Relationship between Glucose-6-Phosphate Dehydrogenase Deficiency, X-Chromosome Inactivation and Inflammatory Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Laboratory
2.3. Determination of G6PD Activity
2.4. Determination Inflammatory Markers
2.5. Identification of G6PD Mutations
2.6. Determination of X-Chromosome Inactivation Pattern
2.7. Statistical Analysis
3. Results
3.1. Hematological Parameters of Study Participants
3.2. Molecular Analysis
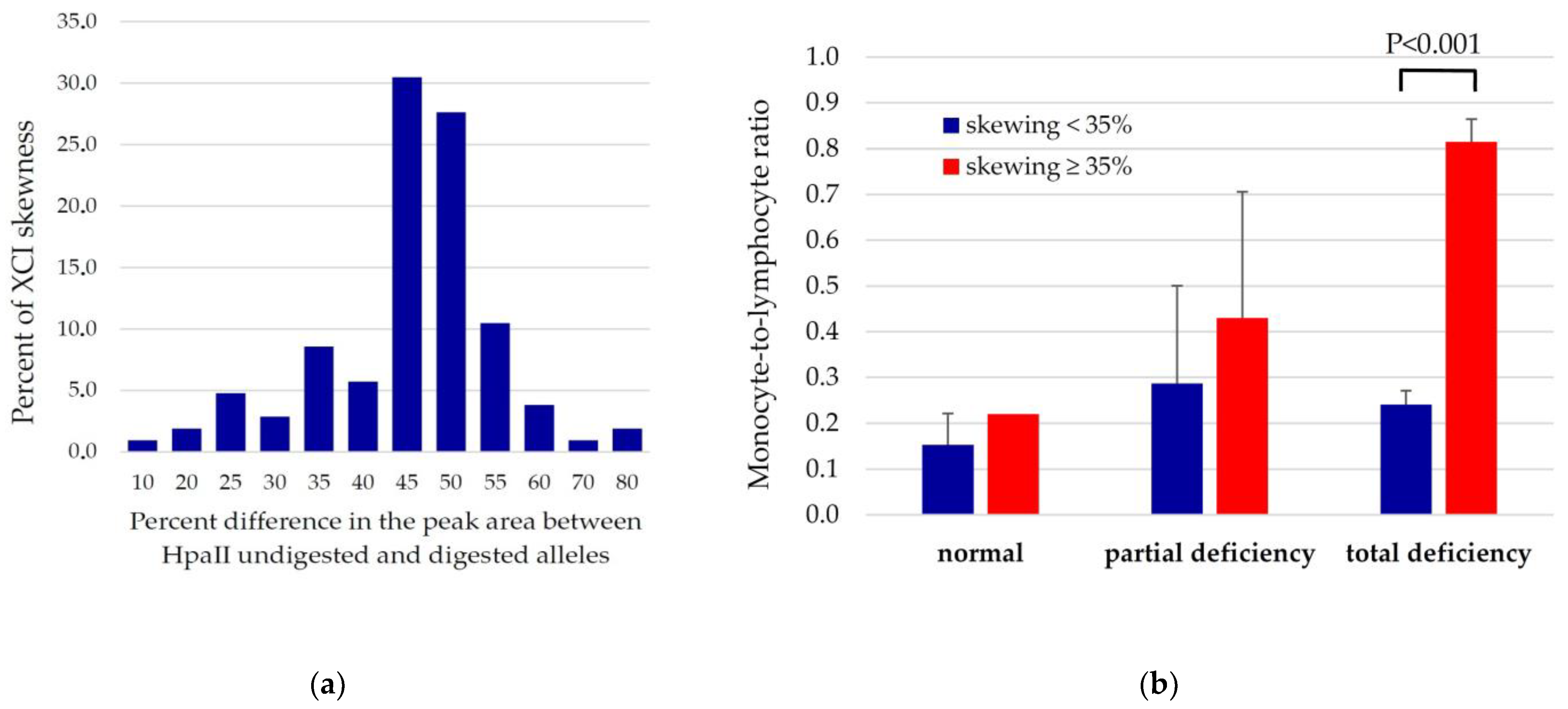
3.3. Relationships between XCI Skewing, G6PD Activity and Systemic Inflammation Markers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luzzatto, L.; Ally, M.; Notaro, R. Glucose-6-phosphate dehydrogenase deficiency. Blood 2020, 136, 1225–1240. [Google Scholar] [CrossRef]
- Beutler, E. G6PD deficiency. Blood 1994, 84, 3613–3636. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.; Dória, S. X-chromosome inactivation: Implications in human disease. J. Genet. 2021, 100, 63. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, Q.-Z.; Chen, Y.-M.; Yi, S.; Liu, D.; Liu, Y.-H.; Zhang, C.-M.; Wei, X.-F.; Zhou, Y.-Q.; Zhong, X.-M.; et al. DNA hypermethylation and X chromosome inactivation are major determinants of phenotypic variation in women heterozygous for G6PD mutations. Blood Cells Mol. Dis. 2014, 53, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Liehr, T.; Ziegler, M.; Löhmer, S.; Weise, A. Assessing Skewed X-Chromosome Inactivation. Curr. Protoc. Hum. Genet. 2018, 98, e66. [Google Scholar] [CrossRef] [PubMed]
- Swastika, M.; Harahap, A.R.; Panggalo, L.V.; Jusman, S.W.A.; Satyagraha, A.W. Determining a critical threshold for G6PD activity below which red blood cell response to oxidative stress is poor. Malar. J. 2020, 19, 208. [Google Scholar] [CrossRef]
- Carrel, L.; Willard, H.F. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 2005, 434, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ochoa, B.; Ortega-Cuellar, D.; González-Valdez, A.; Cárdenas-Rodríguez, N.; Mendoza-Torreblanca, J.G.; Contreras-García, I.J.; Pichardo-Macías, L.A.; Bandala, C.; Gómez-Manzo, S. COVID-19 in G6PD-deficient patients, oxidative stress, and neuropathology. Curr. Top. Med. Chem. 2022, 22, 1307–1325. [Google Scholar] [CrossRef]
- Pes, G.M.; Parodi, G.; Dore, M.P. Glucose-6-phosphate dehydrogenase deficiency and risk of cardiovascular disease: A propensity score-matched study. Atherosclerosis 2019, 282, 148–153. [Google Scholar] [CrossRef]
- Dore, M.P.; Errigo, A.; Bibbò, S.; Manca, A.; Pes, G.M. High Frequency of Glucose-6-Phosphate Dehydrogenase Deficiency in Patients Diagnosed with Celiac Disease. Nutrients 2022, 14, 1815. [Google Scholar] [CrossRef]
- Fois, A.; Dore, M.P.; Manca, A.; Scano, V.; Pirina, P.; Pes, G.M. Association between Glucose-6-Phosphate Dehydrogenase Deficiency and Asthma. J. Clin. Med. 2021, 10, 5639. [Google Scholar] [CrossRef] [PubMed]
- Mengel-From, J.; Lindahl-Jacobsen, R.; Nygaard, M.; Soerensen, M.; Ørstavik, K.H.; Hertz, J.M.; Andersen-Ranberg, K.; Tan, Q.; Christensen, K. Skewness of X-chromosome inactivation increases with age and varies across birth cohorts in elderly Danish women. Sci. Rep. 2021, 11, 4326. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Satta, M.; Berna-Erro, A.; Carrasco-Garcia, E.; Alberro, A.; Saenz-Antoñanzas, A.; Vergara, I.; Otaegui, D.; Matheu, A. Relevance of oxidative stress and inflammation in frailty based on human studies and mouse models. Aging 2020, 12, 9982–9999. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choe, S.S.; Choi, A.H.; Kim, K.H.; Yoon, M.J.; Suganami, T.; Ogawa, Y.; Kim, J.B. Increase in Glucose-6-Phosphate Dehydrogenase in Adipocytes Stimulates Oxidative Stress and Inflammatory Signals. Diabetes 2006, 55, 2939–2949. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Choe, S.S.; Sohn, J.H.; Kim, J.B. The role of glucose-6-phosphate dehydrogenase in adipose tissue inflammation in obesity. Adipocyte 2017, 6, 147–153. [Google Scholar] [CrossRef]
- Yi, H.; Jiang, W.; Yang, F.; Li, F.; Li, Y.; Zhu, W.; Li, Q.; Fakhar, S.H.; Cao, Y.; Luo, L.; et al. G6pd-Deficient Mice Are Protected From Experimental Cerebral Malaria and Liver Injury by Suppressing Proinflammatory Response in the Early Stage of Plasmodium berghei Infection. Front. Immunol. 2021, 12, 719189. [Google Scholar] [CrossRef]
- Martínez-Navarro, F.J.; Martínez-Morcillo, F.J.; López-Muñoz, A.; Pardo-Sánchez, I.; Martínez-Menchón, T.; Corbalán-Vélez, R.; Cayuela, M.L.; Pérez-Oliva, A.B.; García-Moreno, D.; Mulero, V. The vitamin B6-regulated enzymes PYGL and G6PD fuel NADPH oxidases to promote skin inflammation. Dev. Comp. Immunol. 2020, 108, 103666. [Google Scholar] [CrossRef]
- Lakhkar, A.; Dhagia, V.; Joshi, S.R.; Gotlinger, K.; Patel, D.; Sun, D.; Wolin, M.S.; Schwartzman, M.L.; Gupte, S.A. 20-HETE-induced mitochondrial superoxide production and inflammatory phenotype in vascular smooth muscle is prevented by glucose-6-phosphate dehydrogenase inhibition. Am. J. Physiol. Circ. Heart Physiol. 2016, 310, H1107–H1117. [Google Scholar] [CrossRef]
- Yang, H.-C.; Cheng, M.-L.; Hua, Y.-S.; Wu, Y.-H.; Lin, H.-R.; Liu, H.-Y.; Ho, H.-Y.; Chiu, D.T.-Y. Glucose 6-phosphate dehydrogenase knockdown enhances IL-8 expression in HepG2 cells via oxidative stress and NF-κB signaling pathway. J. Inflamm. 2015, 12, 34. [Google Scholar] [CrossRef]
- Mordmüller, B.; Turrini, F.; Long, H.; Kremsner, P.G.; Arese, P. Neutrophils and monocytes from subjects with the Mediterranean G6PD variant: Effect of Plasmodium falciparum hemozoin on G6PD activity, oxidative burst and cytokine production. Eur. Cytokine Netw. 1998, 9, 239–245. [Google Scholar]
- Spolarics, Z.; Siddiqi, M.; Siegel, J.H.; Garcia, Z.C.; Stein, D.S.; Ong, H.; Livingston, D.H.; Denny, T.; Deitch, E.A. Increased incidence of sepsis and altered monocyte functions in severely injured type A− glucose-6-phosphate dehydrogenase-deficient African American trauma patients. Crit. Care Med. 2001, 29, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Ham, M.; Choe, S.S.; Shin, K.C.; Choi, G.; Kim, J.-W.; Noh, J.-R.; Kim, Y.-H.; Ryu, J.-W.; Yoon, K.-H.; Lee, C.-H.; et al. Glucose-6-Phosphate Dehydrogenase Deficiency Improves Insulin Resistance with Reduced Adipose Tissue Inflammation in Obesity. Diabetes 2016, 65, 2624–2638. [Google Scholar] [CrossRef] [PubMed]
- Nobrega-Pereira, S.; Fernandez-Marcos, P.J.; Brioche, T.; Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Viña, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef]
- Minucci, A.; Giardina, B.; Zuppi, C.; Capoluongo, E. Glucose-6-phosphate dehydrogenase laboratory assay: How, when, and why? IUBMB Life 2009, 61, 27–34. [Google Scholar] [CrossRef]
- Roper, D.; Layton, M.; Rees, D.; Lambert, C.; Vulliamy, T.; De la Salle, B.; D’Souza, C.; The British Society for Haematology. Laboratory diagnosis of G6PD deficiency. A British Society for Haematology Guideline. Br. J. Haematol. 2020, 189, 24–38. [Google Scholar] [CrossRef]
- Mosca, A.; Paleari, R.; Rosti, E.; Luzzana, M.; Barella, S.; Sollaino, C.; Galanello, R. Simultaneous Automated Determination of Glucose 6-Phosphate Dehydrogenase and 6-Phosphogluconate Dehydrogenase Activities in Whole Blood. Eur. J. Clin. Chem. Clin. Biochem. 1996, 34, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Blume, K.G.; Kaplan, J.C.; Löhr, G.W.; Ramot, B.; Valentine, W.N. International Committee for Standardization in Haematology: Recommended Methods for Red-Cell Enzyme Analysis. Br. J. Haematol. 1977, 35, 331–340. [Google Scholar] [CrossRef]
- Kratz, A.; Plebani, M.; Peng, M.; Lee, Y.K.; McCafferty, R.; Machin, S.J.; The International Council for Standardization in Haematology (ICSH). ICSH recommendations for modified and alternate methods measuring the erythrocyte sedimentation rate. Int. J. Lab. Hematol. 2017, 39, 448–457. [Google Scholar] [CrossRef]
- Núñez, J.; Nunez, E.; Bodí, V.; Sanchis, J.; Minana, G.; Mainar, L.; Santas, E.; Merlos, P.; Rumiz, E.; Darmofal, H.; et al. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am. J. Cardiol. 2008, 101, 747–752. [Google Scholar] [CrossRef]
- Song, M.; Graubard, B.I.; Rabkin, C.S.; Engels, E.A. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci. Rep. 2021, 11, 464. [Google Scholar] [CrossRef]
- Ji, H.; Li, Y.; Fan, Z.; Zuo, B.; Jian, X.; Li, L.; Liu, T. Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: A syntax score assessment. BMC Cardiovasc. Disord. 2017, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, M.; Yildiz, A.; Oylumlu, M.; Akyuz, A.; Aydin, M.; Kaya, H.; Acet, H.; Polat, N.; Bilik, M.Z.; Alan, S. The association between platelet/lymphocyte ratio and coronary artery disease severity. Anatol. J. Cardiol. 2015, 15, 640–647. [Google Scholar] [CrossRef] [PubMed]
- De Vita, G.; Alcalay, M.; Sampietro, M.; Cappelini, M.D.; Fiorelli, G.; Toniolo, D. Two point mutations are responsible for G6PD polymorphism in Sardinia. Am. J. Hum. Genet. 1989, 44, 233–240. [Google Scholar]
- Fiorelli, G.; Meloni, T.; Palomba, V.; Manoussakis, C.; Villa, S.; Cappellini, M.D. Gene frequency of glucose-6-phosphate dehydrogenase (G6PD) polymorphic variants in Sardinia. Gene Geogr. 1990, 4, 139–142. [Google Scholar] [PubMed]
- Cao, A.; Congiu, R.; Sollaino, M.C.; Desogus, M.F.; Demartis, F.R.; Loi, D.; Cau, M.; Galanello, R. Thalassaemia and Glucose-6-Phosphate Dehydrogenase Screening in 13- to 14-Year-Old Students of the Sardinian Population: Preliminary Findings. Community Genet. 2008, 11, 121–128. [Google Scholar] [CrossRef]
- Allen, R.C.; Zoghbi, H.Y.; Moseley, A.B.; Rosenblatt, H.M.; Belmont, J.W. Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am. J. Hum. Genet. 1992, 51, 1229–1239. [Google Scholar]
- Busque, L.; Zhu, J.; Dehart, D.; Griffith, B.; Willman, C.; Carroll, R.; Black, P.M.; Gilliland, D.G. An expression based clonality assay at the human androgen receptor locus (HUMARA) on chromosome X. Nucleic Acids Res. 1994, 22, 697–698. [Google Scholar] [CrossRef]
- Desai, V.; Donsante, A.; Swoboda, K.J.; Martensen, M.; Thompson, J.; Kaler, S.G. Favorably skewed X-inactivation accounts for neurological sparing in female carriers of Menkes disease. Clin. Genet. 2011, 79, 176–182. [Google Scholar] [CrossRef]
- Ørstavik, K.H. Skewed X inactivation in healthy individuals and in different diseases. Acta Paediatr. 2006, 95, 24–29. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. G6PD deficiency shifts polarization of monocytes/macrophages towards a proinflammatory and profibrotic phenotype. Cell. Mol. Immunol. 2021, 18, 770–772. [Google Scholar] [CrossRef]
- Kalnoky, M.; Bancone, G.; Kahn, M.; Chu, C.S.; Chowwiwat, N.; Wilaisrisak, P.; Pal, S.; LaRue, N.; Leader, B.; Nosten, F.; et al. Cytochemical flow analysis of intracellular G6PD and aggregate analysis of mosaic G6PD expression. Eur. J. Haematol. 2018, 100, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Chen, S.; Shi, J.; Zhu, X.; Ying, H.; Zhang, Y.; Chen, S.; Shen, B.; Li, J. The association between the lymphocyte-monocyte ratio and disease activity in rheumatoid arthritis. Clin. Rheumatol. 2017, 36, 2689–2695. [Google Scholar] [CrossRef] [PubMed]
- Naranbhai, V.; Kim, S.; Fletcher, H.; Cotton, M.F.; Violari, A.; Mitchell, C.; Nachman, S.; McSherry, G.; McShane, H.; Hill, A.V.; et al. The association between the ratio of monocytes:lymphocytes at age 3 months and risk of tuberculosis (TB) in the first two years of life. BMC Med. 2014, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Kadiyoran, C.; Zengin, O.; Cizmecioglu, H.A.; Tufan, A.; Kucuksahin, O.; Cure, M.C.; Cure, E.; Kucuk, A.; Ozturk, M.A. Monocyte to Lymphocyte Ratio, Neutrophil to Lymphocyte Ratio, and Red Cell Distribution Width are the Associates with Gouty Arthritis. Acta Med. 2019, 62, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, M.; Liu, L.; Dang, X.; Zhu, D.; Tian, G. Monocyte/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients with non-ST-elevation myocardial infarction. Medicine 2019, 98, e16267. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, H.; Yang, J.; Lu, Y.; Zhang, D.; Wang, J.; Kuang, C.; Zhu, S.; Wang, M.; Ma, X. Survival and prognostic analysis of preoperative inflammatory markers in patients undergoing surgical resection for laryngeal squamous cell carcinoma. BMC Cancer 2018, 18, 816. [Google Scholar] [CrossRef]
- Yokota, M.; Katoh, H.; Nishimiya, H.; Kikuchi, M.; Kosaka, Y.; Sengoku, N.; Watanabe, M.; Yamashita, K. Lymphocyte-Monocyte Ratio Significantly Predicts Recurrence in Papillary Thyroid Cancer. J. Surg. Res. 2020, 246, 535–543. [Google Scholar] [CrossRef]
- Ham, M.; Lee, J.-W.; Choi, A.H.; Jang, H.; Choi, G.; Park, J.; Kozuka, C.; Sears, D.D.; Masuzaki, H.; Kim, J.B. Macrophage Glucose-6-Phosphate Dehydrogenase Stimulates Proinflammatory Responses with Oxidative Stress. Mol. Cell. Biol. 2013, 33, 2425–2435. [Google Scholar] [CrossRef]
- Liese, A.M.; Siddiqi, M.Q.; Siegel, J.H.; Deitch, E.A.; Spolarics, Z. Attenuated Monocyte Il-10 Production in Glucose-6-Phosphate Dehydrogenase-Deficient Trauma Patients. Shock 2002, 18, 18–23. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Glucose-6-Phosphate Dehydrogenase Deficiency Activates Endothelial Cell and Leukocyte Adhesion Mediated via the TGFβ/NADPH Oxidases/ROS Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 7474. [Google Scholar] [CrossRef]
- Sanna, F.; Bonatesta, R.R.; Frongia, B.; Uda, S.; Banni, S.; Melis, M.P.; Collu, M.; Madeddu, C.; Serpe, R.; Puddu, S.; et al. Production of Inflammatory Molecules in Peripheral Blood Mononuclear Cells from Severely Glucose-6-Phosphate Dehydrogenase-Deficient Subjects. J. Vasc. Res. 2007, 44, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Dore, M.P.; Parodi, G.; Portoghese, M.; Pes, G.M. The Controversial Role of Glucose-6-Phosphate Dehydrogenase Deficiency on Cardiovascular Disease: A Narrative Review. Oxidative Med. Cell. Longev. 2021, 2021, 5529256. [Google Scholar] [CrossRef] [PubMed]
- Au, W.Y.; So, J.C.; Ma, S.K.; Lie, A.K.W. Glucose-6-phosphate-dehydrogenase deficiency and haematopoietic stem cell transplantation in Chinese patients. Hong Kong Med. J. 2009, 15, 35–38. [Google Scholar] [PubMed]
- Pes, G.M.; Dore, M.P. Acquired Glucose-6-Phosphate Dehydrogenase Deficiency. J. Clin. Med. 2022, 11, 6689. [Google Scholar] [CrossRef] [PubMed]
- Dore, M.P.; Vidili, G.; Marras, G.; Assy, S.; Pes, G.M. Inverse Association between Glucose-6-Phosphate Dehydrogenase Deficiency and Hepatocellular Carcinoma. Asian Pac. J. Cancer Prev. 2018, 19, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.G.; Pashko, L.L. Dehydroepiandrosterone, glucose-6-phosphate dehydrogenase, and longevity. Ageing Res. Rev. 2004, 3, 171–187. [Google Scholar] [CrossRef]
- Bittel, D.C.; Theodoro, M.F.; Kibiryeva, N.; Fischer, W.; Talebizadeh, Z.; Butler, M.G. Comparison of X-chromosome inactivation patterns in multiple tissues from human females. J. Med. Genet. 2008, 45, 309–313. [Google Scholar] [CrossRef]
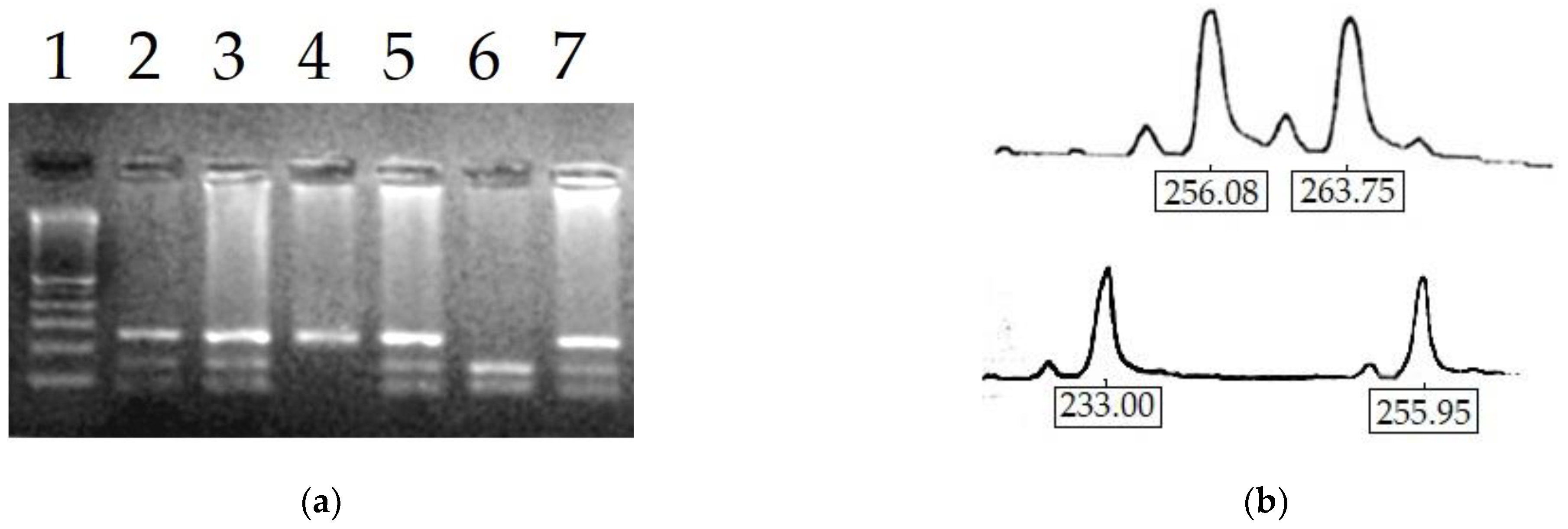
| Parameter | Normal | Partial Deficiency | Total Deficiency | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| No. of cases | 18 | 5 | 7 | 118 | 80 | 4 |
| Age (mean ± SD, years) | 66.8 ± 13.3 | 59.6 ± 10.9 | 66.1 ± 13.6 | 61.9 ± 20.1 | 58.8 ± 24.7 | 69.7 ± 12.8 |
| White blood cells (×109/L) | 7.33 ± 2.04 | 5.67 ± 2.24 | 8.33 ± 3.81 | 7.68 ± 3.77 | 8.18 ± 3.22 | 5.72 ± 2.10 |
| Red blood cells (×1012/L) | 5.34 ± 0.81 | 4.63 ± 0.58 | 4.83 ± 1.02 | 4.39 ± 0.78 | 4.41 ± 0.83 | 4.51 ± 0.46 |
| Hemoglobin (g/L) | 14.8 ± 1.5 | 13.6 ± 1.2 | 14.2 ± 1.2 | 12.3 ± 1.7 | 12.8 ± 2.3 ** | 13.2 ± 0.8 |
| Hematocrit (%) | 43.7 ± 4.4 | 39.5 ± 3.3 | 41.9 ± 3.5 | 37.6 ± 4.8 | 39.5 ± 6.6 * | 40.1 ± 1.7 |
| Mean corpuscular volume (fL) | 83.1 ± 10.6 | 85.6 ± 3.6 | 88.9 ± 12.0 | 86.2 ± 11.7 | 90.4 ± 8.8 * | 89.2 ± 5.4 |
| Mean corpuscular hemoglobin (pg) | 28.2 ± 4.0 | 29.4 ± 1.4 | 30.1 ± 4.0 | 28.4 ± 4.2 | 29.4 ± 3.7 | 29.5 ± 1.2 |
| Mean corpuscular Hb conc. (g/L) | 33.8 ± 0.8 | 34.4 ± 1.8 | 33.9 ± 1.8 | 32.6 ± 1.4 | 32.5 ± 1.4 * | 33.1 ± 2.0 |
| Red cell distribution width (%) | 14.4 ± 1.9 | 13.4 ± 2.1 | 12.9 ± 0.9 | 13.6 ± 1.8 | 13.4 ± 2.4 | 12.5 ± 1.9 |
| Hemoglobin distribution width (g/L) | − | 2.67 ± 0.38 | 3.05 ± 0.92 | 2.64 ± 0.38 | 2.66 ± 0.41 | 2.70 ± 0.6 |
| Platelets (×109/L) | 236 ± 42 | 256 ± 70 | 220 ± 39 | 235 ± 75 | 233 ± 80 | 170 ± 46 |
| Mean platelet volume (fL) | 10.7 ± 1.4 | 9.84 ± 2.67 | 10.1 ± 1.4 | 9.04 ± 1.73 | 8.5 ± 1.3 | 11.15 ± 1.64 |
| White cells count | ||||||
| Neutrophils (×109/L) | 4.30 ± 1.37 | 3.21 ± 0.69 | 5.13 ± 3.61 | 5.16 ± 3.66 | 5.24 ± 2.85 | 3.08 ± 0.31 |
| Lymphocytes (×109/L) | 2.33 ± 0.84 | 1.90 ± 0.77 | 2.28 ± 0.65 | 1.76 ± 0.72 | 2.02 ± 1.04 | 1.83 ± 0.09 |
| Monocytes (×109/L) | 0.42 ± 0.15 | 0.26 ± 0.05 | 0.60 ± 0.44 | 0.45 ± 0.21 | 0.57 ± 0.27 | 0.44 ± 0.08 |
| Eosinophils (×109/L) | 0.20 ± 0.17 | 0.19 ± 0.17 | 0.19 ± 0.21 | 0.13 ± 0.09 | 0.17 ± 0.17 | 0.18 ± 0.17 |
| Basophils (×109/L) | 0.07 ± 0.03 | 0.04 ± 0.04 | 0.09 ± 0.03 | 0.04 ± 0.05 | 0.04 ± 0.05 * | 0.07 ± 0.04 |
| Large unstained cells (×109/L) | − | 0.11 ± 0.03 | 0.10 ± 0.01 | 0.12 ± 0.05 | 0.14 ± 0.09 | 0.10 ± 0.02 |
| G6PD (U/gHb) | 7.2 ± 2.1 | 7.4 ± 0.9 | 4.8 ± 4.6 | 5.9 ± 2.2 | 0.6 ± 0.2 | 0.5 ± 0.8 |
| 6PGD (U/gHb) | 6.6 ± 0.8 | 8.0 ± 1.2 | 7.9 ± 3.2 | 6.8 ± 2.3 | 8.8 ± 3.0 | 7.2 ± 0.5 |
| G6PD/6PGD ratio | 0.877 ± 0.033 | 0.855 ± 0.005 | 0.220 ± 0.056 | 0.521 ± 0.187 | 0.020 ± 0.006 | 0.115 ± 0.110 |
| Inflammation markers | ||||||
| Erythrocyte sedimentation rate (ESR) (mm/h) | 11.9 ± 6.2 | 13.7 ± 8.9 | 14.6 ± 9.2 | 16.1 ± 6.3 | 15.0 ± 7.2 * | 16.0 ± 6.6 |
| High-sensitivity C-reactive protein (hs-CRP) (mg/dL) | 3.95 ± 2.96 | 4.90 ± 3.78 | 4.40 ± 2.93 | 5.02 ± 3.46 | 5.96 ± 3.39 * | 7.1 ± 3.2 |
| Neutrophil-to-lymphocyte ratio (NLR) | 2.00 ± 0.81 | 1.87 ± 0.76 | 2.71 ± 2.93 | 4.01 ± 5.20 | 3.47 ± 3.19 | 1.68 ± 0.08 |
| Monocyte-to-lymphocyte ratio (MLR) | 0.18 ± 0.07 | 0.15 ± 0.07 | 0.31 ± 0.34 | 0.31 ± 0.23 | 0.37 ± 0.26 ** | 0.34 ± 0.03 |
| Platelet-to-lymphocyte ratio (PLR) | 112.8 ± 40.8 | 153.7 ± 73.2 | 103.1 ± 30.3 | 155.7 ± 84.9 | 141.0 ± 77.4 | 93.7 ± 30.5 |
| Parameter | Normal | Partial Deficiency | Total Deficiency | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| No. of cases | 18 | 5 | 7 | 118 | 80 | 4 |
| B * | 18 | − | 0 | − | − | − |
| B/B | − | 4 | − | 10 | − | 0 |
| Med # | 0 | − | 6 | − | 74 | − |
| B/Med | − | 1 | − | 85 | − | 1 |
| Med/Med | − | 0 | − | 9 | − | 3 |
| Union § | 0 | − | 1 | − | 0 | − |
| B/Union | − | 0 | − | 2 | − | 0 |
| Seattle/Seattle ¶ | − | 0 | − | 2 | − | 0 |
| Unknown | − | − | − | 10 | 6 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Errigo, A.; Bitti, A.; Galistu, F.; Salis, R.; Pes, G.M.; Dore, M.P. Relationship between Glucose-6-Phosphate Dehydrogenase Deficiency, X-Chromosome Inactivation and Inflammatory Markers. Antioxidants 2023, 12, 334. https://doi.org/10.3390/antiox12020334
Errigo A, Bitti A, Galistu F, Salis R, Pes GM, Dore MP. Relationship between Glucose-6-Phosphate Dehydrogenase Deficiency, X-Chromosome Inactivation and Inflammatory Markers. Antioxidants. 2023; 12(2):334. https://doi.org/10.3390/antiox12020334
Chicago/Turabian StyleErrigo, Alessandra, Angela Bitti, Franca Galistu, Roberta Salis, Giovanni Mario Pes, and Maria Pina Dore. 2023. "Relationship between Glucose-6-Phosphate Dehydrogenase Deficiency, X-Chromosome Inactivation and Inflammatory Markers" Antioxidants 12, no. 2: 334. https://doi.org/10.3390/antiox12020334
APA StyleErrigo, A., Bitti, A., Galistu, F., Salis, R., Pes, G. M., & Dore, M. P. (2023). Relationship between Glucose-6-Phosphate Dehydrogenase Deficiency, X-Chromosome Inactivation and Inflammatory Markers. Antioxidants, 12(2), 334. https://doi.org/10.3390/antiox12020334








