Abstract
Cognitive impairment is a common non-motor symptom of Parkinson’s disease (PD), which often progresses to PD dementia. PD patients with and without dementia may differ in certain biochemical parameters, which could thus be used as biomarkers for PD dementia. The enzyme paraoxonase 1 (PON1) has previously been investigated as a potential biomarker in the context of other types of dementia. In a cohort of PD patients, we compared a group of 89 patients with cognitive impairment with a group of 118 patients with normal cognition. We determined the kinetic parameters Km and Vmax for PON1 for the reaction with dihydrocoumarin and the genotype of four single nucleotide polymorphisms in PON1. We found that no genotype or kinetic parameter correlated significantly with cognitive impairment in PD patients. However, we observed associations between PON1 rs662 and PON1 Km (p < 10−10), between PON1 rs662 and PON1 Vmax (p = 9.33 × 10−7), and between PON1 rs705379 and PON1 Vmax (p = 2.21 × 10−10). The present study is novel in three main aspects. (1) It is the first study to investigate associations between the PON1 genotype and enzyme kinetics in a large number of subjects. (2) It is the first study to report kinetic parameters of PON1 in a large number of subjects and to use time-concentration progress curves instead of initial velocities to determine Km and Vmax in a clinical context. (3) It is also the first study to calculate enzyme-kinetic parameters in a clinical context with a new algorithm for data point removal from progress curves, dubbed iFIT. Although our results suggest that in the context of PD, there is no clinically useful correlation between cognitive status on the one hand and PON1 genetic and enzyme-kinetic parameters on the other hand, this should not discourage future investigation into PON1’s potential associations with other types of dementia.
1. Introduction
Idiopathic Parkinson’s disease (PD) is the most common parkinsonian syndrome. Although dementia is a defining symptom in some parkinsonisms (e.g., dementia with Lewy bodies), motor symptoms are the cardinal feature of PD. However, non-motor symptoms are nevertheless very important since 22–48% of all PD patients exhibit dementia as a non-motor symptom [1]. PD dementia (PDD) is distinguished from other types of dementia, such as Alzheimer’s disease (AD) and vascular dementia, which may, however, co-occur.
The incidence of cognitive impairment that leads to PDD increases with age and duration of PD. Up to 75% of PD patients will develop cognitive impairment within 10 years of PD onset [1]. The most common pharmacological treatment for PDD consists of acetylcholinesterase inhibitors (e.g., rivastigmine), which were primarily developed for AD but were later shown to be useful in the context of PDD as well. They are also often prescribed to treat visual hallucinations in the context of PD. The other class of drugs for PDD consists of NMDA inhibitors, such as memantine [2]. Nevertheless, similar to other common dementias, PDD is irreversible in most patients [3].
It is known that in the development of PD, oxidative stress plays a large role [4]. In the blood of PD patients, several oxidative-stress-related small molecules, such as homocysteine [5] and coenzyme Q10 [6], as well as enzymes such as superoxide dismutase [7] and xanthine oxidase [8], have been shown to have significantly different concentrations in PD compared to healthy controls. Some of these have also been investigated in relation to the cognition of PD patients, e.g., homocysteine [5,9]. Other serum/plasma factors, such as uric acid [10], epidermal growth factor [11,12], and C-reactive protein [13], have also been associated with PDD. Despite these promising results, there is still no clear biomarker for PDD, and thus the search for better biomarkers continues [5].
Paraoxonase 1 (PON1) is a calcium-dependent enzyme that is present in human blood plasma and associated with high-density lipoprotein particles. Some studies have also investigated PON1 in other body fluids, such as seminal [14,15] and cerebrospinal fluid [16,17]. PON1 is interesting in the context of PD for two main reasons. First, the main function of PON1 in the body is considered to be antioxidative, and oxidative stress has been implicated as a contributing factor to PD [18]. Second, PON1 can hydrolyze organophosphates, which are commonly used as insecticides (e.g., paraoxon), and organophosphate exposure is known to be a potential risk factor for PD [19]. Hence, PON1 has been investigated as a possible protective agent in subjects exposed to organophosphates [20,21].
PON1 can be investigated in several different ways, most notably by (1) genotyping individual single-nucleotide polymorphisms (SNPs) on the PON1 gene, (2) measuring enzyme concentration (which is usually performed with an enzyme-linked immunosorbent assay, i.e., ELISA), and (3) measuring enzyme activity with an artificial substrate of PON1 [22]. Genetic studies on PON1 in PD focused on two SNPs that affect the amino-acid sequence: rs662 (Q192R) and rs854560 (L55M). The L55M polymorphism has particularly been associated with the risk of developing PD in several studies. A meta-analysis by Mota et al. reviewed nine studies [23], of which five identified a higher risk of developing PD for 55MM carriers [24,25,26,27,28], whereas four did not find an association [29,30,31,32]. Another study, performed on a population of agricultural workers, found that among subjects with a history of organophosphate exposure, those with a 55MM-192QQ diplotype showed an increased risk of developing PD compared to 55LL-192RR carriers [33].
PON1 enzymatic activity can be measured using different artificial substrates, which belong to three main groups: lactones (lactonase activity), arylesters (arylesterase activity), and organophosphates (aryldialkylphosphatase, more commonly known as paraoxonase activity) [34,35]. Especially the latter two are commonly used to measure enzymatic activity [36]. The three enzymatic activities do not necessarily correlate with one another, and thus one cannot be extrapolated to the other two [34].
PON1 enzymatic activity has also been investigated in the context of PD. One study found decreased paraoxonase and arylesterase activity in PD patients compared to controls [37]. In another study, decreased paraoxonase activity was associated with PD progression [38]. Decreased paraoxonase activity was also correlated with increased oxidative stress, increased lipid peroxidation, and altered iron metabolism biomarkers in the serum/plasma of PD patients [37,39,40]. PON1 enzymatic activity has also been investigated in the context of Alzheimer’s dementia and vascular dementia, where it has been demonstrated that paraoxonase activity and arylesterase activity are both reduced in dementia patients compared to healthy controls [41,42,43,44]. Several other types of dementia, however, have not yet been investigated in connection with PON1; notable among them is PDD.
Some studies indicate that oxidative stress might affect the Michaelis constant (Km) of PON1 [45]. In the context of catalase and related oxidoreductases, Km has been shown to be a better indicator of oxidative stress than maximal velocity Vmax; the authors of the study suggest that stress results in post-translational modifications of the enzyme, such as oxidation of thiol groups, which then change the enzyme’s affinity for its substrate [46]. Under high-stress conditions, oxidative damage to PON1 can be caused by its neighbor in high-density lipoprotein particles, the enzyme myeloperoxidase [47]. Of note, high oxidative stress has been proposed to be a contributing factor to PDD [48].
The aim of the present study was to investigate whether PDD is associated with the genotype or kinetic activity of PON1. We evaluated whether cognitive test scores, as determined with the mini mental state examination (MMSE) or the binary division of patients (dementia vs. no dementia), are correlated with any of the above-mentioned enzymatic parameters Km and Vmax. Furthermore, we investigated the associations between different PON1 kinetic parameters and PON1 genotypes, as well as between the enzyme kinetics and genetics on the one hand and demographic or clinical parameters on the other hand. Finally, we wanted to assess these associations on our own sample of patients in order to demonstrate the accuracy of our newly developed method that we use for data analysis (e.g., iFIT).
2. Materials and Methods
2.1. Patients
A total of 231 PD patients were recruited from the Neurology Clinic at the University Medical Centre in Ljubljana from October 2016 to April 2018. The study protocol was approved in advance by the Slovenian Ethics Committee for Research in Medicine (0120-268/2016/11 and 0120-296/2016/11). Upon inclusion, all subjects gave written informed consent in accordance with the Declaration of Helsinki. Patient demographic and clinical data were collected during an interview and from the medical documentation; blood samples were also collected. Detailed information about the study cohort is presented in Table 1 and is also available elsewhere [49].

Table 1.
Characteristics of PD patients in the present study.
The general inclusion criteria were as follows: (1) a clinical diagnosis of PD, made by a movement disorder specialist in accordance with the UK Parkinson Disease Society Brain Bank criteria [50], (2) absence of secondary or atypical forms of parkinsonism, (3) the availability of all necessary clinical data, (4) ongoing treatment with levodopa or dopamine agonists that began at least 3 months prior to the inclusion.
Patients were divided into a group with dementia and a group without dementia based on their clinical diagnoses and MMSE scores. The patient inclusion criteria for the dementia group were as follows: (1) an MMSE score of less than 26, (2) a prior diagnosis of PDD or unspecified cognitive decline, or (3) antidementive treatment but lack of visual hallucinations. The patient inclusion criteria for the group without dementia were as follows: (1) an MMSE score of 26 or more and (2) a record that did not indicate a cognitive decline. The exclusion criteria for both groups were as follows: (1) other forms of dementia (e.g., AD); (2) no MMSE in the past 2 years; and (3) a combination of MMSE score of 26 or higher, present visual hallucinations, and antidementive treatment (e.g., rivastigmine). The reason behind criterion (3) was that cognitive decline and visual hallucinations are both indications for prescribing antidementives. We used the most recent MMSE results, which were not more than 2 years old.
Patients who could not be classified into any of the two groups were not accounted for when comparing cognitive status with genetic and enzymatic parameters but were accounted for when comparing different enzymatic parameters and/or genetics with each other.
2.2. DNA Isolation and Genotyping
Peripheral blood samples were collected into EDTA tubes and immediately centrifuged at 2200× g and 4 °C for 10 min. Afterward, the plasma and cellular fractions were stored separately at −80 °C.
Genomic DNA was isolated from white blood cells with the FlexiGene DNA Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Based on the available literature, four different PON1 SNPs (rs662, rs854560, rs705379, and rs75381) were included in the analysis. These four common functional SNPs have already been associated with PON1 activity in several previous studies [32,36,51,52,53,54,55]. SNPs were genotyped using the KASPar assay according to the manufacturer’s instructions. Their additional data are displayed in Supplementary Table S1. A total of 10% of all samples was genotyped twice as a form of quality control; the results were the same as the original measurements. The p-values indicated that all four SNPs were in Hardy–Weinberg equilibrium in our study population.
2.3. Enzyme-Kinetic Measurements
Enzyme activity was measured as previously described [56]. In brief, dihydrocoumarin (DHC) was used as a substrate, prepared as a 25 mM stock solution in methanol. Measurements were performed in a buffer consisting of 50 mM Tris and 1 mM CaCl2, pH = 8, at room temperature. Each reaction had a total volume of 2 mL and was performed in a 1 cm cuvette. For each measurement, 10 μL of plasma and 20 μL of substrate stock solution (final concentration: 250 μM) were added to 1970 μL of the buffer. The substrate was added last, after which measurements were immediately started. Absorbance at 270 nm was measured every second until the progress curve had reached its plateau.
Two different approaches to measuring Km and Vmax are possible to date. Traditionally, initial velocities of an enzymatic reaction are plotted on a MM diagram or its linear derivations, such as the Lineweaver–Burk plot [57]. In recent decades, it has also become increasingly feasible to fit model functions onto entire reaction progress curves, which means that only one enzymatic reaction can be sufficient to acquire an estimate of Km and Vmax [58,59]. Several ways of fitting model function onto progress curves are possible, for example, (1) approximating enzymatic parameters with a system of differential equations (e.g., with the programs ENZO [60] and Dynafit [61]) and (2) modeling the progress curve using a version of the integrated MM equation [62,63].
At least two progress curves were measured for each sample. Afterward, the enzyme-kinetic parameters Km and Vmax were extracted from each progress curve using the program iFIT, which applies the integrated MM equation to the area of maximum curvature of the progress curve, where the most information regarding Km is encoded. The program and its theoretical basis are presented in Petrič et al. (2022a) [64], and a comparison between iFIT and established methods of kinetic data analysis is published in Petrič et al. (2022b) [56]. Vmax was reported as μM/min, where μM refers to the concentration in the final reaction mixture. Km was reported as μM.
2.4. Statistical Analysis
For each patient, we averaged the Km and Vmax values for all measured progress curves. Before averaging, we removed values that were probably a consequence of noisy progress curves, which in turn resulted from insufficiently homogenous plasma samples. The values removed include both very large (Km > 15 μM and Vmax > 200 μM/min) and very small values (Km < 1.5 μM and Vmax < 10 μM/min). We also removed all values that were identified as outliers by the Grubbs test.
When we compared biological parameters (Km, Vmax, and genotype) with each other, we included all patients for whom the relevant parameters were known, including those with unclear dementia status. However, when we compared MMSE or age with physiological parameters or MMSE and age with each other, we only included patients with known dementia status, i.e., with MMSE scores not older than 2 years.
The program SPSS was used for all statistical analyses. The MMSE, Km, and Vmax values were all distributed non-normally, according to the Kolmogorov–Smirnov test (p < 0.05 in all cases). Thus, statistical tests that assume a non-normal distribution were used, and the results were reported as medians and interquartile ranges (IQRs). The Pearson Chi-squared test was used to compare categorical parameters with each other. The Mann–Whitney test (for two groups) or the Kruskal–Wallis test (for more than two groups) were used to compare categorical and continuous parameters. For categorical dependent parameters, logistic regression was used, and the odds ratio (OR) and 95% confidence interval (CI) were reported. The Spearman test was used to compare continuous parameters, and the Spearman correlation coefficient (CC) and p-values were reported. For the Kruskal–Wallis, Mann–Whitney, and Pearson Chi-squared tests, only p-values were reported. A p-value of 0.05 was used as a basic significance threshold. A total of 22 comparisons were made between genetic, biochemical, demographic, and clinical parameters, and thus Bonferroni correction was used to produce a new significance threshold of p = 0.05/22 = 0.0023.
3. Results
3.1. General Characteristics of the Study Population
In total, 89 PD patients were included in the dementia group, and 118 PD patients were included in the group without dementia, which adds up to 207 patients. Although 24 additional PD patients could not be classified into any of the two groups, they were included in the comparisons between genotype and enzyme kinetics. Due to limitations in sample quality, average PON1 Vmax and Km values could only be determined for 159 and 194 patients, respectively. The characteristics of the patients are presented in Table 1.
3.2. Association between Genotype and Kinetic Parameters
When we divided patients into three groups based on their rs662 genotype, PON1 Km values between the three groups were significantly different (p < 10−10). The PON1 rs854560 (L55M) polymorphism also appeared to significantly influence Km (p = 0.005). However, rs662 and rs854560 had a large D’ value, indicating that they were in linkage disequilibrium (see Supplementary Table S2). Thus, we divided patients into three groups based on their rs662 genotype (see Table 2) and assessed the associations between Km and rs854560 while keeping the rs662 genotype constant. Under these conditions, the associations between Km and rs854560 genotypes within each of the three groups were not significant (p > 0.5). Conversely, if we kept rs854560 constant, we could detect significant associations between Km and the rs662 genotype (at constant rs854560 TA: p = 2.53 × 10−8; at constant rs854560 AA: p = 3.44 × 10−10). The other two investigated polymorphisms, rs705379 and rs7538, are located in the non-coding region of the PON1 gene and were not associated with Km.

Table 2.
Average Km values (in μM) for each pair of genotypes of the PON1 SNPs rs662 and rs854560. Some pairs of genotypes were not present in any patient in our study (N = 0). A cell shows the number (N) of patients with a certain pair of genotypes and the median (interquartile range) for Km.
All four SNPs were significantly associated with PON1 Vmax (p < 0.005). The strongest association was observed between Vmax and rs705379 (p = 2.21 × 10−10). As rs705379 and rs705381 also have a high D’ value, we again divided patients into three groups based on their rs705381 genotype (see Table 3) and tested the associations between the rs705379 genotype and Vmax while keeping the rs705381 genotype constant. The association between rs705379 and Vmax was significant within the rs705381 CC subgroup (p = 5.14 × 10−7), whereas it was not significant within the rs705381 TC group (p = 0.148). The associations between Vmax and the rs705381 genotype were non-significant within each of the groups (p = 0.697 for rs705379 = GA; p = 0.612 for GG; the AA group contained only one pair of genotypes).

Table 3.
Average Vmax values (in μM/min) for each pair of genotypes of the PON1 SNPs rs705379 and rs705381. Some pairs of genotypes were not present in any patient in our study (N = 0). A cell shows the number (N) of patients with a certain pair of genotypes and the median (interquartile range) for Vmax.
As rs705379 and rs854560 also have a high D’ value, we divided subjects into three groups based on their rs854560 genotype and tested the association between Vmax and the rs705379 genotype within each of the groups with a specific rs854560 genotype. The association between Vmax and rs705379 was significant for TA (p = 1.3 × 10−5 for rs854560 TA; p = 0.006 for AA; p = 0.36 for TT, which was not significant, possibly due to the small number of subjects in the group). No association was observed between Vmax and rs854560 within any of the groups (p = 0.568 for rs705379 AA; p = 0.136 for GA; p = 0.697 for GG). The results are shown in Table 4.

Table 4.
Average Vmax values (in μM/min) for each pair of genotypes of the PON1 SNPs rs705379 and rs854560. Some pairs of genotypes were not present in any patient in our study (N = 0). A cell shows the number (N) of patients with a certain pair of genotypes and the median (interquartile range) for Vmax.
Both rs662 and rs705379 remained significantly associated with Vmax (p = 9.33 × 10−7 and 2.21 × 10−10, respectively), regardless of which other SNP we set as a constant. Furthermore, rs662 retained a significant association with Km (p < 10−10). These results are presented in Table 5. We also investigated potential correlations between demographic, clinical, and enzyme-kinetic parameters. Two significant associations were observed between (1) Km and Vmax (CC = 0.310, p = 2.75 × 10−5) and (2) age and MMSE (CC = −0.457, p = 1.84 × 10−9). For the latter comparison, we only included subjects with known dementia status (data not shown).

Table 5.
Average Km and Vmax values for each rs662/rs705379 genotype. Genotype-kinetics associations that were below the significance threshold because of linkage disequilibrium are not shown. Median (interquartile range) Km (μM) and Vmax (μM/min) values are shown, as well as N.
3.3. Association between Genetic, Enzyme-Kinetic, and Clinical Parameters
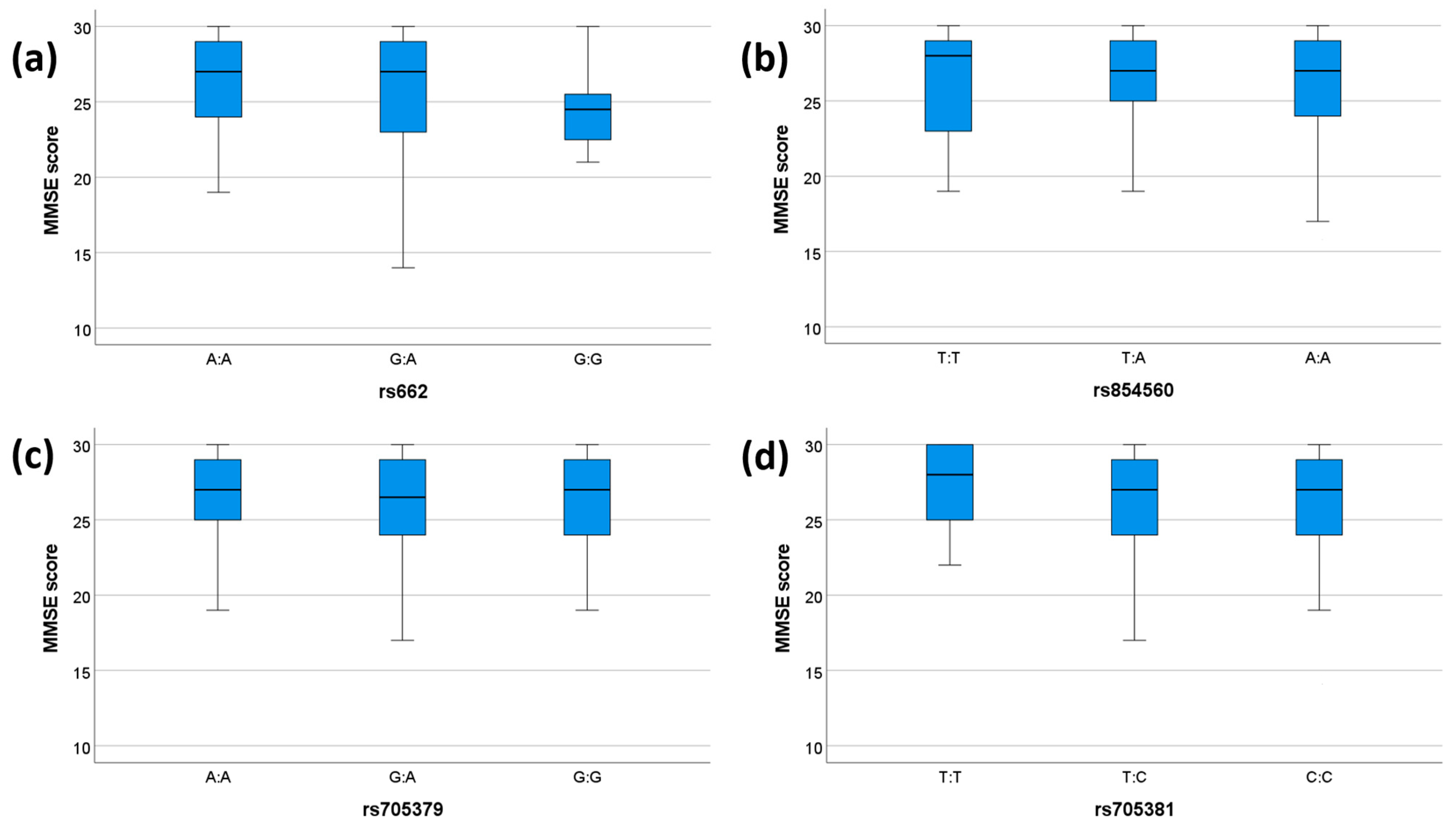
We tested the association between clinical parameters (MMSE and presence/absence of dementia) and genetic variability of PON1. None of the SNPs showed any significant associations with dementia status (Table 6). A significant association was observed between the rs662 genotype and MMSE scores (p = 0.046), which, however, did not pass the Bonferroni correction (Table 7). No other significant association between the investigated SNPs and MMSE was observed (Table 7). The comparisons between genotypes and MMSE scores for each SNP are shown in Figure 1.

Table 6.
Associations between dementia status and PON1 single-nucleotide polymorphisms (SNPs).

Table 7.
The associations between PON1 genetic/enzyme-kinetic parameters (Km, Vmax, and genotype) and cognitive parameters (MMSE and presence of dementia). Correlation coefficients are shown only for comparisons of numeric parameters.
Figure 1.
MMSE scores for each genotype. The box plots display the 5-, 25-, 50-, 75-, and 95-percentage intervals for the genotypes (a) rs662, (b) rs854560, (c) rs705379, and (d) rs705381. The medians (interquartile ranges) are displayed above each box plot. MMSE: mini mental state examination. For (a), N (AA) = 75, N (GA) = 51, N (GG) = 11. For (b), N (TT) = 18, N (TA) = 61, N (AA) = 58. For (c), N (AA) = 37, N (GA) = 55, N (GG) = 45. For (d), N (TT) = 14, N (TC) = 42, N (CC) = 81.
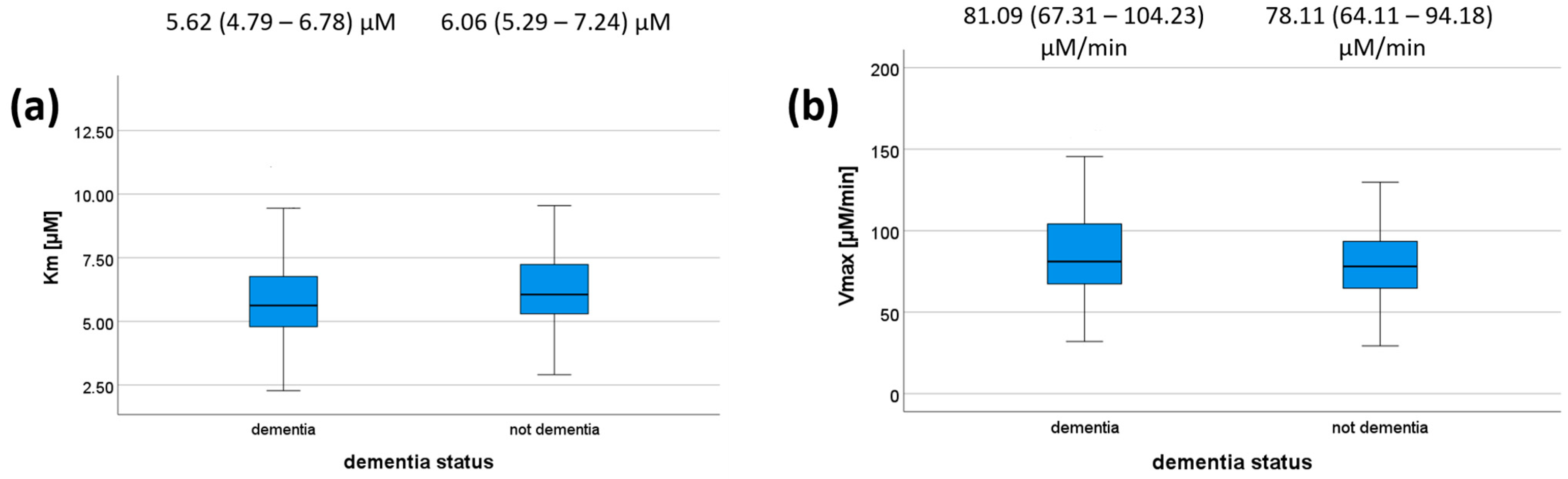
We also tested whether there is any association between dementia status/MMSE on the one hand and PON1 kinetic parameters on the other hand for the reaction with DHC. MMSE scores did not correlate with any of the kinetic parameters (Table 7) or dementia status (Figure 2); however, a trend was observed (p = 0.073 for Km). Patients with dementia had a median Km value of 5.62 μM (IQR = 4.79–6.78 μM), whereas those without dementia had a median of 6.06 μM (IQR = 5.29–7.24 μM). Patients with dementia had a median Vmax value of 81.09 μM/min (IQR = 67.31–104.23 μM/min), whereas those without dementia had a median of 78.11 μM/min (IQR = 64.11–94.18). These results are shown in Figure 2, and their respective p-values are shown in Table 7. We did not compare kinetic parameters and cognitive status for each genotype individually, as all the genotype–cognitive status associations were non-significant to begin with.
Figure 2.
Km (a) and Vmax (b) values according to dementia status. The 5-, 25-, 50-, 75-, and 95-percentage intervals are shown. The medians (interquartile ranges) are displayed above each box plot. For (a), N (dementia) = 112, N (not dementia) = 82. For (b), N (dementia) = 96, N (not dementia) = 63.
4. Discussion
The present study investigated the associations between PON1 genetics and enzyme activity in PDD while also aiming to demonstrate the utility of our new methodological approach for determining PON1 kinetic parameters. The most advantageous part of the reported investigation turned out to be the association between the PON1 genotype and activity. Regarding PDD, our data showed no association between PDD and either PON1 genotype or activity. Thus, we were unable to demonstrate whether this enzyme’s genotype and activity associate better with the MMSE score or the presence/absence of dementia.
4.1. Methodological Improvements
In the present study, we tested several innovations that had not been previously used in medical research on enzyme activity in general or PON1 activity in particular. Enzyme activity, i.e., the amount of substrate consumed or product formed in a unit of time, is most commonly reported in enzyme units [U]; dividing enzyme units by enzyme mass results in specific activity [U/mg]. For clinical samples, the total mass of protein that was present in the enzymatic reaction is usually presented as a proxy for the amount of enzyme when reporting specific activity [65]. Alternatively, enzyme activity can also be divided by the volume of relevant body fluid [U/mL]; in the case of PON1, these measurements are referred to as the “rate of hydrolysis” [22]. Nevertheless, (specific) enzyme activity is a crude metric because differences in activity in impure biological samples can be caused either by a change in enzyme concentration or by a change in the enzyme’s affinity to its substrate [61].
Hence, for our samples, we measured Km and Vmax instead of the rate of hydrolysis for two main reasons. First, the enzyme Km has been proposed as a better indicator of oxidative-stress-induced damage than enzyme-specific activity [46]. Second, while inter-patient differences in Vmax are well-associated with differences in activity, Vmax is independent of parameters such as substrate concentration and more clearly defined than enzyme-specific activity. We also introduced a more precise approach to determining Km, using an approximation of the integrated MM equation [66] as well as an entirely novel improvement of this approach [64], which removes unnecessary points from the reaction progress curve.
Acquiring Km and Vmax from entire progress curves rather than initial velocities has several benefits: fewer experiments are necessary, many more data points are available from a single reaction, and less emphasis is required regarding accurate substrate concentration and the immediate start of the reaction. However, particularly when Km is small compared to [S]0, only a small part of the progress curve will actually encode information about Km, and thus noise in the remainder of the progress curve can easily skew the output Km value. To counter this, Stroberg and Schnell proposed a formula to calculate the time period, denoted tQ, during which the progress curve is at its maximum curvature [67]. Based on their formula, an iterative method was subsequently developed that calculates tQ based on an initial estimate of kinetic parameters and then recalculates these parameters based only on the area of the progress curve denoted by tQ, using a numeric approximation of the integrated MM equation [56]. This iterated method, dubbed “iFIT”, is available online at http://i-fit.si/ (accessed on 1 September 2022) [64].
We only performed our measurements on DHC as a substrate because lactonase activity has been proposed as the native activity of PON1 [68]. The actual substrates of PON1 inside the human body are still unclear, especially in the case of patients with (Parkinson’s) dementia. Thus, it is difficult to predict which artificial substrate would be the most clinically relevant. It has been noted that when comparing PON1 enzyme activities between disease phenotypes, the use of different substrates may produce opposite results within a single study, i.e., one type of substrate activity might increase with disease severity, whereas another one might decrease. Particularly organophosphate substrates might behave differently than lactone and arylester substrates [34]. However, organophosphatase activity is clearly not the enzyme’s native activity and is only interesting in a clinical context for patients who were exposed to organophosphates (e.g., pesticides). As this was not the focus of our study, we did not measure organophosphatase activity.
Our absolute Km values for the reaction of human plasma PON1 with DHC are lower than those published by Billecke et al., which are, to date, the only reported human serum/plasma PON1 Km values [69]. There are different possible explanations for this discrepancy, including the use of different extinction coefficients (1310 M−1 cm−1 in our study vs. 876 M−1 cm−1 in Billecke et al.) and buffer compositions (1 mM Ca2+ in our study, whereas Billecke et al. do not state which Ca2+ concentration they used). However, the quotient between the average Km values for the 192QQ and 192RR genotypes in our study was 1.68 (6.91 vs. 4.15 μM, respectively), equivalent to the one reported by Billecke et al. (1.69, 22 vs. 13 μM, respectively). This strongly indicates that our acquired Km values are reliable for the purpose of inter-patient comparison.
It is already known for PON1 that the rs662 (Q192R) polymorphism affects the PON1 Km value for the substrate DHC [70] and that the rs705379 (−108 C/T) polymorphism affects PON1 concentration. A connection between rs705381 (−162) and enzyme concentration has also been proposed [71]. We were interested in testing these associations on our samples to confirm the accuracy of our method. By determining the association between all other SNPs and both Km and Vmax, we also ensured that any potential association between PON1 kinetics and cognitive status was not confounded by the influence of genetics.
4.2. Associations between PON1 Genotypes and Kinetic Parameters
Our results demonstrate that both rs662 and rs705379 influence enzyme-kinetic properties: rs662 is associated with both Km and Vmax, whereas rs705379 is only associated with Vmax. This implies that the Q192R (rs662) polymorphism changes the affinity of PON1 towards its substrate, as has been suggested before [69]. The −108 C/T (rs705379) polymorphism in the promoter region influences the enzyme concentration but not the enzyme-substrate affinity. This has also been demonstrated before [70,71,72] and can be explained by the fact that the rs705379 site is the binding site of the Sp1 transcription factor [53]. The relationship between rs705381 and Vmax, once linkage disequilibrium with rs705379 is taken into account, does not show any association. This implies that rs705381 is of limited importance in enzyme-kinetic studies, even though other groups have identified a relationship between this genotype and PON1 concentration [70]. Conversely, rs705379 should be genotyped whenever investigating PON1 concentration or Vmax in order to prevent confounding results.
Although these associations between genotype and kinetics have been shown before, they were not investigated using DHC as a substrate, even though lactonase activity is considered the native activity of PON1 [68], whereas certain other commonly used substrates, e.g., paraoxon, are clearly not closely related to PON1’s natural substrates. Only the rs662–Km relation has indeed been investigated with DHC by Billecke et al., but only on the pooled sera of 3–5 individuals [69]. Additionally, DHC has the advantage of having a low Km (in the μM range), which means that hypothetical other enzymes catalyzing the same reaction, but with higher Km’s, will have a small effect on the calculated Km value. We were also the first to investigate the genotype–Vmax association for any substrate of PON1, whereas all other studies either measured enzyme concentration [71,72] or used enzyme activity (in enzyme units) [70] as a substitute for concentration. Of note, all these analyses were performed using iFIT, indicating that iFIT is suitable for work with larger amounts of clinical data.
4.3. Associations between Genetic, Enzyme-Kinetic, and Clinical Parameters
To date, no study has compared PDD patients with cognitively normal PD patients in terms of PON1 genetics, concentration, or activity. Previous studies have reported an association of PON1 status with the presence of PD, compared to healthy controls [37,38]. PON1 SNPs have also been investigated in the context of other dementias (e.g., AD), for which any connection with any SNP is very inconclusive [54,55]. Hence, it was difficult to predict whether any measure of cognitive status would associate with genetic and enzyme-kinetic data and, specifically, whether MMSE score or dementia status was more likely to associate with enzyme genetics or with kinetics. This ties back to one of our aims in the present study: to ascertain whether dementia status, which is the default measure of cognitive status in articles investigating the connection between PON1 and dementia, can also be usefully supplemented by recording the MMSE score.
The only polymorphism for which the association with MMSE score passed the significance threshold of 0.05 was rs662; however, it did not pass the Bonferroni correction. Additionally, none of the polymorphisms were significantly associated with dementia status. It is known that cognitive status decreases with age [73], and we also demonstrated an association between MMSE score and age in this study. A longer average lifespan of 192RR homozygotes could explain this vaguely significant trend. There is, however, no indication of such an effect in the literature. A more plausible explanation would be that the apparent trend is due to the small number of 192RR homozygotes in our study.
We next investigated how cognitive status associates both with Km and with Vmax. The comparison between Km and dementia was much closer to significance than the comparison between Km and MMSE score. This would imply that the division into groups successfully presented the qualitative differences between patients with and without dementia. It would also imply, conversely, that the MMSE score varied too much over time to be useful on its own. Indeed, collecting MMSE scores within a long, 2-year interval around the date of inclusion was a limitation of our study. However, even if PON1 Km affects cognitive status, our results show that this effect is probably very small. Hence, PON1 Km is probably not worth pursuing as a diagnostic or prognostic tool for PDD.
The associations between cognitive status and Vmax were non-significant. Probably similar reasoning holds true as mentioned above for Km. Even if a PD patient’s PON1 Vmax changes alongside a decline in cognitive status, the effect is so small that it is unlikely to be useful for clinical purposes. The ratio Vmax/Km is sometimes used as a kinetic parameter because it can convey more information than either Km or Vmax under certain circumstances (e.g., when substrate concentration is low compared to Km). However, in our study, there were no associations between Vmax/Km and other parameters (which also did not associate with Km or Vmax).
Apart from those already mentioned, several additional limitations of the study should be kept in mind. We did not collect data on motor symptoms and thus could not evaluate any potential connection between PON1 status and motor deficits. We also did not acquire any data on blood oxidative stress biomarkers (other than PON1), which could serve to expand the study and evaluate PON1 as a general proxy for oxidative status. Finally, we did not measure PON1 concentration, which could be used to calculate one more kinetic parameter, kcat, as an addition to the parameters Vmax and Km that we determined. All of the above remain open for future research.
5. Conclusions
Our study helped to clarify the influence of the PON1 genotype on Km and Vmax for the reaction of PON1 with DHC. This has not been previously discussed in the literature. A major novelty of our study was that it demonstrated that the program iFIT, and the integrated MM equation with data point removal that it is based on, is useful for analyzing a large number of clinical samples. Based on our data, we highly advise both using the integrated MM equation and removing surplus data points for routine determination of Km and Vmax values of MM-type enzymatic reactions rather than using older and more time-consuming methods. Although our results suggest that there is no clinically useful correlation between cognitive status and PON1 genetic and enzyme-kinetic parameters in the context of PD, this does not imply that PON1 does not play a role in other kinds of dementia. Therefore, future studies should investigate PON1 in the context of other neurodegenerative diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12020399/s1, Table S1: Single-nucleotide polymorphisms (SNPs) that were analyzed in the present study and their distributions in our population of patients. Median allele frequency (MAF) values were taken from the 1000 Genomes study [70]; Table S2: Linkage disequilibrium (LD) for each pair of the SNPs rs662, rs854560, rs705379, and rs705381, quantified with the parameters D’ and R2, for a European population. Source: LDLink [71]. D’ and R2 are quantitative measures of LD, which both range from 0 (no LD) to 1 (complete LD). D’: (normalized) deviation from an expected distribution of frequencies; R2: the correlation between a pair of loci.
Author Contributions
Conceptualization, A.B. and B.P.; methodology, B.P. and A.B.; software, A.B. and M.G.; formal analysis, B.P., N.M., S.R.T. and A.B.; writing—original draft preparation, B.P.; writing—review and editing, B.P., S.R.T., V.D., M.T., M.G.K., N.M. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Slovenian Research Agency program P1-0170 and project J7-2600.
Institutional Review Board Statement
The study was approved by the Slovenian Ethics Committee for Research in Medicine (0120-268/2016/11 and 0120-296/2016/11), and the experimental protocol for involving patients followed the Declaration of Helsinki.
Informed Consent Statement
Informed consent obtained from study participants was written. It is an obligatory part of the ethics approval issued by the Slovenian Ethics Committee for Research in Medicine.
Data Availability Statement
The data that support the findings of this study, as well as a blank copy of the Informed Consent Statement, are available from the corresponding author.
Acknowledgments
We are thankful to technician Marjan Kužnik, who participated in measuring PON1 activity, and to Katja Goričar for her help with data analysis. We also thank Eva Lasić for reviewing a draft of this manuscript.
Conflicts of Interest
The authors report no conflict of interest related to this study.
References
- Aarsland, D.; Kurz, M.W. The epidemiology of dementia associated with Parkinson disease. J. Neurol. Sci. 2010, 289, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Armstrong, M.J. Treatment of Parkinson’s Disease with Cognitive Impairment: Current Approaches and Future Directions. Behav. Sci. 2021, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Kang, X.; Hu, J.; Zhang, D.; Liang, Z.; Meng, F.; Zhang, X.; Xue, Y.; Maimon, R.; Dowdy, S.F.; et al. Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature 2020, 582, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-H.; Chen, C.-M. The Role of Oxidative Stress in Parkinson’s Disease. Antioxidants 2020, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Irizarry, M.C.; Gurol, M.E.; Raju, S.; Diaz-Arrastia, R.; Locascio, J.J.; Tennis, M.; Hyman, B.T.; Growdon, J.H.; Greenberg, S.M.; Bottiglieri, T. Association of homocysteine with plasma amyloid beta protein in aging and neurodegenerative disease. Neurology 2005, 65, 1402–1408. [Google Scholar] [CrossRef]
- Sohmiya, M.; Tanaka, M.; Tak, N.W.; Yanagisawa, M.; Tanino, Y.; Suzuki, Y.; Okamoto, K.; Yamamoto, Y. Redox status of plasma coenzyme Q10 indicates elevated systemic oxidative stress in Parkinson’s disease. J. Neurol. Sci. 2004, 223, 161–166. [Google Scholar] [CrossRef]
- Sharma, A.; Kaur, P.; Kumar, B.; Prabhakar, S.; Gill, K.D. Plasma lipid peroxidation and antioxidant status of Parkinson’s disease patients in the Indian population. Parkinsonism Relat. Disord. 2008, 14, 52–57. [Google Scholar] [CrossRef]
- Gökçe Çokal, B.; Yurtdaş, M.; Keskin Güler, S.; Güneş, H.N.; Uçar, C.A.; Aytaç, B.; Durak, Z.E.; Yoldaş, T.K.; Durak, I.; Çubukçu, H.C. Serum glutathione peroxidase, xanthine oxidase, and superoxide dismutase activities and malondialdehyde levels in patients with Parkinson’s disease. Neurol. Sci. 2017, 38, 425–431. [Google Scholar] [CrossRef]
- Zoccolella, S.; Lamberti, P.; Iliceto, G.; Dell’Aquila, C.; Diroma, C.; Fraddosio, A.; Lamberti, S.V.; Armenise, E.; DeFazio, G.; De Mari, M.; et al. Elevated plasma homocysteine levels in L-dopa-treated Parkinson’s disease patients with dyskinesias. Clin. Chem. Lab. Med. 2006, 44, 863–866. [Google Scholar] [CrossRef]
- Maetzler, W.; Stapf, A.K.; Schulte, C.; Hauser, A.-K.; Lerche, S.; Wurster, I.; Schleicher, E.; Melms, A.; Berg, D. Serum and cerebrospinal fluid uric acid levels in lewy body disorders: Associations with disease occurrence and amyloid-β pathway. J. Alzheimer’s Dis. 2011, 27, 119–126. [Google Scholar] [CrossRef]
- Pellecchia, M.T.; Santangelo, G.; Picillo, M.; Pivonello, R.; Longo, K.; Pivonello, C.; Vitale, C.; Amboni, M.; De Rosa, A.; Moccia, M.; et al. Serum epidermal growth factor predicts cognitive functions in early, drug-naive Parkinson’s disease patients. J. Neurol. 2013, 260, 438–444. [Google Scholar] [CrossRef]
- Chen-Plotkin, A.S.; Hu, W.T.; Siderowf, A.; Weintraub, D.; Gross, R.G.; Hurtig, H.I.; Xie, S.X.; Arnold, S.E.; Grossman, M.; Clark, C.M.; et al. Plasma epidermal growth factor levels predict cognitive decline in Parkinson disease. Ann. Neurol. 2011, 69, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, D.; Hall, S.; Surova, Y.; Nielsen, H.M.; Janelidze, S.; Brundin, L.; Hansson, O. Cerebrospinal fluid inflammatory markers in Parkinson’s disease--associations with depression, fatigue, and cognitive impairment. Brain, Behav. Immun. 2013, 33, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Marsillach, J.; Lafuente, R.; Checa, M.A.; Maestre-Martínez, C.; Fabián, E.; Brassesco, M.; Beltrán-Debón, R.; Aragonès, G.; Carreras, R.; Pedro-Botet, J.; et al. Paraoxonase-1 is only present in traceable amounts in seminal fluid and does not show any relationship with male subfertility. BJU Int. 2011, 108, 566–570. [Google Scholar] [CrossRef]
- Pradieé, J.; de Campos, F.T.; Rincon, J.; Collares, L.; Goularte, K.; Silveira, P.; Pegoraro, L.; Schneider, A. Paraoxonase 1 (PON1) activity in serum, follicular fluid and seminal plasma of sheep. Reprod. Domest. Anim. 2017, 52, 1142–1144. [Google Scholar] [CrossRef]
- Castellazzi, M.; Trentini, A.; Romani, A.; Valacchi, G.; Bellini, T.; Bonaccorsi, G.; Fainardi, E.; Cavicchio, C.; Passaro, A.; Zuliani, G.; et al. Decreased arylesterase activity of paraoxonase-1 (PON-1) might be a common denominator of neuroinflammatory and neurodegenerative diseases. Int. J. Biochem. Cell Biol. 2016, 81, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Higginbotham, L.; Ping, L.; Dammer, E.B.; Duong, D.M.; Zhou, M.; Gearing, M.; Hurst, C.; Glass, J.D.; Factor, S.A.; Johnson, E.C.B.; et al. Integrated proteomics reveals brain-based cerebrospinal fluid biomarkers in asymptomatic and symptomatic Alzheimer’s disease. Sci. Adv. 2020, 6, eaaz9360. [Google Scholar] [CrossRef]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Androutsopoulos, V.P.; Kanavouras, K.; Tsatsakis, A.M. Role of paraoxonase 1 (PON1) in organophosphate metabolism: Implications in neurodegenerative diseases. Toxicol. Appl. Pharmacol. 2011, 256, 418–424. [Google Scholar] [CrossRef]
- Harley, K.G.; Huen, K.; Aguilar Schall, R.; Holland, N.T.; Bradman, A.; Barr, D.B.; Eskenazi, B. Association of organophosphate pesticide exposure and paraoxonase with birth outcome in Mexican-American women. PLoS ONE 2011, 6, e23923. [Google Scholar] [CrossRef]
- Furlong, C.E.; Suzuki, S.M.; Stevens, R.C.; Marsillach, J.; Richter, R.; Jarvik, G.; Checkoway, H.; Samii, A.; Costa, L.; Griffith, A.; et al. Human PON1, a biomarker of risk of disease and exposure. Chem. Interact. 2010, 187, 355–361. [Google Scholar] [CrossRef]
- Ceron, J.J.; Tecles, F.; Tvarijonaviciute, A. Serum paraoxonase 1 (PON1) measurement: An update. BMC Vet. Res. 2014, 10, 74. [Google Scholar] [CrossRef]
- Mota, A.; Taheraghdam, A.; Valilo, M. Paraoxonase 1 and its relationship with Parkinson’s disease. Brain Nerves 2019, 4. [Google Scholar] [CrossRef]
- Kondo, I.; Yamamoto, M. Genetic polymorphism of paraoxonase 1 (PON1) and susceptibility to Parkinson’s disease. Brain Res. 1998, 806, 271–273. [Google Scholar] [CrossRef]
- Akhmedova, S.N.; Yakimovsky, A.K.; Schwartz, E.I. Paraoxonase 1 Met--Leu 54 polymorphism is associated with Parkinson’s disease. J. Neurol. Sci. 2001, 184, 179–182. [Google Scholar] [CrossRef]
- Carmine, A.; Buervenich, S.; Sydow, O.; Anvret, M.; Olson, L. Further evidence for an association of the paraoxonase 1 (PON1) Met-54 allele with Parkinson’s disease. Mov. Disord. 2002, 17, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Đurić, G.; Svetel, M.; Ilarioškin, S.; Dragađević, N.; Gavrilović, J.; Kostić, V. Polimorfizmi gena citohrom oksidaze P450 2D6 (CYP2D6), paraoksonaze 1 (PON1) iapolipoproteina (APOE) kao faktori rizika od razvoja Parkinsonove bolesti = Polymorphisms in the genes of citohrom oxidase P450 2D6 (CYP2D6), paraxonase 1 (PON1) and apolipoprot. Mil. Med. Pharm. J. Serb. 2007, 64, 25–30. [Google Scholar]
- Manthripragada, A.D.; Costello, S.; Cockburn, M.G.; Bronstein, J.M.; Ritz, B. Paraoxonase 1, agricultural organophosphate exposure, and Parkinson disease. Epidemiology 2010, 21, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Clarimon, J.; Eerola, J.; Hellström, O.; Tienari, P.J.; Singleton, A. Paraoxonase 1 (PON1) gene polymorphisms and Parkinson’s disease in a Finnish population. Neurosci. Lett. 2004, 367, 168–170. [Google Scholar] [CrossRef]
- Fong, C.-S.; Cheng, C.-W.; Wu, R.-M. Pesticides exposure and genetic polymorphism of paraoxonase in the susceptibility of Parkinson’s disease. Acta Neurol. Taiwan 2005, 14, 55–60. [Google Scholar] [PubMed]
- Dick, F.D.; De Palma, G.; Ahmadi, A.; Osborne, A.; Scott, N.W.; Prescott, G.J.; Bennett, J.; Semple, S.; Dick, S.; Mozzoni, P.; et al. Gene-environment interactions in parkinsonism and Parkinson’s disease: The Geoparkinson study. Occup. Environ. Med. 2007, 64, 673–680. [Google Scholar] [CrossRef]
- Wingo, T.S.; Rosen, A.; Cutler, D.J.; Lah, J.J.; Levey, A.I. Paraoxonase-1 polymorphisms in Alzheimer’s disease, Parkinson’s disease, and AD-PD spectrum diseases. Neurobiol. Aging 2012, 33, 204.e13–204.e15. [Google Scholar] [CrossRef]
- Lee, P.-C.; Rhodes, S.L.; Sinsheimer, J.S.; Bronstein, J.; Ritz, B. Functional paraoxonase 1 variants modify the risk of Parkinson’s disease due to organophosphate exposure. Environ. Int. 2013, 56, 42–47. [Google Scholar] [CrossRef]
- Petrič, B.; Kunej, T.; Bavec, A. A Multi-Omics Analysis of PON1 Lactonase Activity in Relation to Human Health and Disease. OMICS A J. Integr. Biol. 2021, 25, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Aharoni, A.; Gaidukov, L.; Brumshtein, B.; Khersonsky, O.; Meged, R.; Dvir, H.; Ravelli, R.; McCarthy, A.; Toker, L.; et al. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat. Struct. Mol. Biol. 2004, 11, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Dardiotis, E.; Aloizou, A.-M.; Siokas, V.; Tsouris, Z.; Rikos, D.; Marogianni, C.; Aschner, M.; Kovatsi, L.; Bogdanos, D.P.; Tsatsakis, A. Paraoxonase-1 genetic polymorphisms in organophosphate metabolism. Toxicology 2019, 411, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kirbas, A.; Kirbas, S.; Cure, M.C.; Tufekci, A. Paraoxonase and arylesterase activity and total oxidative/anti-oxidative status in patients with idiopathic Parkinson’s disease. J. Clin. Neurosci. 2014, 21, 451–455. [Google Scholar] [CrossRef]
- Ikeda, K.; Nakamura, Y.; Kiyozuka, T.; Aoyagi, J.; Hirayama, T.; Nagata, R.; Ito, H.; Iwamoto, K.; Murata, K.; Yoshii, Y.; et al. Serological profiles of urate, paraoxonase-1, ferritin and lipid in Parkinson’s disease: Changes linked to disease progression. Neurodegener. Dis. 2011, 8, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Baltus, T.H.L.; Morelli, N.R.; de Farias, C.C.; Trugilo, K.P.; Okuyama, N.C.M.; de Oliveira, K.B.; de Melo, L.B.; Smaili, S.M.; Barbosa, D.S. Association of -94 ATTG insertion/deletion NFkB1 and c.*126G>A NFkBIA genetic polymorphisms with oxidative and nitrosative stress biomarkers in Brazilian subjects with Parkinson’s Disease. Neurosci. Lett. 2020, 740, 135487. [Google Scholar] [CrossRef] [PubMed]
- de Farias, C.C.; Maes, M.; Bonifacio, K.L.; Matsumoto, A.K.; Bortolasci, C.C.; Nogueira, A.D.S.; Brinholi, F.F.; Morimoto, H.K.; De Melo, L.B.; Moreira, E.; et al. Parkinson’s Disease is Accompanied by Intertwined Alterations in Iron Metabolism and Activated Immune-inflammatory and Oxidative Stress Pathways. CNS Neurol. Disord.-Drug Targets 2017, 16, 484–491. [Google Scholar] [CrossRef]
- Cervellati, C.; Trentini, A.; Romani, A.; Bellini, T.; Bosi, C.; Ortolani, B.; Zurlo, A.; Passaro, A.; Seripa, D.; Zuliani, G. Serum paraoxonase and arylesterase activities of paraoxonase-1 (PON-1), mild cognitive impairment, and 2-year conversion to dementia: A pilot study. J. Neurochem. 2015, 135, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Cervellati, C.; Valacchi, G.; Tisato, V.; Zuliani, G.; Marsillach, J. Evaluating the link between Paraoxonase-1 levels and Alzheimer’s disease development. Minerva Med. 2019, 110, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Trentini, A.; van der Flier, W.M.; Bellini, T.; Zuliani, G.; Cervellati, C.; Teunissen, C.E. Arylesterase Activity of Paraoxonase-1 in Serum and Cerebrospinal Fluid of Patients with Alzheimer’s Disease and Vascular Dementia. Antioxidants 2020, 9, 456. [Google Scholar] [CrossRef]
- Bednarz-Misa, I.; Berdowska, I.; Zboch, M.; Misiak, B.; Zieliński, B.; Płaczkowska, S.; Fleszar, M.; Wiśniewski, J.; Gamian, A.; Krzystek-Korpacka, M. Paraoxonase 1 decline and lipid peroxidation rise reflect a degree of brain atrophy and vascular impairment in dementia. Adv. Clin. Exp. Med. 2020, 29, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Tisato, V.; Romani, A.; Tavanti, E.; Melloni, E.; Milani, D.; Bonaccorsi, G.; Sanz, J.M.; Gemmati, D.; Passaro, A.; Cervellati, C. Crosstalk Between Adipokines and Paraoxonase 1: A New Potential Axis Linking Oxidative Stress and Inflammation. Antioxidants 2019, 8, 287. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Paschalis, V.; Kyparos, A.; Nikolaidis, M.G. Administration of exercise-conditioned plasma alters muscle catalase kinetics in rat: An argument for in vivo-like K(m) instead of in vitro-like V(max). Redox Biol. 2018, 15, 375–379. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Z.; Riwanto, M.; Gao, S.; Levison, B.S.; Gu, X.; Fu, X.; Wagner, M.A.; Besler, C.; Gerstenecker, G.; et al. Myeloperoxidase, paraoxonase-1, and HDL form a functional ternary complex. J. Clin. Investig. 2013, 123, 3815–3828. [Google Scholar] [CrossRef]
- Fan, Y.; Han, J.; Zhao, L.; Wu, C.; Wu, P.; Huang, Z.; Hao, X.; Ji, Y.; Chen, D.; Zhu, M. Experimental Models of Cognitive Impairment for Use in Parkinson’s Disease Research: The Distance Between Reality and Ideal. Front. Aging Neurosci. 2021, 13, 745438. [Google Scholar] [CrossRef] [PubMed]
- Redenšek, S.; Jenko Bizjan, B.; Trošt, M.; Dolžan, V. Clinical-Pharmacogenetic Predictive Models for Time to Occurrence of Levodopa Related Motor Complications in Parkinson’s Disease. Front. Genet. 2019, 10, 461. [Google Scholar] [CrossRef]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Yuzhalin, A.E.; Kutikhin, A.G. Common genetic variants in the myeloperoxidase and paraoxonase genes and the related cancer risk: A review. J. Environ. Sci. Heal. Part C 2012, 30, 287–322. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kim, S.K.; Park, H.K.; Chung, J.H. Association Between Paraoxonase Gene Polymorphisms and Intracerebral Hemorrhage in a Korean Population. J. Mol. Neurosci. 2015, 57, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Kunjantarachot, A.; Pabalan, N.; Jarjanazi, H.; Christofolini, D.M.; Montagna, E.; Barbosa, C.P.; Bianco, B. Paraoxonase single nucleotide variants show associations with polycystic ovary syndrome: A meta-analysis. Reprod. Biol. Endocrinol. 2020, 18, 114. [Google Scholar] [CrossRef]
- Alam, R.; Tripathi, M.; Mansoori, N.; Parveen, S.; Luthra, K.; Lakshmy, R.; Sharma, S.; Arulselvi, S.; Mukhopadhyay, A.K. Synergistic epistasis of paraoxonase 1 (rs662 and rs85460) and apolipoprotein E4 genes in pathogenesis of Alzheimer’s disease and vascular dementia. Am. J. Alzheimer’s Dis. Other Dement. 2014, 29, 769–776. [Google Scholar] [CrossRef]
- Nie, Y.; Luo, D.; Yang, M.; Wang, Y.; Xiong, L.; Gao, L.; Liu, Y.; Liu, H. A Meta-Analysis on the Relationship of the PON Genes and Alzheimer Disease. J. Geriatr. Psychiatry Neurol. 2017, 30, 303–310. [Google Scholar] [CrossRef]
- Petrič, B.; Goličnik, M.; Bavec, A. The Removal of Time-Concentration Data Points from Progress Curves Improves the Determination of K(m): The Example of Paraoxonase 1. Molecules 2022, 27, 1306. [Google Scholar] [CrossRef] [PubMed]
- Lineweaver, H.; Burk, D. The Determination of Enzyme Dissociation Constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Nikolova, N.; Tenekedjiev, K.; Kolev, K. Uses and misuses of progress curve analysis in enzyme kinetics. Open Life Sci. 2008, 3, 345–350. [Google Scholar] [CrossRef]
- Duggleby, R.G.; Clarke, R.B. Experimental designs for estimating the parameters of the Michaelis-Menten equation from progress curves of enzyme-catalyzed reactions. Biochim. et Biophys. Acta (BBA)-Protein Struct. Mol. Enzym. 1991, 1080, 231–236. [Google Scholar] [CrossRef]
- Bevc, S.; Konc, J.; Stojan, J.; Hodošček, M.; Penca, M.; Praprotnik, M.; Janežič, D. {ENZO}: A Web Tool for Derivation and Evaluation of Kinetic Models of Enzyme Catalyzed Reactions. PLoS ONE 2011, 6, e22265. [Google Scholar] [CrossRef]
- Kuzmič, P. Program DYNAFIT for the Analysis of Enzyme Kinetic Data: Application to HIV Proteinase. Anal. Biochem. 1996, 237, 260–273. [Google Scholar] [CrossRef] [PubMed]
- Goličnik, M. Exact and approximate solutions for the decades-old Michaelis-Menten equation: Progress-curve analysis through integrated rate equations. Biochem. Mol. Biol. Educ. 2011, 39, 117–125. [Google Scholar] [CrossRef]
- Schnell, S.; Mendoza, C. Closed Form Solution for Time-dependent Enzyme Kinetics. J. Theor. Biol. 1997, 187, 207–212. [Google Scholar] [CrossRef]
- Petrič, B.; Goličnik, M.; Bavec, A. iFIT: An automated web tool for determining enzyme-kinetic parameters based on the high-curvature region of progress curves. Acta Chim. Slov. 2022, 69, 478–482. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef] [PubMed]
- Goličnik, M. On the Lambert W function and its utility in biochemical kinetics. Biochem. Eng. J. 2012, 63, 116–123. [Google Scholar] [CrossRef]
- Stroberg, W.; Schnell, S. On the estimation errors of KM and V from time-course experiments using the Michaelis–Menten equation. Biophys. Chem. 2016, 219, 17–27. [Google Scholar] [CrossRef]
- Khersonsky, O.; Tawfik, D.S. Structure-reactivity studies of serum paraoxonase PON1 suggest that its native activity is lactonase. Biochemistry 2005, 44, 6371–6382. [Google Scholar] [CrossRef]
- Billecke, S.; Draganov, D.; Counsell, R.; Stetson, P.; Watson, C.; Hsu, C.; La Du, B.N. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab. Dispos. 2000, 28, 1335–1342. [Google Scholar]
- Brophy, V.H.; Jampsa, R.L.; Clendenning, J.B.; McKinstry, L.A.; Jarvik, G.P.; Furlong, C.E. Effects of 5′ regulatory-region polymorphisms on paraoxonase-gene (PON1) expression. Am. J. Hum. Genet. 2001, 68, 1428–1436. [Google Scholar] [CrossRef]
- Suehiro, T.; Nakamura, T.; Inoue, M.; Shiinoki, T.; Ikeda, Y.; Kumon, Y.; Shindo, M.; Tanaka, H.; Hashimoto, K. A polymorphism upstream from the human paraoxonase (PON1) gene and its association with PON1 expression. Atherosclerosis 2000, 150, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Deakin, S.; Leviev, I.; Brulhart-Meynet, M.-C.; James, R.W. Paraoxonase-1 promoter haplotypes and serum paraoxonase: A predominant role for polymorphic position—107, implicating the Sp1 transcription factor. Biochem. J. 2003, 372 Pt 2, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Crum, R.M.; Anthony, J.C.; Bassett, S.S.; Folstein, M.F. Population-Based Norms for the Mini-Mental State Examination by Age and Educational Level. JAMA 1993, 269, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).