An Updated Overview on the Role of Small Molecules and Natural Compounds in the “Young Science” of Rejuvenation
Abstract
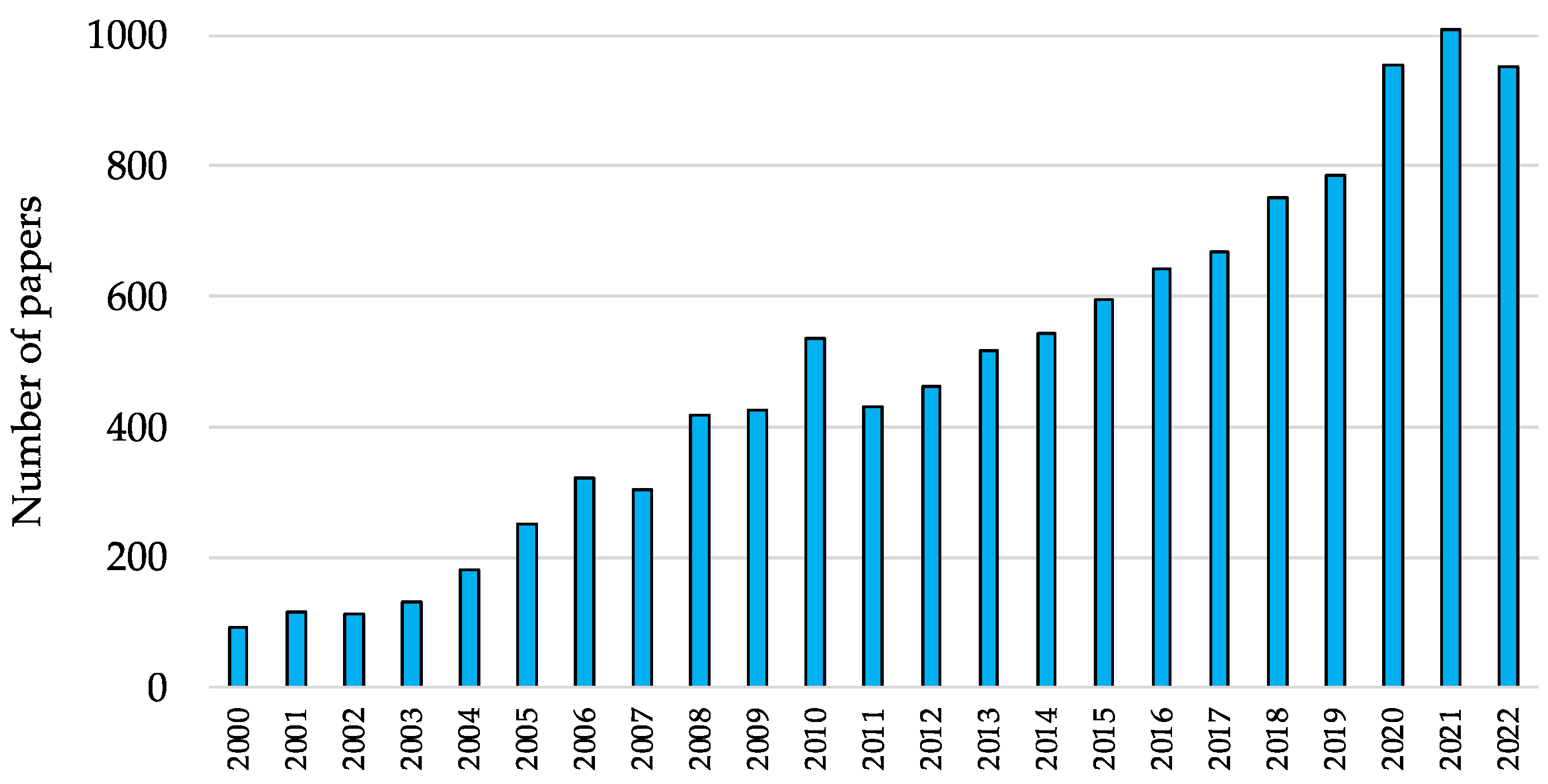
1. Introduction
2. Aging and Rejuvenation
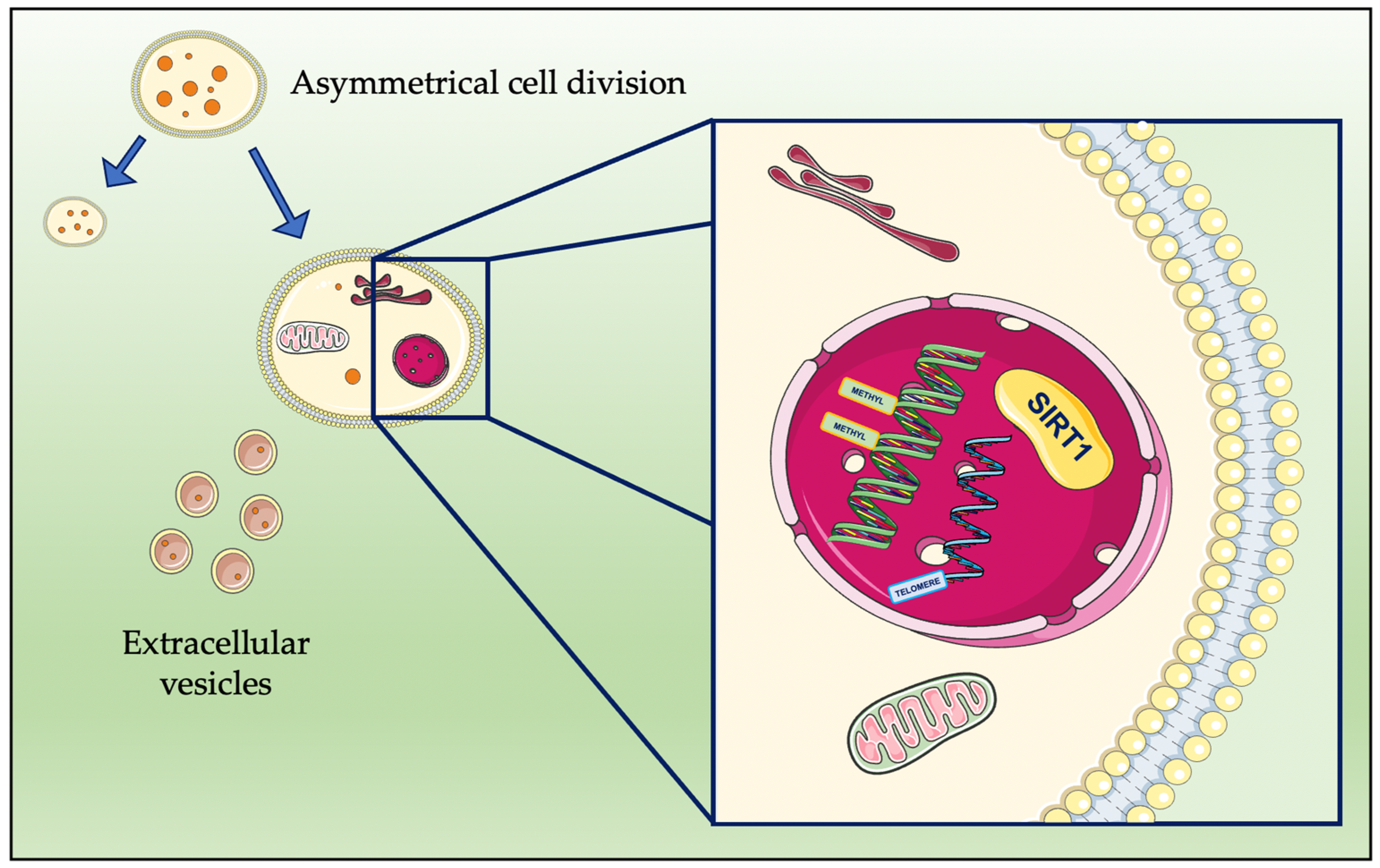
3. Cellular Processes Promoting Rejuvenation
4. Druggable Rejuvenation Mechanisms
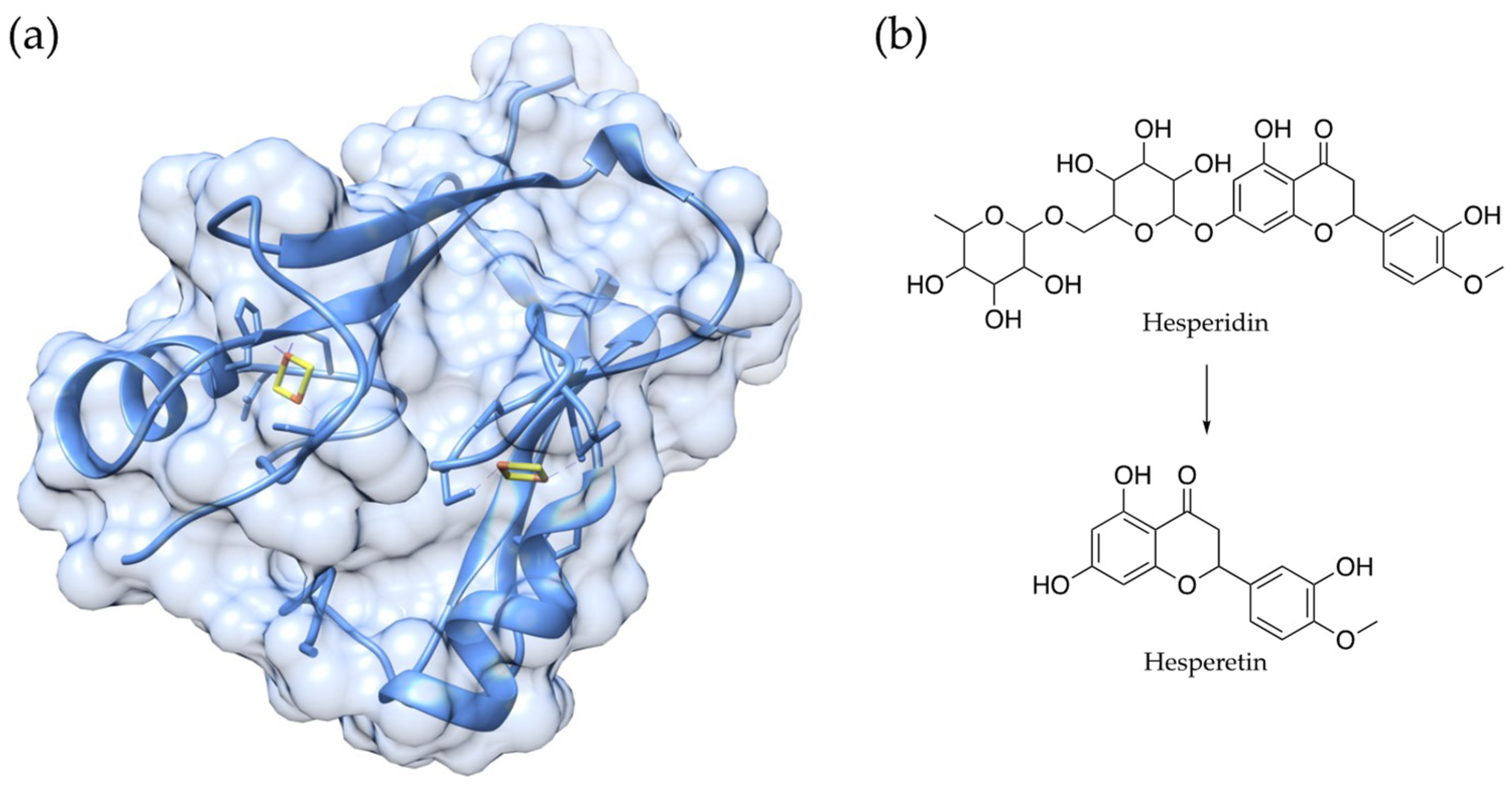
4.1. CDGSH Iron-Sulfur Domain 2 (CISD2)
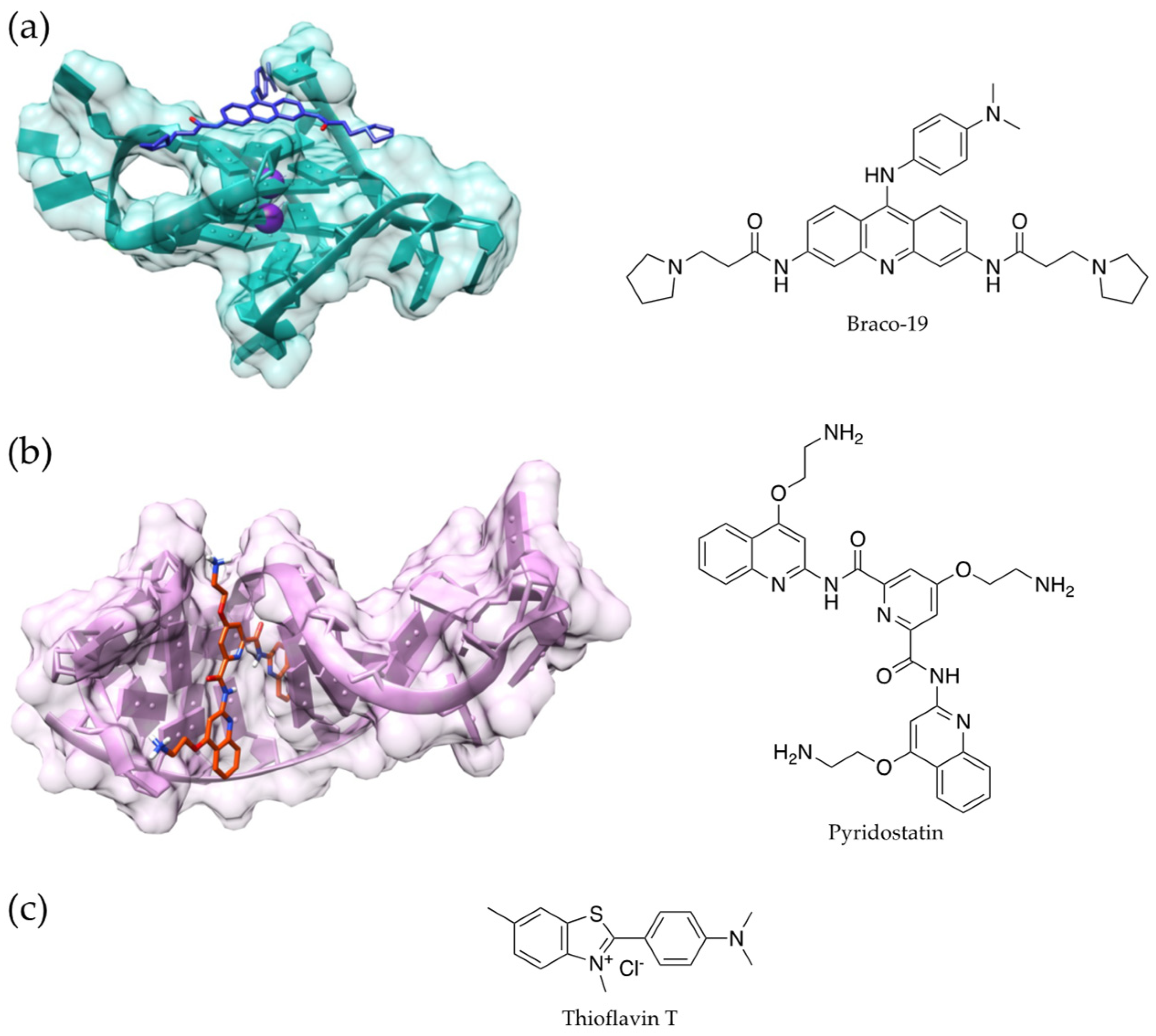
4.2. G-Quadruplexes and DNA Methylation
4.3. Mitochondrial Decay and Antioxidants
4.4. Dietary Compounds Acting through Mixed Mechanisms
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Gill, D.; Parry, A.; Santos, F.; Okkenhaug, H.; Todd, C.D.; Hernando-Herraez, I.; Stubbs, T.M.; Milagre, I.; Reik, W. Multi-omic rejuvenation of human cells by maturation phase transient reprogramming. eLife 2022, 11, e71624. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef] [PubMed]
- Hood, S.; Amir, S. The aging clock: Circadian rhythms and later life. J. Clin. Investig. 2017, 127, 437–446. [Google Scholar] [CrossRef]
- Zhang, B.; Trapp, A.; Kerepesi, C.; Gladyshev, V.N. Emerging rejuvenation strategies—Reducing the biological age. Aging Cell 2022, 21, e13538. [Google Scholar] [CrossRef]
- Eisenstein, M. Rejuvenation by controlled reprogramming is the latest gambit in anti-aging. Nat. Biotechnol. 2022, 40, 144–146. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera?A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- de Magalhães, J.P.; Ocampo, A. Cellular reprogramming and the rise of rejuvenation biotech. Trends Biotechnol. 2022, 40, 639–642. [Google Scholar] [CrossRef] [PubMed]
- O’Loghlen, A. The potential of aging rejuvenation. Cell Cycle 2022, 21, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Galkin, F.; Mamoshina, P.; Aliper, A.; de Magalhães, J.P.; Gladyshev, V.N.; Zhavoronkov, A. Biohorology and biomarkers of aging: Current state-of-the-art, challenges and opportunities. Ageing Res. Rev. 2020, 60, 101050. [Google Scholar] [CrossRef]
- Lu, Y.; Brommer, B.; Tian, X.; Krishnan, A.; Meer, M.; Wang, C.; Vera, D.L.; Zeng, Q.; Yu, D.; Bonkowski, M.S.; et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature 2020, 588, 124–129. [Google Scholar] [CrossRef]
- Zhang, W.-G.; Zhu, S.-Y.; Bai, X.-J.; Zhao, D.-L.; Jian, S.-M.; Li, J.; Li, Z.-X.; Fu, B.; Cai, G.-Y.; Sun, X.-F.; et al. Select aging biomarkers based on telomere length and chronological age to build a biological age equation. Age 2014, 36, 9639. [Google Scholar] [CrossRef]
- Demarchi, B.; Collins, M. Amino Acid Racemization Dating. In Encyclopedia of Scientific Dating Methods; Rink, W.J., Thompson, J., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 1–22. ISBN 978-94-007-6326-5. [Google Scholar]
- Higgins-Chen, A.T.; Thrush, K.L.; Levine, M.E. Aging biomarkers and the brain. Semin. Cell Dev. Biol. 2021, 116, 180–193. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.-B.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.H.; Schumacher, B. BiT age: A transcriptome-based aging clock near the theoretical limit of accuracy. Aging Cell 2021, 20, e13320. [Google Scholar] [CrossRef]
- Zhavoronkov, A.; Li, R.; Ma, C.; Mamoshina, P. Deep biomarkers of aging and longevity: From research to applications. Aging 2019, 11, 10771–10780. [Google Scholar] [CrossRef] [PubMed]
- Sheldrake, A.R. Cellular senescence, rejuvenation and potential immortality. Proc. R. Soc. B 2022, 289, 20212434. [Google Scholar] [CrossRef]
- Heidstra, R.; Sabatini, S. Plant and animal stem cells: Similar yet different. Nat. Rev. Mol. Cell Biol. 2014, 15, 301–312. [Google Scholar] [CrossRef]
- Lindner, A.B.; Madden, R.; Demarez, A.; Stewart, E.J.; Taddei, F. Asymmetric segregation of protein aggregates is associated with cellular aging and rejuvenation. Proc. Natl. Acad. Sci. USA 2008, 105, 3076–3081. [Google Scholar] [CrossRef]
- Spokoini, R.; Moldavski, O.; Nahmias, Y.; England, J.L.; Schuldiner, M.; Kaganovich, D. Confinement to Organelle-Associated Inclusion Structures Mediates Asymmetric Inheritance of Aggregated Protein in Budding Yeast. Cell Rep. 2012, 2, 738–747. [Google Scholar] [CrossRef]
- Ludwig, F.C.; Elashoff, R.M. Mortality in syngeneic rat parabionts of different chronological age. Trans. N. Y. Acad. Sci. 1972, 34, 582–587. [Google Scholar] [CrossRef]
- Krohn, P.L. Review lectures on senescence. II. Heterochronic transplantation in the study of ageing. Proc. R. Soc. Lond. B Biol. Sci. 1962, 157, 128–147. [Google Scholar] [CrossRef] [PubMed]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef]
- Moore, D.L.; Jessberger, S. Creating Age Asymmetry: Consequences of Inheriting Damaged Goods in Mammalian Cells. Trends Cell Biol. 2017, 27, 82–92. [Google Scholar] [CrossRef]
- Takahashi, A.; Okada, R.; Nagao, K.; Kawamata, Y.; Hanyu, A.; Yoshimoto, S.; Takasugi, M.; Watanabe, S.; Kanemaki, M.T.; Obuse, C.; et al. Exosomes maintain cellular homeostasis by excreting harmful DNA from cells. Nat. Commun. 2017, 8, 15287. [Google Scholar] [CrossRef] [PubMed]
- Rang, C.U.; Proenca, A.; Buetz, C.; Shi, C.; Chao, L. Minicells as a Damage Disposal Mechanism in Escherichia coli. mSphere 2018, 3, e00428-18. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, M.S.; Jia, B.; Yan, J.; Zuniga-Hertz, J.P.; Han, C.; Cai, D. Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 2017, 548, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Ritschka, B.; Storer, M.; Mas, A.; Heinzmann, F.; Ortells, M.C.; Morton, J.P.; Sansom, O.J.; Zender, L.; Keyes, W.M. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017, 31, 172–183. [Google Scholar] [CrossRef]
- Sarkar, T.J.; Quarta, M.; Mukherjee, S.; Colville, A.; Paine, P.; Doan, L.; Tran, C.M.; Chu, C.R.; Horvath, S.; Qi, L.S.; et al. Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat. Commun. 2020, 11, 1545. [Google Scholar] [CrossRef]
- Fahy, G.M.; Brooke, R.T.; Watson, J.P.; Good, Z.; Vasanawala, S.S.; Maecker, H.; Leipold, M.D.; Lin, D.T.S.; Kobor, M.S.; Horvath, S. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell 2019, 18, e13028. [Google Scholar] [CrossRef] [PubMed]
- Vitale, G.; Pellegrino, G.; Vollery, M.; Hofland, L.J. ROLE of IGF-1 System in the Modulation of Longevity: Controversies and New Insights from a Centenarians’ Perspective. Front. Endocrinol. 2019, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Mariño, G.; Ugalde, A.P.; Fernández, Á.F.; Osorio, F.G.; Fueyo, A.; Freije, J.M.P.; López-Otín, C. Insulin-like growth factor 1 treatment extends longevity in a mouse model of human premature aging by restoring somatotroph axis function. Proc. Natl. Acad. Sci. USA 2010, 107, 16268–16273. [Google Scholar] [CrossRef] [PubMed]
- Poudel, S.B.; Dixit, M.; Neginskaya, M.; Nagaraj, K.; Pavlov, E.; Werner, H.; Yakar, S. Effects of GH/IGF on the Aging Mitochondria. Cells 2020, 9, 1384. [Google Scholar] [CrossRef] [PubMed]
- Claus Henn, B.; Austin, C.; Coull, B.A.; Schnaas, L.; Gennings, C.; Horton, M.K.; Hernández-Ávila, M.; Hu, H.; Téllez-Rojo, M.M.; Wright, R.O.; et al. Uncovering neurodevelopmental windows of susceptibility to manganese exposure using dentine microspatial analyses. Environ. Res. 2018, 161, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Wong, P.-F.; Dharmani, M.; Ramasamy, T.S. Senotherapeutics for mesenchymal stem cell senescence and rejuvenation. Drug Discov. Today 2023, 28, 103424. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Shen, Z.-Q.; Lin, C.-C.; Lu, C.-K.; Tsai, T.-F. Rejuvenation: Turning Back Time by Enhancing CISD2. IJMS 2022, 23, 14014. [Google Scholar] [CrossRef]
- Shen, Z.-Q.; Huang, Y.-L.; Teng, Y.-C.; Wang, T.-W.; Kao, C.-H.; Yeh, C.-H.; Tsai, T.-F. CISD2 maintains cellular homeostasis. Biochim. Biophys. Acta BBA Mol. Cell Res. 2021, 1868, 118954. [Google Scholar] [CrossRef]
- Conlan, A.R.; Axelrod, H.L.; Cohen, A.E.; Abresch, E.C.; Zuris, J.; Yee, D.; Nechushtai, R.; Jennings, P.A.; Paddock, M.L. Crystal Structure of Miner1: The Redox-active 2Fe-2S Protein Causative in Wolfram Syndrome 2. J. Mol. Biol. 2009, 392, 143–153. [Google Scholar] [CrossRef]
- Lin, C.-C.; Chiang, T.-H.; Sun, Y.-Y.; Lin, M.-S. Protective Effects of CISD2 and Influence of Curcumin on CISD2 Expression in Aged Animals and Inflammatory Cell Model. Nutrients 2019, 11, 700. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Chiang, T.-H.; Chen, W.-J.; Sun, Y.-Y.; Lee, Y.-H.; Lin, M.-S. CISD2 serves a novel role as a suppressor of nitric oxide signalling and curcumin increases CISD2 expression in spinal cord injuries. Injury 2015, 46, 2341–2350. [Google Scholar] [CrossRef] [PubMed]
- Kung, W.-M.; Lin, C.-C.; Kuo, C.-Y.; Juin, Y.-C.; Wu, P.-C.; Lin, M.-S.; Klivényi, P. Wild Bitter Melon Exerts Anti-Inflammatory Effects by Upregulating Injury-Attenuated CISD2 Expression following Spinal Cord Injury. Behav. Neurol. 2020, 2020, 1080521. [Google Scholar] [CrossRef]
- Yeh, C.-H.; Shen, Z.-Q.; Wang, T.-W.; Kao, C.-H.; Teng, Y.-C.; Yeh, T.-K.; Lu, C.-K.; Tsai, T.-F. Hesperetin promotes longevity and delays aging via activation of Cisd2 in naturally aged mice. J. Biomed. Sci. 2022, 29, 53. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Liu, S.; Yang, D.; Yang, L.; Hu, C.-D.; Wan, J. Decoding regulatory associations of G-quadruplex with epigenetic and transcriptomic functional components. Front. Genet. 2022, 13, 957023. [Google Scholar] [CrossRef] [PubMed]
- Rauchhaus, J.; Robinson, J.; Monti, L.; Di Antonio, M. G-quadruplexes Mark Sites of Methylation Instability Associated with Ageing and Cancer. Genes 2022, 13, 1665. [Google Scholar] [CrossRef] [PubMed]
- Kosiol, N.; Juranek, S.; Brossart, P.; Heine, A.; Paeschke, K. G-quadruplexes: A promising target for cancer therapy. Mol. Cancer 2021, 20, 40. [Google Scholar] [CrossRef]
- Ribaudo, G.; Ongaro, A.; Oselladore, E.; Memo, M.; Gianoncelli, A. Combining Electrospray Mass Spectrometry (ESI-MS) and Computational Techniques in the Assessment of G-Quadruplex Ligands: A Hybrid Approach to Optimize Hit Discovery. J. Med. Chem. 2021, 64, 13174–13190. [Google Scholar] [CrossRef]
- Ribaudo, G.; Ongaro, A.; Zagotto, G.; Memo, M.; Gianoncelli, A. Evidence on selective binding to G-quadruplex DNA of isoflavones from Maclura pomifera by mass spectrometry and molecular docking. Nat. Prod. Res. 2021, 35, 2583–2587. [Google Scholar] [CrossRef]
- Ribaudo, G.; Scalabrin, M.; Pavan, V.; Fabris, D.; Zagotto, G. Constrained bisantrene derivatives as G-quadruplex binders. Arkivoc 2016, 2016, 145–160. [Google Scholar] [CrossRef]
- Noh, B.; Blasco-Conesa, M.P.; Lai, Y.-J.; Ganesh, B.P.; Urayama, A.; Moreno-Gonzalez, I.; Marrelli, S.P.; McCullough, L.D.; Moruno-Manchon, J.F. G-quadruplexes Stabilization Upregulates CCN1 and Accelerates Aging in Cultured Cerebral Endothelial Cells. Front. Aging 2022, 2, 797562. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Thombre, R.; Shah, Y.; Latanich, R.; Wang, J. G-Quadruplexes as pathogenic drivers in neurodegenerative disorders. Nucleic Acids Res. 2021, 49, 4816–4830. [Google Scholar] [CrossRef]
- Xu, J.; Huang, H.; Zhou, X. G-Quadruplexes in Neurobiology and Virology: Functional Roles and Potential Therapeutic Approaches. JACS Au 2021, 1, 2146–2161. [Google Scholar] [CrossRef]
- Moruno-Manchon, J.F.; Lejault, P.; Wang, Y.; McCauley, B.; Honarpisheh, P.; Morales Scheihing, D.A.; Singh, S.; Dang, W.; Kim, N.; Urayama, A.; et al. Small-molecule G-quadruplex stabilizers reveal a novel pathway of autophagy regulation in neurons. eLife 2020, 9, e52283. [Google Scholar] [CrossRef]
- Shigenaga, M.K.; Hagen, T.M.; Ames, B.N. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. USA 1994, 91, 10771–10778. [Google Scholar] [CrossRef]
- Visioli, F.; Ingram, A.; Beckman, J.S.; Magnusson, K.R.; Hagen, T.M. Strategies to protect against age-related mitochondrial decay: Do natural products and their derivatives help? Free Radic. Biol. Med. 2022, 178, 330–346. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. N-Acetylcysteine: A Review of Clinical Usefulness (an Old Drug with New Tricks). J. Nutr. Metab. 2021, 2021, 9949453. [Google Scholar] [CrossRef]
- Tsushima, M.; Liu, J.; Hirao, W.; Yamazaki, H.; Tomita, H.; Itoh, K. Emerging evidence for crosstalk between Nrf2 and mitochondria in physiological homeostasis and in heart disease. Arch. Pharm. Res. 2020, 43, 286–296. [Google Scholar] [CrossRef]
- Si, H.; Liu, D. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J. Nutr. Biochem. 2014, 25, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Ovaskainen, M.-L.; Törrönen, R.; Koponen, J.M.; Sinkko, H.; Hellström, J.; Reinivuo, H.; Mattila, P. Dietary intake and major food sources of polyphenols in Finnish adults. J. Nutr. 2008, 138, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiao, D.; Burton-Freeman, B.M.; Edirisinghe, I. Chemical Changes of Bioactive Phytochemicals during Thermal Processing. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; p. B9780081005965031000. ISBN 978-0-08-100596-5. [Google Scholar]
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283. [Google Scholar] [CrossRef]
- Majidinia, M.; Karimian, A.; Alemi, F.; Yousefi, B.; Safa, A. Targeting miRNAs by polyphenols: Novel therapeutic strategy for aging. Biochem. Pharmacol. 2020, 173, 113688. [Google Scholar] [CrossRef]
- Thirumalaisamy, R.; Bhuvaneswari, M.; Haritha, S.; Jeevarathna, S.; Janani, K.S.S.; Suresh, K. Curcumin, Naringenin and Resveratrol from Natural Plant Products Hold Promising Solutions for Modern World Diseases—A Recent Review. S. Afr. J. Bot. 2022, 151, 567–580. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rajabi, S.; Martorell, M.; López, M.D.; Toro, M.T.; Barollo, S.; Armanini, D.; Fokou, P.V.T.; Zagotto, G.; Ribaudo, G.; et al. Plant natural products with anti-thyroid cancer activity. Fitoterapia 2020, 146, 104640. [Google Scholar] [CrossRef]
- Zanforlin, E.; Zagotto, G.; Ribaudo, G. The Medicinal Chemistry of Natural and Semisynthetic Compounds against Parkinson’s and Huntington’s Diseases. ACS Chem. Neurosci. 2017, 8, 2356–2368. [Google Scholar] [CrossRef]
- Zanforlin, E.; Zagotto, G.; Ribaudo, G. An Overview of New Possible Treatments of Alzheimer’s Disease, Based on Natural Products and Semi-Synthetic Compounds. CMC 2017, 24, 3749–3773. [Google Scholar] [CrossRef]
- Pavan, V.; Mucignat-Caretta, C.; Redaelli, M.; Ribaudo, G.; Zagotto, G. The Old Made New: Natural Compounds against Erectile Dysfunction: Natural Compounds against Erectile Dysfunction. Arch. Pharm. Chem. Life Sci. 2015, 348, 607–614. [Google Scholar] [CrossRef]
- Ribaudo, G.; Bortoli, M.; Pavan, C.; Zagotto, G.; Orian, L. Antioxidant Potential of Psychotropic Drugs: From Clinical Evidence to In Vitro and In Vivo Assessment and toward a New Challenge for in Silico Molecular Design. Antioxidants 2020, 9, 714. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, X.; Zhang, D.; Zhou, F.; Wang, D.; Wei, Y.; Gao, F.; Xie, L.; Jia, G.; Wu, W.; et al. Blueberry anthocyanins: Protection against ageing and light-induced damage in retinal pigment epithelial cells. Br. J. Nutr. 2012, 108, 16–27. [Google Scholar] [CrossRef]
- da Luz, P.L.; Tanaka, L.; Brum, P.C.; Dourado, P.M.M.; Favarato, D.; Krieger, J.E.; Laurindo, F.R.M. Red wine and equivalent oral pharmacological doses of resveratrol delay vascular aging but do not extend life span in rats. Atherosclerosis 2012, 224, 136–142. [Google Scholar] [CrossRef]
- Pearson, K.J.; Baur, J.A.; Lewis, K.N.; Peshkin, L.; Price, N.L.; Labinskyy, N.; Swindell, W.R.; Kamara, D.; Minor, R.K.; Perez, E.; et al. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metab. 2008, 8, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A. Anti-inflammatory effects of resveratrol: Possible role in prevention of age-related cardiovascular disease: Anti-inflammatory effects of resveratrol in aging. Ann. N. Y. Acad. Sci. 2011, 1215, 117–122. [Google Scholar] [CrossRef]
- Giuliani, C.; Iezzi, M.; Ciolli, L.; Hysi, A.; Bucci, I.; Di Santo, S.; Rossi, C.; Zucchelli, M.; Napolitano, G. Resveratrol has anti-thyroid effects both in vitro and in vivo. Food Chem. Toxicol. 2017, 107, 237–247. [Google Scholar] [CrossRef]
- Si, H.; Fu, Z.; Babu, P.V.A.; Zhen, W.; Leroith, T.; Meaney, M.P.; Voelker, K.A.; Jia, Z.; Grange, R.W.; Liu, D. Dietary epicatechin promotes survival of obese diabetic mice and Drosophila melanogaster. J. Nutr. 2011, 141, 1095–1100. [Google Scholar] [CrossRef]
- McDonald, C.M.; Ramirez-Sanchez, I.; Oskarsson, B.; Joyce, N.; Aguilar, C.; Nicorici, A.; Dayan, J.; Goude, E.; Abresch, R.T.; Villarreal, F.; et al. (−)-Epicatechin induces mitochondrial biogenesis and markers of muscle regeneration in adults with Becker muscular dystrophy. Muscle Nerve 2021, 63, 239–249. [Google Scholar] [CrossRef]
- Reboul, E. Vitamin E Bioavailability: Mechanisms of Intestinal Absorption in the Spotlight. Antioxidants 2017, 6, 95. [Google Scholar] [CrossRef] [PubMed]
- Valentino, S.; Ghelfi, M.; Zunica, E.; Stamper, M.; Hickman, S.; Hwang, S.; Young, E.; Atkinson, J.; Manor, D. Antioxidant independent actions of vitamin E in modulating gene expression. Free Radic. Biol. Med. 2018, 128, S58–S59. [Google Scholar] [CrossRef]
- Gellert, S.; Schuchardt, J.P.; Hahn, A. Low long chain omega-3 fatty acid status in middle-aged women. Prostaglandins Leukot. Essent. Fat. Acids 2017, 117, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Cutuli, D. Functional and Structural Benefits Induced by Omega-3 Polyunsaturated Fatty Acids During Aging. Curr. Neuropharmacol. 2017, 15, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Chiavellini, P.; Canatelli-Mallat, M.; Lehmann, M.; Gallardo, M.D.; Herenu, C.B.; Cordeiro, J.L.; Clement, J.; Goya, R.G. Aging and rejuvenation—A modular epigenome model. Aging 2021, 13, 4734–4746. [Google Scholar] [CrossRef] [PubMed]
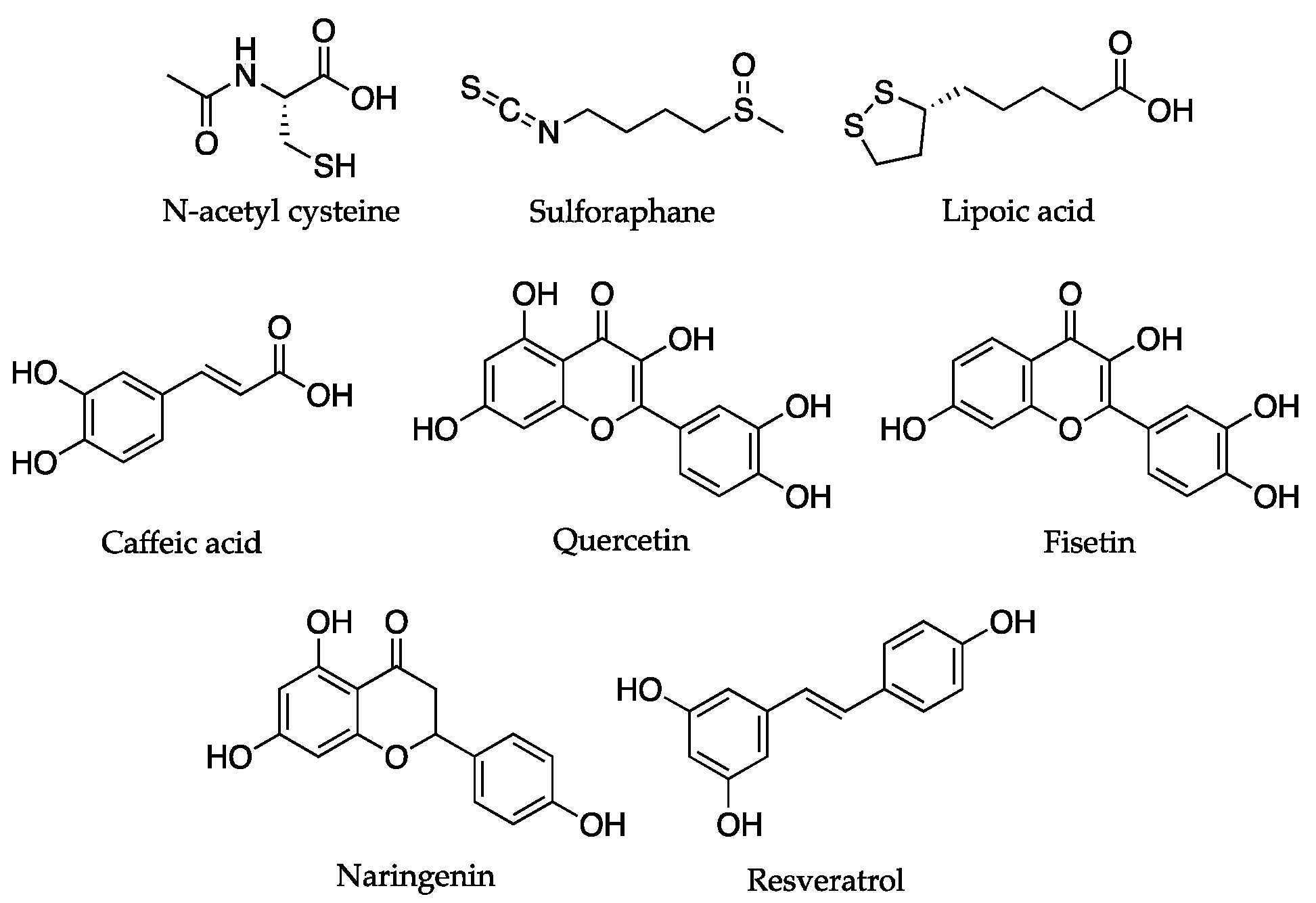
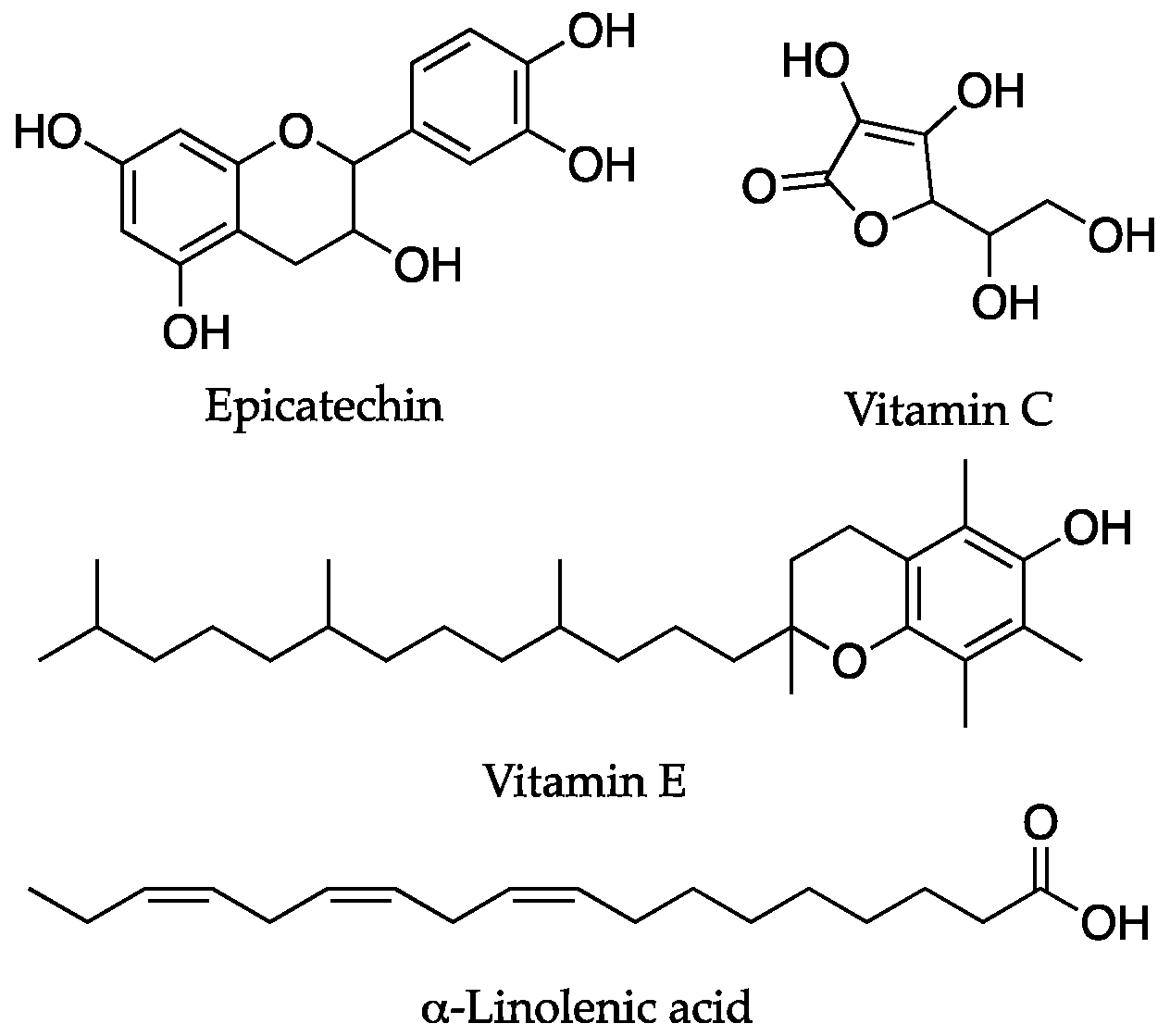
| Compounds | Targeted Mechanisms | Anti-Aging Effects | Rejuvenating Effects | Experimental Model | References |
|---|---|---|---|---|---|
| Growth hormone | GH/IGF-1 pathway, | Decrease in epigenetic age | Mouse, human | [35,36] | |
| Epicatechin | increased glutathione, superoxide dismutase, IGF-1 pathway, protein kinase-α activity, mithocondrial biogenesis | Improvement in tissue biomarkers, increased lifespan | Drosophila, mouse, human | [77,78] | |
| Hesperetin | CISD2 activation | Decrease in muscular aging | Interference with gene expression | Mouse | [45,46] |
| Other polyphenols | Antioxidant activity, miRNA targeting | Reduction in the accumulation of senescent cells and in the expression of oxidative stress markers | Caenorhabditis elegans, Drosophila, rodents | [3,64] | |
| PUFAs | Increase in the levels of signaling factors that are involved in neuronal plasticity | Improved cognitive activity and prevention of degeneration | Hippocampal neurogenesis | Mouse | [81,82] |
| Resveratrol | SIRT1 | Prevention of DNA damage | Improvement in longevity of biomarkers | MRC5 human fibroblasts, invertebrates, mouse, rat | [3,58] |
| Sulforaphane, lipoic acid, caffeic acid, quercetin, fisetin, and naringenin | Nrf2, ROS | Mitochondrial biogenesis, mitophagy, restoration of metabolites | HepG2 cells, HHL-5 cells, neurons, mouse, rat | [58] | |
| Vitamins (C, E) | Direct gene expression, antioxidant activity | Anti-aging activity, prevention of ROS damage | Cultured hepatocytes | [79,80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribaudo, G.; Gianoncelli, A. An Updated Overview on the Role of Small Molecules and Natural Compounds in the “Young Science” of Rejuvenation. Antioxidants 2023, 12, 288. https://doi.org/10.3390/antiox12020288
Ribaudo G, Gianoncelli A. An Updated Overview on the Role of Small Molecules and Natural Compounds in the “Young Science” of Rejuvenation. Antioxidants. 2023; 12(2):288. https://doi.org/10.3390/antiox12020288
Chicago/Turabian StyleRibaudo, Giovanni, and Alessandra Gianoncelli. 2023. "An Updated Overview on the Role of Small Molecules and Natural Compounds in the “Young Science” of Rejuvenation" Antioxidants 12, no. 2: 288. https://doi.org/10.3390/antiox12020288
APA StyleRibaudo, G., & Gianoncelli, A. (2023). An Updated Overview on the Role of Small Molecules and Natural Compounds in the “Young Science” of Rejuvenation. Antioxidants, 12(2), 288. https://doi.org/10.3390/antiox12020288







