Antioxidant Thymoquinone and Its Potential in the Treatment of Neurological Diseases
Abstract
1. Introduction
2. Antioxidant Properties of Thymoquinone in Models of Neurodegeneration Associated with Acute Processes of Brain Damage
2.1. Antioxidant Properties of Thymoquinone in Brain Ischemia Models
2.2. Antioxidant Properties of Thymoquinone in Traumatic Brain Injury Models
2.3. Antioxidant Properties of Thymoquinone in Models of Neurotoxicity of Metals
| Model | The Order of Administration and Form of the Drug | Antioxidant Effect | Reference |
|---|---|---|---|
| Transient cerebral ischemia (10-min bilateral occlusion of both common carotid arteries) in rats | Daily administration of TQ (5 mg/kg) was administered to animals for 5 days before ischemic exposure, as well as 7 days during reperfusion per os. | Normalizes the levels of glutathione, SOD, catalase, and also reduces the level of MDA in hippocampus. | [21] |
| Global ischemia initiated by 20-min four-vessel-occlusion of the carotid arteries in rats | 10 mg/kg of TQ was administered to animals immediately before and in the next 2 days after ischemia i.p. | Decreases the level of MDA in brain. | [22] |
| Permanent bilateral common carotid artery occlusion in rats | Nigella sativa seed extract (400 mg/kg/day) and TQ (40 mg/kg/day) i.p. | Reduction in lipid peroxidation and prevention of a decrease in the SOD activity. | [24] |
| Middle cerebral artery occlusion in rats | Intranasal administration of nanoparticles optimized by the poly(lactide-co-glycolide)+chitosan complex and loaded with TQ for 12 days (50 μL in each nostril). | Reduces the intensity of lipid peroxidation in brain tissue and leads to an increase in the level of glutathione, catalase, and SOD. | [25] |
| Open unilateral rat traumatic brain injury | TQ (5 mg/kg) was administered to animals after traumatic exposure for 7 days i.p. | Reduces the increase in MDA level. | [34] |
| Intraperitoneal administration of AlCl3 (6 weeks, 10 mg/kg) | Intraperitoneal administration of TQ (10 mg/kg) daily. | Reduces the content of MDA in the brain and prevents a decrease in the overall antioxidant capacity. | [49] |
| Lead treatment of pregnant rats. Lead administration (160 and 320 ppm) started from gestation day 1 to day 20. Per os | Pb and TQ co-administration. Females received TQ (10 mg/kg body weight) per os. | Decreases the content of MDA and prevents a decrease in SOD, catalase, glutathione peroxidase level in the fetal, and females brain. | [50] |
| Arsenic in the form of sodium arsenate (10 mg/kg/day; orally) to rats | TQ (2.5 and 5 mg/kg per day, orally) three days before the administration of arsenic. | Decrease in protein carbonyl and lipid peroxidation, prevention of decrease in level of glutathione and SOD. | [53] |
3. Chronic Neurodegenerative Diseases
3.1. Neuroprotective Properties of Thymoquinone in Modeling Alzheimer’s Disease
3.2. Neuroprotective Properties of Thymoquinone in Modeling Epilepsy
3.3. Neuroprotective Properties of Thymoquinone in Modeling Parkinson’s Disease
| Model | The Order of Administration and Form of the Drug | Antioxidant Effect | Reference |
|---|---|---|---|
| Scopolamine-induced model of Alzheimer’s disease in rats | TQ (20 mg/kg, in corn oil) was administered intraperitoneally 1 h before the experiments. | Reduction in lipid peroxidation in the brain. | [61] |
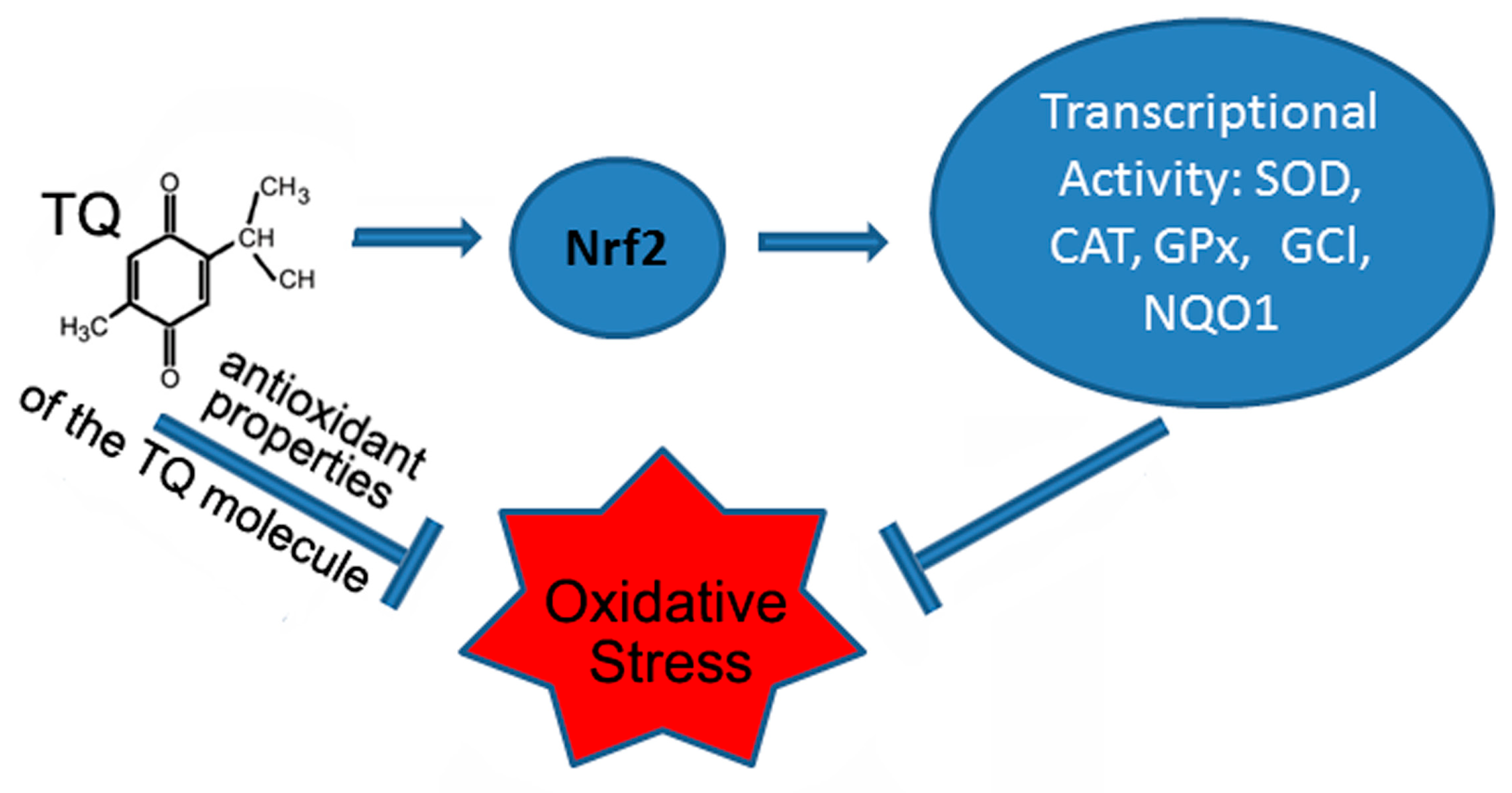
| AlCl3-induced model of Alzheimer’s disease in rats | 10 mg/kg of TQ every day by mouth i.p. | Increases the levels of Nrf2 in brain tissues, recovery of total antioxidant capacity. | [63] |
| Model of Alzheimer’s disease in vitro (administration of beta-amyloid peptide into the culture medium of neurons, cell lines PC 12 and SH–SY5Y) | Medium containing TQ (100 nM) for a period up to 24–48 h. | Reducing oxidative stress, ROS production, and recovery of glutathione level. | [64,65,66] |
| Pentylenetetrazole kindling-animal model of clinical epilepsy | TQ (20 mg/kg intraperitoneally, together with a subconvulsive dose of pentylenetetrazole every other day). | Decrease in malondialdehyde and recovery of glutathione peroxidase activity, and reduction the glutathione level in brain. | [73] |
| The temporal lobe epilepsy model (intrahippocampal administration of kainate to animals) | TQ at a dose of 10 mg/kg per os starting 1 week before surgery. The last treatment was carried out 1 hbefore the surgery. | Decrease in malondialdehyde and recovery of SOD activity in hippocampus. | [74] |
| Lithium-pilocarpine rat model of Status epilepticus | TQ 10 mg/kg twice by intraperitoneal injection 24 h and 1 h prior to injection of pilocarpine. | Increase in Nrf2 and SOD activity in cortex and hippocampus. | [75] |
| The Parkinson’s disease models (animals injected with 6-hydroxydophamine into the striatum) | Pretreatment of animals with TQ (5 or 10 mg/kg) three times with an interval of 24 h per os. | Prevention of an increase in the MDA levels. | [79] |
| The Parkinson’s disease models (animals injected with MPTP to mice) | TQ (once daily for 1 week, 60 min prior to each dose of MPTP administration). i.p. | Decrease in malondialdehyde and recovery of SOD, catalase activity, and glutathione level in brain. | [80] |
4. Further Directions of Research: Limitations of Use of Thymoquinone in Clinical Trials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDA | malondialdehyde |
| MPP+ | 1-methyl-4-phenylpyridinium |
| MPTP | 1-methyl-4-phenyl 1,2,3,6 tetrahydropyridine |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| ROS | Reactive oxygen species |
| SOD | superoxide dismutase |
| TQ | thymoquinone |
References
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J.; Pamplona, R.; Buffenstein, R.; Buttemer, W.A. Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 2007, 87, 1175–1213. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, J.; Wang, R.; Li, S.; Wu, B.; Yuan, Y. Kaempferol Protects Against Cerebral Ischemia Reperfusion Injury Through Intervening Oxidative and Inflammatory Stress Induced Apoptosis. Front. Pharmacol. 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Long, X.; Tang, J.; Li, X.; Zhang, X.; Luo, C.; Zhou, Y.; Zhang, P. The Attenuation of Traumatic Brain Injury via Inhibition of Oxidative Stress and Apoptosis by Tanshinone IIA. Oxid. Med. Cell Longev. 2020, 2020, 4170156. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Alam, M.A.; Samim, M.; Iqbal, Z.; Ahmad, F.J. Quantification and evaluation of thymoquinone loaded mucoadhesive nanoemulsion for treatment of cerebral ischemia. Int. J. Biol. Macromol. 2016, 88, 320–332. [Google Scholar] [CrossRef]
- Myers, A.L.; Zhang, Y.P.; Kramer, M.A.; Bornmann, W.G.; Kaseb, A.; Yang, P.; Tran, H.T. A practical synthesis and X-ray crystallographic analysis of dithymoquinone, a photodimer of thymoquinone. Lett. Org. Chem. 2012, 9, 762–766. [Google Scholar] [CrossRef]
- Jaarin, K.; Foong, W.D.; Yeoh, M.H.; Kamarul, Z.Y.; Qodriyah, H.M.; Azman, A.; Zuhair, J.S.; Juliana, A.H.; Kamisah, Y. Mechanisms of the antihypertensive effects of Nigella sativa oil in L-NAME-induced hypertensive rats. Clinics 2015, 70, 751–757. [Google Scholar] [CrossRef]
- Keyhanmanesh, R.; Boskabady, M.H.; Khamneh, S.; Doostar, Y. Effect of thymoquinone on the lung pathology and cytokine levels of ovalbumin-sensitized guinea pigs. Pharmacol. Rep. 2010, 62, 910–916. [Google Scholar] [CrossRef] [PubMed]
- Bamosa, A.O.; Kaatabi, H.; Lebdaa, F.M.; Elq, A.M.; Al-Sultanb, A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J. Physiol. Pharmacol. 2010, 54, 344–354. [Google Scholar] [PubMed]
- Kaseb, A.O.; Chinnakannu, K.; Chen, D.; Sivanandam, A.; Tejwani, S.; Menon, M.; Dou, Q.P.; Reddy, G.P. Androgen receptor and E2F-1 targeted thymoquinone therapy for hormone-refractory prostate cancer. Cancer Res. 2007, 67, 7782–7788. [Google Scholar] [CrossRef]
- Ragheb, A.; Attia, A.; Eldin, W.S.; Elbarbry, F.; Gazarin, S.; Shoker, A. The protective effect of thymoquinone, an anti-oxidant and anti-inflammatory agent, against renal injury: A review. Saudi J. Kidney Dis. Transpl. 2009, 20, 741–752. [Google Scholar] [PubMed]
- Donnan, G.A.; Fisher, M.; Macleod, M.; Davis, S.M. Stroke. Lancet 2008, 371, 1612–1623. [Google Scholar] [CrossRef] [PubMed]
- Korchagin, V.I.; Mironov, K.O.; Dribnokhodova, O.P.; Maksimova, M.Y.; Illarioshkin, S.N.; Tanashyan, M.M.; Platonov, A.E.; Shipulin, G.A.; Raskurazhev, A.A.; Piradov, M.A. A role of genetic factors in the development of individual predisposition to ischemic stroke. Ann. Clin. Exp. Neurol. 2016, 10, 65–75. [Google Scholar] [CrossRef]
- Orellana-Urzúa, S.; Rojas, I.; Líbano, L.; Rodrigo, R. Pathophysiology of Ischemic Stroke: Role of Oxidative Stress. Curr. Pharm. Des. 2020, 26, 4246–4260. [Google Scholar] [CrossRef]
- Xue, R.; Gao, S.; Zhang, Y.; Cui, X.; Mo, W.; Xu, J.; Yao, M. A meta-analysis of resveratrol protects against cerebral ischemia/reperfusion injury: Evidence from rats studies and insight into molecular mechanisms. Front. Pharmacol. 2022, 13, 988836. [Google Scholar] [CrossRef]
- Mandalaneni, K.; Rayi, A.; Jillella, D.V. Stroke reperfusion injury. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar] [PubMed]
- Isaev, N.K.; Chetverikov, N.S.; Stelmashook, E.V.; Genrikhs, E.E.; Khaspekov, L.G.; Illarioshkin, S.N. Thymoquinone as a Potential Neuroprotector in Acute and Chronic Forms of Cerebral Pathology. Biochemistry 2020, 85, 167–176. [Google Scholar] [CrossRef]
- Al-Majed, A.A.; Al-Omar, F.A.; Nagi, M.N. Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur. J. Pharmacol. 2006, 543, 40–47. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Parvardeh, S.; Asl, M.N.; Sadeghnia, H.R.; Ziaee, T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia_reperfusion injury in rat hippocampus. Phytomedicine 2007, 14, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Du, X.; Chen, S.; Ren, Q.; Hussien, A.B.; Botchway, B.O.A.; Hu, Z.; Fang, M. Therapeutic impact of thymoquninone to alleviate ischemic brain injury via Nrf2/HO-1 pathway. Expert Opin. Ther. Targets 2021, 25, 597–612. [Google Scholar] [CrossRef] [PubMed]
- Fanoudi, S.; Alavi, M.S.; Hosseini, M.; Sadeghnia, H.R. Nigella sativa and thymoquinone attenuate oxidative stress and cognitive impairment following cerebral hypoperfusion in rats. Metab. Brain Dis. 2019, 34, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.Y.; Zhu, Y.X.; Bu, J.Y.; Li, G.W.; Zhou, J.H.; Zhou, S.P. Evaluation of neuroprotective effect of thymoquinone nanoformulation in the rodent cerebral ischemia_reperfusion model. Biomed. Res. Int. 2016, 2016, 2571060. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Ahmed, M.M.; Mohamed, A.S.; Shams-Eldin, E.; Abd El-Daim, T.M.; El-Feky, A.S.; Mustafa, A.B.; Abd Alrahman, M.W.; Mohammed, F.F.; Fathy, M.M. Novel lipid-coated mesoporous silica nanoparticles loaded with thymoquinone formulation to increase its bioavailability in the brain and organs of Wistar rats. BMC Pharmacol. Toxicol. 2022, 23, 71. [Google Scholar] [CrossRef]
- Selassie, A.W.; Zaloshnja, E.; Langlois, J.A.; Miller, T.; Jones, P.; Steiner, C. Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 2008, 23, 123–131. [Google Scholar] [CrossRef]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 130, 1080–1097. [Google Scholar] [CrossRef]
- Fesharaki-Zadeh, A. Oxidative Stress in Traumatic Brain Injury. Int. J. Mol. Sci. 2022, 23, 13000. [Google Scholar] [CrossRef]
- Juurlink, B.H.; Paterson, P.G. Review of oxidative stress in brain and spinal cord injury: Suggestions for pharmacological and nutritional management strategies. J. Spinal Cord Med. 1998, 21, 309–334. [Google Scholar] [CrossRef]
- Bhatti, J.; Nascimento, B.; Akhtar, U.; Rhind, S.G.; Tien, H.; Nathens, A.; da Luz, L.T. Systematic Review of Human and Animal Studies Examining the Efficacy and Safety of N-Acetylcysteine (NAC) and N-Acetylcysteine Amide (NACA) in Traumatic Brain Injury: Impact on Neurofunctional Outcome and Biomarkers of Oxidative Stress and Inflammation. Front. Neurol. 2018, 8, 744. [Google Scholar] [CrossRef]
- Lin, C.J.; Chen, T.H.; Yang, L.Y.; Shih, C.M. Resveratrol protects astrocytes against traumatic brain injury through inhibiting apoptotic and autophagic cell death. Cell Death Dis. 2014, 5, e1147. [Google Scholar] [CrossRef]
- Kalayci, M.; Unal, M.M.; Gul, S.; Acikgoz, S.; Kandemir, N.; Hanci, V.; Edebali, N.; Acikgoz, B. Effect of Coenzyme Q10 on ischemia and neuronal damage in an experimental traumatic brain-injury model in rats. BMC Neurosci. 2011, 12, 75. [Google Scholar] [CrossRef]
- Gülşen, İ.; Ak, H.; Çölçimen, N.; Alp, H.H.; Akyol, M.E.; Demir, İ.; Atalay, T.; Balahroğlu, R.; Rağbetli, M.Ç. Neuroprotective effects of thymoquinone on the hippocampus in a rat model of traumatic brain injury. World Neurosurg. 2016, 86, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Erdivanli, B.; Ozdemir, A.; Sen, A.; Mercantepe, T.; Kazdal, H.; Uydu, H.A.; Tumkaya, L. Protective effect of thymoquinone in preventing trauma-related damage: An experimental study. Biomarkers 2022, 27, 95–100. [Google Scholar] [CrossRef]
- Pointer, C.B.; Klegeris, A. Cardiolipin in central nervous system physiology and pathology. Cell Mol. Neurobiol. 2017, 37, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P.; Antonenko, Y.N.; Cherepanov, D.A.; Chernyak, B.V.; Izyumov, D.S.; Khailova, L.S.; Klishin, S.S.; Korshunova, G.A.; Lyamzaev, K.G.; Pletjushkina, O.Y.; et al. Prevention of cardiolipin oxidation and fatty acid cycling as two antioxidant mechanisms of cationic derivatives of plastoquinone (SkQs). Biochim. Biophys. Acta 2010, 1797, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Severina, I.I.; Severin, F.F.; Korshunova, G.A.; Sumbatyan, N.V.; Ilyasova, T.M.; Simonyan, R.A.; Rogov, A.G.; Trendeleva, T.A.; Zvyagilskaya, R.A.; Dugina, V.B.; et al. In search of novel highly active mitochondria targeted antioxidants: Thymoquinone and its cationic derivatives. FEBS Lett. 2013, 587, 2018–2024. [Google Scholar] [CrossRef]
- Goleva, T.N.; Rogov, A.G.; Korshunova, G.A.; Trendeleva, T.A.; Mamaev, D.V.; Aliverdieva, D.A.; Zvyagilskaya, R.A. SkQThy, a novel and promising mitochondria targeted antioxidant. Mitochondrion 2019, 49, 206–216. [Google Scholar] [CrossRef]
- Genrikhs, E.E.; Stelmashook, E.V.; Popova, O.V.; Kapay, N.A.; Korshunova, G.A.; Sumbatyan, N.V.; Skrebitsky, V.G.; Skulachev, V.P.; Isaev, N.K. Mitochondria targeted antioxidant SkQT1 decreases trauma induced neurological deficit in rat and prevents amyloid β induced impairment of long term potentiation in rat hippocampal slices. J. Drug Target. 2015, 23, 347–352. [Google Scholar] [CrossRef]
- Cardenas-Iniguez, C.; Burnor, E.; Herting, M.M. Neurotoxicants, the Developing Brain, and Mental Health. Biol. Psychiatry Glob. Open Sci. 2022, 2, 223–232. [Google Scholar] [CrossRef]
- Laabbar, W.; Abbaoui, A.; Elgot, A.; Mokni, M.; Amri, M.; Masmoudi-Kouki, O.; Gamrani, H. Aluminum induced oxidative stress, astrogliosis and cell death in rat astrocytes, is prevented by curcumin. J. Chem. Neuroanat. 2021, 112, 101915. [Google Scholar] [CrossRef] [PubMed]
- Shilpa, O.; Anupama, K.P.; Antony, A.; Gurushankara, H.P. Lead (Pb) induced Oxidative Stress as a Mechanism to Cause Neurotoxicity in Drosophila melanogaster. Toxicology 2021, 462, 152959. [Google Scholar] [CrossRef] [PubMed]
- Stelmashook, E.V.; Alexandrova, O.P.; Genrikhs, E.E.; Novikova, S.V.; Salmina, A.B.; Isaev, N.K. Effect of zinc and copper ions on cadmium-induced toxicity in rat cultured cortical neurons. J. Trace Elem. Med. Biol. 2022, 73, 127012. [Google Scholar] [CrossRef] [PubMed]
- Sjogren, B.; Ljunggren, K.G.; Almkvist, O.; Frech, W.; Basun, H. Aluminosis and dementia. Lancet 1994, 344, 1154. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.C.; Piai, K.d.A.; Takayanagui, A.M.; Segura-Muñoz, S.I. Aluminum as a risk factor for Alzheimer’s disease. Rev. Lat. Am. Enferm. 2008, 16, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.S.; Piagette, J.T.; Escobar, A.G.; Martin, A.; Palacios, R.; Pecanha, F.M.; Vassallo, D.V.; Exley, C.; Alonso, M.J.; Miguel, M.; et al. Aluminum exposure at human dietary levels promotes vascular dysfunction and increases blood pressure in rats: A concerted action of NAD(P)H oxidase and COX-2. Toxicology 2017, 390, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Exley, C. The pro-oxidant activity of aluminum. Free Radic. Biol. Med. 2004, 36, 380–387. [Google Scholar] [CrossRef]
- Abbas, F.; Eladl, M.A.; El-Sherbiny, M.; Abozied, N.; Nabil, A.; Mahmoud, S.M.; Mokhtar, H.I.; Zaitone, S.A.; Ibrahim, D. Celastrol and thymoquinone alleviate aluminum chloride-induced neurotoxicity: Behavioral psychomotor performance, neurotransmitter level, oxidative-inflammatory markers, and BDNF expression in rat brain. Biomed. Pharmacother. 2022, 151, 113072. [Google Scholar] [CrossRef]
- Saleh, H.A.; Abd El-Aziz, G.S.; Mustafa, H.N.; El-Fark, M.; Mal, A.; Aburas, M.; Deifalla, A.H. Thymoquinone ameliorates oxidative damage and histopathological changes of developing brain neurotoxicity. J. Histotechnol. 2019, 42, 116–127. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Guidelines for Drinking-water quality: Incorporating First and Second addenda to Third Edition. In E Recommendations; WHO Press: Geneva, Switzerland, 2008; Volume 1, Available online: https://www.who.int/publications/i/item/9789241547611 (accessed on 11 January 2013).
- Tolins, M.; Ruchirawat, M.; Landrigan, P. The developmental neurotoxicity of arsenic: Cognitive and behavioral consequences of early life exposure. Ann. Glob. Health 2014, 80, 303–314. [Google Scholar] [CrossRef]
- Firdaus, F.; Zafeer, M.F.; Ahmad, M.; Afzal, M. Anxiolytic and anti-inflammatory role of thymoquinone in arsenic-induced hippocampal toxicity in Wistar rats. Heliyon 2018, 4, e00650. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, H.; Zhao, N. Thymoquinone protects against cobalt chloride-induced neurotoxicity via Nrf2/GCL-regulated glutathione homeostasis. J. Biol. Regul. Homeost. Agents 2017, 31, 843–853. [Google Scholar] [PubMed]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2018, 14, 367–429. [Google Scholar] [CrossRef]
- Bateman, R.J.; Munsell, L.Y.; Morris, J.C.; Swarm, R.; Yarasheski, K.E.; Holtzman, D.M. Human amyloid-β synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat. Med. 2006, 12, 856–861. [Google Scholar] [CrossRef] [PubMed]
- Marcus, D.L.; Thomas, C.; Rodriguez, C.; Simberkoff, K.; Tsai, J.S.; Strafaci, J.A.; Freedman, M.L. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Exp. Neurol. 1998, 150, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.A.; Chyan, Y.J.; Andorn, A.C.; Poeggeler, B.; Robakis, N.K.; Pappolla, M.A. Increased expression but reduced activity of antioxidant enzymes in Alzheimer’s disease. J. Alzheimers Dis. 1999, 1, 139–145. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Bimonte, S.; Barbieri, A.; Del Vecchio, V.; Muzio, M.R.; Vitale, A.; Benincasa, G.; Ferriello, A.B.; Azzariti, A.; Arra, C.; et al. Dissecting the potential roles of Nigella sativa and its constituent thymoquinone on the prevention and on the progression of Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 16. [Google Scholar] [CrossRef]
- Kantar, D.; Acun, A.D.; Danışman, B. Effects of thymoquinone on scopolamine-induced spatial and echoic memory changes through regulation of lipid peroxidation and cholinergic impairment. Behav. Brain Res. 2022, 431, 113972. [Google Scholar] [CrossRef]
- Song, J. Animal Model of Aluminum-Induced Alzheimer’s Disease. Adv. Exp. Med. Biol. 2018, 1091, 113–127. [Google Scholar] [CrossRef]
- Hamdan, A.M.E.; Alharthi, F.H.J.; Alanazi, A.H.; El-Emam, S.Z.; Zaghlool, S.S.; Metwally, K.; Albalawi, S.A.; Abdu, Y.S.; Mansour, R.E.; Salem, H.A.; et al. Neuroprotective Effects of Phytochemicals against Aluminum Chloride-Induced Alzheimer’s Disease through ApoE4/LRP1, Wnt3/β-Catenin/GSK3β, and TLR4/NLRP3 Pathways with Physical and Mental Activities in a Rat Model. Pharmaceuticals 2022, 15, 1008. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Vaibhav, K.; Javed, H.; Khan, M.M.; Tabassum, R.; Ahmed, M.E.; Srivastava, P.; Khuwaja, G.; Islam, F.; Siddiqui, M.S.; et al. Attenuation of Aβ-induced neurotoxicity by thymoquinone via inhibition of mitochondrial dysfunction and oxidative stress. Mol. Cell Biochem. 2012, 369, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.; Tucci, M.A.; Benghuzzi, H.A. Comparison of potential preventive therapeutic agents green tea, thymoquinone, and dilinoleoylphosphatidylcholine on human neuroblastoma cells. Biomed. Sci. Instrum. 2014, 50, 132–139. [Google Scholar] [PubMed]
- Alhibshi, A.H.; Odawara, A.; Suzuki, I. Neuroprotective efficacy of thymoquinone against amyloid beta-induced neurotoxicity in human induced pluripotent stem cell-derived cholinergic neurons. Biochem. Biophys. Rep. 2019, 17, 122–126. [Google Scholar] [CrossRef]
- Devi, L. Accumulation of Amyloid Precursor Protein in the Mitochondrial Import Channels of Human Alzheimer’s Disease Brain Is Associated with Mitochondrial Dysfunction. J. Neurosci. 2006, 26, 9057–9068. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Hoeffer, C.A.; Wong, H.; Massaad, C.A.; Zhou, P.; Iadecola, C.; Murphy, M.P.; Pautler, R.G.; Klann, E. Amyloid β-induced impairments in hippocampal synaptic plasticity are rescued by decreasing mitochondrial superoxide. J. Neurosci. 2011, 31, 5589–5595. [Google Scholar] [CrossRef]
- Kapay, N.A.; Isaev, N.K.; Stelmashook, E.V.; Popova, O.V.; Zorov, D.B.; Skrebitsky, V.G.; Skulachev, V.P. In vivo injected mitochondria-targeted plastoquinone antioxidant SkQR1 prevents β-amyloid-induced decay of long-term potentiation in rat hippocampal slices. Biochemistry 2011, 76, 1367–1370. [Google Scholar] [CrossRef]
- Beyazcicek, E.; Ankarali, S.; Beyazcicek, O.; Ankarali, H.; Demir, S.; Ozmerdivenli, R. Effects of thymoquinone, the major constituent of Nigella sativa seeds, on penicillin-induced epileptiform activity in rats. Neurosciences 2016, 21, 131–137. [Google Scholar] [CrossRef]
- Pottoo, F.H.; Salahuddin, M.; Khan, F.A.; Alomar, F.; Al Dhamen, M.A.; Alhashim, A.F.; Alqattan, H.H.; Gomaa, M.S.; Alomary, M.N. Thymoquinone Potentiates the Effect of Phenytoin against Electroshock-Induced Convulsions in Rats by Reducing the Hyperactivation of m-TOR Pathway and Neuroinflammation: Evidence from In Vivo, In Vitro and Computational Studies. Pharmaceuticals 2021, 14, 1132. [Google Scholar] [CrossRef]
- Sun, H.; Li, X.; Guo, Q.; Liu, S. Research progress on oxidative stress regulating different types of neuronal death caused by epileptic seizures. Neurol. Sci. 2022, 43, 6279–6298. [Google Scholar] [CrossRef]
- Abdel-Zaher, A.O.; Farghaly, H.S.M.; Farrag, M.M.Y.; Abdel-Rahman, M.S.; Abdel-Wahab, B.A. A potential mechanism for the ameliorative effect of thymoquinone on pentylenetetrazole-induced kindling and cognitive impairments in mice. Biomed. Pharmacother. 2017, 88, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Dariani, S.; Baluchnejadmojarad, T.; Roghani, M. Thymoquinone attenuates astrogliosis, neurodegeneration, mossy fiber sprouting, and oxidative stress in a model of temporal lobe epilepsy. J. Mol. Neurosci. 2013, 51, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.Y.; Li, B.; Huang, Y.M.; Luo, Q.; Xie, Y.M.; Chen, Y.H. Thymoquinone Attenuates Brain Injury via an Anti-oxidative Pathway in a Status Epilepticus Rat Model. Transl. Neurosci. 2017, 8, 9–14. [Google Scholar] [CrossRef]
- Davie, C.A. A review of Parkinson’s disease. Br. Med. Bull. 2008, 86, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Mosley, R.L.; Benner, E.J.; Kadiu, I.; Thomas, M.; Boska, M.D.; Hasan, K.; Laurie, C.; Gendelman, H.E. Neuroinflammation, oxidative stress and the pathogenesis of Parkinson’s disease. Clin. Neurosci. Res. 2006, 6, 261–281. [Google Scholar] [CrossRef] [PubMed]
- Venda, L.L.; Cragg, S.J.; Buchman, V.L.; WadeMartins, R. α-Synuclein and dopamine at the crossroads of Parkinson’s disease. Trends Neurosci. 2010, 33, 559–568. [Google Scholar] [CrossRef]
- Sedaghat, R.; Roghani, M.; Khalili, M. Neuroprotective effect of thymoquinone, the nigella sativa bioactive compound, in 6-hydroxydopamine-induced hemi-parkinsonian rat model. Iran. J. Pharm. Res. 2014, 13, 227–234. [Google Scholar] [PubMed]
- Ardah, M.T.; Merghani, M.M.; Haque, M.E. Thymoquinone prevents neurodegeneration against MPTP in vivo and modulates α-synuclein aggregation in vitro. Neurochem. Int. 2019, 128, 115–126. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, X.; Wang, S.; Xu, C.; Gao, M.; Liu, S.; Li, X.; Cheng, N.; Han, Y.; Wang, X.; et al. Thymoquinone Prevents Dopaminergic Neurodegeneration by Attenuating Oxidative Stress Via the Nrf2/ARE Pathway. Front. Pharmacol. 2021, 11, 615598. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Sheikh, B.Y.; Taha, M.M.E.; How, C.W.; Abdullah, R.; Yagoub, U.; El-Sunousi, R.; Eid, E.E. Thymoquinone-loaded nanostructured lipid carriers: Preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administration. Int. J. Nanomed. 2013, 8, 2163–2172. [Google Scholar] [CrossRef]
- Rathore, C.; Hemrajani, C.; Sharma, A.K.; Gupta, P.K.; Jha, N.K.; Aljabali, A.A.A.; Gupta, G.; Singh, S.K.; Yang, J.C.; Dwivedi, R.P.; et al. Self-nanoemulsifying drug delivery system (SNEDDS) mediated improved oral bioavailability of thymoquinone: Optimization, characterization, pharmacokinetic, and hepatotoxicity studies. Drug Deliv. Transl. Res. 2023, 13, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Talebi, M.; Talebi, M.; Farkhondeh, T.; Samarghandian, S. Biological and therapeutic activities of thymoquinone: Focus on the Nrf2 signaling pathway. Phytother. Res. 2021, 35, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, L.; Chen, Y.; Shi, X. Effects of thymoquinone on oxidative stress and cytokine expression in brain of type 2 diabetic rats. Fudan Univ. J. Med. Sci. 2017, 6, 483–488. [Google Scholar]
- Hafez, A.A.; Jamali, Z.; Khezri, S.; Salimi, A. Thymoquinone reduces mitochondrial damage and death of cardiomyocytes induced by clozapine. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
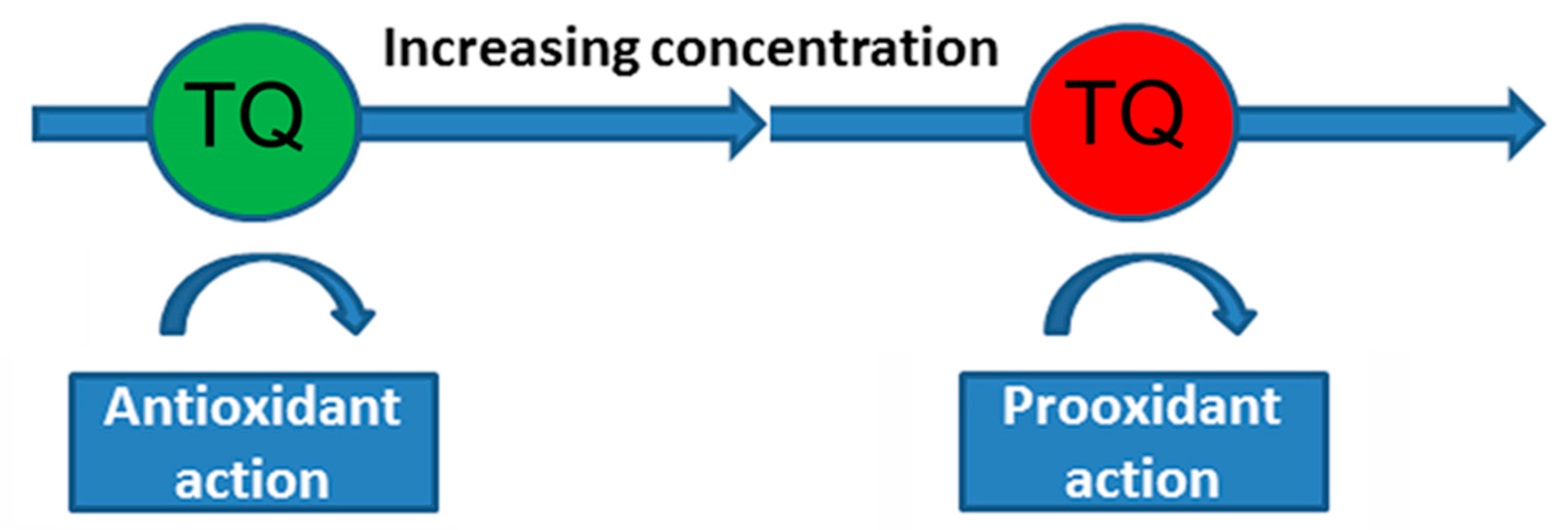
- Mizuno, M.; Fukuhara, K. Antioxidant and Prooxidant Effects of Thymoquinone and Its Hydroquinone Metabolite. Biol. Pharm. Bull. 2022, 45, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.H.; Tayarani-Najaran, Z.; Asghari, M.; Sadeghnia, H.R. Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC12 cells death. Cell Mol. Neurobiol. 2010, 30, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Stelmashook, E.V.; Chetverikov, N.S.; Golyshev, S.A.; Genrikhs, E.E.; Isaev, N.K. Thymoquinone Induces Mitochondrial Damage and Death of Cerebellar Granule Neurons. Biochemistry 2020, 85, 205–212. [Google Scholar] [CrossRef]
- Mansour, M.A.; Nagi, M.N.; El-Khatib, A.S.; Al-Bekairi, A.M. Effects of thymoquinone on antioxidant enzyme activities, lipid peroxidation and DT-diaphorase in different tissues of mice: A possible mechanism of action. Cell Biochem. Funct. 2002, 20, 143–151. [Google Scholar] [CrossRef]
- El-Najjar, N.; Chatila, M.; Moukadem, H.; Vuorela, H.; Ocker, M.; Gandesiri, M.; Schneider-Stock, R.; Gali-Muhtasib, H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis 2010, 15, 183–195. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isaev, N.K.; Genrikhs, E.E.; Stelmashook, E.V. Antioxidant Thymoquinone and Its Potential in the Treatment of Neurological Diseases. Antioxidants 2023, 12, 433. https://doi.org/10.3390/antiox12020433
Isaev NK, Genrikhs EE, Stelmashook EV. Antioxidant Thymoquinone and Its Potential in the Treatment of Neurological Diseases. Antioxidants. 2023; 12(2):433. https://doi.org/10.3390/antiox12020433
Chicago/Turabian StyleIsaev, Nickolay K., Elizaveta E. Genrikhs, and Elena V. Stelmashook. 2023. "Antioxidant Thymoquinone and Its Potential in the Treatment of Neurological Diseases" Antioxidants 12, no. 2: 433. https://doi.org/10.3390/antiox12020433
APA StyleIsaev, N. K., Genrikhs, E. E., & Stelmashook, E. V. (2023). Antioxidant Thymoquinone and Its Potential in the Treatment of Neurological Diseases. Antioxidants, 12(2), 433. https://doi.org/10.3390/antiox12020433






