Abstract
Metal toxicity poses a potential global threat to the environment and living beings. Their numerous agricultural, medical, industrial, domestic, and technological applications result in widespread distribution in the environment which raises concern on the potential effects of metals in terms of health hazards and environmental pollution. Chelation therapy has been the preferred medical treatment for metal poisoning. The chelating agent bounds metal ions to form complex cyclic structures known as ‘chelates’ to intensify their excretion from the body. The main disadvantage of synthetic chelators is that the chelation process removes vital nutrients along with toxic metals. Natural compounds are widely available, economical, and have minimal adverse effects compared to classical chelators. Herbal preparations can bind to the metal, reduce its absorption in the intestines, and facilitate excretion from the body. Curcumin, a bioactive substance in turmeric, is widely used as a dietary supplement. Most studies have shown that curcumin protects against metal-induced lipid peroxidation and mitigates adverse effects on the antioxidant system. This review article provides an analysis to show that curcumin imparts promising metal toxicity-ameliorative effects that are related to its intrinsic antioxidant activity.
1. Introduction
Metals contamination has become an urgent threat due to its persistence, cumulative effect, and specific toxic properties [1]. Most of the metals released into the environment are of anthropogenic origin, but they are also detected in natural sources such as water, soil, and rocks. They are mostly considered as conservative pollutants because they are chemically inert and have no detrimental impact unless disturbed [2]. However, the intensive commercial and industrial development has led to the significant changes in the biogeochemical cycles of metals, which gave rise to the most powerful sources of pollution in the biosphere [3].
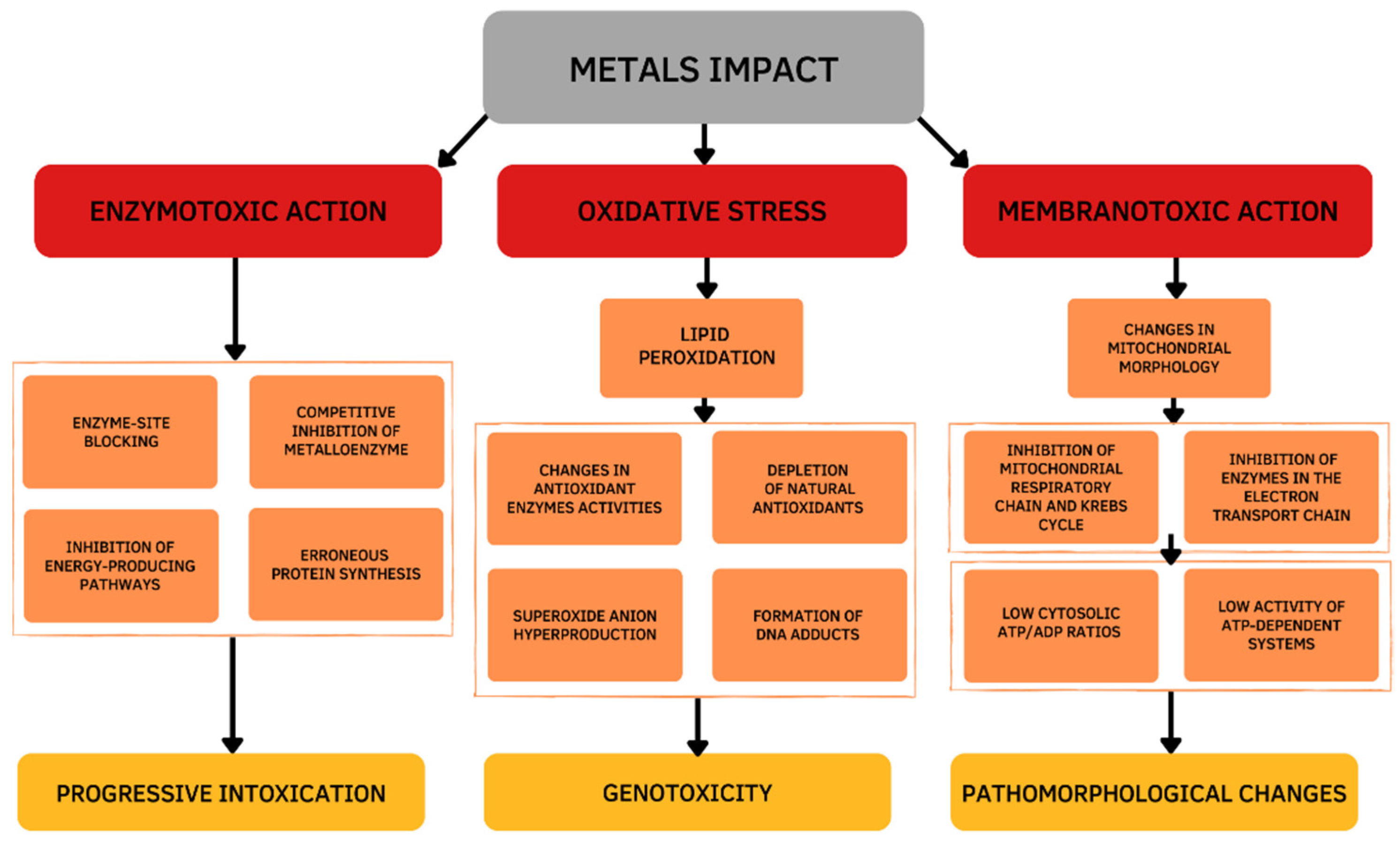
Damage effects and mechanisms of various metals on the human body are similar. Excessive exposure to metals is dangerous and has many several acute and chronic toxic effects on different organs, particularly on the liver. The main changes occur in the oxidative and antioxidant systems under the influence of the metal toxicity are based on three mechanisms (Figure 1). The first mechanism is determined by the ability of metals to bind functional groups of biologically active substances in the body, primarily blocking the sulfhydryl (SH) groups of Cys-containing enzymes. As a result of the reaction of metal ions with SH-groups, weakly dissociating and insoluble compounds are formed such as mercaptides (a metallic salt of methanethiol). The formation of mercaptides is accompanied by damage and dysfunction of proteins, which initiates the development of a toxic process [4]. The second mechanism of the toxic action is based on the displacement of biogenic metals from metal-containing complexes. If the stability of the metal complex is greater than the stability of the biogenic metal complex, the metal is transferred to the more stable complex and metal compounds accumulate in the body, which leads to the disruption of the normal functioning of the body. This mechanism is due to the larger binding strength of toxic vs. biogenic metal ions [5]. The third mechanism is determined by the development of oxidative stress. Active oxidizing-reducing metals (e.g., Fe, Cu, Cr, Co) are directly involved in redox reactions in cells, resulting in the formation of superoxide ions (O2•−). Then, hydrogen peroxide (H2O2) and hydroxyl radical (•OH) are formed during the Fenton reactions and the Haber–Weiss cycle. Exposure to redox inactive metals (e.g., Cd, Zn, Ni, Al) leads to the oxidative stress through the interaction with the antioxidant defense system, disruption of the electron transport chain, or induction of lipid peroxidation [6].
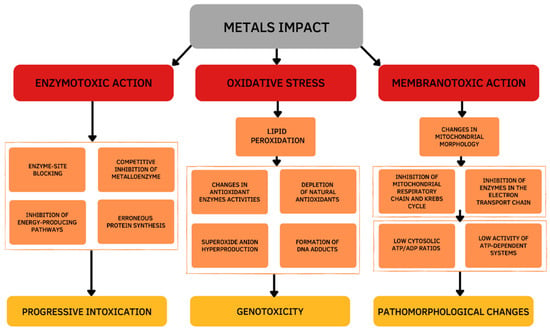
Figure 1.
Cellular mechanisms of the metals toxic action.
Thus, the toxicity of metals is based on enzymatic toxicity due to the inhibitory action of metalloenzymes, membranotropic action, and oxidative stress. The chemical basis of the metal’s toxicity is its ability to bind functional groups of biologically important substances of the body, displace essential metals from metal-containing complexes, and generate reactive oxygen species (ROS). Mechanisms of toxicity are not mutually exclusive and may occur simultaneously.
Due to their bioaccumulation ability, metals pose a severe threat to the body if they exceed acceptable limits (Table 1). Since they are not biodegradable, this only increases affecting health by remaining in the body for a longer time and presenting a long-term risk [3]. To combat organ toxicity caused by metals, scientists are looking for suitable protective agents. The most common chelators are ethylenediaminetetraacetic acid (EDTA), succimer (2,3-dimercaptosuccinic acid, DMSA), unithiol (DMPS), N-acetylcysteine (NAC), alpha-lipoic acid (ALA), etc. [7,8]. In recent years, trends in using nutritional antioxidants have attracted increasing interest. Plant products are well known to protect cells from the aggressive action of free radicals and strengthen the antioxidant defense systems [9]. Certain phytochemicals—including flavonoids and tannins—have metal binding activity for cadmium, nickel, aluminum, and lead in vitro and in vivo. Apart from these, other antioxidants—such as vitamin C, vitamin E, beta-carotene, etc.—may also be useful in combating metal toxicity in animals [10,11]. The beneficial influence of curcumin, a natural bioactive and antioxidant-extracted turmeric spice, on prevention and recovery from metal intoxication, remains to be defined.

Table 1.
Average tolerable intake of metals for a person.
This review analyzes the research concerning the valuable and controversial features of curcumin to determine the effectiveness of its implementation in the therapeutic treatment of metal toxicity and aims to bring researchers’ attention to the most noticeable data obtained in recent years.
2. Curcumin as a Bioactive Compound in Turmeric Plant
Curcumin (CUR) is a hydrophobic low molecular polyphenolic compound [25], a bright yellow color pigment extracted from the rhizome of turmeric (family: Zingiberaceae). It is often considered the most active natural component among other compounds in the plant, comprising about 2–8% of most turmeric formulations [26]. One of the most frequently chosen as an object of study among natural products in different countries [9,27]. Due to its therapeutic and prophylactic properties, it has gained popularity as part of traditional Indian and Chinese medicine [28]. Various studies have presented that the therapeutic potential of CUR does not cause adverse effects in animal models or humans [29]. Clinical and laboratory tests have shown that CUR has a favorable safety profile and can be well tolerated. Treatment even at high doses did not cause negative consequences as well as non-toxic [30,31].
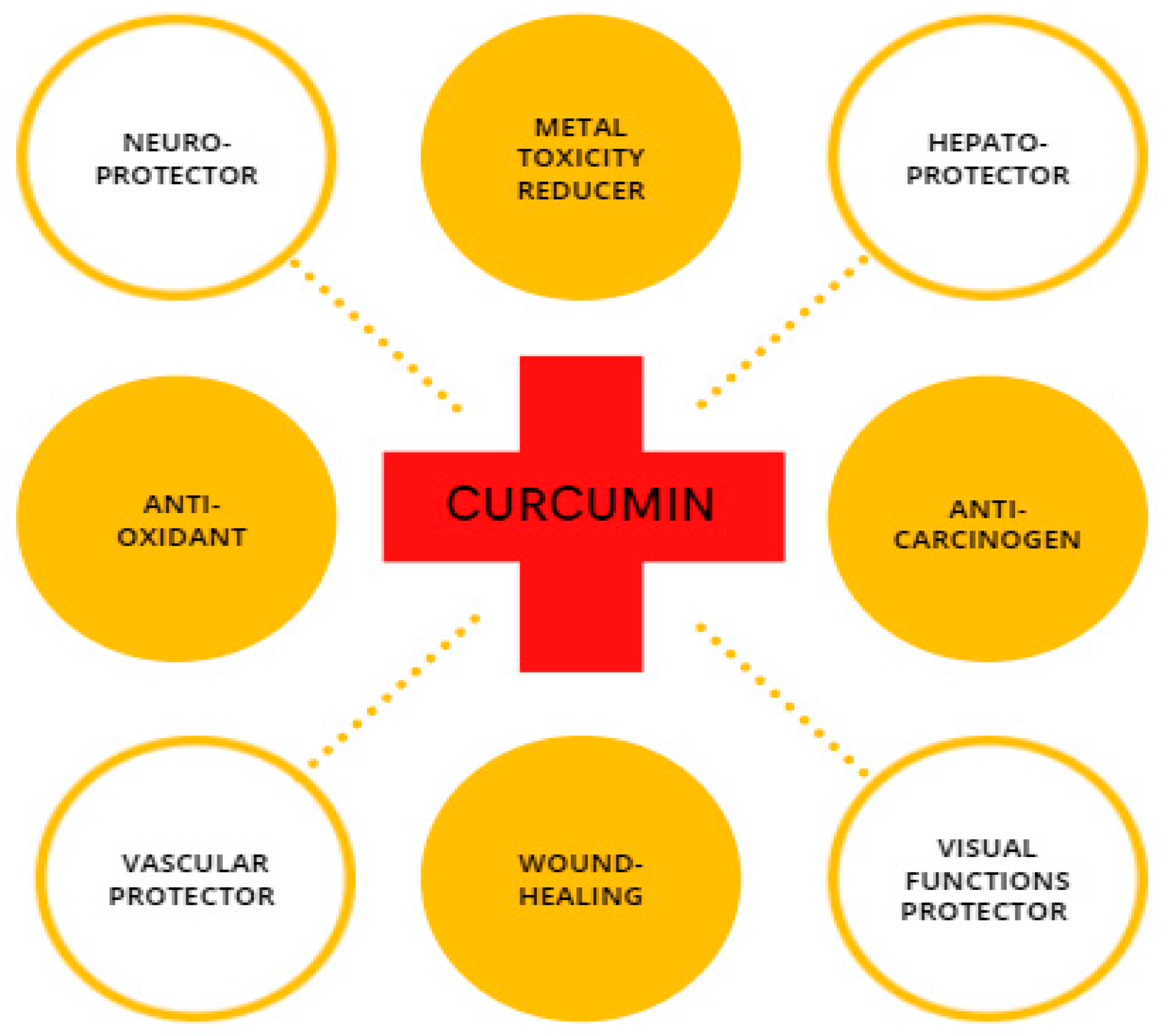
CUR is a compound with high physiological activity, which is intensively studied as a protective element in the field of cancer research [32,33,34]; steatohepatitis [35]; atherosclerosis [36]; neurodegenerative disorders, such as Parkinson’s disease [37,38]; and Alzheimer’s disease [39,40] along with for repairing damaged skin [41,42,43] (Figure 2). Recently, researchers have considered CUR as a neuroprotector in cerebral ischemia-reperfusion. CUR protects the mitochondria of nerve cells from the damaging effects of β-amyloid [44]. Experimental animal studies have shown that turmeric consumption inhibits the process of memory deterioration during brain aging [45]. It also protects the hippocampus from the damaging effects of the long-term use of dexamethasone [46]. Considering the protection effect of CUR against memory impairment [47], it can prevent the development of oxidative stress in the nervous tissue under the influence of sodium nitroprusside [48]. In experimental animals, CUR reduced movement disorders and stiffness caused by haloperidol [49]. Experimental studies have shown that turmeric extract prevents addiction during long-term use of morphine effect in rats [50]. Many studies have demonstrated the antioxidant and anti-apoptotic effects of CUR [51,52]. The involvement of CUR in biological processes mostly arises from its ability to either modulate the intracellular redox state or straight interact with proteins, such as cyclooxygenase 2 (COX-2) [53]. CUR provides forceful antioxidant protection by strongly suppressing the formation of ROS, thereby the organism can counteract oxidative tissue damage [54]. Experiments using animal models for diabetes indicate that the CUR supplement improves microcirculation and decreases blood lipid and glucose levels [55]. In addition to the pharmacological effects of CUR, it has a protective effect against oxidative damage caused by metal toxicity in the reproductive organs [56,57].
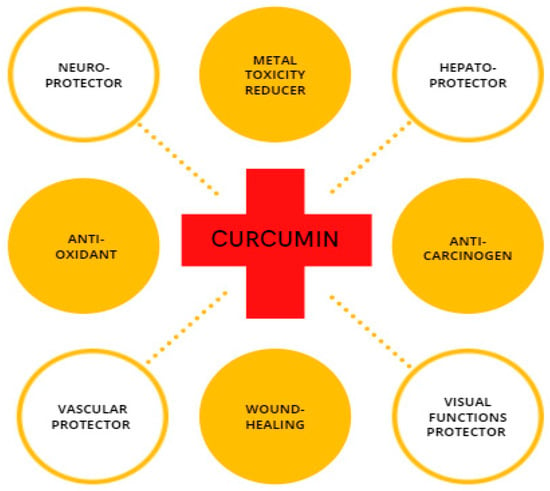
Figure 2.
Schematic illustration of CUR with its biological properties.
3. Chemical Properties of Curcumin
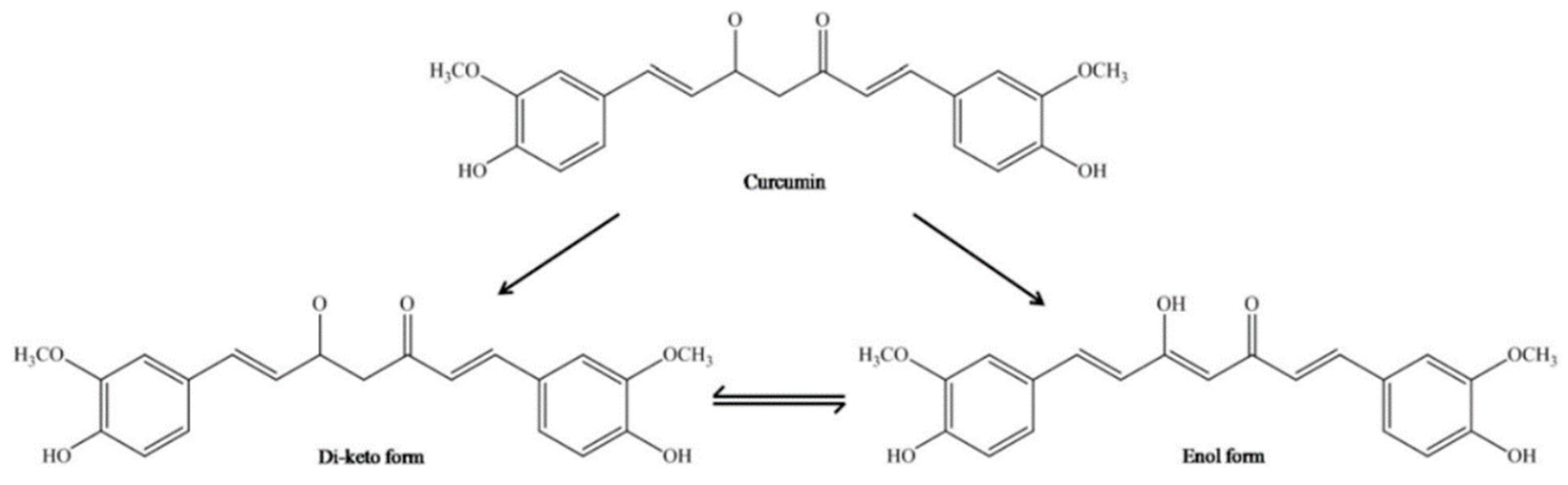
CUR has an original chemical structure that allows it to participate in a range of physical, chemical, and biological processes. Chemically, CUR or diferuloylmethane (1,7-bis-(4-hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione) is a bis-α, β-unsaturated β-diketone, resulting from the conjugation of two ferulic acid molecules connected via a methylene bridge. There are three main functional groups: an aromatic ring, β-diketone, and an alkene bridge. The β-diketone provides flexibility, and the aromatic rings provide hydrophobicity. Conducted research using a molecular modeling technique has shown that CUR may advantageously transform to maximize hydrophobic contact with an interacting protein. The CUR molecule is a diketone that exhibits keto-enol tautomerism. In nonpolar solutions and in a solid phase, CUR exists in the enol form stabilized by hydrogen bonds [58,59]. The interconversion between tautomeric forms of CUR gives supplementary chemical functionality (Figure 3). The most reactive enol form can act both as a donor and an acceptor in the formation of hydrogen bonds. In addition, the keto-enol site determines the advantage of CUR’s chelating activity of divalent ions [60]. It should be noted here that only deprotonated or enolate form of the enol isomer has metal chelating ability through the exhibition of the metal binding properties.
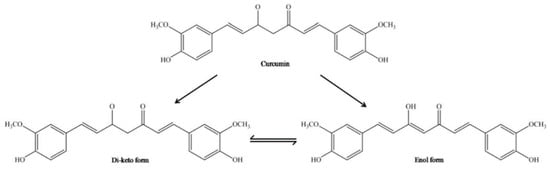
Figure 3.
Chemical structure of enol and keto tautomeric forms of CUR molecule [61].
4. Bioavailability of Curcumin
Despite the powerful pharmacological effects of CUR, it is necessary to point out that its biomedical potential cannot be fully revealed owing to its low bioavailability. The main disadvantages of CUR are the poor oral bioavailability in terms of low intestinal absorption rate, rapid metabolism, and excretion from the body [62] due to the hydrophobic (low solubility in water) and lipophilic (highly soluble in lipid) nature of CUR [63]. It has been reported that about 75% of dietary CUR can be excreted through the feces of animals [64].
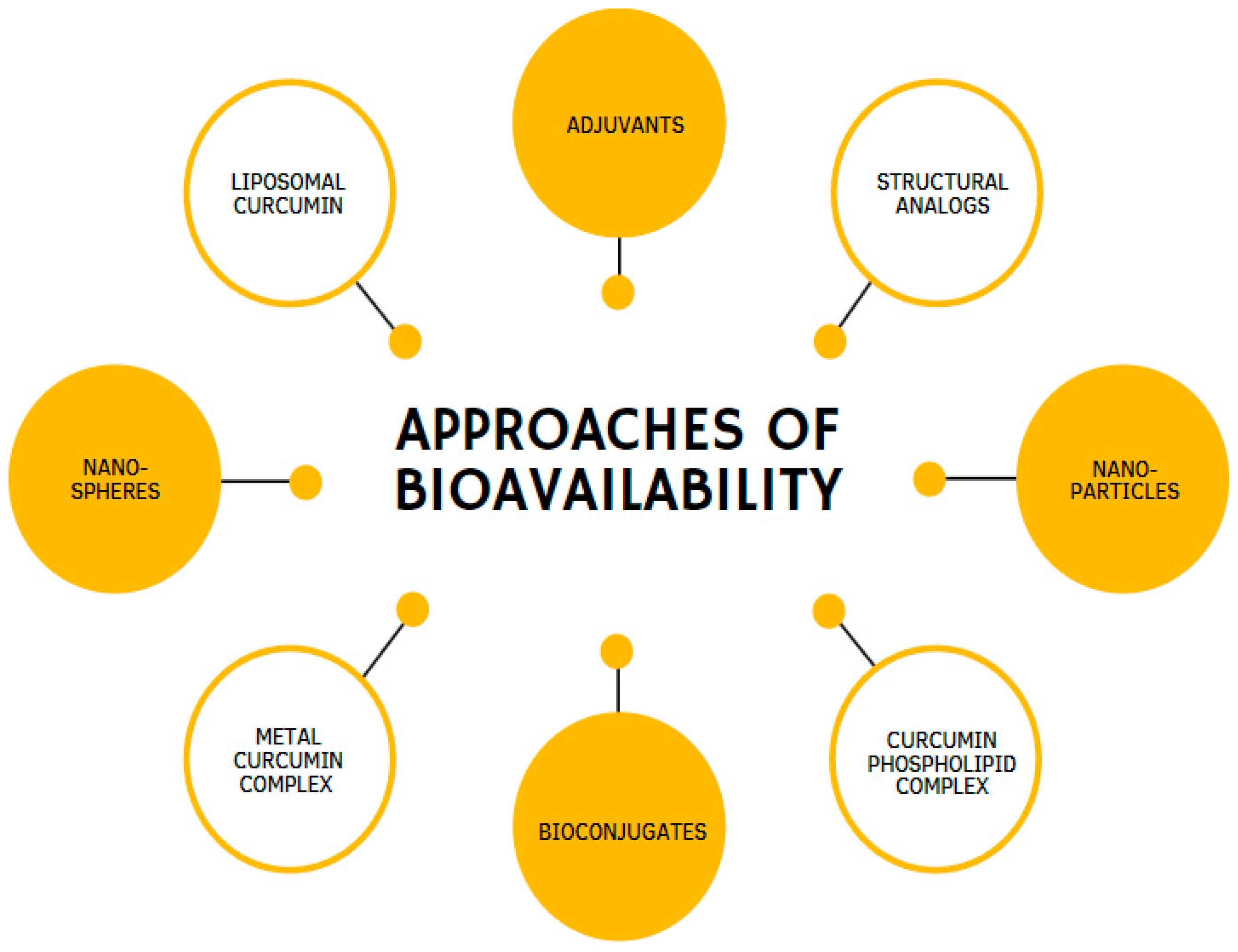
Over the past few years, a considerable number of approaches have been created to improve CUR’s potency and effectiveness. These approaches propose various methods: the use of enhancers, compounds that promote the delivery of biologically active substances, such as the alkaloid piperine [65]; CUR incorporation into liposomes [66]; phospholipids loaded with CUR [67]; the use of analogs that have structural and pharmacological similarities of CUR [68]; and CUR incorporation into nanoparticles [69,70] (Figure 4). Moreover, remarkable results have been achieved by combining CUR in complexes with cyclodextrins [71] or phosphatidylcholine [10,72].
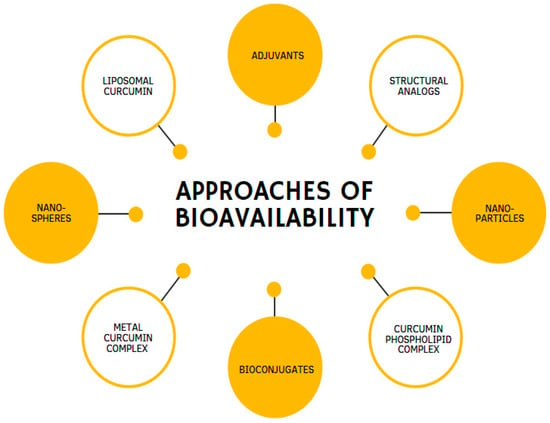
Figure 4.
Different methodologies are used to enhance the bioavailability of curcumin.
Integrating CUR into various nanosystems is the most promising and productive technique to improve the biological activity of poorly water-soluble CUR. With the help of such systems, the highest plasma concentrations, a stable release profile, and significantly increased relative bioavailability have been achieved [73,74,75,76]. The results showed the effectiveness of the obtained nanoparticles compared with pure CUR [77]. It has been reported that PLGA-nanocurcumin is superior to poorly bioavailable native curcumin at 15-fold lower concentration in therapeutic application [78]. Moreover, Shaikh et al. [79] found that encapsulation of nanocurcumin is 9-fold more suitable than native curcumin in terms of oral bioavailability in animals.
A particularly innovative method to address the problem of poor oral absorption is metal complexes with CUR. The enolate form of CUR has ability to bind metal ions enriches the CUR molecule with a chelating mechanism that contributes to the antioxidant activity [80].
5. General Perspective of Curcumin in the Protection of Metal Toxicity
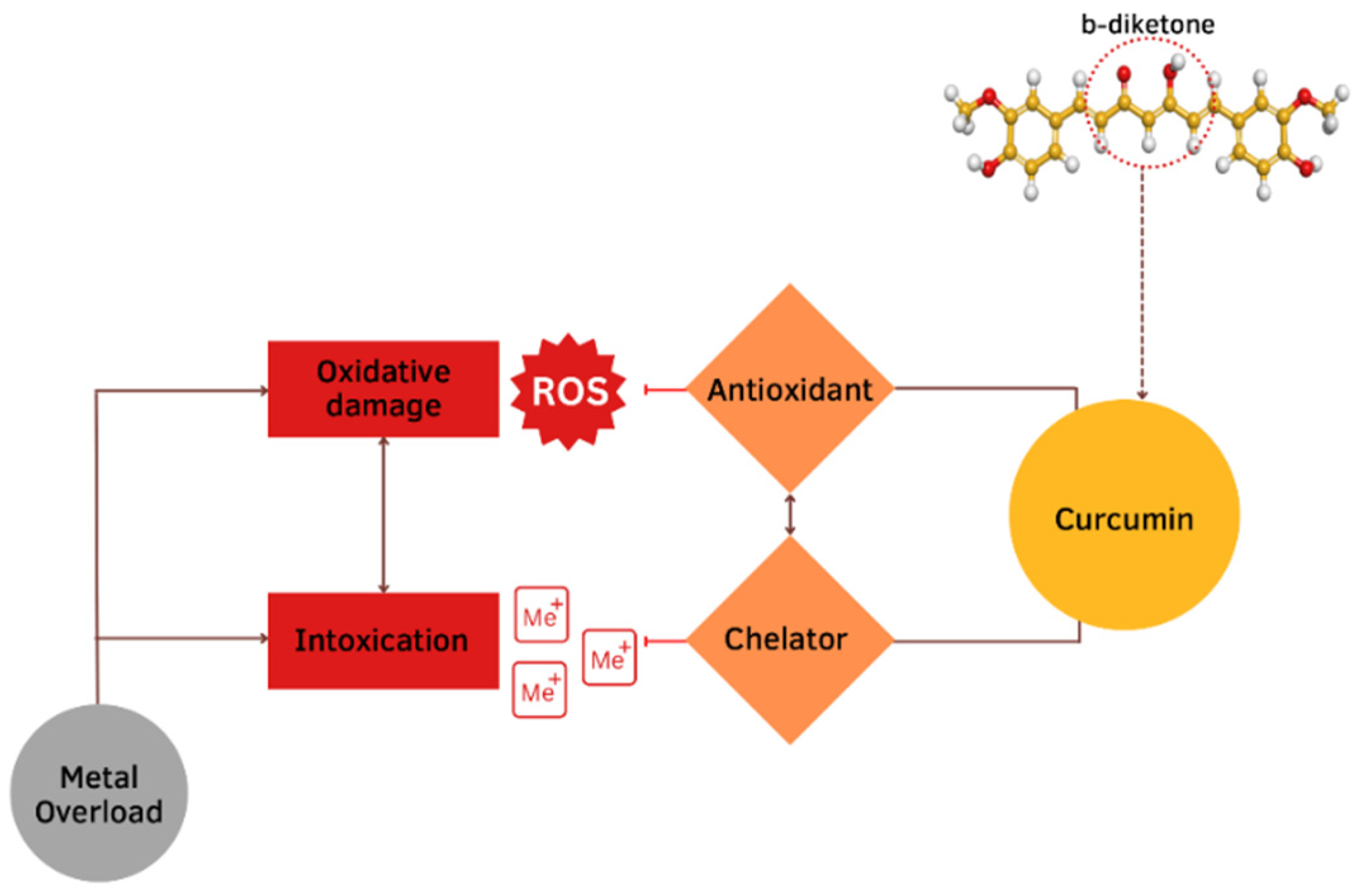
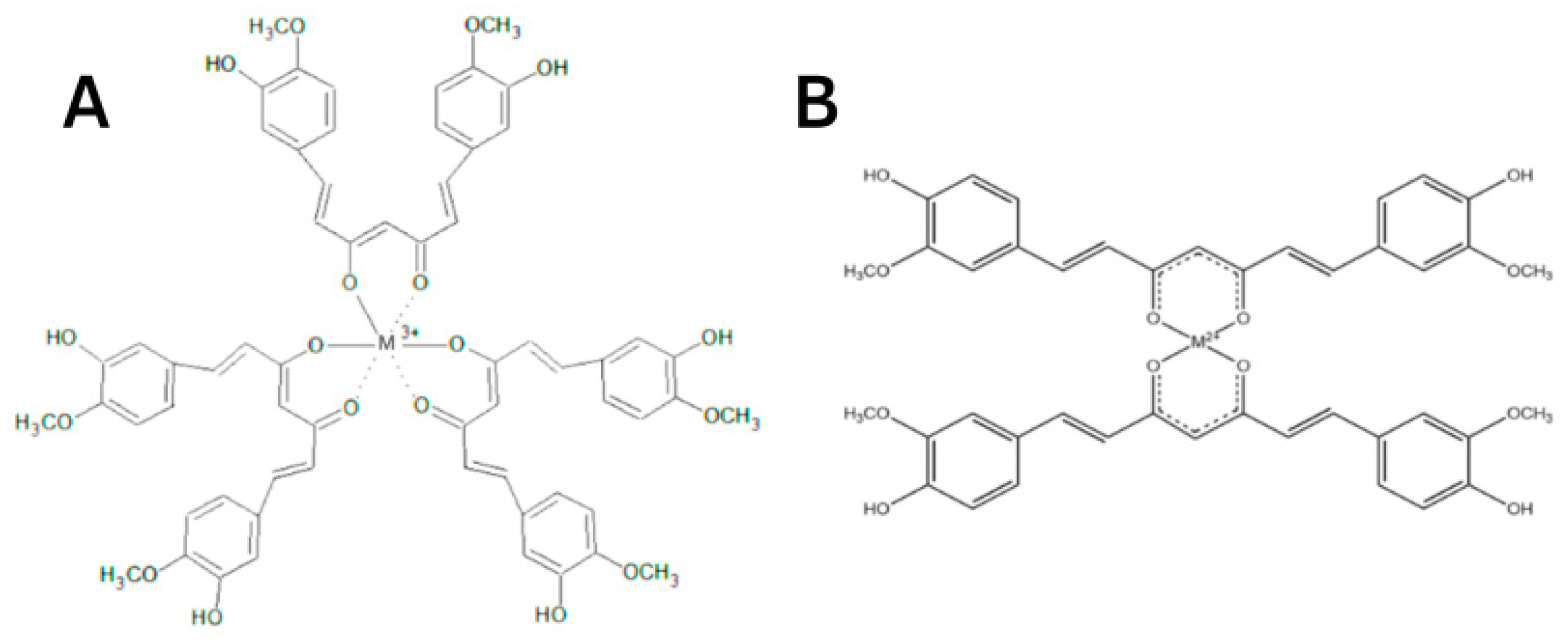
The protective effect of CUR is ascribed to its neutralization of free radicals and chelating properties [81]. CUR can act at the same time as a metal chelator and antioxidant. The CUR chemical structure allows to form chelate complexes with high stability, thereby removing toxic metal ions and inhibiting Aβ polymerization and following the generation of toxic conformations [82]. This is achieved primarily due to the optimal properties of CUR’s natural complexing agent and the presence of a 1,3-diketone moiety that provides the tautomeric form. The more stable enol form acts as a complexing agent [83] with high binding activity and coordinates metal ions around itself [84,85,86], creating complexes [87,88] (Figure 5). The presence of this form makes it an appropriate chelating agent for ingested toxic metals. The keto-enol part is the reaction center [84,85,86,87,88] and provides protection against active free radicals [88]. The enol form of the diketone is better stabilized due to charge delocalization and generates 1:2 and 1:3 type chelates with metal ions [89,90,91,92] (Figure 6). CUR, owing to its clear lipophilicity, easily passes through the blood–brain barrier and cell membranes, which makes it possible to scavenge toxic metals intracellularly [93].

Figure 5.
Mechanisms of the protective effects of CUR against metal toxicity. The protective effect of CUR is ascribed to its neutralization of free radicals and chelating properties. This is achieved primarily due to the presence of a b-diketone.
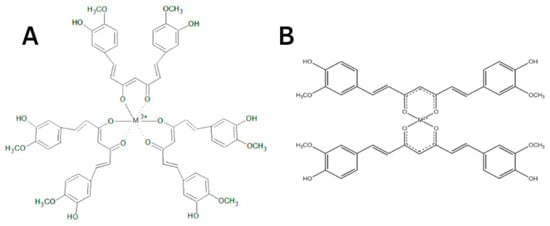
Figure 6.
A simplified illustration of the CUR chelating trivalent metals in a 1:3 configuration (A) and bivalent metals in a 1:2 configuration (B) (adapted from Rainey et al. [94]).
6. Curcumin on Aluminum-Induced Toxicity
Aluminum is ubiquitous in the environment in the form of salts and oxides. Al can enter the organism with drinking water, air, and plant food. One of the Al-specific sources is its ever-increasing use in the food industry (e.g., dishes, packaging material, food additives) and pharmacology [95]. At the molecular level, it can cause protein precipitation and the formation of insoluble protein compounds, which affects reducing the enzyme activity and their systems [96]. Moreover, Al intoxication leads to changes in blood composition, blood disorders (lymphocytosis, eosinopenia, anemia), disorders of calcium-phosphorus metabolism, decreased stability of DNA synthesis, DNA damage, and development of soft tissue fibrosis [97,98]. Furthermore, Al is known as a neurotoxic metal, which may show its negative effects on the nervous system, particularly at higher concentrations; causing movement disorders, seizures, memory loss, psychopathic traits, learning disabilities, depressive tendencies, and encephalopathy [99]. Al is assumed to play a significant role in the occurrence of severe neurodegeneration such as Alzheimer’s disease [95,100]. One of the treatment approaches for Al-induced neurotoxicity is the search for antagonists that halt the absorption of metal [101], replenish nutritional deficiency, and prevent the launching of oxidative processes caused by ROS exposure and disruption or depletion of the antioxidant system during Al intoxication.
It was found that CUR interacts strongly with Al (III), implying that CUR has the potential of removing Al (III) ions and averting interaction between Al (III) and amyloid β-protein (Aβ); thereby, stopping the toxicity of Aβ and detrimental effects of oxidative stress [102] (Table 2). CUR reduced the bonding affinity of Al3+ to DNA [103]. Memory improvement, reversing oxidative damage, and weakening of acetylcholinesterase activity have been observed with CUR long-term administration. Moreover, CUR prevented damage to neurons under oxidative stress conditions, induction of Al3+-caused cognitive impairment, and markedly lowered the Al concentration in Al-treated rats [104]. CUR administration led to a decrease in the expression of NF-κB and TNF-α which are known as the markers of inflammatory reactions [100]. Furthermore, curcumin treatment led to the reduction in ROS and lipid peroxidation in cerebellum as well as improvement of reduced glutathione and glutathione-S-transferase in cerebrum which showed the neuroprotective behavior of curcumin on Al-induced neurodegenerative and behavioral disorders in rats [105]. Recently, it is reported that nano-curcumin has better biological and antioxidant activity than native curcumin on Al-induced toxicity in rats [106] which might be due to the favorable interaction of nanoparticle of curcumin with Al3+ in reducing Al toxicity.

Table 2.
Effects of curcumin on aluminum toxicity based on doses, experimental methods, and findings.
7. Curcumin on Arsenic-Induced Toxicity
Arsenic is a commonly occurring metal in the biosphere, observed in rocks, soils, and water resources. A potential threat to human health occurs when As enters the organism through the contaminated water supply or food. Since As engages in most biological catalytic reactions, its indirect or direct effect spreads to all organs [108]. The liver is the primary target for As poisoning [109]. The results of As toxicity are skin damage, cardiovascular and respiratory diseases, cancer, gangrene, brain dysfunction, etc. Meanwhile, there is currently no remedy endorsed for widespread use in As poisoning [110].
In turn, CUR has been shown to be a satisfactory antioxidant and protector for DNA, regressing As-induced damage in the nucleus (Table 3). Supplementation with CUR has been shown to reduce the total toxic load of As in the liver and assist to increase the As excretion through the urinary tract [111,112]. Moreover, CUR decreased transaminase and phosphatase activity along with the plasma and brain acetylcholinesterase, as well as total protein and albumin levels under As-induced liver injury [113].
Therefore, CUR may have some protective role against biochemical alterations and associated DNA damage caused by As. According to in vitro studies results, CUR modulates autophagy/apoptosis in cells and has a cytoprotective effect against As-induced toxicity, preventing decreased antioxidant levels that cause cell membrane disruption [114].

Table 3.
Effects of curcumin on arsenic toxicity based on doses, experimental methods, and findings.
Table 3.
Effects of curcumin on arsenic toxicity based on doses, experimental methods, and findings.
| Dose/Concentration | Name of Animal Model/Cell Lines | Route of Exposure | Duration of Exposure/Treatment | Results | Source |
|---|---|---|---|---|---|
| Clinical Trial | |||||
| CUR + piperine (20:1) 2 × 500 mg/day | Chronically arsenic-exposed males or females | Orally | 6 months | ↓DNA damage, ↓ROS generation, ↓CAT, SOD enzymes, | [111] |
| CUR + piperine (100:1) 500 mg twice/day | Chronically arsenic-exposed males or females | Orally | 6 months | ↑expression of protein, mRNA of DNA-PK, DNA ligase IV, XRCC4, ↑BER and NHEJ repair pathways, ↓DNA-damaging effect in lymphocytes | [112] |
| In Vivo | |||||
| 5 mg/kg b.w. NaAsO2 + 15 mg/kg b.w. CUR | Male Wistar rats | NaAsO2-orally/CUR-orally | 30 days (co-administration) | ↓transaminases, phosphatases, glucose, urea, creatinine, bilirubin, TL, cholesterol, TG, plasma and brain ache, the levels of TP and Alb | [113] |
| 5 or 300 ppm NaAsO2 + 0.5 mg/kg b.w. nano-CUR | Male Swiss albino mice | NaAsO2-drinking water/CUR-orally | NaAsO2-7 days/CUR-14 days(post-treatment) | ↓histopathological alterations, ↓accumulation of acidic vesicles, ↓apoptotic cells in the thymus and spleen, ↓autophagy, ↓redox imbalance in immune cells | [115] |
| In Vitro | |||||
| 10 μM NaAsO2 + 0, 1, 2.5, 5, 10, 25, 50 or 100 μM CUR | PC12 cells | Cell line | 24 h | ↑membrane integrity, ↓DNA damage, apoptosis rate, ↑protein expressions, ↑cell viability, ↑cytoprotective effect, ↓oxidative stress | [114] |
Abbreviations: ↑ = increase; ↓ = decrease; CUR = curcumin; As = arsenic; IP = intraperitoneal injection; TL = total lipids; TG = triglycerides; TP = total protein; Alb = albumin; ROS = reactive oxygen species; CAT = catalase; SOD = superoxide dismutase; DNA-PK = DNA-dependent protein kinase; XRCC = X ray repair cross complement; BER = base excision repair; NHEJ = nonhomologous end joining; NaAsO2 = sodium arsenite; PC = pheochromocytoma.
8. Curcumin on Cadmium-Induced Toxicity
Cadmium is a carcinogenic metal whose natural content gradually accumulates in the environment, due to anthropogenic emissions. The main route of Cd entry is skin absorption, ingestion, or inhalation. Cd with its wide range of toxic effects has become one of the most critical contaminants in the aquatic toxicology field owing to harmful human activity [116]. The problem that follows Cd accumulation is its competitive behavior with essential metals (Ca, Zn, Fe, Mg). Cd uses the same transport systems as essential metals, which blocks their entry into the cell, inducing toxicity [117,118].
Most studies have shown CUR’s positive healing properties against Cd toxicity (Table 4). Electrochemical studies in mice have predicted a compatible metal-ligand form of Cd and CUR, which could remove metal ions from the body [119] and mitigate the adverse effects of Cd [120]. It is reported that the oral administration of CUR decreased the accumulation of toxic elements in the brain and liver in mice [121]. Co-treatment with CUR significantly reduced the levels of pro-inflammatory cytokines such as tumor necrosis factor-alpha and interleukin-6, as well as biomarkers’ affection of oxidative damage [122]. CUR can inhibit the expression of nuclear factor kappa B, which is responsible for regulating the transcription of genes that control inflammation, immune cell development, and cell death. CUR prevented the release of interleukin-6 and interleukin-8 in response to toxic Cd ions [123]. The studies confirmed the CUR protective effects against Cd-induced nephrotoxicity. It also can be used to prevent respiratory tract injury due to Cd inhalation. Overall, CUR can be added to the diet and can be fairly proposed to be used as a protective factor for Cd-induced nephrotoxic, hepatotoxic, and hematological changes.

Table 4.
Effects of curcumin on cadmium toxicity based on doses, experimental methods, and findings.
9. Curcumin on Copper-Induced Toxicity
Copper’s redox nature makes it essential for many biological processes, but at the same time renders it toxic effects due to the generation of the most dangerous ROS, hydroxyl radical (•OH) [118]. An imbalance of Cu ions in the central nervous system engages in many neurodegenerative diseases pathogenesis, the most common is amyotrophic lateral sclerosis, Alzheimer’s, and Parkinson’s diseases [132,133,134,135]. For Cu toxicity, the same chelating treatment is used as for other metal poisonings [136].
Through quantum chemical computations, it was discovered that due to the proton loss of the CUR enol form, the β-diketone part is the main site of chelation in the CUR-Cu(II) complex [137] (Table 5). In studies conducted with CUR administration, CUR had ameliorating effects on the CuO nanoparticles toxicity in terms of the antioxidant, immunomodulatory, anti-apoptotic, and anti-inflammatory effects on the rats’ kidneys [138]. Moreover, considering the role of oxidative damage in mediating Cu toxicity, CUR suppressed neurotoxicity through its anti-radical and antioxidant properties [82]. CUR’s neuroprotective properties provide the basis for studying its effectiveness in several neurodegenerative diseases involving Cu, such as Alzheimer’s disease. Through the ability of CUR to penetrate the blood–brain barrier, CUR can block amyloid beta-peptide, a key disease factor that accumulates in the brain, and reduce its levels as well as remove the metal ions in the brain [139]. Thus, CUR as a restorative remedy for oxidative stress caused by Cu ions might be useful for therapeutic treatment, including neurodegenerative diseases.

Table 5.
Effects of curcumin on copper toxicity based on doses, experimental methods, and findings.
10. Curcumin on Iron-Induced Toxicity
Iron is an indispensable element in basic life processes such as DNA synthesis, respiration, and a regular participant in biochemical reactions [143]. However, Fe overload in parenchymal organs is associated with degenerative changes in the cellular parenchyma that causes irreversible dysfunction of the most vulnerable vital organs, such as the liver, pancreas, and heart. Chelation therapy is the recommended approach as an antidote for Fe intoxication [144].
Some chelators are responsible both for Fe excretion and may suppress the participation of free labile Fe in oxidation-reduction reactions [94]. Age-related hypokinesia causes muscle atrophy which is related to the Fe accumulation and potentiates the oxidative stress through the Fenton reaction. Thus, the key role of chelating agent is to prevent the Fe participation in the Fenton reaction or the reaction of peroxides decomposition with the formation of hydroxyl radicals in the ROS generation [145].
CUR, an iron-binding antioxidant, prevents the destruction of cell membranes and stimulates the regeneration of hepatocytes, thereby having a protective effect on liver function in Fe overdose (Table 6). According to a study conducted on T51B cells treated with ferric ammonium citrate, CUR bounded but not blocked Fe absorption and did not interfere with its bioavailability [94]. CUR has shown a detoxifying effect by preventing the formation of ROS and abolishing the signaling cascades of Fe-induced stress response. Furthermore, in a study on MIN6 cells, CUR functioned as a moderator of Fe-dependent necrotic cell death, known as ferroptosis, and prevented Fe-induced cell damage during ROS production [146].

Table 6.
Effects of curcumin on iron toxicity based on doses, experimental methods, and findings.
11. Curcumin on Lead-Induced Toxicity
Lead is a toxic metal whose compounds are insoluble in water but highly soluble in stomach acid. The main mechanism of its adverse action is associated with gastrointestinal tract disorders [149]. The Pb study’s importance is explained by its threatening toxicity through extreme spreading in the environment and its multilateral use in industry and everyday life. The toxic mechanism of Pb is associated with the blocking of thiol enzymes, interaction with biopolymers’ carboxyl and phosphate groups, nucleotides, and esterase enzyme inactivation [150,151].
Morphological and functional changes have been revealed that negatively affect the hematopoiesis and the nervous system in the fish body with oversaturated of Pb ions [152]. Fish are more sensitive to toxicity, the key role in the immune response in their body belongs to immunocompetent cells, primarily lymphocytes [153]. It has been reported that Pb led to a shift in white blood cell count and apoptosis has also been recorded [152]. A study conducted over 8 weeks with Pb added to aquarium water has shown an increase in fish mortality and stunting along with a decrease in indicators such as final wet weight and specific growth rate. However, after co-treatment with CUR, DNA damage was markedly reduced, the effects of Pb toxicity in the kidneys were weakened, and the inflammatory process was attenuated [154] (Table 7).
Pb increases lipid peroxidation and reduces the functional abilities of the brain cell, but CUR significantly reduced the Pb toxic effect on nerve cells in rat studies [155]. Furthermore, in rat experiments with Pb accumulation in the liver and kidneys, it was shown that CUR is an effective inducer of the post-accumulation effects of oxidative toxicity. The results have shown a decrease in the Pb amount in the affected organs and the relief of degenerative changes caused by Pb [156].

Table 7.
Effects of curcumin on lead toxicity based on doses, experimental methods, and findings.
Table 7.
Effects of curcumin on lead toxicity based on doses, experimental methods, and findings.
| Dose/Concentration | Name of Animal Model/Cell Lines | Route of Exposure | Duration of Exposure/Treatment | Results | Source |
|---|---|---|---|---|---|
| In Vivo | |||||
| 50 mg/kg Pb(C2H3O2)2 + 100 mg/kg CUR 50 mg/kg Pb(C2H3O2)2 + 200 mg/kg CUR | Male Sprague–Dawley rats | Pb(C2H3O2)2, CUR- orogastric tube | Pb(C2H3O2)2-4 weeks/CUR-4 weeks (post-treatment) | ↓Pb concentration, ↓oxidative stress, ↓cerebellar damage in cerebellum, ↑motor coordination | [155] |
| 50 mg/kg Pb(C2H3O2)2 + 200 mg/kg CUR | Male Wistar rats | Pb(C2H3O2)2 -IP/CUR-orally | 7 days (co-administration) | ↓apoptosis, ↓oxidative stress, ↓inflammation, ↓liver injury, ↑AKT/GSK-3β signaling pathway | [157] |
| 20 mg/kg OD Pb(C2H3O2)2 + 30 mg/kg BD CUR | Male and female Wistar rats | Pb(C2H3O2)2, CUR- IP | 5 days (co-administration) | ↓damage neurons, ↓protein oxidation, lipid peroxidation, ↑GSH | [158] |
| 1 mg/L Pb + 15 g/kg CUR | Cyprinus carpio | Pb-aquarium water/CUR-orally | 8 weeks (co-administration) | ↓mRNA, expression of NF-kB p65, ↓AST, ALT, LDH, ↑protease activity, ↑SOD, GPx, GSH, G-Rd, GST, Nrf2, ↑lysozyme activity, C3, IgM, ↑intestinal microbial abundance, ↑growth parameters, ↑RBC, Hct, Hb, serum protein, albumin, ↑enzymatic activities, ↑IL-10, ↓MDA | [154] |
| In Vitro | |||||
| 10 μM Pb(C2H3O2)2 + 50, 100, or 150 μM CUR | Rat pups’ hippocampi | 3 h | ↓free radicals, ↑cell viability | [158] | |
Abbreviations: ↑ = increase; ↓ = decrease; CUR = curcumin; Pb = lead; IP = intraperitoneal injection; AKT = protein kinase B; GSK-3β = glycogen synthase kinase-3β; OD = once daily; BD = twice daily; SOD = superoxide dismutase; GPx = glutathione peroxidase; GSH = glutathione; IL-10 = interleukin-10; MDA = malondialdehyde; GST = glutathione S-transferase; G-Rd = glutathione reductase; LDH = lactate dehydrogenase; RBC = red blood cells; Hct = hematocrit; Hb = hemoglobin; NF-κB p65 = nuclear factor kappa B p65 subunit; AST = aspartate transaminase; ALT = alanine transaminase; Nrf2 = nuclear factor erythroid 2–related factor 2; C3 = complement component 3; Pb(C2H3O2)2 = lead acetate.
12. Curcumin on Zinc-Induced Toxicity
Zinc is an essential trace element for all life forms. Zn is part of more than 40 enzymes and engages in carbohydrate metabolism and the metabolism of vitamin A. This element is necessary for bone formation. In addition, it exhibits antiviral and antitoxic effects, and Zn toxicity has rarely been reported [159].
Zinc oxide nanoparticles (ZnONP) are of major interest, as they are widely distributed in various fields. ZnONP are used in pharmaceuticals and cosmetology as agents exhibiting antibacterial activity. However, along with beneficial properties, the harmful effect of ZnONP on various body systems has been recorded [160]. Therefore, the toxic effects of Zn and ZnONP require a protective agent. CUR has shown promising results in preventing Zn toxicity in vivo studies (Table 8). Analyzing the content of ZnONP in the brain after administration on rats, it has been found that nanoparticles or its metabolic products cross the blood–brain barrier and cause histological damage to the cerebellum, which in turn was successfully prevented after the addition of CUR [52].
CUR is also proven to be a successful Zn ions chelator and can function as a radical scavenger that accumulates Zn ions [161]. Therefore, apoptosis and necrosis of hepatocytes can be prevented by suppressing the oxidative damage of cells through the CUR treatment [162]. In addition, CUR is a vindicated protector of nephrotoxicity which can reduce kidney damage, and suppresses necrosis factors, and inflammation processes [163].

Table 8.
Effects of curcumin on zinc toxicity based on doses, experimental methods, and findings.
Table 8.
Effects of curcumin on zinc toxicity based on doses, experimental methods, and findings.
| Dose/Concentration | Name of Animal Model/Cell Lines | Route of Exposure | Duration of Exposure/Treatment | Results | Source |
|---|---|---|---|---|---|
| In Vivo | |||||
| 5.6 mg/kg b.w. ZnONP + 200 mg/kg CUR | Male albino rats | ZnONP-IP/CUR-oral gavage | ZnONP-28 days (started on day 7, three times per week)/CUR-28 days (pre-treatment) | ↑cerebellum structure, ↓oxidative stress markers, ↑inflammatory response, ↓COX-2, P53 | [52] |
| 50 mg/kg ZnONP + 200 mg/kg CUR | Male Wistar rats | ZnONP, CUR-orally | ZnONP-14 days (started on day 7)/CUR-14 days (pre-treatment) | ↑body, kidney weight, ↓MDA in the renal tissue, ↑SOD, GPx, ↓histological changes, ↓apoptotic index | [162] |
| 50 mg/kg nano-ZnO + 200 mg/kg CUR | Male Wistar rats | ZnO-gavage method/ CUR- oral gavage | ZnO-21 days (started on day 7)/CUR-21 days (pre-treatment)2 | ↓lipid peroxidation, ↑SOD, GPx ↓ALT, AST, ALP, ↓histology changes, ↓apoptotic index of hepatocytes | [163] |
Abbreviations: ↑ = increase; ↓ = decrease; CUR = curcumin; Zn = zinc; ZnO = zinc oxide; ZnONP = zinc oxide nanoparticles; IP = intraperitoneal injection; P53 = tumor protein P53; COX-2 = prostaglandin-endoperoxide synthase 2; MDA = malondialdehyde; SOD = superoxide dismutase; GPx = glutathione peroxidase; ALT = alanine aminotransferase; AST = aspartate aminotransferase; ALP = alkaline phosphatase.
13. Curcumin on Mercury-Induced Toxicity
Mercury in its natural state at room temperature is a liquid metal. Humans typically contact with Hg from three main sources: dental amalgam fillings, some vaccines, and fish caught in rivers and seas contaminated with Hg waste [10,164]. In addition, Hg-containing devices—such as electric lamps, thermometers, barometers, etc.—are potential sources of Hg in the home [165,166].
Chronic Hg poisoning can lead to ataxia, a lack of coordination that causes a cerebellar gait. It can also cause hand tremors, excessive salivation, and a metallic taste in the mouth. Another representative symptom could be a blue line that appears on the alveolar margin of the gums, as in Pb and bismuth poisoning. The classic symptoms of Hg vapor poisoning include intention tremors, erethism (memory loss, lack of self-control, irritability, excitability, loss of self-confidence, drowsiness, and depression), and gingivitis [167].
CUR has been examined in several Hg-induced toxicity studies (Table 9). CUR treatment provided protective effects on Hg-induced oxidative stress parameters, and it was found to be effective in reversing Hg-induced serum biochemical changes [168]. However, it also reported that CUR supplementation in excess of metal ions from Hg, Al, or As can inhibit utilization of some essential trace minerals such as Se or Zn which may ultimately affect the antioxidant defense system. Similarly, it showed that CUR as a strong antioxidant can limit the levels of other antioxidants in the liver to maintain the optimum antioxidant levels in organisms [168].

Table 9.
Effects of curcumin on mercury toxicity based on doses, experimental methods, and findings.
Pi3K-Akt has been proposed as a key signaling pathway in Hg-induced splenic injury. In vivo experiment in mice has shown that CUR attenuated spleen apoptosis by reversing the same pathway as well as mitigated HgCl2-induced toxicity in the immune system through the Nrf2-ARE activation pathway [169]. Here, the protective role of Nrf2, once released from Keap1, is mainly derived from the activation at the level of the antioxidant response elements (ARE), thereby giving rise to the production of antioxidant enzymes. In another study, it has examined the effects of Hg on neurobehavioral and neurochemical disorders in mouse offspring where CUR increased the levels of cerebral monoamines (dopamine, norepinephrine, and serotonin) in Hg-treated pups. Overall, behavioral disorders, such as anxiety behavior, were suppressed by CUR in treating pups. Conclusively, CUR has shown a desirable mechanism in preventing Hg toxicity and could be recommended for use to avoid toxicants such as Hg [170].
14. Curcumin on Selenium-Induced Toxicity
Selenium is an indispensable, vital trace element for the organisms [151]. It is represented by the active center of many Se-containing proteins involved in the antioxidant defense mechanisms, thyroid hormone metabolism, and performing the immune function [171].
Se supports the Se-containing enzymes function and selenoproteins contained in plasma [10]. Se affects the metabolism of leukotriene, thromboxane, and prostacyclin. Se deficiency disrupts the functioning of the brain [172] and suppresses immune defense reactions, especially nonspecific, cellular, and humoral immunity [173].
In excess concentration, Se can exhibit toxic properties [174]. Symptoms of toxic effects of excess Se include a metallic taste, headaches, nausea, hair loss, and damaged nails. Sensory loss, convulsions, pneumonia, pulmonary edema, and circulatory collapse are also symptoms. Cases of Se toxic effects have been registered not only during exposure to Se associated with industrial production but also during its self-administration [174].
Studies of the role of CUR in combating Se-induced toxicity in the liver and kidneys of Wistar rats have yielded promising results (Table 10). Kidney tissue of Se alone administered rats was critically damaged: cystic degeneration, cytoplasmic vacuolization, cellular proliferation with fibrosis, and vesicle formation. Conversely, CUR pretreatment prevented all degenerative changes caused by Se [175]. There have been several studies of Se-induced cataractogenesis with CUR co-treatment. The findings showed that CUR suppressed Se-induced accumulation of reactive oxygen species and cataract formation in the isolated lens from experimental rat pups, possibly due to inhibition of the enzymatic and non-enzymatic antioxidant defense system and prevention of uncontrolled formation of superoxide radicals, as well as due to inhibition of iNOS activity [176,177].

Table 10.
Effects of curcumin on selenium toxicity based on doses, experimental methods, and findings.
The results implied that CUR might take place as an agent against hepatic and renal toxicities, probably due to its ability to inhibit iNOS levels and proved an antioxidant potential of CUR in preventing Se-exposed toxicity.
15. Curcumin on Chromium-Induced Toxicity
Chromium compounds are highly toxic to humans, primarily hexavalent chromium (Cr+6). The main manifestations of excess Cr are inflammatory diseases with a tendency to ulceration of the mucous membranes, an allergic effect, dermatitis and eczema, bronchial asthma, and risk of cancer [178,179].
The studies reported the effects of CUR against Cr toxicity in the male reproductive system has detected success in the prevention of the Cr destructive action in studied parameters (body weight, the weight of the testis, accessory sex organ weight) (Table 11). CUR through its antioxidant properties protected male germ cells, and testicular histology from oxidative damage by Cr-induced free radicals [180]. The study on Cr-induced renal dysfunction has reported that CUR treatment attenuated tissue damage, decreased free radicals, and reduced antioxidant factors in both kidney tissue and mitochondria. Moreover, the authors highlighted that the protection of mitochondrial function played a crucial role in the defense mechanism of CUR pretreatment against Cr toxicity in the kidneys [181]. Thus, the present studies suggest that CUR may have a protective role against Cr toxicity.

Table 11.
Effects of curcumin on chromium toxicity based on doses, experimental methods, and findings.
16. Conclusions and Future Outlook
The presented literature data provide a wide range of biological activity that determines the various therapeutic properties of curcumin, the polyphenolic compound of the Curcuma longa plant, in relation to metal toxicity. The therapeutic and prophylactic efficacy of curcumin has been found in many inflammatory, oncological, autoimmune, and neurodegenerative diseases. Drugs for the treatment of these diseases are often expensive and have certain side effects. In this regard, the importance of using multi-purpose, harmless, inexpensive, and readily available dietary supplements or nutraceuticals for the prevention and treatment of various human diseases is increasing [60].
Various modern forms (capsules, tablets, lozenges) of dietary supplements containing turmeric, and its bioactive compounds are commercially available. Turmeric powder with glycerin has been used in the treatment of a burning sensation in oral submucous fibrosis [182]. An ointment containing 10% alcohol extract from turmeric roots is effective in the treatment of ulcers caused by aspirin [183]. Turmeric is a safe and secure alternative remedy in the treatment of digestive disorders [184]. It is recommended as a means of stimulating gastric secretion and normalizing the intestines and lungs functioning [185,186,187,188]. Turmeric oil has anti-ulcerogenic potential and prevents the formation of stomach ulcers [189,190,191]. The anti-Helicobacter pylori therapeutic effect was increased with the curcumin supplement therapy [192,193]. Satisfactory results have been obtained using curcumin as a supplement in the treatment of ulcerative colitis, and Crohn’s disease [194]. A mixture of turmeric and ginger oils has a strong hepatoprotective effect in acute alcohol intoxication [195]. Curcumin prevents the development of non-alcoholic fatty liver induced by a high-cholesterol diet [196]. Turmeric is effective in gallbladder diseases [197]. Dietary supplements with curcumin have high bioavailability at recommended dose, good tolerance, and minimal signs of toxicity, which can only appear in individuals with intolerance [54,58].
The low toxicity, even at doses up to 8 g per day [60], availability, and high activity of turmeric and curcumin make it possible to use them for a long time both with food in the form of spice and as a purified biologically active component, which is promising for the prevention of the above diseases and use as an adjuvant in their complex therapy [63,198]. However, this polyphenol has not been approved for clinical use yet.
Numerous studies confirm the effectiveness of curcumin in various pathological processes, however, its pure use is limited by its low bioavailability [199]. Therefore, one of the important technological tasks is the development of effective dosage of curcumin to increase the bioavailability of this compound.
To improve the bioavailability of curcumin, scientists propose various methods. These include the use of enhancers, compounds that promote the delivery of biologically active substances, such as the alkaloid piperine, which prevents glucuronidation; incorporation of curcumin into liposomes and nanoparticles; use of the curcumin-phospholipid complex; complexes with cyclodextrins; etc. By increasing oral bioavailability, curcumin can be proposed as a new orally active metal chelator, such as deferasirox, for the treatment of metal toxicity.
Curcumin is a suitable candidate for metal toxicity treatment given its wide range of biological effects [200]. Detoxification processes triggered by the protective effects of curcumin have been linked to its ability to scavenge free radicals, function as a natural chelating agent, and/or induce antioxidant enzymes by initiating the Keap1/Nrf2/ARE pathway. Even though the molecular mechanisms and primary targets of this compound are still unclear [201]. These circumstances put a barrier to the recognition of curcumin as a potential pharmacological agent, but do not prevent its use as a biologically active agent. Thus, curcumin with an improved form of bioavailability may be used as an adjuvant in the treatment of metal poisoning. The chemical specificity of curcumin may be a useful factor in discovering unknown therapeutic targets in metal toxicology, but more research is needed in this area.
Author Contributions
E.S., M.M. and T.M. developed the idea for this manuscript. E.S., M.M., S.C., A.S. and A.K. developed the search strategy and performed the review and data extraction. E.S. and M.M. wrote the draft manuscript. K.D. review and edit the manuscript. All authors edited and agreed on the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Basic Science Research Program (grant no. 2022R1A2B5B02001711) to Taesun Min (T.M.) through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT and Future Planning. This work was also supported by the Basic Science Research Program (2019R1A6A1A11052070) to T.M. through the National Research Foundation of Korea (NRF) funded by the Ministry of Education. This work was also supported by the Basic Science Research Program (grant no. 2021R1I1A1A01052235) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education to Mohammad Moniruzzaman (M.M.).
Data Availability Statement
All data is reported in this article.
Acknowledgments
E.S. would like to thank the Korean Government Scholarship Program (KGSP) funded by the Ministry of Education for providing the masters research opportunity. M.M. would like to thank the Brain Pool Program (grant no. 2019H1D3A1A01101555) for postdoctoral research under the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning.
Conflicts of Interest
The authors declare that the review article was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Islam, M.S.; Hossain, M.B.; Matin, A.; Sarker, M.S. Assessment of heavy metal pollution, distribution and source apportionment in the sediment from Feni River estuary, Bangladesh. Chemosphere 2018, 202, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Sultana, S.; Habib, A.; Ullah, H.; Musa, N.; Hossain, M.B.; Rahman, M.M.; Sarker, M.S.I. Bioaccumulation of heavy metals in some commercially important fishes from a tropical river estuary suggests higher potential health risk in children than adults. PLoS ONE 2019, 14, e0219336. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Ahmed, A.S.S.; Sarker, M.S.I. Human health risks of Hg, As, Mn, and Cr through consumption of fish, Ticto barb (Puntius ticto) from a tropical river, Bangladesh. Environ. Sci. Pollut. Res. 2018, 25, 31727–31736. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World-New Tricks for an Old Dog; BoD—Books on Demand GmbH: Norderstedt, Germany, 2019; Volume 10, pp. 70–90. [Google Scholar] [CrossRef]
- Sabath, E.; Robles-Osorio, M.L. Renal health and the environment: Heavy metal nephrotoxicity. Nefrología (Engl. Ed.) 2012, 32, 279–286. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Kim, J.J.; Kim, Y.S.; Kumar, V. Heavy metal toxicity: An update of chelating therapeutic strategies. J. Trace Elem. Med. Biol. 2019, 54, 226–231. [Google Scholar] [CrossRef]
- Deore, M.S.; Keerthana, S.; Naqvi, S.; Kumar, A.; Flora, S.J.S. Alpha-Lipoic Acid Protects Co-Exposure to Lead and Zinc Oxide Nanoparticles Induced Neuro, Immuno and Male Reproductive Toxicity in Rats. Front. Pharmacol. 2021, 12, 1210. [Google Scholar] [CrossRef]
- Perrone, D.; Ardito, F.; Giannatempo, G.; Dioguardi, M.; Troiano, G.; Lo Russo, L.; De Lillo, A.; Laino, L.; Muzio, L.L. Biological and therapeutic activities, and anticancer properties of curcumin (Review). Exp. Ther. Med. 2015, 10, 1615–1623. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Lee, S.; Park, Y.; Min, T.; Bai, S.C. Evaluation of dietary selenium, vitamin C and E as the multi-antioxidants on the methylmercury intoxicated mice based on mercury bioaccumulation, antioxidant enzyme activity, lipid peroxidation and mitochondrial oxidative stress. Chemosphere 2021, 273, 129673. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. Metal Ions, Metal Chelators and Metal Chelating Assay as Antioxidant Method. Processes 2022, 10, 132. [Google Scholar] [CrossRef]
- WHO Technical Report Series. Evaluation of Certain Food Additives and Contaminants. Seventy-Fourth Report of the Joint FAO/WHO Expert Committee on Food Additives Food and Agriculture Organization of the United Nations. 2011. Available online: www.who.int (accessed on 26 October 2022).
- Cuciureanu, R.; Urzica, A.; Voitcu, M.; Antoniu, A. Assessment of daily aluminum intake by food consumption. Rev. Med. Chir. Soc. Med. Nat. Iasi 2000, 104, 107–112. Available online: https://pubmed.ncbi.nlm.nih.gov/12089908/ (accessed on 26 October 2022).
- EFSA European Food Safety Authority. Scientific Opinion on Arsenic in Food. EFSA J. 2009, 7, 1351. [Google Scholar] [CrossRef]
- EFSA European Food Safety Authority. Scientific Opinion on the public health hazards to be covered by inspection of meat (solipeds). EFSA J. 2013, 11, 3263. [Google Scholar] [CrossRef]
- EFSA European Food Safety Authority. Scientific Opinion on the risks to public health related to the presence of chromium in food and drinking water. EFSA J. 2014, 12, 3595. [Google Scholar] [CrossRef]
- Baars, A.J.; Theelen, R.M.C.; Janssen, P.J.C.M.; Meijerink, M.C.M.; Verdam, L.; Zeilmaker, M.J. Re-Evaluation of Human-Toxicological Maxi-Mum Permissible Risk Levels; Rijksinstituut voor Volksgezondheid en Milieu RIVM: Utrecht, The Netherlands, 2001.
- Yates, A.A.; Schlicker, S.A.; Suitor, C.W. Dietary Reference Intakes: The new basis for recommendations for calcium and related nutrients, B vitamins, and choline. J. Am. Diet. Assoc. 1998, 98, 699–706. [Google Scholar] [CrossRef]
- WHO Food Additives Series 18. Iron. Available online: https://inchem.org/documents/jecfa/jecmono/v18je18.htm (accessed on 26 October 2022).
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cravedi, J.-P.; Di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; Farmer, P.; et al. Scientific Opinion on Lead in Food. EFSA J. 2010, 8, 1570. [Google Scholar] [CrossRef]
- Chib, S.; Singh, S. Manganese and related neurotoxic pathways: A potential therapeutic target in neurodegenerative diseases. Neurotoxicol. Teratol. 2022, 94, 107124. [Google Scholar] [CrossRef]
- Aubrac, G.; Bastiansz, A.; Basu, N. Systematic Review and Meta-Analysis of Mercury Exposure among Populations and Environments in Contact with Electronic Waste. Int. J. Environ. Res. Public Health 2022, 19, 11843. [Google Scholar] [CrossRef]
- de Almeida, R.P.; Ferrari, R.G.; Kato, L.S.; Hauser-Davis, R.A.; Conte-Junior, C.A. A Systematic Review on Metal Dynamics and Marine Toxicity Risk Assessment Using Crustaceans as Bioindicators. Biol. Trace Elem. Res. 2022, 200, 881–903. [Google Scholar] [CrossRef]
- Johnson, L.J.; Meacham, S.L.; Kruskall, L.J. The antioxidants-vitamin C, vitamin E, selenium, and carotenoids. J. Agromedicine 2003, 9, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget 2017, 8, 66680. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Beevers, C.S.; Huang, S. Targets of curcumin. Curr. Drug Targ. 2011, 12, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Pescosolido, N.; Giannotti, R.; Plateroti, A.M.; Pascarella, A.; Nebbioso, M. Curcumin: Therapeutical potential in ophthalmology. Planta Med. 2014, 80, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: The indian solid gold. Adv. Exp. Med. Biol. 2007, 595, 1–75. [Google Scholar]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Thakaran, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (Congeners) made by man and Mother Nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef]
- Obeid, M.A.; Alsaadi, M.; Aljabali, A.A. Recent updates in curcumin delivery. J. Liposome Res. 2022, 14, 1–12. [Google Scholar] [CrossRef]
- Soleimani, V.; Sahebkar, A.; Hosseinzadeh, H. Turmeric (Curcuma longa) and its major constituent (curcumin) as nontoxic and safe substances: Review. Phyther. Res. 2018, 32, 985–995. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Al Zohairy, M.A.; Aly, S.M.; Khan, M.A. Curcumin: A Potential Candidate in Prevention of Cancer via Modulation of Molecular Pathways. Biomed. Res. Int. 2014, 2014, 761608. [Google Scholar] [CrossRef]
- Howells, L.M.; Iwuji, C.O.O.; Irving, G.R.B.; Barber, S.; Walter, H.; Sidat, Z.; Griffin-Teall, N.; Singh, R.; Foreman, N.; Patel, S.R.; et al. Curcumin combined with FOLFOX chemotherapy is safe and tolerable in patients with metastatic colorectal cancer in a randomized phase IIa trial. J. Nutr. 2019, 149, 1133–1139. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Ramirez-Tortosa, M.C.; Ramirez-Tortosa, C.L.; Mesa, M.D.; Granados, S.; Gil, Á.; Quiles, J.L. Curcumin ameliorates rabbits’s steatohepatitis via respiratory chain, oxidative stress, and TNF-α. Free Radic. Biol. Med. 2009, 47, 924–931. [Google Scholar] [CrossRef]
- Quiles, J.L.; Mesa, M.D.; Ramírez-Tortosa, C.L.; Aguilera, C.M.; Battino, M.; Gil, Á.; Ramirez-Tortosa, M.C. Curcuma longa extract supplementation reduces oxidative stress and attenuates aortic fatty streak development in rabbits. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1225–1231. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Peng, H.; Liu, R.; Ji, W.; Shi, Z.; Shen, J.; Ma, G.; Zhang, X. Targeted exosome coating gene-chem nanocomplex as “nanoscavenger” for clearing α-synuclein and immune activation of Parkinson’s disease. Sci. Adv. 2020, 6, eaba3967. [Google Scholar] [CrossRef]
- Mythri, R.B.; Bharath, M.M.S. Curcumin: A Potential Neuroprotective Agent in Parkinson’s Disease. Curr. Pharm. Des. 2012, 18, 91–99. [Google Scholar] [CrossRef]
- Patil, R.; Gangalum, P.R.; Wagner, S.; Portilla-Arias, J.; Ding, H.; Rekechenetskiy, A.; Konda, B.; Inoue, S.; Black, K.L.; Ljubimova, J.Y.; et al. Curcumin Targeted, Polymalic Acid-Based MRI Contrast Agent for the Detection of Aβ Plaques in Alzheimer’s Disease. Macromol. Biosci. 2012, 15, 1212–1217. [Google Scholar] [CrossRef]
- Liu, Z.J.; Li, Z.H.; Liu, L.; Tang, W.X.; Wang, Y.; Dong, M.R.; Xiao, C. Curcumin attenuates beta-amyloid-induced neuroinflammation via activation of peroxisome proliferator-activated receptor-gamma function in a rat model of Alzheimer’s disease. Front. Pharmacol. 2016, 7, 261. [Google Scholar] [CrossRef]
- Tejada, S.; Manayi, A.; Daglia, M.F.; Nabavi, S.; Sureda, A.; Hajheydari, Z.; Gortzi, O.; Pazoki-Toroudi, H.; Nabavi, S.M. Wound Healing Effects of Curcumin: A Short Review. Curr. Pharm. Biotechnol. 2016, 17, 1002–1007. [Google Scholar] [CrossRef]
- Cheppudira, B.; Fowler, M.; McGhee, L.; Greer, A.; Mares, A.; Petz, L.; Devore, D.; Loyd, D.R.; Clifford, J.L. Curcumin: A novel therapeutic for burn pain and wound healing. Expert Opin. Investig. Drugs 2013, 22, 1295–1303. [Google Scholar] [CrossRef]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a wound healing agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef]
- Huang, H.C.; Xu, K. Curcumin-mediated neuroprotection against amyloid-β-induced mitochondrial dysfunction involves the inhibition of GSK-3β. J. Alzheimers Dis. 2012, 32, 981–996. [Google Scholar] [CrossRef]
- Nam, S.M.; Choi, J.H.; Yoo, D.Y.; Kim, W.; Jung, H.Y.; Kim, J.W.; Yoo, M.; Lee, S.; Kim, C.J.; Yoon, Y.S.; et al. Effects of curcumin (Curcuma longa) on learning and spatial memory as well as cell proliferation and neuroblast differentiation in adult and aged mice by upregulating brain-derived neurotrophic factor and CREB signaling. J. Med. Food 2014, 17, 641–649. [Google Scholar] [CrossRef]
- Issuriya, A.; Kumarnsit, E.; Wattanapiromsakul, C.; Vongvatcharanon, U. Histological studies of neuroprotective effects of Curcuma longa Linn. on neuronal loss induced by dexamethasone treatment in the rat hippocampus. Acta Histochem. 2014, 116, 1443–1453. [Google Scholar] [CrossRef]
- Yu, S.Y.; Gao, R.; Zhang, L.; Luo, J.; Jiang, H.; Wang, S. Curcumin ameliorates ethanol-induced memory deficits and enhanced brain nitric oxide synthase activity in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 44, 210–216. [Google Scholar] [CrossRef]
- Nazari, Q.A.; Kume, T.; Izuo, N.; Takada-Takatori, Y.; Imaizumi, A.; Hashimoto, T.; Izumi, Y.; Akaike, A. Neuroprotective effects of curcumin and highly bioavailable curcumin on oxidative stress induced by sodium nitroprusside in rat striatal cell culture. Biol. Pharm. Bull. 2013, 36, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Sookram, C.; Tan, M.; Daya, R.; Heffernan, S.; Mishra, R.K. Curcumin prevents haloperidol-induced development of abnormal oro-facial movements: Possible implications of Bcl-XL in its mechanism of action. Synapse 2011, 65, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Katsidoni, V.; Alexiou, P.; Fotiadou, M.; Pelecanou, M.; Sagnou, M.; Panagis, G. Curcumin, demethoxycurcumin and bisdemethoxycurcumin differentially inhibit morphine’s rewarding effect in rats. Psychopharmacology 2014, 231, 4467–4478. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.A.; Shahed, A.; Shoskes, D.A. Modulation of apoptotic and inflammatory genes by bioflavonoids and angiotensin II inhibition in ureteral obstruction. Urology 2000, 56, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.G.; Karam, R.A. Morphological and Biochemical Features of Cerebellar Cortex After Exposure to Zinc Oxide Nanoparticles: Possible Protective Role of Curcumin. Anat. Rec. 2018, 301, 1454–1466. [Google Scholar] [CrossRef]
- Hong, J.; Bose, M.; Ju, J.; Ryu, J.H.; Chen, X.; Sang, S.; Lee, M.J.; Yang, C.S. Modulation of arachidonic acid metabolism by curcumin and related β-diketone derivatives: Effects on cytosolic phospholipase A2, cyclooxygenases and 5-lipoxygenase. Carcinogenesis 2004, 25, 1671–1679. [Google Scholar] [CrossRef]
- Tanrikulu-Küçük, S.; Basaran-Küçükgergin, C.; Sögüt, I.; Tunçdemir, M.; Dogru-Abbasoglu, S.; Seyithanoglu, M.; Kocak, H.; Oner-Iyidogan, Y. Dietary curcumin and capsaicin: Relationship with hepatic oxidative stress and apoptosis in rats fed a high fat diet. Adv. Clin. Exp. Med. 2019, 28, 1013–1020. [Google Scholar] [CrossRef]
- Jain, S.K.; Rains, J.; Jones, K. Effect of curcumin on protein glycosylation, lipid peroxidation, and oxygen radical generation in human red blood cells exposed to high glucose levels. Free Radic. Biol. Med. 2006, 41, 92–96. [Google Scholar] [CrossRef]
- Dai, J.; Gu, L.; Su, Y.; Wang, Q.; Zhao, Y.; Chen, X.; Deng, H.; Li, W.; Wang, G.; Li, K. Inhibition of curcumin on influenza A virus infection and influenzal pneumonia via oxidative stress, TLR2/4, p38/JNK MAPK and NF-κB pathways. Int. Immunopharmacol. 2018, 54, 177–187. [Google Scholar] [CrossRef]
- Gupta, A.; Kumar, A.; Naqvi, S.; Flora, S.J.S. Chronic exposure to multi-metals on testicular toxicity in rats. Toxicol. Mech. Methods 2021, 31, 53–66. [Google Scholar] [CrossRef]
- Wright, L.E.; Frye, J.B.; Gorti, B.; Timmermann, B.N.; Funk, J.L. Bioactivity of Turmeric-Derived Curcuminoids and Related Metabolites in Breast Cancer. Curr. Pharm. Des. 2013, 19, 6218–6225. [Google Scholar] [CrossRef]
- Liang, G.; Yang, S.; Zhou, H.; Shao, L.; Huang, K.; Xiao, J.; Huang, Z.; Li, X. Synthesis, crystal structure and anti-inflammatory properties of curcumin analogues. Eur. J. Med. Chem. 2009, 44, 915–919. [Google Scholar] [CrossRef]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef]
- Lee, W.H.; Loo, C.Y.; Bebawy, M.; Luk, F.; Mason, R.S.; Rohanizadeh, R. Curcumin and its Derivatives: Their Applications in Neuropharmacology and Neuroscience in the 21st Century. Curr. Neuropharmacol. 2013, 11, 338–378. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Hekmatdoost, A.; Mirmiran, P. Anti-hyperglycemic and insulin sensitizer effects of turmeric and its principle constituent curcumin. Int. J. Endocrinol. Metab. 2014, 12, e18081. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Min, T. Curcumin, curcumin nanoparticles and curcumin nanospheres: A review on their pharmacodynamics based on monogastric farm animal, poultry and fish nutrition. Pharmaceutics 2020, 12, 447. [Google Scholar] [CrossRef]
- Wahlstrom, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharm. Toxicol. 1978, 43, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Pawar, K.S.; Mastud, R.N.; Pawar, S.K.; Pawar, S.S.; Bhoite, R.R.; Bhoite, R.R.; Kulkarni, M.V.; Deshpande, A.R. Oral Curcumin With Piperine as Adjuvant Therapy for the Treatment of COVID-19: A Randomized Clinical Trial. Front. Pharmacol. 2021, 12, 669362. [Google Scholar] [CrossRef] [PubMed]
- Chiang, P.C.; Lin, S.C.; Pan, S.L.; Kuo, C.H.; Tsai, I.L.; Kuo, M.T.; Wen, W.C.; Chen, P.; Guh, J.H. Antroquinonol displays anticancer potential against human hepatocellular carcinoma cells: A crucial role of AMPK and mTOR pathways. Biochem. Pharmacol. 2010, 79, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Maiti, K.; Mukherjee, K.; Gantait, A.; Saha, B.P.; Mukherjee, P.K. Curcumin-phospholipid complex: Preparation, therapeutic evaluation and pharmacokinetic study in rats. Int. J. Pharm. 2007, 330, 155–163. [Google Scholar] [CrossRef]
- Adams, B.K.; Ferst, E.M.; Davis, M.C.; Herold, M.; Kurtkaya, S.; Camalier, R.F.; Hollingshead, M.G.; Kaur, G.; Sausville, E.A.; Rickles, F.R.; et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorganic Med. Chem. 2004, 12, 3871–3883. [Google Scholar] [CrossRef]
- Kim, J.Y.; Min, T.; Lee, S.J. Nanospheres loaded with curcumin promote gut epithelial motility through F-actin-related migration signaling events. J. Nutr. Biochem. 2021, 88, 108555. [Google Scholar] [CrossRef]
- Kumari, M.; Sharma, N.; Manchanda, R.; Gupta, N.; Syed, A.; Bahkali, A.H.; Nimesh, S. PGMD/curcumin nanoparticles for the treatment of breast cancer. Sci. Rep. 2021, 11, 3824. [Google Scholar] [CrossRef]
- Mihoub, A.B.; Acherar, S.; Frochot, C.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Synthesis of new water soluble β-cyclodextrin@curcumin conjugates and in vitro safety evaluation in primary cultures of rat cortical neurons. Int. J. Mol. Sci. 2021, 22, 3255. [Google Scholar] [CrossRef]
- Marczylo, T.H.; Verschoyle, R.D.; Cooke, D.N.; Morazzoni, P.; Steward, W.P.; Gescher, A.J. Comparison of systemic availability of curcumin with that of curcumin formulated with phosphatidylcholine. Cancer Chemother. Pharmacol. 2007, 60, 171–177. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Shah, A.; Rompicharla, S.V.K.; Ghosh, B.; Biswas, S. Cholesterol-grafted chitosan micelles as a nanocarrier system for drug-siRNA co-delivery to the lung cancer cells. Int. J. Biol. Macromol. 2018, 118, 857–863. [Google Scholar] [CrossRef]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 153–160. [Google Scholar] [CrossRef]
- Bhatia, A.; Flamer, D.; Shah, P.S.; Cohen, S.P. Transforaminal Epidural Steroid Injections for Treating Lumbosacral Radicular Pain from Herniated Intervertebral Discs: A Systematic Review and Meta-Analysis. Anesth. Analg. 2016, 122, 857–870. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; dos Santos, A.M.; Rodero, C.F.; Daflon Gremião, M.P.; Chorilli, M. Design, characterization, and biological evaluation of curcumin-loaded surfactant-based systems for topical drug delivery. Int. J. Nanomed. 2016, 11, 4553–4562. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Dende, C.; Meena, J.; Nagarajan, P.; Nagaraj, V.A.; Panda, A.K.; Padmanaban, G. Nanocurcumin is superior to native curcumin in preventing degenerative changes in Experimental Cerebral Malaria. Sci. Rep. 2017, 7, 10062. [Google Scholar] [CrossRef]
- Shaikh, J.; Ankola, D.D.; Beniwal, V.; Singh, D.; Kumar, M.N.V.R. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. Eur. J. Pharm. Sci. 2009, 37, 223–230. [Google Scholar] [CrossRef]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin—Synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef]
- Farzaei, M.H.; Zobeiri, M.; Parvizi, F.; El-Senduny, F.F.; Marmouzi, I.; Coy-Barrera, E.; Naseri, R.; Nobavi, S.M.; Rahimi, R.; Abdollahi, M. Curcumin in liver diseases: A systematic review of the cellular mechanisms of oxidative stress and clinical perspective. Nutrients 2018, 10, 855. [Google Scholar] [CrossRef]
- Sarawi, W.S.; Alhusaini, A.M.; Fadda, L.M.; Alomar, H.A.; Albaker, A.B.; Aljrboa, A.S.; Alotaibi, A.M.; Hasan, I.H.; Mahmoud, A.M. Curcumin and nano-curcumin mitigate copper neurotoxicity by modulating oxidative stress, inflammation, and akt/gsk-3β signaling. Molecules 2021, 26, 5591. [Google Scholar] [CrossRef]
- Kolev, T.M.; Velcheva, E.A.; Stamboliyska, B.A.; Spiteller, M. DFT and experimental studies of the structure and vibrational spectra of curcumin. Int. J. Quantum Chem. 2005, 102, 1069–1079. [Google Scholar] [CrossRef]
- Barik, A.; Mishra, B.; Shen, L.; Mohan, H.; Kadam, R.M.; Dutta, S.; Zhang, H.Y.; Priyadarsini, K.I. Evaluation of a new copper(II)-curcumin complex as superoxide dismutase mimic and its free radical reactions. Free Radic. Biol. Med. 2005, 39, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019, 93, 2491–2513. [Google Scholar] [CrossRef] [PubMed]
- Borsari, M.; Ferrari, E.; Grandi, R.; Saladini, M. Curcuminoids as potential new iron-chelating agents: Spectroscopic, polarographic and potentiometric study on their Fe(III) complexing ability. Inorganic. Chim. Acta 2002, 328, 61–68. [Google Scholar] [CrossRef]
- Vajragupta, O.; Boonchoong, P.; Watanabe, H.; Tohda, M.; Kummasud, N.; Sumanont, Y. Manganese complexes of curcumin and its derivatives: Evaluation for the radical scavenging ability and neuroprotective activity. Free Radic. Biol. Med. 2003, 35, 1632–1644. [Google Scholar] [CrossRef]
- Priyadarsini, K.I.; Maity, D.K.; Naik, G.H.; Kumar, M.S.; Unnikrishnan, M.K.; Satav, J.G.; Mohan, H. Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin. Free Radic. Biol. Med. 2003, 35, 475–484. [Google Scholar] [CrossRef]
- Jiao, Y.; Wilkinson, I.V.J.; Christine Pietsch, E.; Buss, J.L.; Wang, W.; Planalp, R.; Torti, F.M.; Torti, S.V. Iron chelation in the biological activity of curcumin. Free Radic. Biol. Med. 2006, 40, 1152–1160. [Google Scholar] [CrossRef]
- Zhao, X.Z.; Jiang, T.; Wang, L.; Yang, H.; Zhang, S.; Zhou, P. Interaction of curcumin with Zn(II) and Cu(II) ions based on experiment and theoretical calculation. J. Mol. Struct. 2010, 984, 316–325. [Google Scholar] [CrossRef]
- Schmitz, A.E.; De Oliveira, P.A.; De Souza, L.F.; Da Silva, D.G.H.; Danielski, S.; Santos, D.B.; de Almeira, E.A.; Prediger, R.D.; Fisher, A.; Farina, M.; et al. Interaction of curcumin with manganese may compromise metal and neurotransmitter homeostasis in the hippocampus of young mice. Biol. Trace Elem. Res. 2014, 158, 399–409. [Google Scholar] [CrossRef]
- Balasubramanian, K. Molecular orbital basis for yellow curry spice curcumin’s prevention of Alzheimer’s disease. J. Agric. Food Chem. 2006, 54, 3512–3520. [Google Scholar] [CrossRef]
- Jaruga, E.; Sokal, A.; Chrul, S.; Bartosz, G. Apoptosis-Independent Alterations in Membrane Dynamics Induced by Curcumin. Exp. Cell Res. 1998, 245, 303–312. [Google Scholar] [CrossRef]
- Rainey, N.E.; Moustapha, A.; Saric, A.; Nicolas, G.; Sureau, F.; Petit, P.X. Iron chelation by curcumin suppresses both curcumin-induced autophagy and cell death together with iron overload neoplastic transformation. Cell Death Discov. 2019, 5, 150. [Google Scholar] [CrossRef]
- Łukasz, B.; Rybakowska, I.M.; Krakowiak, A.; Anand, J.S. Skutki zdrowotne środowiskowej i zawodowej ekspozycji na glin. Med. Pr. 2020, 71, 79–88. [Google Scholar] [CrossRef]
- Lukiw, W.J.; Percy, M.E.; Kruck, T.P. Nanomolar aluminum induces pro-inflammatory and pro-apoptotic gene expression in human brain cells in primary culture. J. Inorg. Biochem. 2005, 99, 1895–1898. [Google Scholar] [CrossRef]
- Banasik, A.; Lankoff, A.; Piskulak, A.; Adamowska, K.; Lisowska, H.; Wojcik, A. Aluminum-induced micronuclei and apoptosis in human peripheral-blood lymphocytes treated during different phases of the cell cycle. Environ. Toxicol. 2005, 20, 402–406. [Google Scholar] [CrossRef]
- Walton, J.R. An aluminum-based rat model for Alzheimer’s disease exhibits oxidative damage, inhibition of PP2A activity, hyperphosphorylated tau, and granulovacuolar degeneration. J. Inorg. Biochem. 2007, 101, 1275–1284. [Google Scholar] [CrossRef]
- Drago, D.; Bettella, M.; Bolognin, S.; Cendron, L.; Scancar, J.; Milacic, R.; Ricchelli, F.; Casini, A.; Messori, L.; Tognon, G.; et al. Potential pathogenic role of β-amyloid1-42-aluminum complex in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2008, 40, 731–746. [Google Scholar] [CrossRef]
- Sood, P.K.; Nahar, U.; Nehru, B. Stress proteins and glial cell functions during chronic aluminium exposures: Protective role of curcumin. Neurochem. Res. 2012, 37, 639–646. [Google Scholar] [CrossRef]
- Cherny, R.A.; Barnham, K.J.; Lynch, T.; Volitakis, I.; Li, Q.X.; McLean, C.A.; Multhaup, G.; Beyreuther, K.; Tanzi, R.E.; Masters, C.L.; et al. Chelation and intercalation: Complementary properties in a compound for the treatment of Alzheimer’s disease. J. Struct. Biol. 2000, 130, 209–216. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, L.; Zhang, S.; Sun, P.C.; Ding, C.F.; Chu, Y.Q.; Zhou, P. Interaction of curcumin with Al(III) and its complex strctures based on experiments and theoretical calculations. J. Mol. Struct. 2011, 1004, 163–173. [Google Scholar] [CrossRef]
- Ahmadi, F.; Alizadeh, A.A.; Shahabadi, N.; Rahimi-Nasrabadi, M. Study binding of Al-curcumin complex to ds-DNA, monitoring by multispectroscopic and voltammetric techniques. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1466–1474. [Google Scholar] [CrossRef]
- Kumar, A.; Dogra, S.; Prakash, A. Protective effect of curcumin (Curcuma longa), against aluminium toxicity: Possible behavioral and biochemical alterations in rats. Behav. Brain Res. 2009, 205, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, W.; Malhotra, A. Efficacy of Curcumin in Ameliorating Aluminum- Induced Neurotoxicity. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.M.; Elaal, F.Z.A.; Sahar, Z. Effect of Curcumin and Nano-curcumin on Reduce Aluminum Toxicity in Rats. Int. J. Food Sci. Biotechnol. 2019, 4, 64–73. [Google Scholar] [CrossRef]
- Sharma, D.; Sethi, P.; Hussain, E.; Singh, R. Curcumin counteracts the aluminium-induced ageing-related alterations in oxidative stress, Na+, K+ ATPase and protein kinase C in adult and old rat brain regions. Biogerontology 2009, 10, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.Y.; Yu, S.D.; Hong, Y.S. Environmental source of arsenic exposure. J. Prev. Med. Public Health 2014, 47, 253–257. [Google Scholar] [CrossRef]
- Zhang, A.L.; Tang, S.; Fang, Y.; Li, C.Z.; Ding, X.J.; Zhao, H.; Wang, J.H.; Yang, G.H.; Li, J. Histone demethylase JHDM2A regulates H3K9 dimethylation in response to arsenic-induced DNA damage and repair in normal human liver cells. J. Appl. Toxicol. 2020, 40, 1661–1672. [Google Scholar] [CrossRef]
- Fatoki, J.O.; Badmus, J.A. Arsenic as an environmental and human health antagonist: A review of its toxicity and disease initiation. J. Hazard. Mater. Adv. 2022, 5, 100052. [Google Scholar] [CrossRef]
- Biswas, J.; Sinha, D.; Mukherjee, S.; Roy, S.; Siddiqi, M.; Roy, M. Curcumin protects DNA damage in a chronically arsenic-exposed population of West Bengal. Hum. Exp. Toxicol. 2010, 29, 513–524. [Google Scholar] [CrossRef]
- Roy, M.; Sinha, D.; Mukherjee, S.; Biswas, J. Curcumin prevents DNA damage and enhances the repair potential in a chronically arsenic-exposed human population in West Bengal, India. Eur. J. Cancer Prev. 2011, 20, 123–131. [Google Scholar] [CrossRef]
- Yousef, M.I.; El-Demerdash, F.M.; Radwan, F.M.E. Sodium arsenite induced biochemical perturbations in rats: Ameliorating effect of curcumin. Food Chem. Toxicol. 2008, 46, 3506–3511. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Banik, S.; Akter, M.; Rahman, M.M.; Sikder, M.T.; Hosokawa, T.; Saito, T.; Kurasaki, M. Curcumin alleviates arsenic-induced toxicity in PC12 cells via modulating autophagy/apoptosis. Ecotoxicol. Environ. Saf. 2020, 200, 110756. [Google Scholar] [CrossRef]
- Jamal, Z.; Das, J.; Gupta, P.; Dhar, P.; Chattopadhyay, S.; Chatterji, U. Self Nano-Emulsifying Curcumin (SNEC30) attenuates arsenic-induced cell death in mice. Toxicol. Rep. 2021, 8, 1428–1436. [Google Scholar] [CrossRef]
- Ensibi, C.; Daly, Y.M.N. Toxicity assessment of cadmium chloride on planktonic copepods Centropages ponticus using biochemical markers. Toxicol. Rep. 2017, 4, 83–88. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, B.M.; Kim, H.S. Potential protective roles of curcumin against cadmium-induced toxicity and oxidative stress. J. Toxicol. Environ. Health B Crit. Rev. 2021, 24, 95–118. [Google Scholar] [CrossRef]
- Mohajeri, M.; Rezaee, M.; Sahebkar, A. Cadmium-induced toxicity is rescued by curcumin: A review. Biofactors 2017, 43, 645–661. [Google Scholar] [CrossRef]
- Qayoom, A.; Kazmi, S.; Nadir, A.S. Turmeric Powder as a Natural Heavy Metal Chelating Agent: Surface Characterisation. Pak. J. Sci. Ind. Res. Ser. A Phys. Sci. 2017, 60, 1–8. [Google Scholar] [CrossRef]
- Mehmood, S.; Saeed, D.A.; Rizwan, M.; Khan, M.N.; Aziz, O.; Bashir, S.; Ibrahim, M.; Ditta, A.; Akmal, M.; Mumtaz, M.A.; et al. Impact of different amendments on biochemical responses of sesame (Sesamum indicum L.) plants grown in lead-cadmium contaminated soil. Plant Physiol. Biochem. 2018, 132, 345–355. [Google Scholar] [CrossRef]
- Eybl, V.; Kotyzova, D.; Koutensky, J. Comparative study of natural antioxidants—Curcumin, resveratrol and melatonin–In cadmium-induced oxidative damage in mice. Toxicology 2006, 225, 150–156. [Google Scholar] [CrossRef]
- Alghasham, A.; Salem, T.A.; Meki, A.R.M. Effect of cadmium-polluted water on plasma levels of tumor necrosis factor-α, interleukin-6 and oxidative status biomarkers in rats: Protective effect of curcumin. Food Chem. Toxicol. 2013, 59, 160–164. [Google Scholar] [CrossRef]
- Rennolds, J.; Malireddy, S.; Hassan, F.; Tridandapani, S.; Parinandi, N.; Boyaka, P.N.; Cormet-Boyaka, E. Curcumin regulates airway epithelial cell cytokine responses to the pollutant cadmium. Biochem. Biophys. Res. Commun. 2012, 417, 256–261. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Khalil, A.A.; Abd-Elhakim, Y.M.; Badr, H.A. The potential role of turmeric and black pepper powder diet supplements in reversing cadmium-induced growth retardation, ATP depletion, hepatorenal damage, and testicular toxicity in Clarias gariepinus. Aquaculture 2019, 510, 109–121. [Google Scholar] [CrossRef]
- Deevika, B.; Asha, S.; Taju, G.; Nalini, T. Cadmium acetate induced nephrotoxicity and protective role of curcumin in rats. Asian J. Pharm. Clin. Res. 2012, 5, 186–188. [Google Scholar]
- Aktas, C.; Kanter, M.; Erboga, M.; Ozturk, S. Anti-apoptotic effects of curcumin on cadmium-induced apoptosis in rat testes. Toxicol. Ind. Health 2012, 28, 122–130. [Google Scholar] [CrossRef]
- Oguzturk, H.; Ciftci, O.; Aydin, M.; Timurkaan, N.; Beytur, A.; Yilmaz, F. Ameliorative effects of curcumin against acute cadmium toxicity on male reproductive system in rats. Andrologia 2012, 44, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzová, D.; Bludovská, M. The effect of curcumin on cadmium-induced oxidative damage and trace elements level in the liver of rats and mice. Toxicol. Lett. 2004, 151, 79–85. [Google Scholar] [CrossRef]
- Tarasub, N.; Junseecha, T.; Tarasub, C.; Ayutthaya, W.D.N. Protective Effects of Curcumin, Vitamin C, or their Combination on Cadmium-Induced Hepatotoxicity. J. Basic Clin. Pharm. 2012, 3, 273. [Google Scholar] [CrossRef]
- Kukongviriyapan, U.; Pannangpetch, P.; Kukongviriyapan, V.; Donpunha, W.; Sompamit, K.; Surawattanawan, P. Curcumin protects against cadmium-induced vascular dysfunction, hypertension and tissue cadmium accumulation in mice. Nutrients 2014, 6, 1194–1208. [Google Scholar] [CrossRef]
- Daniel, S.; Limson, J.L.; Dairam, A.; Watkins, G.M.; Daya, S. Through metal binding, curcumin protects against lead- and cadmium-induced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. J. Inorg. Biochem. 2004, 98, 266–275. [Google Scholar] [CrossRef]
- Brewer, G.J. Alzheimer’s disease causation by copper toxicity and treatment with zinc. Front. Aging Neurosci. 2014, 6, 92. [Google Scholar] [CrossRef]
- Montes, S.; Rivera-Mancia, S.; Diaz-Ruiz, A.; Tristan-Lopez, L.; Rios, C. Copper and copper proteins in Parkinson’s disease. Oxid. Med. Cell. Longev. 2014, 2014, 147251. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef]
- Bourassa, M.W.; Brown, H.H.; Borchelt, D.R.; Vogt, S.; Miller, L.M. Metal-deficient aggregates and diminished copper found in cells expressing SOD1 mutations that cause ALS. Front. Aging Neurosci. 2014, 6, 110. [Google Scholar] [CrossRef]
- Tegoni, M.; Valensin, D.; Toso, L.; Remelli, M. Copper chelators: Chemical properties and bio-medical applications. Curr. Med. Chem. 2014, 21, 3785–3818. [Google Scholar] [CrossRef]
- Balasubramanian, K. Quantum chemical insights into Alzheimer’s disease: Curcumin’s chelation with Cu(II), Zn(II), and Pd(II) as a mechanism for its prevention. Int. J. Quantum Chem. 2016, 116, 1107–1119. [Google Scholar] [CrossRef]
- Elkhateeb, S.A.; Ibrahim, T.R.; El-Shal, A.S.; Hamid, O.I.A. Ameliorative role of curcumin on copper oxide nanoparticles-mediated renal toxicity in rats: An investigation of molecular mechanisms. J. Biochem. Mol. Toxicol. 2020, 34, e22593. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, H.Y.; Ji, H.F. A theoretical study on Cu(II)-chelating properties of curcumin and its implications for curcumin as a multipotent agent to combat Alzheimer’s disease. J. Mol. Struct. Theochem. 2005, 757, 199–202. [Google Scholar] [CrossRef]
- Abolaji, A.O.; Fasae, K.D.; Iwezor, C.E.; Aschner, M.; Farombi, E.O. Curcumin attenuates copper-induced oxidative stress and neurotoxicity in Drosophila melanogaster. Toxicol. Rep. 2020, 7, 261–268. [Google Scholar] [CrossRef]
- Hashish, E.A.; Elgaml, S.A. Hepatoprotective and Nephroprotective Effect of Curcumin Against Copper Toxicity in Rats. Indian J. Clin. Biochem. 2016, 31, 270–277. [Google Scholar] [CrossRef]
- Baum, L.; Ng, A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J. Alzheimers Dis. 2004, 6, 367–377. [Google Scholar] [CrossRef]
- Silva, B.; Faustino, P. An overview of molecular basis of iron metabolism regulation and the associated pathologies. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1347–1359. [Google Scholar] [CrossRef]
- Moinipour, N.; Barati, M.; Sahebkar, A.; Iranshahy, M.; Shakeri, A. Protective Effects of Curcumin against Iron-induced Toxicity. Curr. Pharm. Biotechnol. 2021, 23, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Kose, T.; Vera-Aviles, M.; Sharp, P.A.; Latunde-Dada, G.O. Curcumin and (−)-epigallocatechin-3-gallate protect murine MIN6 pancreatic beta-cells against iron toxicity and erastin-induced ferroptosis. Pharmaceuticals 2019, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, H.; Srinivasan, K. Protective effect of dietary curcumin and capsaicin on induced oxidation of low-density lipoprotein, iron-induced hepatotoxicity and carrageenan-induced inflammation in experimental rats. FEBS J. 2006, 273, 4528–4537. [Google Scholar] [CrossRef]
- Messner, D.J.; Sivam, G.; Kowdley, K.V. Curcumin reduces the toxic effects of iron loading in rat liver epithelial cells. Liver Int. 2009, 29, 63–72. [Google Scholar] [CrossRef]
- Wani, A.L.; Ara, A.; Usmani, J.A. Lead toxicity: A review. Interdiscip. Toxicol. 2015, 8, 55–64. [Google Scholar] [CrossRef]
- Patra, R.C.; Rautray, A.K.; Swarup, D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Int. 2011, 2011, 457327. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C. Lipid peroxidation initiated by superoxide-dependent hydroxyl radicals using complexed iron and hydrogen peroxide. FEBS Lett. 1984, 172, 245–249. [Google Scholar] [CrossRef]
- Lee, J.W.; Choi, H.; Hwang, U.K.; Kang, J.C.; Kang, Y.J.; Kim, K.I.; Kim, J.H. Toxic effects of lead exposure on bioaccumulation, oxidative stress, neurotoxicity, and immune responses in fish: A review. Environ. Toxicol. Pharmacol. 2019, 68, 101–108. [Google Scholar] [CrossRef]
- Giri, S.S.; Yun, S.; Jun, J.W.; Kim, H.J.; Kim, S.G.; Kang, J.W.; Kim, S.W.; Han, S.J.; Sukumaran, V.; Park, S.C. Therapeutic effect of intestinal autochthonous Lactobacillus reuteri P16 against waterborne lead toxicity in Cyprinus carpio. Front. Immunol. 2018, 9, 1824. [Google Scholar] [CrossRef]
- Giri, S.S.; Kim, M.J.; Kim, S.G.; Kim, S.W.; Kang, J.W.; Kwon, J.; Lee, S.B.; Jung, W.J.; Sukumaran, V.; Park, S.C. Role of dietary curcumin against waterborne lead toxicity in common carp Cyprinus carpio. Ecotoxicol. Environ. Saf. 2021, 219, 112318. [Google Scholar] [CrossRef]
- Abubakar, K.; Mailafiya, M.M.; Danmaigoro, A.; Chiroma, S.M.; Rahim, E.B.A.; Zakaria, M.Z.A.B. Curcumin attenuates lead-induced cerebellar toxicity in rats via chelating activity and inhibition of oxidative stress. Biomolecules 2019, 9, 453. [Google Scholar] [CrossRef] [PubMed]
- Abubakar, K.; Mailafiya, M.M.; Chiroma, S.M.; Danmaigoro, A.; Zyoud, T.Y.T.; Rahim, E.A.; Zakaria, M.Z.A.B. Ameliorative effect of curcumin on lead-induced hematological and hepatorenal toxicity in a rat model. J. Biochem. Mol. Toxicol. 2020, 34, e22483. [Google Scholar] [CrossRef]
- Alhusaini, A.; Fadda, L.; Hasan, I.H.; Zakaria, E.; Alenazi, A.M.; Mahmoud, A.M. Curcumin ameliorates lead-induced hepatotoxicity by suppressing oxidative stress and inflammation, and modulating akt/gsk-3β signaling pathway. Biomolecules 2019, 9, 703. [Google Scholar] [CrossRef]
- Dairam, A.; Limson, J.L.; Watkins, G.M.; Antunes, E.; Daya, S. Curcuminoids, curcumin, and demethoxycurcumin reduce lead-induced memory deficits in male wistar rats. J. Agric. Food Chem. 2007, 55, 1039–1044. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Romeo, J.; Malavolta, M.; Costarelli, L.; Giacconi, R.; Diaz, L.E.; Marcos, A. Zinc: Dietary intake and impact of supplementation on immune function in elderly. Age 2013, 35, 839–860. [Google Scholar] [CrossRef]
- Keerthana, S.; Kumar, A. Potential risks and benefits of zinc oxide nanoparticles: A systematic review. Crit. Rev. Toxicol. 2020, 50, 47–71. [Google Scholar] [CrossRef]
- Mary, C.P.V.; Vijayakumar, S.; Shankar, R. Metal chelating ability and antioxidant properties of Curcumin-metal complexes—A DFT approach. J. Mol. Graph. Model. 2018, 79, 1–14. [Google Scholar] [CrossRef]
- Heidai-Moghadam, A.; Khorsandi, L.; Jozi, Z. Curcumin attenuates nephrotoxicity induced by zinc oxide nanoparticles in rats. Environ. Sci. Pollut. Res. 2019, 26, 179–187. [Google Scholar] [CrossRef]
- Khorsandi, L.; Mansouri, E.; Orazizadeh, M.; Jozi, Z. Curcumin attenuates hepatotoxicity induced by zinc oxide nanoparticles in rats. Balk. Med. J. 2016, 33, 252–257. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Lee, J.H.; Lee, J.H.; Won, S.H.; Damusaru, J.H.; Bai, S.C. Interactive effect of dietary vitamin E and inorganic mercury on growth performance and bioaccumulation of mercury in juvenile olive flounder, Paralichthys olivaceus treated with mercuric chloride. Anim. Nutr. 2017, 3, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Berlin, M. Mercury in dental amalgam: A risk analysis. Neurotoxicology 2020, 81, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Antonyak, H.; Polishchuk, A.; Semenova, Y.; Lesiv, M.; Lysiuk, R.; Peana, M. Effect of methylmercury on fetal neurobehavioral development: An overview of the possible mechanisms of toxicity and the neuroprotective effect of phytochemicals. Arch. Toxicol. 2022, 96, 3175–3199. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.C.; Zalups, R.K. The aging kidney and the nephrotoxic effects of mercury. J. Toxicol. Environ. Health B Crit. Rev. 2017, 20, 55–80. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Goel, S.K.; Behari, J.R. Detoxification and antioxidant effects of curcumin in rats experimentally exposed to mercury. J. Appl. Toxicol. 2010, 30, 457–468. [Google Scholar] [CrossRef]
- Zhao, G.; Qi, L.; Wang, Y.; Li, X.; Li, Q.; Tang, X.; Wang, X.; Wu, C. Antagonizing effects of curcumin against mercury-induced autophagic death and trace elements disorder by regulating PI3K/AKT and Nrf2 pathway in the spleen. Ecotoxicol. Environ. Saf. 2021, 222, 112529. [Google Scholar] [CrossRef]
- Abu-Taweel, G.M. Neurobehavioral protective properties of curcumin against the mercury chloride treated mice offspring. Saudi J. Biol. Sci. 2019, 26, 736–743. [Google Scholar] [CrossRef]
- Beckett, G.J.; Arthur, J.R. Selenium and endocrine systems. J. Endocrinol. 2005, 184, 455–465. [Google Scholar] [CrossRef]
- Solovyev, N.D. Importance of selenium and selenoprotein for brain function: From antioxidant protection to neuronal signalling. J. Inorg. Biochem. 2015, 153, 1–12. [Google Scholar] [CrossRef]
- Sharma, V.K.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.K.; Pettine, M.; Zboril, R. Assessment of toxicity of selenium and cadmium selenium quantum dots: A review. Chemosphere 2017, 188, 403–413. [Google Scholar] [CrossRef]
- Sun, H.J.; Rathinasabapathi, B.; Wu, B.; Luo, J.; Pu, L.P.; Ma, L.Q. Arsenic and selenium toxicity and their interactive effects in humans. Environ. Int. 2014, 69, 148–158. [Google Scholar] [CrossRef]
- Manikandan, R.; Thiagarajan, R.; Beulaja, S.; Sudhandiran, G.; Arumugam, M. Curcumin protects against hepatic and renal injuries mediated by inducible nitric oxide synthase during selenium-induced toxicity in Wistar rats. Microsc. Res. Tech. 2010, 73, 631–637. [Google Scholar] [CrossRef]
- Manikandan, R.; Thiagarajan, R.; Beulaja, S.; Chindhu, S.; Mariammal, K.; Sudhandiran, G.; Arumugam, M. Anti-cataractogenic effect of curcumin and aminoguanidine against selenium-induced oxidative stress in the eye lens of Wistar rat pups: An in vitro study using isolated lens. Chem. Biol. Interact. 2009, 181, 202–209. [Google Scholar] [CrossRef]
- Manikandan, R.; Thiagarajan, R.; Beulaja, S.; Sudhandiran, G.; Arumugam, M. Effect of curcumin on selenite-induced cataractogenesis in Wistar rat pups. Curr. Eye Res. 2010, 35, 122–129. [Google Scholar] [CrossRef]
- Thompson, C.M.; Aardema, M.J.; Heintz, M.M.; MacGregor, J.T.; Young, R.R. A review of mammalian in vivo genotoxicity of hexavalent chromium: Implications for oral carcinogenicity risk assessment. Crit. Rev. Toxicol. 2021, 51, 820–849. [Google Scholar] [CrossRef]
- Thompson, C.M.; Bichteler, A.; Rager, J.E.; Suh, M.; Proctor, D.M.; Haws, L.C.; Harris, M.A. Comparison of in vivo genotoxic and carcinogenic potency to augment mode of action analysis: Case study with hexavalent chromium. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 800, 28–34. [Google Scholar] [CrossRef]
- Chandra, A.K.; Chatterjee, A.; Ghosh, R.; Sarkar, M. Effect of curcumin on chromium-induced oxidative damage in male reproductive system. Environ. Toxicol. Pharmacol. 2007, 24, 160–166. [Google Scholar] [CrossRef]
- Molina-Jijón, E.; Tapia, E.; Zazueta, C.; El Hafidi, M.; Zatarain-Barrón, Z.L.; Hernández-Pando, R.; Medina-Campos, O.N.; Zarco-Marquez, G.; Torres, I.; Pedraza-Chaverri, J. Curcumin prevents Cr(VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radic. Biol. Med. 2011, 51, 1543–1557. [Google Scholar] [CrossRef]
- Srivastava, A.; Agarwal, R.; Chaturvedi, T.P.; Chandra, A.; Singh, O.P. Clinical evaluation of the role of tulsi and turmeric in the management of oral submucous fibrosis: A pilot, prospective observational study. J. Ayurveda Integr. Med. 2015, 6, 45–49. [Google Scholar] [CrossRef]
- Pawar, R.S.; Toppo, F.A.; Mandloi, A.S.; Shaikh, S. Exploring the role of curcumin containing ethanolic extract obtained from Curcuma longa (rhizomes) against retardation of wound healing process by aspirin. Indian J. Pharmacol. 2015, 47, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Thavorn, K.; Mamdani, M.M.; Straus, S.E. Efficacy of turmeric in the treatment of digestive disorders: A systematic review and meta-analysis protocol. Syst. Rev. 2014, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.C.; Gao, X.; Dulchavsky, S. Immunomodulation by curcumin. Adv. Exp. Med. Biol. 2007, 595, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Gilani, A.H.; Shah, A.J.; Ghayur, M.N.; Majeed, K. Pharmacological basis for the use of turmeric in gastrointestinal and respiratory disorders. Life Sci. 2005, 76, 3089–3105. [Google Scholar] [CrossRef]
- Rajasekaran, S.A. Therapeutic potential of curcumin in gastrointestinal diseases. World J. Gastrointest. Pathophysiol. 2011, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- Dulbecco, P.; Savarino, V. Therapeutic potential of curcumin in digestive diseases. World J. Gastroenterol. 2013, 19, 9256–9270. [Google Scholar] [CrossRef]
- Kim, D.C.; Kim, S.H.; Choi, B.H.; Baek, N.I.; Kim, D.; Kim, M.J.; Kim, K.T. Curcuma longa extract protects against gastric ulcers by blocking H2 histamine receptors. Biol. Pharm. Bull. 2005, 28, 2220–2224. [Google Scholar] [CrossRef]
- Yadav, S.; Sah, A.K.; Jha, R.; Sah, P.; Shah, D. Turmeric (curcumin) remedies gastroprotective action. Pharmacogn. Rev. 2013, 7, 42–46. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. Gastroprotective activity of essential oils from turmeric and ginger. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 95–103. [Google Scholar] [CrossRef]
- Koosirirat, C.; Linpisarn, S.; Changsom, D.; Chawansuntati, K.; Wipasa, J. Investigation of the anti-inflammatory effect of Curcuma longa in Helicobacter pylori-infected patients. Int. Immunopharmacol. 2010, 10, 815–818. [Google Scholar] [CrossRef]
- Huang, X.; Lv, B.; Zhang, S.; Dai, Q.; Chen, B.B.; Meng, L.N. Effects of radix curcumae-derived diterpenoid C on Helicobacter pylori-induced inflammation and nuclear factor kappa B signal pathways. World J. Gastroenterol. 2013, 19, 5085–5093. [Google Scholar] [CrossRef]
- Salomon, N.; Lang, A.; Gamus, D. Curcumin add-on therapy for ulcerative colitis. Harefuah 2015, 154, 56–58. [Google Scholar] [PubMed]
- Nwozo, S.O.; Osunmadewa, D.A.; Oyinloye, B.E. Anti-fatty liver effects of oils from Zingiber officinale and Curcuma longa on ethanol-induced fatty liver in rats. J. Integr. Med. 2014, 12, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Um, M.Y.; Hwang, K.H.; Ahn, J.; Ha, T.Y. Curcumin Attenuates Diet-Induced Hepatic Steatosis byActivating AMP-Activated Protein Kinase. Basic Clin. Pharm. Tox. 2013, 113, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, H.G.; Oh, J.H.; Lee, Y.J. Dramatic increase in hepatic and biliary curcumin exposure by modulation of its elimination pathway in rats. J. Pharm. Pharmacol. 2013, 65, 423–429. [Google Scholar] [CrossRef]
- Desai, K.R.; Rajput, D.K.; Patel, P.B.; Highland, H.N. Ameliorative effects of curcumin on artesunate-Induced subchronic toxicity in testis of swiss albino male mice. Dose-Response 2015, 13, 1559325815592393. [Google Scholar] [CrossRef]
- Beyene, A.M.; Moniruzzaman, M.; Karthikeyan, A.; Min, T. Curcumin nanoformulations with metal oxide nanomaterials for biomedical applications. Nanomaterials 2021, 11, 460. [Google Scholar] [CrossRef]
- Liu, G.; Garrett, M.R.; Men, P.; Zhu, X.; Perry, G.; Smith, M.A. Nanoparticle and other metal chelation therapeutics in Alzheimer disease. Biochim. Biophys. Acta Mol. Basis Dis. 2005, 1741, 246–252. [Google Scholar] [CrossRef]
- Manolova, Y.; Deneva, V.; Antonov, L.; Drakalska, E.; Momekova, D.; Lambov, N. The effect of the water on the curcumin tautomerism: A quantitative approach. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 132, 815–820. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).