlotnik, A.; Mosmann, T.R.; Howard, M.; O’garra, A. IL-10 Inhibits Cytokine Production by Activated Macrophages. J. Immunol. 1991, 147, 3815–3822. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef] [PubMed]
- Likidlilid, A.; Patchanans, M.N.; Poldee, M.S.; Peerapatdit, T. Glutathione and Glutathione Peroxidase in Type 1 Diabetic Patients. J. Med. Assoc. Thai. 2007, 90, 1759–1767. [Google Scholar] [PubMed]
- Stanton, M.C.; Chen, S.C.; Jackson, J.V.; Rojas-Triana, A.; Kinsley, D.; Cui, L.; Fine, J.S.; Greenfeder, S.; Bober, L.A.; Jenh, C.H. Inflammatory Signals shift from adipose to liver during high fat feeding and influence the development of steatohepatitis in mice. J. Inflamm. 2011, 8, 8. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- de Waal Maleyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; de Vries, J.E. Interleukin 10(EL,.10) Inhibits Cytokine Synthesis by Human Monocytes: An Autoregulatory Role of IL-10 Produced by Monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef]
- Unamuno, X.; Gómez-Ambrosi, J.; Rodríguez, A.; Becerril, S.; Frühbeck, G.; Catalán, V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur. J. Clin. Investig. 2018, 48, e12997. [Google Scholar] [CrossRef]
- Wisse, B.E. The inflammatory syndrome: The role of adipose tissue cytokines in metabolic disorders linked to obesity. J. Am. Soc. Nephrol. 2004, 15, 2792–2800. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef]
- Georgiev, P.; Charbonnier, L.M.; Chatila, T.A.; Regulatory, T. Cells: The Many Faces of Foxp3. J. Clin. Immunol. 2019, 39, 623–640. [Google Scholar] [CrossRef]
- Bortolin, R.C.; Vargas, A.R.; Gasparotto, J.; Chaves, P.R.; Schnorr, C.E.; Martinello, K.B.; Silveira, A.K.; Rabelo, T.K.; Gelain, D.P.; Moreira, J.C.F. A new animal diet based on human Western diet is a robust diet-induced obesity model: Comparison to high-fat and cafeteria diets in term of metabolic and gut microbiota disruption. Int. J. Obes. 2018, 42, 525–534. [Google Scholar] [CrossRef]
- Asgary, S.; Karimi, R.; Momtaz, S.; Naseri, R.; Farzaei, M.H. Effect of resveratrol on metabolic syndrome components: A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2019, 20, 173–186. [Google Scholar] [CrossRef]
- Koopmans, S.J.; Schuurman, T. Considerations on pig models for appetite, metabolic syndrome and obese type 2 diabetes: From food intake to metabolic disease. Eur. J. Pharmacol. 2015, 759, 231–239. [Google Scholar] [CrossRef]
- Corpeleijn, E.; Saris, W.H.M.; Blaak, E.E. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: Effects of lifestyle: Etiology and Pathophysiology. Obes. Rev. 2009, 10, 178–193. [Google Scholar] [CrossRef]
- Feng, R.; Du, S.; Chen, Y.; Zheng, S.; Zhang, W.; Na, G.; Li, Y.; Sun, C. High carbohydrate intake from starchy foods is positively associated with metabolic disorders: A Cohort Study from a Chinese population. Sci. Rep. 2015, 5, 16919. [Google Scholar] [CrossRef]
- Green, C.H.; Syn, W.K. Non-nutritive sweeteners and their association with the metabolic syndrome and non-alcoholic fatty liver disease: A review of the literature. Eur. J. Nutr. 2019, 58, 1785–1800. [Google Scholar] [CrossRef]
- Lasker, S.; Rahman, M.M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.B.; Subhan, N.; Ahsan, G.U.; et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Lohsoonthorn, V.; Jiamjarasrangsi, W.; Lertmaharit, S.; Williams, M.A. Association between elevated liver enzymes and metabolic syndrome among Thai adults. Diabetes Metab. Syndr. Clin. Res. Rev. 2008, 2, 171–178. [Google Scholar] [CrossRef] [PubMed]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. EXCLI J. 2016, 15, 817–828. [Google Scholar] [CrossRef]
- Buettner, R.; Parhofer, K.G.; Woenckhaus, M.; Wrede, C.E.; Kunz-Schughart, L.A.; Schölmerich, J.; Bollheimer, L.C. Defining high-fat-diet rat models: Metabolic and molecular effects of different fat types. J. Mol. Endocrinol. 2006, 36, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Candiracci, M.; Justo, M.L.; Castaño, A.; Rodriguez-Rodriguez, R.; Herrera, M.D. Rice bran enzymatic extract-supplemented diets modulate adipose tissue inflammation markers in Zucker rats. Nutrition 2014, 30, 466–472. [Google Scholar] [CrossRef]
- Guerrero, O.A.; Aguilera, A.A.; Quintana Castro, R.; Rodriguez, I.S.; Sanchez Otero, G.; Oliart Ros, R.M. CD36 Gene Expression Induced by Fish Oil in Abdominal Adipose Tissue of Rats with Metabolic Syndrome. J. Food Nutr. Disord. 2017, 6, 2. [Google Scholar] [CrossRef]
- de Luca, C.; Olefsky, J.M. Inflammation and Insulin Resistance. FEBS Lett. 2008, 582, 97–105. [Google Scholar] [CrossRef]
- Preis, S.R.; Massaro, J.M.; Robins, S.J.; Hoffmann, U.; Vasan, R.S.; Irlbeck, T.; Meigs, J.B.; Sutherland, P.; D’Agostino, R.B.; O’Donnell, C.J.; et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the framingham heart study. Obesity 2010, 18, 2191–2198. [Google Scholar] [CrossRef]
- Wei, W.; Hu, M.; Huang, J.; Yu, S.; Li, X.; Li, Y.; Mao, L. Anti-obesity effects of DHA and EPA in high fat-induced insulin resistant mice. Food Funct. 2021, 12, 1614–1625. [Google Scholar] [CrossRef]
- Zhang, H.J.; Gao, X.; Guo, X.F.; Li, K.L.; Li, S.; Sinclair, A.J.; Li, D. Effects of dietary eicosapentaenoic acid and docosahexaenoic acid supplementation on metabolic syndrome: A systematic review and meta-analysis of data from 33 randomized controlled trials. Clin. Nutr. 2021, 40, 4538–4550. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.Y.; Hwang, J.H.; Yang, S.H.; Um, J.I.; Hong, K.W.; Kang, K.; Pan, C.H.; Hwang, K.T.; Kim, S.M. Anti-obesity effect of standardized extract of microalga phaeodactylum tricornutum containing fucoxanthin. Mar. Drugs 2019, 17, 311. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, H.A.; Kang, M.J.; Choi, J.S.; Kim, G.D. Fucosterol, isolated from Ecklonia stolonifera, inhibits adipogenesis through modulation of FoxO1 pathway in 3T3-L1 adipocytes. J. Pharm. Pharmacol. 2017, 69, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.W.; Kao, T.W.; Chang, P.K.; Chen, W.L.; Wu, L.W. Atherogenic index of plasma as predictors for metabolic syndrome, hypertension and diabetes mellitus in Taiwan citizens: A 9-year longitudinal study. Sci. Rep. 2021, 11, 9900. [Google Scholar] [CrossRef]
- Panchal, S.K. Cardioprotective and Hepatoprotective Effects of Natural Products in Metabolic Syndrome; University of Southern Queensland: Toowoomba, Australia, 2012. [Google Scholar]
- Halajzadeh, J.; Milajerdi, A.; Reiner, Ž.; Amirani, E.; Kolahdooz, F.; Barekat, M.; Mirzaei, H.; Mirhashemi, S.M.; Asemi, Z. Effects of resistant starch on glycemic control, serum lipoproteins and systemic inflammation in patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled clinical trials. Crit. Rev. Food Sci. Nutr. 2020, 60, 3172–3184. [Google Scholar] [CrossRef]
- Machado, L.; Carvalho, G.; Pereira, R.N. Effects of Innovative Processing Methods on Microalgae Cell Wall: Prospects towards Digestibility of Protein-Rich Biomass. Biomass 2022, 2, 80–102. [Google Scholar] [CrossRef]
- Grasa-López, A.; Miliar-García, Á.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Reyes-Maldonado, E.; Jaramillo-Flores, M.E. Undaria pinnatifida and fucoxanthin ameliorate lipogenesis and markers of both inflammation and cardiovascular dysfunction in an animal model of diet-induced obesity. Mar. Drugs 2007, 14, 148. [Google Scholar] [CrossRef]
- Devaraj, S.; Jialal, I.; Vega-López, S. Plant sterol-fortified orange juice effectively lowers cholesterol levels in mildly hypercholesterolemic healthy individuals. Arter. Thromb. Vasc. Biol. 2004, 24, e25–e28. [Google Scholar] [CrossRef]
- Dvir, I.; Stark, A.H.; Chayoth, R.; Madar, Z.; Arad, S.M. Hypocholesterolemic effects of nutraceuticals produced from the red microalga Porphyridium sp in rats. Nutrients 2009, 1, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Barragán-Vázquez, S.; Ariza, A.C.; Silva, I.R.; Pedraza, L.S.; Rivera Dommarco, J.A.; Ortiz-Panozo, E.; Zam-brano, E.; Reyes Castro, L.A.; Shivappa, N.; Hébert, J.R.; et al. Pro-inflammatory diet is associated with adiposity during childhood and with adipokines and inflammatory markers at 11 years in mexican children. Nutrients 2020, 12, 3658. [Google Scholar] [CrossRef]
- Lim, S.; Kim, J.W.; Targher, G. Links between metabolic syndrome and metabolic dysfunction-associated fatty liver disease. Trends Endocrinol. Metab. 2021, 32, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Hyde, P.N.; Sapper, T.N.; Crabtree, C.D.; LaFountain, R.A.; Bowling, M.L.; Buga, A.; Fell, B.; McSwiney, F.T.; Dickerson, R.M.; Miller, V.J.; et al. Dietary carbohydrate restriction improves metabolic syndrome independent of weight loss. JCI Insight 2019, 4, e128308. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.T.; Chaves-Filho, A.B.; Yoshinaga, M.Y.; Paiva, N.C.N.; Carneiro, C.M.; Miyamoto, S.; Festuccia, W.T.; Guerra-Sá, R. Liver lipidome signature and metabolic pathways in nonalcoholic fatty liver disease induced by a high-sugar diet. J. Nutr. Biochem. 2021, 87, 108519. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, L.; Mahli, A.; Hellerbrand, C. Development of an in vitro model to study hepatitis C virus effects on hepatocellular lipotoxicity and lipid metabolism. Pathol. Res. Pract. 2018, 214, 1700–1706. [Google Scholar] [CrossRef]
- Mannully, C.T.; Bruck-Haimson, R.; Zacharia, A.; Orih, P.; Shehadeh, A.; Saidemberg, D.; Kogan, N.M.; Alfandary, S.; Serruya, R.; Dagan, A.; et al. Lipid desaturation regulates the balance between self-renewal and differentiation in mouse blastocyst-derived stem cells. Cell Death Dis. 2022, 13, 1027. [Google Scholar] [CrossRef]
- Pang, J.; Xi, C.; Huang, X.; Cui, J.; Gong, H.; Zhang, T. Effects of excess energy intake on glucose and lipid metabolism in C57BL/6 mice. PLoS ONE 2016, 11, e0146675. [Google Scholar] [CrossRef]
- Ehrampoush, E.; Homayounfar, R.; Davoodi, S.H.; Zand, H.; Askari, A.; Kouhpayeh, S.A. Ability of dairy fat in inducing metabolic syndrome in rats. Springerplus 2016, 5, 2020. [Google Scholar] [CrossRef]
- Prasun, P. Mitochondrial dysfunction in metabolic syndrome. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165838. [Google Scholar] [CrossRef]
- García-García, F.J.; Monistrol-Mula, A.; Cardellach, F.; Garrabou, G. Nutrition, bioenergetics, and metabolic syndrome. Nutrients 2020, 12, 2785. [Google Scholar] [CrossRef]
- Sato, A.; Shiraishi, Y.; Kimura, T.; Osaki, A.; Kagami, K.; Ido, Y.; Adachi, T. Resistance to Obesity in SOD1 Deficient Mice with a High-Fat/High-Sucrose Diet. Antioxidants 2022, 11, 1403. [Google Scholar] [CrossRef]
- Vona, R.; Gambardella, L.; Cittadini, C.; Straface, E.; Pietraforte, D. Biomarkers of oxidative stress in metabolic syndrome and associated diseases. Oxidative Med. Cell. Longev. 2019, 2019, 8267234. [Google Scholar] [CrossRef]
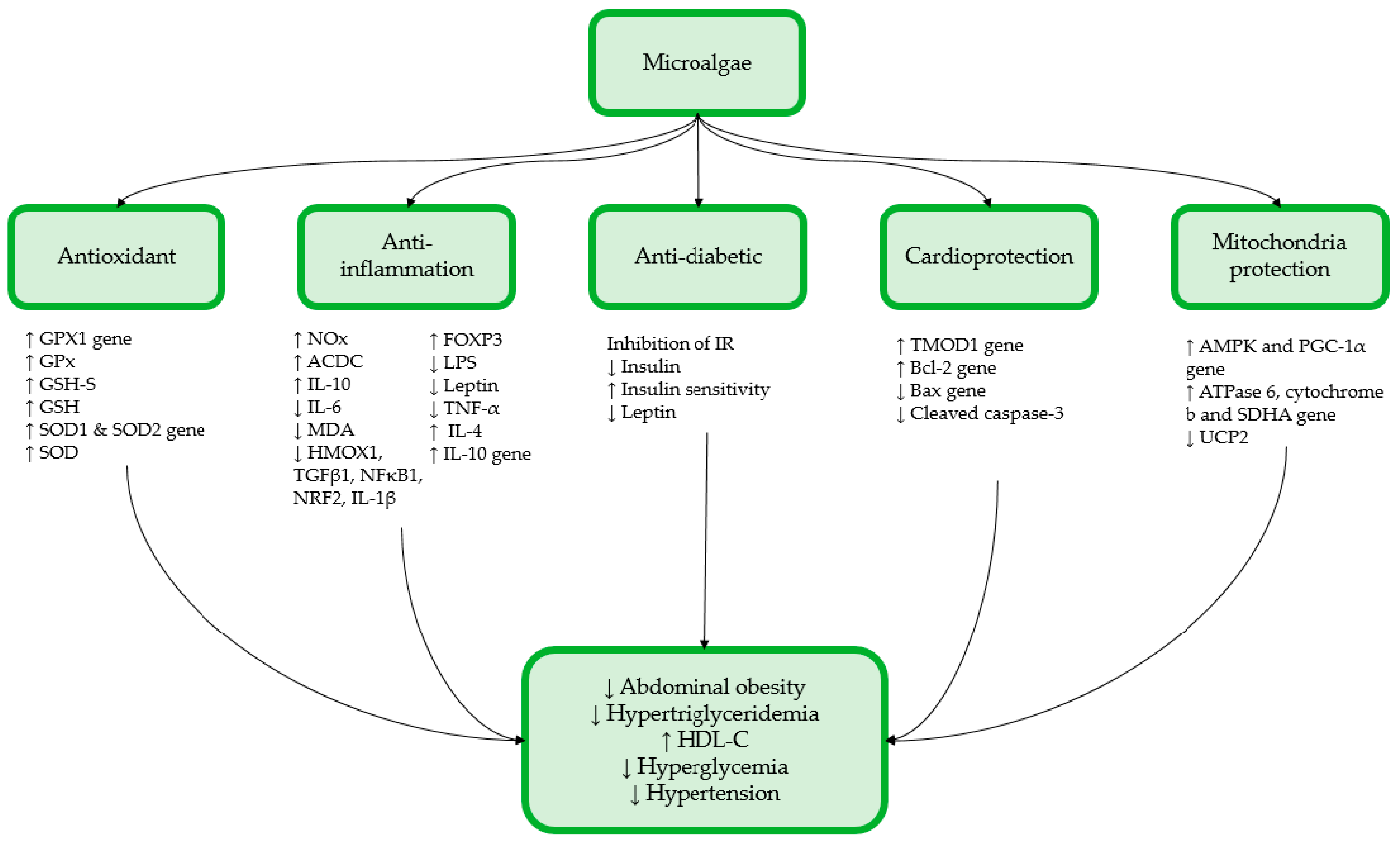
| Experiment | Cell Model | Microalgae and Doses | Experimental Groups | Effects | Mechanisms | Reference Number |
|---|---|---|---|---|---|---|
| Dose-response analysis | COS-1 cells | 0.1% Chaetoceros karianus-derived (7E)-9-OHE or (10E)-9-OHE for 18 h | (1) Positive controls: rosiglitazone or pirinixic acid (2) (7E)-9-OHE or (10E)-9-OHE (3) Negative control: palmitic acid | (7E)-9-OHE or (10E)-9-OHE: Exhibits PPARα/γ agonist activity | - | [38] |
| Endogenous PPAR target genes activation analysis | Huh7 cells SGBS pre-adipocyte cells |
| (1) DMSO (negative controls) (2) 25 µM (7E)-9-OHE or (10E)-9-OHE (3) 50 µM (7E)-9-OHE or (10E)-9-OHE (4) pirinxic acid (positive control) | 25 or 50 µM of (7E)-9-OHE or (10E)-9-OHE: Fatty acid catabolism is activated in Huh-7 and SGBS cells | Huh-7: (7E)-9-OHE: ↑ ACSL3 gene expression (10E)-9-OHE: ↑ PLIN1 gene expression (7E)-9-OHE or (10E)-9-OHE: ↑ CPT1A and ANGPTL4 gene expressions SGBS: (10E)-9-OHE: ↑ ANGPTL4 gene expression (7E)-9-OHE or (10E)-9-OHE: ↑ CPT1A gene expression | |
| Adipocyte differentiation analysis | SGBS pre-adipocyte cells | 25 µM of (7E)-9- OHE or (10E)-9-OHE for 12 days | (1) (7E)-9-OHE or (10E)-9-OHE (2) rosiglitazone (positive control) | Improvement in the regulation of fatty acid metabolism, transport, storage, adipokine signaling and browning | ↑ PPARG, CEBPA, CEBPB, PLIN1, FABP4, CD36, SCD1 and UCP1 expressions | |
| Adipocyte transcriptomics | SGBS pre-adipocyte cells | 25 µM of (7E)-9-OHE or (10E)-9-OHE for 8 days | (1) (7E)-9-OHE or (10E)-9-OHE (2) rosiglitazone (positive control) | ↓ Inflammatory cytokines ↑ Insulin-sensitive adipokines | ↓ IL-6, TNFα, CXCL1, CXCL5 and IL-1B gene expressions ↑ Leptin and insulin sensitizing ADIPOQ genes expressions |
| Animal Model | Microalgae and Doses | Experimental Design | Effects on MetS | Mechanisms | Reference Number |
|---|---|---|---|---|---|
| Male Sprague Dawley rat (7 weeks old) | Tetraselmis chuii powder (0.17, 1.7, 17 mg/kg BW/d) 8 weeks | STD-C: Standard diet-control CAF-C: Cafeteria diet- control CAF + 0.17: CAF + 0.17 mg/kg BW/day of T. chuii powder CAF + 1.7: CAF + 1.7 mg/kg BW/day of T. chuii powder CAF + 17: CAF + 17 mg/kg BW/day of T. chuii powder | CAF + 0.17: ↓ plasma LDL/VLDL-C CAF + 17: ↓ Plasma glucose CAF + 0.17, CAF + 1.7 and CAF + 17: No effects on BW, adiposity index, TG, HOMA-IR index, and HDL-C. | CAF + 0.17: ↑ plasma NOx ↑ GPx activity in liver CAF + 0.17 and CAF + 1.7: ↑ SOD 1 and SOD2 gene expression in liverCAF + 1.7 and CAF + 17: ↑ GPX1 gene expression in liver ↑ GCLm gene expression in liver CAF + 17: ↓ oxLDL levels in plasma ↑ IL-10 levels in plasma ↑ GSH level in liver ↑ SOD1 gene expression in liver ↑ IL-10 gene expression in MWAT ↑ FOXP3 gene expression in spleen CAF + 0.17, CAF + 1.7 and CAF + 17: ↑ GR and GSH-S gene expressions in liver ↑ SOD1 gene expression in liver ↓HMOX1, TGFβ1 and NFκB1 gene expressions in liver ↓ IL-1β, TNFα and IFNG gene expressions in MWAT ↓ IL-1β agene in thymus and spleen ↓ IFNG gene expressions in MWAT, thymus and spleen ↑ ACDC gene expression in MWAT ↓ TNFα, NRF2, HMOX1, NFκB1, IL-1β and IFNG gene expressions in thymus ↑ IL-10 gene expression in thymus and spleen | [59] |
| Female Sus scrofa pigs(5.6 ± 0.8 months old) | Arthrospira platensis (spirulina, Sp) (20 g/d) tablet 25 weeks | CTR−: Control diet CTR+: Control diet + Sp tablet WES−: Western diet WES+: Western diet + Sp tablet | CTR+ and WES+: No effects on BW No effects on visceral adipose tissue proportion No effects on plasma TG No effects on plasma TC ↓ Serum glucose (at late gestation) | CTR+: ↓ ALT levels ↓ Hepatic necrosis gene expression WES+: ↑ Hepatic lipid accumulation gene expression ↓ Hepatic carbohydrate accumulation gene expression ↑ ALT levels ↑ Hepatic necrosis gene expression CTR+ and WES+: ↓ Plasma insulin levels (at slaughter) ↓ Muscular weight gain gene expression ↓ Liver weight ↓ IR gene expression in liver | [60] |
| Male Wistar rat (3 weeks old) | Diacronema lutheri powder (12%) 8 weeks | CTRL: Control diet HF: High fat diet HF-Dia: High fat + D. lutheri powder | HF-Dia: ↓ BW ↓ AAT and EAT weight/BW ratio ↓ Plasma TG levels ↑ HDL levels ↓ HOMA-IR index Improvement in GT & IT No effects on plasma glucose levels | HF-Dia: ↓ Plasma insulin levels ↓ AIP ↑ Plasma IL-4 levels ↑ Adipose IL-10 levels ↓ Leptin ↓ TG in liver ↓ TC in liver ↑ ALT ratio | [61] |
| Male Wistar rat (8–9 weeks old) | Nannochloropsis oceanica powder (5%) 8 weeks | C: Corn starch diet for 16 weeks H: High-carbohydrate, high-fat diet for 16 weeks CN: Corn starch diet for the first 8 weeks + 5% N. oceanica powder for the last 8 weeksHN: High-carbohydrate, high-fat diet for first the 8 weeks + 5% N. oceanica powder for the last 8 weeks | HN: ↓ Total abdominal fat and retroperitoneal fat No effects on BW CN and HN groups: No effects on visceral adiposity % No effects on plasma TG No effects on plasma TCNo improvement in GT & IT No effects on systolic BP | HN: ↓ Hepatic fat vacuole size CN and HN groups: ↑ Abundance of Oxyphotobacteria | [62] |
| Male Wistar rat (3 weeks old) | Tisochrysis lutea powder (12%) 8 weeks | CTRL: Standard diet HF: 260 High fat diet with 10% fructose in drinking water HF-Tiso: HF diet + T. lutea powder | HF-Tiso: ↓ BW ↓ AAT and EAT weight/ BW ratio ↓ Plasma TG ↑ Plasma HDL-C ↓ Plasma LDL-C ↓ Plasma glucose ↓ HOMA-IR index | HF-Tiso: ↓ Plasma insulin ↓ Plasma TNF-α ↑ Adipose tissue anti-inflammatory IL-10 ↓ AIP ↓ Serum LPS ↓ Leptin ↓ TG in liver ↓ TC in liver | [6] |
| Mixed sex and breed of horse (treatment group is 13.2 ± 4.4 years old and control group is 11.5 ± 2.6 years old) | DHA-rich microalgae (110 g/horse/d) 46 days | Control horses Treated horses | Treated horses: No effects on BW No effect on cresty neck score ↓ Plasma TG No effects on glucose tolerance | ↑ Plasma DHA ↓ TNF-α MFI | [63] |
| Male Wistar rat (3 weeks old) | Phaeodactylum tricornutum powder (12%) 8 weeks | CTRL: Control group fed with standard diet HF: 260 High fat diet with 10% fructose in drinking water HF-Phaeo: HF diet + P. tricornutum powder | HF-Phaeo: ↓ BW ↓ AAT and EAT weight/ BW ratio ↓ Plasma TC ↓ Plasma TG ↑ Serum HDL-C ↓ HOMA-IR index No effects on plasma glucose | HF-Phaeo: ↓ Plasma insulin ↑ n-3 LC-PUFA levels in plasma, RBC, and liver lipids ↑ ∆9-Desaturase level in liver lipids ↓ Liver weight/ BW ratio ↓ MUFA levels in plasma lipid and liver phospholipids ↓ TG in liver ↓ TC in liver ↓ AIP ↓ Plasma TNF-α and IL-6 ↑ Plasma IL-4 and IL-10 ↓ Plasma leptin | [10] |
| Sprague Dawley rat(8 weeks old) | Coccomyxa gloeobotrydiformis (CGD) (100 mg/kg BW/d) 12 weeks | Control: Standard chow diet NC: High-energy diet without MetS MS: High-energy diet with MetS MS+CGD: High-energy diet with MetS + CDG MS + CVD: High-energy diet with MetS and CVD MS + CVD + CGD: High-energy diet with MetS and CVD + CGD | MS + CGD: ↓ BW ↓ AC ↓ Serum glucose level ↓ SBP ↓ Serum TG and LDL-C levels ↑ Serum HDL-C levels MS + CVD + CGD: ↑ Left ventricular systolic and end diastolic pressure, and left ventricular systolic pressure maximum increase rate and diastolic pressure maximum decrease rate | MS + CGD: ↑ AMPK and PGC-1α gene expressions in heart, adipose and skeletal muscle tissues ↑ MRC coenzymes (ATPase 6, cytochrome b and SDHA) gene expressions in the liver, heart and skeletal muscle ↓ UCP2 gene expression MS + CVD + CGD: ↓ Pro-inflammatory TNF-α and MDA gene expressions in myocardial tissue ↑ SOD gene expression in myocardial tissue ↑ Bcl-2 gene expression ↓ Bax gene and cleaved caspase-3 gene expressions were decreased ↑ TMOD1 gene expression | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamel Selvan, K.; Goon, J.A.; Makpol, S.; Tan, J.K. Effects of Microalgae on Metabolic Syndrome. Antioxidants 2023, 12, 449. https://doi.org/10.3390/antiox12020449
Tamel Selvan K, Goon JA, Makpol S, Tan JK. Effects of Microalgae on Metabolic Syndrome. Antioxidants. 2023; 12(2):449. https://doi.org/10.3390/antiox12020449
Chicago/Turabian StyleTamel Selvan, Kartthigeen, Jo Aan Goon, Suzana Makpol, and Jen Kit Tan. 2023. "Effects of Microalgae on Metabolic Syndrome" Antioxidants 12, no. 2: 449. https://doi.org/10.3390/antiox12020449
APA StyleTamel Selvan, K., Goon, J. A., Makpol, S., & Tan, J. K. (2023). Effects of Microalgae on Metabolic Syndrome. Antioxidants, 12(2), 449. https://doi.org/10.3390/antiox12020449







