Abstract
This study aimed to analyze the research trends on salivary oxidative stress associated with dental caries and to perform bibliometric approaches for existing publications on this association. A search was performed using the Web of Science Core Collection, without any restriction of language or publication year. The number of periodicals with the most published articles in this theme, most published authors and keywords were mapped; other metrics were also evaluated such as the countries that have more research on the subject and the period in which there were more publications on the subject. During the knowledge mapping, the most frequent experimental designs were analyzed, type of saliva collection, stage of caries disease, evaluated oxidative parameters were retrieved and analyzed from each manuscript. Between the 43 selected articles, the Journal of Clinical Pediatric Dentistry was the periodical appearing the most with 4 published articles. The authors who published the most were Celec, P., Tothova, L., Hegde, A.M., Shetty, S., Antoniali, C., and Pessan, JP with three articles each, and a total of 180 keywords representing the evolution of the theme. India and Asia were found to be the country and continent with most publications, respectively. Most articles collected non-stimulated total saliva, with total antioxidant capacity being the parameter most often evaluated. The type of study that appeared the most was cross-sectional studies, and articles published in the period of 2017–2022 were the most frequent. Studies show that dental caries can be associated to the changes in salivary oxidative biochemistry with an increase in lipid peroxidation, a biomarker of oxidative damage, and an increase in antioxidant capacity in chronic caries, in response to cariogenic challenge. Some studies evidence the reduction of lipid peroxidation after treatment of the carious lesion. Our findings reveal worldwide research trends, as well as a clearer knowledge of the evolution and future scenarios of this issue, also showing the mechanisms associating dental caries with changes in salivary oxidative biochemical parameters are not clear.
1. Introduction
Oxidative stress is caused by an imbalance between the production of free radicals and non-radical species, such as reactive oxygen species (ROS), and the activity of enzymatic and non-enzymatic antioxidant systems, which are robust defense mechanisms against oxidative damage [1,2,3]. Therefore, pro-oxidant species can cause damage to cells and tissue by damaging lipids, proteins, enzymes, and DNA [4,5,6].
Oxidative stress is mainly assessed through the relationship between antioxidants (total antioxidant capacity, reduced glutathione, oxidized glutathione, glutathione peroxidase activity, superoxide dismutase, and catalase) and pro-oxidants (reactive species and levels of nitrates and nitrites), in addition to biomarkers of oxidative damage (lipid peroxidation and protein oxidation) [7,8,9,10,11,12].
By continuously soaking the teeth and oral mucosa, saliva acts as a cleaning solution, a lubricant, and buffer, and storage of calcium and phosphate [13]. These minerals are necessary for the remineralization of the first carious lesions in the oral cavity through the process of remineralization and demineralization of tooth enamel [13]. The biochemical constitution of saliva, which is supersaturated with the existing hydroxyapatite in tooth enamel, aids this action by limiting demineralization and boosting remineralization. Furthermore, saliva dilutes and neutralizes food acids and bacterial metabolism in the biofilm [13,14]. Saliva also plays an important role in protecting the oral cavity, and controlling dental caries due to its buffering effect and antioxidant capacity. The antioxidant of saliva establishes an equilibrium between free radicals, which play an important role in safeguarding the body [15,16]. In addition, salivary biomarkers of oxidative stress have been utilized to diagnose a variety of disorders in children’s oral cavities [17].
Saliva becomes the first line of defense against free radicals because it presents several antioxidant mechanisms (glutamate, ascorbic acid, uric acid, and melatonin), antioxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidase) that will combat the negative effects of reactive oxygen and nitrogen species when found in excess in the oral cavity, beyond the levels necessary for physiological function [18]. Moreover, saliva has been shown to be a means of diagnosing oxidative stress, as these markers cause saliva to reflect changes that occur both in the oral cavity, as well as the pH balance and antioxidant capacity in the oral cavity [16]. It is also considered a fluid with a high capacity to detect molecules that can act as biomarkers for several oral diseases, such as periodontitis and dental caries [16]. Researchers observed a specific pattern of salivary antioxidant responses to oxidative stress in children with caries, with greater total antioxidant capacity (TAC) and superoxide dismutase (SOD) levels in caries-free children that were found lower malondialdehyde (MDA) levels [17,19].
In its turn, dental caries is the most frequent chronic oral disease and is considered a global oral health problem [20]. It is also considered an irreversible microbial disease that affects the hard tissue of teeth and is characterized by the destruction of the inorganic and organic portions of the tooth, leading to cavitation and possible tooth loss [14]. At an early stage of caries formation, signs of demineralization, such as white spotting, are observed in mineralized tooth tissues; however, disease formation occurs within the dental plaque that lies on the surface of the teeth [21]. Early diagnosis of caries is necessary to avoid tooth loss.
Some indicators of imbalance in the redox system were evidenced in the saliva of patients with dental caries, including changes in antioxidant and pro-oxidant parameters and biomarker of oxidative damage [16,22]. Changes in this system, characterized by increased levels of biomarker of oxidative damage and reduced antioxidant capacity are associated with the progression of dental caries [18]. Thus, this study mapped the overview of global scientific research on dental caries and salivary oxidative stress.
2. Materials and Methods
To perform this knowledge mapping we used bibliometric analysis tools, already described in previous studies of our group [23,24].
2.1. Search Strategy
A comprehensive search was performed by two independent examiners in the Web of Science Core Collection (WoS-CC) in November 2022, using the following search key: TS = (caries OR “Dental Decay” OR “Carious Lesion” OR “Dental White Spot” OR “tooth decay” OR “dental cavity” OR Cariology) AND TS = (Saliva).
2.2. Study Selection and Data Collection
Two independent authors selected the articles by reading the title, abstract and then reading the full text; search results were made, without restrictions on publication period and language. The criteria of choice were articles that focused on salivary oxidative stress and dental caries. Editorials, conference papers, letters, and commentaries, as well as studies that did not correspond to the specific theme, were excluded; if they met all eligibility criteria, they were included and in cases of disagreement between the reviewers, a third party resolved the disagreement.
After the selection of articles, TXT and Excel files were extracted from Web of Science. The TXT file was used to extract information such as the countries of the corresponding authors, keywords found in the papers, journals that had the most articles published on this theme, and publication density of the authors of the selected articles. The Excel file was used to extract the following data: authors, year of publication, number of citations, keywords, country, average per year, DOI, caries diagnostic, study design, age of the sample, countries, and continent of the corresponding author, abstract of the study. The number of citations of these articles in Google Scholar and Scopus was also collected to compare the number of citations with WoS-CC.
2.3. Data Analyses
Frequency analysis of descriptive measures and investigation of the authors’ collaboration network and keywords were performed using the VOSviewer software [25,26]. These terms were organized into clusters, and each cluster was represented by a color. The most important terms had larger circles, and the closely related terms were close together. In addition, the lines indicate the relationship between items, with a thicker line indicating a stronger connection. The MapChart tool (https://mapchart.net/ accessed on 30 November 2022) was used to illustrate the global distribution of articles selected.
2.4. Content Analysis
The selected articles were read in their entirety, seeking information about the experimental designs, the biochemical parameters investigated, the method used for saliva collection, the activity of the caries disease, the age of the patients, and the methods used for caries diagnosis, to observe the methodological patterns among the studies. The oxidative parameters that were analyzed by the articles were divided into three categories: antioxidants, pro-oxidants, and biomarkers of oxidative damage.
The study design was categorized as literature review, laboratory research (in vitro, in vivo, in situ, ex vivo), case reports/series, cross-sectional studies, case-control studies, cohort studies, longitudinal studies, clinical trials, and systematic reviews/meta-analyses [27].
3. Results
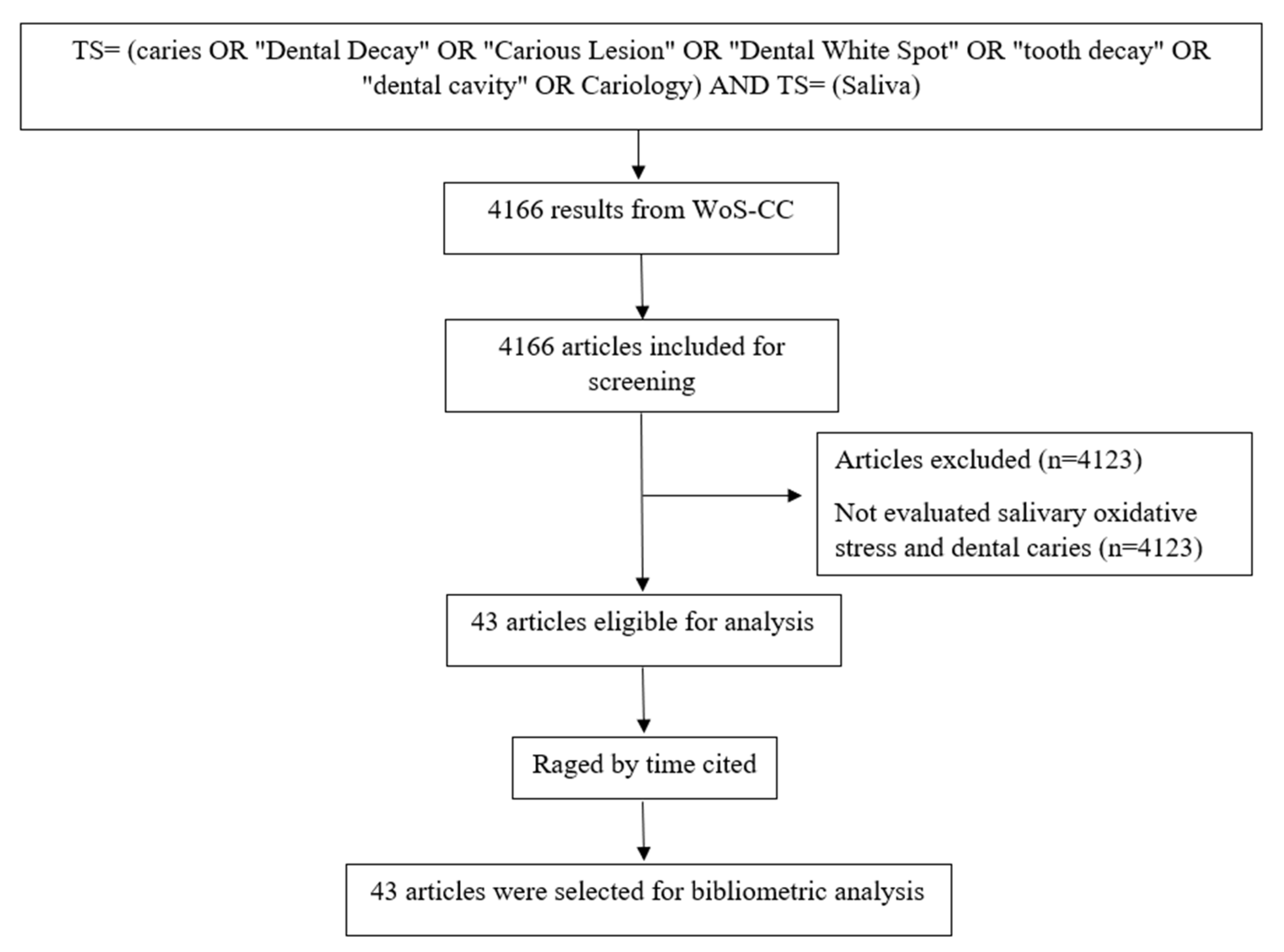
The search, in the WoS-CC, resulted in 4166 articles. A total of 43 articles were selected after reading the title, abstract, and full text (in case of doubt) (Figure 1) and 4123 were excluded.
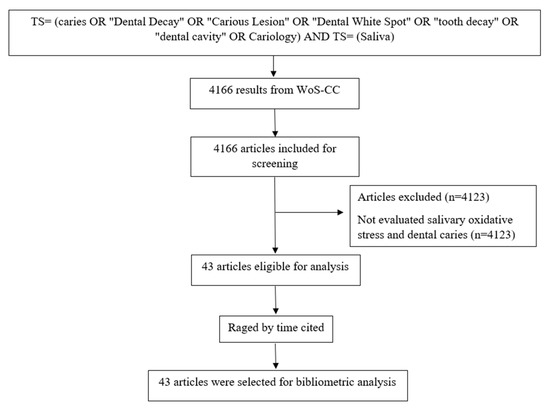
Figure 1.
Flowchart of the search and article selection.
The oldest study was conducted in 2005 [28]. The most recent studies from 2022 sought to group studies that evaluated salivary oxidative biochemical parameters and association with dental caries in children [29] and in children and adolescents [30] (Table 1), The period with the most published articles was between 2017 and 2022 (n = 23) (Table 2).

Table 1.
The articles selected about the relationship between dental caries and salivary oxidative stress.

Table 2.
Publication period of the selected articles.
The journal that published the most articles that related dental caries with salivary oxidative stress was the Journal of Clinical Pediatric Dentistry with a total of 5 articles out of 43, followed by the Archives of Oral Biology and Caries Research with 4 articles each (Figure 2).

Figure 2.
Journals that published at least two articles on dental caries and salivary oxidative stress.
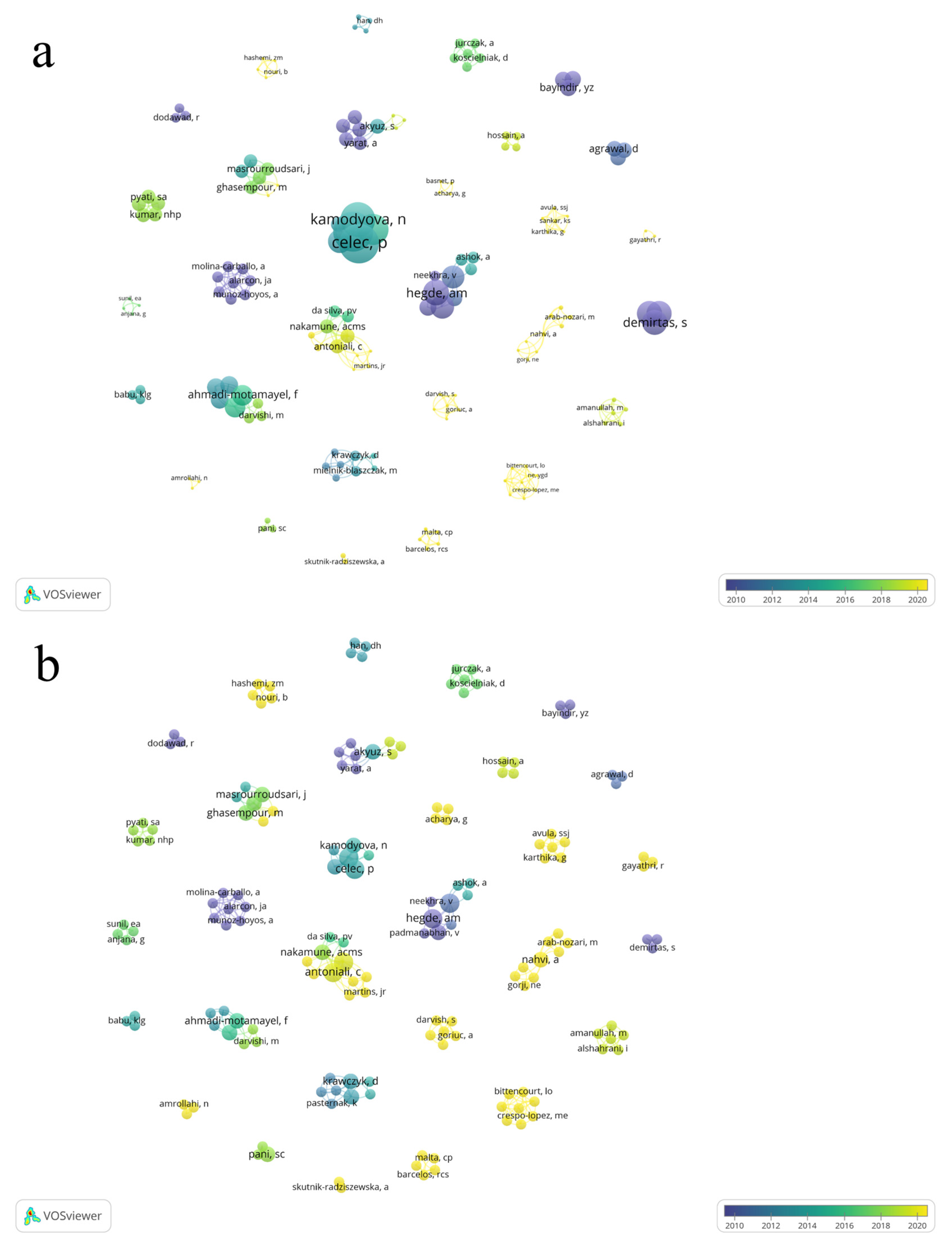
The total number of authors was 164, of which 144 published only one article, 14 published two articles, and six published three articles. The largest contributions regarding the number of published articles were from the authors Celec, P., Tothova, L., Hegde, A.M., Shetty, S., Antoniali, C., and Pessan, JP, each with three published articles (Figure 3a).
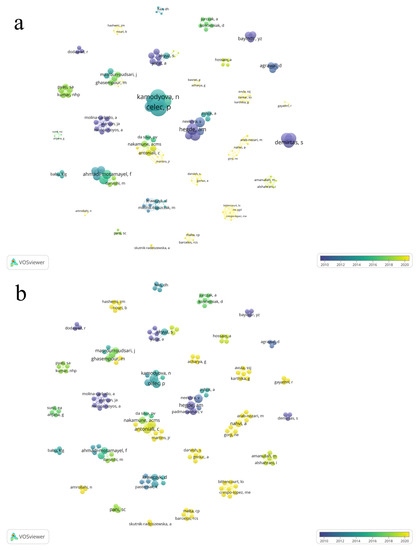
Figure 3.
Co-authorship overlay visualization of the 43 articles showing the collaboration of the authors of the selected articles about the relation of dental caries and salivary oxidative stress. The total of 164 author form 30 clusters. The overlay visualization, the lines connecting authors the size of node denote number of articles (a) and number of citations (b), respectively.
The most cited authors were Celec, P. (166 citations) and Tothova, L. (166 citations), followed by Kamodyova, N. (131 citations), and Cervenka, T. (104 citations) (Figure 4).
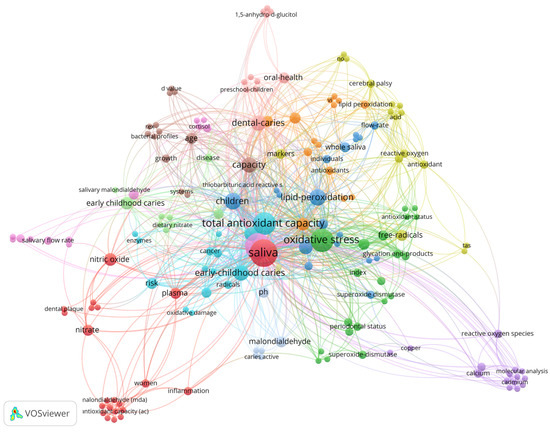
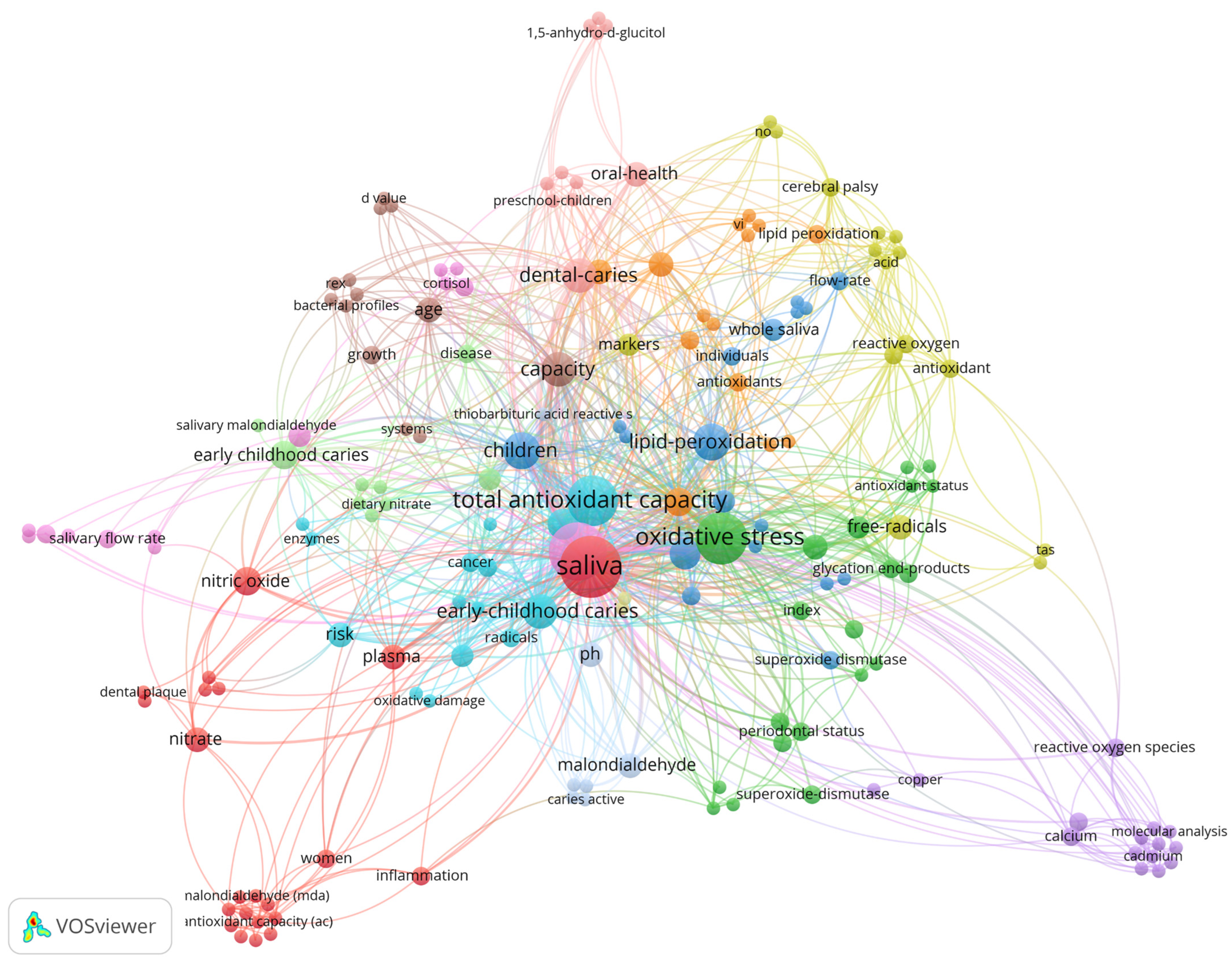
Figure 4.
Network of co-occurrence of all keywords (author and keyword plus). The 180 keywords form 14 clusters. The size of the node represents the frequency of the keyword, with larger nodes indicating higher frequency.
A total of 180 keywords were identified and saliva (n = 27; 250 citations) is the most used and most cited keyword, followed by dental caries (n = 24; 201 citations), oxidative stress (n = 19; 183 citations), and total antioxidant capacity (n = 18; 154 citations). The distribution of the keywords is shown in Figure 4. The size of the node indicates the frequency of the keyword, the larger the node, the higher the frequency. The thickness of the edge is related to the closeness of the interactions between the two nodes. Note that the color of the node indicates the cluster to which the keyword belongs.
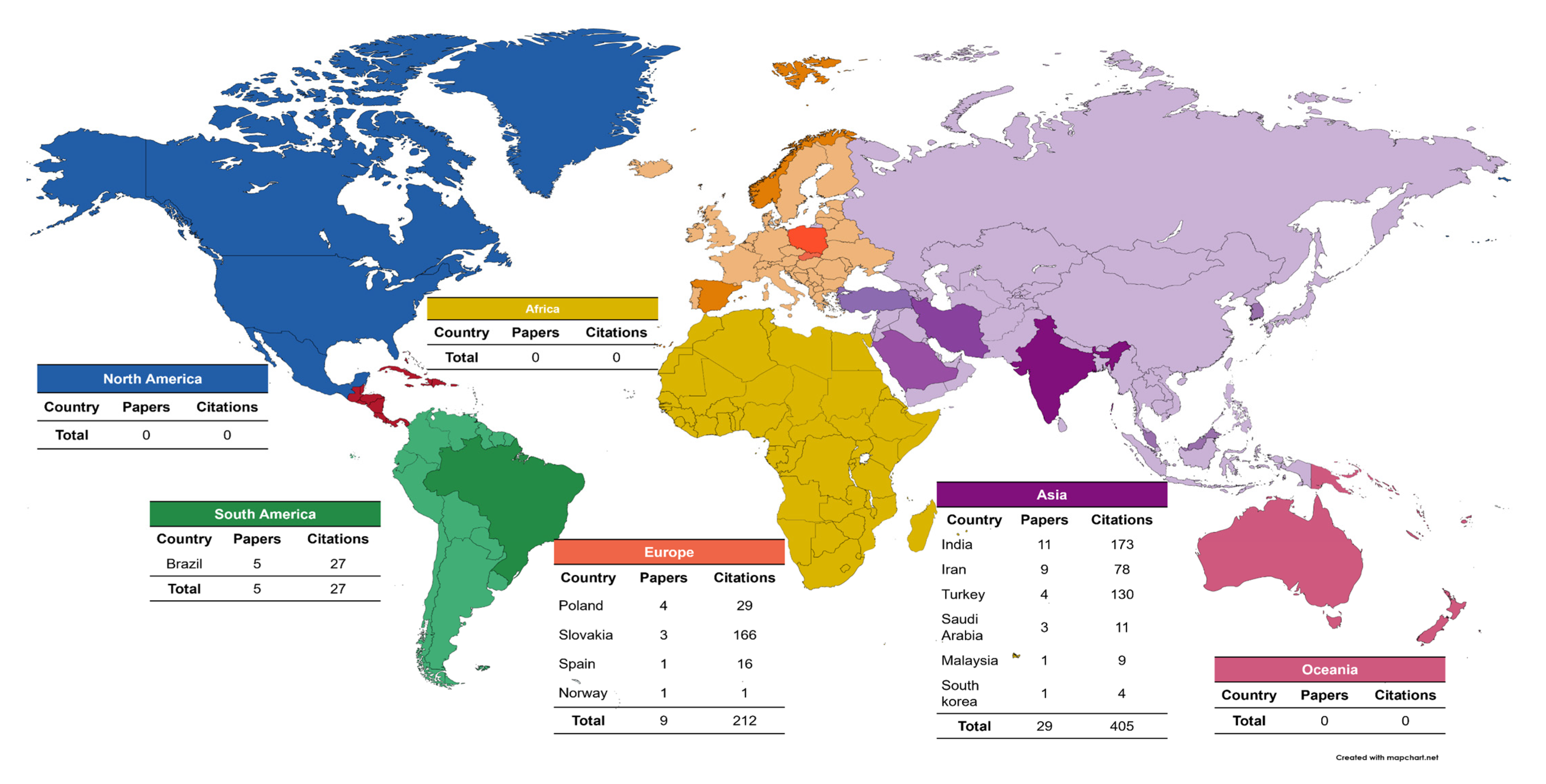
The country with the highest number of publications was India, with a total of 11 published articles and a total of 173 citations. Asia had the most publications on caries and salivary oxidative stress with 29 articles and a total of 405 citations; South America published 5 articles; North America, Africa, and Oceania had no articles published in the area (Figure 5).
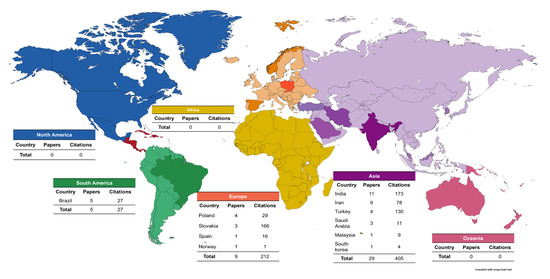
Figure 5.
Geographical distribution of the selected articles.
Among the study types, most were cross-sectional studies (n = 19), as well as case-control articles (n = 14), literature review (n = 4), systematic review (n = 4), experimental in vivo (n = 1) and longitudinal studies (n = 1) (Table 3).

Table 3.
Types of studies of the selected articles.
The most cited article among the 43 selected articles was [18], which is a literature review that aims to gather information on the most frequently used salivary biochemical parameters and analyzes these markers in individuals with dental caries (Table 1).
The most performed form of saliva collection among the articles, for stress analysis, was unstimulated saliva, only two articles used the collection method through stimulated saliva [33,54]. Regarding the evaluation of dental caries, most of the articles did not evaluate according to the depth of caries, evaluating only the number of decayed teeth (Table 3).
Several oxidative parameters were evaluated in saliva, with variation in the evaluation method, and among the primary studies, the most evaluated salivary biochemical parameter was TAC (n = 22 articles) which was evaluated by six different methods, followed by LPO (n = 12 articles) which was evaluated in two different ways, the most common being the quantification of reactive substances to thiobarbituric acid, while the direct quantification of malondialdehyde was evaluated only in one study (Table 4).

Table 4.
Evaluated parameters of saliva collection, caries, and oxidative biochemistry salivary in original articles.
4. Discussion
This study evidenced the scarce knowledge production on the association between dental caries with salivary oxidative stress in the literature. Among the 43 articles included, we obtained a total of 644 citations. The article that obtained the highest number of citations (104 citations) was a literature review, which comprehensively showed the relationship between saliva oxidative biochemical parameters and oral diseases, including caries in children [18]. In addition, most articles have evaluated TAC as the main parameter of biochemical changes in the saliva of patients with dental caries, and parameters such as glutathione, superoxide dismutase, uric acid, catalase, lipid peroxidation, and nitric oxide [7,18,38].
In information science, there are three empirical laws that are used to group empirical relations, namely Bradford’s law of bibliographic dispersion, which shows the relation between the most cited journals, Lotka’s law of scientific productivity, which shows the relation between the most cited authors, and finally, Zipf’s law of word frequency, which shows the relation between the keywords that appear most in bibliometric articles [65]. In our bibliometric review, we used these laws as a tripe, evaluating the metrics most used keywords, journals that published the most, and authors that published the most. When it comes to Bradford’s law, in our study we were able to verify six periodicals that had two or more articles published, and the Journal of Clinical Pediatric Dentistry had the most articles on this subject with a total of five articles.
Lotka’s law cites the importance of observing the network of collaboration among authors who research a given topic. In our study, when we observe the network of authors, we see that 164 participated in the 43 studies included and that a large part of these authors had only one publication in the area. Some authors were highlighted by being in three articles, authors Celec, P., Tothova, L., Hegde, A.M., Shetty, S., Antoniali, C., and Pessan, JP, who were the authors who most contributed to research on dental caries and salivary oxidative stress.
Following Zipf’s law, we analyzed the keywords that appeared most; saliva appeared most often, followed by dental caries, oxidative stress, and total antioxidant capacity. Saliva is a fluid present in the oral cavity responsible for cleaning the teeth and oral mucosa, buffering capacity, pH control, and lubrication, and acts as a reservoir of calcium and phosphate [13] It is a fluid with a high ability to detect biomarkers of various diseases of the oral cavity such as dental caries [13,16]. The included studies showed that any change in the oral cavity ends up unbalancing the levels of biomarkers, as in the case of salivary oxidative stress biomarkers, which in some studies were shown to be altered in the presence of dental caries, for example, total antioxidant capacity and superoxide dismutase were higher in dental caries groups [17,19]
One of the metrics evaluated was the number of citations. The number of citations of an article indicates what a particular study has managed to achieve; in principle, a highly cited article is seen as a watershed moment and may thus have a significant impact on research and practice [66]. In our study, no article reached several citations above 400, with the maximum combined citations of all articles being 644 citations, which suggests that this is not yet a widely studied subject.
When analyzing the country and continent of origin of the articles, Asia was the continent that had the most articles published within the theme of our study; it is possible to observe that the theme is still a little debated since we found only 43 published articles on this subject. It was also possible to observe that no article was retrieved from North America with this theme among the articles selected in the WoS-CC, which is interesting because the USA has the main research centers worldwide with the largest amount of funding for their research [67]. Thus, this may show that this theme is not yet a priority among the main research centers when analyzing the scientific production in WoS, even though saliva is proving to be a diagnostic medium for diseases, such as dental caries.
Dental caries is a multifactorial disease that affects the mineralized tissue of teeth through the metabolism of sugar by bacteria that produce acids that degrade these mineral tissues, altering the natural process of demineralization and remineralization that occurs in the oral cavity [20]. Early detection of dental caries is critical for less intrusive and productive treatment [68]. Thus, based on the International Caries Detection and Assessment System (ICDAS) and the International Caries Classification and Management System (ICCMS TM) procedures contained in the included publications, dentists now have a guide to estimate the risk of caries appropriately. Clinical practice is more successful when knowledge is shared with other experts [69]. As shown by [17], biomarkers of oxidative damage and antioxidants are altered depending on the stage of caries with increased TAC and SOD levels in this group when compared to caries-free children, in an environment where MDA is decreased.
Antioxidant capacity reflects the sum of the effects of all antioxidants, more specifically non-enzymatic antioxidants [17]. In saliva, antioxidant defense is composed of enzymes such as peroxidase, catalase, superoxide dismutase, and glutathione peroxidase, in addition to small molecules such as uric acid, and vitamin E [17,53]. Some studies showed that the level of antioxidants in dental caries children was higher when compared to the control group with no dental caries [17,45,53].
When it comes to antioxidant defense, in this study we found the keyword total antioxidant capacity to be one of the most frequently appearing and the most frequent biochemical parameter in most articles (evaluated in 27 articles). Total antioxidant capacity is the most used parameter because of its rapidity and low cost, and it provides an overview of all the antioxidants [9]. This parameter shows the combined effect of all antioxidants present in plasma and body fluids. However, due to its generalist aspect, total antioxidant capacity has some limitations, as it provides a limited answer about antioxidant defense mechanisms, thus not showing individually how each antioxidant agent acts in the face of increased production of reactive species [9].
The methods used to measure total antioxidant capacity may be sensitive to different types of antioxidants and performed by different methods, making the data between experiments ultimately not comparable [9]. This study identified five different methods for evaluating antioxidant capacity among the selected articles: according to the neutralization capacity of the ABTS radical [7,22,33,50,53,57,64], the capacity to reduce iron ions (FRAP) [7,17,18,19,40,44,48,52,55,56], to reduce phosphomolybdenum [49,58] the capacity to inhibit lipid peroxidation [1,7,59,60] and the capacity to inhibit ORAC [45] and the inhibition of crocin bleaching [62].
Most of the studies included in this review evaluated antioxidant capacity and/or antioxidants; to evaluate the redox system imbalance, malondialdehyde was measured directly or indirectly by measuring thiobarbituric acid-reactive substances, which were conducted in children and/or adolescents. Caries disease increases the levels of malondialdehyde and/or thiobarbituric acid reactive substances, thus demonstrating an association between caries and lipid peroxidation. Moreover, there was an increase in the activity of antioxidants, such as glutathione, glutathione peroxidase, nitric oxide, and vitamin C, as well as an increase in total protein levels. In this sense, the studies evaluated showed an association between caries and the increase in antioxidant capacity evaluated in saliva.
The levels between pro-oxidants and antioxidants end up changing according to the age of the individual. Araujo et al. (2020) [17] showed in their study that there was a change in the levels of total antioxidant capacity depending on the age of the individual: children showed higher total antioxidant capacity compared to that adolescents. Another study by Salman et al. (2021) [32] showed when only adolescents were isolated, a significant decrease in total antioxidant capacity was observed in the dental caries group, showing that antioxidant capacity may vary with age.
We can observe in the articles found, that most of them evaluated caries in children, and few studies were found that had evaluated adult patients. This may be due to the fact that in children it is easier to limit the influence of factors affecting the level of antioxidants, such as smoking, production factors, intake of medicines, and the presence of chronic diseases [70].
When it comes to pro-oxidants, malonaldehyde was the main pro-oxidant biochemical parameter evaluated among the included articles. Malonaldehyde is the most widely used method to indicate damage to biomolecules and can be performed directly or indirectly by testing substances reactive to thiobarbituric acid; the latter method is commonly used in screening procedures because it is a fast and low-cost method [17]. In this aspect, the selected studies showed a negative association between caries and the levels of malonaldehyde or thiobarbituric acid reactive substances.
We observed the great heterogeneity between the articles, which makes it difficult to compare the results since the studies used different ages (albeit in approximate age groups) and different stages of caries disease. Celecová et al. 2012 [55] showed that the effect of age should be considered in the evaluation of salivary markers of oxidative stress in relation to oral health, as it is a predictor for the variability of the analyzed markers.
Another important aspect is the specificity of each method used for evaluating the antioxidant capacity, in addition to this, studies showed values corrected by protein levels, while others express the results in different measures: gross value or by the percentage of the control, some analyses were performed by absorbance, others by fluorimetry, making the comparison between studies sensitive.
Our findings show the need for establishing standard methods and parameters for oral biology analysis in the field of oxidative stress. Differently from periodontology, cariology does not present a robust involvement in oxidative biochemistry fields, which can be visualized by the results described here. The purpose of this review with bibliometric approaches is broader than listing the evidence from peer-reviewed studies; this review brought the fragilities of this field, the research trends, and new perspectives in the field.
Oxidative stress must be understood as more than a biochemical process that may affect biological structures such as cells and tissues; it is also a chemical process that can oxidize other molecules available. Based on that, what are the impacts of oxidative stress on restorative products and techniques? What are the effects of salivary oxidative stress on the enamel quality and demineralization/remineralization process? In the case of salivary oxidative stress being associated with dental caries, is it reasonable to use antioxidant products? These and several other questions are raised after the complete reading and interpretation of our results, contributing to the scientific knowledge production in the field.
5. Conclusions
Our investigation demonstrated that the relationship between salivary oxidative stress and dental caries remains understudied, with the observation that only a few countries are studying this theme, with few studies examining the relationship between these disorders. Although there are some studies that revealed a link between caries and oxidative stress, more research is needed to accurately observe the interaction of salivary oxidative stress with dental caries and clarify the mechanism behind it.
Author Contributions
Y.G.d.S.N., W.F.L. and R.R.L.: study concept and design; Y.G.d.S.N., W.F.L., P.F.S.M., D.C.B.-d.-S., P.C.N. and R.R.L.: analysis and interpretation of data; Y.G.d.S.N., W.F.L., L.O.B., P.F.S.M., R.D.d.S.-R., D.C.B.-d.-S., P.C.N. and R.R.L.: preparation of the manuscript; R.D.d.S.-R., L.R.P., P.A.M.-J. and R.R.L.: critical revision of the manuscript. All authors contributed to the article and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
L.O.B. and P.F.S.M. received a scholarship from FAPESPA—Fundação Amazônia de Amparo a Estudos e Pesquisas. RRL is a researcher from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and received a grant under number 312275/2021-8. The APC was funded by Pró-Reitoria de Pesquisa e Pós-graduação from Federal University of Pará (PROPESP-UFPA).
Data Availability Statement
The data of this manuscript has been included in the main text of this article.
Acknowledgments
We thank the Federal University of Uberlândia and the Federal University of Minas Gerais for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pyati, S.A.; Naveen Kumar, R.; Kumar, V.; Praveen Kumar, N.H.; Reddy, K.M.P. Salivary Flow Rate, pH, Buffering Capacity, Total Protein, Oxidative Stress and Antioxidant Capacity in Children with and without Dental Caries. J. Clin. Pediatr. Dent. 2018, 42, 445–449. [Google Scholar] [CrossRef]
- Kirschvink, N.; de Moffarts, B.; Lekeux, P. The oxidant/antioxidant equilibrium in horses. Vet. J. 2008, 177, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Tunez, I.; Feijoo, M.; Huerta, G.; Montilla, P.; Munoz, E.; Ruiz, A.; Collantes, E. The effect of infliximab on oxidative stress in chronic inflammatory joint disease. Curr. Med. Res. Opin. 2007, 23, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Sheikhi, M.; Bouhafs, R.K.; Hammarström, K.J.; Jarstrand, C. Lipid peroxidation caused by oxygen radicals from Fusobacterium-stimulated neutrophils as a possible model for the emergence of periodontitis. Oral Dis. 2001, 7, 41–46. [Google Scholar] [PubMed]
- Baltacioglu, E.; Akalin, F.A.; Alver, A.; Balaban, F.; Unsal, M.; Karabulut, E. Total antioxidant capacity and superoxide dismutase activity levels in serum and gingival crevicular fluid in post-menopausal women with chronic periodontitis. J. Clin. Periodontol. 2006, 33, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Canakci, V.; Yildirim, A.; Canakci, C.F.; Eltas, A.; Cicek, Y.; Canakci, H. Total antioxidant capacity and antioxidant enzymes in serum, saliva, and gingival crevicular fluid of preeclamptic women with and without periodontal disease. J. Periodontol. 2007, 78, 1602–1611. [Google Scholar] [CrossRef]
- Ahmadi-Motamayel, F.; Goodarzi, M.T.; Mahdavinezhad, A.; Jamshidi, Z.; Darvishi, M. Salivary and Serum Antioxidant and Oxidative Stress Markers in Dental Caries. Caries Res. 2018, 52, 565–569. [Google Scholar] [CrossRef]
- Morris, S.M.; Billiar, T.R. New insights into regulation of inducible nitric oxide synthesis. Am. J. Physiol. 1994, 266, E829–E839. [Google Scholar] [CrossRef]
- Maxwell, S.R.; Dietrich, T.; Chapple, I.L. Prediction of serum total antioxidant activity from the concentration of individual serum antioxidants. Clin. Chim. Acta 2006, 372, 188–194. [Google Scholar] [CrossRef]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Halliwell, B. Antioxidant defence mechanisms: From the beginning to the end (of the beginning). Free Radic. Res. 1999, 31, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Curr. Top. Cell. Regul. 2000, 36, 95–116. [Google Scholar] [PubMed]
- Buzalaf, M.A.; Hannas, A.R.; Kato, M.T. Saliva and dental erosion. J. Appl. Oral Sci. 2012, 20, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Shafer, W.G.; Hine, M.K.; Levy, B.M.E.D. A Text Book of Oral Pathology, 5th ed.; W.B. Saunders Company: Philadelphia, PA, USA, 1993; pp. 567–658. [Google Scholar]
- Edgar, M.; Dawes, C.; O’Mullane, D. Saliva and Oral Health; British Dental Association: London, UK, 2004; 146p. [Google Scholar]
- Lee, Y.H.; Wong, D.T. Saliva: An emerging biofluid for early detection of diseases. Am. J. Dent. 2009, 22, 241–248. [Google Scholar] [PubMed]
- Araujo, H.C.; Nakamune, A.C.M.S.; Garcia, W.G.; Pessan, J.P.; Antoniali, C. Carious Lesion Severity Induces Higher Antioxidant System Activity and Consequently Reduces Oxidative Damage in Children’s Saliva. Oxid. Med. Cell. Longev. 2020, 28, 3695683. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, L.U.; Kamodyová, N.; Červenka, T.; Celec, P. Salivary markers of oxidative stress in oral diseases. Front. Cell. Infect. Microbiol. 2015, 5, 73. [Google Scholar] [CrossRef]
- Da Silva, P.V.; Troiano, J.A.; Nakamune, A.C.M.S.; Pessan, J.P.; Antoniali, C. Increased activity of the antioxidants systems modulate the oxidative stress in saliva of toddlers with early childhood caries. Arch. Oral Biol. 2016, 70, 62–66. [Google Scholar] [CrossRef]
- Mc-Donald, R.E.; Avery, D.R.; Stookey, G.K. Dental caries in the child and adolescent. In Dentistry for the Child and Adolescent, 8th ed.; Mc-Donald, R.E., Avery, D.R., Dean, J.A., Eds.; Elsevier: New Delhi, India, 2005; pp. 203–235. [Google Scholar]
- Mash, P.; Martin, M. Dental plaque. In Oral Microbiology; Springer: Boston, MA, USA, 1992; pp. 98–132. [Google Scholar]
- Krawczyk, D.; Sikorska-Jaroszyńska, M.H.; Mielnik-Błaszczak, M.; Pasternak, K.; Kapeć, E.; Sztanke, M. Dental caries and total antioxidant status of unstimulated mixed whole saliva in patients aged 16-23 years. Adv. Med. Sci. 2012, 57, 163–168. [Google Scholar] [CrossRef]
- Nascimento, P.C.; Ferreira, M.K.M.; Bittencourt, L.O.; Martins-Júnior, P.A.; Lima, R.R. Global research trends on maternal exposure to methylmercury and offspring health outcomes. Front. Pharmacol. 2022, 13, 973118. [Google Scholar] [CrossRef]
- De Lima, W.F.; Né, Y.G.S.; Aragão, W.A.B.; Eiró-Quirino, L.; Baia-da-Silva, D.C.; Cirovic, A.; Lima, R.R. Global Scientific Research Landscape on Aluminum Toxicology. Biol. Trace Elem. Res. 2022, 1–15. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Van Eck, N.J.; Waltman, L.; van Raan, A.F.; Klautz, R.J.; Peul, W.C. Citation analysis may severely underestimate the impact of clinical research as compared to basic research. PLoS ONE 2013, 8, e62395. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Collaboration Glossary. 2019. Available online: https://community.cochrane.org/glossary (accessed on 15 April 2019).
- Bayindir, Y.Z.; Polat, M.F.; Seven, N. Nitric oxide concentrations in saliva and dental plaque in relation to caries experience and oral hygiene. Caries Res. 2005, 39, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.R.; Diaz-Fabregat, B.; Ramirez-Carmona, W.; Monteiro, D.R.; Pessan, J.P.; Antoniali, C. Salivary biomarkers of oxidative stress in children with dental caries: Systematic review and meta-analysis. Arch. Oral Biol. 2022, 139, 105432. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Né, Y.G.; Frazão, D.R.; Bittencourt, L.O.; Fagundes, N.C.F.; Marañón-Vásquez, G.; Crespo-Lopez, M.E.; Maia, L.C.; Lima, R.R. Are Dental Caries Associated with Oxidative Stress in Saliva in Children and Adolescents? A Systematic Review. Metabolites 2022, 12, 858. [Google Scholar] [CrossRef] [PubMed]
- Aliakbarpour, F.; Mahjoub, S.; Masrour-Roudsari, J.; Seyedmajidi, S.; Ghasempour, M. Evaluation of salivary thiobarbituric acid reactive substances, total protein, and pH in children with various degrees of early childhood caries: A case-control study. Eur. Arch. Paediatr. Dent. 2021, 22, 1095–1099. [Google Scholar] [CrossRef]
- Salman, B.N.; Darvish, S.; Goriuc, A.; Mazloomzadeh, S.; Tehrani, M.H.P.; Luchian, I. Salivary Oxidative Stress Markers’ Relation to Oral Diseases in Children and Adolescents. Antioxidants 2021, 10, 1540. [Google Scholar] [CrossRef]
- Wagle, M.; Basnet, P.; Vartun, A.; Acharya, G. Nitric Oxide, Oxidative Stress and Streptococcus mutans and Lactobacillus Bacterial Loads in Saliva during the Different Stages of Pregnancy: A Longitudinal Study. Int. J. Environ. Res. Public Health 2021, 18, 9330. [Google Scholar] [CrossRef]
- Amrollahi, N.; Enshaei, Z.; Kavousi, F. Salivary Malondialdehyde Level as a Lipid Peroxidation Marker in Early Childhood Caries. Iran. J. Pediatr. 2021, 31, 67–70. [Google Scholar] [CrossRef]
- Karthika, G.; Avula, S.S.J.; Sridevi, E.; Pranitha, K.; Sankar, K.S.; Nayak, P. Assessing Vitamin E and Glutathione Peroxidase Levels in Salivary Samples of Children with and without Dental Caries. J. Clin. Diagn. Res. 2021, 15, 4. [Google Scholar] [CrossRef]
- Gorji, N.E.; Shafaroudi, A.M.; Nasiri, P.; Moosazadeh, M.; Nahvi, A. Relationship Between Salivary Nitric Oxide Concentration and Dental Caries in Children: A Systematic Review and Meta-analysis. Iran. J. Pediatr. 2021, 31, e107050. [Google Scholar]
- Ravikumar, D.; Ramani, P.; Gayathri, R. Comparative Evaluation of Salivary Malondialdehyde Levels in Children with Different Caries Status- A Cross-Sectional Observational Study. J. Pharm. Res. Int. 2021, 33, 147–154. [Google Scholar] [CrossRef]
- Vahabzadeh, Z.; Hashemi, Z.M.; Nouri, B.; Zamani, F.; Shafiee, F. Salivary enzymatic antioxidant activity and dental caries: A cross-sectional study. Dent. Med. Probl. 2020, 57, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Skutnik-Radziszewska, A.; Zalewska, A. Salivary Redox Biomarkers in the Course of Caries and Periodontal Disease. Appl. Sci. 2020, 10, 6240. [Google Scholar] [CrossRef]
- Shaki, F.; Arab-Nozari, M.; Maleki, F.; Charati, J.Y.; Nahvi, A. Evaluation of Some Caries-Related Factors in the Saliva of 3-5 Year Old Children in Sari, Northern Iran. Int. J. Pediatr. 2020, 8, 11115–11123. [Google Scholar]
- Malta, C.P.; Barcelos, R.C.S.; Rosa, H.Z.; Burger, M.E.; Bento, L.W. Effect of cerebral palsy and dental caries on dental plaque index, salivary parameters and oxidative stress in children and adolescents. Eur. Arch. Paediatr. Dent. 2021, 22, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Aksit-Bicak, D.; Emekli-Alturfan, E.; Ustundag, U.V.; Akyuz, S. Assessment of dental caries and salivary nitric oxide levels in children with dyspepsia. BMC Oral Health 2019, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Syed, S.; Yassin, S.M.; Dawasaz, A.A.; Amanullah, M.; Alshahrani, I.; Togoo, R.A. Salivary 1,5-Anhydroglucitol and Vitamin Levels in Relation to Caries Risk in Children. Biomed. Res. Int. 2019, 2019, 4503450. [Google Scholar] [CrossRef]
- Rahman, M.T.; Hossain, A.; Pin, C.H.; Yahya, N.A. Zinc and Metallothionein in the Development and Progression of Dental Caries. Biol. Trace Elem. Res. 2019, 187, 51–58. [Google Scholar] [CrossRef]
- Alanazi, G.S.; Pani, S.C.; AlKabbaz, H.J. Salivary antioxidant capacity of children with severe early childhood caries before and after complete dental rehabilitation. Arch. Oral Biol. 2018, 95, 165–169. [Google Scholar] [CrossRef]
- Pani, S.C. The Relationship between Salivary Total Antioxidant Capacity and Dental Caries in Children: A Meta-Analysis with Assessment of Moderators. J. Int. Soc. Prev. Community Dent. 2018, 8, 381–385. [Google Scholar] [CrossRef]
- Mohammed, H.K.; Anjana, G.; Zareena, M.A.; Sunil, E.A. Antioxidant Capacity of Saliva: Effect on Onset and Progression of Dental Caries. Oral Maxillofac. Pathol. J. 2017, 8, 19–22. [Google Scholar]
- Jurczak, A.; Koscielniak, D.; Skalniak, A.; Papiez, M.; Vyhouskaya, P.; Krzysciak, W. The role of the saliva antioxidant barrier to reactive oxygen species with regard to caries development. Redox Rep. 2017, 22, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, P.; Das, L.M.; Babu, K.L.G. Assessment of Salivary Total Antioxidant Levels and Oral Health Status in Children with Cerebral Palsy. J. Clin. Pediatr. Dent. 2014, 38, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, D.; Blaszczak, J.; Borowicz, J.; Mielnik-Blaszczak, M. Life style and risk of development of dental caries in a population of adolescents. Ann. Agric. Environ. Med. 2014, 21, 576–580. [Google Scholar] [CrossRef]
- Hegde, M.N.; Hegde, N.D.; Ashok, A.; Shetty, S. Biochemical Indicators of Dental Caries in Saliva: An in vivo Study. Caries Res. 2014, 48, 170–173. [Google Scholar] [CrossRef]
- Mahjoub, S.; Ghasempour, M.; Gharage, A.; Bijani, A.; Masrourroudsari, J. Comparison of Total Antioxidant Capacity in Saliva of Children with Severe Early Childhood Caries and Caries-Free Children. Caries Res. 2014, 48, 271–275. [Google Scholar] [CrossRef]
- Ahmadi-Motamayel, F.; Goodarzi, M.T.; Hendi, S.S.; Kasraei, S.; Moghimbeigi, A. Total antioxidant capacity of saliva and dental caries. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, E553–E600. [Google Scholar] [CrossRef]
- Han, D.H.; Kim, M.J.; Jun, E.J.; Kim, J.B. The role of glutathione metabolism in cariogenic bacterial growth and caries in Korean children. Arch. Oral Biol. 2013, 58, 493–499. [Google Scholar] [CrossRef]
- Celecová, V.; Kamodyova, N.; Tothova, L.; Kudela, M.; Celec, P. Salivary markers of oxidative stress are related to age and oral health in adult non-smokers. J. Oral Pathol. Med. 2013, 42, 263–266. [Google Scholar] [CrossRef]
- Tóthová, L.; Celecová, V.; Celec, P. Salivary markers of oxidative stress and their relation to periodontal and dental status in children. Dis. Markers 2013, 34, 9–15. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, R.K.; Agrawal, D.; Agrawal, D. An estimation and evaluation of total antioxidant capacity of saliva in children with severe early childhood caries. Int. J. Paediatr. Dent. 2011, 21, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Hegde, A.M.; Joshi, S.; Rai, K.; Shetty, S. Evaluation of Oral Hygiene Status, Salivary Characteristics and Dental Caries Experience in Acute Lymphoblastic Leukemic (ALL) Children. J. Clin. Pediatr. Dent. 2011, 35, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Preethi, B.P.; Pyati, A.; Dodawad, R. Evaluation of flow rate, ph, buffering capacity, calcium, total protein and total antioxidant levels of saliva in caries free and caries active children-An in vivo study. Biomed. Res. 2010, 21, 289–294. [Google Scholar] [CrossRef]
- Hegde, A.M.; Rai, K.; Padmanabhan, V. Total Antioxidant Capacity of Saliva and its Relation with Early Childhood Caries and Rampant Caries. J. Clin. Pediatr. Dent. 2009, 33, 231–234. [Google Scholar] [CrossRef]
- Ozturk, L.K.; Furuncuoglu, H.; Atala, M.H.; Ulukoylu, O.; Akyuz, S.; Yarat, A. Association between dental-oral health in young adults and salivary glutathione, lipid peroxidation and sialic acid levels and carbonic anhydrase activity. Braz. J. Med. Biol. Res. 2008, 41, 956–959. [Google Scholar] [CrossRef] [PubMed]
- Uberos, J.; Alarcon, J.A.; Penãlver, M.A.; Molina-Carballo, A.; Ruiz, M.; Gonzalez, E.; Castejon, J.; Muñoz-Hoyos, A. Influence of the antioxidant content of saliva on dental caries in an at-risk community. Br. Dent. J. 2008, 205, E5. [Google Scholar] [CrossRef]
- Hegde, A.M.; Neekhra, V.; Shetty, S. Evaluation of Levels of Nitric Oxide in Saliva of Children with Rampant Caries and Early Childhood Caries: A Comparative Study. J. Clin. Pediatr. Dent. 2008, 32, 283–286. [Google Scholar] [CrossRef]
- Tulunoglui, O.; Demirtas, S.; Tulunoglu, I. Total antioxidant levels of saliva in children related to caries, age, and gender. Int. J. Paediatr. Dent. 2006, 16, 186–191. [Google Scholar] [CrossRef]
- Chen, Y.S.; Leimkuhler, F.F. A relationship between Lotka’s law, Bradford’s law, and Zipf’s law. JASIST 1986, 37, 307–314. [Google Scholar] [CrossRef]
- Van Noorden, R.; Maher, B.; Nuzzo, R. The top 100 papers. Nature 2014, 514, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Shadgan, B.; Roig, M.; HajGhanbari, B.; Reid, W.D. Top-cited articles in rehabilitation. Arch. Phys. Med. Rehabil. 2010, 91, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Volgenant, C.M.; Zaura, E.; Brandt, B.W.; Buijs, M.J.; Tellez, M.; Malik, G.; Ismail, A.I.; Ten Cate, J.M.; van der Veen, M.H. Red fluorescence of dental plaque in children -A cross-sectional study. J. Dent. 2017, 58, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Griffen, A.L.; Dardis, S.R.; Lee, A.M.; Olsen, I.; Dewhirst, F.E.; Leys, E.J.; Paster, B.J. Bacteria of dental caries in primary and permanent teeth in children and young adults. J. Clin. Microbiol. 2008, 46, 1407–1417. [Google Scholar] [CrossRef]
- Caliri, A.W.; Tommasi, S.; Besaratinia, A. Relationships among smoking, oxidative stress, inflammation, macromolecular damage, and cancer. Mutat. Res. Rev. Mutat. Res. 2021, 787, 108365. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).